Abstract
The mitochondrial quality control system is crucial in maintaining cellular homeostasis during environmental stress. Granulosa cells are the main cells secreting steroid hormones, and mitochondria are the key organelles for steroid hormone synthesis. The impact of the mitochondrial quality control system on granulosa cells’ steroid hormone synthesis and survival under heat stress is still unclear. Here, we showed that acute heat stress induces mitochondrial damage and significantly increases the number of mitophagy-like vesicles in the cytoplasm of duck ovary granulosa cells at the ultra-structural level. Meanwhile, we also found heat stress significantly increased mitochondrial fission and mitophagy-related protein expression levels both in vivo and in vitro. Furthermore, by confocal fluorescence analysis, we discovered that LC3 was distributed spot-like manner near the nucleus in the heat treatment group, and the LC3 spots and lysosomes were colocalized with Mito-Tracker in the heat treatment group. We further detected the mitophagy-related protein in the cytoplasm and mitochondria, respectively. Results showed that the PINK1 protein was significantly increased both in cytoplasm and mitochondria, while the LC3-Ⅱ/LC3-Ⅰ ratio increase only occurred in mitochondrial. In addition, the autophagy protein induced by acute heat treatment was effectively inhibited by the mitophagy inhibitor CysA. Finally, we demonstrated that the alteration of cellular mitophagy by siRNA interference with Drp1 and PINK1 inhibited the steroid synthesis of granulosa cells and increased cell apoptosis. Study provides strong evidence that the Drp1 regulated PINK1-dependent mitophagy pathway protects follicular granulosa cells from acute heat stress-induced injury.
Key words: heat stress, mitophagy, granulosa cells, PINK1, Drp1
INTRODUCTION
Due to global warming, heat stress is one of the most significant environmental issues confronting the global poultry industry (Rostagno, 2020). Laying ducks, which are now kept in cages mostly, are vulnerable to heat stress during summer's high temperatures due to lack of water baths (Ma et al., 2014). It has been extensively demonstrated that heat stress reduced the feeding rate, laying rate, egg quality, and hatching rate of poultry (Goel, 2021). The response of the poultry reproductive system to a heat stress situation is complex and intricate in nature. Heat stress reduced the weight of the ovary and fallopian tube, decreased the number and weight of graded follicles, caused apoptosis of granulosa cells and oxidative stress (Pu et al., 2019; Wang et al., 2019; Xing et al., 2019). Surrounding yolk and oocyte, Granulosa cells are the main secreting cells of steroid hormones, which coordinate the follicular growth. Granulosa cells express steroid hormone synthase such as steroidogenic acute regulatory protein (StAR), 3 beta-hydroxysteroid dehydrogenase (3β-HSD), cytochrome P450 family 11 subfamily A member 1 (CYP11A1), cytochrome P450 family 19 subfamily A member 1 (CYP19A1), etc., thus are the primary secreting cells of progesterone and estradiol in follicles (Zhu et al., 2019). Study indicated that heat stress decreases the expression of steroid hormone synthase genes and inhibit the synthesis and secretion of estradiol and progesterone in granulosa cells (Li et al., 2016; Yang et al., 2021). The underlying regulatory mechanisms of the granulosa cells’ steroid hormones synthesis and survival during heat stress are still not fully understood.
Heat stress altered the granulosa cell cycle, inhibited cell proliferation (Khan et al., 2020), and increased apoptotic genes and proteins, which caused granulosa cells to undergo apoptosis (Luo et al., 2016; Abdelnour et al., 2020). Meanwhile, heat stress led to oxidative stress, which include the accumulation of reactive oxygen species (ROS), DNA damage, and an increase in oxide levels in granulosa cells (Wang et al., 2019; Ho et al., 2021). Mitochondria are key players in cellular metabolism, as well as play important physiological roles in activating oxidative stress and endoplasmic reticulum stress, regulating intrinsic pathways of apoptosis, and participating in the cell cycle (Kim et al., 2016). Significantly, steroid hormone production is also primarily regulated by mitochondria. The first step in the biosynthesis of steroid hormones is the transfer of cholesterol to the mitochondrial outer membrane, which is facilitated by StAR. Then, CYP11A1 initiates steroid genesis by converting cholesterol to pregnenolone at the mitochondrial inner membrane, and the enzyme 3β-HSD binds with CYP11A1 to form a complex inserted into the mitochondrial inner membrane of the mitochondria to synthesize progesterone (Sreerangaraja Urs et al., 2020).
Mitochondria maintain cell homeostasis through a quality control system such as mitochondrial fusion, fission and mitophagy. Mitochondrial quality control has been recognized to play an important role in mitochondrial and cellular health and function. Mitophagy, an autodigestive process that degrades cellular dysfunction mitochondrial, removes ROS, repairs DNA, and folds or degrade proteins, plays an important role in maintaining cellular homeostasis during environmental stress (Scheibye-Knudsen et al., 2015). PTEN Induced Putative Kinase 1 (PINK1)/Parkin pathway is one of the main mitophagy regulation pathways targeting the elimination of damaged mitochondria (Tanaka, 2020). PINK1 enters the inner membrane of healthy mitochondria, where it is cleaved by proteases and eventually degraded (Greene et al., 2012). Damaged mitochondria accumulate PINK1 on the outer membrane were dependent on kinase activity, it recruits and activates Parkin, mitochondrial outer membrane underwent a series of protein ubiquitination, which is introduced into autophagosomes by binding to Microtubule Associated Protein 1 Light Chain 3 (LC3) to induce mitophagy, potentially maintaining organelle fidelity (Geisler et al., 2010; Lazarou et al., 2012). Previous study shows that heat stress impairs mitochondria functions and induces oxidative injury in poultry skeletal muscles (Huang et al., 2015). However, no study has shown the role of mitochondrial quality control system in granulosa cells under heat stress situations.
In the present study, we reveal the relationship between mitophagy and acute heat stress in granulosa cells. By detecting the formation of autophagic vesicle and the expression of key mitophagy molecules, we provide the first evidence that acute heat stress induces mitophagy in granulosa cells both in vivo and in vitro. Moreover, we explored the effect of the mitophagy machinery on granulosa cells steroid hormones synthesis and apoptosis under heat stress situations by disturbing the mitophagy pathway with small interfering RNAs (siRNAs).
MATERIALS AND METHODS
Ethics Approval and Consent to Participate
The experimental protocol was established, according to the Ministry of Agriculture and Food requirements for the Care and Use of Animals for Scientific Purposes. Animal study was approved by the Zhong-kai University of Agriculture and Engineering (ZHKU, China) Animal Care and Ethics Committee (NO. 2020081011).
Animals and Experimental Design
A total of 60 female Shan Ma ducks, aged 60 d, were bred in Zhong-kai Education Scientific Research Base. The ducks were fed with a standard diet and water ad libitum. Animals were kept at 24°C ± 2°C in an environment- tally controlled room. After 15 d, the animals were placed in an artificial climate room at 39°C (without food and water). Twelve ducks of each group were sacrificed using carbon dioxide for sampling at 0, 30, and 60 min, respectively. Ovary samples were carefully dissected, collected, and stored at −80°C for use in subsequent experiments.
Isolation of the Follicular Granulosa Cells and Culture
Follicular granulosa cells were isolated from hierarchical follicles (≥9 mm in diameter) of laying ducks (120–200 d) bred in Zhong-kai Education Scientific Research Base. The layer ducks were sacrificed using carbon dioxide. The granulosa cells were separated from the follicular theca and washed in cold PBS. About 0.2% Collagenase II (Biosharp, BS164) was added for digestion of the cells (37°C for 15 min), then M199 medium (Thermo Scientific, C11150500BT) containing 10% FBS (Thermo Scientific, 10270106) was added to terminate the digestion and the solution was filtered using a 70-μm sieve. Following centrifugation, the granulosa cells were washed twice with a serum-free medium, and then suspended in M199 medium containing 10% FBS, 1% penicillin-streptomycin (Thermo Scientific, 15140122). The granulosa cells were evenly spread in a 6-well plate at a density of 8 × 105 cells/mL and placed in an incubator (37°C, 5% CO2, saturated humidity).
Heat Treatment of Granulosa Cells Cultured In Vitro
Because the anal temperature of laying ducks raised to 43.3°C under heat stress (Zhu et al., 2014). Granulosa cells were placed in an incubator at 43°C for different heat treatments (0 min, 60 min, 90 min, 120 min, and 150 min). After heat treatments, granulosa cells were collected immediately for Western blot analysis and ROS measurement.
Transmission Electron Microscopy
Ovary was fixed with 2.5% glutaraldehyde at 4°C for 1 wk. Granulosa cells are digested by trypsin and collected, and then suspended and fixed in 2.5% glutaraldehyde at 4°C for 2 h. The granulosa cells were washed 3 times in 0.1 M phosphoric acid buffer PB (pH 7.4) and pre-embedded in 1% agarose solution. The ovary and granulosa cells were then incubated at room temperature for 2 h in 1% osmium tetroxide. It is then rinsed 3 times in the buffer and dehydrated during the ethanol grading step. It was then permeated and embedded in the acetone grading step overnight at 37°C. After polymerizing for 48 h at 60°C, 80-nm sections were cut on an Ultra microtome (Leica, Leica UC7) and picked up on copper grids. The grids were poststained in 2% uranyl acetate and 2.6% lead citrate. The sections were observed in a TEM (HITACHI, HT7800).
RNA Extraction and qRT-PCR Analysis
Total RNA was extracted and purified from the ovary using RNAiso Plus (TaKaRa, 9108). Reverse transcription was performed using Prime Script RT reagent Kit with gDNA Eraser (TaKaRa, RR047A) according to the manufacturer's instructions. All primers were designed using Primer 5.0 software (Table 1) and synthesized by Sangon Biotech Co., Ltd. Real-time quantitative RT-PCR was performed in a total reaction volume of 20 mL, which included 10 mL of the 2 × SYBR Premix EX Taq master mix (Genstar, A304), 0.8 mL of each of the forward and reverse primers (10 mM), 1 mL of the cDNA template, and 8.2 mL of sterilized water. The qRT-PCR analysis was performed using the Applied Biosystems Quant Studio 7 Flex Real-Time PCR System (Thermo Fisher, 4485690). Gene expression values were determined using the 2−ΔΔCT method and normalized to the expression levels of β-actin.
Table 1.
Gene primer sequences.
| Genes | Primer sequence (5′–3′) | Annealing temperature (°C) | GenBank no. |
|---|---|---|---|
| β-actin | ATGTCGCCCTGGATTTCG | 60 | XM_038165410.1 |
| CACAGGACTCCATACCCAAGAA | |||
| Drp1 | AGTCTGGAACAGGCAACTGG | 55 | XM_027443342.2 |
| GTTTGCGTGCAACAGGTACA | |||
| OPA1 | AGAGCCTGCTTGGTGAACTC | 55 | XM_027463876.2 |
| TAGTCGCTCCAGCATTCGTT | |||
| Mfn1 | TACCAATCCCTCGTCCTTTA | 55 | XM_005024684.4 |
| TACAACAACCCATACATACTCA | |||
| Mfn2 | TGCTTCACGCTCAGCTACGA | 55 | XM_027443332.2 |
| ACCACTCCACCAACCACGAT | |||
| ACAAGACCAGGACCAGACAG |
Western Blot Analysis
Total protein from the jejunum was extracted using RIPA cell lysis buffer (Beyotime Biotechnology, P0013B). The protein concentration was estimated using BCA Protein Assay Kit (Beyotime Biotechnology, P0012). Samples were mixed with SDS-PAGE Sample Loading Buffer 6× (Beyotime Biotechnology, P0015F), and incubated for 10 min at 100°C. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with phosphate-buffered saline with Tween 20 (PBST) containing 5% fat-free milk, PVDF membranes were coincubated with the antibodies anti-β-Actin (1: 1,000, Proteintech, 66009-1-lg); anti-CYP19A1 (1: 1,000, HUABIO, ER1802-38); anti-CYP11A1 (1: 1,000, CUSABIO, P05108); anti-3β-HSD (1: 1,000, Affinity, DF3653), anti-StAR (1: 1,000, Affinity, DF6192), anti-BCL2 (1: 1,000, Proteintech, 12789-1-AP), anti-Bax (1: 1,000, Wanleibio, WL01637), anti-Caspase3 (1: 1,000, Proteintech, 19677-1-AP), anti-Drp1 (1: 1,000, Proteintech, 12957-1-AP); anti-OPA1 (1: 1,000, Proteintech, 27733-1-AP); anti-Mfn2 (1: 1,000, Proteintech, 12186-1-AP); anti-Mfn1 (1: 1,000, Proteintech, 13798-1-AP); anti-HSP60 (1: 1,000, Proteintech, 15282-1-AP); anti-Tom20 (1: 1,000, Proteintech, 11802-1-AP); anti-PINK1 (1: 1,000, Proteintech, 23274-1-AP); anti-P62 (1: 1,000, Proteintech, 18420-1- AP); anti-Beclin1 (1: 1,000, Proteintech, 11306-1-AP); anti-LC3 (1: 1,000, Proteintech, 14600-1-AP) at 4°C overnight. Subsequently, PVDF membranes were incubated with the corresponding secondary antibody at 37°C for 1 h. Proteins were detected using the ECL kit (GLPBIO, GK10008), and visualized using Chemiluminescence imaging system (Tanon, 5200) device. Densitometry analysis was performed using the Image J software (Bethesda, ImageJ 1.53o).
Mitochondrial and Cytosolic Protein Extraction
Mitochondrial and cytosolic components were extracted using a Mitochondria Isolation Kit (Beyotime Biotechnology, C3601). First, granulosa cells were well-distributed by mitochondria isolation solution containing phenylmethylsulfonylfluoride (PMSF) in an ice bath for 15 min. A glass homogenizer was applied to grind the cells followed by centrifugation with 1,000 × g for 10 min at 4°C. The liquid supernatant was shifted to another tube and centrifuged again with 11,000 × g for 10 min. The sediment was blended with Mitochondrial Lysate Solution to obtain mitochondrial proteins. The supernatant was centrifuged with 12,000 × g for 20 min to obtain cytosolic proteins.
Apoptosis Assay
An Annexin V-EGFP Apoptosis Detection kit (Beyotime Biotechnology, C1062S) and flow cytometry were performed to detect granulosa cells apoptosis.
Biochemical Intervention
Granulosa cells were then treated with M199 medium supplemented with 0 or 20 μM CysA (Aladdin, 59865-13-3) (Li et al., 2017), and incubated for 24 h. After treatment with CysA for 24 h, the Heat treatment groups and the Heat treatment with CysA groups were placed in a constant temperature incubator at 43°C for a 2.5 h period, with the exception of the Control groups and CysA groups.
Transfection and Gene Silencing With siRNAs
Granulosa cells were transfected with riboFECT CP Transfection Kit (RIBOBIO, C10511-05), in the presence of 50 nM (PINK1 or Drp1) of siRNA specific for each of the genes. The si-RNA plasmid targeting duck si-PINK1 (UAUGUUUGGAUGAGGCUGCTT), si-Drp1 (UUAACCCACAGGCAUCAGCTT), and si-NC (UGACACGUUCGGAGAATT) were synthesized by Sanggon Biotech. Knockdown efficiency was confirmed by Western blotting.
Confocal Fluorescence Analysis
Granulosa cells were transfected with pCMV-mCherry-GFP-LC3B (Beyotime Biotechnology, D2816) in a 35-mm cell culture dish using Lipofectamine 3000 (Thermo Scientific, L3000150). DAPI (Beyotime Biotechnology, C1002) (excitation/emission wavelength: 359 nm/461 nm) were used to stain the nucleus foe 15 min. A confocal laser scanning microscope (CLSM) (Zeiss, LSM 780) was used to observe LC3 fluorescence.
MitoTracker Red CMXRos (Beyotime Biotechnology) (excitation/emission wavelength: 579 nm/599 nm) were used to stain the mitochondria for 30 min. DAPI were used to stain the nucleus foe 15 min. A CLSM was used to observe mitochondria fluorescence.
Granulosa cells were transfected with pCMV-GFP-LC3B (Beyotime Biotechnology, D2815) in a 35-mm cell culture dish using Lipofectamine 3000. MitoTracker Red CMXRos were used to stain the mitochondria for 30 min. A CLSM was used to observe colocalization of mitochondria and LC3.
LysoTracker Green DND-26 (Thermo Scientific, L7526) (excitation/emission wavelength: 504 nm/511 nm) were used to stain the lysosomes for 1 h. MitoTracker Red CMXRos were used to stain the mitochondria for 30 min. A CLSM was used to observe colocalization of mitochondria and lysosomes.
Statistical Analysis
GraphPad Prism 7.1 (GraphPad Software Inc.) was used for statistical analysis. The 2-sample t test was conducted the statistical data of autophagosome number. Multiple comparison analysis was performed using a 2-way ANOVA followed by Tukey's post hoc correction for multiple comparisons. All experimental data were analyzed using the means standard deviation (SEM). Specific symbols (*) indicate statistically significant differences for *P < 0.05, **P < 0.01.
RESULTS
Heat Stress Increase the Levels of Mitochondrial Fission and Mitophagy in Ovarian Granulosa Cells
The ultrastructure of ovarian granulosa cells was observed by transmission electron microscopy (TEM). The mitochondria in the control group cells had complete and clear structures. After 30 min of heat treatment, the mitochondria of the granulosa cells became swollen, cristae decreased or disappeared, and some mitochondria shrank and began to form mitochondrial autophagosomes. After 60 min of heat treatment, the morphology of mitochondria had almost completely expanded and rounded, with cristae disappeared and internal vacuolation. Autophagosomes with bilayer membranes were generated in the cells, and mitochondria-like structures could be observed in autophagosomes (→). After 60 min of heat treatment, the number of autophagosomes in a single cell was increased significantly (Figure 1A). The mRNA and protein expression levels of mitochondrial dynamics proteins were detected, and it was found that mitochondrial fusion proteins Mitofusin 1 (Mfn1), Mitofusin2 (Mfn2), and OPA1 Mitochondrial Dynamin Like GTPase (OPA1) were significantly reduced in protein level, and mitochondrial fission protein Dynamin-Related Protein 1 (Drp1) was significantly increased in protein level (Figure 1B and C), which means that the mitochondria of ovarian cells were tend to fission under acute heat stress. In addition, the results showed that the autophagy marker proteins LC3 Ⅱ/LC3 Ⅰ ratio was significantly increased. And, the results indicated that PINK1 protein was significantly increased, while Phosphotyrosine-Independent Ligand for The Lck SH2 Domain of 62 kDa (P62) and Translocase of Outer Mitochondrial Membrane 20 (Tom20) protein was significantly decreased. Meanwhile, no significant changes were found in the protein content of Coiled-coil, moesin-like BCL2-interacting protein (Beclin1) and HSP60 (Figure 1D). This result suggests that acute heat stress increase the level of mitophagy in granulosa cells in vivo.
Figure 1.
Heat treatment causes mitochondrial damage in granulosa cells, promotes mitochondrial fission and mitophagy. (A) The representative figure of ovary ultra-structure. Scale bar: 5 μm, 2 μm, and 1 μm. (B) qRT-PCR was used to identify mitochondrial fission and fusion-related genes mRNA level in ovary. (C) Western blot results showing the protein (Mfn1, Mfn2, OPA1, Drp1) level in ovary. β-Actin was used as an internal reference in whole-tissue lysates. (D) Western blot results showing the protein (LC3-II/LC3-I, Beclin1, p62, PINK1, Tom20, HSP60) level in ovary. β-Actin was used as an internal reference in whole-tissue lysates. Each experiment was repeated 3 times. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
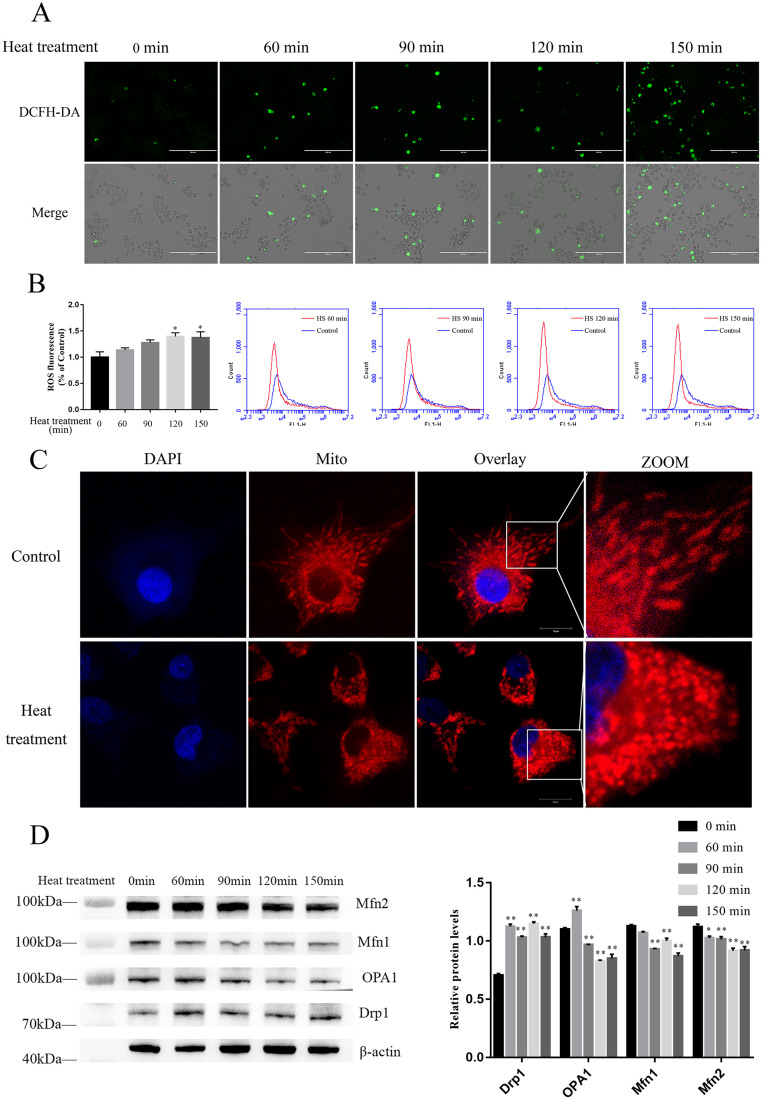
Heat Treatment Induces Granulosa Cells ROS Accumulation and Promotes Mitochondrial Fission In Vitro
ROS is a product of the mitochondrial respiratory electron transport chain. Mitochondrial disorder induces excessive ROS production, which contributes to multiple cellular processes related to cell death, such as apoptosis and necrosis. In our study, Granulosa cells were placed in a constant temperature incubator at 43°C for 0, 60, 90, 120, and 150 min, and then ROS levels were detected, respectively. The result of DCF fluorescence shows that the ROS levels increased with the increase in heat treatment duration (Figure 2A). Quantitative analysis by flow cytometry confirmed that the ROS level of granulosa cells increased significantly after 120 min of heat treatment (Figure 2B). The mitochondrial morphology of granulosa cells, stained with Mito-Tracker Red CMX Ros, was observed by confocal scanning microscopy. Results show that mitochondria appear long and rope-like in the control group but are shortened and rounded in the heat treatment group (Figure 2C). In the heat treatment group, Drp1 protein expression was significantly higher compared to the control group; whereas Mfn1, Mfn2, and OPA1 protein expression were significantly lower (Figure 2D). This indicates that heat treatment of granulosa cells in vitro increased division and prevented the fusion of mitochondria.
Figure 2.
Heat treatment induces ROS accumulation and promotes mitochondrial fission in granulosa cells. (A, B) Cellular ROS levels were detected with fluorescence microscope and flow cytometry. Scale bar: 200 μm. (C) Granulosa cells were stained with MitoTracker Red CMXRos, and images were captured with CLSM. Scale bar: 10 μm. (D) Western blot results showing the protein (Mfn1, Mfn2, OPA1, Drp1) level in granulosa cells. β-Actin was used as an internal reference in whole-cell lysates. Each experiment was repeated 3 times. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
Heat Treatment Increases the Levels of Mitophagy in Granulosa Cells In Vitro
The pCMV-Mcherry-GFP-LC3B plasmid was transfected into granulose cells to observe the fluorescence changes of LC3 fluorescent during heat treatment. The result shows that LC3 was distributed uniformly and diffusely in the control group, while it aggregated in a spot-like manner near the nucleus in the heat treatment group (Figure 3A). The Western blot result shows that the LC3-II level was significantly upregulated in the heat treatment group, with a decrease in LC3-I expression relative to the 0 min heat treatment group. The ratio of LC3-II/LC3-I was the highest in the 150 min heat treatment group. Meanwhile, the protein contents of PINK1 significantly increased, while the protein contents of P62 and Tom20 significantly decreased (Figure 3B). To further determine whether heat treatment regulates cellular mitophagy, we examined the formation of autophagosome-like vesicles in heat-treated granulose cells by using TEM and quantitative analysis (Figure 3C). In accordance with the results of experiments in vivo, the mitochondria of granulosa cells in the heat treatment group were swollen, vacuolated and cristae disappeared, and the presence of autophagosomes in the cells was observed, which surrounded and phagocytic abnormal mitochondria. By counting the number of autophagosomes in a single cell, they increased significantly after heat treatment. In addition, mitochondrial and cytoplasmic proteins of granulosa cells were isolated and detected. The results showed that the PINK1 protein levels were significantly increased both in cytoplasm and mitochondria, while theLC3-Ⅱ/LC3-Ⅰratio increase only occurred in mitochondrial (Figure 3D). These evidences suggest that acute heat treatment increases the levels of mitophagy in granulosa cells.
Figure 3.
Heat treatment increases the levels of mitophagy in granulosa cells. (A) Granulosa cells were transfected with pCMV-mCherry-GFP-LC3B, and images were captured with CLSM. Scale bar: 10 μm. (B) Western blot results showing the protein (LC3-II/LC3-I, Beclin1, p62, PINK1, Tom20) level in granulosa cells. β-Actin was used as an internal reference in whole-cell lysates. (C) The representative figure of granulosa cells ultrastructure. Scale bar: 5 μm, 2 μm, and 1 μm. (D) Western blot results showing the protein (LC3-II/LC3-I and PINK1) level in cytoplasm and mitochondria. β-Actin and HSP60 was used as an internal reference in cytoplasm lysates and mitochondria lysates. Each experiment was repeated 3 times. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
To confirm whether there is a colocalization between autophagy proteins and mitochondrial. After transfection with PCMV-GFP-LC3B for 24 h, granulose cells in the heat treatment group were treated at 43°C, and then mito-Tracker Red CMXRos was used to stain mitochondria. Results of confocal microscopy showed that LC3 spots in the heat treatment group were colocated with Mito-Tracker (Figure 4A). This confirms that LC3 is attached to autophagosomes and is recruited to damaged mitochondria after acute heat treatment. Next, to determine whether the mitochondria wrapped by autophagosome fused with lysosome or not, we stained the cells with LysoTracker Probes and mitochondrial Probes, respectively. Colocalization between lysosome and mitochondria was observed in the heat treatment group (Figure 4B). This confirms that lysosomes are involved in mitophagy.
Figure 4.
Colocalization of LC3 with mitochondria and Lyso with mitochondria under heat treatment. (A) Colocalization of LC3 (green) with mitochondria (red) in granulosa cells. Scale bar: 10 μm. (B) Colocalization of Lyso (green) with mitochondria (red) in granulosa cells. Scale bar: 10 μm. (C) Western blot results showing the protein (LC3-II/LC3-I, Beclin1, p62, PINK1, Tom20) level in granulosa cells treated with CysA. β-actin was used as an internal reference in whole-cell lysates. Each experiment was repeated 3 times. Data are expressed as the mean ± SEM. Different letters indicate a statistical difference P < 0.05.
CysA is a mitophagy inhibiter, which inhibiting the opening of mitochondrial permeability conversion pores. In this study, the mitophagy inhibitor CysA was used to treat granulosa cells to further determine whether mitophagy occurred. The results showed that compared with the heat treatment group, the LC3 Ⅱ/LC3 Ⅰ ratio and PINK1 protein content of granulosa cells treated with 20 μM CysA were significantly decreased (Figure 4C). The results indicate that the autophagy induced by acute heat treatment was effectively inhibited by mitophagy inhibitor CysA. These results further confirm the occurrence of mitophagy under heat treatment in granulosa cells.
Depletion of Endogenous Drp1 and PINK1 Reduces Levels of Mitophagy and Steroid Hormones Synthesis Proteins in Granulosa Cells Under Heat Treatment
When mitochondria are damaged, Drp1 is activated, allowing the damaged mitochondria to be separated. Experiments in vivo and in vitro showed that acute heat treatment increased Drp1 protein. To verify the effect of Drp1 on mitophagy and steroid hormones synthesis of granulosa cells induced by heat treatment, we used si-RNA to interfere Drp1 expression and detected by Western blot. The results showed that after transfection with si-Drp1, the LC3-Ⅱ/LC3-Ⅰ ratio and PINK1 protein were decreased significantly, and P62 and Tom20 protein were significantly increased (Figure 5A). Moreover, after transfection with si-Drp1, StAR, 3β-HSD, and CYP11A1 proteins were reduced significantly (Figure 5B). The results showed that interfering Drp1 inhibited PINK1-dependentmitophagy and steroid hormones synthesis in granulosa cells under heat treatment.
Figure 5.
Inhibited Drp1 and PINK1 expression reduces mitophagy and steroid hormones synthesis in granulosa cells under acute heat treatment. (A, C) Western blot results showing the protein (LC3-II/LC3-I, Beclin1, p62, PINK1, Tom20) level in granulosa cells transfected with si-Drp1 and si-PINK1. (B, D) Western blot results showing the protein (StAR, CYP11A1, 3β-HSD, CYP19A1) level in granulosa cells transfected with si-Drp1 and si-PINK1. β-Actin was used as an internal reference in whole-cell lysates. Each experiment was repeated 3 times. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
PINK1-dependent mitophagy is an important cellular survival mechanism under stress. To verify whether PINK1 is necessary for the process of mitophagy induced by acute heat stress, we used si-RNA to interfere PINK1 expression and detected the protein levels. The results showed that LC3-Ⅱ/LC3-Ⅰ ratio was decreased significantly, and P62 and Tom20 proteins were increased significantly after transfected with si-PINK1 (Figure 5C). Moreover, the StAR and CYP11A1 protein levels increased significantly. However, the levels of 3β-HSD and CYP19A1 protein in granulosa cells were reduced significantly (Figure 5D). These results suggest that interference with PINK1 also inhibits mitophagy and steroid hormones synthesis.
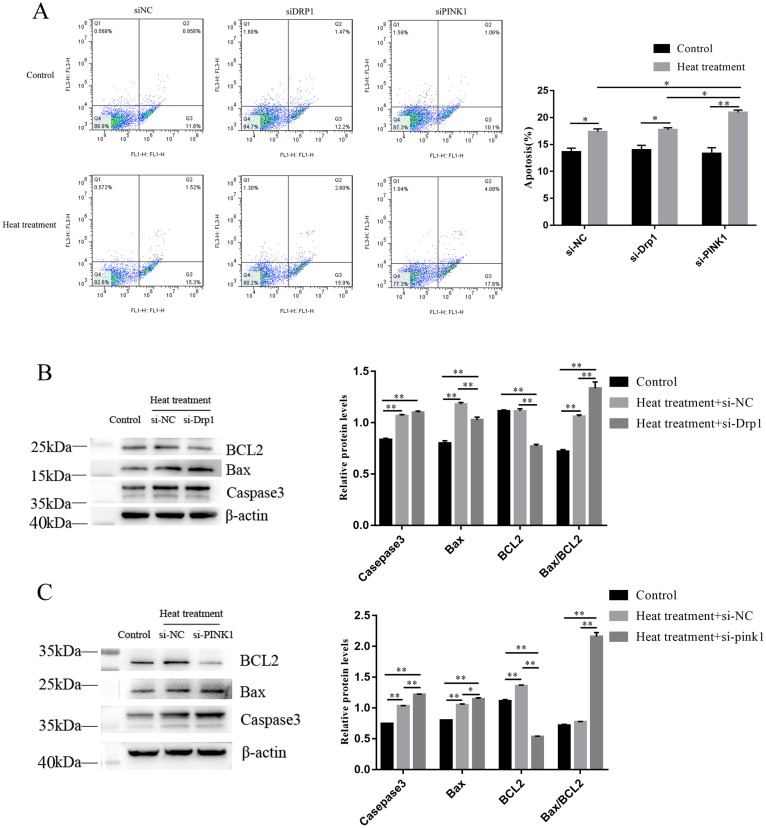
Depletion of Endogenous Drp1 and PINK1 Enhanced Heat Treatment Induced Apoptosis in Granulosa Cells
To further explore the relationship between the mitophagy machinery and apoptosis of granulosa cells under heat treatment, siRNA knockdown experiments were performed to specifically deplete endogenous Drp1 and PINK1 proteins. The apoptosis rate of granulosa cells increased significantly after transfection of si-PINK1 under acute heat treatment, but no significant change was observed after transfection of si-Drp1, which was detected by Flow cytometry (Figure 6A). Western blot analysis showed that the Bax/BCl 2 ratio was increased significantly after transfection with si-Drp1 and si-PINK1 (Figure 6B, C). This indicated that interference with Drp1 and PINK1 increased the level of apoptosis protein, and interference with PINK1 significantly increased the apoptosis rate of granulosa cells.
Figure 6.
Reduced Drp1 and PINK1 expression promotes apoptosis of granulosa cells under acute heat treatment. (A) Apoptosis was detected by flow cytometry. (B, C) Western blot results showing the protein (Caspase3, Bax, BCL2) level in granulosa cells transfected with si-Drp1 and si-PINK1. β-Actin was used as an internal reference in whole-cell lysates. Each experiment was repeated 3 times. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
DISCUSSION
The high-temperature environment is one of the common factors that reduce the reproductive performance of poultry (Li et al., 2020). Granulosa cells are the main cells of steroid hormone biosynthesis, are involved in follicular development, ovulation and regulate reproductive performance. Mitochondria are central sites for the steps of steroid genesis (Miller, 2013). Respiratory-competent mitochondria are required to facilitate steroid genesis in granulosa cells (Miller, 2017). However, Study indicated that mitochondria are one of the earliest organelles that respond to multiple stimuli and environments (Duranova et al., 2020). A previous study in chicken skeletal muscles showed that heat stress impairs mitochondrial functions and induces oxidative injury (Huang et al., 2015). Investigation in chicken spleens also revealed that heat stress decreased the activities of the mitochondrial complex and ATPase as well as the contents of proteins related to mitochondrial fission and fusion (Xu et al., 2017).
Mitophagy is an important mechanism of mitochondria quality control systems to remove damaged mitochondria and ensure cell survival (Pickles et al., 2018). PINK1-mediated mitophagy plays an important role in clearing damaged mitochondria. Previous studies had confirmed that lack of PINK1 led to reduce mitophagy, increase ROS accumulation, decreased mitochondrial membrane potential and ATP levels, increased proapoptotic proteins Bax and Caspase 3, and release of Cytc, which leading to apoptosis ultimately (van der Merwe et al., 2017; Lin et al., 2019; Wang et al., 2021; Xi et al., 2021). Autophagy and lipid autophagy play key roles in cholesterol transport and testosterone production (Ma et al., 2018; Khawar et al., 2021), but the role of mitophagy in steroid synthesis and survival of granulosa cells has not been revealed. By observing the ultrastructure of granulosa cells, we found the mitochondrial structure was damaged under heat stress, and the intracellular mitochondrial fission and mitophagy levels were increased. The Western blot results were also verified at the protein level both in vivo and in vitro. In addition, the confocal microscopy results showed that LC3 spots in the heat treatment group were colocalized with Mito-Tracker. And, colocalization between lysosome and mitochondria was also observed in the heat treatment group. Furthermore, study show that the autophagy-related protein induced by acute heat treatment was effectively inhibited by mitophagy inhibitor CysA. The results indicated acute heat stress increased levels of mitophagy in granulosa cell, which may play an important role in maintain cellular homeostasis (Figure 7).
Figure 7.
Model of the effect of mitophagy on granulosa cells’ survival and steroid hormone synthesis under acute heat stress. In this model, acute heat stress causes mitochondrion damage in granulosa cells. Drp1 regulated PINK1-dependent mitophagy promoted degrades of cellular dysfunction mitochondrial to maintain cellular homeostasis. Downregulated Drp1 or Pink1 inhibited the steroid hormone synthesis of granulosa cells and promoted cell apoptosis.
Mitochondrial fission is associated with mitochondrial dysfunction and is dominant during elevated stress levels and cell death (Zemirli et al., 2018). Drp1 is necessary for heat stress-mediated mitochondrial fracture in nematode larvae, and for functional autophagy after heat stress, it possibly participates in the coordination between mitochondrial fission and autophagosome (Chen et al., 2021). The study indicated that Drp1 knockout significantly inhibits PINK1 translocation induced by CCCP (Park et al., 2018). Drp1, a known core protein involved in mitochondrial fission, is recruited from the cytoplasm to the outer membrane of mitochondria, and Drp1 monomers oligomerize and form contractile rings. Following GTP hydrolysis, conformational changes in Drp1 helices cause a 2-fold decrease in ring diameter, which facilitates membrane constriction (Kraus and Ryan, 2017). Phosphorylation of Drp1 at Ser 616 activates mitochondrial division, whereas phosphorylation at Ser 637 inhibits division (Oliver and Reddy, 2019). Mitochondrial fission induced by heat stress was also observed in the hypothalamus of mice, with increased phosphorylated Drp1 protein level and induced apoptosis and mitophagy (Chen et al., 2021). In mammary epithelial cells of dairy cows, it has been proved that heat stress destroys the balance of mitochondrial fission and fusion, leading to mitochondrial fracture, decreasing of cellular ATP level, membrane potential and antioxidant enzyme activity (Chen et al., 2020). It has also been suggested that short-term heat stress induces mitochondrial biosynthesis through the high expression of SIRT1 and phosphorylation of AMP-activated protein kinase in porcine oocytes, and enhances mitochondrial membrane potential and ATP, thus improving the developmental capacity of oocytes (Itami et al., 2018). Consistent with previous results, we found that Drp1-mediated mitochondrial fission is an important link in heat stress-induced mitophagy. Interference withPINK1 by siRNA reduces mitophagy induced by acute heat stress and promoted apoptosis, and interference with Drp1 also inhibited mitophagy of granulosa cells.
A prerequisite for steroid production is the entry of cholesterol into the mitochondria, which is triggered by StAR. And then cholesterol catalyzed by the cytochrome P450 side chain lysis (CYP11A1) enzyme system in the inner membrane of the mitochondria (Bassi et al., 2021). Mitochondrial fission and fusion are key steps in steroid synthesis. Study indicated that Mfn2-mediated fusion is essential for the protein synthesis of StAR and steroid synthesis (Duarte et al., 2014; Helfenberger et al., 2019). The phosphorylation of Drp1 Ser 637 is essential for steroids synthesis in Leydig cells (Park et al., 2019). StAR protein level and progesterone content were inhibited significantly in dibutyryl cAMP-treated cells, while phosphorylation of Drp1 Ser 637 was specifically inhibited (Park et al., 2019). Drp1 knockout reduced the phosphorylation of Drp1 Ser 637 and Ser 616, and inhibited progesterone production in calf cells and mouse Leydig cell (Plewes et al., 2020). Our experiment confirmed that interfering Drp1expression reduced the protein levels of StAR, 3β-HSD, CYP11A1, CYP19A1 and inhibited the steroid synthesis of granulosa cells under heat stress condition. Interestingly, PINK1 is also a key protein that phosphorylates Drp1Ser 616, PINK1 inactivation suppresses Drp1Ser 616 phosphorylation in cells, mouse, and Drosophila tissues (Han et al., 2020). Meantime, PINK1 degrade Mfn1 and Mfn2 selectively, participating in the control of the balance of mitochondrial fission and fusion (Yamada et al., 2019). In this study, we find that interference PINK1 expression significant downregulated the protein levels of 3β-HSD and CYP19A1, while upregulated the protein levels of StAR and CYP11A1.Whether the increased protein levels of StAR and CYP11A1 were related to the downregulated mitophagy level of cells, and the mechanism underlying the difference between interference with PINK1 and Drp1 needs to be further studied.
In conclusion, acute heat stress causes ovary granulosa cells’ mitochondrial damage and increases the levels of mitochondrial fission and mitophagy. The regulatory mechanism is related to the protein expression of Drp1 increased promoted division of damaged mitochondria and induced PINK1-mediated mitophagy. Interference with Drp1 or PINK1 by siRNA led to the upregulation of apoptosis-related protein expression in granulosa cells under heat treatment. And, interference with Drp1 or PINK1 by siRNA both inhibited the steroid hormones synthesis of granulosa cells under heat exposure.
Acknowledgments
ACKNOWLEDGMENTS
Funding: This work was supported by the National Key Technologies R&D Program of China (No. 2018YFE0128200); National Natural Science Foundation of China (No. 32302750); Natural Science Foundation of Guangdong Province (No. 2020A1515110451); Modern Agriculture Waterfowl Industry Technology System Innovation Team of Guangdong (2023KJ137).
Data Availability Statement: All data generated or analyzed in this study are included in this published article.
Authors’ Contributions: W. J. L., Y. B. T., and C. Y. designed the study. P. L., Y. T. Y., and X. L. F. collected and managed the data and performed the analysis. B. X. L. and X. S. contributed to the interpretation and discussion of results. W. J. L., C. Y., D. N. X., and Y. M. H. wrote and revised the manuscript.
DISCLOSURES
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Abdelnour S.A., Swelum A.A., Abd E.M., Khafaga A.F., Taha A.E., Abdo M. Cellular and functional adaptation to thermal stress in ovarian granulosa cells in mammals. J. Therm. Biol. 2020;92 doi: 10.1016/j.jtherbio.2020.102688. [DOI] [PubMed] [Google Scholar]
- Bassi G., Sidhu S.K., Mishra S. The expanding role of mitochondria, autophagy and lipophagy in steroidogenesis. Cells Basel. 2021;10 doi: 10.3390/cells10081851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Leboutet R., Largeau C., Zentout S., Lefebvre C., Delahodde A., Culetto E., Legouis R. Autophagy facilitates mitochondrial rebuilding after acute heat stress via a DRP-1-dependent process. J. Cell Biol. 2021;220 doi: 10.1083/jcb.201909139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.L., Wang H.L., Jiang L.Z., Qian Y., Yang C.X., Chang W.W., Zhong J.F., Xing G.D. Heat stress induces apoptosis through disruption of dynamic mitochondrial networks in dairy cow mammary epithelial cells. In Vitro Cell Dev. Biol. Anim. 2020;56:322–331. doi: 10.1007/s11626-020-00446-5. [DOI] [PubMed] [Google Scholar]
- Duarte A., Castillo A.F., Podesta E.J., Poderoso C. Mitochondrial fusion and ERK activity regulate steroidogenic acute regulatory protein localization in mitochondria. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranova H., Valkova V., Knazicka Z., Olexikova L., Vasicek J. Mitochondria: a worthwhile object for ultrastructural qualitative characterization and quantification of cells at physiological and pathophysiological states using conventional transmission electron microscopy. Acta Histochem. 2020;122 doi: 10.1016/j.acthis.2020.151646. [DOI] [PubMed] [Google Scholar]
- Geisler S., Holmstrom K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Goel A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. (Berl.) 2021;105:1136–1145. doi: 10.1111/jpn.13496. [DOI] [PubMed] [Google Scholar]
- Greene A.W., Grenier K., Aguileta M.A., Muise S., Farazifard R., Haque M.E., McBride H.M., Park D.S., Fon E.A. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. Embo Rep. 2012;13:378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Tan J., Wang R., Wan H., He Y., Yan X., Guo J., Gao Q., Li J., Shang S., Chen F., Tian R., Liu W., Liao L., Tang B., Zhang Z. PINK1 phosphorylates Drp1(S616) to regulate mitophagy-independent mitochondrial dynamics. Embo Rep. 2020;21:e48686. doi: 10.15252/embr.201948686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfenberger K.E., Castillo A.F., Mele P.G., Fiore A., Herrera L., Finocchietto P., Podesta E.J., Poderoso C. Angiotensin II stimulation promotes mitochondrial fusion as a novel mechanism involved in protein kinase compartmentalization and cholesterol transport in human adrenocortical cells. J. Steroid Biochem. Mol. Biol. 2019;192 doi: 10.1016/j.jsbmb.2019.105413. [DOI] [PubMed] [Google Scholar]
- Ho K.T., Homma K., Takanari J., Bai H., Kawahara M., Nguyen K., Takahashi M. A standardized extract of Asparagus officinalis stem improves HSP70-mediated redox balance and cell functions in bovine cumulus-granulosa cells. Sci. Rep. 2021;11:18175. doi: 10.1038/s41598-021-97632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Itami N., Shirasuna K., Kuwayama T., Iwata H. Short-term heat stress induces mitochondrial degradation and biogenesis and enhances mitochondrial quality in porcine oocytes. J. Therm. Biol. 2018;74:256–263. doi: 10.1016/j.jtherbio.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Khan A., Khan M.Z., Dou J., Umer S., Xu H., Sammad A., Zhu H.B., Wang Y. RNAi-mediated silencing of catalase gene promotes apoptosis and impairs proliferation of bovine granulosa cells under heat stress. Animals (Basel) 2020;10:1060. doi: 10.3390/ani10061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawar M.B., Liu C., Gao F., Gao H., Liu W., Han T., Wang L., Li G., Jiang H., Li W. Sirt1 regulates testosterone biosynthesis in Leydig cells via modulating autophagy. Protein Cell. 2021;12:67–75. doi: 10.1007/s13238-020-00771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.E., Grant A.R., Simic M.S., Kohnz R.A., Nomura D.K., Durieux J., Riera C.E., Sanchez M., Kapernick E., Wolff S., Dillin A. Lipid biosynthesis coordinates a mitochondrial-to-cytosolic stress response. Cell. 2016;166:1539–1552. doi: 10.1016/j.cell.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus F., Ryan M.T. The constriction and scission machineries involved in mitochondrial fission. J. Cell Sci. 2017;130:2953–2960. doi: 10.1242/jcs.199562. [DOI] [PubMed] [Google Scholar]
- Lazarou M., Jin S.M., Kane L.A., Youle R.J. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell. 2012;22:320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.M., Liu L.P., Yin B., Liu Y.Y., Dong W.W., Gong S., Zhang J., Tan J.H. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-alpha systems. Poult. Sci. 2020;99:6084–6093. doi: 10.1016/j.psj.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wu J., Luo M., Sun Y., Wang G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperones. 2016;21:467–475. doi: 10.1007/s12192-016-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zheng M., Xu H., Cui X., Zhang Y., Zhang L., Yang S., Xu Z., Bai R., Sun X. Mitochondrial pathways are involved in Eimeria tenella-induced apoptosis of chick embryo cecal epithelial cells. Parasitol. Res. 2017;116:225–235. doi: 10.1007/s00436-016-5283-z. [DOI] [PubMed] [Google Scholar]
- Lin Q., Li S., Jiang N., Shao X., Zhang M., Jin H., Zhang Z., Shen J., Zhou Y., Zhou W., Gu L., Lu R., Ni Z. PINK1-Parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Li L., Xiao C., Sun Y., Wang G.L. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell. Biochem. 2016;412:81–90. doi: 10.1007/s11010-015-2610-0. [DOI] [PubMed] [Google Scholar]
- Ma X., Lin Y., Zhang H., Chen W., Wang S., Ruan D., Jiang Z. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim. Reprod. Sci. 2014;145:182–190. doi: 10.1016/j.anireprosci.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhou Y., Zhu Y.C., Wang S.Q., Ping P., Chen X.F. Lipophagy contributes to testosterone biosynthesis in male rat Leydig cells. Endocrinology. 2018;159:1119–1129. doi: 10.1210/en.2017-03020. [DOI] [PubMed] [Google Scholar]
- Miller W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013;379:62–73. doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Miller W.L. Disorders in the initial steps of steroid hormone synthesis. J. Steroid Biochem. Mol. Biol. 2017;165(Pt A):18–37. doi: 10.1016/j.jsbmb.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Oliver D., Reddy P.H. Dynamics of dynamin-related protein 1 in Alzheimer’s disease and other neurodegenerative diseases. Cells-Basel. 2019;8:961. doi: 10.3390/cells8090961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.S., Choi S.E., Koh H.C. PGAM5 regulates PINK1/Parkin-mediated mitophagy via DRP1 in CCCP-induced mitochondrial dysfunction. Toxicol. Lett. 2018;284:120–128. doi: 10.1016/j.toxlet.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Park J.E., Kim Y.J., Lee S.G., Kim J.Y., Chung J.Y., Jeong S.Y., Koh H., Yun J., Park H.T., Yoo Y.H., Kim J.M. Drp1 phosphorylation is indispensable for steroidogenesis in Leydig cells. Endocrinology. 2019;160:729–743. doi: 10.1210/en.2019-00029. [DOI] [PubMed] [Google Scholar]
- Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewes M.R., Hou X., Talbott H.A., Zhang P., Wood J.R., Cupp A.S., Davis J.S. Luteinizing hormone regulates the phosphorylation and localization of the mitochondrial effector dynamin-related protein-1 (DRP1) and steroidogenesis in the bovine corpus luteum. FASEB J. 2020;34:5299–5316. doi: 10.1096/fj.201902958R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S., Nagaoka K., Watanabe G. Yolk immunoreactive corticosterone in hierarchical follicles of Japanese quail (Coturnix japonica) exposed to heat challenge. Gen. Comp. Endocrinol. 2019;279:148–153. doi: 10.1016/j.ygcen.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Rostagno M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibye-Knudsen M., Fang E.F., Croteau D.L., Wilson D.R., Bohr V.A. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25:158–170. doi: 10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreerangaraja Urs D.B., Wu W., Komrskova K., Postlerova P., Lin Y., Tzeng C., Kao S. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. Int. J. Mol. Sci. 2020;21:3592. doi: 10.3390/ijms21103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. The PINK1-Parkin axis: an overview. Neurosci. Res. 2020;159:9–15. doi: 10.1016/j.neures.2020.01.006. [DOI] [PubMed] [Google Scholar]
- van der Merwe C., van Dyk H.C., Engelbrecht L., van der Westhuizen F.H., Kinnear C., Loos B., Bardien S. Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson's disease from mitochondrial dysfunction and cell death. Mol. Neurobiol. 2017;54:2752–2762. doi: 10.1007/s12035-016-9843-0. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang C., Elsheikh N., Li C., Yang F., Wang G., Li L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging (Albany NY) 2019;11:5535–5547. doi: 10.18632/aging.102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhu J., Liu Z., Shu S., Fu Y., Liu Y., Cai J., Tang C., Liu Y., Yin X., Dong Z. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J., Rong Y., Zhao Z., Huang Y., Wang P., Luan H., Xing Y., Li S., Liao J., Dai Y., Liang J., Wu F. Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/Parkin-mediated mitophagy. J. Ethnopharmacol. 2021;271 doi: 10.1016/j.jep.2021.113855. [DOI] [PubMed] [Google Scholar]
- Xing S., Wang X., Diao H., Zhang M., Zhou Y., Feng J. Changes in the cecal microbiota of laying hens during heat stress is mainly associated with reduced feed intake. Poult. Sci. 2019;98:5257–5264. doi: 10.3382/ps/pez440. [DOI] [PubMed] [Google Scholar]
- Xu D., Li B., Cao N., Li W., Tian Y., Huang Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget. 2017;8:70394–70405. doi: 10.18632/oncotarget.19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Dawson T.M., Yanagawa T., Iijima M., Sesaki H. SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/Parkin in mitophagy. Autophagy. 2019;15:2012–2018. doi: 10.1080/15548627.2019.1643185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Huang X.B., Chen S.J., Li X.J., Fu X.L., Xu D.N., Tian Y.B., Liu W.J., Huang Y.M. The effect of heat stress on proliferation, synthesis of steroids, and gene expression of duck granulosa cells. Anim. Sci. J. 2021;92 doi: 10.1111/asj.13617. [DOI] [PubMed] [Google Scholar]
- Zemirli N., Morel E., Molino D. Mitochondrial dynamics in basal and stressful conditions. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Fang C., Li J., Mo C., Wang Y., Li J. Transcriptomic diversification of granulosa cells during follicular development in chicken. Sci. Rep. 2019;9:5462. doi: 10.1038/s41598-019-41132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Jiang W., Wu L.Y. Dietary L-arginine supplement alleviates hepatic heat stress and improves feed conversion ratio of Pekin ducks exposed to high environmental temperature. J. Anim. Physiol. Anim. Nutr. 2014;98:1124–1131. doi: 10.1111/jpn.12195. [DOI] [PubMed] [Google Scholar]