1. Introduction
Right ventricular (RV) pacing has been the traditional pacing site for the treatment of symptomatic bradyarrhythmia. RV pacing produces left bundle branch block (LBBB) morphology on the 12-lead electrocardiography (ECG) resulting in dyssynchronous contraction of the left ventricle (LV) similar to that observed in patients with native LBBB [1]. These effects result in myofibrillar disarray, increased fibrosis and reduced LV ejection fraction. Clinically this has translated into frequent heart failure hospitalization, arrhythmias and increased mortality [2].
Three different types of myocardial cells make up the ventricular wall: epicardial, endocardial and M-cells [3]. The depolarization-repolarization process (DRP) differs across the layers and the epicardial cells complete the repolarization process first and the M-cells the last. DRP can be measured on the surface ECG by QT interval which measures both depolarization and repolarization. However, QT interval has to be corrected for heart rate by Bazett's formula as it depends on the length of the previous cardiac cycle. Transmural dispersion of repolarization can be measured by the interval between the peak to the end of T-wave (Tp-Te). Amplification of dispersion of repolarization is a known substrate for ventricular arrhythmias and hence Tp-Te duration can serve as a non-invasive index of arrhythmogenesis [4]. Tp-Te/QTc serves as a more sensitive index as it provides an estimate of dispersion of repolarization relative to the total duration of depolarization eliminating the confounding effects of heart rate [4].
RV pacing results in prolongation of all the markers of depolarization-repolarization process predisposing to ventricular tachyarrhythmias. Lee et al. [5] showed that a prolonged paced QTc interval was independently associated with increase in all-cause mortality (HR 2.08; 95% CI 1.44–3.01; P value <0.001). Since RV pacing has its own limitations, conduction system pacing by capturing the His bundle or left bundle has been suggested as an alternative to provide physiological activation of the ventricles and avoid pacing related complications in patients with AV block and normal LV function [[6], [7], [8], [9], [10], [11]]. The repolarization and depolarization parameters were not studied before in patients with AV block with preserved LV function undergoing LBBP and HBP where the pacing requirement would be more than 40%.
1.1. Aim
To compare the electrocardiographic, depolarization-repolarization parameters and clinical outcomes of patients with AV block with preserved left ventricular (LV) function who had undergone conventional RV pacing (RVP), His bundle pacing (HBP) and left bundle branch pacing (LBBP) and completed at least one year follow-up.
1.2. Methods
This was a retrospective observational study conducted in two centers (A-Geisinger Health System, United states and B-Velammal Medical College Hospital, India). Consecutive patients with AV block requiring pacemaker implantation (RVP, LBBP or HBP) between May 2019 to May 2021 and completed at-least one year follow-up were included in the study. Different types of pacing therapies (RVP, LBBP or HBP) were explained to the patients and patient's choice of selection was considered. Patients with decreased LV function as defined by LV ejection fraction (EF) ≤50% [12], previous history of heart failure (HF) hospitalizations, pregnancy and age less than 10 years were excluded from the study. Included patients were categorized into three groups - group 1- RV pacing (RVP), group 2- LBBP and group 3- HBP. Pacing induced cardiomyopathy (PIC) was defined as reduction in ejection fraction (EF) to <50% or an absolute reduction of >10% from the baseline EF [12,13]. As the study population included only patients with symptomatic AV block, all patients had ventricular pacing >95%. Data collection for the study was approved by institutional review board and adhered to the guidelines of Helsinki declaration. All patients provided written informed consent for the procedure.
1.3. Procedural techniques
For conduction system pacing, the procedure was performed as previously described [[14], [15], [16]]. Briefly, unipolar mapping of His bundle was done with C315 His sheath and 4.1F 3830 Selectsecuretm lead (Medtronic Inc, Minneapolis, MN) and the lead was screwed at the distal His bundle bypassing the diseased segment to obtain a narrow QRS by selective or non-selective capture. Pacing capture threshold of <1.5V at 1 ms pulse-width was accepted. HBP lead position was accepted if complete correction could be achieved with capture threshold <1.5V/1 ms. LBBP was done by placing the sheath at the proximal interventricular septum 1–1.5 cm apical to an imaginary line joining distal His bundle signal to the RV apex. Rapid turns were given in LAO 30⁰ fluoroscopy view to drive the body of the lead behind the helix deep into the septum. LBB capture is confirmed by the presence of right bundle branch conduction delay pattern (qR or QR) in lead V1 along with any one of the following criteria (a) demonstration of LBB potential (b) short and constant (≤80 ms) peak left ventricular activation time (pLVAT) as measured in lead V5-V6 from the onset of the pacing artefact to peak of R-wave both at high and low pacing output (c) demonstration of nonselective-to selective or LV septal pacing-during unipolar threshold testing (d) physiology based ECG criteria [17]. Atrial lead was placed at right atrial appendage or septum as needed. Patients were discharged on the next day if there were no procedure related complications.
1.4. Data collection
Baseline characteristics of the study population were documented. The indication for pacing was symptomatic AV block (nodal or infra-nodal) with normal LV function (LVEF ≥50%) in all patients. Pacing parameters including capture threshold, sensed R wave and pacing impedance were recorded. ECG parameters included QRS duration, QT interval, T peak to T end and Tp-Te/QTc ratio at baseline and 4 weeks after the procedure. Paced QRS duration was measured from the stimulus to the end of the QRS during non-selective capture in patients with HBP and LBBP. T peak to T end (Tp-Te) duration was defined as the interval between the peak of positive or nadir of the negative T-wave to the end of the T-wave in the mid-precordial lead showing the longest value. QT duration was calculated from the onset of Q wave to the end of T wave in the precordial lead showing the longest value for three consecutive beats and rate correction (QTc) was done by Bazett's formula [18]. All the ECG parameters were measured using digital EP caliper at 25 mm sweep speed. Echocardiographic parameters including interventricular septal thickness, LV ejection fraction by modified Simpson's method, regional wall motion abnormalities and valvular regurgitation were recorded before the procedure and during follow-up. ECG measurements were confirmed by 2 cardiologists to confirm accuracy.
1.5. Statistics
Continuous variables were reported as mean ± standard deviation (SD) and compared with two tailed Student's t-test. Analysis of variance (ANOVA) was used for comparing more than two continuous variables. Categorical variables were presented as frequency distributions and compared using Chi-square test. We tested the normality of the continuous variables by Kolmogorov-Smirnoff normality test and they were found to be normally distributed (p > 0.05). All statistical analyses were performed using SPSS software (version 20.0; SPSS Inc). A P value of <0.05 was considered statistically significant.
2. Results
2.1. Baseline characteristics
Overall, 121 patients who had undergone permanent pacemaker implantation for symptomatic AV block were included. Mean age of the study population was 66.5 ± 12.6 years with 53% men. Mean duration of follow-up was 36.7 ± 7.1 months (range 18–42 months). The presence of diabetes mellitus, hypertension and atherosclerotic coronary artery disease were 56%, 41% and 42%, respectively. Baseline characteristics of the study population is shown in the Table 1. Patients were categorized into three groups as described before (RV pacing, LBBP and HBP). There was no significant difference in baseline characteristics between the three groups. Patients with atrial fibrillation at baseline were excluded. All included patients had LV ejection fraction of ≥50% at baseline. The mean baseline LV ejection fraction (LVEF) was 59.4 ± 5.5% (n = 121)
Table 1.
Baseline Characteristics of the study population.
| RVP (n = 41) | LBBP (n = 45) | HBP (n = 35) | P value | |
|---|---|---|---|---|
| Age (years) | 64.6 ± 10.8 | 65.3 ± 13 | 70.8 ± 12.6 | 0.06 |
| Sex | ||||
| Male | 49% | 49% | 63% | 0.2 |
| Female | 51% | 51% | 37% | 0.2 |
| Comorbidities | ||||
| DM | 46% | 44% | 42% | 0.805 |
| HTN | 54% | 56% | 58% | 0.886 |
| CAD | 46% | 44% | 42% | 0.96 |
| Echocardiography | ||||
| LVEF (%) | 61.2 ± 4.0 | 58.3 ± 5.8 | 59.3 ± 6.4 | 0.133 |
| ECG Parameters | ||||
| QRS duration (ms) | 128.7 ± 21.8 | 130.2 ± 27.2 | 127.9 ± 29 | 0.361 |
| QTc duration (ms) | 472.9 ± 58.7 | 456.6 ± 55.5 | 454.3 ± 40.2 | 0.244 |
| Tp-Te duration (ms) | 104.2 ± 13.9 | 106.6 ± 16.6 | 100.1 ± 17.8 | 0.222 |
| Tp-Te/QTc ratio | 0.22 ± 0.04 | 0.23 ± 0.04 | 0.22 ± 0.03 | 0.367 |
2.2. Procedural characteristics
LBBP: 45 patients who had undergone successful LBBP were included. Unipolar pacing threshold was 0.5 ± 0.2V at 0.5 ms pulse-width. The sensed R wave amplitude was 14.2 ± 6.9 mV and the unipolar pacing impedance 677.4 ± 124.2 Ω pLVAT as measured in lead V5 was 67.6 ± 9.3 ms. The lead was deployed deep inside the septum at an average of 9.6±1 mm as measured by echocardiography.
RVP: 41 patients had undergone RVP for symptomatic AV block. The pacing threshold was 0.4 ± 0.1V at 0.4 ms pulse-width, sensed R wave amplitude was 13.9 ± 5.4 mV and the pacing impedance 695.6 ± 107.8 Ω.
HBP: HBP was done successfully in 35 patients. The pacing threshold was 1.1 ± 0.5V at 0.9 ± 0.2 ms pulse-width, sensed R-wave amplitude of 4.8 ± 2.6 mV and pacing impedance of 587.6 ± 100.6 Ω.
2.3. Electrocardiographic parameters
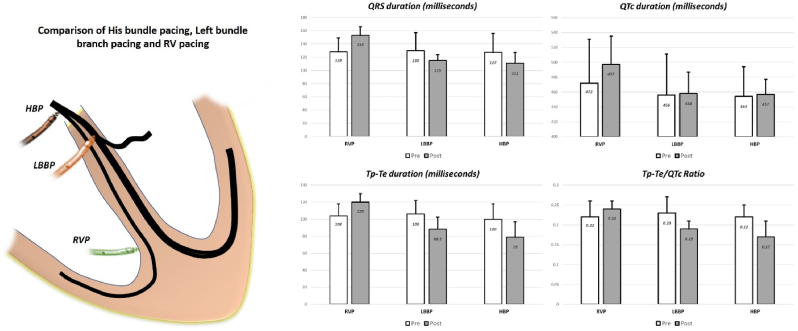
Pre-implantation QRS duration was similar among the three groups (128.7 ± 21.8 ms vs 130.2 ± 27.2 ms vs 127.9 ± 29 ms respectively; p = 0.361). Other ECG parameters are shown in Table 2. The mean QRS duration increased from 128.7 ± 21.8 ms before implantation to 153.6 ± 13.7 ms after RVP (p <0.0001). During LBBP, the baseline QRS duration of 130.2 ± 27.2 ms decreased significantly to 115.8 ± 9.2 ms after LBBP (p value <0.0001), while HBP resulted in reduction in QRS duration from 127.9 ± 29 ms to 111.1 ± 16.5 ms (p = 0.01). (Fig. 1)
Table 2.
Comparison of pre and post ECG parameters after RVA pacing, HBP and LBBP.
| RVP (n = 41) |
LBBP (n = 45) |
HBP (n = 35) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P value | Pre | Post | P value | Pre | Post | P value | |
| QRS duration | 128.7 ± 21.8 | 153.6 ± 13.7 | <0.0001 | 130.2 ± 27.2 | 115.8 ± 9.2 | <0.0001 | 127.9 ± 29 | 111.1 ± 16.5 | 0.01 |
| QTc duration | 472.9 ± 58.7 | 497.4 ± 38.1 | 0.02 | 456.5 ± 55.5 | 458.8 ± 29.1 | 0.799 | 454.3 ± 40.2 | 457.5 ± 20.2 | 0.91 |
| Tp-Te duration | 104.2 ± 13.9 | 120.3 ± 9.9 | <0.0001 | 106.6 ± 16.6 | 88.5 ± 13.8 | <0.0001 | 100.1 ± 17.8 | 79.7 ± 18.6 | 0.003 |
| Tp-Te/QTc | 0.22 ± 0.04 | 0.24 ± 0.02 | 0.005 | 0.23 ± 0.04 | 0.19 ± 0.02 | <0.0001 | 0.22 ± 0.03 | 0.17 ± 0.04 | 0.005 |
| LVEF | 61.2 ± 4.0 | 58.7 ± 8.2 | 0.08 | 58.3 ± 5.8 | 60.3 ± 4.5 | 0.09 | 59.3 ± 6.4 | 61.5 ± 3.8 | 0.08 |
Fig. 1.
Comparison of HBP, LBBP and RVP for symptomatic AV block. Both LBBP and HBP showed reduction in QRS duration, Tp-Te duration and Tp-Te/QTc ratio while RVP increased these durations.
Pre-implantation QTc duration, Tpeak to Tend (Tp-Te) duration and Tp-Te/QTc ratio were comparable. LBBP and HBP resulted in significant reduction in QRS duration (Fig. 1), Tp-Te duration and Tp-Te/QTc ratio after the procedure while RVP was associated with significant prolongation (Table 2). Paced QTc duration at implant was less in LBBP and HBP as compared to RVPg (458.8 ± 29.1 ms vs 457.5 ± 20.2 ms vs 497.4 ± 38.1 ms respectively; p value <0.0001). Tp-Te duration after pacing was less in LBBP and HBP group as compared to RVP (88.5 ± 13.8 ms vs 79.7 ± 18.6 ms vs 120.3 ± 9.9 ms respectively; p value <0.0001). Tp-Te/QTc ratio, a better index of arrhythmogenesis was significantly less in LBBP and HBP as compared to RVP (0.24 ± 0.02 vs 0.19 ± 0.02 vs 0.17 ± 0.04 respectively; p value <0.0001).
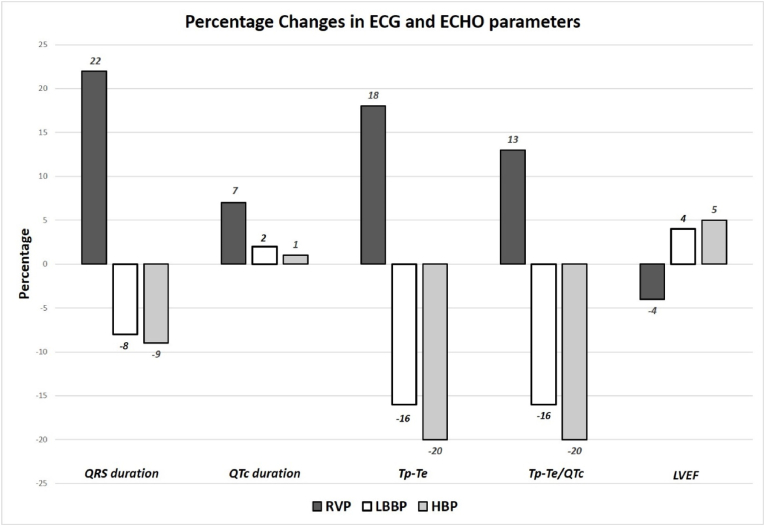
The parameters were also calculated as percentage change from the baseline values (Table 3; Fig. 2). The percent QRS duration reduction was greater with HBP and LBBP while it increased with RVP (−9±23% vs −8±18% vs +23 ± 25% respectively; p value <0.0001). Though the mean QTc was low in LBBP and HBP, post paced ECG showed less increase in QTc from baseline in LBBP and HBP as compared to RVP (+2 ± 12% vs +1 ± 9% vs +7 ± 14% respectively; p value 0.14). There was a significant reduction in Tp-Te duration after pacing (−16 ± 15% vs −20 ± 17% vs +18 ± 20% respectively; p value <0.0001) and Tp-Te/QTc ratio (−16 ± 16% vs −20 ± 19% vs +13 ± 22% respectively; p value <0.0001) in LBBP and HBP group as compared to a significant increase after RVP. Both modalities of physiological pacing (HBP and LBBP) resulted in better depolarization-repolarization parameters as compared to RVP. Along with reduction in QRS duration, there was a significant reduction in Tp-Te duration and Tp-Te/QTc ratio. These changes could possibly translate clinically in a reduction in the incidence of pacing induced cardiomyopathy and ventricular arrhythmias due to transmural dispersion in repolarization.
Table 3.
Change in ECG and echocardiographic parameters after RVA pacing, LBBP and HBP.
| % Change | RVP (n = 41) | LBBP (n = 45) | HBP (n = 35) | P value (ANOVA) | P value (LBB Vs RV) | P value (LBB Vs HB) |
|---|---|---|---|---|---|---|
| QRS duration | +23% ± 25% | −8% ± 18% | −9% ± 23% | <0.0001 | <0.0001 | 0.12 |
| QTc duration | +07% ± 14% | +02% ± 12% | +01% ± 09% | 0.06 | 0.08 | 0.68 |
| Tp-Te | +18% ± 20% | −16% ± 15% | −20% ± 17% | <0.0001 | <0.0001 | 0.26 |
| Tp-Te/QTc | +13% ± 22% | −16% ± 16% | −20% ± 19% | <0.0001 | <0.0001 | 0.31 |
| EF | −04% ± 12% | +04% ± 08% | +05% ± 11% | 0.001 | 0.0006 | 1.0 |
Fig. 2.
Percentage changes in QRS, QTc, Tp-Te duration, Tp-Te/QTc ratio and LVEF. Both LBBP and HBP showed significant reduction in QRS duration, Tp-Te duration, Tp-Te/QTc ratio along with improvement in LVEF during follow-up.
2.4. Depolarization - Repolarization parameters in HBP vs LBBP
Both HBP and LBBP resulted in significant reduction in paced QRS duration from the baseline though there were no significant differences between the 2 groups. QTc duration, Tp-Te duration and Tp-Te/QTc ratio after pacing were not significantly different between the two groups. This suggests LBBP as a comparable alternative to HBP in terms of post pacing depolarization-repolarization parameters.
2.5. Follow-up data
Patients were followed up in the device clinic at the end of 15 days, 3 months and yearly thereafter. Lead parameter analysis and echocardiography were done at one-year follow-up. Mean follow up duration was 36.7 ± 7.1 months. LBBP group showed low and stable threshold during follow up (0.5 ± 0.1V at 0.5 ms pulse-width) as did the RVP group (0.4 ± 0.1V at 0.4 ms pulse-width). 1 patient in HBP group (3%) had progressive increase in capture threshold requiring lead repositioning at 12 months. Two patients had increase in HBP threshold >1V but remained stable. Overall, the pacing threshold remained stable during follow-up (1.1±-0.5 at 0.9 ± 0.2 ms to 1.2 ± 0.6 at 0.7 ± 0.3 ms; p = 0.18). There were no lead related complications in LBBP and RVP groups.
2.6. Echocardiographic parameters
While LV ejection fraction showed a trend towards non-significant improvement after LBBP and HBP (58.3 ± 5.8% to 60.3 ± 4.9%, p = 0.09; 59.3 ± 6.4% to 61.5 ± 3.8%, p = 0.08, respectively), RVP group showed a non-significant reduction (61.2 ± 4% to 58.7 ± 8.2%, p= 0.08) during follow-up. Pacing induced cardiomyopathy (PIC) was not seen in LBBP and HBP group. Five (12%) patients in the RVP group developed PIC during follow-up (p value 0.02) (Table 4). Mean LVEF significantly decreased from 58 ± 5% to 40 ± 4% (p = 0.0002) during follow-up along with increase in LV end diastolic diameter from 51±6 mm to 55±7 mm (p = 0.36). Patients who developed PIC had higher pre-implantation QRS duration, LV end-diastolic diameter and Tpeak to Tend duration. Similarly, post implantation ECG showed higher Tp-Te in patients who developed PIC (132±4 ms vs 120 ± 10 ms; p value 0.01) as compared to those with preserved LV function. Two patients with PIC had undergone upgradation to LBBP and one to HBP. The remaining 2 patients refused for upgradation to either physiological or biventricular pacing. PIC was not seen in LBBP or HBP group despite having comparable baseline ECG and echocardiographic parameters. Physiological pacing resulted in reduction in all depolarization-repolarization parameters along with significant improvement in LV ejection fraction.
Table 4.
Comparison of patients with and without pacing induced cardiomyopathy (PIC) in RV pacing group. Baseline LV dimension, QRS duration and Tp-Te duration were significantly higher in patients who developed PIC. Similarly, these patients had higher paced Tp-Te duration as compared to those without PIC.
| PIC (n = 5) | No PIC (n = 36) | P value | |
|---|---|---|---|
| Age (years) | 59 ± 13 | 64 ± 11 | 0.35 |
| Baseline | |||
| LVEF (%) | 58 ± 5 | 61 ± 3 | 0.06 |
| LVID (mm) | 51 ± 6 | 44 ± 4 | 0.001 |
| QRS duration (ms) | 148 ± 5 | 126 ± 22 | 0.04 |
| QTc (ms) | 468 ± 19 | 476 ± 57 | 0.75 |
| Tp-Te (ms) | 118 ± 4 | 103 ± 13 | 0.02 |
| Tp-Te/QTc ratio | 0.25 ± 0.01 | 0.22 ± 0.04 | 0.10 |
| After Pacing | |||
| LVEF (%) | 40 ± 4 | 61 ± 4 | <0.0001 |
| LVID (mm) | 55 ± 7 | 46 ± 4 | 0.001 |
| QRS duration (ms) | 160 ± 20 | 152 ± 12 | 0.23 |
| QTc (ms) | 509 ± 29 | 496 ± 38 | 0.468 |
| Tp-Te (ms) | 132 ± 4 | 120 ± 10 | 0.01 |
| Tp-Te/QTc ratio | 0.25 ± 0.01 | 0.24 ± 0.02 | 0.28 |
3. Discussion
In our study of 121 patients with preserved LV function undergoing pacing for symptomatic AV block, on-treatment comparison showed (a) LBBP was associated with low and stable pacing thresholds comparable to RVP in patients with AV block and preserved LV function (b) Both LBBP and HBP were associated with significant reduction in paced QRS duration, Tp-Te duration and Tp-Te/QTc ratio while RVP increased these parameters (c) All three modalities increased QTc from the baseline while paced QTc duration was significantly less in LBBP and HBP as compared to RVP (d) As a physiological pacing modality, LBBP was not inferior to HBP in terms of reduction in paced QRS duration and depolarization-repolarization parameters (e) PIC was noted only in RVP group (12%, n = 5) while LBBP and HBP was associated with preserved LV function(f) Patients with PIC had higher baseline QRS duration and LV end-diastolic diameter and greater increase in Tp-Te duration after pacing compared to patients who did not develop PIC with RVP. This is the first study to compare the depolarization-repolarization parameters in patients with AV block and preserved LV function undergoing either physiological or RVA pacing.
RVP produces LBBB pattern on the surface ECG resulting in asynchronous contraction of ventricle similar to that observed in native LBBB [19]. Interventricular and intraventricular dyssynchrony induced by RVP can result in adverse clinical outcomes with chamber dilatation, atrial arrhythmias and heart failure hospitalization (HFH) [20]. Post hoc analysis of both DAVID and MOST trials suggested a threshold of >40% RV pacing in DDD mode predicted HFH [21]. BLOCK-HF trial prospectively randomized high-grade AV block patients with LVEF ≤50% to standard RV vs biventricular pacing and they have demonstrated that RV pacing was associated with 35% higher incidence of composite endpoint of death, HF hospitalization or >15% increase in LV endsystolic volume index [22]. Kiehl EL et al. [23] showed that PIC is not uncommon in patients undergoing RV pacing for AV block with preserved LV function (LVEF>50%) and is strongly associated with pacing burden >20%. Wang et al. [24], showed repolarization and depolarization measures are better in LBBP group as compared to RV pacing among patients undergoing cardiac pacing for various indications.
3.1. Depolarization-Repolarization parameters
Tp-Te duration and Tp-Te/QTc ratio are better measures of transmural dispersion of ventricular repolarization and arrhythmogenesis. Tp-Te/QTc has the added advantage of not being affected by variation in heart rate. Fuenmayor AJ et al. [25] showed that isolated stimulation of both right and left ventricle produced similar increase in QTc, Tp-Te and Tp-Te/QTc ratio in patients without structural heart disease. Left ventricular pacing produced similar perturbation as right ventricular apical pacing. Biventricular pacing also produced a significant increase in all these parameters but shorter than those obtained during isolated LV or RV. In our cohort of patients LBBP resulted in 16% reduction in Tp-Te duration and 16% reduction in Tp-Te/QTc ratio. Similarly, HBP resulted in 20% reduction in Tp-Te duration and 20% reduction in Tp-Te/QTc ratio. HBP showed a trend towards greater reduction as compared to LBBP (Table 3). These findings will place physiological pacing a step above biventricular pacing in terms of stabilizing the ventricular myocardium by causing minimal transmural dispersion of repolarization and likely reduction in incidence of ventricular arrhythmias.
3.2. Ventricular conduction time in HBP, LBBP and RVAP
RVP is associated with long ventricular conduction time as indicated by prolonged QRS duration in the 12 lead ECG. In the MOST trial HF hospitalization increased linearly with paced QRS duration [26]. Prolonged QRS duration after implantation is an indicator that dyssynchrony related remodeling had already occurred rather than just a marker for the development of PIC [27]. Khurshid et al. [28] showed that a paced QRS duration of >150 ms was 95% sensitive for diagnosing PIC and warranted periodic screening in the absence of HF symptoms. In our study, 12% of patients in RVP group showed PIC. Patients who developed PIC had higher baseline QRS duration, LV dimension and Tp-Te duration. Patients who received physiological pacing (LBBP and HBP) had significantly narrower paced QRS duration as compared RVP (115.8 ± 9.2 ms vs 111.1 ± 16.5 ms vs 153.6 ± 13.7 ms respectively; p value <0.0001). Narrow paced QRS duration due to physiological activation of conduction system would confer a benefit of avoiding PIC in this group of patients.
The Pacing to Avoid Cardiac Enlargement trial (PACE) [29] showed in patients with AV block and preserved LVEF, the superiority of biventricular pacing in prevention of adverse LV remodeling and worsening of LV function. At 2-year follow-up the study showed a rate of 15% incidence of PIC after RVP. But compared to conventional pacemaker, biventricular device implantation is associated with two-fold increase of peri-procedural complications [30,31]. CS lead dislodgment at a rate of 3–7% across major trials [32] is another major concern. Given the unpredictable occurrence of PIC along with high cost and complication rates of biventricular pacing, it is not reasonable to propose biventricular pacing therapy for all patients with symptomatic AV block in the absence of structural heart disease or LV dysfunction. Conduction system pacing (LBBP and HBP) has the potential to fill the gap by providing physiological activation of the ventricle, reducing the measures of transmural dispersion of repolarization and narrower paced QRS complexes thereby reducing the risk of pacing induced cardiomyopathy and ventricular tachyarrhythmias.
3.3. HBP vs LBBP
HBP group had better electrocardiographic parameters than LBBP but can be associated with higher capture threshold, lead revision for late rise in threshold, earlier battery depletion, longer learning curve and lower success for correction of distal conduction system disease. In a study by Vijayaraman et al. [33] involving 100 patients, AV nodal block could be corrected in 93% of patients while infra-nodal block had only 76% success rate with HBP. In our study we could show that both HBP and LBBP group showed significant reduction in paced QRS duration. The magnitude of improvement in depolarization-repolarization parameters were comparable between the groups. Pacing thresholds were higher with HBP with increased risk for threshold increase and lead revision. This would make LBBP as an attractive alternative to RVP in patients with normal LV function and AV block.
3.4. Limitations
This is a retrospective, non-randomized, on-treatment comparison of small group of patients with Class I indications for permanent pacemaker implantation with its inherent limitations. Baseline QRS duration was measured during native escape rhythm with RBBB or LBBB morphology in all three groups of the study population which can be considered as a limitation for comparing it with paced QRS duration. As patients were chosen on non-randomized fashion for different types of pacing therapy, the inherent problem of selection bias is another major limitation in our study. The safety of deep septal placement of the lead, the effect of myocardial contraction on the lead insulation and risks of LBBP lead extraction has not been well-studied.
4. Conclusions
Both LBBP and HBP had a favorable effect on transmural dispersion of ventricular repolarization compared to RVP. As LBBP overcomes the limitations of HBP, future studies will help in establishing this therapy to be an effective alternative to routine RVP for AV block with normal LV function. Multicenter, randomized trials are needed to confirm the potential clinical benefits of conduction system pacing.
Funding
None.
Disclosures
SSP – Consultant, Medtronic; PV -Speaker, Consultant, Research, Fellowship support – Medtronic; Consultant – Abbott, Biotronik, Boston Scientific; Patent- HBP delivery tool.
Declaration of competing interest
We wish to submit a research brief article entitled “Pacing for Atrioventricular block with preserved left ventricular function: On-Treatment Comparison between His bundle, left bundle branch, and right ventricular pacing” for consideration by IPEJ for the special issue on conduction system pacing.
We confirm that this work is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere.
Conduction system pacing has gained significant momentum due to availability of better tools. In this study we have compared the electrocardiographic, depolarization-repolarisation parameters and clinical outcomes of patients with AV block and preserved LV function who underwent HBP, LBBP and RVP. Both LBBP and HBP resulted in significant reduction in QRS duration, Tp-Te duration and Tp-Te/QTc ratio as compared to RVP which increased these parameters. 12% of patients in RVP group developed pacing induced cardiomyopathy whereas both HBP and LBBP preserved the LV function during follow-up.
The study was conducted after getting the ethical committee approval.
We have no conflicts of interest to disclose.
The Paper is not under consideration elsewhere. All authors have read and approved the manuscript.
Acknowledgement
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Poole J.E., Singh J.P., Birgersdotter-Green U. QRS duration or QRS morphology: what really matters in cardiac resynchronization therapy? J Am Coll Cardiol. 2016;67:1104–1117. doi: 10.1016/j.jacc.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 2.Tops L.F., Schalij M.J., Bax J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol. 2009;54:764–776. doi: 10.1016/j.jacc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C., Sicouri S., Litovsky S.H., et al. Heterogeneity within the ventricular wall: electrophysiology and pharmacology of epicardial, endocardial and M cells. Circ Res. 1991;69:1427. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 4.Gupta P., Patel C., Patel H., et al. Tp-e/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Lee M., Janardhan A., Kang K., et al. Paced QT interval is a better predictor of mortality than the intrinsic QR interal: long term follow-up study. Heart Rhythm. 2014;11:1184–1189. doi: 10.1016/j.hrthm.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Deshmukh P., Casavant D.A., Romanyshyn M., et al. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normalHis-Purkinje activation. Circulation. 2000;101:869–877. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 7.Vijayaraman P., Chung M., Dandamudi G., et al. His bundle pacing. J Am Coll Cardiol. 2018;72:927–947. doi: 10.1016/j.jacc.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Huang W., Su L., Wu S., et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736. doi: 10.1016/j.cjca.2017.09.013. e1–3. [DOI] [PubMed] [Google Scholar]
- 9.Ponnusamy S.S., Muthu G., Kumar M., et al. Mid-term feasibility, safety and outcomes of left bundle branch pacing – single center experience. J Intervent Card Electrophysiol. 2020 doi: 10.1007/s10840-020-00807-w. [DOI] [PubMed] [Google Scholar]
- 10.Vijayaraman P., Subzposh F.A., Naperkowski A., et al. Prospective evaluation of feasibility, electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019;16:1774–1782. doi: 10.1016/j.hrthm.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Li X., Li H., Ma W., et al. Permanent left bundle branch area pacing for atrioventricular block: feasibility, safety, and acute effect. Heart Rhythm. 2019;16:1766–1773. doi: 10.1016/j.hrthm.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Khurshid S., Epstein A., Verdino R., et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11:1619–1625. doi: 10.1016/j.hrthm.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Kaye G., Ng J.Y., Ahmed S., Valencia D., Harrop D., Ng A.C.T. The prevalence of pacing-induced cardiomyopathy (PICM) in patients with long term right ventricular pacing - is it a matter of definition? Heart Lung Circ. 2018;28:1027–1033. doi: 10.1016/j.hlc.2018.05.196. [DOI] [PubMed] [Google Scholar]
- 14.Ponnusamy S.S., Arora V., Namboodiri N., et al. Left bundle branch pacing: a comprehensive review. J Cardiovasc Electrophysiol. 2020;31(9):2462–2473. doi: 10.1111/jce.14681. [DOI] [PubMed] [Google Scholar]
- 15.Ponnusamy S.S., Vijayaraman P. Electrocardiography guided left bundle branch pacing. J Electrocardiol. 2021;68:11–13. doi: 10.1016/j.jelectrocard.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Ponnusamy S.S., Ganesan V., Syed T., et al. Template Beat: a novel marker for left bundle branch capture during physiological pacing. Circ Arrhythm Electrophysiol. 2021;14(4) doi: 10.1161/CIRCEP.120.009677. [DOI] [PubMed] [Google Scholar]
- 17.Jastrzebski M., Keilbasa G., Curila K., et al. Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm. 2021;18(6):935–943. doi: 10.1016/j.hrthm.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Ananalysis BazettHC. Of the time relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 19.Tops L.F., Schalij M.J., Bax J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony. J Am Coll Cardiol. 2009;54:764–776. doi: 10.1016/j.jacc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen J.C., Kristensen L., Andersen H.R., Mortensen P.T., Pedersen O.L., Pedersen A.K. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003;42:614–623. doi: 10.1016/s0735-1097(03)00757-5. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney M.O., Hellkamp A.S., Ellenbogen K.A., et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 22.Curtis A.B., Worley S.J., Chung E.S., Li P., Christman S.A., St John Sutton M. Improvement in clinical outcomes with biventricular versus right ventricular pacing: the BLOCK HF Study. J Am Coll Cardiol. 2016;67:2148–2157. doi: 10.1016/j.jacc.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Kiehl E.L., Makki T., Kumar R., et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., Liang Y., Wang W., et al. Left bundle branch area pacing is superior to right ventricular septum pacing concerning depolarization-repolarization reserve. J Cardiovasc Electrophysiol. 2020;31:313–322. doi: 10.1111/jce.14295. [DOI] [PubMed] [Google Scholar]
- 25.Fuenmayor A.J., Delgado M.E. Ventricular repolarization during uni and biventricular pacing in normal subjects. Int J Cardiol. 2013;165:72–75. doi: 10.1016/j.ijcard.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 26.Sweeney M.O., Hellkamp A.S., Ellenbogen K.A., et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 27.Merchant F.M., Mittal S. Pacing-induced cardiomyopathy. Cardiac Electrophysiol Clin. 2018;10:437–445. doi: 10.1016/j.ccep.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Khurshid S., Liang J.J., Owens A., et al. Longer paced QRS duration is associated with increased prevalence of right ventricular pacing induced cardiomyopathy. J Cardiovasc Electrophysiol. 2017;27:1174–1179. doi: 10.1111/jce.13045. [DOI] [PubMed] [Google Scholar]
- 29.Chan J.Y.S., Fang F., Zhang Q., et al. Biventricular pacing is superior to right ventricular pacing in bradycardia patients with preserved systolic function: 2-year results of the PACE trial. Eur Heart J. 2011;32:2533–2540. doi: 10.1093/eurheartj/ehr336. [DOI] [PubMed] [Google Scholar]
- 30.Leon A.R., Abraham W.T., Curtis A.B., Daubert J.P., Fisher W.G., Gurley J., et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol. 2005;46:2348–2356. doi: 10.1016/j.jacc.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 31.Pavia S., Wilkoff B. The management of surgical complications of pacemaker and implantable cardioverter-defibrillators. Curr Opin Cardiol. 2001;16:66–71. doi: 10.1097/00001573-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Dreger H., Maethner K., Bondke H., et al. Pacing induced cardiomyopathy in patients with right ventricular stimulation for >15 years. Europace. 2012;14:238–242. doi: 10.1093/europace/eur258. [DOI] [PubMed] [Google Scholar]
- 33.Vijayaraman P., Naperkowski A., Ellenbogen K.A., et al. Electrophysiologic insights into site of atrioventricular block. JACC Clin Electrophysiol. 2015;1:571–581. doi: 10.1016/j.jacep.2015.09.012. [DOI] [PubMed] [Google Scholar]