Abstract
Soil legacy influences plant interactions with antagonists and below-ground mutualists. Plant–antagonist interactions can jeopardize plant–pollinator interactions, while soil mutualists can enhance plant–pollinator interactions. This suggests that soil legacy, either directly or mediated through plant symbionts, affects pollinators. Despite the importance of pollinators to natural and managed ecosystems, information on how soil legacy affects plant–pollinator interactions is limited. We assessed effects of soil management legacy (organic versus conventional) on floral rewards and plant interactions with wild pollinators, herbivores, beneficial fungi and pathogens. We used an observational dataset and structural equation models to evaluate hypothesized relationships between soil and pollinators, then tested observed correlations in a manipulative experiment. Organic legacy increased mycorrhizal fungal colonization and improved resistance to powdery mildew, which promoted pollinator visitation. Further, soil legacy and powdery mildew independently and interactively impacted floral traits and floral reward nutrients, which are important to pollinators. Our results indicate that pollination could be an overlooked consequence of soil legacy and suggests opportunity to develop long-term soil management plans that benefit pollinators and pollination.
Keywords: soil legacy, plant resistance, ecosystem services, aboveground–belowground interactions, floral resources, pollinator nutrition
1. Introduction
Ecological history leaves legacy effects on soil properties and subsequent plants' traits such as resistance to antagonists, including herbivores and pathogens [1–4]. Plant antagonists can negatively influence plant–pollinator interactions, and therefore may mediate soil legacy effects on pollinators [5]. Despite the critical role that pollinators play in ecosystem function [6], the lingering impacts of long-term soil conditioning on pollinators are poorly characterized. Consequently, it is challenging to predict environmental contexts that shift the relationship between antagonists and pollinators, [7] to identify mechanisms by which soil legacies affect plant community composition [1], or to evaluate whether soil management has lasting repercussions on pollinators or pollination services.
Plant antagonists influence the ecological outcomes of plant–pollinator interactions. Herbivory and pathogen infection can affect pollen, nectar quality and floral traits, often jeopardizing plant–pollinator interactions [5,8,9]. For herbivory, factors such as feeding site vary in the strength and direction of their effects on pollinators [10]. The comparatively smaller body of work on plant–pathogen–pollinator interactions focuses disproportionately on floral pathogens rather than foliar pathogens, which may affect floral traits indirectly [11,12]. Moreover, we have a poor understanding of how the outcomes of plant–antagonist–pollinator interactions depend on broader ecological contexts, such as soil environment. While poor soil conditions could amplify the negative effects of antagonists on floral traits, high quality soil could buffer or even reverse these negative effects through overcompensation in the form of increased flower production or pollinator attraction [13–15]. Therefore, the costs and benefits of different agricultural soil management practices may depend on how these practices affect plant biotic interactions.
Long-term soil conditioning affects soil properties, future plant growth and resistance to antagonists, often in ways that are distinct from the effects from short-term conditioning [16–20]. In agriculture, long-term organic management and cover cropping can increase nutrient availability and promote microbial mutualists such as mycorrhizal fungi [21–25]. Crucially, some of the same nutrients and soil microbes affected by soil legacy also shape crop–antagonist interactions. For instance, residual excesses or deficits in soil nutrients [26] can affect leaf composition, and consequently, resistance to herbivores and pathogens [27,28]. Additionally, microbial isolates from soils under long-term organic management enhance plant resistance to herbivores [3]. While not typically considered in this framework, soil legacy likely also affects floral traits and plant–pollinator interactions.
Soil legacy could indirectly or directly influence plant–pollinator interactions. We expect organic soil legacy to benefit floral traits and pollinator attraction by improving plant resistance to antagonists or by preserving microbial mutualisms (e.g. mycorrhizal fungi) that promote floral quality [29,30]. Short-term studies demonstrate that inadequate or excessive soil fertility can negatively impact floral reward nutritional quality [31–33]. However, little work has investigated the legacy effects of long-term management practices on flowers, which may attenuate or magnify with soil conditioning duration or time since application [16,34–39]. Therefore, current evidence is insufficient to develop a predictive framework for how long-term soil management influences floral traits, pollinator fitness, or pollination. Furthermore, soil legacy effects may differ based on pollinator diet breadth because bees that specialize on a narrow range of plant taxa may have decreased ability to forage flexibly in response to changes in diet quality.
Our objective was to understand whether soil legacy affects pollinators, and if so, to identify possible mechanisms. In Experiment 1, we assessed how organic versus conventional soil legacy affects plant interactions with mycorrhizal fungi, powdery mildew, herbivores and pollinators. As soil management practices within organic and conventional management systems vary widely, we also compared the effects of different nutrient regimes within each management type. Within organic systems, we compared a legacy of high- versus low-nutrient amendments. Within conventional systems, we compared a legacy of green manures (tilled-in cover crops) versus synthetic fertilizer inputs. To test direct, indirect and interactive effects of (a) soil legacy, (b) plant antagonists, (c) pollen quality and (d) floral abundance on pollinator abundance, we used structural equation modeling (SEM), a statistical approach that evaluates complex relationships among multiple potentially correlated variables (figure 1).
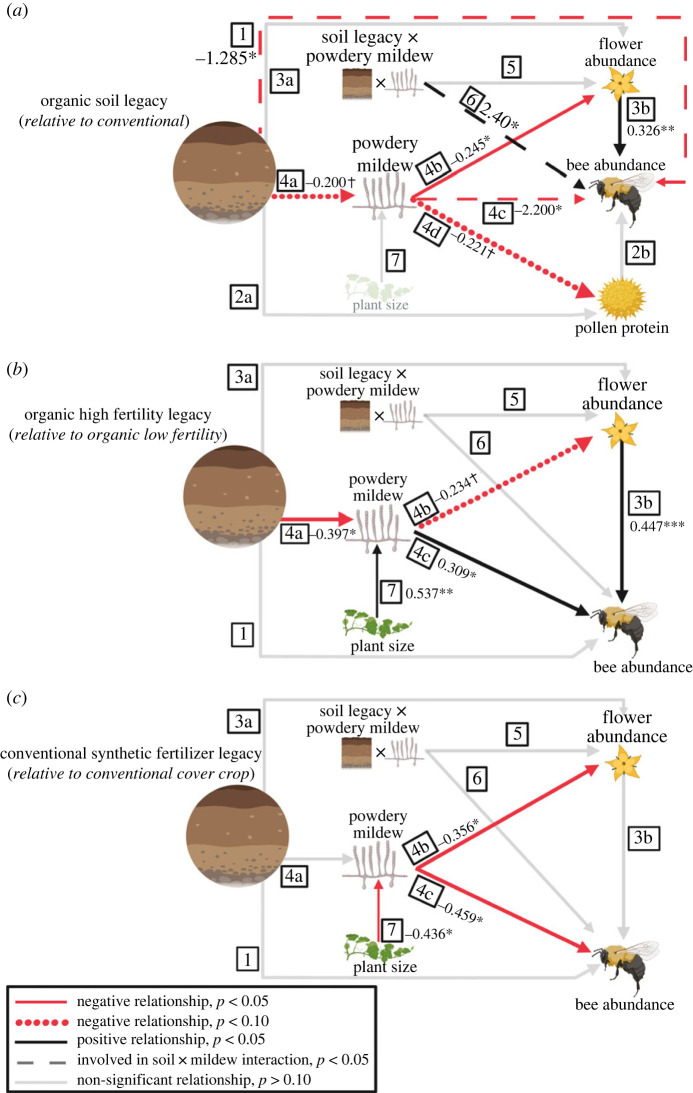
Figure 1.
Hypothesized and observed relationships between soil legacy and pollinator visitation. We tested the hypothesis that an organic compared with conventional management legacy would compromise plant–pollinator interactions. We predicted this would happen directly (H1) and indirectly by increasing pollen quality (H2a, H2b) and/or increasing floral abundance (H3a, H3b). We hypothesized reduced powdery mildew due to soil (H4a) would increase floral abundance (H4b), bee visitation (H4c) and pollen quality (H4d). Additionally, we hypothesized that plants in organic legacy soils would compensate for negative effects of powdery mildew on floral abundance (H5) and bee abundance (H6). All comparisons between soil and response variables are made in reference to (a) conventional soil legacy, (b) organic low fertility and (c) conventional cover crop, and line colour denotes direction of the effect (e.g. a red line between soil and powdery mildew indicates a negative effect of organic compared with conventional soil legacy on powdery mildew infection). Solid lines indicate significant relationships (p < 0.05). Circular-dashed lines indicate relationships where p < 0.1, and faded grey lines indicate nonsignificant relationships (p < 0.10). Rectangular-dashed lines indicate that the variable is involved in a significant soil × powdery mildew interaction; in (a), the positive effect of the interaction term indicates that the negative relationship between powdery mildew and bee abundance in conventional soils was nullified for plants in organic soils. Values represent standardized coefficients, which provide an estimate of the relative effect size of variables measured at different scales. Asterisks represent significance level (*** p < 0.001, ** p < 0.01, * p < 0.05, † p < 0.10).
Because Experiment 1 suggested that soil legacy indirectly affects floral traits and pollinators by changing plant resistance to powdery mildew, we inoculated plants grown in the different legacy soils with powdery mildew in Experiment 2. This manipulation in Experiment 2 tests how soil legacy affects powdery mildew resistance and how soil, powdery mildew, and their interaction affects floral traits important to pollinators. To understand whether soil microbes might mediate soil effects on aboveground interactions, we quantified mycorrhizal colonization. Finally, in Experiment 3, we fed bumble bees with pollen collected from plants grown in Experiment 2 to test whether soil- or powdery mildew-driven changes to pollen quality affect bee colony development.
2. Material and methods
Squashes (genus Cucurbita; Cucurbitaceae) are globally cultivated, pollinator-dependent crops [40,41] with economically important herbivores and pathogens [42,43]. We documented the herbivorous beetles Diabrotica virgifera, Diabrotica barberi, Acalymma vittatum (Chrysomelidae) and the squash bug Anasa tristis (Coreidae). We also documented powdery mildew (Podosphaera xanthii) [44], a primarily wind-borne pathogen of Cucurbits [45]. For pollinators, we observed the specialist squash bee Peponapis pruinosa (Apidae), the generalist bees Apis mellifera, Bombus spp. (Apidae), Lasioglossum spp., Augochlorella spp. and Agapostemon spp. (Halictidae).
(a) . Experiment 1: field mesocosm experiment
We evaluated plant interactions with herbivores, pathogens, and pollinators from 16 June to 19 August 2020, in a mesocosm experiment using potted plants. We collected soils at 15 cm depth from 25 organic and conventional plots at the Musgrave Research Farm (Aurora, NY: 42°44'02.8″N 76°39'04.3″W) and the Homer C. Thompson Research Farm (Freeville, NY: 42°31'07.2″N 76°20'06.2″W) (electronic supplementary material, figure S1). Soils from conventional plots with a synthetic fertilizer legacy had received primarily synthetic fertilizer annually for at least 3 years (N = 5 plots; 3 from Freeville and 2 from Musgrave). Soils from conventional plots with a cover cropping legacy had received primarily green manures (tilled-in cover crops) with 0–1 total applications of synthetic fertilizer for at least 3 years (N = 4 plots; 3 from Freeville and 1 from Musgrave). Soils from organic plots came from two long-term organic management trials that each had low- and high-nutrient regimes comprised of a poultry manure and cover crops (Musgrave), and poultry manure, cover crops and compost (Freeville) (high nutrient: N = 8 plots; 4 plots per site, and low nutrient: N = 8 plots; 4 plots per site). Experimental organic plots were established in 2005 (Musgrave) and 2014 (Freeville) and were certified organic by the Northeast Organic Farming Association of New York (NOFA-NY).
At Freeville, cropping history for the previous three years included Brassica sp., Cucurbita sp. and Lactuca sativa in organic high and low nutrient plots; Cucurbita sp.: Zea mays, Solanum tuberosum, Solanum lycopersicum, Trifolium pratense and Secale cerale in conventional cover crop plots; and Brassica sp., Cucurbita sp., S. tuberosum, Sorghum bicolor (L.) Moench × Sorghum sudanense (Piper) Stapf, S. cerale, and Trifolium repens in conventional synthetic plots. At Musgrave, cropping history for the previous three years included Z. mays, Glycine max, S. cereale, Triticale, Lolium perenne, T. pratense, Melilotus officinalis and S. bicolor in organic high- and low-nutrient plots; Z. mays and G. max in conventional synthetic plots; and T. pratense and Tritucum aestivum in conventional cover crop plots. Therefore, while there were broad differences in management, there was also variation in cropping history (electronic supplementary material, table S1).
Using soils described above, we conducted a common garden mesocosm experiment at Bluegrass Research Center in Ithaca, NY, USA (42°27'34.4″N 76°27'38.7″W). We seeded summer squash (Cucurbita pepo) (‘Success PM’, High Mowing Seeds, Wolcott, VT, USA) in 11.4-litre pots (5–10 pots for each of the 25 plots for a total of 137 replicates, 130 of which survived the entire experiment) in a randomized design on 16 June 2020.
We measured vegetative growth, powdery mildew infection, herbivory, bee visitation, pollen protein and flower abundance. We calculated the proportion of herbivore- and powdery mildew-damaged leaves by dividing the number of damaged or infected leaves by the total number of leaves. To evaluate pollinator visitation, we conducted two-minute timed trials, visiting each plant three times during peak bloom in August between 6.30 am and 12.30 pm, on sunny, low wind days (18°C to 29°C). We recorded the taxa of pollinators that landed on flowers to the highest possible resolution. We collected pollen in July and August by brushing pollen off anthers with a metal spatula, then stored it at −80°C. To measure pollen protein content, we used a Pierce BCA protein quantification kit (electronic supplementary material, material S1).
(b) . Experiment 2: greenhouse manipulative experiment
To pinpoint the role of powdery mildew, we conducted a greenhouse experiment. We manipulated soil type and powdery mildew, then measured powdery mildew infection intensity (proportion leaf area infected), flowering phenology, pollen and nectar quality, and floral morphology. We collected soil from the Freeville organic trials and from adjacent conventional plots (electronic supplementary material, table S2). There were no differences between synthetic fertilizer and cover crop legacy in the mesocosm experiment. Therefore, we did not make this comparison again in the greenhouse experiment. Cropping history for the previous three years in conventional plots included Brassica sp., Cucurbita sp., Beta vulgaris, Spinacia oleracea, Solanum lycopersicum, S. cereale, T. pratense, S. bicolor, Triticum aestivum, Hordeum vulgare, Allium sativum and Z. mays. Cropping history for the previous three years in organic high- and organic low-nutrient plots included Brassica sp., Cucurbita sp., Lactuca sativa, and Beta vulgaris.
On 16 May 2021, we seeded summer squash (Success PM) in 11.4-liter pots in four greenhouses at Cornell University, in a fully factorial design manipulating soil legacy and powdery mildew infection. To prevent contamination, powdery mildew-inoculated plants were kept separate from control plants in two greenhouses each. We collected conventional soils from 7 plots and organic soils from 8 plots (4 high-fertility plots and 4 low-fertility plots) with between 5 and 9 replicate plants for each of the 15 plots. Plants were arranged in a randomized array, and we accounted for spatial position within each greenhouse by assigning plants to spatial blocks.
When plants were three weeks old, we inoculated powdery mildew treatment plants with Podosphaera xanthii (McGrath Laboratory, Cornell Long Island Research and Extension Center, Riverhead, NY, USA). We used a flame-sterilized disposable pipette to transfer fungal spores from the edge of the powdery mildew culture by gently brushing first the sporulating culture and then the leaf with the pipette tip onto three leaves per plant [46]. We repeated the same procedure on control plants using a sterile pipette.
When plants were 6–7 weeks old (approx. 3 weeks post-inoculation), we measured powdery mildew area using calipers. We used LeafByte [47] to calculate average leaf area using a young, middle-aged, and old leaf. To estimate whole plant leaf area, we multiplied average leaf area by the number of leaves on the plant, then calculated the proportion of powdery mildew by dividing powdery mildew area by total leaf area. We measured pollen protein following methods described above, and corolla width using digital calipers (Bioquip Products, Inc., Compton, CA, USA). We measured nectar volume using calipers and microcapillary tubes (Drummond Scientific, Broomall, PA, USA) and sugar concentration with a refractometer (Neta Scientific, Hainesport, NJ, USA). Nectar samples were diluted with 100 uL of distilled water and Brix values back-calculated if volume or sugar concentration were too low or too high respectively for the refractometer to provide an accurate reading. At the end of the experiment on 9 September 2021, we harvested roots for mycorrhizal fungi quantification (electronic supplementary material, material S2).
(c) . Experiment 3: Bee bioassays
We tested whether pollen quality changes due to powdery mildew treatment or soil legacy affect adult bee survival and colony development. We fed bumble bee (Bombus impatiens) micro-colonies pollen from plants grown in Experiment 2. To prepare micro-colonies, we placed five workers of comparable size from three source colonies into 16 oz plastic deli cups with mesh bottoms. We reared micro-colonies for one week on a wildflower pollen diet and 30% sucrose before starting experimental pollen diet treatments. Each micro-colony was fed ad libitum 30% sucrose, plus one of six experimental pollen diets from plants grown in Experiment 2 under the different soil (organic high-fertility, organic low-fertility, or conventional legacy) and powdery mildew (inoculated or control) conditions (N = 3 to 5 micro-colonies per treatment combination). Experimental diets were mixed in a 1 : 1 ratio with wildflower pollen. For one week after initiating the experimental diets, we recorded adult survival, pollen consumption, and new brood cells daily. See electronic supplementary material, material S3 for detailed rearing conditions.
(i) . Statistical analyses
We used RStudio v. 4.2.3 [48] for analyses. We used piecewiseSEM [49] and BioRender (see https://biorender.com/; accessed 29 September 2023) for SEM analyses and diagrams; lme4 [50] and DHARMa [51] for mixed effects models, and ggplot2 [52] to create graphs. In all analyses, we used AIC (Akaike Information Criterion) and parsimony to select the top model. To determine the effect of each predictor variable in mixed effects models, we used a likelihood ratio test to compare the full model to an identical model that excluded the variable of interest. Non-significant variables were removed from the final model if the simpler model yielded a lower AIC value. We evaluated pairwise differences using emmeans [53], correcting for multiple comparisons using false discovery rate. Where necessary, we transformed variables to meet model assumptions (electronic supplementary material, table S3).
(d) . Field mesocosm experiment
All models contained field nested within soil collection site as random effects.
We built three types of SEMs [54] (figure 1). First, we compared organic with conventional management legacy, regardless of nutrient legacy. Then, we examined the effects of nutrient legacy within each management system. For each comparison, we ran two SEMs: one included powdery mildew and the other included herbivory as the ‘plant antagonist’.
The SEMs comparing organic with conventional legacy were composed of the following models: (1) proportion leaf damage from plant antagonist dependent on soil, (2) floral abundance dependent on soil × plant antagonist damage, (3) pollen protein content dependent on soil and plant antagonist damage, and (4) bee visitation dependent on floral abundance, pollen protein and soil × plant antagonist damage. The SEMs comparing fertility legacies contained the same set of paths except for pollen protein due to sample size limitations introduced by the truncated datasets.
Because many plants never developed powdery mildew, we asked whether soil legacy improved resistance to any infection development or improved resistance once infected. We used a binomial model to test whether soil legacy, site or their interaction affected the presence of infection. Next, we used a beta regression to determine predictors of infection intensity only for plants that developed infection.
We tested the significance of soil legacy on abiotic soil traits with a permutation-based multivariate ANOVA using vegan [55] and the random forest classification algorithm [56] to identify variables of importance (electronic supplementary material, material S4).
(e) . Greenhouse manipulative experiments
All models contained greenhouse/field as nested random effects, and where applicable, plant ID and sampling date as random effects (electronic supplementary material, table S3). Percent differences amongst groups were calculated using raw data.
To evaluate nectar volume, sugar concentration, nectar sugar content (volume × sugar concentration) [57], floral width, floral abundance, pollen protein and mycorrhizae, we built mixed effect models with soil legacy, powdery mildew treatment and their interaction, and where applicable, nectar sampling time, as fixed effects. For nectar sugar models we also included whether the sample had been diluted as a random effect. We used a beta distribution for sugar concentration, a Poisson distribution for flower abundance, a binomial distribution for mycorrhizal colonization, and a Gaussian distribution for all other traits. For flowering phenology, we employed a mixed effects survival analysis [58]. In addition to testing whether the binary powdery mildew treatment affected floral traits, we also used these same modeling approaches to test whether infection intensity (proportion leaf area infected) correlated with floral traits for plants inoculated with powdery mildew.
(f) . Bee bioassays
To test whether experimental diet affected adult bee survival, we used mixed effects survival analyses which can be used to model the likelihood that an event has occurred over time. We used soil legacy, powdery mildew treatment, and their interaction as fixed effects and source colony as a random effect.
3. Results
To display the degree to which soil legacy effects were consistent across experiments, we present the results of each comparison (i.e. organic versus conventional, organic high-fertility versus organic low-fertility, etc.) from all three experiments alongside one another.
(a) . Organic versus conventional legacy
(i) . Field mesocosm experiment
The SEM tested indirect and direct relationships between soil legacy and pollinator visitation, and suggested relationships between soil legacy, powdery mildew, flowers, and bees (figure 1a; Fisher's C = 2.936 p = 0.817, 6 d.f.). Plants in organic soils had marginally less powdery mildew than plants in conventional soils (p = 0.093), and plants with higher powdery mildew infection produced fewer flowers (p = 0.030). The effect of soil legacy on bee abundance per plant depended on powdery mildew infection intensity, such that bee abundance negatively correlated with infection intensity only for plants in conventional soils (figure 1a, electronic supplementary material, figure S3; interaction term p = 0.014). Finally, plants with more powdery mildew tended to produce pollen with less protein (p = 0.057).
Plants in organic soils were less likely to develop any powdery mildew infection compared with those in conventional soils (electronic supplementary material, figure S4a; χ2 = 6.676, 1 d.f., p = 0.010). However, organic plants that developed infection had higher proportion leaf area infected when grown in Musgrave soils (electronic supplementary material, figure S5a; χ2 = 9.945, 1 d.f., p = 0.002).
Random forest identified aluminum, organic matter, and potassium content as important variables driving differences in soil composition (electronic supplementary material, material S4). Aluminum was lower in organic soils, while organic matter and potassium were higher in organic soils. Additionally, organic soils had higher phosphorus content (χ2 = 5.642, 1 d.f., p = 0.018).
(ii) . Greenhouse manipulative experiment
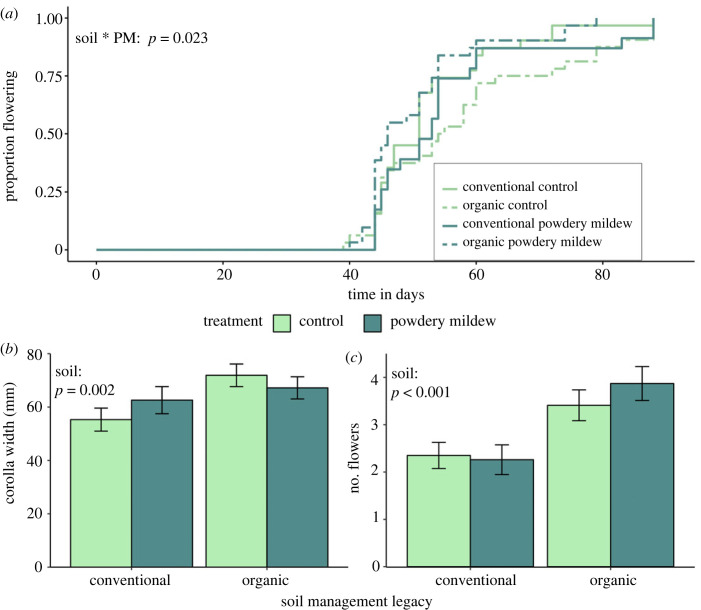
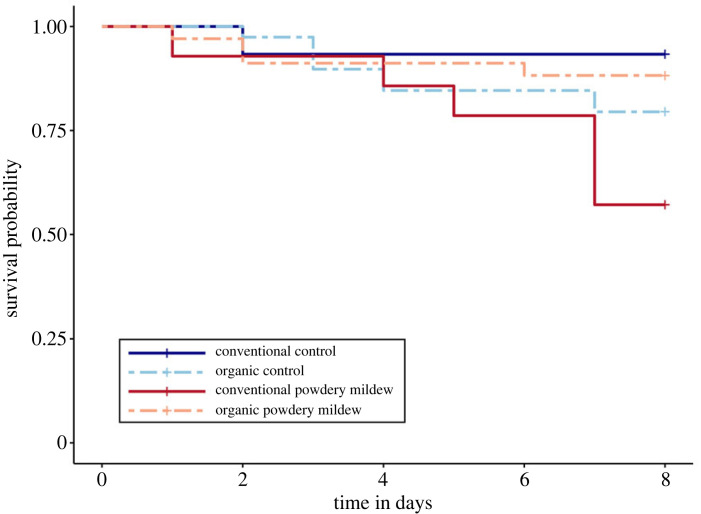
Powdery mildew and soil management legacy interacted to affect flowering phenology (figure 2a; χ2 = 5.202, 1 d.f., p = 0.023); powdery mildew tended to advance bloom only for plants in organic legacy soils (p = 0.08). Soil legacy impacted flower abundance and size (figure 2b,c; χ2 = 14.548, 1 d.f., p < 0.001 and χ2 = 9.837, 1 d.f., p = 0.002); flowers were larger and more abundant in organic soils. There was no effect of powdery mildew or the soil*powdery mildew interaction on flower abundance or width (electronic supplementary material, table S4).
Figure 2.
Effects of soil management legacy and powdery mildew on (a) flowering phenology, (b) flower abundance, and (c) flower size. (a) visualizes results from Cox proportional hazards model predicting soil and powdery mildew effects on plant flowering time. For (b–c), error bars represent model estimated marginal means ± 1 S.E.
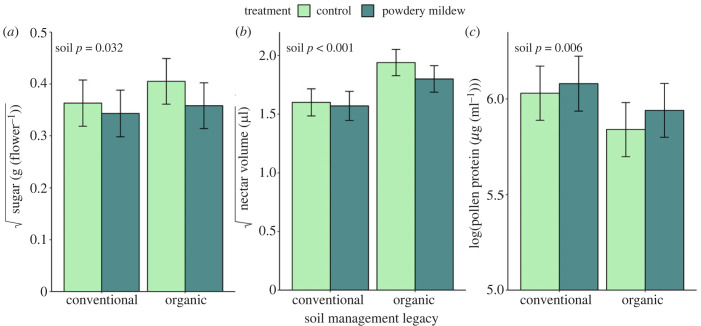
Soil legacy affected nectar sugar content, nectar volume and pollen protein content (figure 3a–c; χ2 = 4.619, 1 d.f., p = 0.032; χ2 = 17.109, 1 d.f., p < 0.001; χ2 = 7.486, 1 d.f., p = 0.006, respectively), but not nectar sugar concentration (χ2 = 1.725, 1 d.f., p = 0.189). Plants in organic legacy soils produced 17% more nectar sugar per flower than those in conventional soils and had 26% higher nectar volume than plants in conventional soils. Plants in organic soils produced pollen with 9% less protein than those in conventional soils. Neither powdery mildew treatment (inoculated versus control) nor its interaction with soil affected pollen or nectar traits (electronic supplementary material, table S4). However, powdery mildew infection intensity (proportion of leaf area infected) was negatively correlated with nectar sugar concentration and sugar content, regardless of soil type (χ2 = 14.382, 1 d.f., p < 0.001 and χ2 = 15.048, 1 d.f., p < 0.001, respectively). Additionally, infection intensity was negatively correlated with nectar volume for plants in conventional, but not in organic soils (interaction term: χ2 = 6.381, 1 d.f., p = 0.011). Neither powdery mildew infection intensity nor its interaction with soil legacy affected pollen protein content (χ2 = 0.3607, 1 d.f., p = 0.548 and χ2 = 1.914, 1 d.f., p < 0.167, respectively).
Figure 3.
Effects of soil management legacy and powdery mildew on (a) total nectar sugar per flower, (b) nectar volume and (c) pollen protein content. Error bars represent model estimated marginal means ± 1 S.E.
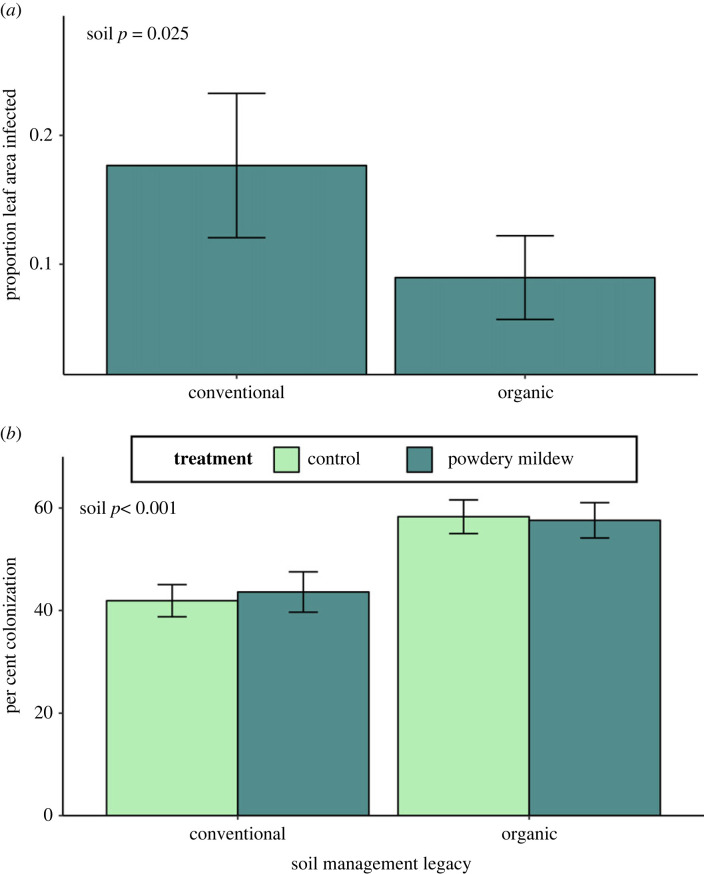
Soil legacy affected powdery mildew infection (figure 4a; χ2 = 4.9831 d.f., p = 0.025). Inoculated plants grown in organic legacy soils had 50% lower infection than those grown in conventional legacy soils. Additionally, plants grown in organic legacy soils had 37% higher mycorrhizal colonization than those grown in conventional legacy soils (figure 4b; χ2 = 17.365, 1 d.f., p < 0.001), likely driven by higher abundance of mycorrhizal vesicles and microsclerotia (χ2 = 10.101, 1 d.f., p = 0.001 and χ2 = 18.994, 1 d.f., p < 0.001, respectively). There was no effect of powdery mildew or its interaction with soil legacy on mycorrhizal colonization (χ2 = 0.014, 1 d.f., p = 0.905 and χ2 = 0.110, 1 d.f., p = 0.740, respectively).
Figure 4.
Effects of soil management legacy on (a) powdery mildew infection of plants inoculated with powdery mildew, and (b) mycorrhizal colonization. Error bars represent model estimated marginal means ± 1 S.E.
(iii) . Bee bioassays
Soil legacy and powdery mildew interacted to shape bee survival (figure 5; χ2 = 4.625 1 d.f., p = 0.032). Bees in micro-colonies fed pollen from powdery mildew-infected plants grown in organic soils had higher survival compared with those fed pollen from powdery mildew-infected plants grown in conventional soils (p = 0.049). Additionally, colonies with higher pollen consumption had higher adult bee survival (χ2 = 3.902, 1 d.f., p = 0.048). There was no effect of soil, powdery mildew, or their interaction on micro-colony pollen consumption (χ2 = 0.141, 1 d.f., p = 0.707, χ2 = 1.3691, 1 d.f., p = 0.242 and χ2 = 1.005, 1 d.f., p = 0.316, respectively) or the time it took for micro-colonies to produce new brood cells (χ2 = 0.460, 1 d.f., p = 0.498, χ2 = 0.022, 1 d.f., p = 0.882 and χ2 = 0.628, 1 d.f., p = 0.428, respectively) (electronic supplementary material, table S5).
Figure 5.
Effects on adult bee survival of pollen from plants grown in organic and conventional soils either infected or not infected with powdery mildew. Plot shows predicted survival of bees based on a Cox proportional hazards test.
(b) . Organic: high- versus low-fertility legacy
(i) . Field mesocosm experiment
The SEM indicated indirect effects of organic fertility legacy on plant–pollinator interactions (figure 1b; Fisher's C = 6.854, p = 0.335, 6 d.f.). Plants in high-fertility soils had lower powdery mildew infection than plants in low-fertility soils (p = 0.012). Bees made more visits to plants with higher powdery mildew infection (p = 0.025). Plants with higher powdery mildew infection tended to produce fewer flowers, though this effect was non-significant (p = 0.061). Floral abundance was positively associated with pollinator visitation (p = 0.002).
Organic fertility legacy did not affect the likelihood of powdery mildew infection (electronic supplementary material, figure S4b; χ2 = 0.0015, 1 d.f., p = 0.943). In contrast, fertility legacy and field site affected infection intensity of infected plants (electronic supplementary material, figure S5b; χ2 = 6.0974, 1 d.f., p = 0.014 and χ2 = 5.7353, 1 d.f., p = 0.017, respectively); plants in low-fertility soils and plants grown in Musgrave soils had higher infection (p = 0.017 and p < 0.001, respectively).
(ii) . Greenhouse manipulative experiment
Organic fertility legacy did not affect powdery mildew resistance (χ2 = 2.089, 1 d.f., p = 0.148). There was no effect of fertility legacy, powdery mildew treatment, or their interaction on total mycorrhizal colonization (χ2 = 2.0514, 1 d.f., p = 0.152, χ2 = 0.177, 1 d.f., p = 0.674, and χ2 = 0.068, 1 d.f., p = 0.749, respectively). However fertility legacy affected microsclerotia and vesicles, which were both more abundant in plants grown in high-fertility legacy soils (χ2 = 57.003, 1 d.f., p < 0.001 and χ2 = 4.5324, 1 d.f., p = 0.033, respectively).
Within organic soils, powdery mildew, but not fertility or their interaction, affected nectar sugar content (χ2 = 3.870, 1 d.f., p = 0.049, χ2 = 0.557, 1 d.f., p = 0.456, and χ2 = 0.350, 1 d.f., p = 0.554, respectively), with infected plants producing less sugar per flower (p = 0.050). Neither fertility legacy, powdery mildew, nor their interaction affected pollen protein, nectar volume, nectar sugar concentration or floral abundance (electronic supplementary material, table S4).
(iii) . Bee bioassays
There was no effect of organic fertility legacy, powdery mildew or their interaction on adult bee survival, pollen consumption, or the time it took for micro-colonies to produce new brood cells (electronic supplementary material, table S5).
(c) . Conventional: synthetic versus. green manure fertility legacy
As shown in figure 1c), conventional fertility legacy had no effect on powdery mildew, flowers, or bee abundance (see also electronic supplementary material, S5). Plants with more powdery mildew produced fewer flowers, and plants with more powdery mildew received fewer bee visits (figure 1c; p = 0.024 and p = 0.007, respectively). Smaller plants had lower powdery mildew infection (p = 0.015).
Soil legacy did not affect herbivore damage, so we present results of those SEM analyses in electronic supplementary material, figure S6. There was no effect of soil legacy on herbivore abundance (electronic supplementary material, S6).
4. Discussion
Our data support the hypothesis that soil legacy has direct and indirect effects on pollinators and traits important to pollinators, including floral display, nectar production and pollen protein content. This was sometimes mediated by powdery mildew, which influenced flowering phenology, nectar production, bee visitation and bee survival, often in negative ways for plants grown in conventional soils. Generally, organic soil legacy benefitted powdery mildew resistance, flower production and nectar sugar rewards. Our work provides new information on how long-term soil management affects mutualistic interactions in an agroecological context and suggests that enduring management practices could have strong effects on crop disease resistance, pollinator diet and crop–pollinator interactions.
Organic soil legacy modified the consequences of powdery mildew for floral traits and pollinators. This is evidenced by the fact that organically grown plants maintained attractiveness to pollinators despite high powdery mildew infection (figure 1a–c). Furthermore, organic soil legacy improved powdery mildew resistance, which may indirectly promote flower production and pollinator attraction. While the relationship between soil and powdery mildew infection intensity was only marginally significant in the SEM (figure 1a), there were general, repeated patterns of reduced powdery mildew in organic soils; powdery mildew incidence was lower in organic plants in the field mesocosm, and greenhouse plants inoculated with powdery mildew better resisted infection (electronic supplementary material, figure S4a; figure 4a). These results imply that continuous organic management may insulate future crops from yield loss both by reducing overall disease intensity and by buffering the consequences of disease for pollinator recruitment. However, more data are needed to assess the pollination consequences of our results.
Soil- and powdery mildew-driven changes to bee foraging behaviour in the field may reflect host plant suitability. Bee visitation negatively correlated with powdery mildew infection intensity for plants grown in conventional soils, even when floral abundance was accounted for. Reflecting this foraging preference, bees had lower survival when fed pollen from powdery mildew-inoculated plants grown in conventional compared with organic soil (figures 1 and 5). These results suggest that bees may detect powdery mildew-induced changes to pollen quality in plants grown in conventional soils [5,59–61]. In conventional soils, powdery mildew likely affected unmeasured components of floral reward chemistry, display, or scent that ultimately influenced bee attraction and survival [33,62].
The effects of soil legacy on floral reward quality and quantity could impact bee nutrition and population dynamics. Plants in organic legacy soils offered more nectar sugar but less pollen protein (figure 3a–c). Carbohydrates and protein are important for fueling bee flight and foraging [63,64], and pollen quality has strong effects on larval development [65,66]. Specialist species may be disproportionately affected by changes to pollen nutritional quality due to their higher reliance on specific host plant resources. However, given inadequate data on bee species- and life stage-specific nutritional requirements, it is hard to predict whether the changes to floral reward nutrients we observed will impact specialist bee larval development in positive or negative ways. Thus, it is important to determine whether soil management effects on floral reward quality impact bee reproduction, with particular focus on specialist species.
Organic and conventional soils varied in their compositions of nutrients and microbes that could have affected plant metabolism, disease resistance and floral traits. Organic soils had higher mycorrhizal colonization, which could have led to primed or induced defense against powdery mildew [67] or promoted floral size and nectar sugar production [30]. Additionally, organic soils had higher phosphorus and potassium (electronic supplementary material S3), which can promote flower production and plant resistance to fungal pathogens, respectively [68,69]. However, soil legacy likely shapes other abiotic and biotic factors that could explain differences in floral traits and disease resistance between organic and conventional soils.
Within organic and conventional soils, effects of fertility legacy on powdery mildew, flowers and pollinators were less consistent. In the mesocosm, plants in organic high-fertility soils had lower powdery mildew infection than plants in organic low-fertility soils (figure 1b). Yet these effects were not repeated in the greenhouse. Within conventional soils, we saw no effect of management on powdery mildew, floral traits or bee visitation, suggesting that benefits of soil fertility practices may not manifest in the context of other conventional practices [70,71]. However, we may have been unable to observe more subtle effects of differences within management systems. Future work should test a broader range of management practices within both organic and conventional systems, ideally with greater replication.
We demonstrate that organic soil legacy promotes floral resources and plant–pollinator interactions directly and, in some cases, by changing interactions with a common plant pathogen. While soil conditions have previously been linked to pollinators [31–33], long- and short-term conditioning effects can differ dramatically. We provide rare documentation showing the lingering effects of long-term soil conditioning on floral traits and pollinators. We also provide one of only a few examples of a foliar plant pathogen affecting pollinator reward quality and behaviour. Our work implies that pollination could be an overlooked consequence of soil legacy with downstream effects on plant community composition and crop yield [1,72] and it suggests an opportunity to develop soil management plans that benefit pollinators and pollination. More broadly, this suggests that agricultural soil legacy could contribute to geographic selection mosaics in natural systems or abandoned agricultural fields by imposing differential selection pressures on plants or their antagonists [73–75]. Thus, the consequences of soil management legacy on crop disease resistance, floral traits and bee behaviour could affect both plant and pollinator population dynamics in natural and managed systems.
Acknowledgements
We thank Kaitlin Deutsch, Laura Figueroa, Katja Poveda, Andre Kessler, Rob Raguso, the Cornell Pollinator & Plant Interactions groups and members of the Thaler lab, particularly Angeliki Cintron, Nathan Mak, and Hannah Tolz, for feedback and logistical support. Thanks also to Stephen Parry at the Cornell Statistical Consulting Unit for SEM advice and Meg McGrath for powdery mildew culture. This work would not have been possible without the dedication of John Putnam, Steve McKay, Gene Sczepanski and Paul Stachowski at Cornell Farm Services.
Ethics
No permits were required to conduct work with the invertebrates in this study.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.f7m0cfz31 [76].
Supplementary material is available online [77].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
J.K.D.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing; A.D.C.: investigation, writing—review and editing; Z.L.G.-P.: conceptualization, methodology, writing—review and editing; H.L.G.: conceptualization, formal analysis, writing—review and editing; B.H.: investigation, writing—review and editing; R.M.M.: conceptualization, project administration, resources, writing—review and editing; C.J.P.: conceptualization, project administration, resources, writing—review and editing; A.R.: conceptualization, resources, writing—review and editing; M.R.R.: conceptualization, resources, writing—review and editing; T.A.U.: conceptualization, formal analysis, methodology, writing—review and editing; J.S.T.: conceptualization, funding acquisition, methodology, resources, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE – 2139899 and an Atkinson Center Sustainable Biodiversity Fund grant, both to J.K.D. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors declare no conflicts of interest.
References
- 1.van der Putten WH, et al. 2013. Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265-276. ( 10.1111/1365-2745.12054) [DOI] [Google Scholar]
- 2.Wurst S, Ohgushi T. 2015. Do plant- and soil-mediated legacy effects impact future biotic interactions? Funct. Ecol. 29, 1373-1382. ( 10.1111/1365-2435.12456) [DOI] [Google Scholar]
- 3.Blundell R, Schmidt JE, Igwe A, Cheung AL, Vannette RL, Gaudin ACM, Casteel CL. 2020. Organic management promotes natural pest control through altered plant resistance to insects. Nat. Plants 6, 483-491. ( 10.1038/s41477-020-0656-9) [DOI] [PubMed] [Google Scholar]
- 4.Xi N, Chen D, Bahn M, Wu H, Chu C, Cadotte MW, Bloor JMG. 2022. Drought soil legacy alters drivers of plant diversity-productivity relationships in oldfield systems. Sci. Adv. 8, eabn3368. ( 10.1126/sciadv.abn3368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler A, Halitschke R. 2009. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Funct. Ecol. 23, 901-912. ( 10.1111/j.1365-2435.2009.01639.x) [DOI] [Google Scholar]
- 6.Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120, 321-326. ( 10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 7.Lucas-Barbosa D. 2016. Integrating studies on plant–pollinator and plant–herbivore interactions. Trends Plant Sci. 21, 125-133. ( 10.1016/j.tplants.2015.10.013) [DOI] [PubMed] [Google Scholar]
- 8.Adler LS, Wink M, Distl M, Lentz AJ. 2006. Leaf herbivory and nutrients increase nectar alkaloids. Ecol. Lett. 9, 960-967. ( 10.1111/j.1461-0248.2006.00944.x) [DOI] [PubMed] [Google Scholar]
- 9.Dötterl S, Jürgens A, Wolfe L, Biere A. 2009. Disease status and population origin effects on floral scent: consequences for oviposition and fruit predation in a complex interaction between a plant, fungus, and noctuid moth. J. Chem. Ecol. 35, 307-319. ( 10.1007/s10886-009-9601-0) [DOI] [PubMed] [Google Scholar]
- 10.Rusman Q, Karssemeijer PN, Lucas-Barbosa D, Poelman EH. 2019. Settling on leaves or flowers: herbivore feeding site determines the outcome of indirect interactions between herbivores and pollinators. Oecologia 191, 887-896. ( 10.1007/s00442-019-04539-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cellini A, Giacomuzzi V, Donati I, Farneti B, Rodriguez-Estrada MT, Savioli S, Angeli S, Spinelli F. 2019. Pathogen-induced changes in floral scent may increase honeybee-mediated dispersal of Erwinia amylovora. ISME J. 13, 847-859. ( 10.1038/s41396-018-0319-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida W, Matsunaga S, Sugiyama R, Kazama Y, Kawano S. 2003. Morphological development of anthers induced by the dimorphic smut fungus Microbotryum violaceum in female flowers of the dioecious plant Silene latifolia. Planta 218, 240-248. ( 10.1007/s00425-003-1110-8) [DOI] [PubMed] [Google Scholar]
- 13.Barragán-Fonseca KY, Greenberg LO, Gort G, Dicke M, van Loon JJA. 2023. Amending soil with insect exuviae improves herbivore tolerance, pollinator attraction and seed yield of Brassica nigra plants. Agricult. Ecosyst. Environ. 342, 108219. ( 10.1016/j.agee.2022.108219) [DOI] [Google Scholar]
- 14.Barragán-Fonseca KY, Rusman Q, Mertens D, Weldegergis BT, Peller J, Polder G, van Loon JJA, Dicke M. 2023. Insect exuviae as soil amendment affect flower reflectance and increase flower production and plant volatile emission. Plant Cell Environ. 46, 931-945. ( 10.1111/pce.14516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gils S, van der Putten WH, Kleijn D. 2016. Can above-ground ecosystem services compensate for reduced fertilizer input and soil organic matter in annual crops? J. Appl. Ecol. 53, 1186-1194. ( 10.1111/1365-2664.12652) [DOI] [Google Scholar]
- 16.Diez JM, Dickie I, Edwards G, Hulme PE, Sullivan JJ, Duncan RP. 2010. Negative soil feedbacks accumulate over time for non-native plant species. Ecol. Lett. 13, 803-809. ( 10.1111/j.1461-0248.2010.01474.x) [DOI] [PubMed] [Google Scholar]
- 17.Heinen R, Hannula SE, De Long JR, Huberty M, Jongen R, Kielak A, Steinauer K, Zhu F, Bezemer TM. 2020. Plant community composition steers grassland vegetation via soil legacy effects. Ecol. Lett. 23, 973-982. ( 10.1111/ele.13497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klironomos JN. 2002. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417, 67-70. ( 10.1038/417067a) [DOI] [PubMed] [Google Scholar]
- 19.Heinen R, van der Sluijs M, Biere A, Harvey JA, Bezemer TM. 2018. Plant community composition but not plant traits determine the outcome of soil legacy effects on plants and insects. J. Ecol. 106, 1217-1229. ( 10.1111/1365-2745.12907) [DOI] [Google Scholar]
- 20.Kos M, Tuijl MAB, de Roo J, Mulder PPJ, Bezemer TM. 2015. Species-specific plant–soil feedback effects on above-ground plant–insect interactions. J. Ecol. 103, 904-914. ( 10.1111/1365-2745.12402) [DOI] [Google Scholar]
- 21.Barel JM, Kuyper TW, de Boer W, Douma JC, De Deyn GB. 2018. Legacy effects of diversity in space and time driven by winter cover crop biomass and nitrogen concentration. J. Appl. Ecol. 55, 299-310. ( 10.1111/1365-2664.12929) [DOI] [Google Scholar]
- 22.García-González I, Quemada M, Gabriel JL, Alonso-Ayuso M, Hontoria C. 2018. Legacy of eight-year cover cropping on mycorrhizae, soil, and plants. J. Plant Nutr. Soil Sci. 181, 818-826. ( 10.1002/jpln.201700591) [DOI] [Google Scholar]
- 23.Gosling P, Hodge A, Goodlass G, Bending GD. 2006. Arbuscular mycorrhizal fungi and organic farming. Agricult. Ecosyst. Environ. 113, 17-35. ( 10.1016/j.agee.2005.09.009) [DOI] [Google Scholar]
- 24.Oehl F, Sieverding E, Mäder P, Dubois D, Ineichen K, Boller T, Wiemken A. 2004. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138, 574-583. ( 10.1007/s00442-003-1458-2) [DOI] [PubMed] [Google Scholar]
- 25.Schrama M, de Haan JJ, Kroonen M, Verstegen H, Van der Putten WH. 2018. Crop yield gap and stability in organic and conventional farming systems. Agricult. Ecosyst. Environ. 256, 123-130. ( 10.1016/j.agee.2017.12.023) [DOI] [Google Scholar]
- 26.McLauchlan K. 2006. The nature and longevity of agricultural impacts on soil carbon and nutrients: a review. Ecosystems 9, 1364-1382. ( 10.1007/s10021-005-0135-1) [DOI] [Google Scholar]
- 27.Altieri MA, Nicholls CI. 2003. Soil fertility management and insect pests: harmonizing soil and plant health in agroecosystems. Soil Tillage Res. 72, 203-211. ( 10.1016/S0167-1987(03)00089-8) [DOI] [Google Scholar]
- 28.Ding S, Shao X, Li J, Ahammed GJ, Yao Y, Ding J, Hu Z, Yu J, Shi K. 2021. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 44, 1596-1610. ( 10.1111/pce.14019) [DOI] [PubMed] [Google Scholar]
- 29.Gange AC, Smith AK. 2005. Arbuscular mycorrhizal fungi influence visitation rates of pollinating insects. Ecol. Entomol. 30, 600-606. ( 10.1111/j.0307-6946.2005.00732.x) [DOI] [Google Scholar]
- 30.Bennett AE, Meek HC. 2020. The influence of arbuscular mycorrhizal fungi on plant reproduction. J. Chem. Ecol. 46, 707-721. ( 10.1007/s10886-020-01192-4) [DOI] [PubMed] [Google Scholar]
- 31.Hoover SER, Ladley JJ, Shchepetkina AA, Tisch M, Gieseg SP, Tylianakis JM. 2012. Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecol. Lett. 15, 227-234. ( 10.1111/j.1461-0248.2011.01729.x) [DOI] [PubMed] [Google Scholar]
- 32.Ceulemans T, Hulsmans E, Ende WV, Honnay O. 2017. Nutrient enrichment is associated with altered nectar and pollen chemical composition in Succisa pratensis Moench and increased larval mortality of its pollinator Bombus terrestris L. PLoS ONE 12, e0175160. ( 10.1371/journal.pone.0175160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JK, Aguirre LA, Barber NA, Stevenson PC, Adler LS. 2019. From plant fungi to bee parasites: mycorrhizae and soil nutrients shape floral chemistry and bee pathogens. Ecology 100, e02801. ( 10.1002/ecy.2801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mårtensson AM, Carlgren K. 1994. Impact of phosphorus fertilization on VAM diaspores in two Swedish long-term field experiment. Agricult. Ecosyst. Environ. 47, 327-334. ( 10.1016/0167-8809(94)90099-X) [DOI] [Google Scholar]
- 35.Verbruggen E, Röling WFM, Gamper HA, Kowalchuk GA, Verhoef HA, van der Heijden MGA. 2010. Positive effects of organic farming on below-ground mutualists: large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 186, 968-979. ( 10.1111/j.1469-8137.2010.03230.x) [DOI] [PubMed] [Google Scholar]
- 36.Hawkes CV, Kivlin SN, Du J, Eviner VT. 2013. The temporal development and additivity of plant–soil feedback in perennial grasses. Plant Soil 369, 141-150. ( 10.1007/s11104-012-1557-0) [DOI] [Google Scholar]
- 37.Lepinay C, Vondráková Z, Dostálek T, Münzbergová Z. 2018. Duration of the conditioning phase affects the results of plant–soil feedback experiments via soil chemical properties. Oecologia 186, 459-470. ( 10.1007/s00442-017-4033-y) [DOI] [PubMed] [Google Scholar]
- 38.Merot A, Fermaud M, Gosme M, Smits N. 2020. Effect of conversion to organic farming on pest and disease control in French vineyards. Agronomy 10, 1047. ( 10.3390/agronomy10071047) [DOI] [Google Scholar]
- 39.Jalli M, Huusela E, Jalli H, Kauppi K, Neimi M, Himanen S, Jauhiainen L. 2021. Effects of crop rotation on spring wheat yield and pest occurrence in different tillage systems: a multi-year experiment in Finnish growing conditions. Front. Sustain. Food Syst. 5, 647335. ( 10.3389/fsufs.2021.647335) [DOI] [Google Scholar]
- 40.Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I. 2008. Functional group diversity of bee pollinators increases crop yield. Proc. R. Soc. B 275, 2283-2291. ( 10.1098/rspb.2008.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Artz DR, Nault BA. 2011. Performance of Apis mellifera, Bombus impatiens, and Peponapis pruinosa (Hymenoptera: Apidae) as pollinators of pumpkin. J. Econ. Entomol. 104, 1153-1161. ( 10.1603/ec10431) [DOI] [PubMed] [Google Scholar]
- 42.Keinath AP, Wintermantel WM, Zitter TA. 2017. Compendium of cucurbit diseases and pests, 2nd edn. St. Paul, MN: APS Press. [Google Scholar]
- 43.University of Maine Cooperative Extension. 2010. Powdery Mildew of Cucurbits: Pest Management [Fact Sheet]. Orono, MN: University of Maine Cooperative Extension. (See https://extension.umaine.edu/ipm/ipddl/publications/5085e/ .)
- 44.McGrath M. 2021. Cucurbit Powdery Mildew (accessed on 9 May 2022). See https://www.vegetables.cornell.edu/pest-management/disease-factsheets/cucurbit-powdery-mildew/.
- 45.Gent DH, Bhattacharyya S, Ruiz T. 2019. Prediction of spread and regional development of hop powdery mildew: a network analysis. Phytopathology 109, 1392-1403. ( 10.1094/PHYTO-12-18-0483-R) [DOI] [PubMed] [Google Scholar]
- 46.Xiang Y, Miller AN, McGrath M, Babadoost M. 2020. Genotyping-by-sequencing for analysis of the genetic variation of Podosphaera xanthii, incitant of cucurbit powdery mildew. Plant Dis. 104, 951-957. ( 10.1094/PDIS-03-19-0513-RE) [DOI] [PubMed] [Google Scholar]
- 47.Getman-Pickering ZL, Campbell A, Aflitto N, Grele A, Davis JK, Ugine TA. 2020. LeafByte: a mobile application that measures leaf area and herbivory quickly and accurately. Methods Ecol. Evol. 11, 215-221. ( 10.1111/2041-210X.13340) [DOI] [Google Scholar]
- 48.R Core Team 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 49.Lefcheck JS. 2016. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573-579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 50.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 51.Hartig F. 2022. DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package v. 0.4.5. See https://CRAN.R-project.org/package=DHARMa.
- 52.Pedersen HW, Navarro D, Lin T. 2016. Elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 53.Lenth RV, Buerkner P, Maxime H, Love J, Miguez F, Riebl H, Singmann H. 2022. emmeans: Estimated marginal means, aka least-squares means. R package v. 1.8.9. (See https://cran.r3.project.org/web/packages/emmeans/index.html.)
- 54.Shipley B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90, 363-368. ( 10.1890/08-1034.1) [DOI] [PubMed] [Google Scholar]
- 55.Dixon P. 2003. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927-930. ( 10.1111/j.1654-1103.2003.tb02228.x) [DOI] [Google Scholar]
- 56.Liaw A, Wiener M. 2002. Classification and regression by randomForest. R News 2, 18-22. (See https://journal.r-project.org/articles/RN-2002-022/.) [Google Scholar]
- 57.Bolten AB, Feinsinger P, Baker HG, Baker I. 1979. On the calculation of sugar concentration in flower nectar. Oecologia 41, 301-304. ( 10.1007/BF00377434) [DOI] [PubMed] [Google Scholar]
- 58.Therneau T. 2022. coxme: Mixed Effects Cox Models_. R package version 2.2-18.1. (See https://cran.r-project.org/web/packages/coxme/index.html.)
- 59.Aguirre LA, Davis JK, Stevenson PC, Adler LS. 2020. Herbivory and time since flowering shape floral rewards and pollinator-pathogen interactions. J. Chem. Ecol. 46, 978-986. ( 10.1007/s10886-020-01213-2) [DOI] [PubMed] [Google Scholar]
- 60.Bruinsma M, Lucas-Barbosa D, ten Broeke CJM, van Dam NM, van Beek TA, Dicke M, van Loon JJA. 2014. Folivory affects composition of nectar, floral odor and modifies pollinator behavior. J. Chem. Ecol. 40, 39-49. ( 10.1007/s10886-013-0369-x) [DOI] [PubMed] [Google Scholar]
- 61.Antico CJ, Colon C, Banks T, Ramonell KM. 2012. Insights into the role of jasmonic acid-mediated defenses against necrotrophic and biotrophic fungal pathogens. Front. Biol. 7, 48-56. ( 10.1007/s11515-011-1171-1) [DOI] [Google Scholar]
- 62.Becklin KM, Gamez G, Uelk B, Raguso RA, Galen C. 2011. Soil fungal effects on floral signals, rewards, and aboveground interactions in an alpine pollination web. Am. J. Bot. 98, 1299-1308. ( 10.3732/ajb.1000450) [DOI] [PubMed] [Google Scholar]
- 63.Heinrich B. 1975. Energetics of pollination. Annu. Rev. Ecol. Syst. 6, 139-170. ( 10.1146/annurev.es.06.110175.001035) [DOI] [Google Scholar]
- 64.Vaudo AD, Patch HM, Mortensen DA, Tooker JF, Grozinger CM. 2016. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. PNAS 113, E4035-E4042. ( 10.1073/pnas.1606101113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roulston TH, Cane JH. 2002. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 16, 49-65. ( 10.1023/A:1016048526475) [DOI] [Google Scholar]
- 66.Burkle LA, Irwin RE. 2010. Beyond biomass: measuring the effects of community-level nitrogen enrichment on floral traits, pollinator visitation and plant reproduction. J. Ecol. 98, 705-717. ( 10.1111/j.1365-2745.2010.01648.x) [DOI] [Google Scholar]
- 67.Qu L, Wang M, Biere A. 2021. Interactive effects of mycorrhizae, soil phosphorus and light on growth and induction and priming of defense in Plantago lanceolata. Front. Plant Sci. 12, 647372. ( 10.3389/fpls.2021.647372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poulton JL, Bryla D, Koide RT, Stephenson AG. 2002. Mycorrhizal infection and high soil phosphorus improve vegetative growth and the female and male functions in tomato. New Phytol. 154, 255-264. ( 10.1046/j.1469-8137.2002.00366.x) [DOI] [Google Scholar]
- 69.Amtmann A, Troufflard S, Armengaud P. 2008. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 133, 682-691. ( 10.1111/j.1399-3054.2008.01075.x) [DOI] [PubMed] [Google Scholar]
- 70.Güereña DT, Kimetu J, Riha S, Neufeldt H, Lehmann J. 2016. Maize productivity dynamics in response to mineral nutrient additions and legacy organic soil inputs of contrasting quality. Field Crops Res. 188, 113-120. ( 10.1016/j.fcr.2015.12.017) [DOI] [Google Scholar]
- 71.Riedo J, et al. 2021. Widespread occurrence of pesticides in organically managed agricultural soils—the ghost of a conventional agricultural past? Environ. Sci. Technol. 55, 2919-2928. ( 10.1021/acs.est.0c06405) [DOI] [PubMed] [Google Scholar]
- 72.Kaplan I, Bokulich NA, Caporaso JG, Enders LS, Ghanem W, Ingerslew KS. 2020. Phylogenetic farming: can evolutionary history predict crop rotation via the soil microbiome? Evol. Appl. 13, 1984-1999. ( 10.1111/eva.12956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groen SC, et al. 2016. Virus infection of plants alters pollinator preference: a payback for susceptible hosts? PLoS Pathog. 12, e1005790. ( 10.1371/journal.ppat.1005790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mursinoff S, Tack AJM. 2017. Spatial variation in soil biota mediates plant adaptation to a foliar pathogen. New Phytol. 214, 644-654. ( 10.1111/nph.14402) [DOI] [PubMed] [Google Scholar]
- 75.Tack AJM, Laine A-L, Burdon JJ, Bissett A, Thrall PH. 2015. Belowground abiotic and biotic heterogeneity shapes aboveground infection outcomes and spatial divergence in a host–parasite interaction. New Phytol. 207, 1159-1169. ( 10.1111/nph.13408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis JK, et al. 2023. Data from: Agricultural soil legacy influences multitrophic interactions between crops, their pathogens and pollinators. Dryad Digital Repository. ( 10.5061/dryad.f7m0cfz31) [DOI] [PMC free article] [PubMed]
- 77.Davis JK, et al. 2023. Agricultural soil legacy influences multitrophic interactions between crops, their pathogens and pollinators. Figshare. ( 10.6084/m9.figshare.c.6927460) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Davis JK, et al. 2023. Data from: Agricultural soil legacy influences multitrophic interactions between crops, their pathogens and pollinators. Dryad Digital Repository. ( 10.5061/dryad.f7m0cfz31) [DOI] [PMC free article] [PubMed]
- Davis JK, et al. 2023. Agricultural soil legacy influences multitrophic interactions between crops, their pathogens and pollinators. Figshare. ( 10.6084/m9.figshare.c.6927460) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.f7m0cfz31 [76].
Supplementary material is available online [77].