Abstract
Mosquito-borne diseases (MBDs) threaten public health and food security globally. We provide the first biogeographic description of the African mosquito fauna (677 species) and the 151 mosquito-borne pathogens (MBPs) they transmit. While mosquito species richness agrees with expectations based on Africa's land surface, African arboviruses and mammalian plasmodia are more speciose than expected. Species assemblages of mosquitoes and MBPs similarly separate sub-Saharan Africa from North Africa, and those in West and Central Africa from eastern and southern Africa. Similarities between mosquitoes and MBPs in diversity and range size suggest that mosquitoes are key in delimiting the range of MBPs. With approximately 25% endemicity, approximately 50% occupying one to three countries and less than 5% occupying greater than 25 countries, the ranges of mosquitoes and MBPs are surprisingly small, suggesting that most MBPs are transmitted by a single mosquito species. Exceptionally widespread mosquito species feed on people and livestock, and most are high-altitude-windborne migrants. Likewise, widespread MBPs are transmitted among people or livestock by widespread mosquitoes, suggesting that adapting to people or livestock and to widespread mosquito species promote range expansion in MBPs. Range size may predict range expansion and emergence risk. We highlight key knowledge gaps that impede prediction and mitigation of future emergence of local and global MBDs.
Keywords: arbovirus, biodiversity, disease ecology, global health, one health, vector-borne diseases
1. Introduction
Africa carries the heaviest global burden of mosquito-borne diseases (MBDs), with more than 400 000 deaths attributable to malaria out of the total 700 000 deaths caused by vector-borne diseases annually [1]. At least 8 of the 11 most impactful global mosquito-borne pathogens (MBPs) originated in Africa—namely yellow fever virus (YFV), West Nile virus (WNV), chikungunya virus (CHIKV), Rift Valley fever virus (RVFV), Zika virus (ZIKV) and three human Plasmodium species (falciparum, malariae, ovale) [1]. Excluding its islands, Africa comprises only 20% of the Earth's land surface, but is the origin of 73% (8/11) of these global MBPs. Based on the species richness–area relationship [2], this excess is highly significant (p < 0.001, exact binomial test), corroborating a recent literature review that reached a similar conclusion using different data [3]. The reasons for Africa's disproportionate role as the origin of so many global MBPs may include being the only continent that extends from the northern to southern temperate zones, covering greater than 70° of latitude (37°N–34°S), and straddles several biomes including the outstandingly diverse equatorial forest [2,4,5]. As the homeland of the hominids and extant apes, we expected that Africa would contain more human MBPs [6]; yet, five of the eight global MBDs are zoonotic (YF, WN, RVF, CHIK and ZIK), yielding a new hypothesis that Africa has more MBDs altogether, not only those affecting humans. Africa is also home to the largest number of megafauna species, and thus it poses a greater risk to many phylogenetically related domestic animals. Knowledge and understanding of the MBDs of Africa, therefore, would be valuable for global health and food security. As Africa undergoes dramatic perturbations due to deforestation, urbanization and climate change (e.g. warming and desertification), coupled with food and water scarcity, the risk for the emergence/re-emergence of MBDs needs to be closely monitored. The projected growth of the African population over future decades (from 1.4 to 4 billion) surpasses that of all other continents [7] and is expected to result in a higher rate of disease emergence and spread unless humanity is better prepared. Knowledge of the natural transmission cycles of pathogens is a prerequisite to successful mitigation of MBDs; however, this information is vastly lacking.
The study of MBDs has traditionally been fragmented into separate fields (virology, parasitology, entomology, etc.) and most studies have focused on one or a few pathogens and/or their vectors in a limited region. Excepting a few reviews of certain MBDs [8,9], the ensemble of MBDs as a biological system composed of mosquitoes and pathogens has never before been holistically studied to our knowledge. On the other hand, the increasing frequency of disease emergence in humans has deservedly been the focus of extensive study [10–16], yet their broad scope may have precluded inferences into commonalities shared among certain groups of diseases. We present a dedicated database constructed based on a comprehensive literature search (electronic supplementary material) focusing on all known pathogens transmitted by mosquitoes between terrestrial tetrapods in continental Africa. Using this database, we describe the composition and geographical organization of the mosquito species and MBPs in Africa to better understand the process of MBD range expansion, which is key to disease emergence. Specifically, we evaluate the hypothesis that Africa has exceptionally high mosquito and MBP diversities and map the landscapes of their species richness, endemicity and composition. The results provide insights into the role of mosquito and MBP dispersal, the nature of barriers to their spread and the future of MBD surveillance in Africa. We propose a process for range expansion of MBPs and accordingly rank the African MBPs, as to their expected risk for disease emergence.
In this exploratory analysis, we summarize trends based on knowledge that has been accumulated over at least 120 years. Thus, it may come as a surprise that the main vector species of MBPs of vertebrates are still largely unknown, including most sylvatic vectors (transmitting among wild animals) of the better-studied pathogens [17–26]. This is also the case for many MBP species of vertebrates [8,17,19,21,27]. Therefore, it is likely that the role of many mosquito species as vectors of known and unknown pathogens is yet to be discovered. Moreover, biogeographic patterns that apply to non-vector species may equally pertain to vector species. Accordingly, we include all known African mosquito species in the current analysis.
2. Results
As many of the records on mosquito and MBP distribution were collected before 1980, localization of a large portion of these records is only available at the country level [9,17,22,26,28–31]. Because many African countries cover multiple ecozones [4], and given that biogeographic regions as defined for various animal classes [32] vary in size, our units of analysis—countries—form fuzzy eco-geographic units.
(a) . What are the African mosquitoes and mosquito-borne pathogens?
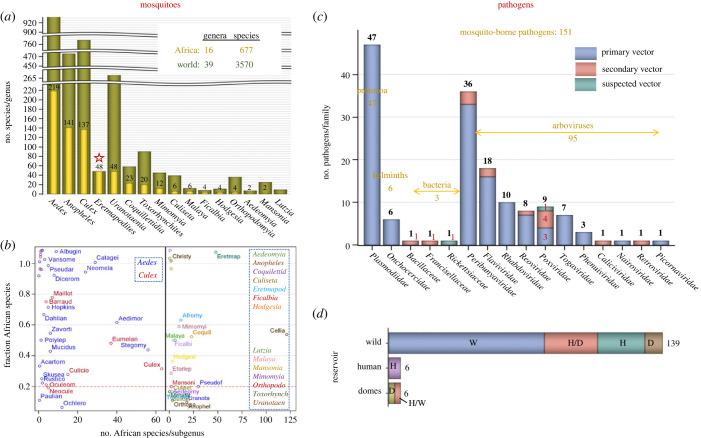
Continental Africa, which comprises 20% of the world's land surface, supports 19% of all known global mosquito species (N = 3570, see Methods) [26]. The African mosquito fauna includes 677 species spanning 16 genera and 53 subgenera, with Aedes comprising the largest number of species, followed by Anopheles and Culex (figure 1a). Goodness of fit tests contrasting global diversity by genus with an expected fraction of 20% (in genera where N > 15 species/genus) reveal higher fractions of African species in Aedes (23%, p < 0.01, ), Anopheles (30%, p < 0.0001, ) and Coquillettidia (40%, p < 0.0002, ), but are insignificant in the other genera. The highest fraction of African species (100%) is found in the genus Eretmapodites (n = 48), which is endemic to Africa (figure 1a). Genera whose share of African species is greater than 20% (of their global diversity) likely reflect disproportional larger local speciation (or fewer extinctions) on the continent. Among the 53 mosquito subgenera in Africa, Anopheles (Cellia) is by far the most speciose (n = 121, figure 1b; electronic supplementary material, table S1). Several subgenera have a high proportion of African species (figure 1b), although most of these have a small number of species in total, such as Anopheles (Christya) (n = 2). Nonetheless, all 29 Aedes (Catageiomyia) species are exclusively African species and 24 of the 28 species of Aedes (Neomelaniconion) are African (figure 1b; electronic supplementary material, table S1). While not precluding that some of the species also occur outside Africa, a high fraction of species found in Africa, especially in taxa with a large number of species, highlights the fauna's characteristic elements.
Figure 1.
Taxonomical profile of African mosquitoes and MBPs. (a) Number of African species/genera (gold and numerals) compared with the total number worldwide (green). Star denotes entirely African genus. Note: breaks in the y-axis. (b) The fraction of African mosquito species per subgenus (y-axis) of their worldwide total in relation to their number in Africa (x-axis). To minimize label overlap, values near 1 were jittered. Subgenera labels (abbreviated) are shown if they have two or more species. Where no subgenera are known, e.g. Ficalbia (electronic supplementary material, table S1), genus names were used. Corresponding genera (bold italic font) of the same colour are listed in the dotted frame. Red line marks expected 20% based on Africa's share of land surface (see text). (c) Taxonomic composition of African MBPs of vertebrates by family and importance of mosquito-borne transmission (see legend). Suspected mosquito transmission reflects compelling, yet non-definitive evidence (electronic supplementary Data File 2). The number of pathogens in each family is shown above bars (black) and the total by taxonomic group shown across (gold). The number of pathogens transmitted mechanically are listed (red). (d) Division of MBPs by group of vertebrate hosts acting as reservoir (y-axis) and by the host group impacted by the pathogen (subgroups in colour). Key: W, H and D denote wild, human and domestic animal (those raised by people, Domes), respectively, and H/D denotes that humans and domestic animals are impacted by MBPs whose reservoir are wild animals.
A total of 151 known mosquito-borne-pathogen (MBP) species affecting vertebrates have been reported from continental Africa (figure 1c). These include 95 viruses, 47 protozoans, 6 helminths and 3 bacteria, comprising a total of 16 families and 30 genera (figure 1c; including 3 unclassified genera). These 95 arboviruses represent a significantly higher share than expected from the known global total based on the surface land area of Africa (32% versus 20%, p < 0.0001, ). The fraction of mosquito-borne arboviruses is likely even higher because among the 300 total arboviruses that have been isolated from mosquito pools worldwide [26], some are probably not vectored by mosquitoes. Likewise, of the 60 mammalian plasmodia [21], 27 species (40%) are reported from Africa, which is larger than expected based on the continental/global land mass area (p < 0.0001, ). Plasmodia of birds are not considered here because recent molecular analyses reveal many novel species awaiting formal descriptions [19,21,33].
The vast majority of MBPs are maintained in wild host reservoirs (figure 1d), although a few can be transmitted for short periods between humans, e.g. YFV and O'nyong'nyong virus (ONNV), or between domestic animals e.g. RVFV. Mosquito transmission is the primary route of vertebrate infection in most MBPs (figure 1c), whereas 16 pathogens rely on other arthropods or direct transmission as their primary mode of transmission and mosquitoes represent a secondary route (figure 1c). At least eight of the nine poxviruses are transmitted mechanically by mosquitoes, as are the three bacteria [34] (figure 1c; electronic supplementary material, file S2). Mechanical transmission appears to be linked to secondary transmission (figure 1c; electronic supplementary material, file S2), although certain poxviruses can be transmitted several weeks post single exposure [35,36].
(b) . Range size occupied by the African mosquitoes and mosquito-borne pathogens
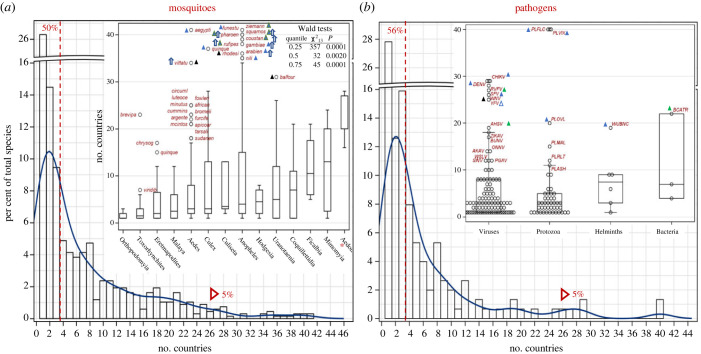
Range size evaluations reveal that 26% of all African mosquito species are endemic (known from a single country), and that over 50% are restricted to at most three countries (median = 3.0, figure 2a). The L-shape distribution reveals that only 5% of the total number of species are found across over half of the continent (more than 25 countries, figure 2a). The Pearson correlation coefficient between the number of countries and total area (sum over countries areas) occupied by each species is 0.966 (N = 677, p < 0.0001), indicating that the number of countries approximates range size well. The median area occupied by a mosquito species is 3.09 × 106 km2 (95% CI: 2.72–3.42 km2). Range size varies among genera (p < 0.05, quantile regression, figure 2a: inset).
Figure 2.
Geographic range size of mosquitoes (a) and MBPs (b) based on the number of countries per species overall and by taxonomical groups (insets). Note the break in the y-axis. The fraction of species occupying 1–3 countries and over 25 countries are shown to the left of the red broken line and red triangle, respectively. Insets: number of countries per species across mosquito genera (a) and taxonomic group of MBPs (b). Genera represented by less than three species (Lutzia (N = 1), Mansonia (N = 2) and Aedeomyia (N = 2)) were pooled (red asterisk). The box shows the 25th, 50th and 75th quantiles of the distribution and the whiskers extend to the extreme observations up to 1.5× the interquartile range (75th–25th quantiles). Outliers exceeding the whiskers are shown by abbreviated species name (a) and acronym (b) in red; triangles indicate preference to blood feeding on (a) and transmission between (b) humans (blue) domestic animals (green) and wild hosts (black). Empty triangle (b) indicates transmission to humans but no persistent transmission from humans. Blue arrows indicate high-altitude windborne species (see text). Table (a) summarizes results of the quantile regression across quartiles (see text).
Species of Lutzia, Mansonia, Aedeomyia, Mimomiya, Ficalbia and Coquillettidia exhibit the largest ranges (figure 2a). However, the most widespread mosquito species that are found in greater than 30 countries (14 of 677 species, figure 2) include the most important (human) disease vectors: An. gambiae, An. arabiensis, An. funestus, Ae. aegypti and Cx. quinquefasciatus, less important vectors: An. pharoensis, An. squamosus, An. coustani, An. ziemanni, An. rufipes, An. rhodesiensis, An. nili, and Ae. vitattus, and Ur. balfouri, which is not known as a vector. Anopheles predominates this group of exceptionally widespread species. Eleven of these species thrive in domestic environments and feed on people or domestic animals (figure 2a), and at least seven engage in high-altitude windborne migration [37–39]. Surprisingly, the distribution of range size in African MBPs is remarkably similar to that of the mosquitoes (figure 2b), with 28% being single country-endemic, 56% found in one to three countries (median = 3.0), and 5% in greater than 25 countries. The Pearson correlation coefficient between the number of countries and total area occupied by each MBP species is 0.967 (N = 151, p < 0.0001), corroborating that the number of countries is a proxy of range size. The median area occupied by an MBP is 2.15 × 106 km2 (95% CI: 1.64–2.65 × 106 km2). The most widespread MBPs (40 countries) are Pl. falciparum and Pl. vivax (PLFLC, PLVIX) with only nine MBPs being reported in 20 or more countries (figure 2b). Excepting WNV, which is primarily transmitted among birds (including migratory birds), all of these remarkably widespread MBPs are transmitted among humans (6) or domestic animals (2), and all are vectored by at least one of the most widespread mosquito species mentioned above (figure 2a). For example, PLFLC, PLVIX and Pl. ovale (PLOVL) are transmitted by some or all of the above Anopheles species, and DENV, CHIKV and YFV are transmitted by Ae. aegypti in urban and semi-urban settings [9,22,40–44]. Similarly, WNV is transmitted by Cx. quinquefasciatus and YFV and ZIKV are transmitted by Ae. vittatus [9,45–47].
(c) . Diversity and endemism across the continent
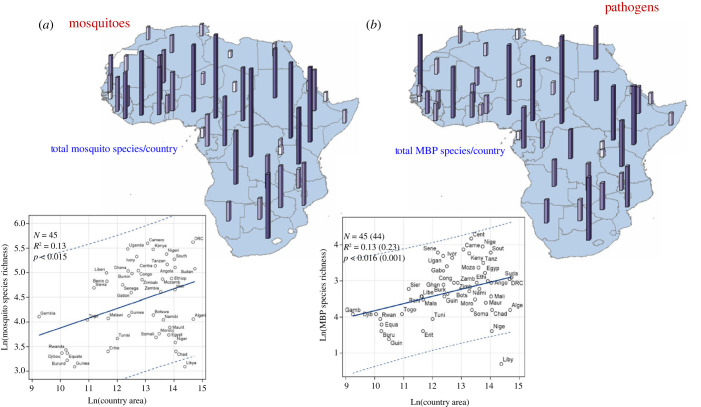
According to the area–species richness principle, mosquito species richness has been found to increase with a country's area in a worldwide analysis [29]. In Africa, this relationship accounted for only 13% of the variance compared with 42% worldwide (figure 3a). The equatorial forest possesses the highest species richness (figure 3a), with the Democratic Republic of the Congo (DRC), Cameroon, Uganda, Kenya, Nigeria and Ivory Coast showing the highest values. Adjusting for area minimally changes these countries' ranking (figure 3a). North Africa represents a uniform belt of lowest mosquito diversity, with Libya being an outlier that exceeds the 95% confidence limits (CL), given its area (figure 3a). A corridor of modest diversity exists along the Sahel (from Mauritania to Chad) in addition to another potential corridor between Central and East Africa including countries from Namibia and Botswana to Rwanda, which remained stable after accounting for country area (figure 3a).
Figure 3.
Maps showing mosquito (a) and MBP (b) country diversity (top) and the country's area–species richness relationship (bottom). Bar height and colour shows total endemic species per country (top). Linear regression (solid line) shows the increase in expected number of species per country given its area with 95% confidence limit for individual countries (broken lines). Values in parenthesis (b) show the change in the regression's summary statistics after exclusion of outliers. Abbreviated country names are used. Note: countries with insufficient information are excluded (e.g. Eswatini), or pooled together (e.g. South and North Sudan) to reflect available information (Methods).
Similarly to mosquitoes, the species richness of African MBPs is highest in Central Africa, followed by an East African zone stretching from Kenya to South Africa (figure 3b). Except for Senegal and the Ivory Coast, West Africa exhibits lower diversity of MBPs than East Africa. Sahelian countries and those between Central and East Africa exhibit lower MBP richness than the surrounding regions, whereas North Africa exhibits the lowest MBP richness (figure 3b). Species richness increases with country size, but this relationship accounts for only 13% of the variance among countries (excluding outliers: Libya, increased R2 to 23%, figure 3b).
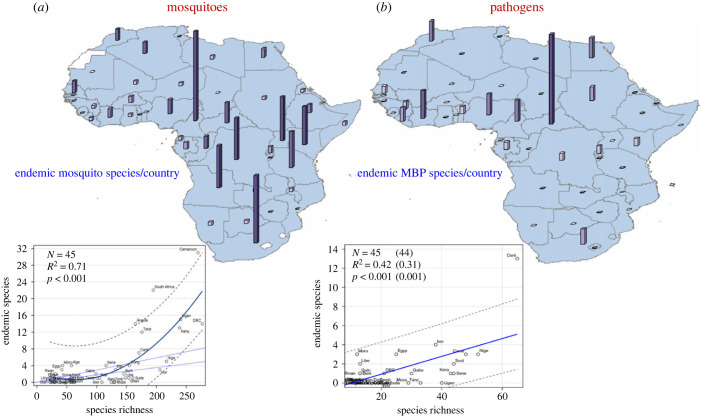
The distribution of country-endemic mosquito species reveals greater heterogeneity than species richness, with the highest endemicity in Equatorial Central Africa, especially Cameroon (31), followed by South Africa and Angola (22, figure 4a). These three countries represent outliers after accounting for species richness and, indirectly, country area (captured by species richness, figure 3a). Unlike with species richness, the lowest mosquito endemicity is found across the Sahel from Mauritania to Somalia and, notably, extending to equatorial West Africa. Additionally, the secondary ‘corridor’ of low species richness separating South Africa from Central and East Africa (figure 3a) appears to be wider for endemicity. Countries without known endemic mosquito species include Chad and Mozambique (figure 4a). The number of endemic species per country is correlated with its species richness (r = 0.7, N = 45, p < 0.001), but visual inspection suggests a higher slope after species richness exceeds approximately 100 species per country (figure 3a and figure 4a).
Figure 4.
Maps showing endemic species of mosquito (a) and MBP (b) per country (top) and the relationship between species endemicity and richness (bottom). Bar height and colour shows total endemic species per country (top). Quadratic (a, bottom) and linear regression (b, bottom) solid lines show the increase in expected mean number of endemic species given species richness with 95% confidence limits for individual countries (broken lines). Dotted lines show expected linear trends assuming monotonic increase predicted by the mean (higher) and median (lower) ratio of endemics to total species. Values in parenthesis (b) show the regression summary statistics after exclusion of outliers. Abbreviated country names are used.
Country-endemic MBPs comprise 25 arboviruses, 11 plasmodia and 1 nematode, reflecting a similar proportion of endemicity across taxa: 27.5%, 25.6% and 16.7%, respectively. Endemic MBPs show an extreme hotspot in the Central African Republic (CAR) and moderate endemism in the Ivory Coast, followed by Nigeria and then Cameroon, Egypt and Morocco (figure 4b). Endemic MBP species per country also increases with species richness and indirectly with country area (figure 3b). After accounting for species richness, the CAR remains an outlier endemic hotspot.
(d) . Heterogeneity in species composition across regional and country scales
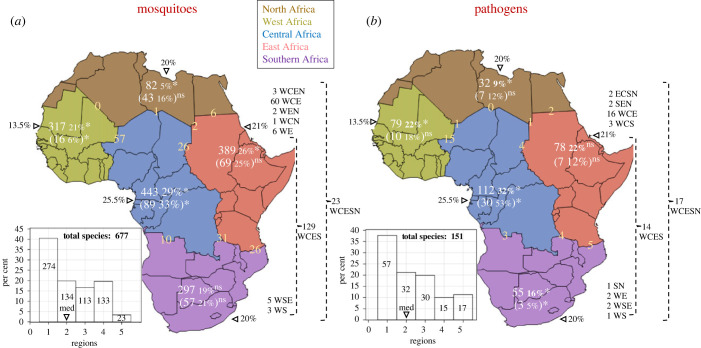
Because countries differ considerably in surveillance effort, a regional analysis, wherein each region consists of multiple countries, exhibits less variability in surveillance effort and can be used to ascertain the patterns noted at the country level. Here, five regions have been defined to maximize distances between regions, accommodate latitudinal variation, and minimize inter-region enclaves (without regard for political regions, see Methods). Over 40% of mosquito species are region endemic and 60% of mosquito species are found in one to two regions. Only 19% are found across sub-Saharan Africa and merely 3% are distributed across all five regions (figure 5a). Consistent with country results (figure 4a), the highest mosquito richness and endemicity are found in Central Africa and the lowest species richness is in North Africa. Notably, endemicity is lowest in West Africa (figure 5a). Excesses of species richness based on region area are found in West and Central Africa, whereas North Africa exhibits a deficit (p < 0.01, Z > 3.1, Exact Binomial tests, figure 6a). Excess of endemic species is detected in Central Africa, whereas West Africa exhibits the lowest endemicity and largest deficit of endemic species, reflecting the large number of species it shares with Central Africa (N = 57), as well as with both East and Central Africa (N = 60, figure 5a).
Figure 5.
Composition of mosquitoes (a) and MBP species (b) across five regions in Africa (North, West, Central, East and Southern Africa). Regional species richness and (region endemic) in absolute and percentage numbers (white font) against each region's relative landmass in per cent (black font behind triangles). Statistically significant departures (p < 0.05) of the actual species richness or endemicity from expectations based on the region area is marked by ‘*’; ‘ns’ denotes insignificant departure. The number of species between two and three adjacent regions are shown at the border between regions (yellow font). Number of species shared between disjoined regions are shown on the right (black) before the regions' acronym. Histogram showing the number of species occupying different number of regions (median = 2, marked by a black triangle, mean = 2.25).
Figure 6.
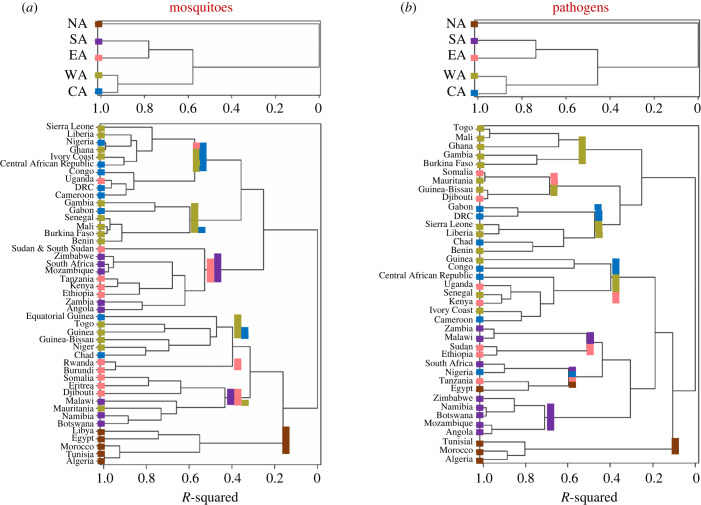
Dendrograms showing clustering of regions (above) and countries (below) based on species composition of mosquitoes (a) and MBPs (b). Region and country colour follows colour of the regions in figure 5 (North Africa (brown), West Africa (green), Central Africa (blue), East Africa (pink), Southern Africa (purple)).
Similarly to trends observed in mosquitoes, 38% of MBP species are region endemic and 60% are found in one to two regions, while only 10% are found across sub-Saharan Africa and 11% are found across the continent (figure 5b). MBP richness is highest in Central Africa and lowest in North Africa, whereas West and East Africa both have similar MBP richness which appears to be higher than that of Southern Africa (figure 5b). Considering the region's area, an excess of MBPs is detected in Central Africa and West Africa, whereas a deficit is detected in North Africa (figure 5b, p < 0.01, |Z| > 2.6, Binomial test). MBP endemicity is also highest in Central Africa, but lowest in Southern Africa, showing corresponding sharp departures from expectations based on the region's area (figure 5b, p < 0.01, |Z| > 2.5, Binomial test). Similar numbers of region-endemic MBPs are found in West, North and East Africa, in accordance with expectations based on area (figure 5b, p > 0.05). West and Central Africa share more MBPs with one another than other region pairs, whereas North Africa shares the fewest MBPs with all adjoining regions (figure 5b). Overlapping MBPs between three regions is highest between West, Central and East Africa (N = 16) compared with other combinations (1–4, figure 5).
The regional mosquito fauna is split into sub-Sahara and North Africa—the two most distinct divisions in terms of mosquito species composition—followed by the further split of sub-Saharan Africa into West-Central and East-Southern fauna (figure 6a, top). A country-based dendrogram reveals a more complex picture (figure 6a, bottom). Most countries from West and Central Africa are grouped together, as are countries from East and Southern Africa (figure 6a). Nine of the twelve high similarity clusters (R2 > 0.9) group countries from the same region, with only three exceptions (Nigeria–Ghana, Ivory Coast–CAR and DRC–Uganda, figure 6a), which share ecological similarity if not geographic continuity. The country dendrogram suggests substantive differences in mosquito faunas between Sahelian and equatorial West Africa countries, which is further supported by the grouping of Chad with Niger, as well as Nigeria with Liberia. Assemblages of mosquitoes defined by their significant co-occurrence in particular areas, independently from the regions defined above, are illustrated in electronic supplementary material, figure S1a.
The composition of MBPs at the regional scale closely follows that of the mosquitoes, splitting sub-Sahara from North Africa, followed by a further split of sub-Saharan Africa into West-Central Africa and the East-Southern African fauna (figure 6b, top). The country-level dendrogram based on MBP composition reveals more pervasive cross-regional and cross-sub-divisional clusters. For example, North African countries are clustered together, but Egypt is clustered with Tanzania (figure 6b). Nonetheless, most countries are grouped by their region or subdivision. Departures from regional clustering often follows ecological similarity between countries in the equatorial forests, such as with Cameroon and the Ivory Coast. Assemblages of MBPs defined by their significant co-occurrence in particular areas, independently from the regions defined above, are illustrated in electronic supplementary material, figure S1b (electronic supplementary material, Results and Discussion).
3. Discussion
Africa is facing changes in human density, accompanied by ecosystem destruction—processes that are projected to increase the exposure of humans and domestic animals to wildlife diseases [5,12,14,48]. Given Africa's high burden of MBDs, an understanding of the African mosquito-borne diseasosome is essential for improving local and global health and food security. This study provides the first holistic description of the joint biodiversity of mosquitoes and MBPs. Despite scarce/incomplete information and historically unbalanced sampling efforts of these taxa across Africa and globally (below), the data recovered herein summarizes over a century of surveillance and is worthy of exploration to guide future risk management and surveillance by recognizing key patterns and knowledge gaps. Our results identify regions harbouring more sylvatic vectors of both known and yet-to-be discovered MBPs, and advance understanding of the factors that have shaped the diversity of African MBDs. We consider the process of MBDs range expansion key for disease emergence and address the following questions: (i) does exceptional biodiversity of mosquitoes and MBPs in Africa account for its disproportionally large role in the origin of global MBDs, and whether this trend will continue? (ii) What is the geographical organization of mosquitoes and MBPs in Africa, and has the former structured the latter? and, (iii) what are the roles of domestication, dispersal, and adaptation to new vectors and hosts as drivers of MBD range expansion? Finally, we call attention to the need for surveillance and monitoring changes in MBPs' transmission patterns, as well as the development of new tools for translation of these data into risk maps and mitigation options.
A caveat of our analysis is the low resolution of the country-based distributional data. As explained above, a substantial part of the records on mosquito and MBP distribution is only available at the country level. Our analysis and interpretation of the results accommodate these limitations, although obtaining higher-resolution records will improve future biogeographical investigations.
(a) . Global mosquito-borne diseases that originated from Africa: past and future
The disproportionately large share of global MBDs originating from Africa, despite lower sampling effort of vector-borne diseases compared with other continents [3], along with the zoonotic nature of most of these diseases (Introduction), led to our hypothesis that the African mosquito and/or MBP faunas are especially diverse. The share of the African mosquito fauna (677 species) in the global culicid diversity closely aligns with expectations based on continental land area (19% versus 20%). Dominance of cosmopolitan genera, such as Aedes, Culex and Anopheles (figure 1a) further supports that the African fauna is not distinct at the genus level. However, it has a distinct assemblage of subgenera (figure 1b). The diversity of African arboviruses and (mammalian) plasmodia—the largest taxonomic groups of MBPs (figure 1c)—are considerably greater than expected by land mass at 30% and 40%, respectively (p < 0.0001). The higher diversity of African MBPs (but not mosquitoes) may account for the larger share of global MBDs originating in Africa and in part for its disproportionally heavy burden of MBDs. Moreover, this excess MBP diversity predicts that new global MBPs will continue to emerge from Africa at a higher rate than from any other continent.
Unlike the mosquito fauna, which is mostly well described, African MBPs remain poorly known, given that 47% of MBPs have been found in humans and domestic animals while at least 92% are maintained in wild species reservoirs (figure 1c). A conservative estimate of the African MBPs of vertebrates can be derived assuming that humans represent a typical host for vertebrate-specific MBPs of African origin. Humans, the most studied vertebrate are known as the only natural host for at least three and possibly five African plasmodia [49–52], as well as possibly one nematode [53,54]. This may be an underestimate since humans are among the youngest species. With over 5000 vertebrate species in Africa (approx. 1400 mammals [55], 2401 birds [56], 1648 reptiles [57] and approx. 600 amphibians [58]), a conservative estimate would be around 15 000 MBPs, suggesting that only approximately 1% of the total African MBP diversity is currently known. Thus, further pathogen and vector discovery and the identification of their reservoir hosts would be a productive area of future study, especially if targeting lesser-known vertebrates and mosquitoes. The emergence of previously considered ‘benign’ zoonotic pathogens such as ZIKV, CHIKV and WNV illustrates the need for comprehensive knowledge of MBPs, including those transmitted among wild animals by sylvatic vectors. Targeting mosquito subgenera with a high fraction of African species that have presumably had more time to be co-opted as vectors by African pathogens, such as species of Eretmapodites, Aedes (Catageiomyia), Aedes (Neomelaniconion), Culex (Maillotia) and Culex (Barraudius), might yield many new MBPs. The network of mosquitoes and MBPs defined by their significant co-occurrence in the same countries (electronic supplementary material, figure S2) identifies putative sylvatic vectors (electronic supplementary material, Results and Discussion).
(b) . The area occupied by African mosquitoes and mosquito-borne pathogens: drivers and implications
Most mosquitoes and MBPs occupy relatively small geographic ranges (figure 2). Endemicity in African mosquitoes is lower than that reported globally (50%) [29], likely because islands were excluded from our analysis. Based on the median area occupied by mosquito and MBP species (see Results), their typical range sizes cover 10% and 7% of continental Africa, respectively, that can be approximated by squares with sides of approximately 1500 km. The distributions of range size in African mosquitoes and MBPs are strikingly similar, suggesting that most African MBPs are transmitted by one or just a few mosquitoes in sylvatic cycles among their wild host species. As loyal association of MBPs with one or few mosquito vectors limits pathogen range, adapting to multiple vector species is likely a prerequisite for range expansion in MBPs (more below). Today's most widespread MBPs may have undergone range expansion from an original state that was similar to the majority of MBPs: circulating among wild vertebrates in a relatively small area and this can be expected for MBPs that will emerge in the future (below).
Whereas most mosquito species occupy a small area (one to three countries), some are significantly widespread (greater than 30 countries), including Ae. aegypti, Ae. vitattus, Cx. quinquefasciatus and 10 Anopheles species (figure 2a). These species feed preferentially on people and/or domestic animals and are well adapted to the domestic environment. Notably, at least 7 of these 13 species have been intercepted at altitude (40–290 m above ground) [37–39], indicating that windborne long-range migration is common among these species, as for other insects [59–62]. The high proportion of widespread Anopheles species suggests increased dispersal capacity and faster adaptation to domestic environments, perhaps reflecting their preference to feed on medium and large mammals. These traits may mutually reinforce each other because the widespread presence of domestic settings minimizes the risk of ending long-range migration in an inhospitable habitat [63].
There are only eight MBPs whose range exceeds 25 countries (figure 2b)—PLFLC, PLVIX, PLOVL, DENV, YFV, CHIKV, WNV and RVFV—all of which are vectored by one or more of the most widespread mosquitoes (above). These results suggest that the features of the exceptionally widespread MBPs include transmission among people or domestic animals and adaptation for being transmitted by at least one of the exceptionally widespread mosquitoes apart from vectors that maintain the virus in its sylvatic cycle.
(c) . Diversity, endemism and composition of mosquitoes and mosquito-borne pathogens across the continent
Consistent with ample evidence relating the decrease of species richness with latitude [5,26,29], African mosquito and MBP diversities measured by species richness are similarly concentrated along the equatorial forest peaking in Central Africa. Mosquitoes and MBPs exhibit corridors of moderate species richness along the Sahel (Mauritania to Chad), as well as between Central Africa and both East and Southern Africa (figure 3). These corridors' continuity and association to areas of seasonal aridity, which generally are inhospitable to mosquitoes, attest that they represent natural features. Unlike species richness, mosquito endemicity reveals two or three hotspots, whereas surrounding countries possess few or no endemic species (figure 4). The African equatorial forest, which is known for its high biodiversity, combines stable conditions with diverse habitats, a large area and mountains (greater than 1000 m above sea level) that promote speciation and the accumulation of species adapted to cooler habitats at higher elevation [2]. Thus, higher rates of speciation, lower rates of extinction, and high ecosystem diversity can explain the high richness and endemicity of mosquitoes, MBPs (figures 3 and 4) and vertebrate species [4] in this region. Somewhat different constellations of these factors extend into East and Southern Africa surrounding the Rift System, which similarly explain this area's high biodiversity [4].
In Sahelian countries, the markedly low ratio of endemicity to richness (figures 3 and 4) suggests a fauna with high propensity for long-range migration, enabling these mosquitoes to benefit from the ephemeral habitats that provide ideal conditions during the short Sahelian wet season [37,38,59,62,64]. Indeed, 50 species of mosquitoes have been intercepted at high altitudes (40–290 m above ground) in the Sahel of Mali alone, representing approximately 50% of the documented ground mosquito fauna in Mali [38]. This is compared with 31 species in the equatorial region in Kenya [39], representing only 13%, consistent with predictions that migratory strategies are more common around seasonal ecosystems [64–66]. The high endemicity/richness ratio in equatorial regions (figures 3 and 4) indicates a lower propensity for long-range migration, in support of predictions identifying resource tracking as the key driver of long-range migration. Windborne movement of infected mosquitoes may initiate outbreaks hundreds of kilometres away from sylvatic cycles. These results suggest that such outbreaks are more common at ecozones around the Sahel (e.g. [20,67]). The landscape of endemicity among MBPs shows a focal hotspot in CAR—one that is difficult to reconcile solely by the effect of species richness and country area (figure 4), even given the high biodiversity of the equatorial forest. In part, it might be explained by biased sampling; for example, research centres on yellow fever were established over 90 years ago in Nigeria and Uganda, leading to the discovery of new viruses such as WNV, ZIKV and Semliki Forest virus (SFV). Additional virus research centres were later established in South Africa, Egypt, Ivory Coast, Senegal, CAR, Kenya, Tanzania, DRC and Sudan [68,69], concentrating arbovirus surveillance in these areas. Regional differences in diversity, however, are minimal because the centres were distributed across all regions. Thus, regional analysis stands as a validation test of the main country-based results. For example, mosquito and MBP species display a higher proportion of region versus country endemicity (40% versus 25%, respectively), compared with only 3% (mosquitoes) and 11% (MBPs) across all regions of the continent (figure 5), which are consistent patterns with country-based results. Additionally, despite sharing ecozones and biomes between regions [4], the regional analysis revealed compositional heterogeneity in mosquitoes and MBPs across the continent (figures 5 and 6). Remarkably, the clustering of regions into subdivisions based on the composition of the mosquito and MBP faunas were nearly identical (figure 6). Similar to plant and vertebrate biogeographical results [4], our sub-Saharan Africa and North Africa divisions match the Palearctic and the Afrotropical faunal realms and highlight the Sahara as a geographic barrier. Clustering West and Central Africa regions together, separately from the cluster of East and Southern Africa (figure 6), aligns with the biogeographical landscapes of mammals and birds [4]. Our West and Central regions share the Sudanian, Sahelian and Equatorial (Guinean-Congolian) zoogeographic zones whereas our East and Southern Africa regions overlap with the Zambezian and South African zoogeographic zones [32]. Additionally, the high mountains along the Rift System probably contribute to separation between the East and Central regions. The clustering of countries based on mosquito composition indicated a subdivision of our West and Central African regions into Sudano-Sahelian and Equatorial subregions, as indicated by the grouping of Chad with Niger and Ivory Coast with CAR (figure 6); this showed correspondence between our results and the zoogeographical zones identified by Linder et al. [32]. Collectively, these corresponding patterns add support for a strong bio-geographical signal in our results.
Vertebrate hosts and vectors likely delimit MBPs' range; however, there is evidence supporting a greater role for the mosquito vectors. Both mammals and birds exhibit areas of high species richness and endemicity in East Africa and in smaller areas in Central, West and Southern Africa [4], unlike African mosquitoes and MBPs. Likewise, the Sudano-Sahelian area from Senegal to Sudan exhibits the second highest level of African mammals endemicity [4]. In birds, aside from a hyper-endemic area in East Africa, the remainder of the continent is nearly devoid of endemics [4]. Although salient biodiversity features of MBPs are more similar to mosquitoes than to mammals and birds, resolving this question requires data on species richness and endemicity using the same unit area, which is beyond the scope of our data and analysis.
(d) . A model of mosquito-borne disease range expansion and predicting emerging mosquito-borne diseases of the future
Our results suggest that the vast majority of MBPs are vectored by one or few mosquito species among wild vertebrates within a narrow range. Following Wolfe and colleagues [6], the biogeographical differences observed between the ‘original’ and the ‘emergent states’, represented by a handful of African MBDs (e.g. human malaria, YFV) points to a plausible process of range expansion. The last phase of this expansion includes continuous transmission (i) between people or domestic animals, (ii) by vectors that feed preferentially on these hosts and are themselves exceptionally widespread. The preceding state would include a capacity to circulate in humans or domestic animals for a single season or a few years, depending on whether vector populations are perennial or seasonal [67,70–72]. Unlike pathogens that are directly transmitted among hosts, the dependence of MBPs on both wild vertebrate and mosquito vector species (that blood-feed preferentially on reservoir species) implies that range expansion requires sequential adaptations to achieve transmissibility by new vectors, expanding the host-species breadth of the pathogen. Host and vector switching typically faces fitness tradeoffs linked to specialization in utilization of particular host and vector species [73–80]; thus, we expect that a typical range expansion of MBP requires a longer intermediate phase than a directly transmitted pathogen. Evidence in support of such a slow process is found in the rare occasions in which avian plasmodia (and other haemosporidia) found in migrant birds can be established in resident birds in both the Northern and Southern Hemispheres [73,80]. During the intermediate phase, the number of vectors and host species slowly increase, facilitating a gradual enlargement of the geographical range of the MBP. Once the MBP attains transmissibility into and from human or domestic animals by at least one of the domesticated vectors, the transition into the last states is complete and a rapid final range expansion is expected worldwide.
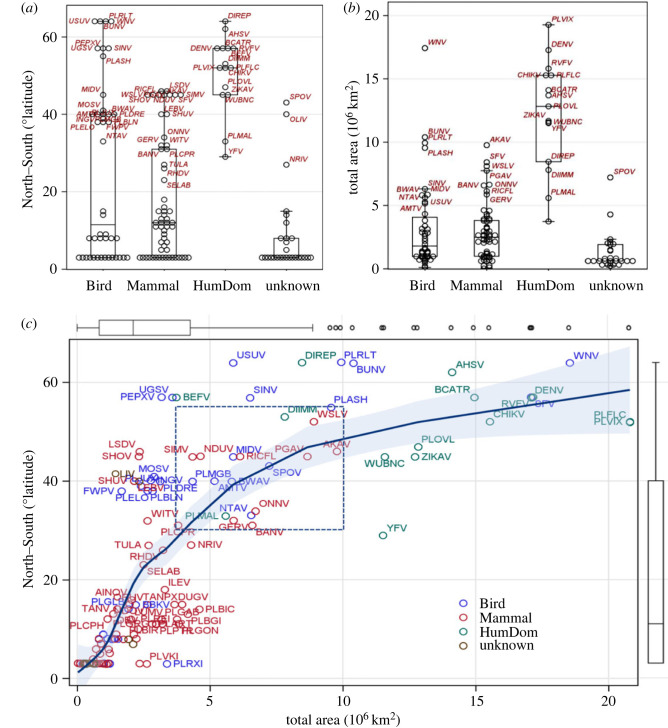
Accordingly, a larger-than-typical geographical range would be a marker of an MBP in the intermediate phase, in which it has adapted to be transmitted by multiple mosquito vector species and possibly between additional sylvatic vertebrate species. Except for small coastal ecozones, the African ecozones are typically wider across their east–west axis than across their north–south axis [4], suggesting that the longer the north–south dimension of an MBP's range, the more likely it is to be transmitted by multiple vector and host species. Hence, we propose that an MBP's total range size, estimated as the sum of the area (of countries, in our data) covered by its range and the maximal north–south length of its range, be used to gauge its range expansion phase. While these range size measures are expected to be largest in MBPs circulating among humans and domestic animals and smallest for those circulating in wild mammals, it is less clear if MBPs circulating in wild birds are larger than those of wild mammals. Both measures were found to be larger for MBPs circulating in humans and domestic animals for the median and the 75th quantile (area: p < 0.0001, td.f. = 116 > 8, north–south: p < 0.0001, td.f. = 116 > 5, figure 7), but no significant differences were found between MBPs circulating in mammals and birds even by using a one-sided test (area: p > 0.31, td.f. = 116 < 0.11, north–south: p > 0.11, td.f. = 116 < 1.2, figure 7). Contrary to our expectation, the north–south distance seems to ‘saturate’ faster than the total area (figure 7), suggesting that it may be more sensitive to early range expansion than to later stages of MBD range expansion. The total area of most MBPs transmitted among humans or domestic animals (undergone range expansion, N = 15) covers an area of 10–20 × 106 km2 and their north–south distance spans 40–60°, whereas the overall majority of MBPs (N > 90) cover area less than 4 × 106 km2 and north–south distance less than 15° (figure 7c). Except for eight MBPs transmitted among wild birds that have a long north–south distance, 30 MBPs occupy intermediate ranges covering area 4–10 × 106 km2 and north–south distance of 20–50° (figure 7c). Accordingly, this group is enriched with species that are currently at the intermediate phase of range expansion that pose elevated risk for disease emergence. These include Usutu (USUV), Wesselsbron (WSLV), Akabane (AKAV), Spondweni virus (SPOV) and ONNV (figure 7c). This approach putatively identifies pathogens during their intermediate phase of range expansion before they infect humans or domestic animals. Monitoring changes in geographical range as well as the MBP host and vector ranges will further validatate these predictions. For example, the number of vectors and hosts in which a pathogen is found and the numbers by which it can be transmitted may be used as independent markers of the MBP's prospects to undergo range expansion. Experimental evidence about the pathogen compatibility and capacity for transmission, e.g. [81] with the most widespread vectors and domestic hosts, will further augment its risk assessment. Evaluation of the pathogenicity and impact that an MBP would have on human and domestic animals is beyond the scope of this analysis, but the possibility of increased virulence linked to transmissibility in these new hosts by domesticated vectors—for instance, ZIKV—should not be ignored.
Figure 7.
Ranking of African MBPs by their range area and maximum north–south distance to estimate their phase of range expansion. (a) Variation between host groups (HumDom denotes pathogens transmitted by vector among humans or domestic animals) in north–south distance (latitude degrees) and (b) variation between host groups total range area (106 km2). (c) Relationship between MBP's total area (106 km2; x-axis) and the maximum north–south distance (degrees latitude; y-axis) using local regression (locally estimated scatterplot smoothing (loess) and 95% confidence limits of the mean (response) (CLM) on all MBDs (N = 150). Box plots along axes display distributions of corresponding variables. Acronym of MBPs are given for those with total area larger than 2.5 or north–south longer than 5° and colours denote host group (birds were used if birds and mammals are thought to act as natural hosts). Box draws attention to putative MBPs at intermediate phase of range expansion (excluding MBPs of birds and domestic animals, see text).
(e) . Conclusion and policy implications
MBDs such malaria, West Nile and Zika have emerged from the African continent at a disproportionally high rate, reflecting Africa's exceptionally diverse arboviral and plasmodia faunas and highlighting that attention to African MBDs is key for predicting and mitigating future threats to global public health and food security. Here, we aimed to better understand MBP range expansion by analysis of the geographical organization of mosquitoes and MBPs on the continent. We find that most MBPs circulate in sylvatic cycles between wild host species and vector species, which remain mostly unknown. We estimate that only 1% of the African MBPs are presently known. Mosquito and MBP diversities across the continent reveal major divisions and hotspots. Striking similarities between mosquitoes and MBPs in their spatial diversities and range sizes suggest that mosquitoes play a dominant role in delimiting the range of MBPs. Extending the theory which explains pathogen emergence in relation to regional and global spread, our results highlight drivers that promote range expansion via adaptations to new vector and host species and allow us to rank the African MBPs by their emergence risk for further tracking and validation.
The projected growth of the African human population will speed up anthropogenic changes on the continent including fragmentation of natural ecosystems; biological invasions; and warming, floods and droughts caused by climate change. These changes will likely increase the risk of MBD emergence unless health science capacity in Africa is strengthened. Improving disease surveillance in Africa, capacity to predict and effectively respond to MBD outbreaks will strengthen public health, veterinary science, food security and conservation, promoting cross-disciplinary collaboration between vector biologists, parasitologists, virologists, zoologists, taxonomists, ecologists, meteorologists, conservation and agricultural experts, sociologists and land-use planners, among others. Such investments will tackle the poor state of knowledge of MBPs, enable data integration and modelling of outbreaks, empower preparedness and effective mitigation strategies that are urgently needed in Africa. Given the accelerating pace of pathogen emergence and rapid spread across borders, implementing trans-continental investments in Africa is a priority we cannot afford to postpone.
4. Methods
The database and our analysis refer to continental Africa (surrounded by the Mediterranean Sea to the north, the Indian Ocean to the east and the Atlantic Ocean to the west), excluding all islands (e.g. Cape Verde, Comoros, Madagascar, Mauritius, Seychelles, São Tomé and Príncipe) because island biogeography requires consideration of multiple factors, such as distances to the nearest mainland and to other islands, historical formation of the island, existence of past terrestrial bridges, etc., which deserve separate treatment. Very few records of mosquitoes and MBPs can be found for Eswatini, Lesotho, South Sudan and Western Sahara. Moreover, parts of their records are included in their previous political affiliations, e.g. South Sudan in Sudan. Therefore, these countries are not listed in our analysis; instead, our analysis, pertains to 45 countries, with few countries that subsumed those in the past and still ‘contain’ their records, e.g. ‘Sudan and South Sudan’ being used (electronic supplementary material, table S1). Because countries differ in surveillance effort, grouping neighbouring countries into regions minimizes variation in surveillance effort variability and was used to test country-based patterns. Unlike the geopolitical regions with the same names, our five regions were defined to maximize distances among regions, accommodate latitudinal variation and minimize inter-region enclaves (figure 5).
Our African mosquito distribution data (electronic supplementary material, file S1) was initially generated based on the global distribution lists, updated to 2017 [26]. We updated records of anophelines in sub-Saharan countries [31], and culicines following country-specific lists recently published for Mali [82], Mauritania [83], Morocco [84] and incorporated records for southern African countries [85]. Information on global diversity of mosquitoes was recently updated [26] and allowing reconciliation of species identifications that were later revised, e.g. Culex tigripes/Lutzia tigripes or An. arabiensis and An. gambiae. Subspecies were not included in our data. To our knowledge, these sources represent the most comprehensive and updated information on the African mosquito fauna.
The MBP distribution data was generated based on hundreds of references listed in electronic supplementary material, file S2, providing they met the three criteria as follows: a peer-reviewed scientific source (or a source listed in peer-reviewed sources, e.g. the Centers for Disease Control (CDC) arbovirus catalogue) reported that the MBP has been: (i) naturally transmitted in continental Africa, (ii) to a terrestrial vertebrate host, (iii) by mosquito vector, to the extent that this mode of transmission is recognized to have an epidemiological role, even if other mode(s) of transmission play a greater role. Repeated searches in PubMed, Web of Science, Google scholar and Biosis were carried out using these basic terms: ‘Africa’ AND ‘pathogen*’ AND ‘mosquito*’, with the following permutations. The term ‘pathogen’ was changed into virus, arbovirus, protozoa, plasmodium, nematode, filaria and bacteria and the term ‘mosquito*’ was changed to ‘vector*’. For each MBP, additional searches with its name (e.g. ‘Plasmodium vivax’, ‘Zika virus’) were carried out with the term ‘Africa*’. The literature cited in publications that met our criteria (above) were examined to expand the sources. The additional sources included books, catalogues/databases and older publications that were not available in the electronic literature databases (above). The title/abstract of each paper were read to select putatively relevant sources with respect to the localization of African MBPs, their vectors and their natural vertebrate hosts. A more detailed reading allowed us to extract the relevant information about isolation of MBPs and add the reference to our database.
Our database includes information whether mosquito role in the MBP transmission is secondary or primary and whether it is biological or mechanical. Strains or any sub-species definitions were not included. To ascertain accuracy of our MBP records, we compared our data with the CRORA database (Centre de Référence OMS sur la Recherche des Arbovirus et des Fièvres Hémorragiques (CRORA)) (last updated in 2015) and the EID2 database [86] (as of September 2021) among other sources. Only records that met our above criteria were included in our database. Although redundant and laborious, the construction process of this database (above) ensured exhaustive and comprehensive information that was cross validated between us to minimize errors and bias. The comparisons with independent databases, e.g. CRORA and EID2 have corroborated its completeness with respect to the criteria defined above. By confining our records to continental Africa, the term endemic refers to a species found in one African country (or region, when specified), however, although less common, the species may be also found outside continental Africa.
Information on land mass of the world and of continental African countries [87] were used to calculate the proportion of area of continental Africa from the land worldwide and total area per species. Accordingly, the total area of the worldwide and continental Africa we used are 148,568,946.1 and 296.63,582.0. Global coordinates central position of each African country [88] were used to compute maximum north–south range distances for each MBP.
(a) . Data analysis
Goodness of fit χ2-tests implemented by Proc Freq [89] were used to assess if diversity in a particular area was higher than predicted by the relative size of the area. Exact tests were used if expected values were smaller than 5. Confidence intervals (distribution free) of medians were computed using Proc Univariate [89] based on ranks. Person correlation, linear and quadratic regression models to relate biodiversity measures with country area were implemented by Proc Reg [89]. Quantile regression implemented by Proc Quantreg [89] extends the general linear model for estimating conditional change in the response variable across its distribution as expressed by quantiles, rather than its mean (though the median is similar to the mean in symmetric distributions). It does not assume parametric distribution (e.g. normal) of the random error part of the model, thus it is considered semiparametric. The value of this analysis is that it allows to address variation among the medians of various groups and also across quantiles even when the mean and the median are unchanging. The parameters estimates in linear quantile regression models are interpreted as in typical general linear models, as rates of change adjusted for the effects of the other variables in the model for a specified quantile [90]. We used matrices of presence absence of mosquitoes or MBPs to compute matrices of Jaccard distances between regions or countries (separately), using Proc Distance [89] and used the Ward method in Proc Cluster with height measured by R2 (the proportion of variance accounted by the clusters) to produce and plot dendrograms
Acknowledgements
We thank Roy Faiman, Alvaro Molina-Cruz, Jose’ MC Ribeiro (NIH/NIAID), Don R. Reynolds (University of Greenwich, UK), Tom Burkot (James Cook University, Australia), and Phil Lounibos (University of Florida) for providing helpful comments based on earlier versions of this manuscript and for exciting discussions.
Data accessibility
The data are provided in electronic supplementary material [91].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
T.L.: conceptualization, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing; C.K.: data curation, methodology, validation, writing—original draft, writing—review and editing; J.W.: data curation, methodology, writing—original draft, writing—review and editing; M.D.: methodology, validation, writing—review and editing; R.W.: data curation, writing—review and editing; Y.L.: data curation, methodology, resources, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda MD (AI001196-06). Y.-M.L. was supported by the Global Emerging Infections Surveillance Branch of the Armed Forces Health Surveillance Division (AFHSD-GEIS) (WRBU Sustainment Award, P0050_23_WR). Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
References
- 1.W.H.O. 2020. Vector-borne diseases [WWW Document] [accessed on 2020]. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
- 2.Lomolino MV. 2020. Biogeography a very short introduction, 1st edn. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Swei A, Couper LI, Coffey LL, Kapan D, Bennett S. 2020. Patterns, drivers, and challenges of vector-borne disease emergence. Vector-Borne Zoonot. Dis. 20, 159-170. ( 10.1089/vbz.2018.2432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess ND, Hales JD, Underwood E, Dinerstein E, Olson D, Itoua I, Schipper J, Rickkets T, Newman K. 2004. Terrestrial eco-regions of Africa and Madagascar: A conservation assessment. Washington, DC: Island Press. [Google Scholar]
- 5.Guernier V, Hochberg ME, Guégan JF. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2, e141. ( 10.1371/journal.pbio.0020141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe ND, Dunavan CP, Diamond J. 2007. Origins of major human infectious diseases. Nature 447, 279-283. ( 10.1038/nature05775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritchie H, Rodes-Guirao L, Mathieu E, Gerber M, Ortiz-Ozpina E, Hasell J, Roser M. 2021. Population Growth. Published online at at OurWorldInData.org (Online Resource). See https://ourworldindata.org/future-population-growth.
- 8.Weaver SC, Winegar R, Manger ID, Forrester NL. 2012. Alphaviruses: population genetics and determinants of emergence. Antiviral Res. 94, 242-257. ( 10.1016/j.antiviral.2012.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braack L, Gouveia De Almeida AP, Cornel AJ, Swanepoel R, De Jager C. 2018. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasites and Vectors 11, 29. ( 10.1186/s13071-017-2559-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke D. 1998. The evolvability of emerging viruses. In Pathology of emerging infections 2 (eds Nelson AM, Horsburg RCJ), pp. 1-12. Washington, DC: American Society for Microbiology. [Google Scholar]
- 11.Binder S, Levitt AM, Sacks JJ, Hughes JM. 1999. Emerging infectious diseases: public health issues for the 21st century. Science 284, 1311-1313. ( 10.1126/science.284.5418.1311) [DOI] [PubMed] [Google Scholar]
- 12.Taylor LH, Latham SM, Woolhouse MEJ. 2001. Risk factors for human disease emergence. Philos. Trans. R. Soc. B 356, 983-989. ( 10.1098/rstb.2001.0888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolhouse MEJ, Gowtage-Sequeria S. 2005. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 11, 1842-1847. ( 10.3201/eid1112.050997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morse SS, Mazet JAK, Woolhouse M, Parrish CR, Carroll D, Karesh WB, Zambrana-Torrelio C, Lipkin WI, Daszak P. 2012. Prediction and prevention of the next pandemic zoonosis. The Lancet 380, 1956-1965. ( 10.1016/S0140-6736(12)61684-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg R. 2015. Detecting the emergence of novel, zoonotic viruses pathogenic to humans. Cell. Mol. Life Sci. 72, 1115-1125. ( 10.1007/s00018-014-1785-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karabatsos N. 1985. International catalogue of arboviruses, including certain other viruses of vertebrates, 3rd edn. San Antonio, TX: American Society for Tropical Medicine and Hygiene for the Subcommittee on Information Exchange of the American Committee on Arthropod-borne viruses. [Google Scholar]
- 18.Service MW. 2001. Encyclopedia of arthropod-transmitted infections, 1st edn. New York, NY: CAB International. [Google Scholar]
- 19.Njabo KY, Cornel AJ, Sehgal RNM, Loiseau C, Buermann W, Harrigan RJ, Pollinger J, Valkiūnas G, Smith TB. 2009. Coquillettidia (Culicidae, Diptera) mosquitoes are natural vectors of avian malaria in Africa. Malar. J. 8, 1-12. ( 10.1186/1475-2875-8-193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diallo D, et al. 2012. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Neglected Tropical Diseases 6, 1-14. ( 10.1371/journal.pntd.0001649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins SL. 2014. Malaria's many mates: past, present, and future of the systematics of the Order Haemosporida. J. Parasitol. 100, 11-25. ( 10.1645/13-362.1) [DOI] [PubMed] [Google Scholar]
- 22.Kyalo D, Amratia P, Mundia CW, Mbogo CM, Coetzee M, Snow RW. 2017. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898-2016. Welcome Open Res. 2, 57. ( 10.12688/wellcomeopenres.12187.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villinger J, Mbaya MK, Ouso D, Kipanga PN, Lutomiah J, Masiga DK. 2017. Arbovirus and insect-specific virus discovery in Kenya by novel six genera multiplex high-resolution melting analysis. Mol. Ecol. Resour. 17, 466-480. ( 10.1111/1755-0998.12584) [DOI] [PubMed] [Google Scholar]
- 24.Nanfack Minkeu F, Vernick KD. 2018. A systematic review of the natural virome of anopheles mosquitoes. Viruses 10, 222. ( 10.3390/v10050222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver SC, Chen R, Diallo M. 2020. Chikungunya virus: role of vectors in emergence from enzootic cycles. Annu. Rev. Entomol. 65, 313-332. ( 10.1146/annurev-ento-011019-025207) [DOI] [PubMed] [Google Scholar]
- 26.Wilkerson RC, Linton Y-M, Strickman D. 2021. Mosquitoes of the world. Vols. 1 & 2. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 27.Perkins SL. 2018. Malaria in farmed ungulates: an exciting new system for comparative parasitology. mSphere 3, e00161-18. ( 10.1128/mSphere.00161-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG. 1998. New vectors of Rift Valley fever in West Africa. Emerg. Infect. Dis. 4, 289-293. ( 10.3201/eid0402.980218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley DH, Rueda LM, Wilkerson RC. 2007. Insight into global mosquito biogeography from country species records. J. Med. Entomol. 44, 554-567. ( 10.1093/jmedent/44.4.554) [DOI] [PubMed] [Google Scholar]
- 30.CDC. 2019. Arbovirus Catalog (WWW Document) (last accessed on 18 December 2021). See https://wwwn.cdc.gov/arbocat/VirusBrowser.aspx.
- 31.Irish SR, Kyalo D, Snow RW, Coetzee M. 2020. Updated list of Anopheles species (Diptera: Culicidae) by country in the Afrotropical Region and associated islands. Zootaxa 4747, 401-449. ( 10.11646/zootaxa.4747.3.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linder HP, De Klerk HM, Born J, Burgess ND, Fjeldså J, Rahbek C. 2012. The partitioning of Africa: statistically defined biogeographical regions in sub-Saharan Africa. J. Biogeogr. 39, 1189-1205. ( 10.1111/j.1365-2699.2012.02728.x) [DOI] [Google Scholar]
- 33.Bensch S, Hellgren O, Perez-Tris J. 2009. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 9, 1353-1358. ( 10.1111/j.1755-0998.2009.02692.x) [DOI] [PubMed] [Google Scholar]
- 34.Turell MJ, Knudson GB. 1987. Mechanical transmission of Bacillus anthracis by stable flies (Stomoxys calcitrans) and mosquitoes (Aedes aegypti and Aedes taeniorhynchus). Infect. Immun. 55, 1859-1861. ( 10.1128/iai.55.8.1859-1861.1987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kligler IJ, Muckenfuss RS, Rivers TM. 1928. Transmission of fowl-pox by mosquitoes. Proc. Soc. Exp. Biol. Med. 26, 128-129. ( 10.3181/00379727-26-4175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damassa AJ. 1966. The role of Culex tarsalis in the transmission of fowl pox virus. Avian Dis. 10, 57. ( 10.2307/1588207) [DOI] [Google Scholar]
- 37.Huestis DL, et al. 2019. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 574, 404-408. ( 10.1038/s41586-019-1622-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaro AS, et al. 2022. Diversity, composition, altitude, and seasonality of high-altitude windborne migrating mosquitoes in the Sahel: implications for disease transmission. Front. Epidemiol. 2, e1001782. ( 10.3389/fepid.2022.1001782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atieli HE, et al. 2023. Wind-assisted high-altitude dispersal of mosquitoes and other insects in East Africa. J. Med. Entomol. 60, 698-707. ( 10.1093/jme/tjad033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jupp P, Mcintosh B. 1990. Aedes furcifer and other mosquitoes as vectors of chikungunya virus at Mica, northeastern Transvaal, South Africa. J. Am. Mosq. Control Assoc. 6, 415-420. [PubMed] [Google Scholar]
- 41.Collins WE, Jeffery GM. 2005. Plasmodium ovale: parasite and disease. Clin. Microbiol. Rev. 18, 570-581. ( 10.1128/CMR.18.3.570-581.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diallo D, et al. 2014. Zika virus emergence in mosquitoes in Southeastern Senegal, 2011. PLoS ONE 9, e109442. ( 10.1371/journal.pone.0109442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faye O, Ba Y, Faye O, Talla C, Diallo D, Chen Ret al. 2014. Urban epidemic of Dengue virus serotype 3 infection, Senegal, 2009. Emerg. Infect. Dis. 20, 456-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Twohig KA, Pfeffer DA, Baird JK, Price RN, Zimmerman PA, Hay SI, Gething PW, Battle KE, Howes RE. 2019. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl. Trop. Dis. 13, e0007140. ( 10.1371/journal.pntd.0007140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faulde MK, Spiesberger M, Abbas B. 2012. Sentinel site-enhanced near-real time surveillance documenting West Nile virus circulation in two Culex mosquito species indicating different transmission characteristics, Djibouti City, Djibouti. J. Egypt Soc. Parasitol. 42, 461-474. ( 10.12816/0006332) [DOI] [PubMed] [Google Scholar]
- 46.Epelboin Y, Talaga S, Epelboin L, Dusfour I. 2017. Zika virus: an updated review of competent or naturally infected mosquitoes. PLoS Negl. Trop. Dis. 11, e0005933. ( 10.1371/journal.pntd.0005933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diagne MMÃ, et al. 2021. Yellow fever outbreak in eastern Senegal, 2020–2021. Viruses 13, 1475. ( 10.3390/v13081475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fenollar F, Mediannikov O. 2018. Emerging infectious diseases in Africa in the 21st century. New Microb. New Infect. 26, S10. ( 10.1016/j.nmni.2018.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, et al. 2010. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature 467, 420-425. ( 10.1038/nature09442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutledge GG, et al. 2017. Plasmodium malariae and P. ovale genomes provide insights into malaria parasite evolution. Nature 542, 101-104. ( 10.1038/nature21038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arisue N, Honma H, Kume K, Hashimoto T. 2021. Progress in understanding the phylogeny of the Plasmodium vivax lineage. Parasitol. Int. 87, 102507. ( 10.1016/j.parint.2021.102507) [DOI] [PubMed] [Google Scholar]
- 52.Daron J, et al. 2021. Population genomic evidence of Plasmodium vivax Southeast Asian origin. Sci. Adv. 7, eabc3713. ( 10.1126/sciadv.abc3713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurence BR. 1989. The global dispersal of bancroftian filariasis. Parasitol. Today 5, 260-264. ( 10.1016/0169-4758(89)90260-3) [DOI] [PubMed] [Google Scholar]
- 54.Small ST, Labbé F, Coulibaly YI, Nutman TB, King CL, Serre D, Rogers R. 2019. Human migration and the spread of the nematode parasite Wuchereria bancrofti. Mol. Biol. Evol. 36, 1931-1941. ( 10.1093/molbev/msz116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgin CJ, Colella JP, Kahn PL, Upham NS. 2018. How many species of mammals are there? J. Mammal. 99, 1-14. ( 10.1093/jmammal/gyx147) [DOI] [Google Scholar]
- 56.Lepage D. 2021. Avibase - The World Bird Database (WWW Document) (last accessed on 17 May 2021). See https://avibase.bsc-eoc.org/checklist.jsp?region=AFC.
- 57.Tolley KA, Alexander GJ, Branch WR, Bowles P, Maritz B. 2016. Conservation status and threats for African reptiles. Biol. Conserv. 204, 63-71. ( 10.1016/j.biocon.2016.04.006) [DOI] [Google Scholar]
- 58.Channing A, Rodel M-O. 2019. Field guide to the frogs & other amphibians of Africa. Cape Town, South Africa: Penguin Random House. [Google Scholar]
- 59.Pedgley DE, Reynolds DR, Tatchell GM. 1995. Long-range insect migration in relation to climate and weather: Africa and Europe. In Insect migration: tracking resources through space and time (eds Drake VA, Gatehouse AG), pp. 3-30. New York, NY: Cambridge University Press. [Google Scholar]
- 60.Reynolds DR, Chapman JW, Harrington R. 2006. The migration of insect vectors of plant and animal viruses. Adv. Virus Res. 67, 453-517. ( 10.1016/S0065-3527(06)67012-7) [DOI] [PubMed] [Google Scholar]
- 61.Chapman JW, Bell JR, Burgin LE, Reynolds DR, Pettersson LB, Hill JK, Bonsall MB, Thomas JA. 2012. Seasonal migration to high latitudes results in major reproductive benefits in an insect. Proc. Natl Acad. Sci. USA 109, 14 924-14 929. ( 10.1073/pnas.1207255109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drake VA, Reynolds DR. 2012. Radar entomology: observing insect flight and migration. Wallingford, UK: CAB International. [Google Scholar]
- 63.Lehmann T, Bamou R, Chapman JW, Reynolds DR, Armbruster PA, Dao A, Yaro AS, Burkot TR, Linton Y-M. 2023. Urban malaria may be spreading via the wind—here's why that's important. Proc. Natl Acad. Sci. USA 20, e2301666120. ( 10.1073/pnas.2301666120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Florio J, et al. 2020. Diversity, dynamics, direction, and magnitude of high-altitude migrating insects in the Sahel. Sci. Rep. 10, 1-14. ( 10.1038/s41598-020-77196-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Southwood TRE. 1962. Migration of terrestrial arthropods in relation to habitat. Biol. Rev. 37, 171-211. ( 10.1111/j.1469-185X.1962.tb01609.x) [DOI] [Google Scholar]
- 66.Drake VA, Gatehouse AG. 1995. Insect migration: tracking resources through space and time. New York, NY: Cambridge University Press. [Google Scholar]
- 67.Diallo M, Nabeth P, Ba K, Sall AA, Ba Y, Mondo M, Girault L, Abdalahi MO, Mathiot C. 2005. Mosquito vectors of the 1998–1999 outbreak of Rift Valley Fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med. Vet. Entomol. 19, 119-126. ( 10.1111/j.0269-283X.2005.00564.x) [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg R, Johansson MA, Powers AM, Miller BR. 2013. Search strategy has influenced the discovery rate of human viruses. Proc. Natl Acad. Sci. USA 110, 13 961-13 964. ( 10.1073/pnas.1307243110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasilakis N, et al. 2019. Exploiting the legacy of the arbovirus hunters. Viruses 11, 471. ( 10.3390/v11050471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sellers RF. 1980. Weather, host and vector–their interplay in the spread of insect-borne animal virus diseases. J. Hyg. 85, 65-102. ( 10.1017/S0022172400027108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. 1999. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science (New York, N.Y.) 285, 397-400. ( 10.1126/science.285.5426.397) [DOI] [PubMed] [Google Scholar]
- 72.Hanafi H, et al. 2010. Rift Valley Fever virus epidemic in Kenya, 2006/2007: the entomologic investigations. Am. J. Trop. Med. Hyg. 83, 28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hellgren O, Waldenström J, Peréz-Tris JA, Szöll E, Si Ö, Hasselquist D, Krizanauskiene A, Ottosson U, Bensch S. 2007. Detecting shifts of transmission areas in avian blood parasites—a phylogenetic approach. Mol. Ecol. 16, 1281-1290. ( 10.1111/j.1365-294X.2007.03227.x) [DOI] [PubMed] [Google Scholar]
- 74.Joy DA, Gonzalez-Ceron L, Carlton JM, Gueye A, Fay M, Mccutchan TF, Su X-. 2008. Local adaptation and vector-mediated population structure in Plasmodium vivax malaria. Mol. Biol. Evol. 25, 1245-1252. ( 10.1093/molbev/msn073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasilakis N, Deardorff ER, Kenney JL, Rossi SL, Hanley KA, Weaver SC. 2009. Mosquitoes put the brake on arbovirus evolution: experimental evolution reveals slower mutation accumulation in mosquito than vertebrate cells. PLoS Pathog. 5, e1000467. ( 10.1371/journal.ppat.1000467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molina-Cruz A, Lehmann T, Knöckel J. 2013. Could culicine mosquitoes transmit human malaria? Trends Parasitol. 29, 530-537. ( 10.1016/j.pt.2013.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Molina-Cruz A, et al. 2013. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340, 984-987. ( 10.1126/science.1235264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. 2015. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc. Natl Acad. Sci. USA 112, 15 178-15 183. ( 10.1073/pnas.1520426112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molina-Cruz A, et al. 2020. Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc. Natl Acad. Sci. USA 117, 2597-2605. ( 10.1073/pnas.1917042117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ricklefs RE, et al. 2017. Avian migration and the distribution of malaria parasites in New World passerine birds. J. Biogeogr. 44, 1113-1123. ( 10.1111/jbi.12928) [DOI] [Google Scholar]
- 81.Haddow AD, Nasar F, Guzman H, Ponlawat A, Jarman RG, Tesh RB, Weaver SC. 2016. Genetic characterization of Spondweni and Zika viruses and susceptibility of geographically distinct strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni virus. PLoS Negl. Trop. Dis. 10, e0005083. ( 10.1371/journal.pntd.0005083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tandina F, Doumbo O, Yaro AS, Traoré SF, Parola P, Robert V. 2018. Mosquitoes (Diptera: Culicidae) and mosquito-borne diseases in Mali, West Africa. Parasites & Vectors 11, 467. ( 10.1186/s13071-018-3045-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mint Mohamed Lemine A, et al. 2017. Mosquitoes (Diptera: Culicidae) in Mauritania: a review of their biodiversity, distribution and medical importance. Parasites and Vectors 10, 35. ( 10.1186/s13071-017-1978-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trari B, Dakki M, Harbach RE. 2017. An updated checklist of the Culicidae (Diptera) of Morocco, with notes on species of historical and current medical importance. J. Vector Ecol. 42, 94-104. ( 10.1111/jvec.12243) [DOI] [PubMed] [Google Scholar]
- 85.Jupp PG. 1996. Mosquitoes of Southern Africa: culicinae and toxorhynchitinae. Hartebeespoort, Republic of South Africa: Ekogilde Publishers. [Google Scholar]
- 86.Wardeh M, Risley C, Mcintire MK, Setzkorn C, Baylis M. 2015. Database of host-pathogen and related species interactions, and their global distribution. Sci. Data 2, 1-11. ( 10.1038/sdata.2015.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.The-World-Bank. 2021. Data: Land Area (WWW Document) (last accessed on 9 February 2021). See https://data.worldbank.org/indicator/AG.LND.TOTL.K2.
- 88.Google Developers. 2021. Countries: Public data (WWW Document) (last accessed on 6 October 2021). See https://developers.google.com/public-data/docs/canonical/countries_csv.
- 89.SAS Institute. 2012. SAS software for Windows Version 9.4. See www.sas.com
- 90.Cade BS, Noon BR. 2003. A gentle introduction to quantile regression for ecologists. Frontiers Ecol. Environ. 1, 412–420.
- 91.Lehmann T, Kouam C, Woo J, Diallo M, Wilkerson R, Linton Y-M. 2023. The African mosquito-borne diseasosome: geographical patterns, range expansion and future disease emergence. Figshare. ( 10.6084/m9.figshare.c.6922283) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lehmann T, Kouam C, Woo J, Diallo M, Wilkerson R, Linton Y-M. 2023. The African mosquito-borne diseasosome: geographical patterns, range expansion and future disease emergence. Figshare. ( 10.6084/m9.figshare.c.6922283) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in electronic supplementary material [91].