Summary
Background
Patients with chronic lymphocytic leukemia (CLL) have a higher risk of developing other malignancies (OMs) compared to the general population. However, the impact of CLL-related risk factors and CLL-directed treatment is still unclear and represents the focus of this work.
Methods
We conducted a retrospective international multicenter study to assess the incidence of OMs and detect potential risk factors in 19,705 patients with CLL, small lymphocytic lymphoma, or high-count CLL-like monoclonal B-cell lymphocytosis, diagnosed between 2000 and 2016. Data collection took place between October 2020 and March 2022.
Findings
In 129,254 years of follow-up after CLL diagnosis, 3513 OMs were diagnosed (27.2 OMs/1000 person-years). The most common hematological OMs were Richter transformation, myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Non-melanoma skin (NMSC) and prostate cancers were the most common solid tumors (STs).
The only predictor for MDS and AML development was treatment with fludarabine and cyclophosphamide with/without rituximab (FC ± R) (OR = 3.7; 95% CI = 2.79–4.91; p < 0.001). STs were more frequent in males and patients with unmutated immunoglobulin heavy variable genes (OR = 1.77; 95% CI = 1.49–2.11; p < 0.001/OR = 1.89; 95% CI = 1.6–2.24; p < 0.001).
CLL-directed treatment was associated with non-melanoma skin and prostate cancers (OR = 1.8; 95% CI = 1.36–2.41; p < 0.001/OR = 2.11; 95% CI = 1.12–3.97; p = 0.021). In contrast, breast cancers were more frequent in untreated patients (OR = 0.17; 95% CI = 0.08–0.33; p < 0.001).
Patients with CLL and an OM had inferior overall survival (OS) than those without. AML and MDS conferred the worst OS (p < 0.001).
Interpretation
OMs in CLL impact on OS. Treatment for CLL increased the risk for AML/MDS, prostate cancer, and NMSC. FCR was associated with increased risk for AML/MDS.
Funding
AbbVie, and EU/EFPIAInnovative Medicines Initiative Joint Undertaking HARMONY grant n° 116026.
Keywords: Chronic lymphocytic leukemia, Other malignancies, Other cancers, Second primary malignancies
Research in context.
Evidence before this study
Two authors independently reviewed the published literature in PubMed using “chronic lymphocytic leukemia,” “second malignancies,” “other malignancies,” “second cancers,” and “other cancers” as search items with no restrictions on the date of publication or the language.
The available evidence indicates that patients with chronic lymphocytic leukemia (CLL) have a higher risk of developing a second, other malignancy (OM) than the general population. Large registry studies from the United States, Denmark, and the Netherlands found an increased incidence of both hematological and non-hematological OMs in patients with CLL. In addition, several studies showed that the overall survival (OS) of patients with OMs and CLL was worse than those with CLL but without an OM. In these studies, in parallel with the general population, older age, and male sex remained important risk factors for the increased risk of OMs. However, the CLL-associated risk factors for OMs remain elusive. CLL-directed treatment has been linked with the occurrence of OMs, although these findings are inconsistent between studies.
The current study is an international multicenter retrospective study aimed at finding potential risk factors for the occurrence of OMs in patients with CLL.
Added value of this study
Our study highlights for the first time in a large cohort the clear association of the FC ± R regimen with AML and MDS.
In addition, the varied effect of CLL-directed treatment between different non-hematological OMs reflects their heterogeneity and partly explains the conflicting results of the literature. Finally, the association between unmutated immunoglobulin heavy variable (IGHV) genes and certain solid tumors (not previously explored in the literature) provides new insights into the potential mechanisms of the increased susceptibility to OMs occurrence.
Implications of all the available evidence
The existing literature illustrates the burden of OMs in patients with CLL. Across different studies, patients with CLL and OMs have consistently a worse OS than patients with CLL but without OMs. In our study, the FC ± R regimen was associated with AML and MDS occurrence, while no events occurred in patients treated exclusively with novel agents. This finding provides valuable information on treatment selection in patients with CLL, particularly in younger patients with favorable disease characteristics.
Despite this evidence, studies with long follow-ups of patients treated only with novel agents are needed to reveal the true impact of these treatments on the occurrence of OMs.
Introduction
Patients with chronic lymphocytic leukemia (CLL) have a higher risk of developing a second malignancy. Several retrospective studies reported that other malignancies (OMs), either hematological and/or solid, occur more frequently in patients with CLL versus the general population.1, 2, 3, 4
Several CLL-related factors could explain this association. Firstly, patients with CLL have inherent and therapy-related immune defects that heavily impact their long-term health.5, 6, 7, 8 This CLL-associated immunodeficiency may also render patients more susceptible to developing an OM. Secondly, the close follow-up of patients with CLL increases the probability of detecting an indolent OM.9 Finally, CLL and certain OMs have higher frequencies in older patients and might share common genetic and environmental predisposing factors.
CLL can transform into an aggressive lymphoma [described as Richter transformation (RT)] in 2–9% of patients with CLL. Numerous clinical and genetic risk factors have been associated with RT.10, 11, 12, 13, 14, 15 Diffuse large B cell lymphoma (DLBCL) is the most common histologic type, although Hodgkin lymphoma and other aggressive lymphomas can also arise in the context of RT.16 In addition, other hematological malignancies occur more frequently in patients with CLL. In particular, several studies have emphasized the association of fludarabine-based regimens with treatment-related myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).1,17, 18, 19, 20
The incidence of solid tumors is also higher in patients with CLL. A retrospective study of 16,367 patients with CLL/SLL enrolled in the Surveillance, Epidemiology, and End Results (SEER) program found an increased risk of solid malignancies. Kaposi sarcoma, melanoma, and cancers of the larynx and the lung were significantly more frequent in patients with CLL versus the general population2; however, CLL-directed treatment had no impact on the occurrence of solid tumors. Subsequently, a report from Denmark confirmed the higher incidence of OMs in patients with CLL and found that not only MDS but also lower respiratory and skin cancers occurred more frequently in treated patients.1 In a recent large retrospective study of patients included in the SEER program from 1973 to 2015, most OMs were diagnosed early after CLL diagnosis, and their incidence was higher in men, those treated with chemotherapy, older, and non-White patients.3
Despite this evidence, several questions remain open regarding the development of OMs throughout the natural history of CLL. First, the impact of CLL-directed treatment on the occurrence of OMs remains to be determined, especially considering conflicting reports in the literature.1, 2, 3,21 Moreover, similar to the general population, age and sex dictate OM occurrence in patients with CLL; however, it remains unclear if and how CLL clinical and biological characteristics can also impact the development of OMs.3,22 Finally, risk factors for AML or MDS development, besides treatment with fludarabine-based regimens, remain elusive.
We conducted an international multicenter retrospective study focusing on OM risk factors associated with CLL inherent characteristics or CLL-directed therapy. We aimed to (i) determine the incidence of OMs in a population of patients with CLL, (ii) assess the overall survival of patients with CLL and OM, and (iii) find potential risk factors for the occurrence of OM in patients with CLL. In contrast to cancer registries, we specifically designed this study to fill essential knowledge gaps about OMs in patients with CLL.
Methods
Data collection
This is a retrospective international multicenter study conducted by ERIC, the European Research Initiative on CLL, in the context of HARMONY, the Healthcare alliance for resourceful medicines offensive against neoplasms in hematology (https://www.harmony-alliance.eu/). Investigators at each participating site provided data on consecutive sets of patients diagnosed with CLL/small lymphocytic lymphoma (SLL) or high-count CLL-like monoclonal B-cell lymphocytosis (MBL) between 2000 and 2016.23 CLL diagnosis, treatment decisions, and review of medical history were performed by the local teams following international guidelines.24
Collected data included baseline demographics; date of CLL diagnosis; immunoglobulin heavy variable (IGHV) gene somatic hypermutation status; cytogenetic status for chromosomes 11q, 13q 17p, and 12 determined by fluorescence in situ hybridization; TP53 gene mutation status assessed by Sanger sequencing or next-generation sequencing; CLL treatment status; type of treatment; presence of comorbidities, Cumulative illness rating scale (CIRS); date and type of other malignancy, date of last follow-up and outcome.
The investigators reviewed the medical records and assessed information related to anatomical and chromosomal characteristics (when available) to ascertain participants' assigned sex at birth. Cases diagnosed with CLL/SLL or MBL before 2000 or after 2016 and those missing information on the date of CLL diagnosis and/or OM occurrence were excluded from the study. Cases missing information on other mandatory variables were included only in the calculation of the incidence.
Depending on the context, Overall Survival (OS) was defined as the time from CLL diagnosis to death or last follow-up date or as the time from OM diagnosis to death or last follow-up date.
Statistical analysis
For descriptive statistics, frequencies and relative frequencies were used to describe categorical variables, whereas median and interquartile range (IQR) were used for the numeric ones.
Univariable and multivariable analyses (MVA) were performed, having the development of OMs after CLL diagnosis and OS as outcomes. Concerning the development of OMs, the dataset contains censored data (with different follow-up times) for these outcomes. In addition, OMs are relatively rare events, hence a significant proportion of patients will never experience them: this means that not all of the censored data are due to follow-up incompleteness, leading to a plateau in the Kaplan–Meier curves (Supplemental Material). On these grounds, Mixture Cure models were applied in order to examine both the risk of experiencing the outcome and the time until that happens.25 All Mixture Cure models were semiparametric, using the logit link function for the cure/long-term part (risk of eventually developing the outcome), but no specific distribution for the time until the outcome development. Odds ratio (OR) was used to examine the risk factors associated with the risk of eventually developing the outcome of interest (independently of the time this happens), whereas hazard ratio (HR) was used to examine the risk factors associated with the time of outcome development between susceptible subjects. Of note, a risk factor may affect the risk of developing the outcome but not the time until that happens, and vice versa. Recognizing the importance of aging in the occurrence of OMs, we obtained crude and age-adjusted estimates for all analyses.26,27
Since the outcomes of interest are relatively rare events, Firth’s bias reduction method was also applied.28 The estimates are reported along with their confidence intervals. The standard errors of the coefficient estimates were estimated via bootstrap sampling, and Wald-type confidence intervals were constructed. No corrections for multiple comparisons were conducted.
Clinical and biological characteristics, treatment status (before OM occurrence), total lines of treatment until malignancy occurrence, and the type of treatment were examined as potential risk factors for the occurrence of OMs after CLL diagnosis.
As for OS, it was calculated either from the time of CLL diagnosis or from the time of OM development. The risk factors examined were the development of OM or not, as well as the type of OM. The analyses were performed using the log-rank test.
For the MVA, we performed a two-level variable selection approach. At first, we obtained the risk factors with p-value ≤0.20 from the univariable analyses and used them as risk factors for a multivariable model. In that model, LASSO penalty was used.29
Concerning handling missing data, pairwise deletion was performed for the univariable analyses. For the multivariable analyses, listwise deletion was performed only for the variables entered in the model.
All statistical analyses were conducted in R 4.1.3. We used dplyr package for data manipulation,30 ggplot2 for data visualization,31 and the intsurv package for the Mixture Cure model.32
Ethics statement
The study was approved by the local institutional ethics committees, and the investigators obtained informed consent from alive patients.
Role of the funding source
The study sponsors did not have access to the data and were not involved in the study design, collection, analysis, or interpretation of data, the writing of the article, and the decision to submit it for publication. The views expressed in the article reflect the author’s view, and neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained herein.
Paolo Ghia, Kostas Stamatopoulos, Lydia Scarfò, and Thomas Chatzikonstantinou had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Results
Patient characteristics
We assessed data on 19,705 patients from 85 different centers in 28 countries [the flow diagram of the study is provided in the Supplemental Material]. The median age at CLL/SLL or MBL diagnosis was 65 years (IQR 57–73), and the median follow-up from CLL diagnosis was 6.2 years (IQR 3.7–9.6). The majority of patients had CLL (18,386, 93.3%), while 528 (2.7%) and 791 (4%) were diagnosed with SLL and high-count CLL-like MBL, respectively. Baseline characteristics and disease-specific biomarkers are listed for all patients and specific subgroups in Table 1 and Supplemental Material, respectively.
Table 1.
Patient characteristics.
| Patient characteristics | Results | N = 19,705 | % | Missing | % |
|---|---|---|---|---|---|
| Sex assigned at birth | Female | 7626 | 38.7 | 0 | 0 |
| Male | 12,079 | 61.3 | 0 | 0 | |
| Diagnosis | CLL | 18,386 | 93.3 | 0 | 0 |
| MBL | 791 | 4 | 0 | 0 | |
| SLL | 528 | 2.7 | 0 | 0 | |
| Survival status | Alive | 13,524 | 68.6 | 0 | 0 |
| Dead | 5984 | 31.4 | |||
| Comorbidities at diagnosis | No | 4271 | 30 | 5489 | 27.9 |
| Yes | 9945 | 70 | |||
| Treatment status | Treated | 10,146 | 52.6 | 433 | 2.2 |
| Untreated | 9126 | 47.4 | |||
| Type of treatment | CIT or/and chemotherapy | 7128 | 71.3 | 147 | 1.4 |
| CIT or/and chemotherapy and novel agents | 1940 | 19.4 | |||
| Only novel agents | 600 | 6 | |||
| Other | 331 | 3.3 | |||
| IGHV gene status | Mutatedb | 4256 | 50.5 | 11,269 | 57.2 |
| Unmutatedc | 4180 | 49.5 | |||
| del(13q) | Negative | 4851 | 52.7 | 10,507 | 53.3 |
| Positive | 4347 | 47.3 | |||
| del(11q) | Negative | 8334 | 86.5 | 10,068 | 51.1 |
| Positive | 1303 | 13.5 | |||
| Trisomy 12 | Negative | 7531 | 82.5 | 10,578 | 53.7 |
| Positive | 1596 | 17.5 | |||
| del(17p) | Negative | 8992 | 90.4 | 9755 | 49.5 |
| Positive | 958 | 9.6 | |||
| TP53 mutation status | Mutated | 604 | 13.5 | 15,244 | 77.4 |
| Unmutated | 3857 | 86.5 | |||
| Karyotypea | Normal | 2107 | 52.2 | 15,668 | 79.5 |
| Abnormal | 1930 | 47.8 | |||
| Other malignant neoplasms | No | 15,571 | 79 | 0 | 0 |
| Yes | 4134 | 21 | |||
| Time of other malignant neoplasms | Before CLL diagnosis | 872 | 21.1 | 0 | 0 |
| After CLL diagnosis | 2940 | 71.1 | |||
| Before and after CLL | 322 | 7.8 | |||
| Multiple other malignant neoplasms | Two or more non-hematological | 464 | 2.4 | 0 | 0 |
| Both non-hematological and hematological | 212 | 1 | |||
| Two hematological | 16 | 0.08 | |||
| Transformation | No | 19,072 | 96.8 | 0 | 0 |
| Yes | 633 | 3.2 | |||
| Type of transformation | DLBCL | 559 | 88.3 | 0 | 0 |
| HL | 35 | 5.5 | |||
| PLL | 18 | 2.8 | |||
| Burkitt lymphoma | 3 | 0.5 | |||
| Other | 18 | 2.8 |
CIT, chemoimmunotherapy; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; MBL, monoclonal B lymphocytosis; IGHV, immunoglobulin heavy variable; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin's lymphoma; PLL, B cell prolymphocytic leukemia.
Karyotype specific abnormalities: Complex Karyotype (≥3 abnormalities): 384 (9.5%), Trisomies 12 & 19: 50 (1.2%), 6q deletions: 62 (1.5%), Translocations of 14q: 54 (1.3%), Translocation (14; 19): 17 (0.4%).
Mutated: <98% germline identity.
Unmutated: ≥98% germline identity.
At last follow-up, 10,146 (52.6%) patients had received at least one line of treatment (median 1, IQR 1–2). Most patients (7,128, 73.7%) received only chemoimmunotherapy or/and chemotherapy. Only 600 (6.2%) were treated exclusively with novel agents (i.e., Bruton’s tyrosine kinase and PI3Kδ inhibitors and the BCL2 inhibitor venetoclax), while both treatment types (i.e., chemotherapy/chemoimmunotherapy followed by novel agents) were used in 1940 (20.1%) patients (Table 1 & Supplemental Material).
Other malignancies in patients with CLL
Diagnosis of OM was reported in 4134/19,705 (21%) patients with CLL; of these, 633 (3.2%) had RT or B cell prolymphocytic leukemia (B-PLL). Most patients (3088, 15.7%) were diagnosed with one or more solid tumors, 834 (4.2%) patients had a second hematological malignancy, and 212 (1%) patients had both.
In 2940/4134 (71.1%) patients, the OM postdated the diagnosis of CLL; in 872/4134 (21.1%), the OM either antedated or was diagnosed concurrently with CLL; finally, in 322/4134 (7.8%), an OM was diagnosed both before and after CLL diagnosis (Table 1). Overall, 3513 OMs were diagnosed in 129,254 years of follow-up after CLL diagnosis (27.2 OMs/1000 person-years). Supplemental Table S2 and Fig. 1 depict the most common hematological and non-hematological OMs, respectively. Detailed information on non-hematological OMs is provided in Supplemental Material.
Fig. 1.
Other hematological and non-hematological malignancies and their relation to CLL diagnosis and CLL therapy initiation. ∗Including Richter transformation. AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
Other hematological malignancies excluding RT
The median time from CLL diagnosis to the diagnosis of hematological OM was 4.71 years (IQR = 2.06–7.77). The most common hematological OMs were MDS (84/19,705, 0.43%), followed by AML (39/19,705, 0.2%) and multiple myeloma (26/19,705, 0.13%) (Supplemental Table S2).
CLL-directed treatment was the only risk factor for developing a hematological malignancy after CLL diagnosis. However, when we excluded AML or MDS from the analysis, only 13q deletions remained as a risk factor in the MVA.
Several risk factors [including CLL-directed treatment, treatment with bendamustine-based regimens, and treatment with fludarabine and cyclophosphamide with or without rituximab (FC ± R)] were associated with the development of AML or MDS in the univariable analyses (Supplemental Material). However, treatment with FC ± R was the only statistically significant risk factor in the MVA (Table 2). We confirmed this result in separate analyses for AML and MDS and when we restricted the analyses to two subgroups of interest (i) only treated patients and (ii) only treated patients with mutated IGHV genes without known TP53 aberrations (n = 1697) (a group of patients for which FCR is still recommended as a valid option) (Supplemental Material & Table 2).33 The median time from the start of FC ± R until AML or MDS development was 3.65 years (IQR = 2.41–4.97). Twenty-one (44%) new AML or MDS diagnoses were made within 3 years of follow-up after FC ± R, while 36/48 (75%) and 46/48 (96%) cases were diagnosed within 5 and 10 years, respectively. Within 5 years of follow-up from FC ± R treatment, 2.6% (36/1419) of patients developed AML or MDS. Finally, the median survival from MDS/AML diagnosis for all patients was 13 months (95% CI = 10–30).
Table 2.
Multivariate analyses of risk factors for different other malignancies after CLL diagnosis.
| Multivariate analyses for different OMs | |||||
|---|---|---|---|---|---|
| Risk factor | Category | ORb (95% CI) | p value | HRc (95% CI) | p value |
| Hematological malignancies (excluding Richter transformation, AML and MDS) | |||||
| del(13q) | Positive | 1.67 (0.98–2.85) | 0.06 | – | – |
| AML or MDS | |||||
| FC ± R at any line (before MDS or AML) | Yes | 3.7 (2.79–4.91) | <0.001 | – | – |
| AML or MDS (including only treated patients) | |||||
| FC ± R at any line (before MDS or AML) | Yes | 4.57 (2.19–9.53) | 0.006 | 1.6 (1.12–2.29) | 0.01 |
| Age at diagnosis | 1.04 (1.01–1.08) | <0.001 | – | – | |
| AML or MDS (including only treated patients with mutated IGHV and excluding those with known TP53 aberrations) | |||||
| FC ± R at any line (before MDS or AML) | Yes | 3.59 (1.09–11.81) | 0.035 | – | – |
| All non-Hematological malignancies (excluding non-melanoma skin cancers) | |||||
| Sex assigned at birth | Male | 1.77 (1.49–2.11) | <0.001 | – | – |
| IGHV gene status | Unmutateda | 1.89 (1.6–2.24) | <0.001 | – | – |
| Treatment status (before malignancy) | Treated | – | – | 0.4 (0.3–0.48) | <0.001 |
| Age at diagnosis | – | – | 1.03 (1.03–1.04) | <0.001 | |
| Bladder cancer | |||||
| Sex assigned at birth | Male | 6.36 (3.33–12.16) | <0.001 | – | – |
| Treatment status (before bladder) | Treated | – | – | 0.37 (0.21–0.67) | <0.001 |
| Age at diagnosis | – | – | 1.03 (1.02–1.05) | <0.001 | |
| Breast cancer | |||||
| Sex assigned at birth | Male | 0.3 (0.22–0.4) | <0.001 | – | – |
| Type of treatment (before breast cancer) | Chemotherapy or CIT | 0.55 (0.34–0.89) | <0.001 | – | – |
| IGHV gene status | Unmutateda | 1.83 (1.02–3.26) | 0.042 | – | – |
| Colon cancer | |||||
| Sex assigned at birth | Male | 1.03 (1.01–1.05) | 0.001 | – | – |
| Bronchus and lung cancer | |||||
| IGHV gene status | Unmutateda | 2.09 (1.07–4.09) | 0.03 | – | – |
| Sex assigned at birth | Male | 2.23 (0.84–5.88) | 0.11 | – | – |
| Melanoma | |||||
| IGHV gene status | Unmutateda | 2.41 (1.16–5.01) | 0.02 | – | – |
| Non-melanoma skin cancers | |||||
| Sex assigned at birth | Male | 2.53 (1.81–3.55) | <0.001 | – | – |
| Age at diagnosis | 1.04 (1.02–1.07) | 0.001 | 1.04 (1.02–1.06) | <0.001 | |
| FC ± R | Yes | 1.8 (1.36–2.41) | <0.001 | – | – |
| Prostate cancer | |||||
| Treatment status (before prostate) | Treated | 2.11 (1.12–3.97) | 0.021 | – | – |
OMs, other malignancies; OR, odds ratio; HR, hazard ratio; CI, confidence interval; FC ± R, fludarabine, cyclophosphamide with or without rituximab; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; IGHV, immunoglobulin heavy variable.
Empty cells denote that the respective risk factor was not included in the cure or survival part of the model.
Unmutated: ≥98% germline identity.
OR was used to examine the risk factors associated with the risk of eventually developing the outcome of interest (independently of the time this happens).
HR was used to examine the risk factors associated with the time of outcome development between susceptible subjects.
Other non-hematological malignancies
Solid tumors occurred at a median of 4.36 years (IQR = 2.05–7.56) after CLL diagnosis. Male patients and those with unmutated IGHV gene status had a higher risk of developing a non-hematological OM [excluding non-melanoma skin cancers (NMSC)] (Table 2). NMSC (986/19,705, 5%) and prostate cancers (530/19,705, 2.7%) were the most frequent, followed by colon (382/19,705, 1.9%) and breast cancers (343/19,705, 1.7%) (Fig. 1 & Supplemental Material).
Next, we focused on identifying potential risk factors for solid tumors detected in more than 150 patients (i.e., NMSC, melanoma, prostate, breast, lung, colon, and bladder cancers). Statistically significant associations were found between: (i) NMSC development and male sex, older age at diagnosis, and FC ± R treatment; (ii) male sex and colorectal, lung, and bladder cancers; (iii) unmutated IGHV gene status and melanoma, lung and breast cancers (Table 2).
CLL-directed treatment and other non-hematological malignancies
Initially, we analyzed solid tumors as a group and found that CLL–directed treatment was associated with a higher incidence of non-hematological OMs in the univariable but not in the multivariable analysis. That notwithstanding, the impact of treatment was not uniform when we analyzed the single most common solid tumors (Table 2). In detail, for prostate cancer, CLL treatment of any type was the only statistically significant risk factor in the MVA, while for NMSC, only the treatment with FC ± R remained significant. On the contrary, CLL–directed treatment was not a risk factor for lung cancer in the MVA. Conversely, CLL-directed treatment appeared to exert a protective effect on breast and colon cancers. Of note, the hazard of developing breast cancer for untreated patients was also higher in the MVA (Table 2).
Overall survival
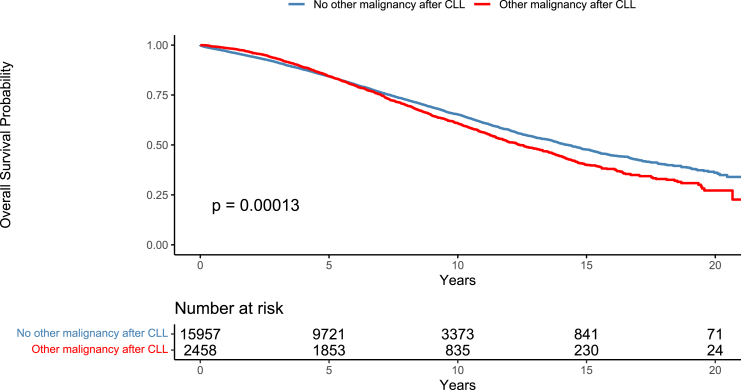
Patients with OM had worse OS than those without [median OS 12.41 (95% CI = 11.81–13.29) versus 14.28 (95% CI = 13.86–14.77) years] (Fig. 2). We also performed an OS analysis (from CLL diagnosis until death or last follow-up) to compare four different groups: patients with AML or MDS, hematological OM (excluding RT, AML, and MDS), solid tumors (excluding NMSC) and patients without OMs or with only NMSC. We found that patients with solid tumors had a worse OS than patients without OMs or with only NMSC (p < 0.001). Finally, patients with AML or MDS had the worst OS, regardless of the timing of AML or MDS diagnosis (p < 0.001) (Fig. 3).
Fig. 2.
Overall survival from the time of CLL diagnosis. Comparison between patients with at least one other malignancy after CLL diagnosis versus patients without a second malignancy after CLL diagnosis (Patients with Richter transformation were excluded).
Fig. 3.
Overall survival from the time of CLL diagnosis. Comparison between patients without a second malignancy after CLL diagnosis or only non-melanoma skin cancers versus patients diagnosed with MDS or AML after CLL diagnosis, versus patients diagnosed with a hematological malignancy (excluding Richter transformation, B cell prolymphocytic leukemia, AML, and MDS) after CLL diagnosis versus patients diagnosed with a non-hematological malignancy (excluding non-melanoma skin cancers) after CLL diagnosis. Hem, hematological malignancy; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; skin, non-melanoma skin cancers.
Discussion
We here present the largest non-registry-based study on OMs in patients with CLL. In this dedicated effort, we found that 16.6% of all patients with CLL developed at least one OM after CLL diagnosis (27.2 new OMs per 1000 person-years): this group had inferior OS than patients without OMs, particularly if concerning AML or MDS. Moreover, we report that the occurrence of AML or MDS was associated only with the administration of the FC ± R regimen, hence significantly strengthening the evidence from previous albeit considerably smaller series. Finally, we highlight for the first time that the impact of CLL-directed treatment on the type of OM is highly variable and that CLL-related features (i.e., the somatic hypermutation status of the clonotypic IGHV genes) are linked with the occurrence of certain solid tumors.
Our findings align with the literature regarding the high risk of OMs for patients with CLL.1, 2, 3, 4 The largest study on patients enrolled in the SEER program, an Australian, a Dutch and a Danish study, respectively, reported a rate between 24 and 36 new OMs per 1000 person-years.1,3,22,34 These studies have also shown that patients with CLL have a higher risk of developing OMs compared with the general population by calculating the absolute excess risk (AER). We chose not to perform AER analysis due to our cohort's heterogeneity; instead, we focused on the risk factors of OMs.
Similar to previous studies, patients with CLL and OMs had an inferior OS than those without.4,21,35 Despite the changes in the incidence and the improvements in OS of patients with many cancers, including CLL, the impact of OMs on the OS of patients with CLL remained constant across studies conducted in different periods.36, 37, 38, 39 The co-occurrence of CLL and OMs poses several challenges. First, these patients often tolerate chemotherapy poorly due to the added CLL-related immunosuppression in addition to the older age, comorbidities and polypharmacy that characterize a significant proportion of patients with CLL. Second, these tumors may fare more aggressively in patients with CLL due to the well-known CLL-associated immune dysfunction. Interestingly, a SEER Population-Based Study found that patients with CLL have an inferior OS when diagnosed with specific types of cancer than those with the same cancers but without CLL.40 Finally, CLL-directed treatment could interact with specific OM treatments, limiting the available options. Regardless the underlying causes, this finding underlines the importance of identifying and potentially altering the risk factors for the occurrence of OMs, also through periodic screening.
Of note, we chose to include all patients in the OS analysis regardless of the reason of death for two main reasons. Firstly, some patients may have died due to other causes that nonetheless were associated with their OM (e.g., infections, treatment toxicity, and limited treatment options due to concurrent OM treatment). Secondly, detailed information about the reason of death may be unknown since different physicians usually treat these patients after the diagnosis of OM.
The development of MDS or AML was associated with certain biological features and treatment for CLL. However, the only risk factor in the MVA was treatment with the FC ± R regimen. In keeping with the literature, in our cohort, 2.6% of patients treated with FC ± R developed AML or MDS within 5 years after treatment.17,20 This finding, combined with the dismal outcomes of these patients, poses essential questions about the role of CIT in CLL in the era of chemo-free treatment, when this is available. Relevant to mention in that regard, even in the most updated recommendations by several organizations (e.g., NCCN, ESMO), CIT is proposed as a valid option for young patients with mutated IGHV genes without other adverse prognostic features on the grounds that such patients may enjoy prolonged responses when treated with FCR.33,41 This is particularly relevant as young patients are those who more likely will live long enough to develop such tumors. Furthermore, FCR should be particularly avoided in patients with clonal hematopoiesis of indeterminate potential (CHIP), as they have an even higher risk of developing myeloid malignancies.42 In the current era, patients without adverse prognostic features could experience an excellent prognosis also with novel agents without the risk of a treatment-related myeloid malignancy.41,43 Our findings indicate that we could reduce the incidence of treatment-related myeloid malignancies by simply omitting FCR from CLL treatment options. Intriguingly, the need for this approach is further supported by a SEER Cancer registry study reporting an improvement in the incidence of treatment-related MDS and AML in patients treated with CIT for many common lymphoid malignancies but not for CLL.39
Of note, none of the patients treated with only novel agents developed a myeloid malignancy. However, larger series and longer follow-up are imperative before definitive conclusions can be drawn. That said, interestingly, studies on novel agents report treatment-related myeloid malignancies only in patients previously exposed to cytotoxic drugs.44
Hematological malignancies, excluding AML, MDS, and RT, appeared unaffected by CLL-directed treatment. Only 13q deletions were associated with their occurrence, albeit without reaching statistical significance. Patients with 13q deletions -especially if lacking adverse prognostic features-experienced a prolonged survival, conceivably allowing for more hematological OMs to occur.45
The types of solid tumors diagnosed more frequently in our cohort mirror other studies in patients with CLL.1,3,4,27 Unsurprisingly, most solid tumors were diagnosed after CLL, except for breast and thyroid cancers, which typically affect younger patients. Similar to the general population, males had an increased risk of solid tumors.27 The only CLL-related risk factor associated with solid tumors was the presence of unmutated IGHV genes, although with the caveat that for a significant proportion of the patients the IGHV gene somatic hypermutation status was not available. That notwithstanding, the precise links and causality, if any, of this association currently remain elusive.
The effect of CLL-directed treatment on solid tumors differed depending on the type of malignancy. Interestingly, previous studies also reported that NMSCs were more frequent in chemotherapy-treated patients.1,21,22 The closer follow-up of treated patients may have led to prompt dermatology referrals, while the effects of chemotherapy on the skin could render it susceptible to ultraviolet radiation. Prostate cancers were also more frequent in treated patients in a population of long-term CLL survivors.21 While chemotherapy is not a traditional risk factor for prostate cancer, however, treated patients are followed closely and, thus, are more likely to be screened for prostate cancer.9 In addition, both the prevalence of prostate cancer and the likelihood of treatment for CLL increase in parallel with age.27 Finally, in the univariable analyses, melanoma and lung cancers were more frequent in patients with unmutated IGHV genes and were associated with FC ± R and CLL treatment, respectively. Nonetheless, it is relevant to mention that the majority of treated patients carry unmutated IGHV genes, which may influence these findings.1
Chemotherapy for CLL exerted a protective effect on breast cancer. Theoretically, chemotherapy could eradicate the cancer cells of a chemosensitive tumor.46 Similar results in the Dutch study and in our univariable analysis on colon cancer, another chemosensitive tumor, appear in support of this concept.22 Although this finding poses interesting questions, only further research could reveal its actual relevance and implicated mechanisms.
Among the merits of our study stands that, unlike any previous study, we collected detailed information about CLL treatment and biomarkers that led to unique findings. That notwithstanding, we acknowledge certain limitations. Firstly, we did not collect data on some known environmental/lifestyle risk factors for certain malignancies (e.g., occupational and smoking habits) that may have confounded our results. Secondly, malignancies with an indolent course (e.g., NMSC, prostate cancer, CLL) may run for quite some time (even years) before establishing a formal diagnosis,9,47 reducing the validity of comparisons about their timing. Furthermore, we included patients from different continents with different predispositions for specific cancer types.27 Finally, missing information, sampling and attrition bias-inherent problems of retrospective studies-could also apply to our study. Somehow mitigating the latter two limitations, we (i) collected data on consecutively diagnosed patients with CLL in each participating center, (ii) used curation mechanisms that minimized missing information and ensured data quality, and (iii) applied Mixture Cure models considering differences in follow-up times.
Most participating centers in our study are tertiary referral centers for CLL. Thus, our cohort may also suffer from selection bias towards younger treated patients. On the contrary, the short follow-up of some cases led to an imbalance in the expected ratio between treated and untreated patients. Acknowledging this, we performed subanalyses to confirm major findings within the treated population (e.g., FC ± R association with AML or MDS). Finally, we did not collect information on the progression of MBL cases to CLL and the clonal relationship of RT cases with CLL. Thus, we avoided comparing the OMs risk between CLL and MBL cases and exploring the risk factors for RT.
Taken together, our findings illustrate the burden of OMs and their impact on OS in a large collection of non-registry patients with CLL. In addition, the strong association between FCR and AML or MDS is a crucial point to discuss when counseling young patients needing treatment, considering that FCR is still mentioned as an option for certain subgroups of patients with CLL.
Contributors
TC, LS, CD collected data, coordinated the study, wrote the paper, assessed and verified the data, and performed the analysis; GK wrote the paper and performed the analysis; GI and JK collected data, coordinated the study, and contributed to interpretation and manuscript editing; EM and DC coordinated the data collection, curate the data, and contributed to interpretation and manuscript editing; LSm, SMu, MA, SA, TAS, FBa, MB, FBi, ACa, AM, AKM, RC, SC, RCo, ACh, ZD, MDi, DD, GDS, BD, ME, SEA, AE, AF, SG, AG, RGS, EG, IGGM, AGo, RG, AjG, RGu, SH, EH, YH, JAHR, LI, OJ, SJ, EKa, LK, VK, APK, BK, MK, EK, MKM, IKo, RJK, JL, DL, M-DL, IL, TL, ALG, JM, LMR, MMa, SM, CMB, BM, IM, FM, RM, MMo, RMu, UKN, ANB, ACO, JO, DO, IPS, MP, TP, ZK, PP, CP, AP, LR, GR, GMR, RR, MDDS, MS, AS, YS, MSi, SS, DSAS, MSp, TT, KT, ET, TV, AV, CV, JVT, GV, VV, RW, EWS, ZX, MYa, LY, MY, JZ, MAn, DA, BB, MCa, RCl, MC, ACu, FD, BE, GG, OBK, LL, EN, GP, PPa, VMP, SP, PS, NS, CT, LT, AC, FB, MDo collected data and contributed to interpretation and manuscript editing; PG and KS designed and coordinated the study, and wrote the paper.
Data sharing statement
Aggregated data used in this study can be made available upon reasonable request from the corresponding author.
Declaration of interests
TC received honoraria from AbbVie. LS received consulting fees from AbbVie, BeiGene, AstraZeneca, Lilly, and Janssen, honoraria from Janssen and Octapharma, support for attending meetings from BeiGene and Janssen and advisory board fees from Merck. SMu received advisory board fees from AbbVie, AstraZeneca, Janssen, Roche and BeiGene. JAHR received honoraria as a consultant from Janssen, AbbVie, AstraZeneca, Lilly, and BeiGene and support for attending meetings from Janssen, AbbVie, AstraZeneca, and BeiGene. LI received honoraria from AbbVie, Roche, and Janssen-Cilag. APK received advisory board fees and research money from Janssen, AbbVie, BMS, AstraZeneca, Roche/Genentech, support for attending meetings from Janssen and AbbVie. M-DL received travel expenses from Janssen and AbbVie. GMR received honoraria for participation to speaker's bureau from AbbVie, Astra Zeneca, Beigene, and Janssen, and support for attending meetings from Janssen. JVT received consulting fees from AbbVie, AstraZeneca, BeiGene, and Janssen, honoraria for scientific talks from AbbVie, AstraZeneca, BeiGene, Janssen, Lilly, and Roche, travel support from AbbVie, AstraZeneca, BeiGene, Janssen, Roche, and Lilly, and advisory boards fees for AbbVie, Amgen, AstraZeneca, BeiGene. GI received honoraria from Novartis, BMS, Sandoz, AstraZeneca, Janssen, Kite/Gilead, and Miltenyi, support for attending meetings from Kite/Gilead, AstraZeneca, and AbbVie and advisory board fees from Kite/Gilead, Novartis, BMS, and Autolus. FBi received support for attending meetings from AbbVie. RCo received support for attending meetings from Janssen-Cilag and S.A. SG received honoraria support for attending meetings from AbbVie, AstraZeneca, Jazz, Novartis, and Incyte, honoraria from Roche, Celgene, Pfizer, and Janssen, and support for attending meetings from Jazz, AstraZeneca, and Roche. RGS received support for attending meetings from AbbVie and S.L.U. RG received honoraria, consulting fees, and support for attending meetings from AbbVie, Beigene, Roche, Janssen, AstraZeneca. YH received honoraria from Janssen, AbbVie, Roche, AstraZeneca, Medion, and Lilly. OJ received honoraria from Johnson and Johnson, AstraZeneca, and Lilly, honoraria from Johnson and Johnson, AbbVie, AstraZeneca, and Lilly, and support for attending meetings from Johnson and Johnson, and AbbVie. LK received consulting fees from AbbVie, AstraZeneca, Janssen, Beigene, Takeda, and Novartis. MKM received honoraria from Novartis, Pfizer, and Gad Medical LTD and support for attending meetings from Novartis. IKo received honoraria and consulting fees from AbbVie and Janssen. IM received honoraria from AbbVie, Roche, Sandoz, AstraZeneca, and Janssen, and support for attending meetings from AbbVie, Roche, and Takeda. ANB received honoraria, advisory board fees and support for attending meetings from AbbVie, AstraZeneca, Takeda, Janssen, and Beigene. JO received honoraria from AbbVie, AstraZeneca, and Janssen. GR received consulting fees from AbbVie, AstraZeneca, Janssen, and Beigene, and is currently employed by AstraZeneca. TP received honoraria and advisory board fees from AbbVie, Janssen-Cilag, and AstraZeneca and support for attending meeting from AstraZeneca. MS received honoraria and support for attending meeting from AstraZeneca, AbbVie, and Janssen-Cilag and owns shares of stock in AbbVie, AstraZeneca, Merck, Eli Lilly, Sanofi, Johnson and Johnson, Pfizer, Gilead, and GSK. MSp received honoraria and consulting and advisory board fees, and support for attending meeting from AbbVie, AstraZeneca, and Janssen. ET received support for attending meetings from Takeda. TV received honoraria from Takeda, Roche, Genesis Pharma, Merck, Novartis, Gilead, Sandoz, AstraZeneca, Integris, and Servier and support for attending meetings from Takeda, Roche, Genesis Pharma, Merck, Pfizer, and Winmedica. CV received honoraria from AbbVie, consulting fees from AstraZeneca and support for attending meeting from AstraZeneca, Takeda, and Janssen. RW received honoraria from AbbVie, AstraZeneca, and Beigene, support for attending meetings from Janssen, AbbVie, and AstraZeneca and advisory board fees from AbbVie, AstraZeneca, Janssen, Beigene, and SecuraBio. EWS received honoraria from AbbVie, Roche, and Janssen-Cilag and support for attending meetings from AbbVie. LY received honoraria from AbbVie, AstraZeneca, Novartis, Gilead, Janssen, Jazz, MSD, and Pfizer, support for attending meetings from AbbVie, AstraZeneca, Gilead, Janssen, and Pfizer, and advisory board fees from AbbVie, AstraZeneca, Jazz, Janssen, Beigene, and Celgene. MAn received consulting fees from AbbVie, Takeda, Janssen, Roche, Genesis, Gilead, and Amgen and honoraria from AbbVie, Takeda, Roche, Genesis, Gilead, and Novartis. MC received honoraria, advisory board fees, and support for attending meetings from AbbVie, AstraZeneca, and Janssen. ACu received honoraria, advisory board fees, and support for attending meetings from AbbVie, AstraZeneca, Beigene, Janssen, and Lilly. FD support for attending meetings from Janssen and AbbVie. GG received honoraria, and advisory board fees from AbbVie, AstraZeneca, Beigene, Janssen and advisory board fees from Lilly. EN received honoraria from AbbVie. LSm received consulting fees, honoraria and support for attending meetings from AbbVie, AstraZeneca, and Janssen and advisory board fees from AbbVie and AstraZeneca. NS received honoraria from Janssen, AbbVie, AstraZeneca, and Lilly, and support for attending meetings from Janssen and AstraZeneca. CT received honoraria from AbbVie, Beigene, Janssen, and LOXO. FB received consulting fees, honoraria and payment for expert testimony from AbbVie, Genentech, Novartis, Takeda, Janssen, Roche, Mundipharma, Celgene/BMS, AstraZeneca, Lilly, Beigene, Gilead and TG Therapeutics, Advantage Allogene, Lava Therapeutics, and Enterome. MDo received honoraria and advisory board fees from AbbVie, AstraZeneca and Janssen, advisory board fees from Swixx, and support for attending meetings from Janssen. PG received honoraria and consulting fees from AbbVie, AstraZeneca, BMS, Janssen, Lilly/Loxo Oncology, MSD, and Roche; grant support from AbbVie, AstraZeneca, BMS, Janssen. KS received honoraria from Janssen, AbbVie, Lilly and AstraZeneca, consulting fees and support for attending meetings from Janssen and AstraZeneca. GK, EM, DC, JK, CD, MA, SA, TAS, FBa, MB, ACa, AM, AKM, RC, SC, ACh, ZD, MDi, DD, GDS, BD, ME, SEA, AE, AF, AG, EG, IGGM, AGo, AjG, RGu, SH, EH, SJ, EKa, VK, BK, MK, EK, RJK, JL, DL, IL, TL, ALG, JM, LMR, MMa, SM, CMB, BM, FM, RM, MMo, RMu, UKN, ACO, DO, IPS, MP, ZK, PP, CP, AP, LR, RR, MDDS, AS, YS, MSi, SS, DSAS, TT, KT, AV, GV, VV, ZX, MYa, MY, JZ, DA, BB, MCa, RCl, BE, OBK, LL, GP, PPa, VMP, SP, PS, LT, AC, have no conflict of interest to disclose.
Acknowledgements
This project was supported in part by AbbVie; EU/EFPIA Innovative Medicines Initiative [2] Joint Undertaking HARMONY grant n° 116026; the Hellenic Precision Medicine Network in Oncology; MH-CZ_AZV_NU23-03-00401; MH CZ DRO (FNBr, 65269705); MH CZ DRO (FNOl, 00098892), program COOPERATIO (research area ONCO), and NPO-NUVR LX22NPO5102. Munci Yagci was provided with study materials.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102307.
Appendix A. Supplementary data
References
- 1.da Cunha-Bang C., Rostgaard K., Andersen M.A., et al. Risk of new malignancies among patients with cll treated with chemotherapy: results of a Danish population-based study. Br J Haematol. 2021;193:339–345. doi: 10.1111/bjh.17337. [DOI] [PubMed] [Google Scholar]
- 2.Hisada M., Biggar R.J., Greene M.H., Fraumeni J.F., Jr., Travis L.B. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–1981. doi: 10.1182/blood.v98.6.1979. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V., Ailawadhi S., Bojanini L., et al. Trends in the risk of second primary malignancies among survivors of chronic lymphocytic leukemia. Blood Cancer J. 2019;9:75. doi: 10.1038/s41408-019-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsimberidou A.M., Wen S., McLaughlin P., et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forconi F., Moss P. Perturbation of the normal immune system in patients with cll. Blood. 2015;126:573–581. doi: 10.1182/blood-2015-03-567388. [DOI] [PubMed] [Google Scholar]
- 6.Hilal T., Gea-Banacloche J.C., Leis J.F. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: linking mechanisms with infections. Blood Rev. 2018;32:387–399. doi: 10.1016/j.blre.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F., O'Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother. 2006;55:197–209. doi: 10.1007/s00262-005-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadhwa P.D., Morrison V.A. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:240–249. doi: 10.1053/j.seminoncol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Bell K.J., Del Mar C., Wright G., Dickinson J., Glasziou P. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int J Cancer. 2015;137:1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabbri G., Rasi S., Rossi D., et al. Analysis of the chronic lymphocytic leukemia coding genome: role of Notch1 mutational activation. J Exp Med. 2011;208:1389–1401. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauro F.R., Foa R., Giannarelli D., et al. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood. 1999;94:448–454. [PubMed] [Google Scholar]
- 12.Rossi D., Rasi S., Spina V., et al. The genome of chemorefractory chronic lymphocytic leukemia reveals frequent mutations of Notch1 and SF3B1. Leuk Suppl. 2012;1:S26–S28. doi: 10.1038/leusup.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi D., Spina V., Cerri M., et al. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin Cancer Res. 2009;15:4415–4422. doi: 10.1158/1078-0432.CCR-08-3266. [DOI] [PubMed] [Google Scholar]
- 14.Sutton L.A., Young E., Baliakas P., et al. Different spectra of recurrent gene mutations in subsets of chronic lymphocytic leukemia harboring stereotyped B-cell receptors. Haematologica. 2016;101:959–967. doi: 10.3324/haematol.2016.141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsimberidou A.M., O'Brien S., Khouri I., et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24:2343–2351. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sawaf O., Robrecht S., Bahlo J., et al. Richter transformation in chronic lymphocytic leukemia (CLL)-a pooled analysis of German CLL study group (GCLLSG) front line treatment trials. Leukemia. 2021;35:169–176. doi: 10.1038/s41375-020-0797-x. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini O., Jain P., Trinh L., et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–1650. doi: 10.3109/10428194.2014.957203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colovic M., Suvajdzic N., Jankovic G., et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia in patients with chronic lymphocytic leukemia treated with fludarabine and cyclophosphamide. Biomed Pharmacother. 2011;65:319–321. doi: 10.1016/j.biopha.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Morrison V.A., Rai K.R., Peterson B.L., et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y., Tang G., Medeiros L.J., et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25:237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falchi L., Vitale C., Keating M.J., et al. Incidence and prognostic impact of other cancers in a population of long-term survivors of chronic lymphocytic leukemia. Ann Oncol. 2016;27:1100–1106. doi: 10.1093/annonc/mdw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Straten L., Levin M.D., Dinnessen M.A.W., et al. Risk of second primary malignancies in patients with chronic lymphocytic leukemia: a population-based study in the Netherlands, 1989-2019. Blood Cancer J. 2023;13:15. doi: 10.1038/s41408-023-00784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon B.M., Chaffee K.G., Moreira J., et al. Risk of non-hematologic cancer in individuals with high-count monoclonal B-cell lymphocytosis. Leukemia. 2016;30:331–336. doi: 10.1038/leu.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallek M., Cheson B.D., Catovsky D., et al. Iwcll guidelines for diagnosis, indications for treatment, response assessment, and supportive management of cll. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 25.Amico M., Van Keilegom I. Cure models in survival analysis. Annu Rev Stat Appl. 2018;5:311–342. [Google Scholar]
- 26.Moser E.C., Noordijk E.M., van Leeuwen F.E., et al. Risk of second cancer after treatment of aggressive non-Hodgkin's lymphoma; an EORTC cohort study. Haematologica. 2006;91:1481–1488. [PubMed] [Google Scholar]
- 27.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 28.Kosmidis I., Firth D. Bias reduction in exponential family nonlinear models. Biometrika. 2009;96:793–804. [Google Scholar]
- 29.Zou H., Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc B. 2005;67:301–320. [Google Scholar]
- 30.Wickham H., François R., Henry L., Müller K., Vaughan D. 2022. Dplyr: a grammar of data manipulation.Https://dplyr.Tidyverse.Orghttps://github.Com/tidyverse/dplyr [Google Scholar]
- 31.Wickham H. Springer-verlag new york; 2016. Ggplot2: elegant graphics for data analysis.https://ggplot2.Tidyverse.Org [Google Scholar]
- 32.Wang W., Chen K., Yan J. 2021. Intsurv: integrative survival models. R package version 0.2.2.https://github.Com/wenjie2wang/intsurv [Google Scholar]
- 33.Eichhorst B., Robak T., Montserrat E., et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:23–33. doi: 10.1016/j.annonc.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Royle J.A., Baade P.D., Joske D., Girschik J., Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105:1076–1081. doi: 10.1038/bjc.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visentin A., Imbergamo S., Gurrieri C., et al. Major infections, secondary cancers and autoimmune diseases occur in different clinical subsets of chronic lymphocytic leukaemia patients. Eur J Cancer. 2017;72:103–111. doi: 10.1016/j.ejca.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Dong Y., Shi O., Zeng Q., et al. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020;9:14. doi: 10.1186/s40164-020-00170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maclachlan K., Diamond B., Maura F., et al. Second malignancies in multiple myeloma; emerging patterns and future directions. Best Pract Res Clin Haematol. 2020;33 doi: 10.1016/j.beha.2020.101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNerney M.E., Godley L.A., Le Beau M.M. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513–527. doi: 10.1038/nrc.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton L.M., Curtis R.E., Linet M.S., et al. Trends in risk for therapy-related myelodysplastic syndrome/acute myeloid leukemia after initial chemo/immunotherapy for common and rare lymphoid neoplasms, 2000-2018. EClinicalMedicine. 2023;61 doi: 10.1016/j.eclinm.2023.102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon B.M., Rabe K.G., Slager S.L., Brewer J.D., Cerhan J.R., Shanafelt T.D. Overall and cancer-specific survival of patients with breast, colon, kidney, and lung cancers with and without chronic lymphocytic leukemia: a seer population-based study. J Clin Oncol. 2013;31:930–937. doi: 10.1200/JCO.2012.43.4449. [DOI] [PubMed] [Google Scholar]
- 41.Shanafelt T.D., Wang X.V., Hanson C.A., et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140:112–120. doi: 10.1182/blood.2021014960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voso M.T., Pandzic T., Falconi G., et al. Clonal haematopoiesis as a risk factor for therapy-related myeloid neoplasms in patients with chronic lymphocytic leukaemia treated with chemo-(immuno)therapy. Br J Haematol. 2022;198:103–113. doi: 10.1111/bjh.18129. [DOI] [PubMed] [Google Scholar]
- 43.Al-Sawaf O., Zhang C., Lu T., et al. Minimal residual disease dynamics after venetoclax-obinutuzumab treatment: extended off-treatment follow-up from the randomized CLL14 study. J Clin Oncol. 2021;39:4049–4060. doi: 10.1200/JCO.21.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bond D.A., Huang Y., Fisher J.L., et al. Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia. 2020;34:3197–3205. doi: 10.1038/s41375-020-0987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi D., Rasi S., Spina V., et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013;121:1403–1412. doi: 10.1182/blood-2012-09-458265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg H., Zandbank J., Kent V., et al. Chemotherapy may eradicate ductal carcinoma in situ (DCIS) but not the associated microcalcifications. Eur J Surg Oncol. 2017;43:1415–1420. doi: 10.1016/j.ejso.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Delongchamps N.B., Singh A., Haas G.P. The role of prevalence in the diagnosis of prostate cancer. Cancer Control. 2006;13:158–168. doi: 10.1177/107327480601300302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.