Abstract
Background
Implantable cardioverter-defibrillation (ICD) shocks after left ventricular assist device therapy (LVAD) are associated with adverse clinical outcomes. Little is known about the association of pre-LVAD ICD shocks on post-LVAD clinical outcomes and whether LVAD therapy affects the prevalence of ICD shocks.
Objectives
The purpose of this study was to determine whether pre-LVAD ICD shocks are associated with adverse clinical outcomes post-LVAD and to compare the prevalence of ICD shocks before and after LVAD therapy
Methods
Patients 18 years or older with continuous-flow LVADs and ICDs were retrospectively identified within the University of Pittsburgh Medical Center system from 2006–2020. We analyzed the association between appropriate ICD shocks within 1 year pre-LVAD with a primary composite outcome of death, stroke, and pump thrombosis and secondary outcomes of post-LVAD ICD shocks and ICD shock hospitalizations.
Results
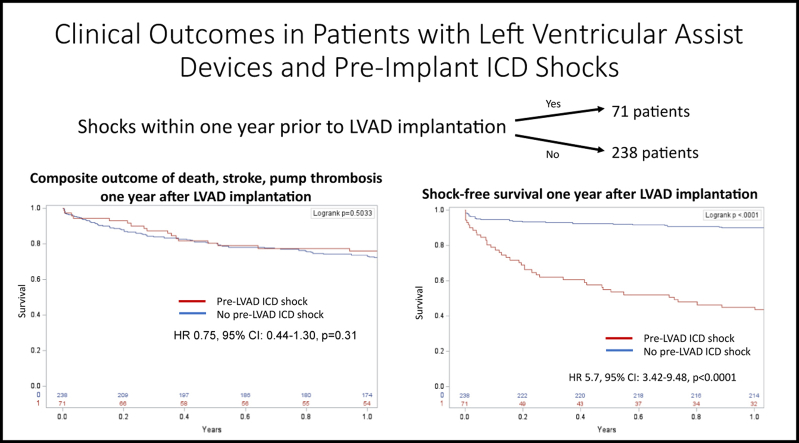
Among 309 individuals, average age was 57 ± 12 years, 87% were male, 80% had ischemic cardiomyopathy, and 42% were bridge to transplantation. Seventy-one patients (23%) experienced pre-LVAD shocks, and 69 (22%) experienced post-LVAD shocks. The overall prevalence of shocks pre-LVAD and post-LVAD were not different. Pre-LVAD ICD shocks were not associated with the composite outcome. Pre-LVAD ICD shocks were found to predict post-LVAD shocks (hazard ratio [HR] 5.7; 95% confidence interval [CI] 3.42–9.48; P <.0001) and hospitalizations related to ICD shocks from ventricular arrhythmia (HR 10.34; 95% CI 4.1–25.7; P <.0001).
Conclusion
Pre-LVAD ICD shocks predicted post-LVAD ICD shocks and hospitalizations but were not associated with the composite outcome of death, pump thrombosis, or stroke at 1 year. The prevalence of appropriate ICD shocks was similar before and after LVAD implantation in the entire cohort.
Keywords: Ventricular tachycardia, Heart failure, Assist device, Mechanical support, Defibrillation
Graphical abstract
Key Findings.
-
▪
Implantable cardioverter-defibrillator (ICD) shocks up to 1 year before left ventricular assist device implantation (LVAD) did not predict death, stroke, or pump thrombosis risk 1 year after implant.
-
▪
Pre-LVAD ICD shocks were a significant predictor of post-LVAD ICD shocks.
-
▪
The prevalence of ICD shocks within 1 year before LVAD implantation was not different than the prevalence 1 year after implantation.
Introduction
In the United States, approximately 1% of more than 6 million patients with heart failure progress toward advanced disease. A significant subset of these patients receives left ventricular assist device (LVAD) implantation either as destination therapy or as a bridge to transplantation.1,2 LVAD therapy improves both quality of life and survival. However, complications including bleeding events, driveline infections, pump thrombosis, stroke, right-sided heart failure, and ventricular arrhythmias (VAs) are common.3 As the prevalence of cardiovascular disease continues to climb and the demand for transplantation outpaces availability, it has become increasingly important to identify predictors of adverse postimplantation complications and to characterize their impact on outcome measures.
Approximately one-third of patients experience VAs after mechanical support implantation.4 Postimplant VAs are associated with painful implantable cardioverter-defibrillator (ICD) shocks and posttraumatic stress disorder, increased hospitalizations, and right ventricular failure. Studies evaluating mortality risk have shown mixed results.5, 6, 7, 8, 9, 10, 11 Preimplantation VAs have been reported as a risk factor for postimplantation VAs; however, little is known about the impact of pre-LVAD ICD shocks for VAs on postimplant outcomes and whether LVAD therapy affects their prevalence.8,12 Not uncommonly, destination LVAD therapy is declined for patients with pre-existing VAs. An improved understanding of the effect of pre-LVAD ICD shocks on post-LVAD outcomes will allow us to better care for this complex patient population. We conducted a retrospective cohort study of patients at a single academic medical center involving ischemic and nonischemic cardiomyopathy who had an ICD and underwent LVAD implantation. We sought to describe the association of appropriate ICD shocks before LVAD implantation with post-LVAD clinical outcomes.
Methods
Study population
A retrospective cohort study was performed to identify patients 18 years of age or older with a history of ICD implantation who later underwent durable continuous-flow LVAD implantation at the University of Pittsburgh Medical Center between 2006 and 2020. For patients with multiple LVAD implantations, the time of the first LVAD implantation was considered for the study. The study was approved by the University of Pittsburgh Medical Center institutional review board. Patient consent was waived due to the retrospective nature of this study with review of de-identified data.
Study variables
Baseline data, including demographic variables, medical history, medications, echocardiographic, and arrhythmia data, were collected from hospital records before LVAD implantation. The type of VA (monomorphic vs polymorphic) and number of ICD shocks were determined based on device interrogation and documentation data. The total number of appropriate shocks received was collected. Outcome variables of post-LVAD appropriate shocks, shock-related hospitalizations, stroke, death, and pump thrombosis were collected.
The primary outcome of the study was a composite outcome of death, stroke, and pump thrombosis within 1 year post-LVAD implantation. Secondary outcomes included the individual components of the composite outcome, the association between pre-LVAD ICD shocks and post-LVAD ICD shocks, and hospitalizations.
Statistical analysis
Statistical analysis was performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC). Descriptive categorical variables are reported as frequency (percentage) and continuous variables as mean ± SD or median [interquartile range]. The Fisher exact test was used to compare categorical variables. Univariate analysis was performed using all baseline variables. Significant baseline variables were chosen for multivariate Cox regression analysis. Preimplant ventricular tachycardia (VT) ablation and Class III antiarrhythmic drug use were not included in the multivariate analysis to avoid confounding with a diagnosis of VT. History of cardiac resynchronization therapy (CRT) device implantation also was not included because the left ventricular lead was turned off after LVAD implant. Backward elimination method was used to select the final model. Kaplan-Meier analysis was used for time to outcome analysis. All tests of significance were 2-sided, and P <.05 was considered significant.
Results
A total of 614 patients with LVAD implanted between 2006 and 2020 were reviewed (Figure 1). Of these patients, 221 without a durable continuous-flow LVAD, 45 with significant missing data, and 39 patients without an ICD were excluded. A total of 309 patients were included in the study. Baseline variables are summarized in Table 1. Average age was 57.4 ± 11.9 years; 86.7% were male; and 39.8% had nonischemic cardiomyopathy, with the remaining patients having ischemic cardiomyopathy. A total of 119 patients (39.7%) were treated with Class I or III antiarrhythmic drugs before implantation, and 21 patients (6.8%) had previously undergone ablation for VA before LVAD. All patients had an ICD implanted before LVAD, and 149 (48.2%) had cardiac resynchronization. Of the 309 patients in the study population, 71 (23.0%) had ICD shocks that occurred within the 1 year preceding LVAD implantation. The first shock was due to monomorphic VT in 60 patients (19.4%) and polymorphic VT or ventricular fibrillation in 11 patients (3.5%). Patients with an appropriate ICD shock before LVAD implantation had significantly higher comorbidities, with higher proportions of obesity, hypertension, chronic kidney disease, chronic obstructive pulmonary disease, previous stroke history, and higher left ventricular end-diastolic diameter (Table 1). These significant variables were included in the multivariate Cox regression analysis (Table 2). Patients with pre-LVAD shocks also had a higher usage of CRT devices, higher rate of HeartMate II (CF-LVAD; St. Jude Medical, Minneapolis, MN) devices, usage of Class III antiarrhythmic drug therapy, and preimplant VT ablation history as expected. CRT therapy was routinely turned off post-LVAD implantation at our center.
Figure 1.
Flowchart outlining study inclusion. ICD = implantable cardioverter-defibrillator; LVAD = left ventricular assist device.
Table 1.
Baseline demographic data before LVAD implantation
| Pre-LVAD ICD shock (n = 71) | No Pre-LVAD ICD shock (n = 238) | P value | |
|---|---|---|---|
| Average age (y) | 57 ± 11 | 57 ± 12 | 1 |
| Male | 64 (90) | 204 (86) | .43 |
| Obesity (BMI ≥30 kg/m2) | 41 (58) | 98 (41) | .015 |
| Nonischemic cardiomyopathy | 29 (41) | 94 (40) | .90 |
| HeartMate II | 16 (23) | 84 (35) | .045 |
| HeartMate III | 18 (25) | 40 (17) | .12 |
| HeartWare | 29 (41) | 102 (43) | .79 |
| Other continuous-flow LVAD | 8 (12) | 13 (5) | .11 |
| INTERMACS score | 2.3 ± 0.8 | 2.3 ± 0.8 | 1 |
| Bridge to transplant indication | 28 (39) | 98 (41) | .90 |
| Preimplant VT ablation | 21 (30) | 0 | <.0001 |
| Hypertension | 61 (86) | 172 (72) | .04 |
| Diabetes | 29 (41) | 102 (43) | .79 |
| Creatinine | 1.3 ± 0.4 [0.5–2.7] | 1.5 ± 0.7 [0.4–4.4] | .02 |
| Chronic kidney disease | 29 (41) | 81 (34) | .32 |
| Hemodialysis | 2 (3) | 2 (1) | .23 |
| Obstructive sleep apnea | 26 (37) | 63 (26) | .10 |
| Chronic obstructive pulmonary disease | 29 (41) | 46 (19) | .0004 |
| Atrial fibrillation | 45 (63) | 132 (55) | .27 |
| Previous stroke | 20 (28) | 30 (13) | .003 |
| History of smoking | 48 (68) | 155 (65) | .78 |
| Left ventricular ejection fraction (%) | 16 ± 5 | 17 ± 8 | .32 |
| Left ventricular end-diastolic diameter (cm) | 6.5 ± 1.1 [4.3–9.2] | 6.1 ± 1.2 [1.8–10.1] | .01 |
| Left ventricular end-diastolic diameter >7 cm before LVAD | 39 (55) | 101 (42) | .08 |
| Cardiac resynchronization therapy | 45 (63) | 104 (44) | .004 |
| Preimplant beta-blocker use | 60 (85) | 196 (82) | .72 |
| Preimplant ACEi/ARB/ARNI use | 46 (65) | 152 (64) | 1 |
| Preimplant mineralocorticoid | 33 (46) | 100 (42) | .59 |
| Preimplant Class III antiarrhythmic drug | 41 (58) | 74 (31) | .0001 |
Patient subsets divided between those receiving pre-LVAD ICD shocks and those not receiving pre-LVAD ICD shocks.
Value are given as n (%), mean ± SD, or mean ± SD [interquartile range] unless otherwise indicated.
ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BMI = body mass index; ICD = implantable cardioverter-defibrillator; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; LVAD = left ventricular assist device; VT = ventricular tachycardia.
Table 2.
Multivariate Cox regression analysis for the primary composite outcome of stroke, pump thrombosis, and death
| Adjusted hazard ratio (95% confidence interval) | P value | |
|---|---|---|
| Pre-LVAD ICD shock | 0.75 (0.44– 1.3) | .31 |
| Hypertension | 0.91 (0.52–1.6) | .74 |
| COPD | 1.41 (0.77–2.56) | .26 |
| Obesity | 0.61 (0.39–0.95) | .03 |
| History of stroke | 0.09 (0.04–0.18) | <.0001 |
| LV end-diastolic diameter | 1.45 (0.89–2.34) | .13 |
| Chronic kidney disease | 1.08 (0.68–1.73) | .74 |
COPD = chronic obstructive pulmonary disease; LV = left ventricle; other abbreviations as in Table 1.
During the year after LVAD implantation, the composite outcome of death, pump thrombosis, and stroke occurred in 17 patients (24%) with pre-LVAD ICD shocks and in 67 patients (28%) without pre-LVAD ICD shocks (hazard ratio [HR] 0.84; 95% confidence interval [CI] 0.48–1.51; P = .57) (Table 3 and Figure 2). Pre-LVAD shocks were not associated with the individual secondary outcome variables of death, pump thrombosis, and stroke. Among patients with pre-LVAD ICD shocks, 4 died with progressive heart failure, 2 from infection, 2 from VAs, and 4 from an unidentifiable cause of death. In the patients without pre-LVAD ICD shocks, 16 died from progressive heart failure, 6 from infection, 7 from stroke, 5 from hemorrhage, 5 from VAs, 3 from renal failure, 2 from pump thrombosis, 1 from cancer, 1 from probable suicide, and 6 from an unidentifiable cause of death . Sixteen patients (23%) with pre-LVAD ICD shocks underwent heart transplantation within 1 year postimplantation compared with 66 patients (28%) without pre-LVAD ICD shocks (HR 0.92; 95% CI 0.57–1.50; P = .74).
Table 3.
Clinical outcomes up to 1 year after LVAD implantation
| Pre-LVAD ICD shock (n = 71 patients) | No Pre-LVAD ICD shock (n = 238 patients) | Adjusted hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|
| Composite outcome of stroke, pump thrombosis, or death | 17 (24) | 67 (28) | 0.75 (0.44–1.3) | .31 |
| Death within 1 year | 12 (17) | 52 (22) | 1.18 (0.63–2.23) | .61 |
| Pump thrombosis | 6 (8) | 7 (7) | 0.83 (0.32–2.16) | .70 |
| Stroke | 2 (3) | 9 (4) | 2.77 (0.34–22.7) | .34 |
| Shock after LVAD implant | 41 (58) | 28 (12) | 5.7 (3.42–9.48) | <.0001 |
| Hospitalization for ICD shocks | 17 (24) | 7 (3) | 10.34 (4.1–25.7) | <.0001 |
| Heart transplantation | 16 (23) | 66 (28) | 0.92 (0.57–1.5) | .74 |
Patient cohort divided between those experiencing pre-LVAD ICD shocks and those without history of pre-LVAD ICD shocks.
Values are given as n (%) unless otherwise indicated.
Abbreviations as in Table 1.
Figure 2.
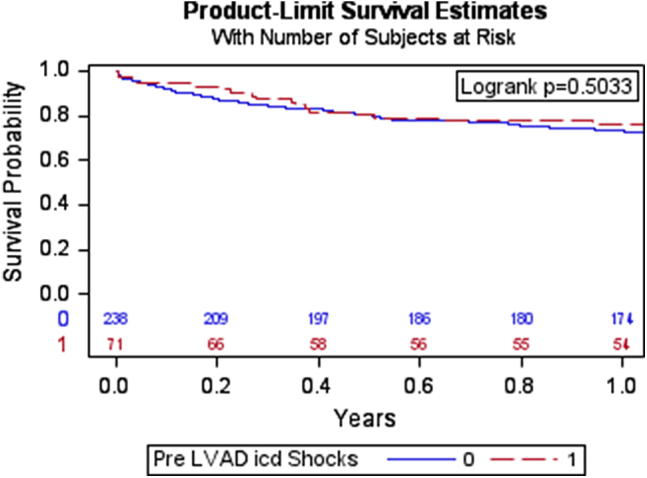
Kaplan-Meier survival curve showing the composite outcome of death, pump thrombosis, and stroke in patients with pre-LVAD ICD shocks compared to patients without pre-LVAD ICD shocks. Abbreviations as in Figure 1.
Shocks after LVAD implantation occurred in 41 patients (58%) with a history of pre-LVAD ICD shocks and in 28 patients (12%) without pre-LVAD ICD shocks (HR 5.7; 95% CI 3.42–9.48; P <.0001) (Table 3 and Figure 3). Notably, the prevalence of ICD shocks before and after LVAD were similar in the entire cohort, with 71 patients receiving shocks before LVAD and 69 receiving ICD shocks after LVAD (P = .924). Amiodarone was frequently used empirically post-LVAD implantation at our institution for arrhythmia prevention. This protocol included initiation of intravenous amiodarone, followed by transition to oral amiodarone and discontinuation at 1 month for patients without a history of arrhythmia. Sixty-six patients (93%) with pre-LVAD ICD shocks received Class III antiarrhythmic drugs post-LVAD compared to 178 patients (75%) without pre-LVAD ICD shocks (P = .007). Hospitalization for ICD shock occurred in 17 patients (24%) with pre-LVAD ICD shocks and in 7 patients (3%) without pre-LVAD ICD shocks (HR 10.34; 95% CI 4.1–25.7; P <.0001).
Figure 3.
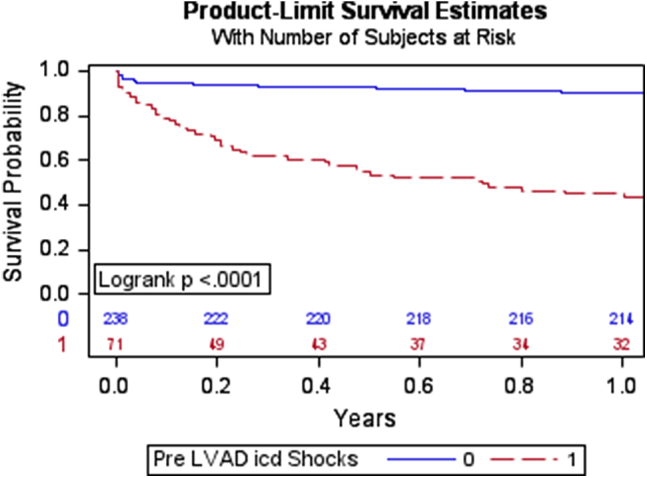
Kaplan-Meier survival curve showing shock-free survival 1 year after LVAD implantation. Abbreviations as in Figure 1.
Further subgroups analysis was performed based on the presence or absence of ICD shocks pre- and post-LVAD implantation. Baseline variables for the subgroups are given in Tables 4 and 5. In subgroup analysis, patients with new incidence of shock after LVAD implantation had a higher prevalence of preimplant left ventricular end-diastolic diameter >7 cm compared to patients with no ICD shock incidence (61% vs 40%; P = .04) (Table 5). Patients with pre-LVAD shocks but no incidence of shocks post-LVAD had a significantly higher prevalence of females and lower prevalences of hypertension and chronic kidney disease.
Table 4.
Baseline demographic data of patients with pre-LVAD ICD shocks with and without post-LVAD ICD shocks
| +Pre-LVAD shocks +Post-LVAD shocks (N = 41) |
+Pre-LVAD shocks –Post-LVAD shocks (N = 30) |
P value | |
|---|---|---|---|
| Average age (y) | 58 ± 9 | 57 ± 13 | .80 |
| Male | 41 (100) | 23 (77) | .002 |
| Obesity (BMI ≥30 kg/m2) | 25 (61) | 16 (53) | .63 |
| Nonischemic cardiomyopathy | 25 (61) | 16 (53) | .63 |
| HeartMate II | 9 (21) | 7 (23) | 1 |
| HeartMate III | 9 (21) | 9 (30) | .58 |
| HeartWare | 20 (48) | 9 (30) | .14 |
| INTERMACS score | 2.4 ± 0.7 | 2.2 ± 0.9 | .21 |
| Bridge to transplant indication | 18 (44) | 10 (33) | .46 |
| Preimplant VT ablation | 3 (7) | 3 (10) | .69 |
| Hypertension | 38 (93) | 23 (77) | .08 |
| Diabetes | 17 (41) | 12 (40) | 1 |
| Creatinine | 1.2 ± 1.7 | 1.4 ± 0.5 | .42 |
| Chronic kidney disease | 22 (54) | 7 (23) | .01 |
| Hemodialysis | 2 (5) | 0 (0) | .51 |
| Obstructive sleep apnea | 17 (41) | 12 (40) | 1 |
| Chronic obstructive pulmonary disease | 10 (24) | 5 (17) | .56 |
| Atrial fibrillation | 30 (73) | 15 (50) | .05 |
| Previous stroke | 20 (49) | 12 (40) | .48 |
| History of smoking | 30 (73) | 18 (60) | .43 |
| Left ventricular ejection fraction (%) | 16 ± 5 | 16 ± 5 | .62 |
| Left ventricular end-diastolic diameter (cm) | 5.8 ± 3.4 | 6.4 ± 1.1 | .37 |
| Left ventricular end-diastolic diameter >7 cm before LVAD | 23 (56) | 16 (53) | 1 |
| Preimplant beta-blocker use | 35 (85) | 25 (83) | 1 |
| Preimplant ACEi/ARB/ARNI use | 26 (63) | 20 (67) | .81 |
| Preimplant mineralocorticoid | 17 (41) | 16 (53) | .35 |
| Preimplant Class III antiarrhythmic drug | 26 (63) | 15 (50) | .30 |
Values are given as no. of patients (%) or mean ± SD unless otherwise indicated.
Abbreviations as in Table 1.
Table 5.
Baseline demographic date of patients without pre-LVAD ICD shocks with and without post-LVAD ICD shocks
| –Pre-LVAD shocks +Post-LVAD shocks (N = 28) |
–Pre-LVAD shocks –Post-LVAD shocks (N = 210) |
P value | |
|---|---|---|---|
| Average age (y) | 61 ± 13 | 57 ± 12 | .13 |
| Male | 27 (96) | 176 (84) | .20 |
| Obesity (BMI ≥30 kg/m2) | 13 (46) | 88 (42) | .69 |
| Nonischemic cardiomyopathy | 15 (54) | 117 (56) | .84 |
| HeartMate II | 10 (36) | 73 (35) | 1 |
| HeartMate III | 5 (18) | 35 (17) | .79 |
| HeartWare | 13 (46) | 88 (42) | .69 |
| INTERMACS score | 2.3 ± 0.9 | 2.2 ± 1.1 | .96 |
| Bridge to transplant indication | 11 (39) | 87 (42) | .84 |
| Preimplant VT ablation | 0 (0) | 0 (0) | 1 |
| Hypertension | 22 (79) | 150 (72) | .51 |
| Diabetes | 9 (32) | 93 (45) | .23 |
| Creatinine | 1.2 ± 2.1 | 1.4 ± 1.4 | .53 |
| Chronic kidney disease | 10 (36) | 71 (34) | .84 |
| Hemodialysis | 1 (4) | 1 (1) | .22 |
| Obstructive sleep apnea | 6 (21) | 57 (27) | .65 |
| Chronic obstructive pulmonary disease | 6 (21) | 40 (19) | .80 |
| Atrial fibrillation | 15 (54) | 117 (56) | .84 |
| Previous stroke | 17 (61) | 94 (45) | .16 |
| History of smoking | 17 (61) | 137 (66) | .83 |
| Left ventricular ejection fraction (%) | 16 ± 11 | 16 ± 8 | .95 |
| Left ventricular end-diastolic diameter (cm) | 5.8 ± 3.1 | 5.4 ± 3.3 | .52 |
| Left ventricular end-diastolic diameter >7 cm before LVAD | 17 (61) | 83 (40) | .04 |
| Preimplant beta-blocker use | 24 (86) | 171 (82) | .79 |
| Preimplant ACEi/ARB/ARNI use | 16 (57) | 136 (65) | .41 |
| Preimplant mineralocorticoid | 10 (36) | 89 (43) | .1166 |
| Preimplant Class III antiarrhythmic drug | 12 (43) | 62 (30) | .1925 |
Values are given as no. of patients (%) or mean ± SD unless otherwise indicated.
Abbreviations as in Table 1.
Discussion
The current study is the first to comprehensively evaluate pre-LVAD ICD shocks on post-LVAD clinical and mortality outcomes. The study shows that ICD shocks for VAs occurring within 1 year before LVAD implantation are not associated with a composite outcome of mortality, stroke, and pump thrombosis within 1 year postimplant after adjusting for comorbidities, despite an increased prevalence of post–LVAD ICD shocks in this subgroup.
VAs are a common complication of advanced heart failure and are a major cause of morbidity and mortality.1,2 The advent of the ICD for prevention of sudden cardiac death has led to a significant reduction in arrhythmic death in these high-risk patients; however, ICD shocks themselves are associated with several adverse outcomes, including increased hospitalizations and mortality.13 Mechanical support with a continuous-flow LVAD is now a mainstay of therapy in patients with refractory heart failure, leading to improved quality of life and longevity.14,15 Several previous studies have evaluated predictors of post-LVAD mortality. Brenyo et al8 retrospectively evaluated 61 patients with ICDs and LVAD implantation and found that pre-LVAD ICD therapy was not associated with post-LVAD mortality. Similarly, Efimova et al12 retrospectively studied 98 patients receiving an LVAD and found that sustained pre-LVAD VAs were not associated with post-LVAD mortality. A 2015 meta-analysis by Makki et al16 of 9 studies including 1179 patients found that a history of VAs preceding LVAD implantation was an independent risk factor for mortality in patients with post-LVAD VAs but not in those without post-LVAD VAs. These findings suggest that pre-LVAD ICD shocks for VAs may not be a significant factor in pursuing LVAD implantation because post-LVAD clinical outcomes are similar to those without pre-LVAD ICD shocks.
The prevalence of VAs after LVAD ranges between 22% and 59% depending on the presence of VAs before LVAD implantation and other patient characteristics.6 In our study, the prevalence of ICD shocks in the entire group was not different before vs after LVAD implantation. However, like previous studies, the current study also shows that pre-LVAD ICD shocks for VA predicted the occurrence of post-LVAD ICD shocks and hospitalizations.8,9,12 Although unloading of the left ventricle may reduce the risk of VAs through a reduction in myocardial stress and ischemia, the LVAD itself may lead to VAs in some cases through scar generated by the insertion of the apical LVAD inflow cannula, or mechanical stimulation of the myocardium by the inflow cannula, or through suction events.17, 18 Of interest, in this study, patients with new ICD shock incidence after LVAD implantation had a higher prevalence of pre-LVAD left ventricular dilation >7 cm. In addition to the effect of the LVAD itself on arrhythmia, patients with pre-existing scar based VA have an untreated substrate with continued potential for arrhythmogenesis. This is an important issue, and therapies to reduce VA incidence can improve morbidity. These patients might benefit from additional independent interventions such as catheter ablation or intraoperative ablation during LVAD implantation for VA.
Study limitations
The study was a single-center retrospective study, and important confounding factors may be unaccounted for. Our study cohort was relatively large but was not powered to detect small differences in mortality between the 2 groups. Subgroups analysis is significantly limited, with a small number of patients in each group. We did not include data on antitachycardia pacing events due to missing data; however, our study was focused on ICD shocks given their clinical importance compared to antitachycardia pacing therapy.19 We only included appropriate ICD shocks as a means of identifying the impact of VAs on post-LVAD outcomes. In addition, the study included patients over a 14-year timespan over which device and medication therapy approaches for heart failure have evolved. Therefore, device settings post-LVAD may have varied over time. Amiodarone usage was frequent in the acute postoperative period at our center and could have affected the arrhythmia incidence and clinical outcomes post LVAD implantation.
Conclusion
In patients with an ICD, pre-LVAD ICD shocks for VAs predicted post-LVAD ICD shocks and hospitalizations but were not associated with the composite outcome of death, pump thrombosis, or stroke at 1 year after implantation. Pre-LVAD ICD shocks did not increase the risk of several common and devastating post-LVAD complications, and LVAD implantation did not affect the ICD shock prevalence.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Suresh R. Mulukutla reports research support from Boston Scientific and Medtronic. Sandeep K. Jain reports research support from Boston Scientific and Medtronic; and being a research trial investigator for Abbott, Boston Scientific, and Medtronic. Samir F. Saba reports research support from Abbott and Boston Scientific; and being a consultant for Boston Scientific, Medtronic, and Sensydia. N. A. Mark Estes reports being a consultant for Boston Scientific and Medtronic. Krishna Kancharla reports being a consultant for Boston Scientific and Varian Medical Systems. All other authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Patient consent was waived due to the retrospective nature of this study with review of de-identified data.
Ethics Statement
The research reported in this article adhered to Helsinki Declaration guidelines and was approved by University of Pittsburgh Institutional Review Board.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., et al. Heart Disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Han J.J., Acker M.A., Atluri P. Left ventricular assist devices. Circulation. 2018;138:2841–2851. doi: 10.1161/CIRCULATIONAHA.118.035566. [DOI] [PubMed] [Google Scholar]
- 3.Kilic A., Acker M.A., Atluri P. Dealing with surgical left ventricular assist device complications. J Thorac Dis. 2015;7:2158–2164. doi: 10.3978/j.issn.2072-1439.2015.10.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon J.S., Maynes E.J., Choi J.H., et al. Ventricular arrhythmias following continuous-flow left ventricular assist device implantation: a systematic review. Artif Organs. 2020;44:E313–E325. doi: 10.1111/aor.13665. [DOI] [PubMed] [Google Scholar]
- 5.Enriquez A.D., Calenda B., Miller M.A., Anyanwu A.C., Pinney S.P. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol. 2013;6:668–674. doi: 10.1161/CIRCEP.113.000457. [DOI] [PubMed] [Google Scholar]
- 6.Nakahara S., Chien C., Gelow J., et al. Ventricular arrhythmias after left ventricular assist device. Circ Arrhythm Electrophysiol. 2013;6:648–654. doi: 10.1161/CIRCEP.113.000113. [DOI] [PubMed] [Google Scholar]
- 7.Ambardekar A.V., Allen L.A., Lindenfeld J., et al. Implantable cardioverter-defibrillator shocks in patients with a left ventricular assist device. J Heart Lung Transplant. 2010;29:771–776. doi: 10.1016/j.healun.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Brenyo A., Rao M., Koneru S., et al. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J Cardiovasc Electrophysiol. 2012;23:515–520. doi: 10.1111/j.1540-8167.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 9.Greet B.D., Pujara D., Burkland D., et al. Incidence, predictors, and significance of ventricular arrhythmias in patients with continuous-flow left ventricular assist devices: a 15-year institutional experience. JACC Clin Electrophysiol. 2018;4:257–264. doi: 10.1016/j.jacep.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Galand V., Flécher E., Auffret V., et al. ASSIST-ICD Investigators Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. JACC Clin Electrophysiol. 2018;4:1166–1175. doi: 10.1016/j.jacep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A., Tandon V., O'Sullivan D.M., et al. ICD shocks in LVAD patients are not associated with increased subsequent mortality risk. J Interv Card Electrophysiol. 2019;56:341–348. doi: 10.1007/s10840-019-00619-7. [DOI] [PubMed] [Google Scholar]
- 12.Efimova E., Fischer J., Bertagnolli L., et al. Predictors of ventricular arrhythmia after left ventricular assist device implantation: a large single-center observational study. Heart Rhythm. 2017;14:1812–1819. doi: 10.1016/j.hrthm.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Borne R.T., Varosy P.D., Masoudi F.A. Implantable cardioverter-defibrillator shocks: epidemiology, outcomes, and therapeutic approaches. JAMA Intern Med. 2013;173:859–865. doi: 10.1001/jamainternmed.2013.428. [DOI] [PubMed] [Google Scholar]
- 14.Slaughter M.S., Rogers J.G., Milano C.A., et al. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 15.Mehra M.R., Goldstein D.J., Uriel N., et al. MOMENTUM 3 Investigators. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–1395. doi: 10.1056/NEJMoa1800866. [DOI] [PubMed] [Google Scholar]
- 16.Makki N., Mesubi O., Steyers C., Olshansky B., Abraham W.T. Meta-analysis of the relation of ventricular arrhythmias to all-cause mortality after implantation of a left ventricular assist device. Am J Cardiol. 2015;116:1385–1390. doi: 10.1016/j.amjcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 17.Monreal G., Gerhardt M.A. Left ventricular assist device support induces acute changes in myocardial electrolytes in heart failure. ASAIO J. 2007;53:152–158. doi: 10.1097/MAT.0b013e3180302a8b. [DOI] [PubMed] [Google Scholar]
- 18.Zabel M., Koller B., Sachs F., Franz M. Stretch-induced voltage changes in the isolated beating heart: Importance of the timing of stretch and implication for stretch-activated ion channels. Cardiovasc Res. 1996;32:120–130. [PubMed] [Google Scholar]
- 19.Sweeney M.O., Sherfesee L., DeGroot P.J., Wathen M.S., Wilkoff B.L. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:353–360. doi: 10.1016/j.hrthm.2009.11.027. [DOI] [PubMed] [Google Scholar]