Abstract
Premature Ventricular Complexes (PVCs) refer to electrical activity arising from ventricles resulting in ventricular contraction independent of the native rhythm. PVCs by themselves are common in the general population but based on the origin of the PVCs, either related to anatomical or electrical substrate, the disease process has a widely varied presentation and prognosis. The clinical presentation of symptoms may vary from being extremely benign, or very severe (malignant). Benign PVCs include those that are asymptomatic or induce very mild symptoms including palpitations, lightheadedness, chest discomfort, or the sensation of skipped beats. The middle range of PVCs present as heart failure or heart failure complicated by PVCs. The malignant variety may present as syncope, or sudden cardiac death. In this review we describe the multiple facets of PVC presentation and strategies of clinical management.
Keywords: PVCs, Benign, Malignant, Cardiomyopathy, Sudden cardiac death
Abbreviations:
- ECG
Electrocardiogram
- PVC
Premature Ventricular Complexes
- RV
Right Ventricle
- LV
Left Ventricle
- CAD
Coronary artery disease
- LVEF
Left Ventricular Ejection Fraction
- CMRI
Cardiac Magnetic Resonance Imaging
1. Introduction
Premature Ventricular Contractions (PVCs) are an arrhythmia that occurs when the ventricle spontaneously depolarizes independent of the native underlying rhythm. PVCs can be considered as a part of a presentation of a syndrome which may be a single entity that is isolated and benign or as a part of a disease process.
Idiopathic PVCs are those found in patients who present with isolated PVCs without any obvious heart disease. The clinical presentation may be an incidental finding noticed during routine clinical assessment. Another group of patients with benign include those with idiopathic PVCs who present with mild symptoms. The symptoms may comprise of palpitations, chest discomfort, light headedness, shortness of breath, or skipped beats.
The spectrum of clinical presentation of PVCs may extend further, with PVCs presenting as cardiomyopathy which may be detected without obvious symptoms of PVCs or with symptoms of heart failure. Patients with structural heart disease may also demonstrate PVCs and its role clinically may have different implication.
The group of patients who present with serious ventricular arrhythmias including sudden cardiac death, without any obvious structural heart disease and noticed to have a PVC trigger for ventricular fibrillation is grouped under idiopathic ventricular fibrillation.
The goal of this review is to summarize the spectrum of PVC presentation from benign to malignant and to differentiate between the two entities, as well as outline an approach in managing these patients.
2. Epidemiology
The prevalence of PVCs ranges from 3 to 40% across different cohorts of healthy individuals [1,2]. Kim YG et al. have described PVCs as a common arrhythmia affecting 1–2% of the general population and it has been considered to have a benign clinical course [3]. In their cohort of newly diagnosed PVCs identified in 4515 people among 9,743,582 people without prior history of PVC, heart failure or ventricular arrhythmias, patients with newly diagnosed PVCS carry a higher incidence of heart failure or ventricular arrhythmias [3].
It is important to note that the detection of PVCs increases with the use of outpatient monitoring devices. Additionally, absence of PVCs in the elderly is uncommon. For example, in a multiethnic cohort of men and women between 75 and 85 years old who wore a Zio XT patch (a 2-week continuous ambulatory ECG recording device), PVCs were detected in 98.9% of participants [4]. Beyond age, several other risk factors have been identified and are thought to contribute to an increased prevalence of PVCs including male sex, increased height, smoking, black race, history of CAD, lower LVEF, and hypertension [1,5,6].
3. Benign premature ventricular contractions
3.1. Asymptomatic
Isolated clinical presentations of PVC are often an incidental discovery of PVCs on a surface electrocardiogram obtained for reasons unrelated to arrhythmia. This group of patients may not have symptoms at presentation but probing clinically patients may describe vague symptoms of fatigue. Largely the symptoms are simply correlated to PVCs and there is no clear clinical cause and effect and are therefore considered an asymptomatic presentation.
Typically, the assessment of PVCs includes characterization of PVC location and assessing relative burden using ambulatory monitoring. Monitoring should be completed on a yearly basis if the PVC burden is significant (i.e. at least 8%) as these patients are at risk for development of PVC-related pathologies should the burden increase. The exact clinical course is hard to predict but the potential increase of burden can't be ignored. Echocardiogram should include global longitudinal strain as an early sign for development of LV dysfunction. Additional work-up includes assessment for structural heart disease with a transthoracic echocardiogram and in some instances a cardiac MRI. In the absence of structural heart disease even with the presence of PVCs the management is predominantly reassurance and follow up. Asymptomatic PVCs noted on a negative treadmill stress test should not be ignored as Califf and colleagues noted a higher incidence of coronary artery disease and LV dysfunction in those with PVCs observed compared to those without [7]. Athletes with incidentally noted PVCs should undergo a thorough evaluation for presence of existing heart disease including assessment of burden with ambulatory monitoring, structure with echo and/or cardiac MRI, and depending upon these results, invasive EP study if there are malignant features revealed in evaluation.
3.2. Symptomatic
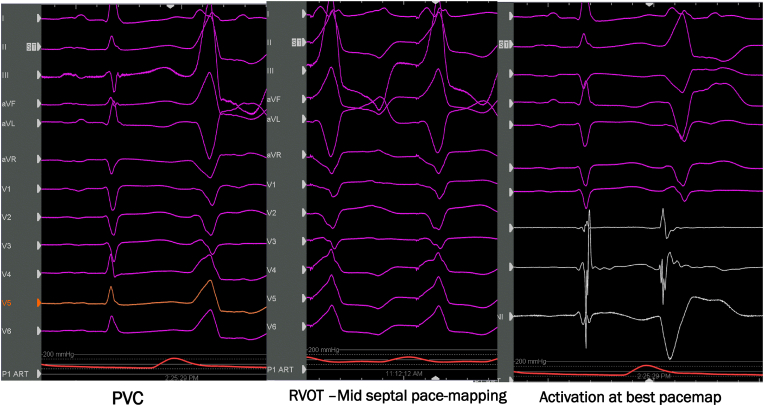
The common location of symptomatic PVCs is from outflow tracts, but any location either right or left ventricle may present with symptoms (Fig. 1). Commonly patients with symptomatic PVCs present with palpitations, skipped beats, chest discomfort, or lightheadedness. Some patients are quite incapacitated with functional limitation due to symptoms. Isolated symptomatic PVCs are idiopathic and usually arise from right ventricular outflow tract, left ventricular outflow tract, aortic cusps, fascicular branches, or papillary muscles. Although fascicular PVCs mostly may be benign, one should be cautious as it may potentially trigger VF in certain situations. The work up includes PVC burden assessment as well as assessment of LV and RV function with a transthoracic echocardiogram. Depending upon the findings on the echocardiogram further assessment may be needed to obtain a complete clinical assessment. CMRI including late gadolinium enhancement may be needed to further characterize structural heart disease. The LV ejection fraction alone may not provide the whole picture as presence of substrate in the form of either inflammation or scar may result in PVCs [8]. A composite end point of age, male gender, family history of sudden cardiac death, multifocal PVCs, right bundle inferior axis PVC morphology was found to be present in 16% of the patient screened with normal ejection fraction in this study. The location of this PVCS can occur anywhere but commonly in the outflow tracts and coronary cusps in the absence of structural heart disease.
Fig. 1.
68-year-old male patient with symptomatic PVCs with palpitations and lightheadedness. This figure demonstrates PVC arising from right ventricular outflow tract activation and pace mapping at the successful ablation location.
Treatment includes risk stratification as described with assessment of PVC burden using ambulatory ECG monitoring, structural assessment in the form of echocardiography and possible MRI if there is a suspicion based on history and clinical presentation to assess presence of scar burden. First line therapies include medications, primarily targeting symptoms. Beta blockers and calcium channel blockers have been shown to have modest effects on symptoms and PVC burden. Beta blockers and calcium channel blockers are often limited by relative bradycardia that is frequent seen in patients with high PVC burden. Additionally side effects of fatigue frequently limit their clinical utility. Other drugs may be helpful to treat symptoms include Class 1C and Class 3 and anti-arrhythmic medications. These are much more effective than beta blockers and calcium channel blockers if there are no contraindications to use in this group of patients [9].
4. PVCs and cardiomyopathy
PVCs and cardiomyopathy are grouped under arrhythmia-induced cardiomyopathy. Niwano et al. described high PVC prevalence and LV dysfunction in long term follow up of patients [10]. The association of these two entities is very important in the evaluation of non-ischemic cardiomyopathy. In the absence of any specific etiology of cardiomyopathy, the association of PVCs if present becomes an important and potentially treatable form of cardiomyopathy.
The mechanisms of PVC induced cardiomyopathy are quite complex as many potential explanations have been described. The cardiomyopathy may be reduced LVEF or heart failure with preserved ejection fraction.
4.1. Mechanisms
In general, it has been observed that the higher the PVC burden is, the more likely the PVC will lead to a cardiomyopathy. The common observation clinical threshold for pursuing PVC as the cause of a non-ischemic cardiomyopathy is a burden of 10% or more, which comes from several studies including Baman et al. that demonstrated a PVC burden of 24% or higher with single morphology are associated with high-risk of developing cardiomyopathy [11].
Other PVC characteristics that have been thought to be including interpolated PVCs [12] and PVC coupling interval of more than 375 ms [13]. Similarly, Kawamura et al., have demonstrated high risk of development of PVC induced cardiomyopathy when the PVC coupling interval dispersion is greater than 115 ms [14]. Carballeira et al. noted PVC QRS duration of more than 153 ms seemed to have higher risk of developing cardiomyopathy [15]. Post extra systolic potentiation (PESP) as a predictor of recovery of left ventricular dysfunction was observed by Krishnan B, Tholakanahalli et al. [16] In this series, it was noted that patients with more than 8 mmHg, PESP was associated with higher chance of recovery of left ventricular systolic function following ablation. It was also noted that if PESP less than 8 mmHg even after suppressing PVCs the cardiomyopathy seemed to be not reversible (Figure-2). Several groups and previous studies have discussed how frequent PVCs alter intra-cellular calcium handling and sympathetic tone leading to further LV dysfunction [[17], [18], [19], [20]]. Additional risk factors for developing PVC-induced cardiomyopathy include increased duration [21] of PVC exposure with asymptotic PVCs making this more likely [22]. Additional risk factors include those that are epicardial in nature [23], males [24], and those with a lack of diurnal variation [25].
Fig. 2.
This figure demonstrates a patient's electrocardiogram with simultaneous intraarterial blood pressure tracing showing post-extra systolic potentiation.
There are a number of underlying mechanism that need to be understood when dealing with patients with frequent PVCs which include PVCs that initiate re-entrant tachycardia's, for example Qu et al. discussed how PVCs can trigger re-entrant arrhythmias when propagation of the wave front is blocked (due to the T-wave of a previous beat) in one direction ultimately resulting in initiation of reentry tachycardia (R-to-T mechanism). However, there are also instances where the T-wave itself can initiate reentrant tachycardia due to the substrate alone (R-from-T) [26]. This is an important distinction because depending upon the mechanism, the treatment will vary from medical management to substrate modification (i.e. ablation). Other extra-cardiac mechanisms should be considered as well, for example Tan and colleagues demonstrated in a canine model of PVC induced cardiomyopathy that in spite of LVEF recovery that there are residual changes to the cardiac sympathetic/parasympathetic balance that serve as important triggers for ventricular proarrhythmias [27]. Finally, it should be known that there are PVCs that the underlying trigger can be enhanced or inhibited by the underlying heart rate. For example Pitzalis and colleagues investigated those that are bradycardia-enhanced (higher PVC burden with bradycardia) versus tachycardia-enhanced (higher PVC burden with tachycardia). For those that were bradycardia-enhanced, beta blockade with nadolol worsened PVC burden but improved it in those that were tachycardia-enhanced. There is a third subset that seems to be indifferent and nadolol administration did not affect this group [28].
Often PVC ablation has been associated with substantial improvement in symptoms with relatively normal LV function. This concept was noted by Akkaya M, Tholakanahalli V et al., and have observed that patients after undergoing PVC ablation have improvement in left ventricular diastolic function in the form of improvement in the tissue Doppler indices and left atrial volume indices [29]. The cellular mechanisms have been described by Wang Y et al. showing reduction in Ito, Ik1, ICaL in PVC myocytes. Heterogeneity in action potential duration and configuration as well as possible impaired calcium release from sarcoplasmic reticulum [30].
5. Management
Evaluation for PVCs as a potential non-ischemic cardiomyopathy mechanism must be entertained as often this reversible entity may be missed. Initial evaluation should include a 12 lead ECG (or 12-lead holter monitor if it is not possible to record one in this manner) for initial localization and a cutaneous patch monitor for assessing overall burden. Further work up includes assessment of structural heart disease or any form of reversible cardiomyopathy (most frequently ischemia or coronary artery disease) before considering PVC as the etiology for causing ventricular dysfunction. To begin with an echocardiogram assessing function as well as any valvular heart disease. Ischemic work-up including stress imaging or coronary angiogram must be obtained as coronary artery disease is the most common cardiomyopathy. CMRI should be obtained if there is any suspicion for potential scar, infiltrative, or inflammatory cardiomyopathy. In instances where there is a strong clinical suspicion for inflammatory cardiomyopathy but there is still a negative CMRI evaluation, one can consider an FDG PET scan to evaluation for occult inflammation that may be driving ventricular arrhythmias [31].
According to AHA/ACC/HRS guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death, the class-I indication for arrhythmia suppression includes declining left ventricular function for frequent PVCs of more than 15% and predominantly one morphology for which antiarrhythmic medications are ineffective or intolerable or patient preference, ablation is recommended. It is a class IIa indication to pursue pharmacologic treatment with beta blockers or amiodarone to reduce recurrent arrhythmias and improve symptoms and possibly left ventricular systolic function [32]. By reversing a non-ischemic cardiomyopathy via reducing PVC burden via ablation or pharmacologic therapy, defibrillator implantation as a part of primary prevention of sudden cardiac death is not needed with improvement of ventricular function.
Based on the description of mechanisms associated with PVCs, it is also noted that PVCs may arise from any location which could potentially cause cardiomyopathy. Therefore, all the suspected reasoning as described above need to be considered or potentially, when PVCs are addressed. Ablation therapy is often successful more than 80% of the time in many cohorts based on the accessibility of the PVCs for ablation. (Figure-3).
Fig. 3.
This patient was a 52-year-old male who had and left ventricular ejection fraction of 20%, with a PVC burden of 21%. The PVC was successfully ablated and the ejection fraction improved to 55% in a 6-month follow-up echocardiogram. This figure demonstrates PVC mapping shown on (A) fluoroscopy in left anterior and right anterior oblique views. The earliest activation timing at the right coronary cusp was noted in the (B) intracardiac tracing. The 12-lead electrocardiogram demonstrates (C) PVC morphology.
In a large retrospective cohort study involving 1185 patients, PVC ablation was successful 84% of the patients. Overall complication rate was 5.2% with major complications being 2.4%. Hence ablation is effective treatment strategy with low risk [33]. In a cohort of 510 patients Radiofrequency ablation was more effective than anti arrhythmic drug therapy. Beta blockers and calcium channel blockers had modest effects on the reduction of PVCs [9]. Interestingly, and frequently confusing for clinicians, are patients who have PVCs secondary to a cardiomyopathy but the PVC is not causative of the cardiomyopathy. In this instance, it is frequently appropriate to do a trial of anti-arrhythmic therapy (i.e. amiodarone) in an effort to suppress ventricular ectopy and assess its effect on systolic function prior to pursuing ablation therapy. Nonetheless, there may still be instances where it is unclear if PVCs are cause or effect of the cardiomyopathy and it is appropriate to attempt an ablation. If successful PVC ablation is unable to restore left ventricular function, a patient should have an ICD placed in absence of recovery of LVEF.
6. Other categories of PVCs and cardiomyopathy: Not so benign
The other form of PVC's which may potentially affect cardiomyopathy are those which interfere with biventricular pacing. In this instance, frequent PVCs results in a significant drop in the percentage of biventricular paced beats decreasing the effectiveness in cardiac resynchronization therapy (Figure-4). Relatively very low percentage of ectopic beats greater than or equal to 0.1% dramatically drops biventricular pacing less than 97%. This was observed in multi-center automatic defibrillator implementation with cardiac resynchronization therapy cohort (MADIT-CRT). The risk of heart failure/death was increased significantly in those with 0.1–1.5% (Hazard ratio:3.13 and 1.84) and greater than 1.5% (2.38 and 2.74) [34].
Fig. 4.
This figure demonstrates PVC arising from septal location whose biventricular pacing percentage dropped to <90% with 19% PVC burden. Bipolar ablation resulted in elimination of PVCs with improvement of biventricular pacing to 99%.
Patients with PVCs associated with post infarction usually arise from infarct scar and often seemed to arise from the exit side of the reentrant tachycardia. Hence ablation of these PVCs may have implications of potentially reducing ventricular arrhythmias associated with infarct substrate [35]. Finally, it should be noted that the percent of BiV pacing reported by an implanted device could be over-reported in the setting of pseudo-fusion of a PVC and paced beat and the reason for ineffective CRT therapy [36].
7. Malignant PVC's
While PVCs frequently present as asymptomatic and benign can also present in a malignant form with sudden cardiac death. The true incidence of PVC induced VF is unclear as autopsy will not reveal the cause of death. PVCs triggering ventricular fibrillation (VF) and sudden cardiac death have been observed to have certain anatomic features that are high-risk. For example, PVCs arising from the distal Purkinje system have been frequently observed to induce VF. This can be seen in patients with normal hearts, inherited arrhythmognic disease, and other non-ischemic cardiomyopathies. The eventual perpetuation of VF may be a reentrant mechanism among this group of patients but primarily initiated by malignant PVCs. In this population, in addition to ablation, treatment may also requiring anti-arrhythmic therapy in addition to ICD therapy.
Initial description of PVCs as triggers causing VF were systematically described by Haissaguerre et al. [37] In this study it was noticed that among 23 patients VF occurred during daily activity but none during effort. Four patients in this cohort had VF during sleep. The PVC morphologies in this cohort were noted to be right or left bundle branch block pattern in 10 and 13 patients, and both patterns among 4 patients. Although the coupling interval between the sinus and the PVC beats or fixed in 4, it varied from 20 to 160 ms. Most of the patients had VF noticed at the shortest coupling interval of 297 ± 41 ms (Figure-5). It was noted in this cohort that PVCs originated from the Purkinje fibers in 23 out of 27 patients, 10 from the left ventricular septum, 9 from the right ventricle and 4 from both [37].
Fig. 5.
Fascicular PVC
This figure demonstrates a telemetry strip of an 84-year-old male patient who presented with type II second degree atrioventricular block and received pacemaker. The following day he had a cardiac arrest demonstrated in the strip showing triggered PVC resulting in polymorphic Ventricular tachycardia.
The origination of triggered PVCS causing VF has been described by Sadek MM et al., originating from the moderator band the characteristic left bundle branch block QRS morphology with the late precordial transition > V4 [38]. The mechanisms have been proposed as early afterdepolarization attributed to inward calcium currents. The triggered PVCs seem to have coupling interval commonly of <300 ms and this was first earlier observed by Lienhardt et al. [38,39] This was subsequently also noted in several observations and concept of short-coupled PVCs (Figure-4) as triggers from Purkinje mediated VF has been postulated. Igarashi M et al. proposed prematurity index defined as ratio of coupling interval of the trigger PVC to preceding RR interval of sinus cycle. An index of 0.73 has a sensitivity of 91% and specificity of only 44% for identifying malignant PVCs. Li CO et al. described in their case series intermediate-coupled PVCs with coupling intervals <400 ms with normal QT intervals, initiation of VF/VT in early recovery from exercise [40,41].
8. Management
First line for management of VF is to rule out myocardial ischemia prior to pursuing additional management options. After ruling out ischemic triggers, most of the management strategies described in the literature seemed to be suppressing VF episodes after multiple defibrillator therapies. Although there are distinctive entities where VF triggers may be primarily treated with ablative therapy. Malignant PVCS may occur with or without structural heart disease.
Since PVC induced VF results in sudden cardiac death, treatment is usually multi-modality including medications, ablation, and ICD implantation for secondary prevention of sudden cardiac death. Identification of Purkinje potential was noticed 38 ± 28 ms ahead of local activation a in cohort described by Haissaguerre et al. [37] Patient with these malignant PVCS almost always need rescue defibrillator, but ablative therapy is extremely helpful suppressing the event rate. The recurrence rate of VF was 18% among 38 patients over a median follow-up of 24 months in a cohort described by Knecht S et al. [42] Among these patients 33 out of 38 arose from Purkinje fibers. There is some debate regarding use of Verapamil in suppressing short-coupled variant of torsades de pointes.
Prior to undertaking a PVC ablation in this clinical situation, one should have evidence that the PVC trigger is responsible for the malignant arrhythmia. This should be in the form of a hospital telemetry monitoring system demonstrating this in addition to a 12 lead ECG of the particular PVC of interest. Assessment of these PVCs in an electrophysiological study may be augmented by intraprocedural imaging to identify intracavitary structures that could potentially be the origin of the PVC. This may include the moderator band where right bundle is inserted, left ventricular false tendon, papillary muscles of both right ventricle and left ventricle. Since the His-Purkinje system is endocardial in location and often associated with intracavitary structures, multi-modality imaging is frequently beneficial at identifying a PVC origin by combining electroanatomic mapping with intracardiac echocardiogram.
9. Conclusion
PVCs present clinically from benign to malignant variants. Management of these PVCs depends upon symptoms, development, or progression of cardiomyopathy, or sudden cardiac death. This includes treatments ranging from reassurance, conservative pharmacological management, ablation and even implantable defibrillator therapy for certain malignant variants.
Declaration of competing interest
No relevant disclosures for all authors.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Simpson R.J., Jr., Cascio W.E., Schreiner P.J., Crow R.S., Rautaharju P.M., Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2002;143:535–540. doi: 10.1067/mhj.2002.120298. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy H.L., Whitlock J.A., Sprague M.K., Kennedy L.J., Buckingham T.A., Goldberg R.J. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193–197. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.G., Choi Y.Y., Han K.-D., Min K.J., Choi H.Y., Shim J., Choi J.-I., Kim Y.-H. Premature ventricular contraction increases the risk of heart failure and ventricular tachyarrhythmia. Sci Rep. 2021;11:1–8. doi: 10.1038/s41598-021-92088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norby F.L., Alonso A., Rooney M.R., Maheshwari A., Koene R.J., Zhang M., Soliman E.Z., Loehr L.R., Mosley T., Gottesman R.F. Association of ventricular arrhythmias with dementia: the atherosclerosis risk in communities (ARIC) study. Neurology. 2021;96:e926–e936. doi: 10.1212/WNL.0000000000011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerola T., Dewland T.A., Vittinghoff E., Heckbert S.R., Stein P.K., Marcus G.M. Modifiable predictors of ventricular ectopy in the community. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Rotz M., Aeschbacher S., Bossard M., Schoen T., Blum S., Schneider S., Estis J., Todd J., Risch M., Risch L. Risk factors for premature ventricular contractions in young and healthy adults. Heart. 2017;103:702–707. doi: 10.1136/heartjnl-2016-309632. [DOI] [PubMed] [Google Scholar]
- 7.Califf R.M., McKinnis R.A., McNeer J.F., Harrell F.E., Jr., Lee K.L., Pryor D.B., Waugh R.A., Harris P.J., Rosati R.A., Wagner G.S. Prognostic value of ventricular arrhythmias associated with treadmill exercise testing in patients studied with cardiac catheterization for suspected ischemic heart disease. J Am Coll Cardiol. 1983;2:1060–1067. doi: 10.1016/s0735-1097(83)80330-1. [DOI] [PubMed] [Google Scholar]
- 8.Muser D., Santangeli P., Castro S.A., Casado Arroyo R., Maeda S., Benhayon D.A., Liuba I., Liang J.J., Sadek M.M., Chahal A. Risk stratification of patients with apparently idiopathic premature ventricular contractions: a multicenter international CMR registry. Clin Electrophysiol. 2020;6:722–735. doi: 10.1016/j.jacep.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Zhong L., Lee Y.-H., Huang X.-M., Asirvatham S.J., Shen W.-K., Friedman P.A., Hodge D.O., Slusser J.P., Song Z.-Y., Packer D.L. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm. 2014;11:187–193. doi: 10.1016/j.hrthm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Niwano S., Wakisaka Y., Niwano H., Fukaya H., Kurokawa S., Kiryu M., Hatakeyama Y., Izumi T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009;95:1230–1237. doi: 10.1136/hrt.2008.159558. [DOI] [PubMed] [Google Scholar]
- 11.Baman T.S., Lange D.C., Ilg K.J., Gupta S.K., Liu T.-Y., Alguire C., Armstrong W., Good E., Chugh A., Jongnarangsin K. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Olgun H., Yokokawa M., Baman T., Kim H.M., Armstrong W., Good E., Chugh A., Pelosi F., Jr., Crawford T., Oral H. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011;8:1046–1049. doi: 10.1016/j.hrthm.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Potfay J., Kaszala K., Tan A.Y., Sima A.P., Gorcsan J., III, Ellenbogen K.A., Huizar J.F. Abnormal left ventricular mechanics of ventricular ectopic beats: insights into origin and coupling interval in premature ventricular contraction–induced cardiomyopathy. Circulation: Arrhythmia and Electrophysiology. 2015;8:1194–1200. doi: 10.1161/CIRCEP.115.003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura M., Badhwar N., Vedantham V., Tseng Z.H., Lee B.K., Lee R.J., Marcus G.M., Olgin J.E., Gerstenfeld E.P., Scheinman M.M. Coupling interval dispersion and body mass index are independent predictors of idiopathic premature ventricular complex-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25:756–762. doi: 10.1111/jce.12391. [DOI] [PubMed] [Google Scholar]
- 15.Pol L.C., Deyell M.W., Frankel D.S., Benhayon D., Squara F., Chik W., Kohari M., Deo R., Marchlinski F.E. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization–induced cardiomyopathy. Heart Rhythm. 2014;11:299–306. doi: 10.1016/j.hrthm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan B., Sankar A., Anand I., Adabag S., Li J.-M., McFalls E.O., Benditt D.G., Shivkumar K., Tholakanahalli V.N. Post-extrasystolic potentiation as a predictor of recovery of left ventricular dysfunction after radiofrequency catheter ablation. JACC (J Am Coll Cardiol): Clin Electrophysiol. 2017;3:1283–1291. doi: 10.1016/j.jacep.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Takada H., Takeuchi S., Ando K., Kaito A., Yoshida S., Hisada S., Mizuno Y. Experimental studies on myocardial contractility and hemodynamics in extrasystoles. Jpn Circ J. 1970;34:419–430. doi: 10.1253/jcj.34.419. [DOI] [PubMed] [Google Scholar]
- 18.Segerson N.M., Wasmund S.L., Abedin M., Pai R.K., Daccarett M., Akoum N., Wall T.S., Klein R.C., Freedman R.A., Hamdan M.H. Heart rate turbulence parameters correlate with post–premature ventricular contraction changes in muscle sympathetic activity. Heart Rhythm. 2007;4:284–289. doi: 10.1016/j.hrthm.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichterle D., Melenovsky V., Simek J., Malik J., Malik M. Hemodynamics and autonomic control of heart rate turbulence. J Cardiovasc Electrophysiol. 2006;17:286–291. doi: 10.1111/j.1540-8167.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 20.Cooper M.W. Postextrasystolic potentiation. Do we really know what it means and how to use it? Circulation. 1993;88:2962–2971. doi: 10.1161/01.cir.88.6.2962. [DOI] [PubMed] [Google Scholar]
- 21.Walters T.E., Rahmutula D., Szilagyi J., Alhede C., Sievers R., Fang Q., Olgin J., Gerstenfeld E.P. Left ventricular dyssynchrony predicts the cardiomyopathy associated with premature ventricular contractions. J Am Coll Cardiol. 2018;72:2870–2882. doi: 10.1016/j.jacc.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 22.Yokokawa M., Kim H.M., Good E., Chugh A., Pelosi F., Jr., Alguire C., Armstrong W., Crawford T., Jongnarangsin K., Oral H. Relation of symptoms and symptom duration to premature ventricular complex–induced cardiomyopathy. Heart Rhythm. 2012;9:92–95. doi: 10.1016/j.hrthm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Blaye-Felice M.S., Hamon D., Sacher F., Pascale P., Rollin A., Duparc A., Mondoly P., Derval N., Denis A., Cardin C. Premature ventricular contraction-induced cardiomyopathy: related clinical and electrophysiologic parameters. Heart Rhythm. 2016;13:103–110. doi: 10.1016/j.hrthm.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Park K.-M., Im S.I., Park S.-J., Kim J.S., On Y.K. Risk factor algorithm used to predict frequent premature ventricular contraction-induced cardiomyopathy. Int J Cardiol. 2017;233:37–42. doi: 10.1016/j.ijcard.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Hamon D., Swid M.A., Rajendran P.S., Liu A., Boyle N.G., Shivkumar K., Bradfield J.S. Premature ventricular contraction diurnal profiles predict distinct clinical characteristics and beta-blocker responses. J Cardiovasc Electrophysiol. 2019;30:836–843. doi: 10.1111/jce.13944. [DOI] [PubMed] [Google Scholar]
- 26.Qu Z., Liu M.B., Olcese R., Karagueuzian H., Garfinkel A., Chen P.-S., Weiss J.N. R-on-T and the initiation of reentry revisited: integrating old and new concepts. Heart Rhythm. 2022;19:1369–1383. doi: 10.1016/j.hrthm.2022.03.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan A.Y., Elharrif K., Cardona-Guarache R., Mankad P., Ayers O., Joslyn M., Das A., Kaszala K., Lin S.-F., Ellenbogen K.A. Persistent proarrhythmic neural remodeling despite recovery from premature ventricular contraction-induced cardiomyopathy. J Am Coll Cardiol. 2020;75:1–13. doi: 10.1016/j.jacc.2019.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitzalis M.V., Mastropasqua F., Massari F., Totaro P., Di Maggio M., Rizzon P. Holter-guided identification of premature ventricular contractions susceptible to suppression by β-blockers. Am Heart J. 1996;131:508–515. doi: 10.1016/s0002-8703(96)90529-3. [DOI] [PubMed] [Google Scholar]
- 29.Akkaya M., Roukoz H., Adabag S., Benditt D.G., Anand I., Li J.M., Zakharova M., Tholakanahalli V. Improvement of left ventricular diastolic function and left atrial reverse remodeling after catheter ablation of premature ventricular complexes. J Intervent Card Electrophysiol. 2013;38:179–185. doi: 10.1007/s10840-013-9836-0. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Eltit J.M., Kaszala K., Tan A., Jiang M., Zhang M., Tseng G.-N., Huizar J.F. Cellular mechanism of premature ventricular contraction–induced cardiomyopathy. Heart Rhythm. 2014;11:2064–2072. doi: 10.1016/j.hrthm.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tung R., Bauer B., Schelbert H., Lynch J.P., III, Auerbach M., Gupta P., Schiepers C., Chan S., Ferris J., Barrio M. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–2498. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., Bryant W.J., Callans D.J., Curtis A.B., Deal B.J., Dickfeld T., Field M.E., Fonarow G.C. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 33.Latchamsetty R., Yokokawa M., Morady F., Kim H.M., Mathew S., Tilz R., Kuck K.-H., Nagashima K., Tedrow U., Stevenson W.G. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC (J Am Coll Cardiol): Clin Electrophysiol. 2015;1:116–123. doi: 10.1016/j.jacep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Ruwald M.H., Mittal S., Ruwald A.-C., Aktas M.K., Daubert J.P., McNitt S., Al-Ahmad A., Jons C., Kutyifa V., Steinberg J.S. Association between frequency of atrial and ventricular ectopic beats and biventricular pacing percentage and outcomes in patients with cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:971–981. doi: 10.1016/j.jacc.2014.06.1177. [DOI] [PubMed] [Google Scholar]
- 35.Bogun F., Crawford T., Chalfoun N., Kuhne M., Sarrazin J.F., Wells D., Good E., Jongnarangsin K., Oral H., Chugh A. Relationship of frequent postinfarction premature ventricular complexes to the reentry circuit of scar-related ventricular tachycardia. Heart Rhythm. 2008;5:367–374. doi: 10.1016/j.hrthm.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Lakkireddy D., Di Biase L., Ryschon K., Biria M., Swarup V., Reddy Y.M., Verma A., Bommana S., Burkhardt D., Dendi R. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J Am Coll Cardiol. 2012;60:1531–1539. doi: 10.1016/j.jacc.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 37.Haïssaguerre M., Shoda M., Jaïs P., Nogami A., Shah D.C., Kautzner J., Arentz T., Kalushe D., Lamaison D., Griffith M. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 38.Sadek M.M., Benhayon D., Sureddi R., Chik W., Santangeli P., Supple G.E., Hutchinson M.D., Bala R., Carballeira L., Zado E.S. Idiopathic ventricular arrhythmias originating from the moderator band: electrocardiographic characteristics and treatment by catheter ablation. Heart Rhythm. 2015;12:67–75. doi: 10.1016/j.hrthm.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Leenhardt A., Glaser E., Burguera M., Nürnberg M., Maison-Blanche P., Coumel P. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–215. doi: 10.1161/01.cir.89.1.206. [DOI] [PubMed] [Google Scholar]
- 40.Igarashi M., Tada H., Kurosaki K., Yamasaki H., Akiyama D., Sekiguchi Y., Kuroki K., Machino T., Murakoshi N., Nakata Y. Electrocardiographic determinants of the polymorphic QRS morphology in idiopathic right ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2012;23:521–526. doi: 10.1111/j.1540-8167.2011.02232.x. [DOI] [PubMed] [Google Scholar]
- 41.Li C.O., Franciosi S., Deyell M.W., Sanatani S. Intermediate-coupled premature ventricular complexes and ventricular tachycardia during exercise recovery. HeartRhythm Case Reports. 2021;7:127–130. doi: 10.1016/j.hrcr.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knecht S., Sacher F., Wright M., Hocini M., Nogami A., Arentz T., Petit B., Franck R., De Chillou C., Lamaison D. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol. 2009;54:522–528. doi: 10.1016/j.jacc.2009.03.065. [DOI] [PubMed] [Google Scholar]