Abstract
Tuberculous meningitis treatment outcomes are poor and alternative regimens are under investigation. Reliable methods to measure drug concentrations in cerebrospinal fluid are required to evaluate distribution into the cerebrospinal fluid. A simple and quick method was developed and validated to analyse linezolid in human cerebrospinal fluid. Samples were prepared by protein precipitation followed by isocratic liquid chromatography and tandem mass spectrometry. The run time was 3.5 min. Accuracy and precision were assessed in three independent validation batches with a calibration range of 0.100–20.0 μg/mL. The method was used to analyse cerebrospinal fluid samples from patients with tuberculous meningitis enrolled in a clinical trial. Potentially infective patient samples could be decontaminated using Nanosep® nylon and Costar® nylon filter tubes under biosafety level 3 conditions before analysis. The filtration process did not significantly affect the quantification of linezolid. Linezolid concentration in cerebrospinal fluid obtained from tuberculous meningitis patients ranged from 0.197 μg/mL to 15.0 μg/mL. The ratio between average CSF and plasma linezolid concentrations varied with time, reaching a maximum of 0.9 at 6 h after dosing.
Keywords: Linezolid, Cerebrospinal fluid, LC-MS/MS, TB-Meningitis
Highlights
-
•
Samples from tubercular meningitis patients decontaminated by filtration.
-
•
Non-specific adsorption of linezolid insignificant on nylon filter membranes.
-
•
Linezolid ranged from 0.197 μg/mL to 15.0 μg/mL in cerebrospinal fluid.
-
•
Linezolid cerebrospinal fluid to plasma concentration ratio varies with time.
1. Introduction
Tuberculous meningitis (TBM) is the most severe form of tuberculosis (TB) affecting the central nervous system (CNS) and surrounding structures. It occurs in approximately 1–2 % of all TB cases [[1], [2], [3]] and is associated with a high mortality and frequent disability [[3], [4], [5]]. Greater than 90 % of TBM deaths occur in the first three months [6], despite the use of anti-TB drugs.
Infection with the Human Immunodeficiency Virus (HIV) increases the likelihood of getting TBM and dying from it [5,7]. Currently recommended TBM treatment is the same as pulmonary TB treatment, differing only in duration. However, some drugs in the standard regimen penetrate poorly into the cerebrospinal fluid (CSF), a proxy for exposures at the site of disease in TBM, hence the need to investigate alternative drugs to improve outcomes. Linezolid is an oxazolidinone drug that has been repurposed for use in pulmonary TB. There is evidence of good linezolid CSF penetration among patients receiving 600 mg intravenously twice daily to treat CNS bacterial infections [[8], [9], [10], [11]]. In addition, the use of linezolid showed promising results in an observational study in children [12] and a retrospective cohort study in adults [13] with drug-sensitive TBM. Linezolid is being evaluated in several clinical trials for TBM and it is important to quantify CSF exposures to optimise dosing for patients with this condition.
Most published analytical methods for linezolid in biomatrices are in plasma. Methods for the quantification of linezolid in CSF have also been reported but most measured the drug using spectrophotometric detection [11,[14], [15], [16]]. The quantification of linezolid in CSF using Liquid chromatography – tandem mass spectrometry was reported by Yogev et al. (2010) [17] and Cazorla-Reyes et al. (2014) [18]. These methods were used to analyse CSF samples from patients treated for Gram positive infections. The current method was developed and validated to analyse CSF samples from a phase 2 clinical trial, called LASER-TBM, that evaluated the safety of intensified antituberculosis therapy, including high dose rifampicin plus linezolid 1200 mg daily, for adult patients with HIV-associated TBM [19]. Results from this study will contribute towards formulating improved treatment regimens for TBM.
2. Materials and methods
2.1. Chemicals and materials
Linezolid and linezolid-d3 were purchased from Toronto Research Chemicals (purity 98.0 % and 100 %, respectively). Acetonitrile, methanol, and dimethyl sulfoxide (DMSO) (HPLC grade) were purchased from Honeywell Burdick & Jackson™. Formic acid was purchased from Merck. LC-MS grade water was produced in-house using a Synergy S kit Millipore Water Purification System (Merck Millipore, Darmstadt, Germany). Pooled human CSF was purchased from BioIVT (West Sussex, United Kingdom). The collection and use of CSF from trial participants was approved and overseen by the University of Cape Town Department of Medicine Human Research Ethics Committee (125/2021). Written informed consent was obtained from all study participants according to the LASER-TBM study protocol. All procedures involving human subjects were conducted according to the Helsinki Declaration. Clinical trial sample quality and stability were ensured by storing at −80 °C within 1 h of collection and long term and transporting on dry ice in temperature-monitored containers.
2.2. Instrumentation
Liquid chromatography was carried out on an Agilent 1290 chromatography system. It comprised of an Agilent 1290 binary pump, an Agilent 1290 autosampler and an Agilent 1290 column oven. The LC system was coupled to an AB Sciex API 3200 triple quadrupole mass spectrometer for detection, through an electrospray ionization source. Chromatography was achieved using an Agilent Poroshell 120 EC C18, 4.6 mm × 50 mm, 2.7 μm column.
2.3. Method development and LC-MS/MS conditions
Optimization of compound specific mass spectrometer parameters was achieved by infusing 250 ng/mL solutions of linezolid and linezolid-d3 using a syringe pump (Harvard Scientific). Each compound was dissolved in a 1:1 (v/v) mixture of methanol and 0.1 % formic acid solution and infused at a flow rate of 10 μL/min. An isocratic method which was also used in the analysis of linezolid in plasma was chosen as it does not require column re-equilibration after each injection, thus reducing the run time. A 4:6 (v/v) mixture of mobile phases A and B allowed analyte elution within 2.4 min. The Agilent Poroshell 120 EC C18, 4.6 mm × 50 mm, 2.7 μm column produced symmetrical peaks and good signal-to-noise ratios. The presence of phospholipids in CSF and its potential interference with the quantification of linezolid was assessed by adding phospholipid transitions in the method and no phospholipids were detected. Ion source parameters (temperature, ion-spray voltage, gases) were optimized by flow injection analysis.
Chromatography was performed by applying an isocratic mobile phase consisting of a mixture of 0.1 % (v/v) formic acid solution and methanol (4:6, v/v) at a flow rate of 300 μL/min and run time of 3.5 min. The autosampler and column oven were set at 8 °C and 40 °C, respectively. Electrospray ionization in the positive mode was used as interface between the chromatographic system and the mass spectrometer. The ion source temperature was set at 500 °C, and the ion-spray voltage was 3000 V. The nebulizer gas, turbo gas and curtain gas were each set at 30 arbitrary units, while the collision gas was set at 8 arbitrary units. Ions were monitored in the multiple reaction monitoring mode. Monitored transitions for linezolid were 338.3 → 296.3 and 338.3 → 148.1 as quantifier and qualifier respectively. A deuterium-labelled analogue of linezolid (linezolid-d3) was used as the internal standard (ISTD) which was monitored with a transition of 341.1 → 297.1. Both compounds were monitored with a dwell time of 150 ms. For linezolid, the declustering potential, entrance potential, collision energy, and collision cell exit potential were: 81.0 V, 7.0 V, 25.0 eV, and 2.0 V, respectively. For linezolid-d3, these values were: 91.0 V, 8.0 V, 29 eV, and 2. V, respectively.
2.4. Preparation of stock and working solutions, calibration standards and quality controls
Stock solutions (1.00 mg/mL) of linezolid and linezolid-d3 were prepared by dissolving reference material in DMSO. These were stored in 1.5 mL polypropylene microcentrifuge tubes at −80 °C. Linezolid stock solutions were used to prepare working solutions by serial dilution with DMSO. Working solutions used to prepare calibration standards were diluted to the following concentrations: 2.00, 4.00, 10.0, 36.0, 90.0, 180, 300, and 400 μg/mL. Working solutions used to prepare quality control samples (QCs) were diluted to 2.00, 5.00, 160, and 320 μg/mL. Calibration standards and QCs were prepared by spiking 50 μL working solution into 950 μL blank CSF. Concentrations of calibration standards were 0.100, 0.200, 0.500, 1.80, 4.50, 9.00, 15.0, and 20.0 μg/mL. Concentrations of QCs were 0.100, 0.250, 8.00, and 16.0 μg/mL.
2.5. Extraction procedure
Twenty microlitres of calibration standard, QC, or clinical sample was mixed with 100 μL of acetonitrile, in which 0.100 μg/mL internal standard was dissolved. The mixture was centrifuged at 20 238×g for 5 min. A 100 μL aliquot of the supernatant was transferred to a 96-well plate before diluting with an equal volume of 0.1 % (v/v) formic acid solution. Two μL of the prepared sample was injected onto the chromatography system for analysis.
2.6. Validation
2.6.1. Linearity, accuracy, and precision
The assay was validated according to the Food and Drug Administration (FDA) Guidance for Bioanalytical Method Validation [20]. Calibration curves were constructed with duplicate points ranging from 0.100 to 20.0 μg/mL. The linear regression equations and correlation coefficients were obtained from the calibration standard/ISTD peak area ratios plotted against the respective CSF concentrations. Accuracy and precision were evaluated by intra- and inter-assay validation experiments. Three accuracy and precision batches were run over three days. Four QCs were used to assess accuracy and precision, namely: Lower Limit of Quantification (LLOQ), QC Low (QCL), QC Medium (QCM), and QC High (QCH). Six aliquots of each QC were analysed in each batch. Accuracy was calculated as the percentage of the calculated concentration relative to the nominal concentration and was acceptable if it was between 85 and 115 % or 80 and 120 % at the LLOQ. Precision was expressed as the percentage coefficient of variation (%CV) among the six replica of QCs and was acceptable if it was not more than 15 % or 20 % at the LLOQ.
2.6.2. Sensitivity and specificity
Six blank CSF samples from six different sources were extracted with and without ISTD. The same six lots were also extracted at the LLOQ level with and without ISTD. To measure sensitivity, chromatograms of LLOQs extracted with ISTD were qualitatively compared to those of blank CSF extracted with ISTD. The signal to noise ratio of peaks in the LLOQs was also measured. Specificity was assessed by comparing chromatograms of blank CSF, extracted with and without internal standard, with chromatograms of LLOQ samples extracted without internal standards. Any peaks in chromatograms of blank CSF at the linezolid retention time should not exceed 20 % of LLOQ peak area and any peaks in chromatograms of blank samples extracted without ISTD at the linezolid-d3 retention time should not exceed 5 % of the average ISTD peak areas in the blank samples extracted with ISTD.
2.6.3. Dilutions
To test whether samples that have concentrations above the upper limit of quantification may be diluted to within the calibration range and retested, samples were prepared at a concentration twice the upper limit of quantification (40 μg/mL). Six replicas of these samples were diluted 1:4 with blank CSF before extraction using the validated method. A dilution factor of 5 was applied to the analytical response and the calculated concentration compared to the nominal concentration. The accuracy should be within 15 % of the nominal concentration, and the coefficient of variation (precision) below 15 %.
2.6.4. The effect of decontamination of CSF
Potentially infected CSF samples from patients with TBM should be routinely decontaminated. Samples were decontaminated by filtration in filter tubes through membranes with pore diameters of 0.2 μm, made of cellulose acetate (Costar Spin-X®) or nylon (Costar Spin-X® and Nanosep®). To test the possible influence this process may have on the analysis of linezolid in the CSF, three different tube types were assessed. Samples were aliquoted into the filter chamber of the filter tubes and filtered by centrifugation for 5 min at 20 238×g. The filtrate was transferred to a second filter tube and filtered again for 5 min at 20 238×g. The analytical response of unfiltered samples was used as reference against which the response of filtered samples was compared. Reference and filtered samples were analysed in six replicates. Analyte/ISTD peak area ratios of filtered QCH and QCL were compared to those of unfiltered QCH and QCL. The %Difference was acceptable if it was less than 15 %.
2.6.5. Effect of concomitant medication
Medications that were co-administered in the LASER-TBM clinical trial, or those expected to be co-administered with linezolid for TB treatment, were assessed for their potential to interfere with the analysis of linezolid. Samples at QCH and QCL concentrations were prepared using CSF containing the following drugs: isoniazid, rifampicin, pyrazinamide, ethambutol, aspirin, dexamethasone, dolutegravir, lamivudine, emtricitabine, efavirenz, tenofovir, zidovudine, abacavir, omeprazole, and pyridoxine. The analytical responses of QCs prepared with CSF containing concomitant medications were compared to the responses of QCs prepared with blank CSF. The %Difference should be less than 15 %.
2.6.6. Carryover and crosstalk
Carryover was assessed by injecting a blank CSF sample extracted without ISTD immediately after the highest calibration standard. A peak at the linezolid retention time in the resulting chromatogram was regarded as carryover, and acceptable if the peak area was less than 20 % of the LLOQ peak area. Crosstalk was assessed by monitoring the linezolid response when blank CSF extracted with ISTD was injected, and by monitoring the ISTD response when CSF with linezolid at the upper limit of quantification was extracted without ISTD. Crosstalk was acceptable if the peak area of linezolid from the blank CSF sample was less than 20 % of the LLOQ peak area. In addition, the linezolid-d3 peak area in the sample with linezolid at the upper limit of quantification extracted without ISTD was acceptable if it was less than 5 % of the average ISTD response.
2.6.7. Matrix effects
The matrix effect was evaluated using the method first described by Matuszewski which attempts to quantify matrix effects across the calibration range [21]. Six blank CSF samples from different sources were extracted without internal standard. For each extracted CSF source, the analyte was added to achieve theoretical final concentrations of QCL, QCM, and QCH and the internal standard was added at a uniform concentration. The analyte/ISTD peak area ratios of each CSF source at QCL, QCM, and QCH, were plotted against the nominal concentrations to produce a simple linear regression. Variability of the slopes of regressions from the six CSF sources was used to estimate matrix effects. The acceptable variability was a coefficient of variation less than 5 %.
2.6.8. Recovery and process efficiency
Recovery was determined by comparing analytical responses of six CSF samples from different sources, spiked with the analyte before extraction (pre-extraction spiked), with responses of the same six CSF samples, extracted blank and spiked with the analyte post-extraction (post-extraction spiked). Process efficiency was determined by comparing pre-extraction spiked samples with neat solutions of analyte in a 4:1 (v/v) mixture of 0.1 % (v/v) formic acid in water and acetonitrile. Post-extraction spiked samples and neat solutions were spiked to the post-extraction theoretical concentration. This was done for QCL, QCM, and QCH concentrations. Recovery was expressed as the percentage of pre-extraction spiked average response relative to post-extraction spiked average response. Process efficiency was expressed as the percentage of pre-extraction spiked average response relative to the average response of neat solutions. The variability of recovery and process efficiency across the concentration levels was acceptable if the coefficient of variation was less than 15 %.
2.6.9. Stability
Linezolid stability in stock and working solutions was assessed at room temperature, 4 °C, −20 °C, and −80 °C. Working solution stability was assessed at the highest and lowest working solution concentrations. Analytical responses of test solutions were compared to those of fresh solutions.
Linezolid stability in CSF was assessed at room temperature and −80 °C. Stability in CSF was tested by quantifying linezolid in test samples using freshly prepared calibration standards and comparing the calculated concentration to the nominal concentration.
To assess the effect of using previously frozen samples on the accuracy and precision, frozen QCH and QCL samples were thawed and analysed with freshly prepared calibration standards. In addition, the effect of repeated freezing and thawing of samples on linezolid stability was assessed by subjecting QCL and QCH samples to five freeze-thaw cycles and testing the accuracy and precision using fresh calibration standards.
Stability of linezolid in extracted samples was assessed by leaving extracted samples that were injected in the first validation run, in the autosampler for approximately 147 h before re-injecting them. Accuracy and precision of the reinjection were assessed. Additionally, peak area ratios of analyte/ISTD of the reinjection were compared to those of the first run.
All stability experiments were conducted using six replicates of test and reference samples.
2.7. Statistical analysis
Analytical data acquisition, processing and analysis were performed using Analyst® software version 1.6.3. Results of linezolid concentrations in patient samples were analysed using R statistical software version 4.2.2.
2.8. Clinical application
Adults with HIV-associated TBM were recruited from several South African hospitals according to the LASER-TBM clinical study protocol [22]. Plasma and lumber CSF samples were collected on day 3 and 28 after trial enrolment and initiation of linezolid therapy. All CSF samples were obtained between 2- and 7- hours post-dose. Each CSF sample was drawn at approximately the same time as one of the plasma samples for each trial participant. Plasma samples were analysed according to a previously validated, unpublished method. Summary statistics for CSF concentrations and CSF to plasma ratios were calculated. The correlation between CSF and plasma linezolid concentrations was assessed.
3. Results and discussion
3.1. Mass spectra and chromatograms
We developed a simple and rapid method which required simple protein precipitation for sample pre-treatment and a 3.5-min isocratic chromatographic process. Mass spectra of linezolid and linezolid-d3 produced after infusion of the compounds are shown in Fig. 1. The mass spectra show the proposed collision-induced fragmentation of the linezolid and linezolid-d3 precursor ions, to the quantifier ions. The most abundant product ions were chosen for quantifying the analyte. The isocratic chromatography method was adapted from a previously validated, unpublished method that was used to analyse linezolid in plasma samples. Assessment of potential phospholipid interference was done using phospholipid transitions previously used in our laboratory [23]. Phospholipids were not detected, and this was not surprising since phospholipid concentrations in CSF are relatively low, and the mass spectrometer used in this method was not sensitive enough to detect them. We speculated that the low phospholipid concentrations would not interfere with quantification of the analyte since the analyte concentrations were much higher. This method is shorter than other published methods for quantifying linezolid and allows high throughput analysis. Typical chromatograms of blank, LLOQ and representative patient samples are presented in Fig. 2.
Fig. 1.
Product ion mass spectra and proposed fragmentation of linezolid and linezolid-d3.
Fig. 2.
Typical chromatograms of a blank, LLOQ and a representative patient sample.
3.2. Validation results
3.2.1. Linearity, accuracy, and precision
The method was accurate and consistent, with a linear calibration curve ranging from 0.100 to 20.0 μg/mL. Correlation between peak area ratios and concentration was characterised by a quadratic regression, weighted by 1/x, where x = linezolid concentration. The correlation coefficient (r) was greater than 0.999 in all three validation runs. Accuracy (%Nominal) of QCs ranged from 97.8 to 100.4 and the coefficient of variation ranged from 3.0 to 5.5 % in the three validation runs. A summary of accuracy and precision results is presented in Table 1.
Table 1.
Summary of accuracy and precision of quality control samples from three validation runs, N = 6 for each day and N = 18 for inter-day.
| QC | Nominal concentration (μg/mL) | Mean observed concentration (μg/mL) | %CV | %Accuracy | |
|---|---|---|---|---|---|
| Day 1 | LLOQ | 0.100 | 0.0950 | 5.5 | 95.0 |
| QCL | 0.250 | 0.240 | 0.4 | 96.2 | |
| QCM | 8.00 | 7.94 | 3.2 | 99.3 | |
| QCH | 16.0 | 15.9 | 4.0 | 99.4 | |
| Day 2 | LLOQ | 0.100 | 0.100 | 4.4 | 99.6 |
| QCL | 0.250 | 0.251 | 2.0 | 100.4 | |
| QCM | 8.00 | 7.94 | 3.3 | 99.3 | |
| QCH | 16.0 | 16.5 | 2.6 | 103.1 | |
| Day 3 | LLOQ | 0.100 | 0.101 | 3.0 | 101.1 |
| QCL | 0.250 | 0.242 | 3.7 | 96.7 | |
| QCM | 8.00 | 8.12 | 3.4 | 101.5 | |
| QCH | 16.0 | 15.8 | 3.1 | 98.7 | |
| Inter-day | LLOQ | 0.100 | 0.0985 | 4.9 | 98.5 |
| QCL | 0.250 | 0.244 | 3.0 | 97.8 | |
| QCM | 8.00 | 8.00 | 3.3 | 100.1 | |
| QCH | 16.0 | 16.1 | 3.7 | 100.4 |
3.2.2. Sensitivity and specificity
The method was sensitive, allowing reliable quantitation using a sample volume of 20 μL. The average signal to noise ratio of six chromatograms produced when samples from six different sources spiked at the LLOQ (0.100 μg/mL) were injected was 32.3. Fig. 3 shows a representative chromatogram of an LLOQ sample in blue, overlaid with a blank sample that was extracted with internal standard in red.
Fig. 3.
Representative overlay of LLOQ and blank chromatograms. The LLOQ is shown in blue and the blank in red, monitored for the linezolid mass transition m/z 338.2 → 296.3.
Other methods for quantifying linezolid in CSF have used at least 100 μL samples [11,[16], [17], [18]]. Smaller sample volumes are particularly advantageous in neonates and pre-term babies, if CSF samples must be drawn for pharmacokinetic analysis.
To demonstrate specificity, six different lots of blank CSF samples extracted without internal standards showed no analyte or internal standard peaks in the chromatograms. In addition, the same lots of blank CSF extracted with internal standard showed peaks only for the internal standard and no interference peaks in the analyte channel.
3.2.3. Dilutions
Samples above the upper limit of quantification up to 40 μg/mL could be diluted with blank CSF by a factor of five and be accurately and consistently analysed. The accuracy of six replicas of samples at 40 μg/mL was 99.1 % and the coefficient of variation was 3.3 %.
3.2.4. Decontamination of CSF potentially containing M. tuberculosis
Linezolid concentrations in CSF have been measured mostly in patients with confirmed or suspected gram-positive CNS infections [8,9,11,16,17,[26], [27], [28]]. These measurements are scarce in patients with mycobacterial CNS infections. Handling CSF from patients with CNS TB poses a biohazard as samples may carry live bacilli which can easily be transmitted by inhalation, in the unlikely event that aerosols are generated in processes such as vortex mixing and pipetting. According to international classification, M. tuberculosis is a Risk Group 3 pathogen and manipulation of samples suspected of containing it should be conducted in a biosafety level 3 (BSL-3) facility. The current method validated the pre-filtration of samples using filters with 0.2 μm pore diameters for decontamination. Validation was necessary to ensure that the analyte was not lost due to non-specific adsorption to the filter. The percentage difference between filtered and non-filtered quality controls was less than 15 % for samples filtered using nylon membranes from two different suppliers and was slightly above 15 % for samples filtered with the cellulose acetate membrane. The Nanosep® nylon membrane showed the least loss of analyte. Detailed results are presented in Table 2.
Table 2.
Percentage differences in peak area ratios between samples filtered using various filters and unfiltered samples.
| %Difference against Reference |
||
|---|---|---|
| QCH | QCL | |
| Nanosep® nylon | −0.9 | −4.7 |
| Costar Spin-X® nylon | −8.6 | −11.6 |
| Costar Spin-X® cellulose acetate | −14.3 | −16.8 |
The cellulose acetate filter was found to cause analyte loss exceeding the acceptable limit of 15 % (16.8 %) at QCL. Therefore, only the nylon filters could be used.
Heat treatment is an alternative decontamination method. Linezolid was stable after two heat treatment cycles at 56 °C for 45 min in a study by Tobin et al. (2001) [29]. However, heat treatment is more time consuming and requires the installation of additional heating equipment in the Biosafety Level-3 facility.
3.2.5. Concomitant medications
QCL and QCH samples prepared with CSF spiked with concomitant medications showed no significant difference from those prepared with blank CSF. Percentage differences of −3.2 % and −5.3 % were observed with coefficient of variation of 4.3 % and 4.1 % for the High and Low QCs, respectively. Additionally, blank CSF samples containing concomitant medications did not show interfering peaks for the analyte and internal standard. Therefore, the concomitant medications tested did not interfere with the performance of the assay.
3.2.6. Carryover and crosstalk
No analyte or internal standard peaks were observed when blank samples extracted without internal standard were injected following samples with high linezolid concentrations; therefore, carryover was negligible. No interfering peaks were observed in the analyte channel for the blank sample from three injections of a sample containing ISTD. Small interfering peaks (0.6 % of the mean ISTD response in the blank sample extracted with ISTD) were observed in the ISTD channel when samples with linezolid at the upper limit of quantification extracted without ISTD were injected.
3.2.7. Matrix effects
The coefficient of variation of slopes of six regressions constructed from injecting CSF from six different sources spiked post-extraction with analyte at three concentration levels and ISTD was 2.1 %, as shown in Table 3. This showed that the matrix effects did not differ significantly among CSF samples from different sources. Matrix effects did not affect the reliability of the assay; use of different CSF sources to prepare calibration standards does not have an adverse effect on the accurate quantification of linezolid. Matrix-matched calibration standards and QCs may have been used in some published methods, but these used spectrophotometric detection. Examples include Luque et al. (2014) and Myrianthefs et al. (2006) [11,26]. Both methods were only briefly described, and validation details were not reported.
Table 3.
Regression results for six different CSF sources spiked with linezolid.
| High Concentration (16.0 μg/mL) Peak Area Ratio | Medium Concentration (8.00 μg/mL) Peak Area Ratio | Low Concentration (0.250 μg/mL) Peak Area Ratio | Area Ratio vs. Conc. Regression Slope | |
|---|---|---|---|---|
| Matrix lot A | 12.8 | 6.50 | 0.216 | 0.797 |
| Matrix lot B | 13.1 | 6.78 | 0.219 | 0.816 |
| Matrix lot C | 13.0 | 6.68 | 0.207 | 0.813 |
| Matrix lot D | 13.0 | 6.55 | 0.210 | 0.809 |
| Matrix lot E | 13.5 | 6.66 | 0.215 | 0.847 |
| Matrix lot F | 12.9 | 6.95 | 0.210 | 0.803 |
| Average | 13.0 | 6.69 | 0.213 | 0.814 |
| STDEV | 0.270 | 0.165 | 0.00434 | 0.0172 |
| CV(%) | 2.1 | 2.5 | 2.0 | 2.1 |
3.2.8. Recovery and process efficiency
The %Recovery of linezolid was 81.1 %, 80.0 %, and 80.0 % for QCH, QCM, and QCL, respectively. The average recovery over three concentration levels was 80.4 % with a coefficient of variation of 0.8 %. The process efficiency was 89.3 %, 99.2 %, and 93.3 %, and the average process efficiency was 93.9 with a coefficient of variation of 5.3 %.
3.2.9. Stability
Stability was demonstrated in solution in DMSO and in CSF. A linezolid solution at 1 mg/mL in DMSO was stable at room temperature, 4 °C, and −20 °C for 24 h, and at −80 °C for 87 days. Working solutions in DMSO tested at 2.00 and 800 μg/mL were stable at room temperature, 4 °C, and −20 °C for 24 h, and at −80 °C for 85 days. In CSF, linezolid was stable at room temperature for 4 h, at −80 °C for 110 days and after freezing and thawing five times. Extracted samples were stable in the autosampler at 8 °C for 116 h. No stability concerns were observed under all tested conditions, in agreement with linezolid stability widely reported in plasma and solutions.
3.3. Clinical application
CSF samples were collected from 22 trial participants, who provided a total of 28 CSF samples for analysis. Linezolid concentrations were above the LLOQ for 21 samples; median 4.52 μg/mL, range 0.197 μg/mL to 15.0 μg/mL. This range is similar to ranges that have been reported when linezolid was used for prophylaxis or treatment of gram-positive infections as twice daily 600 mg intravenous doses [11,16,17]. Linezolid concentrations in CSF were higher at day 3 of treatment than at day 28 (Table 4). Potential explanations include dynamic CSF protein concentrations, with higher concentrations earlier in the illness influencing drug exposure [30] or an induction effect from use of concomitant rifampicin.
Table 4.
Summary statistics of linezolid concentration (μg/mL) in CSF samples.
| Day | Number of samples | Mean μg/mL | Standard deviation | Median | Minimum | Maximum | Interquartile range |
|---|---|---|---|---|---|---|---|
| 3 | 15 | 5.76 | 4.85 | 4.70 | 0.266 | 15 | 6.46 |
| 28 | 6 | 3.96 | 4.12 | 2.74 | 0.197 | 10.7 | 5.22 |
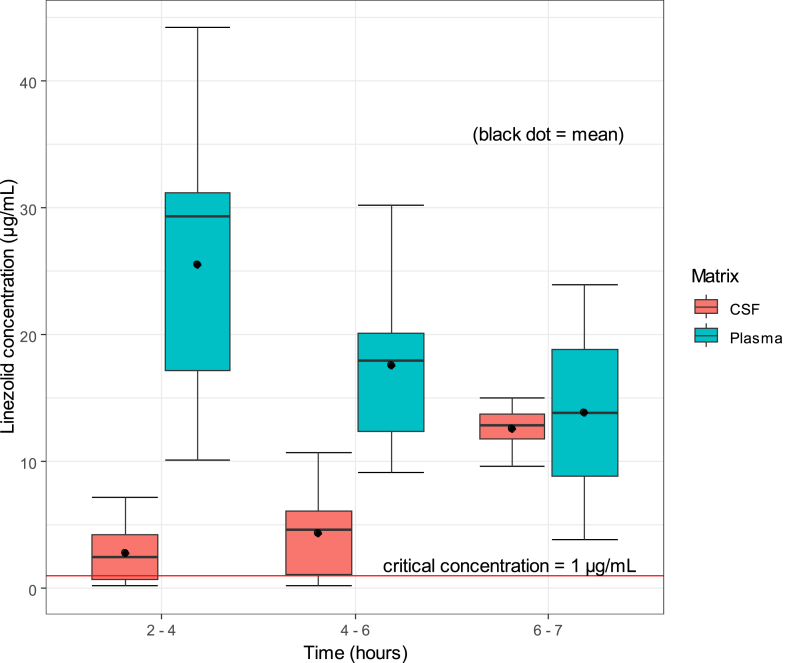
While linezolid concentrations peaked between two and 4 h in plasma, concentrations in CSF only peaked after 6 h, indicating a delay in equilibration between plasma and CSF. The ratio between average CSF and plasma linezolid concentrations varied at different time points post dose; 0.1 between two and 4 h, 0.2 between four and 6 h, and 0.9 between six and 7 h. Although linezolid concentrations in CSF were lower than in plasma at earlier timepoints, they mostly exceeded the critical concentration of 1 μg/mL against M. tuberculosis (Fig. 4). Results of safety, tolerability, and efficacy of the linezolid-containing regimen were reported elsewhere.
Fig. 4.
Linezolid concentrations in CSF and plasma at various time ranges post dose.
3.4. Comparison with other published methods
Although linezolid concentrations in CSF have been widely reported in literature, in patients treated for gram-positive bacterial infections, the methods used for quantification have been less widely reported [24,25]. Many of the methods used ultraviolet–visible (UV–vis) spectrophotometry for detection, which is less sensitive than the method described in this article. To the knowledge of the authors, none of the published methods reported the use of filter tubes to decontaminate samples that were potentially infected with bacteria, and the evaluation of non-specific adsorption of linezolid in CSF to filter membranes.
LC-MS/MS was used in a study on plasma and CSF pharmacokinetics of linezolid in paediatric patients with hydrocephaly [17]. The use of isocratic chromatography and linezolid-d3 as internal standard as well as the transitions used for linezolid and linezolid-d3 were the same as our current method. However, analyte ionization was achieved using atmospheric pressure chemical ionization in contrast to the more sensitive electrospray ionization we used. In addition, they used liquid-liquid extraction for sample pre-treatment, which is more laborious and time consuming than the protein precipitation used in this method. It is unclear whether their calibration standards and quality controls were matrix-matched for CSF samples, as much of the validation details were not described.
Linezolid in CSF was also analysed using ultra-high-performance LC-MS/MS along with twenty other antibacterial drugs [18]. The method used a simple dilution of the CSF samples prior to analysis using a 6-min gradient chromatography method, unlike the isocratic elution used in the current study. The authors did not state the calibration range and the method was not used to analyse linezolid in clinical samples.
A limitation of this method is that it measured the concentrations of a single analyte. The specificity of LC-MS/MS analysis allows for the simultaneous analysis of multiple drugs in the same matrix, however in this study the emphasis is on the investigational stage of the use of linezolid in TBM. Applying this analytical method will result in better understanding of the effect of linezolid but can easily be adapted to analyse multiple drugs to study combination drug treatment of TBM as new regimens become defined.
4. Conclusion
A simple and rapid method was developed and validated for the quantification of linezolid in CSF. This method was used to analyse CSF samples from patients with TB meningitis. The method was accurate, precise, and reproducible, and could be conducted partly under biosafety level 3 conditions to analyse samples potentially infected with M. tuberculosis. Linezolid was stable in solution in DMSO and in CSF.
Data availability statement
Data associated with this study has not been deposited into a publicly available repository. Data will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Marian Mazanhanga: Writing – original draft, Validation, Methodology, Formal analysis, Data curation. Anton Joubert: Writing – review & editing, Supervision, Methodology. Sandra Castel: Writing – review & editing, Supervision, Project administration. Marthinus Van de Merwe: Writing – review & editing, Supervision. Gary Maartens: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Sean Wasserman: Writing – review & editing, Resources, Investigation, Funding acquisition, Conceptualization. Lubbe Wiesner: Writing – review & editing, Visualization, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The University of Cape Town Clinical PK Laboratory is supported in part via the Adult Clinical Trial Group (ACTG), by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701; as well as the Infant Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT), funding provided by National Institute of Allergy and Infectious Diseases (U01 AI068632), The Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of Mental Health grant AI068632. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. SW is supported by the National Institutes of Health (K43TW011421 and U01AI170426). For the purposes of open access, the authors have applied a CC-BY public copyright to any author-accepted manuscript arising from this submission.
References
- 1.Dodd P.J., et al. The global burden of tuberculous meningitis in adults: a modelling study. PLOS Glob Public Health. 2021;1(12) doi: 10.1371/journal.pgph.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducomble T., et al. The burden of extrapulmonary and meningitis tuberculosis: an investigation of national surveillance data, Germany, 2002 to 2009. Euro Surveill. 2013;18(12) [PubMed] [Google Scholar]
- 3.Phypers M., Harris T., Power C. CNS tuberculosis: a longitudinal analysis of epidemiological and clinical features. Int. J. Tubercul. Lung Dis. 2006;10(1):99–103. [PubMed] [Google Scholar]
- 4.Davis A.G., et al. Cognitive impairment in tuberculous meningitis. Clin. Infect. Dis. 2023;76(5):842–849. doi: 10.1093/cid/ciac831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson R.J., et al. Tuberculous meningitis. Nat. Rev. Neurol. 2017;13(10):581–598. doi: 10.1038/nrneurol.2017.120. [DOI] [PubMed] [Google Scholar]
- 6.Stadelman A.M., et al. Treatment outcomes in adult tuberculous meningitis: a systematic review and meta-analysis. Open Forum Infect. Dis. 2020;7(8) doi: 10.1093/ofid/ofaa257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonyagars L., Sangketchon C., Pholtawornkulchai K. Presentation, clinical characteristics, and treatment outcomes among tuberculous meningitis patients with and without HIV infection at vajira hospital, Thailand: a retrospective cohort study. J. Int. Assoc. Phys. AIDS Care. 2021;20 doi: 10.1177/23259582211045551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auriti C., et al. Staphylococcal meningitis therapy with linezolid in a young infant: efficacy, CSF levels and side effects. Ital. J. Pediatr. 2020;46(1):90. doi: 10.1186/s13052-020-00854-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beer R., et al. Pharmacokinetics of intravenous linezolid in cerebrospinal fluid and plasma in neurointensive care patients with staphylococcal ventriculitis associated with external ventricular drains. Antimicrob. Agents Chemother. 2007;51(1):379–382. doi: 10.1128/AAC.00515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boak L.M., et al. Successful treatment and cerebrospinal fluid penetration of oral linezolid in a patient with coagulase-negative Staphylococcus ventriculitis. Ann. Pharmacother. 2006;40(7–8):1451–1455. doi: 10.1345/aph.1H029. [DOI] [PubMed] [Google Scholar]
- 11.Luque S., et al. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int. J. Antimicrob. Agents. 2014;44(5):409–415. doi: 10.1016/j.ijantimicag.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Li H., et al. Linezolid is associated with improved early outcomes of childhood tuberculous meningitis. Pediatr. Infect. Dis. J. 2016;35(6):607–610. doi: 10.1097/INF.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 13.Sun F., et al. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis. Antimicrob. Agents Chemother. 2014;58(10):6297–6301. doi: 10.1128/AAC.02784-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Günther S., et al. Therapeutic drug monitoring of linezolid: HPLC-based assays for routine quantification of linezolid in human serum and cerebrospinal fluid. Eur J Hosp Pharm. 2023;30(6):353–358. doi: 10.1136/ejhpharm-2021-003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y., et al. Pharmacokinetics of linezolid in plasma and cerebrospinal fluid in patients with cerebral hemorrhage post-surgical intervention. Eur. J. Clin. Pharmacol. 2017;73(7):919–921. doi: 10.1007/s00228-017-2239-x. [DOI] [PubMed] [Google Scholar]
- 16.Tsona A., et al. Linezolid penetration into cerebrospinal fluid and brain tissue. J. Chemother. 2010;22(1):17–19. doi: 10.1179/joc.2010.22.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Yogev R., et al. Pharmacokinetics and distribution of linezolid in cerebrospinal fluid in children and adolescents. Pediatr. Infect. Dis. J. 2010;29(9):827–830. doi: 10.1097/INF.0b013e3181df4b9a. [DOI] [PubMed] [Google Scholar]
- 18.Cazorla-Reyes R., et al. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014;89:203–212. doi: 10.1016/j.jpba.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Davis A.G., et al. A phase 2A trial of the safety and tolerability of increased dose rifampicin and adjunctive linezolid, with or without aspirin, for human immunodeficiency virus–associated tuberculous meningitis: the LASER-TBM trial. Clin. Infect. Dis. 2022;76(8):1412–1422. doi: 10.1093/cid/ciac932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA . FDA; Maryland: 2018. Bioanalytical Method Validation Guidance for Industry 2018, U.S.D.o.H.a.H. Services. Editor. [Google Scholar]
- 21.Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC−MS/MS. Anal. Chem. 2003;75(13):3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 22.Davis A.G., et al. Study protocol for a phase 2A trial of the safety and tolerability of increased dose rifampicin and adjunctive linezolid, with or without aspirin, for HIV-associated tuberculous meningitis [LASER-TBM] Wellcome Open Res. 2021;6:136. doi: 10.12688/wellcomeopenres.16783.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazanhanga M.T., et al. Liquid chromatography-tandem mass spectrometry analysis of delamanid and its metabolite in human cerebrospinal fluid using protein precipitation and on-line solid-phase extraction. J. Pharmaceut. Biomed. Anal. 2023;227 doi: 10.1016/j.jpba.2023.115281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes G., Salgado H.R.N., Santos J.L.D. A critical review of HPLC-based analytical methods for quantification of Linezolid. Crit. Rev. Anal. Chem. 2020;50(3):196–211. doi: 10.1080/10408347.2019.1605876. [DOI] [PubMed] [Google Scholar]
- 25.Kokilambigai K.S., et al. Linezolid-A review of analytical methods in pharmaceuticals and biological matrices. Crit. Rev. Anal. Chem. 2020;50(2):179–188. doi: 10.1080/10408347.2019.1599709. [DOI] [PubMed] [Google Scholar]
- 26.Myrianthefs P., et al. Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob. Agents Chemother. 2006;50(12):3971–3976. doi: 10.1128/AAC.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji Y., et al. Pharmacokinetics and protein binding of linezolid in cerebrospinal fluid and serum in a case of post-neurosurgical bacterial meningitis. Scand. J. Infect. Dis. 2011;43(11–12):982–985. doi: 10.3109/00365548.2011.600327. [DOI] [PubMed] [Google Scholar]
- 28.Villani P., et al. Cerebrospinal fluid linezolid concentrations in postneurosurgical central nervous system infections. Antimicrob. Agents Chemother. 2002;46(3):936–937. doi: 10.1128/AAC.46.3.936-937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobin C.M., et al. A simple, isocratic high-performance liquid chromatography assay for linezolid in human serum. J. Antimicrob. Chemother. 2001;48(5):605–608. doi: 10.1093/jac/48.5.605. [DOI] [PubMed] [Google Scholar]
- 30.Abdelgawad N., et al. medRxiv; 2023. Linezolid Population Pharmacokinetic Model in Plasma and Cerebrospinal Fluid Among Patients with Tuberculosis Meningitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has not been deposited into a publicly available repository. Data will be made available on request.