Abstract
Use of montelukast, as a cause of neuropsychiatric events, in patients with asthma or allergic rhinitis is controversial, and comprehensive statistical analyses verifying this relationship remain lacking. To better understand the relationship between montelukast and neuropsychiatric events, it is vital to guide patients in the effective use of the drug, especially in children whose mothers are concerned about its side effects. In this study, randomized controlled trials (RCTs) investigating montelukast and neuropsychiatric events were retrieved from a literature search of the Medline (1966 to February 2023), Embase (1974 to February 2023), Web of Science, and other related databases. After screening, 18 RCTs were ultimately included in a meta-analysis to merge statistics, which demonstrated no significant increase in neuropsychiatric events compared with placebo. A similar pattern of adverse neuropsychiatric events was observed in patients grouped according to age, with headache being the most common adverse neuropsychiatric event. Overall, montelukast did not significantly increase neuropsychiatric events in patients with allergic rhinitis and/or asthma compared with placebo. Large-sample RCTs are needed to verify the association between neuropsychiatric events and montelukast use in children, and attention is also devoted to FDA warnings.
Keywords: Allergic airway disease, Montelukast, Neuropsychiatric event, Children, Allergic rhinitis, Asthma
1. Introduction
As a widely used leukotriene receptor antagonist, montelukast can alleviate the symptoms of allergic diseases, including asthma and allergic rhinitis (AR), and improve patient prognosis by reducing the production of inflammatory mediators [[1], [2], [3]]. Moreover, montelukast can be administered to both adults and children. However, with its widespread use, neuropsychiatric events (NEs) in patients receiving montelukast have significantly increased [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]].
Considering pharmacovigilance studies [[4], [5], [6],8,[11], [12], [13], [14]] and case reports [7,9,10] addressing neuropsychiatric adverse reactions, the United States Food and Drug Administration (FDA) has added a warning about NEs to the montelukast label. Before prescribing montelukast, patient condition should be comprehensively assessed, including whether the patient has depression, headache, anxiety, insomnia, aggression, suicidal ideation, or behavioral neuropsychiatric adverse reactions [15,16]. In 2020, the FDA issued the following black box warning: “limit the use of allergic rhinitis unless the patient is irresponsive to or unable to tolerate other clinical drugs and comprehensively consider asthma patients before using montelukast” [17]. This has raised serious concerns about NEs among mothers, especially those whose children have allergic diseases, such as asthma and/or AR.
The relationship between NEs and montelukast currently remains controversial. Some studies have reported that patients using montelukast experience a high incidence of NEs, whereas others have concluded that the occurrence of NEs is not related to montelukast [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. In addition, statistical analyses of the relationship between montelukast and NEs remain lacking [18]. As such, this study aimed to clarify the relationship between montelukast and the occurrence of NEs in adults and children with asthma and AR through a meta-analysis of high-quality randomized controlled trials (RCTs).
2. Materials and methods
Registration for the current project was completed on the INPLASY platform after the search (INPLASY202310051).
2.1. Search strategy
To analyze the possibility of NEs, a comprehensive survey of patients with AR or asthma who received montelukast was performed. A literature search of the Medline (1966 to February 2023), Embase (1974 to February 2023), and Web of Science databases for studies investigating montelukast and neuropsychiatric events was performed. Key words used in the literature search included: ((((((((montelukast) OR (MK 0476)) OR (MK-0476)) OR (Singulair)) OR (montelukast sodium)) OR (sodium 1-(((1-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropylacetate))) OR (leukotriene receptor antagonists) AND ((((((((asthma) OR (Asthmas)) OR (Bronchial Asthma)) OR (Asthma, Bronchial)) OR (Rhinitis, Allergic)) OR (Allergic Rhinitides)) OR (Rhinitides, Allergic)) OR (Allergic Rhinitis))) AND ((((((randomized controlled trial) OR (randomized)) OR (placebo)) OR (random and allocation)) OR (random allocation)) OR (randomized and controlled and trial)). In addition, the reference lists of the retrieved studies were manually searched for additional, potentially eligible, relevant studies to ensure comprehensive inclusion.
2.2. Inclusion and exclusion criteria
Studies were included according to the “PICOS” criteria, as follows: population of interest (P), patients with AR and/or asthma; intervention (I), patients using montelukast; comparison (C), placebo group(s) included as comparators; outcomes of interest (O), adverse drug reactions (ADRs) of montelukast; and study design (S), RCTs.
Studies involving patients with other diseases, those for which full text versions were not available online, those not published in English, and those that did not report available data for analysis were excluded.
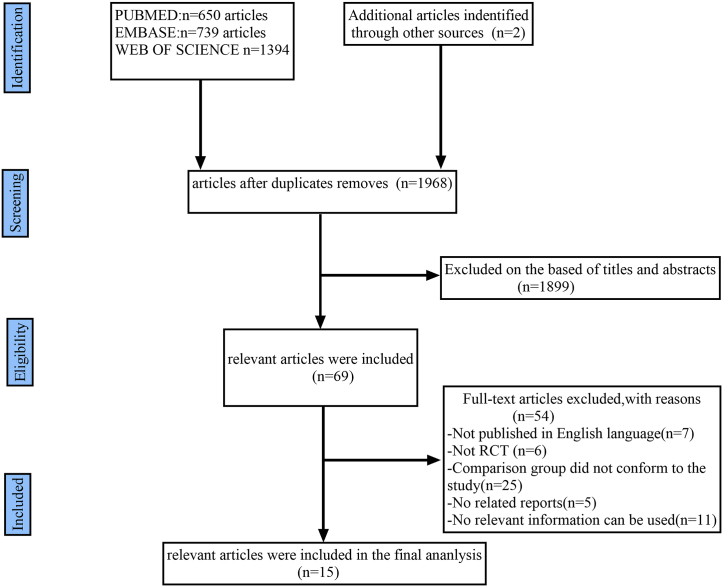
A flow-diagram illustrating the study inclusion process is presented in Fig. 1.
Fig. 1.
PRISMA flow diagram demonstrating the progress of study evaluation throughout the review.
2.3. Quality assessment
The quality of the included studies was assessed using the Jadad Scale [19]. Each study was assessed by certain methods (method of patient random allocation, concealment of allocation, blinding, and data loss to follow). The Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [20] was used to grade individual studies. Each study was assigned a category, as follows: A (low risk of bias, study met almost all criteria); B (moderate risk of bias, study met part or was unclear for ≥1 quality criteria); and C (high risk of bias, study did not meet or included the criteria). Differences were resolved through discussion among the authors.
2.4. Data extraction
Measurable data were independently extracted and summarized by two researchers. The following data were extracted from the included studies: first author's name; publication year; names of diseases and type(s) of design; sample size(s) and patient age; therapy patients in the experimental group received; number and type of NE(s) occurrences; and follow-up period.
2.5. Outcome measures
The outcome measures are described in Appendix A.
2.6. Statistical analysis
Statistical analysis was performed using STATA version 12.0 (StataCorp LLC, College Station, TX, USA). Fixed- or random-effects models were used to combine statistics. The relative risk (RR) with corresponding 95 % confidence interval (CI) was used to estimate dichotomous outcomes. The I2 test and χ2-based Q statistics were used to assess study heterogeneity. Studies with an I2 value > 50 % or p < 0.1, were considered to have significant heterogeneity, and the fixed-effects model was used; otherwise, a random effects model was used. Differences and associations with P < 0.05 were considered to be statistically significant.
3. Results
3.1. Characteristics of the included studies and participants
The initial literature search retrieved 2785 studies from the databases and reference lists of retrieved studies. After screening, 15 studies were ultimately included [1,[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]] containing 18 RCTs for the meta-analysis after reading the full text. The screening process and characteristics of each study are reported in Fig. 1 and Table 1, respectively.
Table 1.
Characteristics of the included studies.
| Study | Design | Disease | Age (Range, years) | Therapy in experimental group |
Therapy in control group | Dosage | Sample size |
NEa sample size |
NEa type and sample size | Follow up period (wk) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | |||||||||
| Leff 1998 |
RCTb | asthma | 15–45 years | Montelukast | Placebo | 10 mg | 54 | 56 | 11 | 18 | Headache11/18 | 12 |
| Knorr 1998 |
RCTb | asthma | 6- 14-years | Montelukast | Placebo | 5 mg | 201 | 135 | 38 | 29 | Headache38/29 | 8 |
| Noonan 1998 |
RCTb | asthma | 18–65 years | Montelukast | Placebo | 2mg/10mg/50 mg | 212 | 69 | 18 | 10 | Headache18/10 | 3 |
| Reiss 1998 |
RCTb | asthma | ≥15 years | Montelukast | Placebo | 10 mg | 408 | 273 | 75 | 61 | Headache73/57; depression1/4; anxiety1/0 | 12 |
| Malmstrom 1999 | RCTb | asthma | ≥15 years | Montelukast | Placebo | 10 mg | 387 | 257 | 68 | 40 | Headache68/40 | 12 |
| Meltzer 2000–1 |
RCTb | Allergic rhinitis | 15–75 years | Montelukast | Placebo | 10 mg | 185 | 91 | 12 | 6 | Headache12/6 | 2 |
| Meltzer 2000–2 |
RCTb | Allergic rhinitis | 15–75 years | Montelukast/Loratadine | Loratadine | 10 mg + 10 mg:10 mg | 90 | 92 | 1 | 8 | Headache1/8 | 2 |
| Nayak 2002–1 |
RCTb | Allergic rhinitis | 15–85 years | Montelukast | placebo | 10 mg | 155 | 149 | 5 | 7 | Headache5/7 | 2 |
| Nayak 2002–2 |
RCTb | Allergic rhinitis | 15–85 years | Montelukast/Loratadine | Loratadine | 10 mg + 10 mg:10 mg | 302 | 301 | 9 | 12 | Headache9/12 | 2 |
| Baumgartner 2003 |
RCTb | asthma | ≥15 years | Montelukast | Placebo | 10 mg | 313 | 103 | 31 | 18 | Headache31/18 | 6 |
| Baena-Cagnani 2003 |
RCTb | Allergic rhinitis and asthma | ≥15 years | Montelukast | Placebo | 10 mg | 311 | 302 | 11 | 11 | Headache11/11 | 4 |
| Yildirim 2004 |
RCTb | asthma | mean:36.93 ± 2.98 years | Montelukast/Budesonide | Budesonide | 10 mg + 400 mg:800 mg | 15 | 15 | 2 | 0 | Headache2/0 | 6 |
| Asthma linical Research enters 2007 |
RCTb | asthma | ≥15 years | Montelukast | Placebo | 10 mg | 164 | 164 | 34 | 36 | nervousness34/36 | 24 |
| Shah 2006 |
RCTb | asthma | 18–60 years | Montelukast/Budesonide | Budesonide | 10 mg + 200 mg:400 mg | 30 | 30 | 1 | 0 | Headache1/0 | 8 |
| Bisgaard 2009 |
RCTb | Allergic rhinitis | 2–14 years | Montelukast | Placebo | 5 mg | 280 | 133 | 9 | 2 | Headache9/2 | 8 |
| Chen 2021 |
RCTb | Allergic rhinitis | 18–60 years | Montelukast/Budesonide | Budesonide | 10 mg + 256ug:256ug | 23 | 23 | 0 | 0 | No | 2 |
| Prenner 2010–1 |
RCTb | asthma | 15–92 years | Montelukast | Placebo | 10 mg or 20 mg | 555 | 1111 | 6 | 19 | Headache6/19 | 2 |
| Prenner 2010–2 |
RCTb | asthma | 15–92 years | Montelukast/Loratadine | Loratadine | 10 mg + 10 mg:10 mg | 969 | 971 | 66 | 85 | Nervous system disorders66/85; Headache34/29; Somnolence25/10 |
2 |
NE: Neuropsychiatric event.
RCT: randomized controlled trial.
3.2. Quality of individual studies
All studies were RCTs; the quality of individual studies is summarized in Table 2. The study by Prenner [32] had a Jadad level C, which indicated a high risk and may have introduced bias in the results if included; as such, it was excluded in the analysis. The quality level of four studies [22,28,31,34] were rated B, and the remaining studies [1,21,[23], [24], [25], [26], [27],29,30,33] were rated A (11 RCTs [1,[21], [22], [23], [24], [25], [26], [27],30,31,33] that enrolled patients in montelukast or placebo groups; and five RCTs [25,26,28,29,34] that enrolled patients in experimental and control groups). These RCT trials were included.
Table 2.
Quality assessment of individual study.
| Study | Allocation sequence generation | Allocation concealment | Blinding | Lost to follow-up | Calculation of sample size | Statistical analysis | Level of quality | ITT‡ analysis |
|---|---|---|---|---|---|---|---|---|
| Leff 1998 | B | A | A | 1 | Yes | ANOVA † Fisher's exact test |
B | Yes |
| Knorr 1998 | A | A | A | 2 | Yes | ANOVA † Shapiro-Wilks test Cochran-Mantel-Haenszel test |
A | Yes |
| Noonan 1998 | A | A | A | 0 | Yes | ANOVA † Fisher's exact Cochran-Mantel-Haenszel test Tukey's modified linear trend test (stepwise trend test) |
A | Yes |
| Reiss 1998 | A | A | A | 11 | Yes | ANOVA † Cochran-Mantel-Haenszel test |
A | Yes |
| Malmstrom 1999 | A | A | A | 13 | Yes | ANOVA † Dunnet–Tamhane approach, Shapiro–Wilk statistic, Levene test |
A | No |
| Meltzer 2000-1 | A | A | A | 4 | Yes | ANOVA † Fisher exact test Cochran-Mantel-Haenszel test; |
A | No |
| Meltzer 2000-2 | A | A | A | 4 | Yes | ANOVA † Fisher exact test Cochran-Mantel-Haenszel test; |
A | No |
| Nayak 2002-1 | A | A | A | 3 | Yes | ANOVA † Wilcoxon rank sum test |
A | Yes |
| Nayak 2002-2 | A | A | A | 3 | Yes | ANOVA † Wilcoxon rank sum test |
A | Yes |
| Baumgartner 2003 | A | A | A | NA | Yes | ANOVA † | A | Yes |
| Baena-Cagnani 2003 | A | A | A | 0 | Yes | ANOVA † | A | Yes |
| Yildirim 2004 | A | A | B | 0 | Yes | Mann–Whitney U test | B | No |
| Asthma Clinical Research Centers 2007 | A | A | A | NA | Yes | Huber-White variance; Linear and logistic regression models |
A | Yes |
| Shah 2006 | A | A | A | 0 | Yes | ANOVA † | A | Yes |
| Bisgaard 2009 | B | B | A | 0 | Yes | Fisher's exact test | B | No |
| Chen 2021 | A | B | B | 5 | Yes | χ2 tests Pearson correlation test T test |
B | No |
| Prenner 2010-1 | B | B | C | 9 | Yes | NA§ | C | No |
| Prenner 2010-2 | B | B | C | 13 | Yes | NA§ | C | No |
A, low risk of bias: the study met almost criteria; B, moderate risk of bias: the study met part or unclear for one or more quality criteria; C, high risk of bias: the study was not met or included criteria; †ANOVA: analysis of variance; ‡ITT: Intention-to-treat; §NA: not available.
3.3. NEs
3.3.1. The occurrence of NEs in randomized placebo-controlled studies
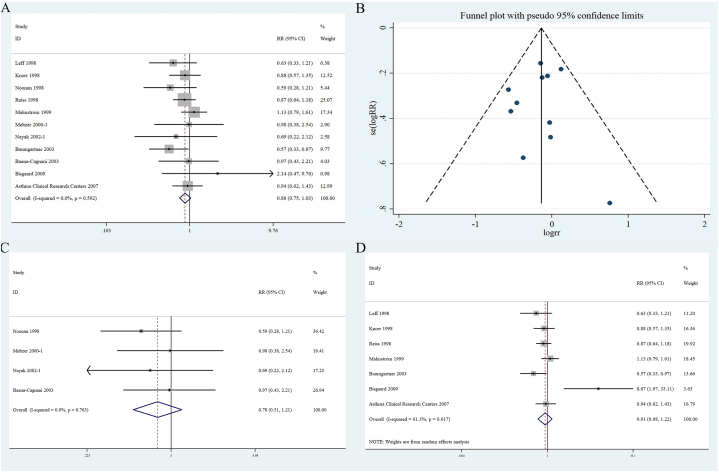
Eleven RCTs [1,[21], [22], [23], [24], [25], [26], [27],30,31,33] that enrolled 4402 patients (montelukast, n = 2670; placebo, n = 1732) were used to assess the occurrence of NEs in patients with AR and/or asthma. No significant heterogeneity (I2 < 50 %, P > 0.1) was found. Therefore, a fixed-effects model was used to consolidate the statistics and RR was used to assess the effect size (Fig. 2A). The forest plot revealed no statistical significance (RR 0.88 [95 % CI 0.75–1.03]; P = 0.592) in the difference between NEs in the experimental and control groups. A funnel plot developed to assess the publication bias of NEs in patients receiving montelukast or placebo presented a symmetrical appearance suggesting low publication bias (i.e., not statistically significant [Begg test, P = 1.00; Egger's test, P = 0.70]) (Fig. 2B).
Fig. 2.
Forest plots of (A):the relationship between patient with asthma or allergic rhinitis use montelukast and the occurrence of neuropsychiatric events; (B): funnel plot of the included studies; (C):the relationship between montelukast and neuropsychiatric events in <1 month group; (D):the relationship between montelukast and neuropsychiatric events in≤ 1 month group.
To further evaluate the effect of follow-up on NE occurrence, patients were further divided into two groups (<1 month and ≥1 month). The <1 month group included 4 RCTs [1,23,25,26] with 1474 patients (montelukast, n = 863; placebo, n = 611). The forest plot revealed no statistically significant association between the NEs and montelukast (RR 0.78 [95 % CI 0.51–1.21]; P = 0.763). The ≥1 month group included 7 RCTs [21,22,24,27,30,31,33] with 2928 patients (montelukast, n = 1807; placebo, n = 1121). The forest plot demonstrated a statistically significant association between NEs and montelukast (RR 0.91 [95 % CI 0.68–1.22]; P = 0.017) (Fig. 2C and D).
3.3.2. Headache-related NEs
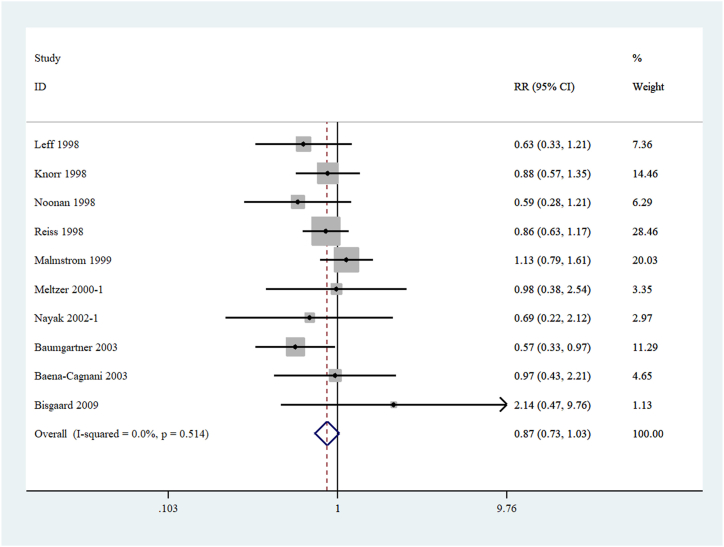
Ten RCTs [1,[21], [22], [23], [24], [25], [26], [27],31,33] that enrolled 4074 patients (montelukast, n = 2506; placebo, n = 1568) were used to assess the occurrence of headache, the most common event in NEs. No significant heterogeneity was found; thus, a fixed-effects model was used. The results indicated no statistically significant difference in the occurrence of headaches between the montelukast and placebo groups (RR 0.87 [95 % CI 0.73–1.03]; P = 0.514) (Fig. 3).
Fig. 3.
Forest plots of patients with asthma or rhinitis have been linked to a neuropsychiatric event called headache.
3.3.3. NEs in patients with asthma or AR
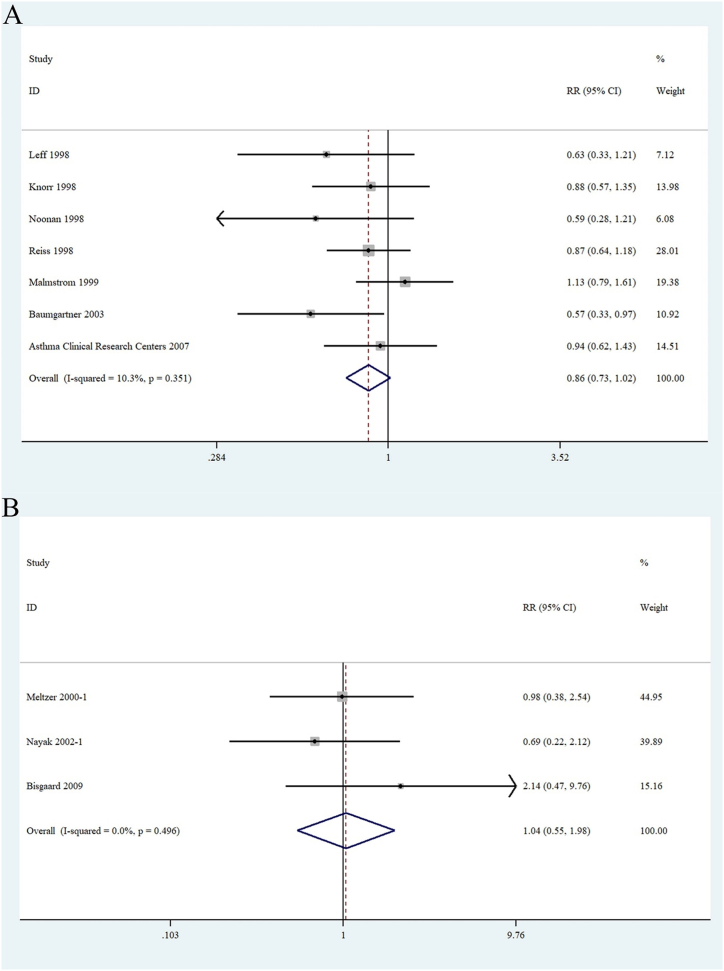
Asthma and AR are common chronic inflammatory diseases. Seven RCTs [[21], [22], [23], [24],27,30,33] that enrolled 2796 patients (montelukast, n = 1739; placebo, n = 1057) reported data regarding the occurrence of NEs in patients with asthma. The study found no statistically significant differences between the montelukast and placebo group (RR 0.86 [95 % CI 0.73–1.02]; P = 0.35) (Fig. 4A). Three RCTs [25,26,31] evaluated ADRs to montelukast in 993 patients with AR (montelukast, n = 620; placebo, n = 373). Results of analysis suggested that there was no increase in NEs in patients with AR who received montelukast compared with the placebo group (RR 1.04 [95 % CI 0.55–1.98]; P = 0.496) (Fig. 4B).
Fig. 4.
Forest plots of (A): the relationship between the use of montelukast and neuropsychiatric events in asthma patients and (B) the relationship between the use of montelukast and neuropsychiatric events in patients with rhinitis.
3.3.4. NE in children or adults
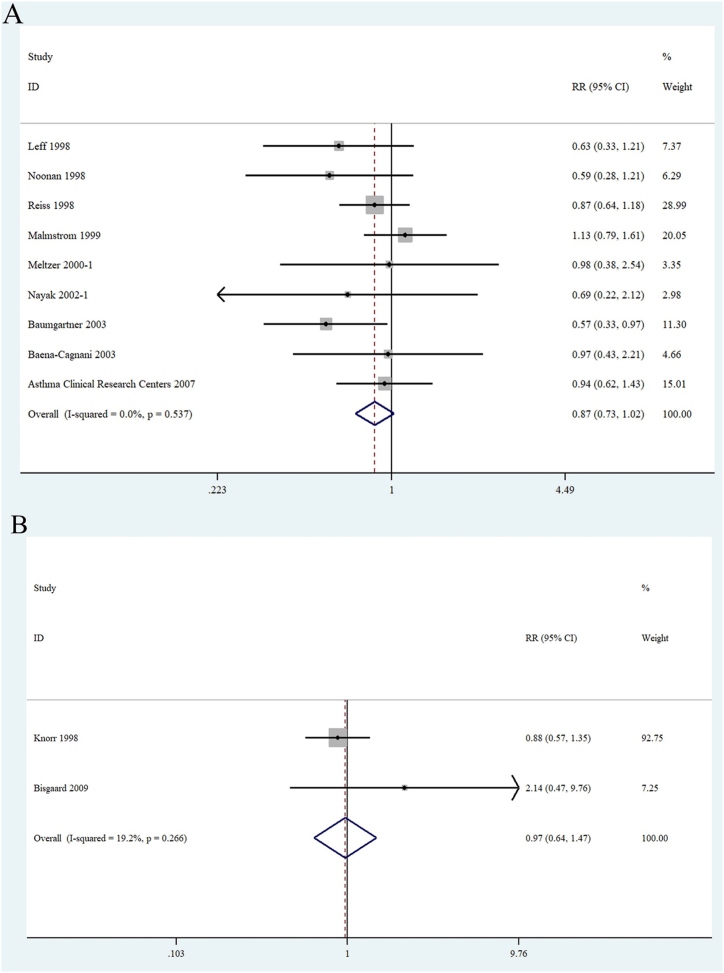
Nine RCTs [1,[22], [23], [24], [25], [26], [27],30,33] that enrolled adult 3653 patients (≥15 years of age) (montelukast, n = 2189; placebo, n = 1464) were used to estimate the occurrence of NEs. Forest plots for the fixed-effect model indicated an RR of 0.87 (95 % CI 0.73–1.02; P = 0.537) (Fig. 5A), with no statical associations between the two groups. Two RCTs [21,31] with 749 child patients (≤14 years of age) evaluated the occurrence of NEs (montelukast, n = 481; placebo, n = 268). Based on results from the fixed-effect model, the result revealed no increase of the number of NEs in child patients taking montelukast compared with placebo (RR 0.97 [95 % CI 0.64–1.47]; P = 0.266) (Fig. 5B).
Fig. 5.
Forest plots of (A):the relationship between montelukast and neuropsychiatric events in adult patients with asthma or rhinitis and (B): the relationship between montelukast and neuropsychiatric events in child's patients with asthma or rhinitis.
3.3.5. Relationship between NE and montelukast or common clinical drugs
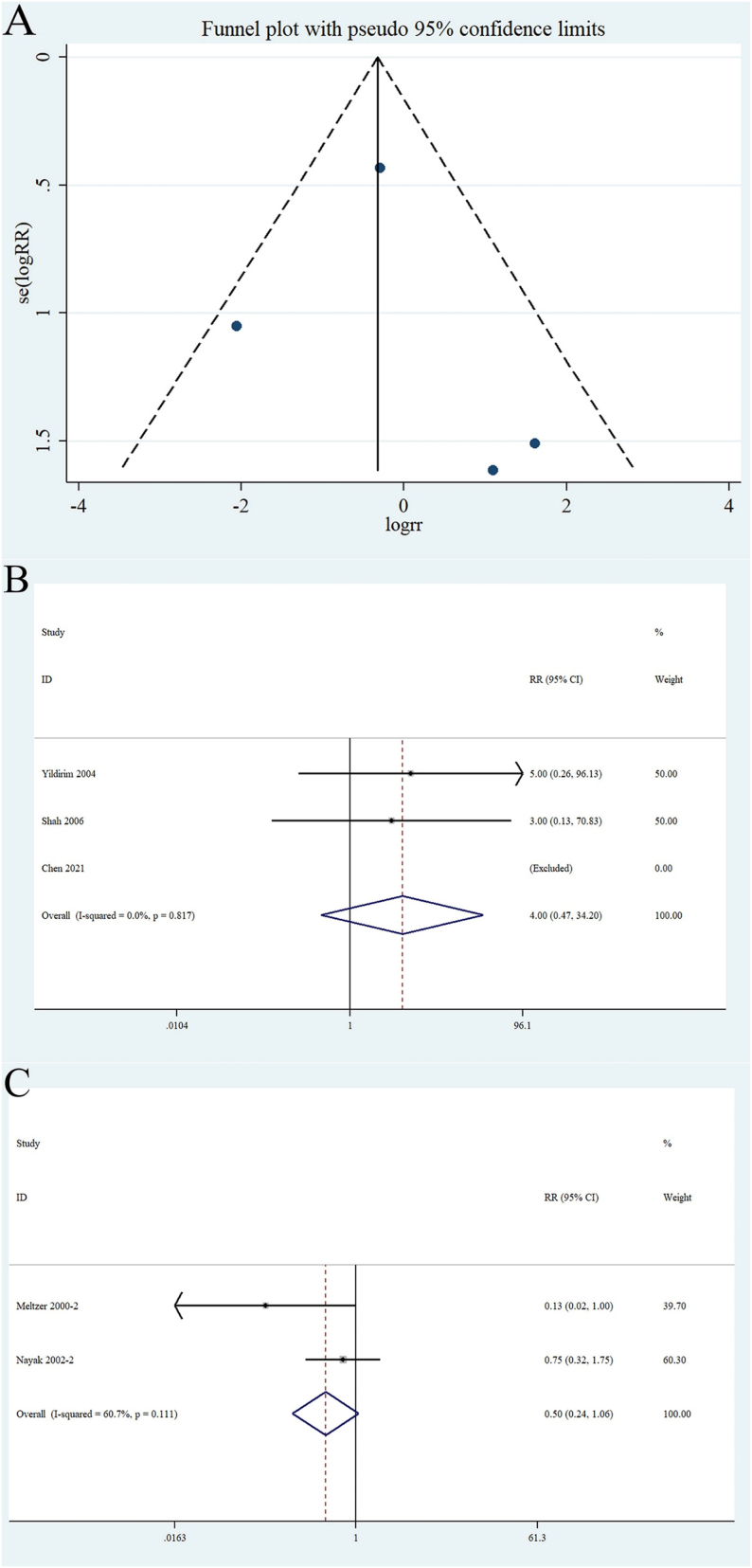
Five RCTs [25,26,28,29,34] enrolling 916 patients (experimental, n = 458; control, n = 458) assessed the occurrence of NE in patients with AR and/or asthma. A funnel plot was created to evaluate publication bias. A symmetrical appearance indicated low publication bias (Begg's test, P = 0.73; Egger test, P = 0.75) (Fig. 6A). Three RCTs [28,29,34] compared NEs between patients taking budesonide and those taking montelukast. No heterogeneity was observed (I2 < 50 %); therefore, a fixed effects model was used. This result revealed no statistically significant difference (RR 4.0 [95 % CI 0.47–34.20]; P = 0.817) (Fig. 6B). The remaining two RCTs [25,26] with 785 patients (experimental, n = 392; control, n = 393) were used to compare the incidence of NEs between patients taking loratadine and those taking montelukast. The heterogeneity test results were I2 = 60.7 %, P > 0.1. A random-effect model was used to combine the effect, with an RR of 0.50 (95 % CI 0.24–1.06; P = 0.111) (Fig. 6C). Furthermore, montelukast combined with loratadine likely caused more NEs than loratadine alone.
Fig. 6.
Funnel plot of (A): the included studies based on the patient with montelukast or common clinical drugs analysis and forest plots of (B):the neuropsychiatric events between montelukast and budesonide versus budesonide alone in asthmatic patients and (C): the occurrence of neuropsychiatric events in patients with allergic rhinitis compared with montelukast combined with loratadine versus loratadine alone.
4. Discussion
Asthma and AR are common allergic diseases with high occurrence rates. Clinically, montelukast is used to treat asthma and AR [35,36]. Montelukast was approved by the FDA in 1998 for the treatment of asthma and, in 2002, for the treatment of AR; however, long-term use of montelukast can cause adverse neuropsychiatric reactions [35]. In 2009, the FDA added NEs to treat ADRs. With the increase in reports of NEs, the FDA strengthened existing warnings about serious behavior- and mood-related changes in montelukast on March 4, 2020 [[15], [16], [17]].
However, only pharmacovigilance studies [[4], [5], [6],8,[11], [12], [13], [14]] and case reports [7,9,10] have shown that montelukast can cause adverse neuropsychiatric reactions. High-quality randomized controlled studies have not been comprehensively analyzed. Therefore, this meta-analysis aimed to compare whether montelukast causes NEs in patients with asthma, rhinitis, or both. Eleven RCTs [1,[21], [22], [23], [24], [25], [26], [27],30,31,33] comprising 4402 patients were included in our meta-analysis. The results indicated no statistically significant differences between montelukast and NEs in either asthma or AR. In addition, the most common neuropsychiatric adverse reaction―namely, headache―was also analyzed. The analysis revealed that montelukast did not significantly increase the incidence of headaches. These results were consistent with the overall results of the included studies.
Aldea-Perona et al. [5] found that age differences in the occurrence of NEs and neuropsychiatric diseases are more common in children than in adults. Therefore, we grouped patients according to age to analyze its role in the occurrence of NEs. The relationship between montelukast and NEs in children (≤14 years of age) and adults (≥15 years of age) was analyzed using the medical concept of adult age. The results yielded an RR of 0.87 (95 % CI of 0.73–1.02; P = 0.537). This indicated that there was no significant difference between children and adults, and no obvious correlation between montelukast and NEs in children. This is consistent with the results of a large, nested, case-control study with 1920 patients with asthma matched for age, sex, and geographical region [8], which demonstrated no significant positive association between montelukast use and NEs among children. However, the results differed from those of other reports and databases. Approximately 23 % of the reported ADRs to montelukast occur in patients <11 years of age on the World Health Organization's (WHO) VigiAccess database (https://www.vigiaccess.org/). NE events mentioned in the WHO's VigiAccess database include nervous system disorders (headache, dizziness, somnolence, tremor, etc.) and psychiatric disorders (depression, anxiety, insomnia, suicidal ideation, etc.), with headache being the most common nervous system disorder. The FDA has boxed warnings about serious mental health side effects (risks may include suicidal thoughts or actions) for asthma and the allergy drug montelukast, mainly based on case reports submitted to the FDA. In this study, we screened all NEs associated with montelukast and found that headache was the most common NE in all RCT studies, although our analysis did not reveal a relationship between NE and montelukast. In addition, the high incidence of such NEs among adults and children in the United States, Canada, and Europe [37] suggests that montelukast may lead to NEs. Consequently, montelukast-associated NEs have drawn much attention from practicing pediatricians in the United States, Australia, and the United Kingdom. The risk for depression associated with montelukast use has been reported in the United States [38] and Turkish [39] guidelines. Dixon et al. reported that montelukast causes ADRs, such as neuropsychiatric and gastrointestinal disorders, in children and young individuals with asthma; however, this systematic review was mainly based on case reports, case-control studies, and cohort studies (only 1 RCT) [40]. It should be noted that most reported NE cases were from retrospective studies; furthermore, the results of this study based on RCTs could not fully cover NEs because there were too few RCTs addressing ADRs in children. Nevertheless, assessment before medication and monitoring during drug administration are essential in some special and sensitive populations [41]. Given the inconsistency between our results, based on RCTs, and the current large number of case reports, we further grouped the follow-up time and found a correlation between NEs and montelukast in the group with a follow-up > 1 month, with a small bias (Bisgaard, 2009). We speculate that with longer follow-up periods and more RCTs, the relationship between NEs and montelukast may become clearer. This also heightens the importance of explaining the potential adverse effects to parents before prescribing montelukast to their children. Risk management plans should be carefully explained. Pediatric healthcare providers should be aware of the risk for NEs associated with montelukast use and advise patients to observe, monitor, and report possible NEs.
We also analyzed the occurrence of adverse events associated with montelukast combined with commonly used drugs for AR or asthma, such as loratadine and budesonide. Loratadine is an H1 receptor antagonist that is used to treat rhinitis and asthma. Our analysis demonstrated that loratadine combined with montelukast significantly relieved rhinitis and asthma [42]. When combined with montelukast or double doses, the hormone medication budesonide demonstrated similar improvement among patients [28]. To clarify the relationship between montelukast and the occurrence of adverse mental events, we analyzed AR and asthma. Taking loratadine or inhalation of budesonide and montelukast in combination or alone, the occurrence of NEs was not statistically significant (P > 0.05) in the montelukast group compared with the control group. Combining medications may be more effective, considering the effects of medication and the side effects of hormone drugs.
Our results revealed no significant association between montelukast use and NEs in patients with rhinitis or asthma. We hypothesized that NE is associated with this disease. Timonen et al. [43] and Kovacs et al. [44] found that individuals with allergies were more likely to develop depression than healthy individuals. This finding may be due to disorders of the hypothalamic-pituitary-adrenal axis and sympathetic adrenal medulla systems, and changes in the secretion of cytokines in the body, which cause patients to be affected by emotional and behavioral factors in daily life, depression, and other neuropsychiatric adverse events. Asthma is a chronic disease and repeated attacks can result in severe mental disorders. The inflammatory state of the body during the onset of asthma leads to an increase in proinflammatory cytokines, which are associated with the development of depression [45]. In addition, animal studies have shown that after increasing the cholinergic response in rats, tracheal constriction and airway inflammation may develop, thereby increasing the occurrence of NEs [46,47]. The above-mentioned research indicates that NEs may be caused by airway allergic diseases. However, we were unable to conduct further research owing to insufficient data.
All except 1 of the studies [32] were of good quality according to Begg and Egger tests. The major funnel plots were symmetrical, indicating no evidence of publication bias. Our meta-analysis revealed no significant increase in the incidence of NEs in patients with AR and asthma compared with the placebo group (P > 0.05).
Findings of the present study have practical implications for physicians, patients (especially parents of child patients), and regulatory agencies. As a leukotriene receptor antagonist, montelukast has been warned by the FDA; however, it is still of great value in the treatment of AR and asthma, and there is currently no ideal alternative medication. It must be emphasized that this drug should be used with strict indications and for an appropriate duration. At the same time, adverse effects of NE must be strictly monitored during montelukast use.
5. Strengths and limitations
A major strength of our study was that it was the first RCT-based systematic review of the correlation between montelukast and NEs. It has been established that RCTs represent the highest level of original research because they use random assignment methods that control for confounding factors and provide strong evidence supporting cause and effect.
We must, however, acknowledge that the present study also had some limitations. First, the effect of disease on the results should not be excluded. Second, because the study was not designed to report ADRs, no detailed records of their occurrence were available. Third, there are too few RCTs addressing ADRs in children to fully cover all NEs. Finally, observational studies were excluded from the analyses. Data from some pharmacovigilance research databases [6,7,[11], [12], [13], [14], [15],17] could not be combined and analyzed, which further reduced the reliability of the studies’ results and affected study quality.
6. Conclusions
In conclusion, the present study found no cause-and-effect relationship between montelukast and NEs in patients with asthma and AR based on current evidence from RCTs. However, there is an increased incidence of NEs when montelukast was combined with loratadine, or when the follow-up was >1 month (with a little bias). To our knowledge, this is the first meta-analysis-based RCT to provide evidence of this aspect, and our results are not consistent with those of some current cohort-based studies. Whether this is because RCT studies, as high-level evidence-based medical research, remain few in number. Anyway, it definitely does not mean we disagree with the reports of others, it only indicates that the issue should be taken more seriously. Therefore, large-sample RCTs are needed to verify the association between NEs and montelukast use in patients with allergic airway disease, and attention should also be devoted to FDA warnings.
Funding
This work was supported by the National Natural Science Foundation of China (82071021) and Shandong Provincial Postdoctoral Foundation Project (Innovation Project) (SDCX-ZG-202203077).
Ethics approval and consent to participate
Informed consent was not needed for this study because all analyses were based on previous published studies.
Consent for publication
Not applicable.
Data availability statement
Data included in article/supp. Material/referenced in article.
CRediT authorship contribution statement
Yakui Mou: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Qing Song: Writing – original draft, Data curation. Chunying Zhao: Writing – original draft, Data curation. Han Fang: Methodology, Formal analysis. Chao Ren: Methodology, Formal analysis, Conceptualization. Xicheng Song: Writing – review & editing, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21842.
Contributor Information
Chao Ren, Email: renchaotg@126.com.
Xicheng Song, Email: drxchsong@163.com.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Baena-Cagnani C.E., Berger W.E., DuBuske L.M., et al. Comparative effects of desloratadine versus montelukast on asthma symptoms and use of beta 2-agonists in patients with seasonal allergic rhinitis and asthma. Int. Arch. Allergy Immunol. 2003;130(4):307–313. doi: 10.1159/000070218. [DOI] [PubMed] [Google Scholar]
- 2.Price D.B., Hernandez D., Magyar P., et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211–216. doi: 10.1136/thorax.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiss T.F., Chervinsky P., Dockhorn R.J., Shingo S., Seidenberg B., Edwards T.B. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch. Intern. Med. 1998;158(11):1213–1220. doi: 10.1001/archinte.158.11.1213. [DOI] [PubMed] [Google Scholar]
- 4.Aagaard L., Hansen E.H. Paediatric adverse drug reactions following use of asthma medications in Europe from 2007 to 2011. Int. J. Clin. Pharm. 2014;36(6):1222–1229. doi: 10.1007/s11096-014-0020-0. [DOI] [PubMed] [Google Scholar]
- 5.Aldea Perona A., Garcia-Saiz M., Sanz Alvarez E. Psychiatric disorders and montelukast in children: a disproportionality analysis of the VigiBase((R)) Drug Saf. 2016;39(1):69–78. doi: 10.1007/s40264-015-0360-2. [DOI] [PubMed] [Google Scholar]
- 6.Bygdell M., Brunlof G., Wallerstedt S.M., Kindblom J.M. Psychiatric adverse drug reactions reported during a 10-year period in the Swedish pediatric population. Pharmacoepidemiol. Drug Saf. 2012;21(1):79–86. doi: 10.1002/pds.2265. [DOI] [PubMed] [Google Scholar]
- 7.Callero-Viera A., Infante S., Fuentes-Aparicio V., Zapatero L., Alonso-Lebrero E. Neuropsychiatric reactions to montelukast. J Investig. Allergol. Clin. Immunol. 2012;22(6):452–453. [PubMed] [Google Scholar]
- 8.Cereza G., Garcia Dolade N., Laporte J.R. Nightmares induced by montelukast in children and adults. Eur. Respir. J. 2012;40(6):1574–1575. doi: 10.1183/09031936.00092812. [DOI] [PubMed] [Google Scholar]
- 9.Ibarra-Barrueta O., Palacios-Zabalza I., Mora-Atorrasagasti O., Mayo-Suarez J. Effect of concomitant use of montelukast and efavirenz on neuropsychiatric adverse events. Ann. Pharmacother. 2014;48(1):145–148. doi: 10.1177/1060028013510396. [DOI] [PubMed] [Google Scholar]
- 10.Kocyigit A., Gulcan Oksuz B., Yarar F., Uzun F., Igde M., Islek I. Hallucination development with montelukast in a child with asthma: case presentation. Iran. J. Allergy, Asthma Immunol. 2013;12(4):397–399. [PubMed] [Google Scholar]
- 11.Lafay-Chebassier C., Chavant F., Favreliere S., Pizzoglio V., Perault-Pochat M.C. Drug-induced depression: a case/non case study in the French pharmacovigilance database. Therapie. 2015;70(5):425–432. doi: 10.2515/therapie/2015026. [DOI] [PubMed] [Google Scholar]
- 12.B P., W L., P G., F S., S S., Mann R.D. Pharmacosurveillance and safety of the leukotriene receptor antagonist (LTRA), montelukast. Clin. Exp. Allergy Rev. 2001:300–304. [Google Scholar]
- 13.Marchand M.S., Jonville-Bera A.P., Autret-Leca E. [Psychiatric disorders associated with montelukast: data from the National Pharmacovigilance Database] Arch. Pediatr. : organe officiel de la Societe francaise de pediatrie. 2013;20(3):269–273. doi: 10.1016/j.arcped.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Wallerstedt S.M., Brunlof G., Sundstrom A., Eriksson A.L. Montelukast and psychiatric disorders in children. Pharmacoepidemiol. Drug Saf. 2009;18(9):858–864. doi: 10.1002/pds.1794. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration . 2020. FDA Requires Boxed Warning about Serious Mental Health Side Effects for Asthma and Allergy Drug Montelukast (Singulair)https://www.fda.gov/drugs [Google Scholar]
- 16.US Food and Drug Administration . 2008. Early Communication about an Ongoing Safety Review of Montelukast (Singulair)http://www.fda.gov/Drugs [Google Scholar]
- 17.US Food and Drug Administration . 2009. Updated Information on Leukotriene Inhibitors: Montelukast (Marketed as Singulair), Zafirlukast (Marketed as Accolate), and Zileuton (Marketed as Zyflo and Zyflo CR)http://www.fda.gov/Drugs [Google Scholar]
- 18.Narang R., Narang S., Narang D., Udeani G. Contemporary use of montelukast and its association with depression in asthma and allergic rhinitis patients. Chest. 2014;146(4):13A. [Google Scholar]
- 19.Jadad A.R. BMJ Publishing Group; London: 1998. Randomised Controlled Trials. [Google Scholar]
- 20.Higgins J., Green S. Cochrane Collaboration Web site; 2011. Cochrane Handbook for Systematic Reviews of interventions,v.5.1. [Google Scholar]
- 21.Knorr B., Matz J., Bernstein J.A., et al. Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. Pediatric Montelukast Study Group. JAMA. 1998;279(15):1181–1186. doi: 10.1001/jama.279.15.1181. [DOI] [PubMed] [Google Scholar]
- 22.Leff J.A., Busse W.W., Pearlman D., et al. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N. Engl. J. Med. 1998;339(3):147–152. doi: 10.1056/NEJM199807163390302. [DOI] [PubMed] [Google Scholar]
- 23.Noonan M.J., Chervinsky P., Brandon M., et al. Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Montelukast Asthma Study Group. Eur. Respir. J. 1998;11(6):1232–1239. doi: 10.1183/09031936.98.11061232. [DOI] [PubMed] [Google Scholar]
- 24.Malmstrom K., Rodriguez-Gomez G., Guerra J., et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann. Intern. Med. 1999;130(6):487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 25.Meltzer E.O., Malmstrom K., Lu S., et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: a randomized, placebo-controlled clinical trial. J. Allergy Clin. Immunol. 2000;105(5):917–922. doi: 10.1067/mai.2000.106040. [DOI] [PubMed] [Google Scholar]
- 26.Nayak A.S., Philip G., Lu S., Malice M.P., Reiss T.F. Efficacy and tolerability of montelukast alone or in combination with lo-ratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann. Allergy Asthma Immunol.: official publication of the American College of Allergy, Asthma, & Immunology. 2002;88(6):592–600. doi: 10.1016/S1081-1206(10)61891-1. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner R.A., Martinez G., Edelman J.M., et al. Distribution of therapeutic response in asthma control between oral montelukast and inhaled beclomethasone. Eur. Respir. J. 2003;21(1):123–128. doi: 10.1183/09031936.03.00028803. [DOI] [PubMed] [Google Scholar]
- 28.Yildirim Z., Ozlu T., Bulbul Y., Bayram H. Addition of montelukast versus double dose of inhaled budesonide in moderate persistent asthma. Respirology. 2004;9(2):243–248. doi: 10.1111/j.1440-1843.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 29.Shah A.R., Sharples L.D., Solanki R.N., Shah K.V. Double-blind, randomised, controlled trial assessing controller medications in asthma. Respiration; international review of thoracic diseases. 2006;73(4):449–456. doi: 10.1159/000090898. [DOI] [PubMed] [Google Scholar]
- 30.Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am. J. Respir. Crit. Care Med. 2007;175(3):235–242. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 31.Bisgaard H., Skoner D., Boza M.L., et al. Safety and tolerability of montelukast in placebo-controlled pediatric studies and their open-label extensions. Pediatr. Pulmonol. 2009;44(6):568–579. doi: 10.1002/ppul.21018. [DOI] [PubMed] [Google Scholar]
- 32.Prenner B.M., Lu S., Danzig M.R. Safety of fixed-dose loratadine/montelukast in subjects with allergic rhinitis. Allergy Asthma Proc. 2010;31(6):493–498. doi: 10.2500/aap.2010.31.3401. [DOI] [PubMed] [Google Scholar]
- 33.Reiss T.F., Chervinsky P., Dockhorn R.J., et al. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch. Intern. Med. 1998;158(11):1213–1220. doi: 10.1001/archinte.158.11.1213. [DOI] [PubMed] [Google Scholar]
- 34.Chen H., Zhang L., Lou H.F., et al. A randomized trial of comparing a combination of montelukast and budesonide with budesonide in allergic rhinitis. The laryngoscope. 2021;131(4):E1054–E1061. doi: 10.1002/lary.28433. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein J.A. Allergic and mixed rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2010;31(5):365–369. doi: 10.2500/aap.2010.31.3380. [DOI] [PubMed] [Google Scholar]
- 36.Martinez F.D., Vercelli D. Asthma. Lancet (London, England) 2013;382(9901):1360–1372. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 37.Al-Shamrani A., Alharbi S., Kobeisy S., et al. Adverse drug reactions (ADRs) of montelukast in children. Children. 2022 Nov 21;9(11):1783. doi: 10.3390/children9111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seidman M.D., Gurgel R.K., Lin S.Y., et al. AAO-HNSF. Clinical practice guideline: allergic rhinitis. Otolaryngol. Head Neck Surg. 2015;152(1 Suppl):S1–S43. doi: 10.1177/0194599814561600. [DOI] [PubMed] [Google Scholar]
- 39.Ecevit M.C., Özcan M., Haberal Can I., et al. Turkish guideline for diagnosis and treatment of allergic rhinitis (ART) Turk. Arch. Otolaryngol. 2021;59:1–157. doi: 10.4274/tao.2021.suppl.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon E.G., Rugg-Gunn C.E., Sellick V., et al. Adverse drug reactions of leukotriene receptor antagonists in children with asthma: a systematic review. BMJ Paediatr Open. 2021 Oct 13;5(1) doi: 10.1136/bmjpo-2021-001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mou Y.K., Wang H.R., Zhang W.B., et al. Allergic rhinitis and depression: profile and proposal. Front. Psychiatr. 2022 Jan 4;12 doi: 10.3389/fpsyt.2021.820497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dockhorn R.J., Bergner A., Connell J.T., et al. Safety and efficacy of loratadine (Sch-29851): a new non-sedating antihistamine in seasonal allergic rhinitis. Ann. Allergy. 1987;58(6):407–411. [PubMed] [Google Scholar]
- 43.Timonen M., Jokelainen J., Silvennoinen-Kassinen S., et al. Association between skin test diagnosed atopy and professionally diagnosed depression: a Northern Finland 1966 Birth Cohort study. Biol. Psychiatr. 2002;52(4):349–355. doi: 10.1016/s0006-3223(01)01364-6. [DOI] [PubMed] [Google Scholar]
- 44.Kovacs M., Stauder A., Szedmak S. Severity of allergic complaints: the importance of depressed mood. J. Psychosom. Res. 2003;54(6):549–557. doi: 10.1016/s0022-3999(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 45.Eyre H., Baune B.T. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37(9):1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Djuric V.J., Cox G., Overstreet D.H., Smith L., Dragomir A., Steiner M. Genetically transmitted cholinergic hyperresponsiveness predisposes to experimental asthma. Brain Behav. Immun. 1998;12(4):272–284. doi: 10.1006/brbi.1998.0538. [DOI] [PubMed] [Google Scholar]
- 47.Overstreet D.H., Daws L.C., Schiller G.D., Orbach J., Janowsky D.S. Cholinergic/serotonergic interactions in hypothermia: im-plications for rat models of depression. Pharmacol., Biochem. Behav. 1998;59(4):777–785. doi: 10.1016/s0091-3057(97)00514-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. Material/referenced in article.