Abstract
The streptococcal plasmid pMV158 replicates by the rolling-circle mechanism. One feature of this replication mechanism is the generation of single-stranded DNA intermediates which are converted to double-stranded molecules. Lagging-strand synthesis initiates from the plasmid single-stranded origin, sso. We have used the pMV158-derivative plasmid pLS1 (containing the ssoA type of lagging-strand origin) and a set of pLS1 derivatives with mutations in two conserved regions of the ssoA (the recombination site B [RSB] and a conserved 6-nucleotide sequence [CS-6]) to identify sequences important for plasmid lagging-strand replication in Streptococcus pneumoniae. Cells containing plasmids with mutations in the RSB accumulated 30-fold more single-stranded DNA than cells containing plasmids with mutations in the CS-6 sequence. Specificity of lagging-strand synthesis was tested by the development of a new in vitro replication system with pneumococcal cell extracts. Four major initiation sites of lagging-strand DNA synthesis were observed. The specificity of initiation was maintained in plasmids with mutations in the CS-6 region. Mutations in the RSB region, on the other hand, resulted in the loss of specific initiation of lagging-strand synthesis and also severely reduced the efficiency of replication.
Plasmid replication by the rolling-circle (RC) mode has two initiation stages for the synthesis of the leading and the lagging strands. Leading-strand replication initiates through a nucleophilic attack exerted by the plasmid-encoded Rep protein on a specific dinucleotide, after recognition of a strand-specific sequence, the double-strand origin (dso). The 3′-OH end generated by Rep cleavage is thought to be elongated by conserved host-encoded proteins, such as DNA polymerase III and a helicase (reviewed in references 7, 11, 13, and 21). Termination of leading-strand synthesis leads to the generation of single-stranded plasmid DNA (ssDNA) intermediates which are the hallmark of this type of replicon; such plasmids are generically termed RCR (rolling-circle-replicating) plasmids (11, 20, 26). Lagging-strand synthesis usually starts from the single-strand origin, sso, which is a noncoding DNA region physically separate from the dso. The plasmid ssos operate in an orientation-dependent manner and have a high potential for intrastrand pairing (8, 10). The ssDNA intermediates are later converted to the double-stranded DNA (dsDNA) form solely by the use of the host machinery (1, 11, 13, 21).
Based on their sequence homologies, four groups of sso have been described so far: (i) ssoA, present in several plasmids from Staphylococcus aureus, such as pE194, pT181, and pSN2 (21); (ii) ssoU, described for pUB110 (2); (iii) ssoT, found in Bacillus plasmids such as pBAA1 and pT4010 (29); and (iv) ssoW, present in the lactococcal plasmid pWV01 (17, 28). The different types of sso can be distinguished not only by their nucleotide sequence and structure but also by the host range in which they are functional and by the effect of their deletion on plasmid copy number and segregational stability. In vivo and in vitro studies have shown either partial or total dependence on the host RNA polymerase (RNAP) in the first step of plasmid lagging-strand synthesis (2, 9, 14, 28, 29). In vitro, RNAP directs the synthesis of a 20-nucleotide (nt)-long primer RNA (pRNA) from within the ssoA of pMV158, which is later elongated by the host-encoded DNA polymerase I (15).
The streptococcal plasmid pMV158 is a promiscuous mobilizable multicopy RCR plasmid, originally isolated from Streptococcus agalactiae (3). It has the unique feature of having two ssos, namely ssoA and ssoU. This latter sso was removed when the pMV158-derivative plasmid pLS1 was constructed (8, 16, 23, 30). The pLS1-ssoA is included within a region of 199 bp, in which palindromic sequences able to generate a long stem-loop structure within the ssDNA are found (6, 14). Two sequences within this hairpin are conserved among ssoA-containing plasmids: (i) the recombination site B (RSB), a 14-nt-long sequence located at the 5′ end of the stem (22, 25), and (ii) a 6-nt conserved sequence, termed CS-6, located in the central loop (8, 10). The function of each of these conserved sequences has been assessed previously by construction of a set of mutants in the pLS1-ssoA. Changes in the RSB region affect the binding of RNAP to the ssoA, while mutations in CS-6 interfered with pRNA termination without altering RNAP binding to the ssDNA (15). In the present work we have analyzed the in vivo effect of both types of mutations, carried by pLS1-derivative plasmids, in Streptococcus pneumoniae. We have also developed a cell-free system from S. pneumoniae and used it to study the effects of these mutations on the efficiency and specificity of lagging-strand initiation in vitro. The results obtained in vitro are consistent with those seen in vivo and demonstrate that RSB plays a major role in lagging-strand replication while CS-6 has a minor influence on the ssoA activity in S. pneumoniae.
MATERIALS AND METHODS
Bacterial strains.
S. pneumoniae 708 (end-1 exo-2 trt-1 hex-4 malM594) was employed as host of plasmids with the pMV158 replicon and as the source of cell extracts. Pneumococcal cells were grown and transformed with plasmid DNA as previously described (5, 16). Escherichia coli JM109 {[endA1 recA1 gyrA96 thi hsdR17 (rK− mK+) relA1 supE44 Δ(lac-proAB) F′(traD36 proA+B+ lacIq lacZΔM15)] (31) was used to prepare ssDNA from the recombinant phagemids based on the pALTER-1 (Promega) vector. Selective pressure was exerted by using tetracycline at concentrations of 1 μg/ml (S. pneumoniae containing pMV158-based replicons) and 15 μg/ml (Escherichia coli containing phagemids).
Construction of pLS1 derivatives and recombinant phagemids.
Plasmids, phagemids, and the M13 coliphage containing the ssoA region of pE194 are listed in Tables 1 and 2. To construct pLS1 derivatives with mutations in the ssoA, site-directed mutagenesis in the pLS1-ssoA was performed by the use of the Altered Sites kit (Promega) designed for in vitro mutagenesis. The nucleotide changes introduced in conserved sequences CS-6 and RSB are described in Table 1. The 1,243-bp EcoRI-PstI fragment of pLS1, containing the ssoA (the enzymes cut at pMV158 coordinates 3170 and 5, respectively) (16, 23), was cloned into pALTER-1 to perform the mutagenesis (14, 15). The changes introduced new restriction sites, which were used to select the desired recombinants (15). The altered EcoRI-PstI fragments were inserted back into pLS1 by swapping the same restriction fragment. The resulting pLS1-derivative plasmids containing each of the mutations (Table 1) were rescued by transformation of pneumococcal cells. For each plasmid, the entire nucleotide sequence of the region encompassing the altered ssoA was determined by using the T7 sequencing kit (Pharmacia). To construct recombinant phagemids from which ssDNA was isolated for the in vitro replication assays, the 1,134-bp HindIII-PstI fragments of the pLS1 derivatives (coordinates of cleavage 4407 and 5, respectively) containing the mutated ssoA were cloned into the phagemid pALTER-1 digested with the same enzymes (Table 2). The recombinant phagemids were transferred to E. coli JM109 cells by transformation (27).
TABLE 1.
Plasmids used in the in vivo studies
| Plasmid | Size (bp) | Relevant property(-ies) in the ssoA region | Nucleotide sequence of the ssoA subregion (5′-3′)a | Reference or source |
|---|---|---|---|---|
| pLS1 | 4,408 | wt | CCGAAAGGCTTTAGCGTTTCGG | 16 |
| pLS1CM | 4,408 | Totally unpaired CS-6, 5-bp change that generates a weak consensus | CCGAAAGGCTTATCGATTTCGG | 14 |
| pLS1G1 | 4,408 | Totally unpaired CS-6, contains the wt CS-6 sequence | CCGAAAATATTTAGCGTTTCGG | This work |
| pLS1G3 | 4,408 | Total change of the CS-6 sequence, structure is similar to the wt | CCGAAGGATTTGGATCCTTCGG | This work |
| pLS1G4 | 4,408 | Totally unpaired and changed CS-6 | CCGAAGGGCTTGGATCCTTCGG | This work |
| pLS1G5 | 4,408 | RSB almost totally unpaired, maintains the wt sequence | GAAATATTAATAAb | This work |
| pLS1G6 | 4,411 | RSB totally paired, maintains the wt sequence | GCTTTCATGGCATAAc | This work |
| pLS1G7 | 4,408 | 6-bp change in the unpaired RSB sequence, structure is similar to the wt | CGATTTATGCCAGATCTGCTATTT | This work |
| pLS1G3G7 | 4,408 | Double mutant containing the G3 and G7 mutations | This work | |
| pLS1Δ13 | 4,267 | Deleted RSB, wt CS-6 sequence | 14 | |
| pLS1Δ14 | 4,202 | Deleted RSB, wt CS-6 sequence | 14 | |
| pLS1ΔNA | 4,209 | Deletion of the pLS1 AflII-NcoI small fragment, entire ssoA deleted | 14 | |
| pE194 | 3,728 | wt ssoA, homologous to pLS1-ssoA | 12 |
Changes introduced in the wt conserved CS-6 and RSB sequences are underlined, and changes introduced in the RSB complementary sequence are in boldface.
Complementary sequence of RSB modified to reduce base pairing.
Complementary sequence of RSB modified to increase base pairing.
TABLE 2.
Phagemids used in the in vitro studies
| Phagemid | Size (bp) | Relevant property(-ies) | Source or reference |
|---|---|---|---|
| pALTER-1 | 5,680 | Phagemid vector | Promega |
| pA-pLS1ssoA | 6,806 | HindIII-PstI fragment of pLS1 cloned into pALTER-1, ssoA-wt in functional orientation | 15 |
| pA-pLS1ssoA− | 6,880 | EcoRI-PstI fragment (pLS1) cloned in pALTER-1, ssoA-wt in the nonfunctional orientation | 15 |
| pA-CM | 6,806 | HindIII-PstI fragment (pLS1CM), mutant in CS-6 | This work |
| pA-G1 | 6,806 | HindIII-PstI fragment (pLS1G1), mutant in CS-6 | This work |
| pA-G3 | 6,806 | HindIII-PstI fragment (pLS1G3), mutant in CS-6 | This work |
| pA-G4 | 6,806 | HindIII-PstI fragment (pLS1G4), mutant in CS-6 | This work |
| pA-G5 | 6,806 | HindIII-PstI fragment (pLS1G5), mutant in RSB | This work |
| pA-G6 | 6,809 | HindIII-PstI fragment (pLS1G6), mutant in RSB | This work |
| pA-G7 | 6,806 | HindIII-PstI fragment (pLS1G7), mutant in RSB | This work |
| pA-G3G7 | 6,806 | HindIII-PstI fragment (pLS1G3G7), double mutant in CS-6 and RSB | This work |
| pA-Δ13 | 6,665 | HindIII-PstI fragment (pLS1Δ13), partial ssoA deletion | This work |
| pA-Δ14 | 6,806 | HindIII-PstI fragment (pLS1Δ14), partial ssoA deletion | This work |
| pA-ΔNA | 6,607 | HindIII-PstI fragment (pLS1ΔNA), total ssoA deletion | This work |
| M13-pE194ssoA | 7,792 | Phage M13 containing the ssoA region of pE194 in its functional orientation | 9 |
Detection of ssDNA accumulated in vivo and measurement of plasmid copy number.
Pneumococcal cultures containing plasmids were grown to mid-exponential phase (about 2 × 108 CFU per ml of culture), and total DNA was prepared (8). DNAs were analyzed by electrophoresis on 0.7% agarose gels, followed by transfer to nitrocellulose filters with or without prior denaturation (26). DNA on the filters was hybridized by using 32P-labeled pLS1 DNA as the probe. The amount of ss- and dsDNA transferred to the filters was directly quantified with the aid of a PhosphorImager and ImageQuant software (Molecular Dynamics). Plasmid copy number was determined by agarose gel electrophoresis of sheared whole-cell lysates, followed by ethidium bromide staining and fluorescence densitometry as previously described (24).
Isolation of ssDNA from phagemids.
E. coli JM109 cells containing the recombinant phagemids were grown at 37°C to an optical density at 600 nm of 0.7 and infected with the RK408 helper bacteriophage at a multiplicity of infection of 10. Cultures were grown for 13 to 16 h, and the encapsidated ssDNA was purified as previously described (27). The DNA products were analyzed on 1% agarose gels, and the amount of ssDNA was directly quantified with the Gel Doc system and software (Bio-Rad Laboratories). When the recombinant pALTER-1-based phagemid contained the wild type (wt) or mutated pLS1-ssoA in the functional orientation, the ssDNAs obtained had the initiation signals properly positioned. ssDNA molecules from pA-pLS1ssoA− contained the complementary sequence of the replication origin, i.e., the ssoA in the nonfunctional orientation.
Cell-free replication extracts from S. pneumoniae.
Cell extracts were prepared essentially as described previously for Staphylococcus aureus (1). One liter of exponentially growing S. pneumoniae culture (about 2 × 108 CFU per ml) was centrifuged (5,000 × g for 10 min at 4°C), and the cell pellet was washed in buffer (50 mM Na-phosphate [pH 6.9], 1 mM EDTA, 5 mM EGTA, 0.1 mM phenylmethylsulfonyl fluoride). Cells were resuspended in 4 ml of the same buffer to which KCl was added (final concentration, 150 mM). After two rounds of freezing (−70°C) and thawing (10°C), autolysin was added to a final concentration of 10 μg/ml, and the mixtures were incubated at 37°C for 7 min. Cell debris was removed by centrifugation (100,000 × g for 10 min at 4°C). To remove nucleic acids, 400 μl of streptomycin sulfate (30% in distilled water) was slowly added to the supernatant (3.6 ml) in an ice-chilled tube. After 30 min on ice, the precipitate was separated by centrifugation (20,000 × g), and ammonium sulfate (0.472 g/ml) was added to the supernatant. The protein precipitate was collected by centrifugation (20,000 × g) and dissolved in the assay buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 100 mM KCl, 1 mM dithiothreitol, 10% ethylene glycol). After dialysis against the same buffer, proteins were stored at −70°C. Protein concentration (usually 55 to 60 mg/ml) was measured by the bicinchoninic acid protein assay (Pierce).
In vitro lagging-strand synthesis.
The in vitro replication assays were performed essentially as previously described (1). Reaction mixtures (30 μl) contained 150 ng of ssDNA in buffer (40 mM Tris-HCl [pH 8.0], 100 mM KCl, 12 mM magnesium acetate, 1 mM dithiothreitol), 50 μM NAD, 50 μM cyclic AMP, 2 mM ATP, 0.5 mM (each) UTP, CTP, and GTP, 50 μM (each) dCTP, dGTP, and dTTP, 20 μM [α-32P]dCTP (3,000 Ci/mmol), and 240 μg of cell extract proteins (unless otherwise stated). Mixtures were incubated at 32°C for 20 min in the experiments designed to map the lagging-strand initiation sites or for 60 min when total DNA replication was tested. DNA was recovered by phenol-chloroform extraction and ethanol precipitation. The replication products were separated by electrophoresis on 1% agarose gels in the presence of ethidium bromide (0.5 μg/ml) or on 8% acrylamide–8 M urea sequencing gels. When total replication levels were to be determined, the DNA products were linearized with HindIII (which cuts approximately 1,000 nt downstream from the ssoA, in the direction of replication) to convert the various forms of DNA (double-stranded open circles and covalently closed supercoiled and replicative intermediates) to a single band before electrophoresis. Bands were visualized under UV irradiation and by autoradiography of the dried gels. As size markers, linearized dsDNA phagemids were run on the same gel. To map the initiation sites of lagging-strand synthesis, the procedure described by Dempsey et al. (9) was followed. Partially replicated ssDNA samples (20-min reaction) were treated with AflII (which cuts approximately 100 bp downstream from the expected initiation site of DNA synthesis) before electrophoresis. To determine the distance from the lagging-strand initiation start points to the restriction site, known nucleotide sequencing reactions were run as size markers.
RESULTS AND DISCUSSION
In vivo characterization of ssoA mutants.
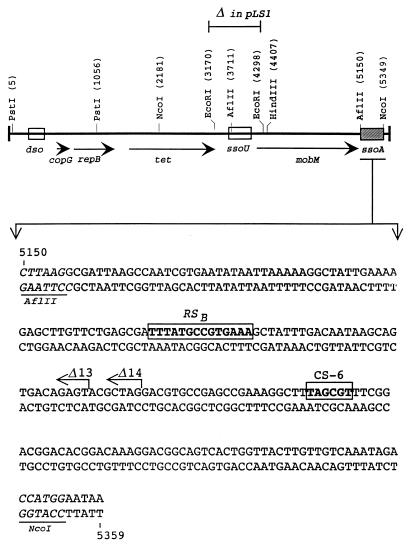
The boundaries of the pLS1-ssoA have been defined between the recognition sites for AflII and NcoI (pMV158 coordinates 5150 and 5349, respectively) (Fig. 1). Deletion of this fragment results in plasmids which accumulate large amounts of ssDNA in S. pneumoniae (14). The two conserved sequences of the ssoA, RSB and CS-6, are located within this 199-bp region (Fig. 1). This region is predicted to fold into an extensive secondary structure (Fig. 2). The functionality of the ssoA of RCR plasmids is orientation dependent, indicating that sequences important in their function are located in the unpaired regions within the ssoA secondary structure (8, 10). The CS-6 and part of the RSB region comply with these requirements. Mutations were introduced in these two regions to change the sequence or the local structure of the RSB (mutations G5, G6, and G7), the CS-6 (mutations CM, G1, G3, and G4), or both (G3G7 double mutant), without a gross alteration of the global pLS1-ssoA structure (15) (see Table 1). When ssDNA preparations containing these mutations were tested in vitro for their interactions with the Bacillus subtilis RNAP, two types of results were obtained: mutations in the RSB severely impaired the binding of the enzyme to the DNA, whereas alterations in the CS-6 led to a twofold reduction in the total amount of primer RNA (pRNA) synthesized and resulted in the synthesis of pRNAs longer than 20 nt (size of the pRNA from the wt substrate [15]).
FIG. 1.
Plasmid pMV158 and its ssoA. (Top) Schematic map of pMV158 with some of the plasmid-encoded genes, relevant restriction sites (coordinates of cleavage sites in parentheses), and the relative positions of the dso and the two ssos (ssoU and ssoA). The position of the small EcoRI fragment (deleted in plasmid pLS1) is indicated. (Bottom) Nucleotide sequence of the plasmid ssoA between restriction sites AflII and NcoI (underlined). The two conserved sequences, RSB and CS-6, are boxed. The 3′ ends of the deletions in plasmids pLS1Δ13 and pLS1Δ14 are indicated (arrows).
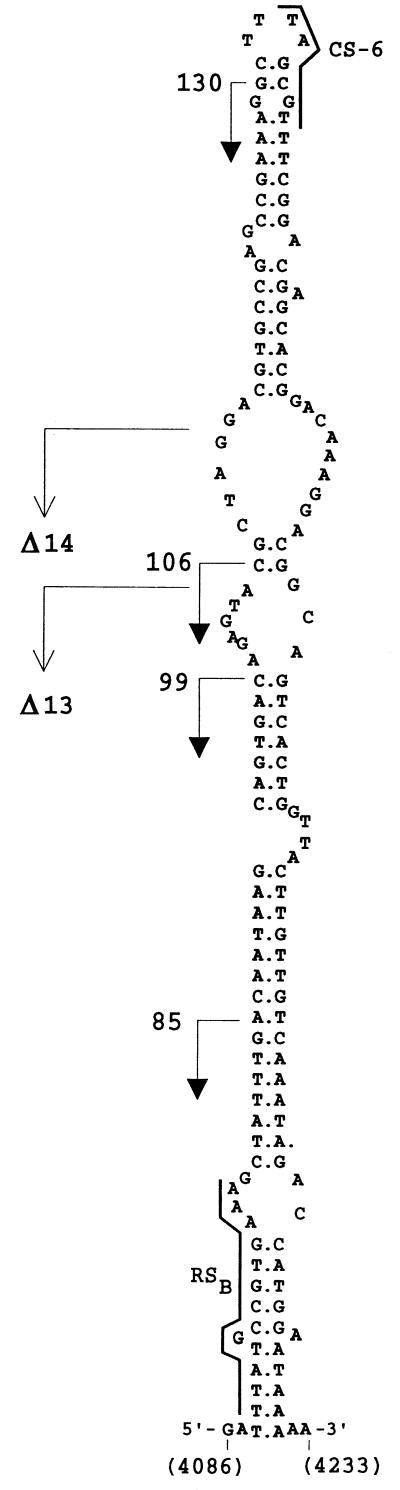
FIG. 2.
Predicted secondary structure of the pLS1-ssoA and initiation of lagging-strand synthesis in the wt pLS1 plasmid. The proposed structure of the pMV158-ssoA between the indicated coordinates, and the positions of the CS-6 and RSB sequences, and of the 3′ end of the deletions in plasmids pLS1Δ13 and pLS1Δ14 are shown. The initiation points and direction of lagging-strand synthesis, mapped in Fig. 6, are indicated by filled arrowheads along with the sizes of the bands obtained after digestion with AflII. The same bands were observed in a time course analysis from the pLS1-ssoA (15).
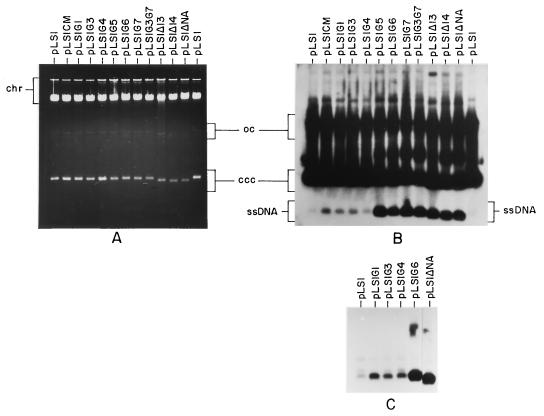
We have analyzed the roles of the RSB and CS-6 sequences in the function of the ssoA in S. pneumoniae. In this host, defects in the functionality of the pLS1-ssoA are revealed by three phenotypic features: (i) reduction in plasmid copy number measured as dsDNA, (ii) accumulation of large amounts of ssDNA, and (iii) plasmid-segregational instability is usually observed (6, 8). To determine whether any of these features were affected by the above-mentioned mutations, pLS1 derivatives containing each of the mutations were constructed, and the plasmids were transferred into S. pneumoniae. In addition, we used three previously constructed plasmids in which the pLS1-ssoA had been partially or totally deleted (Table 1 and Fig. 1): pLS1Δ13 and pLS1Δ14 (lacking the RSB but containing the CS-6) and pLS1ΔNA (total ssoA deletion by removal of the AflII-NcoI small fragment). The low amount of ssDNA accumulated in pneumococcal cells harboring the wt pLS1 ssoA allowed us to determine the variations in the amount of ssDNA generated by the various mutants. Total DNA preparations from pneumococcal cells containing the different plasmid species were separated by electrophoresis in a 0.7% agarose gel, and the DNA was transferred to nitrocellulose filters with or without prior denaturation (in the latter case, only ssDNA is transferred and detected). The results showed that, with the exception of the deletion derivatives pLS1ΔNA, pLS1Δ13, and pLS1Δ14 in which a twofold reduction in the copy number was observed (from 22 copies per chromosome in pLS1-wt to 12), no significant reduction in the copy number of the other pLS1 mutants was found (around 20) (Fig. 3A). When the levels of ssDNA accumulated were quantified, the mutated plasmids fell into two categories (Fig. 3B). First, plasmids having mutations in the CS-6 region (CM and G1 to G4) accumulated twice as much ssDNA as the wt, regardless of the nature of the mutation introduced. Second, RSB plasmid mutants (G5 to G7) or the double mutant (G3G7) exhibited a phenotype similar to plasmids either lacking this region (pLS1Δ13 and pLS1Δ14) or carrying a complete ssoA deletion (pLS1ΔNA). Cells harboring plasmids with mutations in the RSB accumulated 30-fold more ssDNA than those with mutations in the CS-6. These differences were more clearly visible when the DNAs were transferred without prior denaturation because of lack of interference with the plasmid dsDNA forms (Fig. 3C). Finally, stability tests showed that the plasmid mutants assayed (CM, G3, and G6) were as stable as pLS1 (wt ssoA) since they were stably maintained for 60 generations in the absence of selective pressure (the frequency of plasmid loss per cell per generation was ≤0.004; data not shown). On the other hand, pLS1ΔNA lacking the ssoA showed a plasmid loss per cell per generation of 0.036 (11a). From these results, we can draw the following conclusions: (i) the sequence and structure of the RSB plays a major role as a signal for ss- → dsDNA conversion in S. pneumoniae, (ii) defects in the functionality of the pLS1-ssoA, measured as large increases in the intracellular amount of ssDNA, do not necessarily lead to a reduction in the plasmid copy number, and (iii) the CS-6 also has an influence, albeit minor, on the ssoA activity. In addition, since the CM, G3, and G6 mutant plasmids were stable, we conclude that more than one region of the ssoA is involved in the stable maintenance of pLS1 and ss- → dsDNA conversion, confirming our previous results (6). Determination of the intracellular levels of ssDNA in S. pneumoniae cells containing the staphylococcal plasmid pE194 (which also contains an ssoA element [12, 21]) could not be performed since we have been unable to establish pE194 in this host.
FIG. 3.
Copy number and intracellular ssDNA accumulation of pLS1 and its derivatives in S. pneumoniae. Total DNA was isolated from the indicated plasmid-containing cultures, electrophoresed on 0.7% agarose gels in the presence of ethidium bromide (A), and transferred to nitrocellulose with (B) or without (C) prior denaturation. Note that in the latter case, only ssDNA is visible. The different plasmid forms were detected by using radiolabeled pLS1 as a probe. Both autoradiograms were overexposed to reveal the weak bands corresponding to ssDNA from plasmids with mutations in the CS-6 region. The positions of the supercoiled monomeric plasmid form (ccc), open circular dsDNA (oc) and ssDNA are indicated. chr, chromosomal DNA.
Development of an S. pneumoniae replication system for in vitro DNA synthesis.
Plasmid pLS1 accumulated large amounts of ssDNA when transferred to S. aureus (not shown), as was the case with E. coli and B. subtilis (6, 8), indicating that the pLS1-ssoA was poorly functional in these hosts. Consequently, we decided to develop an in vitro replication system from S. pneumoniae, the host in which the pLS1-ssoA is fully functional. Based on previously described cell-free plasmid replication systems from E. coli (5) and S. aureus (1), we set up a new cell-free system from S. pneumoniae. Pneumococcal cells were subjected to lysis by the use of purified pneumococcal autolysin, since neither lysozyme nor lysostaphin (used for E. coli and S. aureus, respectively) acts on the pneumococcal cell wall. To optimize the lysis conditions, we tested different amounts of autolysin, reaction times, and temperatures of assay. Optimal conditions were as described in Materials and Methods.
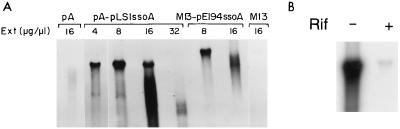
To analyze whether the pneumococcal cell extracts were able to support DNA replication from the pLS1- and pE194-ssoAs, de novo synthesis of dsDNA from ssDNA substrates was assayed (9). The sources of ssDNAs were phagemid pALTER-1 or pA-pLS1ssoA and coliphage M13 or M13-pE194ssoA (Table 2). Various amounts of extract were tested (Fig. 4). The assays were performed at 32°C for 60 min, conditions which allowed completion of lagging-strand synthesis (15). DNA in the reaction mixtures was purified and linearized with HindIII (to obtain a single band of dsDNA). Newly synthesized DNA was visualized by agarose gel electrophoresis and autoradiography. Optimal DNA synthesis was obtained with 8 μg of protein/μl of reaction mixture (about 240 μg of cell protein), whereas higher amounts resulted in DNA degradation (Fig. 4A). DNA synthesis from ssDNA substrates lacking ssoA (pALTER-1 and M13) was negligible. Furthermore, DNA synthesis was inhibited by the presence of the RNAP-inhibitor rifampin (Fig. 4B), indicating the involvement of this enzyme in synthesis from the ssoA. We conclude that the newly developed cell-free replication system prepared from S. pneumoniae supports efficient and specific DNA synthesis from ssDNAs containing the ssoA of pLS1 or pE194. In addition, synthesis from the pE194-ssoA seemed to be less efficient than that from the pLS1-ssoA.
FIG. 4.
In vitro replication from pLS1-ssoA and pE194-ssoA in the pneumococcal cell-free system (A) and inhibition of replication from the pLS1-ssoA by rifampin (B). (A) A total of 150 ng of ssDNA isolated from pA-pLS1ssoA and M13-pE194ssoA was used as the template. As negative controls, ssDNAs from both vectors pALTER-1 and M13mp19 (pA and M13, respectively) were employed. Each reaction was carried out in a volume of 30 μl, incubated for 60 min at 32°C with different concentrations of proteins from the cell extract (Ext). The reaction products were digested with HindIII and electrophoresed on a 1% agarose gel for 12 h at 2.5 V/cm. The autoradiogram of the dried gel is shown. (B) Similar assays were performed with DNA from pA-pLS1ssoA in the absence (−) or in the presence (+) of rifampin (Rif) (100 μg/ml).
Influence of ssoA mutations on in vitro DNA synthesis.
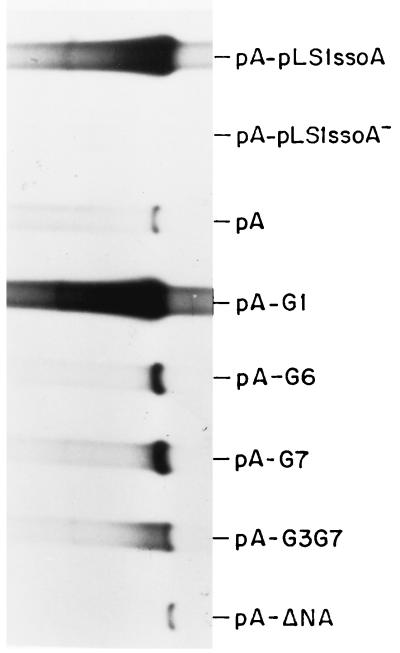
Cells containing pLS1 derivatives with mutations in the ssoA exhibited two categories of phenotypes related to the amount of intracellular ssDNA accumulated (Fig. 3): moderate (CS-6 mutants) or high (mutations in the RSB). To test whether this situation could be reproduced in the in vitro replication system, we constructed phagemids with some of the ssoA mutations representing each category (Tables 1 and 2). Thus, the following phagemids were constructed: pA-G1, changes in the CS-6; pA-G6, changes in the local structure of RSB; pA-G7, changes in the conserved sequence of the RSB; and pA-G3G7, changes in both the conserved sequences. In addition, we cloned a DNA region encompassing the pLS1-ssoA in the nonfunctional orientation (pA-pLS1ssoA−) or a DNA region from a plasmid lacking ssoA (pA-ΔNA) in the vector pALTER-1. From the various phagemids, ssDNA was generated and used as the substrate for in vitro DNA synthesis in the pneumococcal extracts as described above. The results (Fig. 5) can be summarized as follows. The efficiency of in vitro ss- → dsDNA conversion obtained with the G1 mutant was very similar to that with the wt origin. However, alterations in the RSB region led to 17- and 20-fold decreases in the level of lagging-strand synthesis for the G7 and G6 mutants, respectively. These results agree with those obtained in vivo, supporting a major role of the RSB region in the activity of the ssoA. No DNA synthesis was observed when the substrates lacked ssoA (pA-ΔNA) or contained it in a nonfunctional orientation (pA-pLS1ssoA−), confirming the specificity and the orientation dependence of the pLS1-ssoA in the in vitro pneumococcal system.
FIG. 5.
Lagging-strand synthesis from the mutagenized ssoA in S. pneumoniae. Reactions were performed as described in the legend for Fig. 4 but 8 μg of protein per 30 μl of reaction mixture (240 μg) and the ssDNA generated from the indicated recombinant phagemids were used. The autoradiogram was overexposed to visualize the bands corresponding to the RSB mutants (pA-G6 and pA-G7) and to the double mutant (pA-G3G7).
What is the role of RSB in the lagging-strand replication in S. pneumoniae? In vitro pRNA synthesis from the pLS1-ssoA containing mutations in the RSB is abolished due to the failure of the RNAP to bind to its target (15). In addition, pLS1 derivatives containing the same mutations behaved in vivo like plasmids lacking ssoA, as evidenced by the accumulation of intracellular ssDNA intermediates (Fig. 3). Taking these results together, we propose that both in vitro and in vivo binding of the RNAP to the RSB and the subsequent RNAP-directed synthesis of the pRNA are essential steps in the initiation of lagging-strand DNA synthesis. Due to the fact that (i) replication is inhibited by the RNAP-inhibitor rifampin both in vitro (9) (Fig. 4B) and in vivo (2, 14, 28, 29) and (ii) in vitro synthesis of the pRNA absolutely requires an intact RSB for RNAP binding (15), we conclude that synthesis of the pRNA in the ssoA-containing plasmids is achieved by a main pathway involving the host RNAP and that other host-encoded functions (DNA primase or primosome) do not seem to be involved in this primary pathway. We do not rule out the possibility that alternative mechanisms for ss- → dsDNA conversion exist. In fact, three observations point to the existence of secondary pathways of conversion: (i) DNA primase may participate in lagging-strand synthesis in ssoW-containing plasmids (28), (ii) plasmids lacking ssoA are able to replicate if selective pressure is maintained (14, 18, 19), and (iii) this replication is insensitive to RNAP inhibitors (14).
Specificity of in vitro lagging-strand synthesis: influence of ssoA mutations.
In vitro DNA synthesis from the pLS1-ssoA initiates specifically at four major positions in S. pneumoniae (15). These sites were mapped by employment of partially replicated molecules obtained in the in vitro extracts which were then treated or not treated with a restriction enzyme that cleaves downstream of the expected initiation sites of DNA replication from the ssoA (9, 15). In the case of the pLS1-ssoA, the enzyme chosen was AflII, which cleaves downstream of the replication start points (Fig. 1). Denaturation of the DNA releases replication products whose sizes correspond to the distance between the initiation sites of lagging-strand synthesis and the enzyme cleavage site. Lack of specificity in initiation of DNA synthesis is revealed either by lack of defined bands in the reaction products or by the presence of the same bands in both the digested and undigested samples. Bands that are present in the undigested samples indicate random initiation and/or end points of ss- → dsDNA synthesis and do not reveal the specific initiation sites (9).
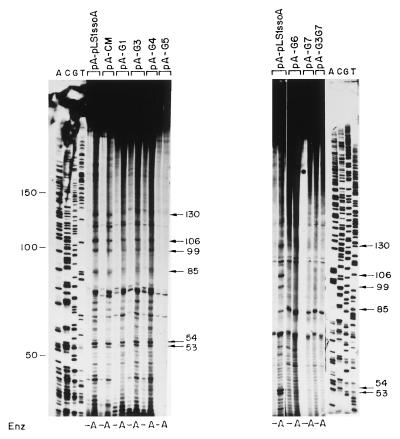
To determine if the specificity of initiation of lagging-strand synthesis was maintained when mutations were introduced in the ssoA, we determined the initiation points of DNA synthesis by using ssDNA substrates containing the mutated ssoA and compared these with those obtained with the wt ssoA (Fig. 6). We used the S. pneumoniae extracts, and ssDNA samples were allowed to partially replicate for 20 min. In the case of the wt DNA samples digested with AflII, four main bands of 130, 106, 99, and 85 nt were observed (Fig. 6). RNA primers were already removed from these products since treatment of the samples with RNase A after denaturation of the samples did not change the sizes of these bands (data not shown). Since a 20-nt RNA primer is synthesized from the pLS1 ssoA (15), the above-mentioned bands likely represent initiation sites of DNA synthesis (RNA-DNA transition points). The sizes of these bands position the first RNA-DNA transition point near the CS-6 sequence (Fig. 2). Results obtained with the ssoA mutants fell into two categories (Fig. 6). Alterations in the CS-6 (CM, G1, G3, and G4) resulted in ssDNA substrates with initiations at positions identical to the wt ssoA. Nevertheless, the 130-nt band (and to a lesser extent the other three bands), which was clearly visible in ssDNAs from the wt and CM mutant, was slightly reduced (1.4-fold) in the reactions performed with the G1, G3, and G4 mutants. DNA synthesis from ssDNA with mutations in the RSB (G5 to G7 and G3G7) was mostly nonspecific, since the same bands were generally seen in the AflII-treated and untreated samples. Two additional bands of 53 and 54 nt were observed after AflII digestion, and they were more intense in the substrates containing the ssoA wt and CS-6 mutations than in those with mutations in the RSB (Fig. 6). Although the origin of these bands is not clear, these small products have been interpreted as precursors to the larger replicated products since their levels were reduced upon an increase in the incubation time (9). Another major band migrating between the 85- and the 53/54-nt bands probably does not correspond to a specific initiation point since this band was frequently seen in samples untreated with AflII (Fig. 6, pA-pLS1ssoA [right]; also data not shown). The above results suggest that the specificity of lagging-strand initiation is severely reduced or abolished when the RSB region is altered. Changes in the CS-6 lead to a reduction in the levels of the specific bands, but the specificity of initiation is maintained. The start sites of lagging-strand synthesis from the pE194-ssoA have previously been determined in S. aureus (9). When the wt pE194-ssoA was tested in the pneumococcal extracts, the results resembled those obtained with pLS1-ssoA with mutations in the RSB (not shown). This behavior could be due to at least two reasons: (i) replication from the pE194-ssoA starts at multiple nonspecific positions or (ii) specific initiation from the pE194-ssoA does not occur from the same positions as that in pLS1-ssoA, and perhaps the specific replication products were not detected due to the particular restriction enzymes used.
FIG. 6.
Determination of the initiation points of lagging-strand synthesis from the mutagenized pLS1-ssoA in S. pneumoniae. The ssDNAs generated from the indicated recombinant phagemids were employed as replication templates. After a 20-min reaction, the DNA was purified and one half of each sample was digested with AflII (A) or left undigested (−). DNA in the samples was denatured by boiling prior to separation of the reaction products on an 8% polyacrylamide-urea gel. ACGT, sequencing ladder generated by the Sanger method (27). Numbers indicate sizes of bands in nucleotides.
In conclusion, here we have shown a correlation between the in vitro and the in vivo results in S. pneumoniae. Furthermore, results obtained with strains containing plasmid derivatives with mutations in the conserved regions of the pLS1-ssoA demonstrated that RSB acts as the primary signal for lagging-strand synthesis, which agrees with this region being the place where RNAP binds (15). It is likely that the poor ss- → dsDNA conversion observed in vivo in pLS1 derivatives affected in the RSB is the consequence of initiation of lagging-strand replication at multiple, nonspecific sites. Alterations in the CS-6 region result in (i) a twofold reduction in the level of pRNA synthesis and (ii) synthesis of pRNAs longer than 20 nt (15). This would lead to a moderate increase of intracellular ssDNA accumulation (also twofold), but the specificity of initiation would be maintained. In spite of the homologies between the ssoA regions of the streptococcal (pLS1) and staphylococcal (pE194) plasmids, the differences in the ability of their ssoA sequences to support replication in S. pneumoniae are intriguing. This observation is consistent with the results of previous studies showing that the ssoAs are generally functional only in their natural hosts (11, 13, 21). Perhaps different host factors may be responsible for the recognition of closely related ssoA sequences in bacteria, and this may play an important role in determining the host range of RCR plasmids (4). Since there are several RCR plasmids containing ssoAs homologous to that of pLS1, it would be interesting to perform experiments similar to those reported here but using different hosts in which ssoA-containing plasmids have been isolated (11, 17, 21, 32). This should provide information on the factors involved in host and/or sso recognition and on the generality of the initiation mechanisms of lagging-strand replication in RCR plasmids in different hosts.
ACKNOWLEDGMENTS
Thanks are due to R. López for his gift of pneumococcal autolysin, to G. del Solar and P. López for helpful discussions and comments, and to M. T. Alda for her help in preparation of DNA samples.
This research was supported by CICYT grant BIO97-0347 (to M.E.) and by National Institutes of Health grant GM31685 (to S.A.K.).
REFERENCES
- 1.Birch P, Khan S A. Replication of single-stranded plasmid pT181 DNA in vitro. Proc Natl Acad Sci USA. 1992;89:290–294. doi: 10.1073/pnas.89.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boe L, Gros M F, te Riele H, Ehrlich S D, Gruss A D. Replication origins of single-stranded DNA plasmid pUB110. J Bacteriol. 1989;171:3366–3372. doi: 10.1128/jb.171.6.3366-3372.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B) Antimicrob Agents Chemother. 1980;18:753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Solar G, Alonso J C, Espinosa M, Díaz-Orejas R. Broad host range plasmid replication: an open question. Mol Microbiol. 1996;21:661–666. doi: 10.1046/j.1365-2958.1996.6611376.x. [DOI] [PubMed] [Google Scholar]
- 5.del Solar G, Díaz R, Espinosa M. Replication of the streptococcal plasmid pMV158 and derivatives in cell-free extracts of Escherichia coli. Mol Gen Genet. 1987;206:428–435. doi: 10.1007/BF00428882. [DOI] [PubMed] [Google Scholar]
- 6.del Solar G, Kramer M G, Ballester S, Espinosa M. Replication of the promiscuous plasmid pLS1: a region encompassing the minus origin of replication is associated with stable plasmid inheritance. Mol Gen Genet. 1993;241:97–105. doi: 10.1007/BF00280206. [DOI] [PubMed] [Google Scholar]
- 7.del Solar G, Moscoso M, Espinosa M. Rolling circle-replicating plasmids from Gram-positive and Gram-negative bacteria: a wall falls. Mol Microbiol. 1993;8:789–796. doi: 10.1111/j.1365-2958.1993.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 8.del Solar G, Puyet A, Espinosa M. Initiation signals for the conversion of single stranded to double stranded DNA forms in the streptococcal plasmid pLS1. Nucleic Acids Res. 1987;15:5561–5580. doi: 10.1093/nar/15.14.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey L A, Zhao A C, Khan S A. Localization of the start sites of lagging-strand replication of rolling circle plasmids from Gram-positive bacteria. Mol Microbiol. 1995;15:679–687. doi: 10.1111/j.1365-2958.1995.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 10.Gruss A D, Ross H F, Novick R P. Functional analysis of a palindromic sequence required for normal replication of several staphylococcal plasmids. Proc Natl Acad Sci USA. 1987;84:2165–2169. doi: 10.1073/pnas.84.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruss A D, Ehrlich S D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989;53:231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Hernandez-Arriaga, A. M. Personal communication.
- 12.Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamine, and streptogramin type B antibiotics. J Bacteriol. 1982;150:804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan S A. Mechanism of replication and copy number control of plasmids in Gram-positive bacteria. In: Setlow J K, editor. Genetic engineering. Vol. 18. New York, N.Y: Plenum Press; 1996. pp. 183–201. [DOI] [PubMed] [Google Scholar]
- 14.Kramer M G, del Solar G, Espinosa M. Lagging-strand origins of the promiscuous plasmid pMV158: physical and functional characterization. Microbiology. 1995;141:655–662. doi: 10.1099/13500872-141-3-655. [DOI] [PubMed] [Google Scholar]
- 15.Kramer M G, Khan S A, Espinosa M. Plasmid rolling circle replication: identification of the RNA polymerase-directed primer RNA and requirement of DNA polymerase I for lagging strand synthesis. EMBO J. 1997;16:5784–5795. doi: 10.1093/emboj/16.18.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacks S A, López P, Geenberg B, Espinosa M. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J Mol Biol. 1986;192:753–765. doi: 10.1016/0022-2836(86)90026-4. [DOI] [PubMed] [Google Scholar]
- 17.Leenhouts K J, Tolner B, Bron S, Kok J, Venema G, Seegers J F M L. Nucleotide sequence and characterization of the broad-host-range lactococcal plasmid pWV01. Plasmid. 1991;26:55–66. doi: 10.1016/0147-619x(91)90036-v. [DOI] [PubMed] [Google Scholar]
- 18.Meijer W J J, van der Lelie D, Venema G, Bron S. Effects of the generation of single-stranded DNA on the maintenance of plasmid pMV158 and derivatives in different Bacillus subtilis strains. Plasmid. 1995;33:79–89. doi: 10.1006/plas.1995.1010. [DOI] [PubMed] [Google Scholar]
- 19.Meijer W J J, van der Lelie D, Venema G, Bron S. Effects of the generation of single-stranded DNA on the maintenance of plasmid pMV158 and derivatives in Lacotococcus lactis. Plasmid. 1995;33:91–99. doi: 10.1006/plas.1995.1011. [DOI] [PubMed] [Google Scholar]
- 20.Murray R W, Koepsel R R, Khan S A. Synthesis of single-stranded plasmid pT181 DNA in vitro: initiation and termination of DNA replication. J Biol Chem. 1989;264:1051–1057. [PubMed] [Google Scholar]
- 21.Novick R P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- 22.Novick R P, Projan S J, Rosenblum W, Edelman I. Staphylococcal plasmid cointegrates are formed by host- and phage-mediated general rec systems that act on short regions of homology. Mol Gen Genet. 1984;195:374–377. doi: 10.1007/BF00332777. [DOI] [PubMed] [Google Scholar]
- 23.Priebe S D, Lacks S A. Region of the streptococcal plasmid pMV158 required for conjugative mobilization. J Bacteriol. 1989;171:4778–4784. doi: 10.1128/jb.171.9.4778-4784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Projan S J, Carleton S, Novick R P. Determination of plasmid copy number by fluorescence densitometry. Plasmid. 1983;9:182–190. doi: 10.1016/0147-619x(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 25.Projan S J, Novick R P. Comparative analysis of five related staphylococcal plasmids. Plasmid. 1988;19:203–221. doi: 10.1016/0147-619x(88)90039-x. [DOI] [PubMed] [Google Scholar]
- 26.te Riele H, Michel B, Ehrlich S D. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986;5:631–637. doi: 10.1002/j.1460-2075.1986.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Seegers J F M L, Zhao A C, Meijer W J J, Khan S A, Venema G, Bron S. Structural and functional analysis of the single-strand origin of replication from the lactococcal plasmid pWV01. Mol Gen Genet. 1995;249:43–50. doi: 10.1007/BF00290234. [DOI] [PubMed] [Google Scholar]
- 29.Seery L T, Devine K M. Analysis of features contributing to activity of the single-stranded origin of Bacillus plasmid pBAA1. J Bacteriol. 1993;175:1988–1994. doi: 10.1128/jb.175.7.1988-1994.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Lelie D, Bron S, Venema G, Oskam L. Similarity of minus origins of replication and flanking open reading frames of plasmids pUB110, pTB913 and pMV158. Nucleic Acids Res. 1989;17:7283–7294. doi: 10.1093/nar/17.18.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 32.Zaman S, Radnedge L, Richards H, Ward J M. Analysis of the site for second-strand initiation during replication of the Streptomyces plasmid pIJ101. J Gen Microbiol. 1993;139:669–676. doi: 10.1099/00221287-139-4-669. [DOI] [PubMed] [Google Scholar]