Abstract
Background:
Previous studies have shown that the perioperative and postoperative chemotherapy can lead to an improvement in the prognosis of patients with resectable gastric cancer (GC). There is no preference for postoperative chemotherapy with the two common treatment regimens, FLOT and FOLFOX, in these patients. The aim of this study was to compare FOLFOX and FLOT regimens in perioperative chemotherapy in resectable GC based on pathological response and complications.
Methods:
This prospective cohort study was conducted on 112 patients with resectable GC who were admitted to Firozgar Hospital affiliated with Iran University of Medical Sciences, Tehran, Iran between 2021 to 2022. Given the inclusion criteria, 80 patients were enrolled in the present study. Patients were divided into 2 groups based on the type of treatment regimen, FOLFOX (40 patients) and FLOT (40 patients). Tumor response was classified using Mandard Tumor regression grading system criteria into five categories of TRG1 to 5. Also, the side effects were classified according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 5.0.
Results:
The rate of complete pathological response in FOLT group was significantly higher than FOLFOX group (35.0% vs 2.5%, p: 0.001). The frequency of neurological complications and hair loss in the FOLT group was significantly higher than the FOLFOX group (P<0.05). While no significant difference was observed in the frequency of hematological, Gastroenterological, hepatic, renal and stomatitis complications in the both groups (p>0.05).
Conclusion:
Our study showed that perioperative FLOT regimen has a better pathological response than FOLFOX regimen. The frequency of neurological complications and hair loss was significantly higher in patients treated with FLOT regimen. Thus, perioperative FLOT regimen may be recommended for treating GC patients.
Key Words: Gastric cancer, perioperative chemotherapy, FLOT regimen, FOLFOX regimen
Introduction
Gastric cancer (GC) is one of the fatal cancers in the world. GC is the fifth and third cause of cancer-related death in women and men, respectively (Talebi et al., 2020; Thrift and El-Serag, 2020). GC accounts for 10.4% of all cancer deaths, and every year about 930,000 people in the world are diagnosed as a new case of this disease, and at least 700,000 of them die because of this disease (Parkin et al., 2005). The survival rate GC depends on various factors such as stage of the disease, condition of the disease, access to medical services and treatment method (Talebi et al., 2020). Also weight loss , leukocytosis and asthenia are poor prognostic factor (Trujillo-Rivera et al., 2021). Despite the progress made in the field of treatment, the five-year survival rate is low. The survival rates of patients have been reported based on the type of treatment methods (Eusebi et al., 2020). Cancer treatment is usually done by three methods: surgery, radiotherapy and chemotherapy. The main treatment for the GC in the early stages is surgery. Perhaps the most important measure necessary to increase the success of this type of cancer treatment is early diagnosis and the application of combined methods (Talebi et al., 2020). Previous studies have reported the positive effects of the perioperative and postoperative chemotherapy compared to surgery alone in increasing the success rate of treatment in GC. The perioperative and postoperative chemotherapy leads to an increase in overall survival (OS), progression-free survival (PFS) and curative resection rate (Cunningham et al., 2006; Petrillo et al., 2019; Charruf et al., 2020; Dos Santos et al., 2022). A systematic review and meta-analysis showed that the perioperative and postoperative chemotherapy significantly increased the five-year OS rate compared to surgery alone (Jiang et al., 2015).
The choice of the best perioperative and postoperative chemotherapy for the better management of patients with gastric cancer is still debated. Based on previous studies, FOLFOX regimen (combination of 5-fluorouracil, leucovorin, and oxaliplatin) seems to be an effective and tolerable regimen as perioperative chemotherapy in the treatment of resectable GC (Wang et al., 2019b). Studies have shown that the use of newer treatment regimens including the FLOT regimen (including 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel) increase the rate of treatment success and the overall survival of patients compared to surgery alone (Al-Batran et al., 2004).
The superiority of the FLOT regimen compared to the ECF regimen (including 5-fluorouracil, epirubicin, and cisplatin) has also been noted in clinical trials (Al-Batran et al., 2019; Rivera et al., 2021; Farrokhi et al., 2022a). These findings have made the FLOT diet to be considered as one of the first-line treatments for patients suffering from operable gastric cancer (Tastekin et al., 2023). The treatment of GC patients is associated with various complications, and the selection of the appropriate type of treatment in these patients should be based on the patient’s clinical condition (Li et al., 2011). The FOLFOX regimen can be considered a safe regimen for elderly patients or patients who cannot tolerate a more intense regimen (Al-Batran et al., 2004; Wang et al., 2016; Patil et al., 2020; Farrokhi et al., 2022b). While complications such as severe neutropenia or mucositis, nausea, vomiting and diarrhea have been reported in patients receiving the FLOT regimen with a higher prevalence compared to the ECF regimen (Schulz et al., 2012; Al-Batran et al., 2019; Wang et al., 2019b; Watson et al., 2019; Ganschow et al., 2021). Therefore, although the available evidence has shown that the perioperative and postoperative chemotherapy can lead to an improvement in the prognosis of patients who suffer from operable tumors of the stomach, but studies to compare these regimens in terms of benefits and toxicity are lacking. It is needed to enable doctors to choose the appropriate treatment regimen. Therefore, considering the high prevalence of gastric cancer and the limited number of studies conducted in this field, the aim of this study was to compare perioperative FOLFOX and FLOT regimens in operable gastric cancers based on pathologic response. The results of this study can help to choose the right treatment with a higher success rate and less complications for patients with GC.
Materials and Methods
Design, patients and study setting
The present study was approved by the ethics committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1400.194). This prospective cohort study was conducted on 112 patients with resectable GC who were treated at Firouzgar Hospital affiliated with Iran University of Medical Sciences between 2021 and 2022. Given the inclusion criteria, 80 patients were included in the study. The treatment was done routinely for the patients and the researcher had no intervention in the treatment process of the patients. Patients were divided into two groups based on the type of treatment received. Group 1 included 40 patients who underwent perioperative FLOT regimen, and group 2 included 40 patients who underwent perioperative FOLFOX regimen. Informed consent was obtained from all patients.
Inclusion and exclusion criteria
Inclusion criteria included: age 20 to 80 years, definitive diagnosis of advanced primary gastric adenocarcinoma through Histopathological examination, endoscopic ultrasound and CT scan before surgery and acceptance and tolerance of chemotherapy. Presence of distant metastasis or intolerance to surgery, active bleeding or complete pyloric obstruction, history of chemotherapy, radiotherapy, immunotherapy or targeted treatments, patients with a history of malignant tumors except basal cell carcinoma of the skin treated and carcinoma in situ of the cervix and a history of total gastrectomy were defined as exclusion criteria.
Treatment method
For the patients who were included in the FLOT group, chemotherapy was prescribed for 4 cycles before surgery and 4 cycles after surgery. For these patients, treatment was combination of 3 drugs including docetaxel at the rate of 50 mg/m2 on day 1, oxaliplatin 85 mg/m2 on day 1 and 5-fluorouracil 2,600 mg/m2 as continuous injection over 24 hours on day 1 along with prescription of leucovorin 200 mg/m2 on day 1 to reduce side effects. For the patients who were placed in the FOLFOX group, chemotherapy was prescribed for 6 cycles before surgery and 6 cycles after surgery. For these patients, treatment with oxaliplatin 85 mg/m2 as an intravenous injection for 2 hours , 5-fluorouracil 400 mg/m2 bolus then 2,400 mg/m2 as a continuous injection over 46 hours and leucovorin 200 mg/m2 on the first day was administrated. Endoscopic ultrasound and contrast-enhanced CT scan were performed before the fourth cycle of chemotherapy for comprehensive evaluation.
Surgery was performed for patients after 4 weeks of neoadjuvant chemotherapy. At this stage, patients underwent total or distal gastrectomy based on the location or size of the tumor. After distal gastrectomy, Billrouth II reconstruction was used, and after total gastrectomy, Roux-en-Y anastomosis was used. Also, patients received intravenous nutritional support and antibiotic treatment after gastrointestinal decompression surgery. In addition, chemotherapy regimens were continued after surgery.
Outcome
Tumor response was classified according to Mandard Tumor regression grading (TRG) system criteria into five categories as follows: complete regression (TRG1), fibrosis with scattered tumor cell (TRG2), fibrosis and tumor cells with a dominance of fibrosis (TRG3), fibrosis and tumor cells with a dominance of tumor cells (TRG4) and tumor without evidence of regression (TRG5). All pathology findings were evaluated by an oncologist. Also, the side effects were classified according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 5, which includes hematological, gastrointestinal, liver, kidney, neurological, and hair loss complications during the course of chemotherapy which were recorded for each patient. In cases of unbearable side effects and the patient’s lack of tolerance and acceptance, neoadjuvant chemotherapy was stopped and the patient was excluded from the study. Finally, the tumor pathological response and side effects were compared between the two groups treated with FLOT and FOLFOX regimens.
Statistical analyses
The data was analyzed using SPSS version 22 statistical software. Descriptive statistics (frequency and %) were used to report qualitative variables. Quantitative variables were reported with mean and standard deviation. Normality of quantitative variables was tested by Kolmogorov -Smirnov test. T-test was used to compare quantitative variables in the two treatment groups. Chi-square test was used to compare qualitative variables in the two groups. Multivariate logistic regression analysis was used to control confounding variables in two groups. The effect size in two groups was reported with the adjusted odds ratio (Adj OR) in the 95% confidence interval (95% CI). A P value <0.05 was considered as the level of statistical significance.
Results
Comparison of demographic characteristics and tumor characteristics
The mean age of patients in FOLFOX group and FOLT group was 63.2 ±6.59 and 61.3 ±8.21years, respectively (P: 0.18). Respectively, 60% and 70% of patients in FOLFOX group and FOLT group were male. In terms of tumor size, the majority of patients in both groups were larger than T2. No significant difference was observed for demographic characteristics and tumor characteristics in the two groups (p>0.05) Table 1.
Table 1.
Comparison of Demographic Characteristics and Tumor Characteristics in Two Groups
| Variable | Group | P value | ||
|---|---|---|---|---|
| Total (N:80) |
FOLFOX (N:40) |
FOLT (N:40) |
||
| Age (Year) | 62.27±8.22 | 63.2±6.59 | 61.3±8.21 | 0.18* |
| Sex (n%) | 0.34** | |||
| Male | 52 (65.0) | 24 (60) | 28 (70) | |
| Female | 28 (35.0) | 16 (40) | 12 (30) | |
| BMI (Kg/m2) | 23.95±3.4 | 24.53±3.2 | 23.61±2.9 | 0.57* |
| History of smoking | 40 (50.0) | 21 (52.5) | 19 (47.5) | 0.16** |
| Tumor size | 0.23** | |||
| T1 | 7 (8.75) | 4 (10) | 3 (7.5) | |
| T2 | 8 (10.0) | 4 (10) | 4 (40) | |
| T3 | 48 (60.0) | 24 (60) | 24 (60) | |
| T4 | 17 (21.25) | 8 (20) | 9 (22.5) | |
| Degree of differentiation Tumor | 0.14** | |||
| Poor | 41 (51.25) | 23 (57.5) | 18 (45) | |
| Moderate | 27 (33.75) | 9 (22.5) | 18 (45) | |
| Well | 11 (13.75) | 7 (17.5) | 4 (10) | |
*, t-test; **, chi-square
Comparison of response to treatment and degree of improvement in the two treatment groups
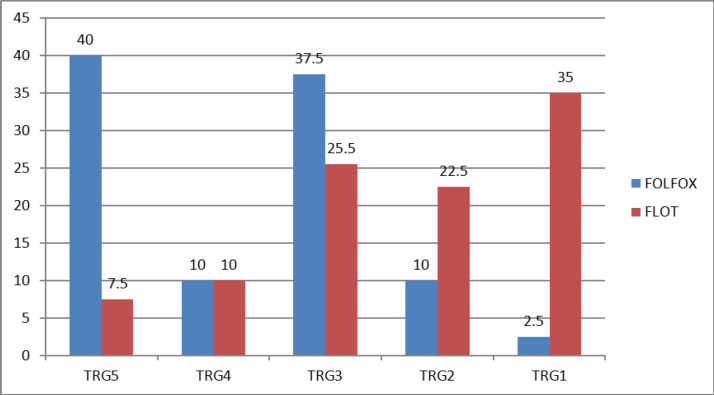
The rate of complete pathological response in FOLT group was significantly higher than FOLFOX group (35.0% vs. 2.5%, p: 0.001) (Figure 1).
Figure 1.
Comparison of Improve Degree or Response to Treatment in Two Treatment Groups
Comparison of the frequency of complications in two groups
Although the frequency of hematological complications (G2 and G3) was slightly higher in the FOLT group compared to the FOLFOX group, this difference was not statistically significant (p: 0.061). No significant difference was observed in the frequency of gastrointestinal (Vomiting, Diarrhea and Nausea), liver, kidney and stomatitis complications in the two groups (p>0.05). In general, 3 deaths were reported (1 case in the FOLT group and 2 cases in the FOLFOX group), and this difference was not statistically significant (P: 0.56) Table 2.
Table 2.
Comparison of the Frequency of Complications in Two Groups
| Side complications | Group | P value | |
|---|---|---|---|
| FOLFOX (N:40) |
FOLT (N:40) |
||
| Hematological (N %) | 0.061 | ||
| G1 | 30 (75) | 28 (70) | |
| G2 | 8 (20) | 6 (15) | |
| G3 | 1 (2.5) | 6 (15) | |
| Metastasis | 1 (2.5) | 0 | |
| Gastroenterological (Vomiting, Diarrhea &Nausea ((N %) | 10 (25) | 14 (35) | 0.36 |
| Liver (N %) | 1 (2.5) | 3 (7.5) | 0.081 |
| Kidney (N %) | 3 (7.5) | 1 (2.5) | 0.15 |
| Stomatitis (N %) | 8 (20) | 6 (15) | 0.41 |
| Hair loss (N %) | 3 (7.5) | 18 (45) | 0.001 |
| Neurological (N %) | 12 (30) | 26 (65) | 0.002 |
| Death (N %) | 2 (5) | 1 (2.5) | 0.56 |
**, chi-square
Factors predicting response to treatment in two groups based on multivariate analysis
The results of multivariate logistic regression analysis showed that the level of complete pathological response and significance (TRG1,2) to FOLFOX regimen was significantly better with younger age (adj OR: 0.91, 95% CI: 0.84, 0.99, P: 0.022), in women (adj OR: 5.74, 95% CI: 1.23, 18.56, P: 0.028) and in patients with tumor size lower than T2 (adj OR: 3.08, 95% CI: 1.21, 5.11, P: 0.037). Moreover, in patients treated with FOLT, the improvement rate was better in patients with tumor size lower than T2 (adj OR: 2.84, 95% CI: 1.29, 5.66, P: 0.036). While there was no significant relationship between the response rate and other background variables and tumor characteristics in the two treatment groups (P>0.05) Table 3.
Table 3.
Factors Predicting Response to Treatment in Two Groups based on Multivariate Analysis
| Variable | adj OR | 95% CI | P value |
| FOLFOX | |||
| Age | 0.91 | 0.84, 0.99 | 0.022 |
| Sex ( male vs female) | 5.74 | 1.23, 18.56 | 0.028 |
| Size ( >T2 vs =<T2) | 3.08 | 1.21,5.11 | 0.037 |
| FOLT | |||
| Size (>T2 vs =<T2) | 2.84 | 1.29,5.66 | 0.036 |
Discussion
According to cancer treatment guidelines, there is no preference for perioperative chemotherapy with two common treatment regimens, FLOT and FOLFOX, in patients with resectable GC, and both treatments are routinely used in Iranian patients. Despite conducting studies in this field, the rate of response to treatment and the complications of using these treatments have not been clearly determined. Therefore, considering the importance of this issue and also preventing the occurrence of serious complications and choosing the appropriate treatment in these patients, with the knowledge of the outcomes of these treatment methods, in this prospective study, we evaluated the rate of pathological response to treatment and the frequency of side effects following perioperative chemotherapy with two common treatment regimens, FLOT and FOLFOX, in patients with operable gastric cancer.
Our study showed that the mean age of the patients was 62.27 years and more than half of the patients were less than 63 years old. No significant difference was observed in the mean age of patients in the FOLFOX and FOLT treatment groups. Nearly two-thirds of the patients were male, and the tumor size was greater than T2 in the majority of patients. No significant difference was observed in tumor size, degree of tumor differentiation, sex and other characteristics of patients. The degree of complete improvement and significance (complete pathological response) in the treatment with FOLT was significantly higher than the treatment with FOLFOX. No response to treatment was observed in 40% of patients treated with FOLFOX, while this rate was only 7.5% for FOLT treatment. In line with the results of our study, Schulz et al. showed in the phase two of a clinical trial by examining 50 patients who received the FLOT regimen preoperatively that this regimen significantly increased the survival rate without disease progression compared to surgery (Schulz et al., 2012). The benefits of this treatment include reducing the stage of the disease, increasing the amount of R0 resection (without microscopic involvement of the surgical margin), tumor regression, and avoiding unnecessary surgery. Therefore, a significant pathological response to chemotherapy can play a significant role in improving the prognosis of patients (Boige et al., 2007; Schuhmacher et al., 2010). Al-Batran et al. by comparing FLOT and ECF chemotherapy regimens on 356 and 360 patients with resectable GC, showed the superior efficacy of perioperative FLOT regimen in the management of patients with resectable GC (Al-Batran et al., 2019). Farrokhi et al. Showed that the FLOT chemotherapy regimen in patients with resectable GC led to a significant improvement in OS and progression-free survival compared to other chemotherapy regimens including the FOLFOX regimen, which was consistent with the results of our study (Farrokhi et al., 2022a). While contrary to the results of our study, Beliak et al. By examining 79 patients including 44 patients treated with FLOT regimen and 35 patients treated with FOLFOX regimen preoperatively, did not report a significant difference in the degree of tumor histopathological regression between the two groups (Beliak et al., 2021). In this study, the tumor regression grade (TRG) factor was used to evaluate the response to treatment, which may be effective in the difference observed in the results of the two studies. Chen et al. By comparing two chemotherapy regimens FOLFOX and EOX in patients with advanced gastric cancer, showed only 19.5% of patients treated with FOLFOX regimen had significant tumor regression and complete tumor regression was observed in only 4.6% of patients (Chen et al., 2014) which these results confirmed the findings of our study. Pourghasemian et al. reported a complete response rate to chemotherapy with FOLFOX regimen of 4.4%, and about half of the patients did not show any pathological response to the treatment (Pourghasemian et al., 2020), which was in line with our results.
Our study also showed the frequency of neurological complications and hair loss in the FOLT group was significantly higher than that of the FOLFOX group. There was no statistically significant difference in the frequency of hematological, liver, kidney, and Gastroenterological and stomatitis complications between the two groups. Regarding hematological complications, grade 3 complications were observed in only 2.5% of patients treated with FOLFOX regimen, while the frequency of this complication was 15% in the FOLT group, however, this difference was not statistically significant that these results were consistent with the findings of studies conducted in this field. In 2022, Farrokhi et al., (2022a) from Iran, by examining 37 patients treated with FOLFOX and 32 patients treated with FLOT, the incidence of hematological complications was reported as 16% and 37%, respectively, this difference can be justified due to the difference in the design as well as the difference in the treatment regimen under investigation. In this study, the highest rate of hematological complications before the FLOT regimen was related to DCF regimen treatments, which can suggest the role of docetaxel in causing more hematological complications in these chemotherapy regimens. In the study of Al-Batran et al., (2019) grade 3 and 4 neutropenia was observed in about 50% of patients treated with FLOT regimen, which was higher than the results of our study, which can be justified due to the difference in the sample size of the two studies. Also, in line with the results of our study, Chen et al., (2014) reported the incidence of grade 3 neutropenia as only 4.6%. The low rate of hematological complications of the FOLFOX regimen has been confirmed in other studies as well (Liu, 2009; Wang et al., 2019a). The frequency of gastrointestinal complications including nausea, vomiting and diarrhea for FLOT and FOLFOX regimens was 35% and 25%, respectively, and this difference was not statistically significant. These findings were consistent with the results of the study by Farrokhi et al., 2022a) who did not report a significant difference in the incidence of gastrointestinal symptoms in patients treated with FOLFOX and FLOT chemotherapy regimens. Chen et al., (2014) reported the frequency of nausea and vomiting in patients treated with the FOLFOX regimen to be 21%. The rate of nausea and vomiting reported in patients treated with FOLFOX regimen was 25.5% and 19%, respectively in Pourghasemian et al.,(2020)’s study and Beliak et al., (2021)’s study.
Also, in our study, we examined the predictive factors of treatment response in both groups. The results of logistic regression analysis showed that the rate of complete response to FOLFOX treatment was significantly better in younger patients, women, and in patients with lower tumor size. Moreover, in patients treated with FOLT, the complete response rate was lower in patients with tumor size higher than T2. These results were in line with the results of studies conducted in this field (Al-Batran et al., 2019; Wang et al., 2019a; Beliak et al., 2021; Farrokhi et al., 2022a). Accordingly, younger patients and women seem to show a better response to perioperative chemotherapy with the FOLFOX regimen. The reason for this could be the better anti-tumor immunological response in these people, although due to the lack of investigation of this relationship with previous studies, there is a need to conduct more studies to prove this relationship. In the case of FLOT chemotherapy regimen, there was no significant relationship between age and gender of patients with pathological response to treatment.
Our study has strengths and weaknesses that should be noted. The most important weakness of the present study was the observational design of the study and only the findings recorded in the patients were used, which can affect the results. Also, in this study, due to the short period of the study and the lack of follow-up of the patients, we were not able to estimate the survival of the patients in the treatment methods. The design of randomized controlled clinical trial studies with proper follow-up can help to estimate the results more accurately. The most important strength of our study was to compare the efficacy and side effects of FOLFOX and FLOT regimens in perioperative chemotherapy in resectable GC based on pathological response in a suitable sample size of patients in a prospective cohort study.
In conclusion, our study showed that perioperative FLOT regimen has a better pathological response than FOLFOX regimen in patients with resectable GC. The frequency of neurological complications and hair loss was significantly higher in patients treated with FLOT regimen, while no significant difference was observed for other complications and death between the two groups. Perioperative FLOT regimen can be recommended as the preferred chemotherapy regimen for the treatment of patients with GC due to better response to treatment and no severe and fatal side effects compared to FOLFOX regimen.
Author Contribution Statement
Study concept and design: S.H. and A.M.A.; acquisition of data: A.F.; analysis and interpretation of data: S.H.; drafting of the manuscript: K.N..; critical revision of the manuscript for important intellectual content: A.B..; statistical analysis: biostatistician. M.G.; critical revision of manuscript..
Acknowledgements
This research was funded by Iran University of Medical Sciences (grant number 20837 to A.M.A.). Authors would like to thank all patients who participated in the project.
Ethical approval statement
The protocol of the study was approved by the Ethics Committee of Iran University of Medical Sciences, Iran (IR.IUMS.FMD.REC.1400.194); a written informed consent form was also obtained from the patients or their legal guardian.
Clinical trial registration number
Not applicable.
Data availability statement
All data generated and analyzed during this study can be accessed through direct communication with the corresponding author and the agreement of all research team members.
Conflict of interest statement
The authors report no conflicts of interest.
References
- Al-Batran SE, Atmaca A, Hegewisch-Becker S, et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol. 2004;22:658–63. doi: 10.1200/JCO.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- Boige V, Pignon J, Saint-Aubert B, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25:4510. [Google Scholar]
- Charruf AZ, Ramos MFKP, Pereira MA, et al. Impact of neoadjuvant chemotherapy on surgical and pathological results of gastric cancer patients: A case‐control study. J Surg Oncol. 2020;121:833–9. doi: 10.1002/jso.25839. [DOI] [PubMed] [Google Scholar]
- Chen W, Shen J, Pan T, et al. FOLFOX versus EOX as a neoadjuvant chemotherapy regimen for patients with advanced gastric cancer. Exp Ther Med. 2014;7:461–7. doi: 10.3892/etm.2013.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- Dos Santos M, Lequesne J, Leconte A, et al. Perioperative treatment in resectable gastric cancer with spartalizumab in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT): a phase II study (GASPAR) BMC Cancer. 2022;22:537. doi: 10.1186/s12885-022-09623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi LH, Telese A, Marasco G, et al. Gastric cancer prevention strategies: a global perspective. J Gastroenterol Hepatol. 2020;35:1495–502. doi: 10.1111/jgh.15037. [DOI] [PubMed] [Google Scholar]
- Farrokhi P, Sadeghi A, Sharifi M, et al. Efficacy and safety of FLOT regimen vs DCF, FOLFOX, and ECF regimens as perioperative chemotherapy treatments for resectable gastric cancer patients; a report from the middle east. Res Pharm Sci. 2022a;17:621–34. doi: 10.4103/1735-5362.359430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhi P, Sadeghi A, Sharifi M, et al. Efficacy and safety of FLOT regimen vs DCF, FOLFOX, and ECF regimens as perioperative chemotherapy treatments for resectable gastric cancer patients; a report from the middle east. Res Pharm Sci. 2022b;17:621–34. doi: 10.4103/1735-5362.359430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganschow P, Hofmann L, Stintzing S, et al. Operative results and perioperative morbidity after intensified neoadjuvant chemotherapy with FLOT for gastroesophageal adenocarcinoma impact of intensified neoadjuvant treatment. J Gastrointest Surg. 2021;25:58–66. doi: 10.1007/s11605-019-04511-7. [DOI] [PubMed] [Google Scholar]
- Jiang L, Yang Kh, Guan Ql, et al. Survival benefit of neoadjuvant chemotherapy for resectable cancer of the gastric and gastroesophageal junction. J Clin Gastroenterol. 2015;49:387–94. doi: 10.1097/MCG.0000000000000212. [DOI] [PubMed] [Google Scholar]
- Li ZY, Shan F, Zhang LH, et al. Complications after radical gastrectomy following FOLFOX7 neoadjuvant chemotherapy for gastric cancer. World J Surg Oncol. 2011;9:1–7. doi: 10.1186/1477-7819-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. A prospective study of FOLFOX7 scheme as neoadjuvant chemotherapy for stage III gastric adenocarcinoma. Zhonghua Wai Ke Za Zhi. 2009;47:1305–8. [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Patil P, Gupta VG, Rangaraju RR, et al. Survival outcomes with docetaxel, oxaliplatin, and fluorouracil regimen for the treatment of metastatic gastric adenocarcinoma: A single-center experience. Oncol J Indian. 2020;4:110. [Google Scholar]
- Petrillo A, Pompella L, Tirino G, et al. Perioperative treatment in resectable gastric cancer: current perspectives and future directions. Cancers. 2019;11:399. doi: 10.3390/cancers11030399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourghasemian M, Mehr AD, Molaei M, et al. Outcome of FOLFOX and modified DCF chemotherapy regimen in patients with advanced gastric adenocarcinoma. Asian Pac J Cancer Prev. 2020;21:2337. doi: 10.31557/APJCP.2020.21.8.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera F, Izquierdo-Manuel M, García-Alfonso P, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158–67. doi: 10.1016/j.ejca.2020.12.005. [DOI] [PubMed] [Google Scholar]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–8. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi A, Mohammadnejad A, Akbari A, et al. Survival analysis in gastric cancer: a multi-center study among Iranian patients. BMC Surg. 2020;20:1–8. doi: 10.1186/s12893-020-00816-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastekin D, Paksoy N, Dogan I, et al. Fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) regimen in the first-line treatment of metastatic gastric cancer: A single-center experience. J Cancer Res Ther. 2023;19:253–8. doi: 10.4103/jcrt.jcrt_672_22. [DOI] [PubMed] [Google Scholar]
- Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–42. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Rivera A, Sampieri CL, Morales L, et al. Prognostic Factors for Survival in Patients with Gastric Cancer Treated at Two Public Health Institutions in Mexico. Asian Pac J Cancer Care. 2021;6:429–40. [Google Scholar]
- Wang G, Yang B, Fu Z, et al. Efficacy and safety of oxaliplatin-based regimen versus cisplatin-based regimen in the treatment of gastric cancer: a meta-analysis of randomized controlled trials. Int J Clin Oncol. 2019a;24:614–23. doi: 10.1007/s10147-019-01425-x. [DOI] [PubMed] [Google Scholar]
- Wang K, Ren Y, Ma Z, et al. Docetaxel, oxaliplatin, leucovorin, and 5-fluorouracil (FLOT) as preoperative and postoperative chemotherapy compared with surgery followed by chemotherapy for patients with locally advanced gastric cancer: a propensity score-based analysis. Cancer Manag Res. 2019b;11:3009. doi: 10.2147/CMAR.S200883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao L, Liu H, et al. A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer. 2016;114:1326–33. doi: 10.1038/bjc.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S, De La Fouchardiere C, Kim S, et al. Oxaliplatin, 5-fluorouracil and nab-paclitaxel as perioperative regimen in patients with resectable gastric adenocarcinoma: a GERCOR phase II study (FOXAGAST) Eur J Cancer. 2019;107:46–52. doi: 10.1016/j.ejca.2018.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study can be accessed through direct communication with the corresponding author and the agreement of all research team members.