Abstract
Background:
Natural treatment of cancer has received a lot of attention recently due to its advantages including low cost, and fewer side effects. In this study, we aimed to investigate the antimetastatic properties of Cyrtopodion scabrum, a common home gecko, through Epithelial-Mesenchymal Transition (EMT) process.
Methods:
Human colon cancer HCT116 cell line was selected and allocated into the following experimental groups: untreated control, vehicle control (DMSO), Retinoic acid (RA), and two treatment groups including aqueous C.scabrum Whole Extract (CWE) and C.scabrum Cell Extract (CCE) groups. The effects of the two different extracts on the viability, migration, and morphology of HCT116 cells were investigated using MTT, colony formation, and wound healing assay as well as microscopic evaluation. We also investigated the gene expression of E-cad, N-cad, and Snail genes using Real-Time PCR analysis.
Results:
Our findings revealed that CWE and CCE were toxic to the HCT116 cell line with IC50 values of 590 and 680 µg/mL, respectively. Colony formation and migration ability of cancer cells were also inhibited by the two extracts, and the morphology of the cells were determined as epithelial phenotype. Moreover, the expression of N-cad and Snail were remarkably decreased in CWE and CCE, and RA groups, while E-cad didn’t change significantly as compared to the control.
Conclusion:
The results suggest that C. scabrum extract (CsE) may induce its anti-cancer activity through the inhibition of cancer cell growth and the EMT process. CCE, as a valuable natural source, could be also suggested, to be used as an alternative/complementary medicine for the treatment of cancer, in clinical trials.
Key Words: Alternative medicine, cyrtopodion scabrum, epithelial-mesenchymal transition, metastasis, natural products
Introduction
Cancer is one of the major public health problems in the world (Torre et al., 2016). Based on the statistics, there were 19.3 million new cases and 10 million cancer deaths worldwide in 2020, and a global cancer burden of 28.4 million cases in 2040 is expected, a 47% increase from 2020. Despite recent advances in different treatment strategies such as surgery, chemotherapy, and radiation therapy, unfortunately, the burden of cancer mortality is rapidly growing worldwide (Sung et al., 2021). One of the most important reasons for this occurrence is cancer metastasis. Metastasis, defined as the ability to disseminate to distant organs, is the cause of 90% of all cancer-related deaths (Riggi et al., 2018). It has been proposed that Epithelial-Mesenchymal Transition (EMT) might be closely related to the acquisition of invasive traits by tumor cells, thereby triggering the dissemination of carcinoma cells and subsequent metastasis (Kamal et al., 2022; Tsai and Yang, 2013). EMT is a process during which the epithelial cells lose their epithelial features such as cell-cell adhesion and phenotypic characteristics and instead acquire mesenchymal traits such as increased mobility (Acharya et al., 2022). This transition is characterized by changes in the expression pattern of specific genes known as EMT markers including N-cadherin and Snail (mesenchymal marker), and E-cadherin (epithelial marker) (Riggi et al., 2018). In this way, the development of novel treatment strategies targeting EMT may serve as an efficient strategy for the treatment of malignant and metastatic tumors.
Many scientists have turned their attention to the use of natural agents such as herbal extracts or animal products to treat diseases such as cancer due to further advantages of easy administration, low cost, and fewer side effects than other treatment strategies (C. H. Song et al., 2017). For almost thousands of years, the traditional use of natural products including zoo and herbal therapy has provided a valuable source of effective drugs (Dias et al., 2012). Cyrtopodion scabrum (so-called rough-tailed home gecko) is a kind of gecko that is spread around the Indus Valley to the eastern borders of the Caspian Sea, including Iran, Turkey, Pakistan, Iraq, etc. (Rastegar-Pouyani et al., 2010). Recently, we have reported the antiproliferative effects of its extract on the human breast, colorectal, stomach, and liver cancer cells, with the highest inhibitory effect on the colorectal cancer cells without any significant harmful effect on the normal cells (Amiri et al., 2015; Rashidi et al., 2017). By obtaining the evidence from in-vivo studies, using colorectal tumor-bearing mice, we also have demonstrated that C. scabrum extract (CCE) significantly decreased the mean scores of tumor weight and volume as well as 5-fluorouracil (5-FU) chemotherapy drug, while interestingly, unlike 5-FU, hemato-immunological indices and liver function were maintained as normal; also, the treated mice demonstrated good physical activity and normal weight gain (Babaei et al., 2023). Moreover, we have shown that C. scabrum homogenate and extract significantly attenuated the 5-FU-induced liver dysfunction in rats through strengthening antioxidant defense system, resulting in liver function improvement (Diba et al., 2021).
According to all the above-mentioned findings, obtained from both in-vitro and in-vivo studies, regarding the high specificity and sensitivity of C. scabrum extract in the treatment of colorectal cancer, we designed the present study using HCT116 cells, as a poorly differentiated and highly metastatic human colorectal cell line, to investigate the potential antimetastatic and differentiating properties of C. scabrum extracts through EMT process.
Materials and Methods
Preparation of aqueous extract from Cyrtopodion scabrum
C. scabrum was provided by the Razi Research Institute of Vaccine & Serum, Shiraz, Iran, and was identified and approved by a taxonomist (F.Torki, the head of the department at FTEHCR). The aqueous C. scabrum Whole Extract (CWE) was obtained as described previously by Amiri et al. with minor modifications (Amiri et al., 2015). In brief, all animals were cleaned, ground, and homogenized by a homogenizer (Bodine Electric Company, Chicago, USA). The homogenate was then defatted by methanol at room temperature (RT) and boiled three times in distilled water for 2 hrs. The aqueous homogenate was then centrifuged and the supernatants were added to 3 volumes of absolute ethanol for 24 hrs at 4°C. The ethanol precipitate was collected, centrifuged, dialyzed against DW, and then freeze-dried in the Laboratory freeze dryer Alpha 1-2/LD plus (Christ, Germany). The powder was kept at -20°C until use.
The appropriate procedures for experimental animal manipulation outlined by the Institutional Animal Ethics Committee of Shiraz University of Medical Sciences were followed. The University/Regional Research Ethics Committee of Shiraz University of Medical Sciences reviewed, approved, and authorized this study (Ethics ID: IR.SUMS.REC.1398.816).
Preparation of cell extract from Cyrtopodion scabrum
C. scabrum Cell Extract (CCE) was obtained from the C. scabrum cell line established previously in our laboratory. Briefly, the cells were seeded in T75 cell culture flasks to reach 70% confluency. Then, they were washed with ice-cold PBS, collected by scraping, and transferred to 15 ml tubes on ice while pipetting up and down with insulin and a 5 ml syringe. Subsequently, sonication was performed by Ultrasonic Homogenizer (Hielscher UP200H, Germany). The final lysate was freeze-dried and the powder was kept at -20°C until use.
Cell culture
The human HCT116 colorectal cancer cell line was purchased from the Pasteur Institute of Iran. To avoid mycoplasma contamination the cells were checked with a PCR-based test, and then cultured in RPMI 1640 cell culture medium supplemented with 10% FBS (Fetal bovine serum) and 1% Pen-Strep (Penicillin 100 unit/ml, Streptomycin 100mg/ ml); then, they were incubated in a 37°C and 5% CO2 incubator.
MTT assay
2.5×103 number of HCT116 were seeded in each well of 96-well flat-bottom plates and allowed to adhere for 12 hrs at 37°C with a 5% CO2 atmosphere. The experimental groups were as follows: negative untreated control, vehicle control (DMSO), Retinoic acid (RA) as a positive control, CWE, and CCE. Different concentrations of 25-150 µM of RA and 100-1000 µg/mL of CWE and CCE were added to each corresponding well and incubated further for 72 hrs. Tetrazolium bromide solution (Sigma, USA) was then added at the final concentration of 0.5 mg/mL to each well and incubated in the dark for 4 hrs. The media were then aspirated from each well and 100 µL DMSO (SINACLON, Iran) was added and shaken gently to solve the formazan crystals and the absorbance of each well was measured at 570 nm using a microplate reader (Mikura, England). Cell viability percentage was determined with respect to the control. All concentrations were tested in triplicate wells and the experiment was repeated two times.
Colony Formation Assay
The capability of a single cell to grow into a large colony through clonal expansion was investigated by colony formation assay (Liu et al., 2018). HCT116 cells were seeded in 6-well plates at 2000 cells/well for each treatment group and incubated for 24 hrs. Then, the treatment with DMSO (vehicle control), RA as the positive control (100µM), and 1000 µg/ml of each of the extracts (CWE and CCE) was done, and the results were compared to the untreated control cells. The cells were then incubated for at least 7 days to form colonies and the media were changed twice a week. Colonies were then fixed with ice-cold methanol for 15 minutes and stained with 0.1% crystal violet for 30 min and photographed. For quantitative analysis, 1mL of 33% acetic acid was added to each well, and then the plates were shaken for 1 hrs. The absorbance of each well at 560nm was measured by the microplate reader (Mikura, England).
Colony formation % = (A560 test/A560 control) × 100
The results were presented as means and standard deviations of two independent experiments with quadruplicate samples for every treatment condition.
Wound-healing assay
Since cell migration is one of the key steps in the wound-healing process, we used a wound-healing assay to investigate cell migration capability (Grada et al., 2017). Briefly, HCT116 (75×103) cells were plated onto 24-well plates and grown to reach 80–90% confluency. Then, the cell monolayer was scratched (CsE) manually with a plastic 10µl pipette tip, and the cellular debris was subsequently washed with PBS. Different treatments with DMSO (vehicle control), RA as the positive control (100µM), and 1000 µg/ml of each of the extracts (CWE and CCE) for each corresponding well were performed. An inverted microscope (OLYMPUS, Japan) equipped with a digital camera was used to capture images at 40X magnification. The wound closure was photographed at the period of 0, 24, and 48 hrs. to evaluate the wound healing efficiency. Six different fields from each sample were documented for the quantitative estimation of the distance between the borderlines. The scratch area was determined by ImageJ software. The migration rate was expressed as the percentage of area reduction or wound closure. Two independent series of experiments were conducted in quadruplicate.
(1)
To evaluate the morphological changes induced by different treatments, the cells in all the treatment groups after 72 hrs of incubation were also observed using the same microscope, and the images were obtained at 100X magnification.
RNA Isolation, cDNA Synthesis, and quantitative RT- PCR Analysis for EMT gene markers
3×105 HCT116 cells were seeded into 25 cm2 (T-25) cell culture flasks, and after 24 hrs of incubation, the cells were treated with DMSO (vehicle control), RA as the positive control (100µM) and 1000 µg/ml of each of the extracts (CWE and CCE) for 72 hrs. Following the 72 hrs treatment of the cells, the total RNA was extracted by RNX-Plus solution (Sinaclon, Iran) according to the manufacturer’s Instructions. The yield and purity of the isolated RNA were determined by spectrophotometer, and RNA integrity was checked by visual inspection of the two ribosomal RNAs 18S and 28S, using a 1% agarose gel. cDNA synthesis was performed by Revert Aid First Strand cDNA Synthesis Kit (Fermentase), following the manufacturer’s instructions starting from 2μg RNA/sample. Quantitative RT- PCR reactions were performed with high ROX Ampliqon SYBR Green Master Mix and specific primers of target genes: Snail family transcriptional repressor 1 (Snail1), N-cadherin (N-cad) and E-cadherin (E-cad) using Applied Biosystems™ QuantStudio™ 3 Real-Time PCR System. Expression values for all transcripts were normalized to the endogenous control of GUSB (β- Glucuronidase) as a reference gene, and ratios relative to non-treated samples were generated. The fold change for all the samples was calculated using the 2-ΔΔCt method. Using AlleleID software, we designed unique exon-exon junction or intron-spanning primers to remove contaminating genomic DNA. The primer sequences are presented in Table 1.
Tables 1.
The List of Primers
| Gene name | Forward | Reverse |
|---|---|---|
| GUSB | 5'-TCGCTCACACCAAATCCTT -3' | 5'- GGCTTCTGATACTTCTTATACCA -3' |
| N-cad | 5'- ACAGGG TAGACATCATAGTAGC -3' | 5'-GCAGCAACAGTAAGGACA-3' |
| E-cad | 5'- TCCAACAAAGACAAAGAAGGCAAG- 3' | 5'- ACAGCGTGAGAGAAGAGAGTG- 3' |
| Snail | 5'- CCTGCGTCTGCGGAACCTG- 3' | 5'- ACATCTGAGTGGGTCT GGAGG- 3' |
Statistical analysis
All the experiments were carried out in two independent experiments, and the data were presented as Mean±SEM. Statistical analyses were performed using SPSS 15 software (Chicago, USA). One-way ANOVA and LSD post hoc tests were used to compare the groups. Graphs were plotted using Graph pad Prism software (V.8.0). P values≤ 0.05 were considered significant.
Results
Effect of C. scabrum extracts on cell proliferation
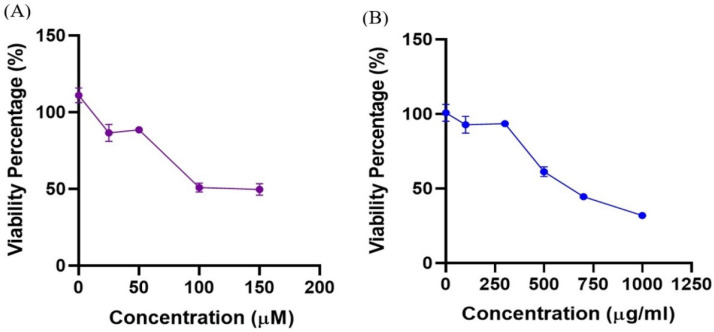
The effect of C. scabrum extracts on the viability of the HCT116 cell line was analyzed by treating the cells with different concentrations of CWE and CCE. As seen in Figure 1, the cell viability was decreased in the treatment groups in a dose-dependent manner. Based on the results obtained from this part of the study, the IC50 value for RA, as a potent inducer of cell differentiation and growth arrest, was 126 µM (Figure 1A), and this value for CWE and CCE was 590 and 680 µg/mL, respectively (Figure 1B).
Figure 1.
Cell Viability Percentage of HCT116 Treated Ccells. A, RA treatment; B, CWE and CCE treatments. Data are presented as Mean ± SEM of two independent experiments performed in triplicate
Effect of C. scabrum extracts on the colony formation capacity
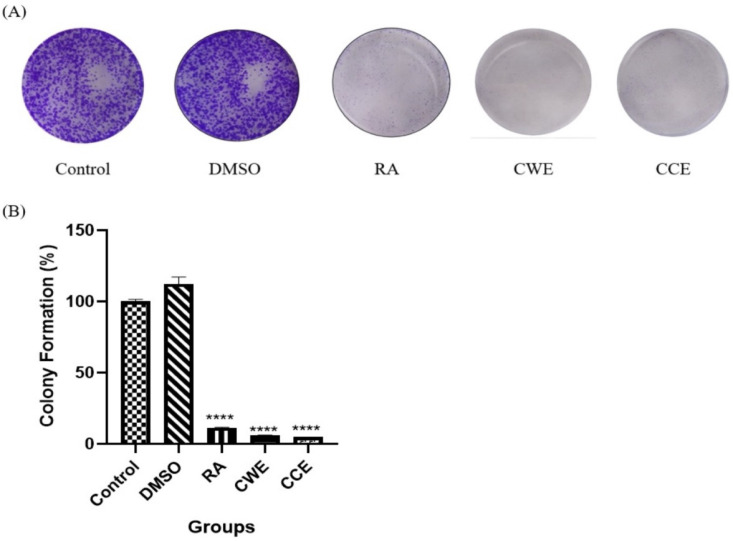
The effect of CWE and CCE on the replicative capability of HCT116 colorectal cancer cells was also evaluated by colony formation assay, and the results are presented in Figure 2. As shown in Figure 2A, in the presence of CWE and CCE, colony formation was inhibited compared to the negative control, and these effects were comparable with RA treatment as the positive control. The quantitative measurement and statistical analysis of colony formation percentage revealed that the colony formation ability of HCT116 cells was significantly suppressed by CWE and CCE as well as RA, as demonstrated in Figure 2B.
Figure 2.
Colony Formation Assay of HCT116 Cell Line. HCT116 cells treated with DMSO (vehicle control), RA as positive control (100µM), 1000 µg/ml of each extract (CWE and CCE) for 7-days. A) The colonies were stained with crystal violet and photographed B) Quantification of HCT116 cancer cell colonies estimated by measuring the absorbance at 560nm. Data are presented as Mean ± SEM of two independent assays conducted in quadruplicate. Significant results are represented as **** p ≤ 0.0001 versus untreated control cells
Effect of C. scabrum extracts on migration capability
To determine the potential effect of C. scabrum extracts on cell migration, a wound-healing assay was carried out. Figure 3 shows the images of HCT116 cells at 0, 24, and 48 hrs post-scratching and after treatment with DMSO (vehicle control), RA as the positive control (100µM), and 1000 µg/ml of each extract (CWE and CCE). Analysis of the images obtained from different treatment groups showed that 24 hrs after treatment, only the inhibitory effects of RA on the migration ability were observed, while after 48 hrs of treatment, wound closure was significantly inhibited in all the experimental groups compared to the negative control (Figure 3A). The quantitative evaluation and statistical analysis of the wound closure percentage measured by ImageJ software (Figure 3B) demonstrated that no significant difference was observed between the results of CWE and CCE-treated cells and the negative control group after 24 hrs. After 48 hrs post-scratching, the closure rate of CWE and CCE group significantly decreased compared to the negative control, and these effects were comparable with RA, as the positive control. These results showed that the extracts had obvious inhibitory effects on the migration and wound closure capabilities of HCT116 cells.
Figure 3.
Wound Healing Assay of HCT116 Cell Line after 24 and 48 hrs of Treatment with C.scabrum Extracts. A, Migration of HCT116 cells treated with DMSO (vehicle control), RA as positive control (100µM), 1000 µg/ml of each extract (CWE and CCE) was monitored with an optical microscope equipped with a digital camera at 40X magnification at the indicated time intervals; B, Quantification of the wound closure percentage estimated by ImageJ software analysis. Data are presented as Mean ± SEM of two independent assays conducted in quadruplicate. Significant results are represented as *p ≤ 0.05, *** p ≤ 0.001 and **** p ≤ 0.0001 versus untreated control cells
Effect of C. scabrum extracts on cell morphology
Since EMT plays an important role in the migration and metastasis of cancer cells, we explored whether EMT and invasion capacity are regulated by C. scabrum extracts in HCT116 cells. Comparing the cells treated with both C. scabrum extracts to the RA-treated cells after 72 hrs showed that the extracts changed the morphology of HCT116 cells towards the epithelial phenotype more effectively. We found that the treatment with CWE and CCE changed the cellular morphology from spindle-like mesenchymal and pseudopodium stretching to pebble-like epithelial (Figure 4).
Figure 4.
Morphological Changes of HCT116 Cells after 72 hrs of Treatment with C.scabrum Extracts. CWE and CCE changed the cellular morphology from dispersed, spindle-like mesenchymal and pseudopodium stretching to pebble-like epithelial (Original magnification: 100X)
Effect of C. scabrum extracts on the expression of EMT markers
We further studied the possible changes in the invasive traits of HCT116 cancer cells through the EMT process in response to the C. scabrum extracts. We evaluated the expression pattern of specific EMT marker genes including N-cad, Snail (mesenchymal marker), and E-cad (epithelial marker) after 72 hrs. As shown in Figure 5A, N-cad mesenchymal marker gene expression significantly decreased after treatment with both extracts as well as RA, as a positive control of differentiation. The results of another mesenchymal marker, Snail, showed that only CCE treatment significantly decreased its expression in the same manner as the RA group, while it was not affected by CWE treatment compared to the negative control group (Figure 5B). As displayed in Figure 5C, gene expression of E-cad, as an epithelial marker, in CWE and CCE treatment groups did not change significantly in comparison to the negative control group, while RA treatment significantly increased the expression of this marker.
Figure 5.
Gene Expression of EMT Markers in HCT116 Cell Line Treated by C.scabrum Extracts. A, Ncad; B, Snail; C, Ecad. Data are presented as Mean ± SEM of two independent assays conducted in triplicate. Significant results are represented as **p ≤ 0.01, *** p ≤ 0.001 and **** p ≤ 0.0001 versus untreated control cells
Discussion
The anticancer properties and growth inhibitory effects of C. scabrum, a genus of the rough-tailed geckonid lizard of Iran, were approved for the first time in our laboratory. Evidence from our in-vivo studies demonstrated that C. scabrum extract exerted its tumor suppressive effects in CT 26 tumor-bearing mice without any side effects on the hemato-immunological indices and liver function compared with a 5-FU chemotherapy drug (Babaei et al., 2023). Furthermore, it attenuated 5-FU-induced liver (Diba et al., 2021), kidney, and heart (unpublished data) dysfunction by strengthening the antioxidant defense system. Our previous studies which investigated the antitumor mechanism of C. scabrum showed that the observed anti-cancer effect was associated with apoptosis induction through TP53 up-regulation, but P53-independent transcriptional activity (Rashidi and Seghatoleslam, 2021). Since one of the most important reasons for cancer mortality is metastasis, the present study was designed to have a better understanding of the role of C. scabrum and its potential differentiating and anti-metastatic properties. Since the greatest inhibitory effect of C. scabrum was observed in colorectal cancer cells, according to our previous studies, we selected a colon cancer cell line, HCT116, which has been recognized in the literature as a model of poorly differentiated and highly metastatic colorectal cancer cell line (Flatmark et al., 2004). Firstly, we evaluated the effect of C. scabrum extract on HCT116 cell viability via MTT assay. The results obtained from this part of the study indicated that CWE and CCE could significantly inhibit the proliferation of HCT116 cells as well as RA as a positive control in a dose-dependent manner. These results were further approved by colony formation assay, which revealed a statistically significant decrease in the number of colonies in CWE- and CCE- treated cells as well as the RA group compared to the negative control cells. These results are consistent with several studies which revealed that C. scabrum and other species of Gecko could inhibit proliferation in different cancer cells including liver and cervical cancers (Amiri et al., 2015; Ge et al., 2016; Rashidi et al., 2017; Y. Song et al., 2012).
In the process of cancer development, metastasis is a complicated event that starts with the migration of tumor cells from their primary site to other tissues. To investigate the migration ability of cancer cells in response to C. scabrum extracts, we performed a wound-healing assay. Comparison of the wound closure area at the initial and final time points exhibited significant inhibitory effects of both extracts on the migration of the HCT116 cell line as well as RA as a positive control. In close agreement with these results, a reduced migration rate of MCF-7 (breast cancer) and SW-742 (colorectal cancer) cell lines following C. scabrum extract treatment has also been observed in the findings of Amiri et al. (Amiri et al., 2015).
Although the molecular principles of metastasis have been extensively studied, the exact mechanisms are not properly understood. Activation of EMT is considered one of the most important hallmarks of cancer metastasis responsible for more than 90% of cancer deaths (Tsai and Yang, 2013). Since the EMT process is characterized by switching in the epithelial phenotype to mesenchymal form, morphological changes of HCT116 cells in response to C. scabrum extracts were also evaluated. The microscopic results showed that both extracts changed the morphology of HCT116 cells from spindle-like mesenchymal and pseudopodium stretching towards pebble-like epithelial phenotype and were even more effective than RA as the inducer of the epithelial phenotype. To study the anti-migratory effect of C. scabrum further through the regulation of the EMT process, we evaluated the expression pattern of EMT marker genes. The results indicated that the gene expression of N-cad, as an important mesenchymal marker, was dramatically suppressed by the extracts even more than RA as the inhibitor of the EMT process when compared to the control untreated cells. Similarly, the expression of another mesenchymal marker, Snail, was also suppressed by the extracts and the changes were highly significant in the CCE group, as well as the RA group, compared to the negative control. These results are in the same line with some previous studies on natural compounds with anticancer activity which have reported the inhibition of EMT through the downregulation of mesenchymal markers (Zhang et al., 2015). Based on the findings of this part of the experiment, E-cad epithelial marker gene expression changed significantly after treatment with neither CWE nor CCE in comparison to the negative control. Up-regulation of the E-cad epithelial marker in response to natural compounds with anticancer properties has been reported in some studies. Nevertheless, in close agreement with our study, Hollestelle et al. in a large study on 41 human breast cancer cell lines revealed a lack of reliable correlation between E-cad loss and expression of EMT markers. They also showed that restoring E-cad expression could not affect the mesenchymal-like traits of E-cad-negative cell lines (Hollestelle et al., 2010). Furthermore, Nilsson et al. detected that E-cad was expressed at the early stages of EMT events and concluded that loss of E-cad was not a causal factor in EMT (Nilsson et al., 2014).
Because of the effectiveness of C. scabrum extract as an anticancer natural agent, we explored its chemical composition and active ingredients in a parallel study for the first time. We detected considerable amounts of polyphenols and flavonoids in C. scabrum extracts (unpublished data). Polyphenols are a class of organic compounds which contain aromatic rings directly bonded to one or more hydroxyl groups, and flavonoids are one of the most numerous subclasses. Although polyphenolic compounds are extensively dispersed in the plant kingdom, the presence of these compounds has been shown in some animal extracts (Aldarraji et al., 2013; Anjana et al., 2013). Numerous studies have documented that these compounds could fight cancer through different mechanisms, namely an antioxidant potential, induction of apoptosis and cell cycle arrest, modulation of intracellular signaling cascades, enhancement of the ability of cancer cells to differentiate by targeting EMT, and other miscellaneous mechanisms (Ko et al., 2015; Lin et al., 2012; Mustafa et al., 2020; Vergara et al., 2011). Since C. scabrum high antioxidants potential was mentioned in our previous report (Diba et al., 2021) and its anti-migratory ability by targeting EMT was observed in the current study, it is suggested that the antitumor properties of C. scabrum can be mainly attributed to its polyphenols and flavonoids content.
However, further biochemical and Molecular studies are required to clarify the exact anticancer mechanisms of CCE treatment. In addition, more extensive research should be conducted in clinical trials to optimize CCE for use in the treatment of patients who suffer from cancer.
In conclusion, taken together, this study, as the first experimental research, examined the effects of C. scabrum on the migration and differentiation of HCT116 human colorectal cancer cells. We revealed that C. scabrum inhibited cell proliferation, colony formation, and wound healing ability, and also induced gene expression pattern changes are associated with more differentiated forms of HCT116 cells. The most remarkable point emerging from our results is that C. scabrum is a valuable natural source of anti-cancer materials possibly exerting its anti-tumor effects through the reversion of the EMT process. These results together with the previous results from our laboratory could also suggest it be used as an alternative/complementary medicine for the treatment of cancer, in clinical trials.
Author Contribution Statement
A.S. and F.Kh. participated in the design of the work. F.Kh. and A.R. conducted the experiments and analyzed the data. A.S., Z.M.P., and H.Gh. provided conceptual and technical guidance for all aspects of the study. A.S. and F.Kh. wrote the manuscript with input from all authors. All authors contributed to the manuscript revisions..
Acknowledgements
The authors would all like to express their gratitude to the Vice-chancellor of the Research and Computer Consultation Center of Shiraz University of Medical Sciences for the English editing of the manuscript. This article was extracted from the Ph.D. thesis written by the first author, Fatemeh Khademi.
The present study follows the development of a Critical Appraisal Tool (AIMRDA) standard reporting recommendation (Ahmad et al., 2021).
Funding
The present study was financially supported by research grant (Grant No:. 97-01-01-19397), from Shiraz University of Medical Sciences, Shiraz, Iran.
Ethical approval
All the procedures were approved by the Institutional Animal Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Ethical code: IR.SUMS.REC.1398.816).
Availability of data
Data are available on the corresponding request.
Disclosure of interest
The authors report no conflict of interest.
References
- Acharya B, Chajaroenkul W, Na-Bangchang K. β-Eudesmol Inhibits the Migration of Cholangiocarcinoma Cells by Suppressing Epithelial-Mesenchymal Transition via PI3K/AKT and p38MAPK Modulation. Asian Pac J Cancer Prev. 2022;23:2573–81. doi: 10.31557/APJCP.2022.23.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R, Riaz M, Aldholmi M, et al. Development of a Critical Appraisal Tool (AIMRDA) for the Peer-Review of Studies Assessing the Anticancer Activity of Natural Products: A Step towards Reproducibility. Asian Pac J Cancer Prev. 2021;22:3735. doi: 10.31557/APJCP.2021.22.12.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldarraji QM, Halimoon N, Majid NM. Antioxidant activity and total phenolic content of earthworm paste of Lumbricus rubellus (red worm) and Eudrilus eugenia (African night crawler) J Entomol Nematol. 2013;5:33–7. [Google Scholar]
- Amiri A, Namavari M, Rashidi M, Fahmidehkar MA, Seghatoleslam A. Inhibitory effects of Cyrtopodion scabrum extract on growth of human breast and colorectal cancer cells. Asian Pac J Cancer Prev. 2015;16:565–70. doi: 10.7314/apjcp.2015.16.2.565. [DOI] [PubMed] [Google Scholar]
- Anjana J, Sruthy P, Rathinamala J, Jayashree S. Evaluation of earthworm powder (Eudrilus eugeniae) and its application in cotton crepe bandage. Int J Bioassays. 2013;2:1250–5. [Google Scholar]
- Babaei Z, Namavari G, Khademi F, et al. Potential Anti-Inflammatory and Growth Inhibitory Effect of Cyrtopodion scabrum Extract on Colon Cancer; An in vivo Study. Asian Pac J Cancer Prev. 2023;24:1209–16. doi: 10.31557/APJCP.2023.24.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–36. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba M, Seghatoleslam A, Namavari M, et al. Potential Protective Role of Cyrtopodion Scabrum in Antioxidant Parameters in Serum and Liver of Rats with 5-FU-Induced Oxidative Damage. Arch Razi Inst. 2021;76 doi: 10.22092/ari.2019.126702.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatmark K, Mælandsmo GM, Martinsen M, Rasmussen H, Fodstad Ø. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593–8. doi: 10.1016/j.ejca.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Ge W-J, Liu L, Li R-F, et al. Anti-tumor effects of Gecko ethanol extract on human cervical cancer SiHa cells. Int J Clin Exp Med. 2016;9:18962–71. [Google Scholar]
- Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol. 2017;137:11–6. doi: 10.1016/j.jid.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Hollestelle A, Nagel JH, Smid M, et al. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat. 2010;121:53–64. doi: 10.1007/s10549-009-0460-8. [DOI] [PubMed] [Google Scholar]
- Kamal IM, Temerik DF, Yassin EH, Mosad E, Hussien MT. Prognostic Outcome of Mesenchymal Transition Biomarkers in Correlation with EGFR Expression in Epithelial Ovarian Carcinoma Patients. Asian Pac J Cancer Prev. 2022;23:4213–25. doi: 10.31557/APJCP.2022.23.12.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YS, Lee WS, Joo YN, et al. Polyphenol mixtures of Euphorbia supina the inhibit invasion and metastasis of highly metastatic breast cancer MDA-MB-231 cells. Oncol Rep. 2015;34:3035–42. doi: 10.3892/or.2015.4304. [DOI] [PubMed] [Google Scholar]
- Lin C-H, Shen Y-A, Hung P-H, Yu Y-B, Chen Y-J. Epigallocathechin gallate, polyphenol present in green tea, inhibits stem-like characteristics and epithelial-mesenchymal transition in nasopharyngeal cancer cell lines. BMC Complement Altern Med. 2012;12:1–12. doi: 10.1186/1472-6882-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wang W, Zhou Z, et al. Chemopreventive activities of sulforaphane and its metabolites in human hepatoma HepG2 cells. Nutrients. 2018;10:585. doi: 10.3390/nu10050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa SK, Oyouni A, Aljohani MM, Ahmad MA. Polyphenols more than an antioxidant: Role and scope. J Pure Appl Microbiol. 2020;14:47–61. [Google Scholar]
- Nilsson G, Akhtar N, Kannius-Janson M, Baeckström D. Loss of E-cadherin expression is not a prerequisite for c-erbB2-induced epithelial-mesenchymal transition. Int J Oncol. 2014;45:82–94. doi: 10.3892/ijo.2014.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidi M, Seghatoleslam A. Induction of Apoptosis with Cyrtopodion Scabrum Extract in Colon Cancer Cells: A Preliminary Study on Targeting P53 Signaling Pathway. Middle East J Cancer. 2021;12:466–72. [Google Scholar]
- Rashidi M, Seghatoleslam A, Namavari M, et al. Selective Cytotoxicity and apoptosis-induction of Cyrtopodion scabrum extract against digestive cancer cell lines. Int J Cancer Manag. 2017;10:7. [Google Scholar]
- Rastegar-Pouyani N, Khosravani A, Oraie H. A new record of Cyrtopodion scabrum (Heyden, 1827) from the Caspian Sea Coastal Region, Guilan Province, Northern Iran. Herpetol Notes. 2010;3:61–3. [Google Scholar]
- Riggi N, Aguet M, Stamenkovic I. Cancer metastasis: a reappraisal of its underlying mechanisms and their relevance to treatment. Annu Rev Pathol Mech Dis. 2018;13:117–40. doi: 10.1146/annurev-pathol-020117-044127. [DOI] [PubMed] [Google Scholar]
- Song CH, Han J-W, Jeong B, Yoon J. Mapping the Patent Landscape in the Field of Personalized Medicine. J Pharm Innov. 2017;12:238–48. [Google Scholar]
- Song Y, Wang J-G, Li R-F, et al. Gecko crude peptides induce apoptosis in human liver carcinoma cells in vitro and exert antitumor activity in a mouse ascites H22 xenograft model. J Biotechnol Biomed. 2012:2012. doi: 10.1155/2012/743573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Yang J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara D, Valente CM, Tinelli A, et al. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 2011;310:1–8. doi: 10.1016/j.canlet.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang X, Lai M. Modulation of epithelial-to-mesenchymal cancerous transition by natural products. Fitoterapia. 2015;106:247–55. doi: 10.1016/j.fitote.2015.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on the corresponding request.