Abstract
Background:
Programmed death ligand 1 (PD-L1) plays critical role in PD-1-dependent immunity suppress. Abnormal PD-L1 expression has shown to be directly related to poor prognosis and drug resistance in cancer patients. Hence, we aimed to evaluate PD-L1 expression in relapsing and remitting Hodgkin lymphoma (HL) as a prognostic factor.
Methods:
In this cross-sectional study, 100 patients with HL between 2007 and 2015, were included. A thin section of tumor tissue fixed and processed on slides, stained by immunohistochemistry (IHC) PD-L1 specific antibodies. The clinical, imaging and pathology information of patients were obtained using case reading and by retrospective follow-up. The status of recurrence or improvement was determined after 5 years of diagnosis. GraphPad Prism v.8 was used for analysis.
Results:
of 100 HL cases, the mean age of 33 relapsed group cases was significantly higher than remission group (p-value = 0.006), and gender was not significant however majority of cases in both groups were male. The frequency of PD-L1 expression found in 49% of all patients. A significant relationship was found between the expression of PD-L1 and disease progression, HL subtype, stage of tumor (p-value<0.05). High expression of PD-L1 found in majority of relapse group and low expression in remission group.
Conclusion:
PD-L1 expression assessment in HL patients is a valuable tool for prediction of the disease subtype, progression, stage, and treatment outcome. IHC method as an available, simple, rather cheap, and efficient tool could use for evaluation of PD-L1 expression and predicting the prognosis of HL disease, elsewhere.
Key Words: Hodgkin’s Lymphoma, Immunohistochemistry, PD-L1- gene expression
Introduction
Hodgkin’s lymphoma (HL) is a rare disease that affects B lymphocyte cells. It causes around 11% of all lymphomas and 0.5% of all malignancies (Weniger and Küppers, 2021). New treatment strategy for HL is using chemotherapy combined with radiotherapy depending on the stage of the disease and the risk of recurrence. Meanwhile, more than 80% of treatments are successful (Adams et al., 2020). Therefore, around 20% of HL patients are at risk for recurrence or drug resistance (Othman et al., 2021; Weniger and Küppers, 2021). In this regards, high-dose chemotherapy (HDC) and autologous stem cell transplantation (Auto-SCT) combination therapy is the second line of treatment which are successful in 50% of resistant cases (Castagna et al., 2020). Additionally, drug-conjugated antibodies (anti-CD30) and checkpoint blockers (anti-PD-1 and anti-PD-L1) are applicable for those resistant cases (Huang and Huang, 2022; Zhang et al., 2022). In this regards, identification of HL patients at higher risk of recurrence is critical for patient management and choice of treatment (Othman et al., 2021).
PD-1 is a protein on the surface of T and B cells that has a role in regulating the immune system’s response. PD-1 ligands (PD-L1 and PD-L2), could inhibit TCR mediated signal transduction and anti-tumor activity of immune cells (Yi et al., 2022). PD-1 blocker antibodies can boost immunity and cause extended clinical responses in solid tumors and hematological cancers (Hayashi and Nakagawa, 2020; Johansen et al., 2019). Nivolumab and Pembrolizumab as two FDA approved PD-1 inhibitor antibodies for HL patients (Cencini et al., 2021; Johansen et al., 2019). It is documented that, elevated PD-L1 expression in the majority of solid tumors and hematologic cancers accompanied by poor survival (Cencini et al., 2021; Vassilakopoulos et al., 2020). However, the significance of PD-L1 as a prognostic factor remains uncertain. In classical HL (cHL), there is a link between PD-L1 protein expression and corresponding genetic alterations. Patients with 9p24.1, which is linked to higher PD-L1 expression, have a considerably worse progression-free survival (PFS) (Brice et al., 2021; von Keudell and Younes, 2019).
PD-L1 expression could be identified by various method such as immunohistochemistry (IHC) and ELISA (Li et al., 2019). A higher serum PD-L1 levels (sPD-L1) could distinguish solid cancer patients but it has poor predictive indicator for blood cancers (Shimada et al., 2021). Moreover, the sPD-L1 levels and its prognosis in HL patients is under investigation (Jalali et al., 2019).
Therefore, in the present study we decided to compare the expression of PD-L1 expression in Hodgkin’s lymphoma (HL) FFPE samples into two recurrent group (cases) and remission group as control.
Materials and Methods
Study Population
In this retrospective cross-sectional study, 100 patients with Hodgkin’s lymphoma referred to Firoozgar Hospital, affiliated to Iran University of Medical Sciences (IUMS), Tehran, Iran, between 2007 and 2015, whose clinical, imaging and pathological data in hospital repository was available recruited. Inclusion criteria: 1) Diagnosis of Hodgkin’s lymphoma in the pathology report, 2) availability of pathology paraffin embedded block 3) suitability for further IHC analysis using their slide. Exclusion criteria: 1) Positive medical history for other cancers 2) Presence of cancers other than Hodgkin’s lymphoma at the same time 3) Patients had not complete data sheets 4) Bone marrow aspiration (BMA) samples 5) Patient with history of treatment with checkpoint inhibitors or T-cell costimulatory blockade. In a five-year follow-up schedule, we collect the patient’s data including clinical, imaging and pathology. Then, based on hospital repository reports, we categorized patients in two groups: 1) Recurrence (relapse) group in which the patient’s disease relapsed in five years after HL diagnosis, and 2) Remission (improved) group in which the patients with rehabilitation in five years after HL diagnosis.
A checklist prepared for data import which include sex, age, duration of disease, clinical manifestations, tumor characteristics such as tumor size and location, stage and subtype of the cancer, CT scan reports and etc. WHO classification for HL type was used in which they classified into four stages of HL disease based on CT scan report.
Immunohistochemistry (IHC)
The formalin-fixed paraffin-embedded (FFPE) tissue 3-4 μ thick sections were placed on positive charged slides, then, dried in oven for 1 hours. Slides were processed by polyol lysine coating, xylene deparaffinization and serial dilution of ethanol.
Monoclonal rabbit anti-PD-L1 (CD274) (Zytomed Systems, GmbH, Berlin, Germany) was used for IHC by staining protocol which performed in several stages. First, 100 ul peroxidase blocking reagent used in darkness for 10 minutes and wash by TBS for blocking of endogenous peroxidase. Second, primary antibody incubation by using 100 ul primary antibody amplifier for 15 minutes at room temperature (RT) and then, washing by TBS. Third, incubation with master polymer plus HRP by using 100 ul of the solution and put at RT for 30 minutes and wash by TBS. Forth, immunostaining visualization via chromogen solution incubation. Chromogen solution prepared by adding 1 drop DAB into 1 ml DAB substrate buffer and then, the solution added to each sample and incubate 5 minutes at RT. Fifth, staining intensifier used by apply DAB enhancer for 1-2 minutes at RT. Sixth, counterstaining by using hematoxylin to cover the samples for 1 min and finally, rinse and mount by washing with water, dehydrate by increasing alcohol concentration, clearing in xylene and mounting with permanent mounting medium.
Scoring was performed by two pathologists. All slides underwent scoring separately by two pathologists and reviewed again if there was not similar result. The positive stained samples were scored using tumor proportion score (TPS). TPS is calculated as follows:
TPS<1% was considered as no PD-L1 expression while 1≤TPS<50 and TPS≥50 were considered as intermediate and high PD-L1 expression, respectively. Intensity of cytoplasmic and/or membranous positivity ranked as 0 (no staining), 1+ (weak or equivocal staining), 2+ (moderate staining), or 3+ (strong staining).
Statistical Analysis
Microsoft Excell and Graphpad Prism 8 were utilized for all statistical analyses and designing plots and figures. Chi-square test was used for contingency analyses and Mann-Whitney U test and ANOVA were employed to compare quantitative variables. P-value < 0.05 was considered as significant.
Results
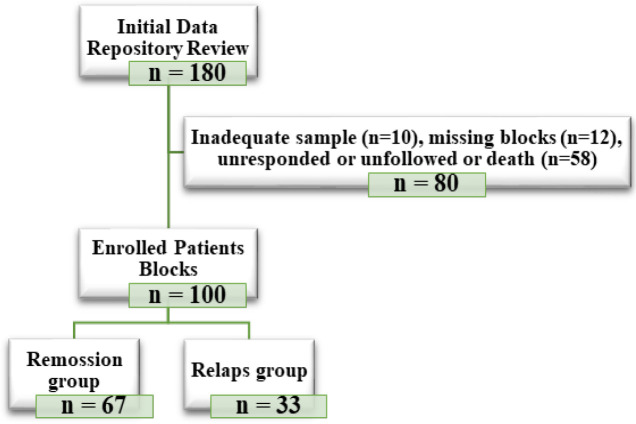
A total of 100 patients with confirmed Hodgkin’s lymphoma were included in the present study. Figure 1 showed the flowchart of enrolled HL patients by the present study. Of them, 79 (79%) were male and 21 (21%) were female. The mean age ± SD of patients was 34.78 ± 15.78 years in the range of 8 to 76 years (Table 1).
Figure 1.
Flowchart of Enrolled Patients with Hodgkin's Lymphoma
Table 1.
Demographic Characteristics of Studied Hodgkin’s Lymphoma Cases
| Variable | Male N (%) | Female N (%) | Total N (%) | P-value |
|---|---|---|---|---|
| Age (Mean ± SD) | 34.72±16.46 | 31.37±13.21 | 34.03±15.78 | 0.3343 |
| Sex | 79 (79%) | 21 (21%) | 100 (100) | - |
| Tumor Type | ||||
| Classic | 19 (24%) | 5 (23.8%) | 24 (24%) | 0.6498 |
| Nodular Sclerosis | 38 (48.1%) | 8 (38%) | 46 (46%) | |
| Mixed Cellularity | 18 (22.8%) | 5 (23.8%) | 23 (23%) | |
| Lymphocyte-Rich | 3 (3.8%) | 2 (9.6%) | 5 (5%) | |
| Lymphocyte-Depleted | 1 (1.3%) | 1 (4.8%) | 2 (2%) | |
| 5-year Disease Progression | ||||
| Relapse | 28 (35.5%) | 5 (23.9%) | 33 (33%) | 0.4352 |
| Remission | 51 (64.5%) | 16 (76.1%) | 67 (67%) | |
| Stage of HL | ||||
| Stage I | 18 (22.8%) | 4 (19.1%) | 22 (22%) | 0.7853 |
| Stage II | 32 (40.5%) | 7 (33.3%) | 39 (39%) | |
| Stage III | 14 (17.7%) | 4 (19.1%) | 18 (18%) | |
| Stage IV | 15 (19%) | 6 (28.5%) | 21 (21%) | |
By the Hodgkin’s lymphoma type, majority of 46 cases (46%) were Nodular Sclerosis type, and minority of 2 cases (2%) were Lymphocyte-Rich type. Tumor type was not significant statistically (p = 0.6). Among 100 patients, 33 (33%) have experienced HL relapse or have not responded to treatment within 5 years, and 67 (67%) were in remission phase of HL within 5 years after diagnosis (p = 0.4). By the stage of HL at the time of initial diagnosis, according to the CT-scan imaging report, majority of 39 patients (39%) were in stage II of the disease, and minority of 18 patients (18%) were in stage III (p = 0.7) (Table 1).
Out of 100 patients, in 49 cases (49%) PD-L1 expression was positive using IHC analysis. IHC analysis of PD-L1 protein expression is shown in (Supplementary Figure 1 and Table 2). Statistical analysis using Mann-Whitney U test showed a significant difference between the age of the relapse against remission group (p = 0.006). Hence, HL cases with higher age at initial diagnosis are more probable to enter the HL relapse phase (Figure 2A and Table 2). Statistical analysis using Chi-square test (Fisher test) indicated that there is no significant interaction between sex and disease status (p = 0.47) (Figure 2B and Table 2). Statistical analysis using Chi-square test showed no significant relationship between the type of Hodgkin’s lymphoma and the state of disease progression (p = 0.17). However, Lymphocyte-Rich and Lymphocyte-Depleted groups did not participate in the statistical test due to the very small population (Figure 2C and Table 2). Statistical analysis using Mann-Whitney U test demonstrated significant relationship between HL stage and disease progression status (p = 0.004). The patients with higher stages of HL are more probably to experience relapse of HL (Figure 2D and Table 2). Statistical analysis using Chi-square test (Fisher test) represented a significant difference between the frequency of PD-L1 expression in relapse versus remission groups (p = 0.03). Also, statistical analysis using Mann-Whitney U test showed a significant difference between PD-L1 expression in TPS score between relapse and remission groups (p = 0.003) (Figure 2A, 2B, Table 2).
Table 2.
Detail Characteristics of Patients based on Relapse and Remission Groups
| Scale/variable | Relapse group | Remission group | Total N (%) | p-value | |
|---|---|---|---|---|---|
| Age | Mean ± SD | 41 ± 15.65 | 26 ± 14.72 | 34.03 ± 15.78 | 0.006 |
| Gender N (%) | Male | 28 (35.5) | 51 (64.5) | 79 (79) | 0.47 |
| Female | 5 (23.9) | 16 (76.1) | 21 (21) | ||
| Tumor type % | Classic | 29.20% | 70.80% | 24 (24) | 0.17 |
| Nodular Sclerosis | 26.10% | 73.90% | 46 (46) | ||
| Mixed Cellularity | 47.80% | 52.20% | 23 (23) | ||
| Lymphocyte-Rich | 60% | 40% | 5 (5) | ||
| Lymphocyte-Depleted | 50% | 50% | 2 (2) | ||
| Numerical stage | Mean ± SD | 2.8±0.92 | 2.2±1.01 | 2.4±1.04 | 0.004 |
| PD-L1 expression | Positive N (%) | 23 (69.7%) | 26 (38.8%) | 49 (49) | 0.03 |
| Negative N (%) | 10 (19.6%) | 41 (80.4%) | 51 (51) | ||
| TPS scoring | Mean ± SD | 42.68±33.23 | 19.2±27.86 | 27.2±31.76 | 0.003 |
| Not expressed N (%) | 10 (19.6) | 41 (80.4) | 51 (51) | - | |
| Low expression N (%) | 5 (21.7) | 18 (78.3) | 23 (23) | ||
| High expression N (%) | 18 (69.2) | 8 (31.8) | 26 (26) |
Figure 2.
Analysis of Patients’ Demographic Data in Two Relapse and Remission Groups. A, age differences; B, gender differences; C, frequency of different types of HL; D, frequency of different HL stages. NS: not significant; ***: significant p-value < 0.01
No significant difference was observed between PD-L1 expression frequency in different types of tumors using Chi-square test (P = 0.2) (Figure 3A, 3B, Table 3). Statistical analysis using ANOVA (Brown-Forsythe & Welch`s Test) showed a significant relationship between PD-L1 expression and TPS score in different tumor types (P = 0.009; Brown-Forsythe, P = 0.012; Welch`s Test). According to the results, PD-L1 expression is the lowest in Nodular Sclerosis and the highest in Mixed Cellularity type. Lymphocyte-Rich and Lymphocyte-Depleted types were not included in the statistical analysis due to low sample size (Figure 3C, 3D, Table 3). The frequency of PD-L1 expression was higher in patients with higher stage of Hodgkin’s lymphoma, but statistical analysis using Chi-square test did not show a significant relationship between the frequency of PD-L1 expression and the stage of Hodgkin’s lymphoma (P = 0.15). The results showed that PD-L1 expression TPS score was higher in superior stages of Hodgkin’s lymphoma. This relationship was shown to be significant using ANOVA (Brown-Forsythe & Welch`s Test) (P = 0.016; Brown-Forsythe, P = 0.0018; Welch`s Test) (Figure 3E, 3F, Table 3).
Figure 3.
Analysis of Patients’ Data based on PD-L1 Expression and TPS Score. A, frequency of PD-L1 expression in remission and relapse groups; B, TPS score in relapse and remission groups; C, PD-L1 expression in different types of HL; D, TPS score in different types of HL; E, PD-L1 expression in different stages of HL; F, TPS score in different stages of HL. NS: not significant; ***: significant p-value < 0.01; **: significant p-value <0.05
Table 3.
Results of PD-L1 Expression and TPS Score Based on IHC
| Variables | Classification | PD-L1 expression | p-value | TPS score | p-value |
|---|---|---|---|---|---|
| N (%) | Mean ± SD | ||||
| Tumor groups | Classic | 11 (45.8%) | 0.2 | 23.25 ± 31.47 | 0.009 B |
| Nodular Sclerosis | 17 (36.9%) | 14.08 ± 22.89 | 0.012 W | ||
| Mixed Cellularity | 14 (60.9%) | 42.11 ± 35.05 | |||
| HL stage | Stage I | 6 (27.3%) | 0.15 | 8.33 ± 16.89 | 0.016 B & W |
| Stage II | 20 (51.3%) | 26.67 ± 32.44 | 0.0018 W | ||
| Stage III | 10 (55.5%) | 35.63 ± 33.86 | |||
| Stage IV | 13 (61.9%) | 39.41 ± 34.36 |
HL, Hodgkin's lymphoma; B, Brown-Forsythe; W, Welch`s Test
Discussion
Generally, the incidence of Hodgkin’s lymphoma (HL) in Iran by recent study on 126,000 cases was estimated 4 cases per 1,000 people (Shamloo et al., 2017). By the type of HL, the most common type reported nodular sclerosis type which is estimated 62.3% of all cases. Also, the mean age ± SD of HL cases calculated 46 ± 6.2 y in which it seems to be more in male (73%) than female (27%) (Jung et al., 2022; Vassilakopoulos et al., 2020). Cytotoxic therapy is the main strategy of treatment for majority of HL cases (80-90%) however 15-20% of them became resistant and disease relapse could be seen in these patients (Weniger and Küppers, 2021). Having a biomarker for resistance prediction or disease prognostic factor to change the treatment choices, may reduce the disease burden and related long-term complications(Cencini et al., 2021). Currently, positron emission tomography (PET) imaging, tissue biomarkers, tumor-infiltrated macrophage population, cytokines, and circulating nucleic acids are used to determine the prognosis of Hodgkin’s lymphoma (29). PD-L1 has been studied as a prognostic marker in various malignancies, but the importance and significance of using the IHC in assessment of PD-L1 expression in Hodgkin’s lymphoma rarely studied(Annibali et al., 2018; Castagna et al., 2020; Huang and Huang, 2022).
In the present study, a total of 100 HL patients based on pathology data, laboratory tests and imaging reports underwent IHC staining against PD-L1 for assessment of its expression in two groups of relapse and remission. By a 5-year follow-up for all patients, we found 33 (33%) of our HL patients experienced relapse. IHC results showed 49 (49%) of patients were positive for PD-L1 expression and based on TPS scoring system, 23 specimens classified as low expression and 26 as high expression. Of 33 relapsed cases, 23 had positive PD-L1 expression which identified statistically significant (P = 0.03). Also, PD-L1 expression was significantly related to the probability of disease recurrence in 5 years (P = 0.003). By comparing the degree of significance of the relationship between the frequency and expression of PD-L1 with the probability of recurrence, it is observed that the expression of PD-L1 is a much more valuable factor in estimating the prognosis of HL disease.
In this study, it was shown that age is a variable that is significantly higher in the group of recurrent than in the remission patients. The mean age of recurrent HL patients was 41±15.65, which can be considered as a possible factor in predicting the prognosis of HL. Thus, higher age in HL patients predisposed as cases with more probability to enter the HL relapse phase.
In general, in terms of patients’ background characteristics, HL type and patient’s gender had no significant relationship with the likelihood of disease recurrence, but age and stage of the disease had a significant relationship with the probability of disease recurrence. The mean age of total patients in the study was 34.03±15.78 years, ranging from 8 years to 76 years. This age was lower than previous studies (Jalili et al., 2022; Jung et al., 2022), but another study showed a bimodal age curve (a mode at 15 ~ 35 years of age and the second mode in elderly) (Nakatsuka and Aozasa, 2006). These differences may note the various age specific relation for the HL however a study in Iran (Monabati et al., 2020) and China (Jalili et al., 2022) reported a rather similar age. Also, due to the much larger standard deviation of the result, this difference can be attributed to the limited number of samples.
Also, the most common type of HL in this study was Nodular Sclerosis, which is consistent with previous finding (Jung et al., 2022). In examining the relationship between HL type and other variables, two types of Lymphocyte-Rich and Lymphocyte-Depleted were not included due to the very small sample size.
Our results showed that the presence of PD-L1 expression seen in various stages or tumor types of the HL disease, but it was not statistically significant. In contrast, PD-L1 expression levels was significantly related to the tumor types as well as stage of the disease. This is a very important finding that shows the presence of PD-L1 expression has less prognostic value, but the level of expression is valuable factor for assessment and prediction of the stage and type of HL disease. This finding is in line with the Paydas et al. (Paydas et al., 2015) which reported PD-L1 expression is highly variable from 5% to 90%.
In a study by Chen et al. (Chen et al., 2013), 53 HL samples analyzed for PD-L1 expression by IHC. They reported strong PD-L1 expression by higher levels in in nodular sclerosis and mixed cellularity compared with Non-Hodgkin Lymphoma samples (82% of cases were positive for PD-L1). Menter et al. (Menter et al., 2016) studied 280 HL cases in which they reported PD-L1 is overexpressed in most cases including 65% in the nodular sclerosis subtype, 67% in the lymphocyte-deplete subtype, 81% in the mixed cellularity subtype and 90% in lymphocyte-rich subtype. They emphasize that PD-L1 assessment can be helpful in the differential diagnosis of HL. Compared with our study results we have found the levels of PD-L1 was significantly higher in nodular sclerosis but lower in mixed cellularity subtypes. Also, by the higher stages of lymphoma, the expression levels were significantly higher. These findings showed the prognostic value of the PD-L1 expression assessment in HL patients that is related to the stage and subtype of tumor. Additionally, we have found the higher expression of PD-L1 in relapse group than remission group which noted the significance of PD-L1 high expression (69%) as a prognostic test for prediction of relapse and low expression (78%) for remission group diagnosis in HL patients. Another study by Roemer et al (Roemer et al., 2016) in 108 HL cases showed 97% of them had significantly increased PD-L1 and PD-L2 expression in patients with advanced stage and their assessment have prognostic relevance. That is in line with our study results and emphasized on the prognostic value of PD-L1 assessment in HL patients.
There were some limitations by our study which are including the small sample size and no history of therapy procedure to describe their value in remission or relapse outcome. Also, the patient’s survival could not be calculated due to the recall bias and other missing data causes.
In conclusion, the relapse group has significantly higher ages than remission, and the frequency and levels of PD-L1 expression in HL patients has significant and direct relationship with the recurrence of HL within 5 years after diagnosis. On the other hand, HL remission could be distinguished by low expression of PD-L1. Additionally, the level of expression is significantly differentiating the stage and subtype of HL. Although further studies by the greater sample size could define the results better, based on our findings PD-L1 expression assessment has prognostic value which can be a valuable predictor of HL disease prognosis. IHC method as a applicable tool, elsewhere, could help experts to diagnose the HL cases subtype, stage, treatment outcome and disease prognosis, appropriately.
Author Contribution Statement
The authors confirm contribution to the paper as follows: study conception and design: B.B., S.D.; data collection: S.M., M.P., S.D.; analysis and interpretation of results: B.B., S.D., A.B.; draft manuscript preparation: A.B., S.D. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgements
We wish to express our special thanks to Firoozgar hospital staff and patients for their cooperation and voluntary efforts. This study was done as an approved residency student thesis (Shadi Dadkhah) at Iran University of Medical Sciences. The present study granted by Iran University of Medical Sciences by the grant number 19422.
Ethical Approval
The Ethics Committee of Iran University of Medical Sciences, School of Medicine, approved the present study with the reference number IR.IUMS.FMD.REC.1399.587. All collected information was kept confidential and analyzed without the patient`s name. The participants adhered to all the moral principles of Helsinki.
Availability of data
The data that support the findings of this study are available on request from the corresponding author.
Conflict of Interest
The authors declare no conflict of interest.
References
- Adams CM, Mitra R, Vogel AN, et al. Targeting BCL-W and BCL-XL as a therapeutic strategy for Hodgkin lymphoma. Leukemia. 2020;34:947–52. doi: 10.1038/s41375-019-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annibali O, Crescenzi A, Tomarchio V, et al. PD-1/PD-L1 checkpoint in hematological malignancies. Leuk Res. 2018;67:45–55. doi: 10.1016/j.leukres.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Brice P, de Kerviler E, Friedberg JW. Classical Hodgkin lymphoma. Lancet. 2021;398:1518–27. doi: 10.1016/S0140-6736(20)32207-8. [DOI] [PubMed] [Google Scholar]
- Castagna L, Santoro A, Carlo-Stella C. Salvage therapy for Hodgkin’s lymphoma: a review of current regimens and outcomes. J Blood Med. 2020;2020:389–403. doi: 10.2147/JBM.S250581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencini E, Bocchia M, Fabbri A. Nivolumab in relapsed/refractory Hodgkin lymphoma: towards a new treatment strategy? Am J Blood Res. 2021;11:261. [PMC free article] [PubMed] [Google Scholar]
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–73. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Nakagawa K. Combination therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol. 2020;25:818–30. doi: 10.1007/s10147-019-01548-1. [DOI] [PubMed] [Google Scholar]
- Huang J, Huang J. Immune Checkpoint Inhibitors in Hodgkin Lymphoma and Non-Hodgkin Lymphoma. Ann Transl Med. 2022;9:PMC8267255. [Google Scholar]
- Jalali S, Price-Troska T, Bothun C, et al. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 2019;9:22. doi: 10.1038/s41408-019-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili J, Vahedi A, Danandehmehr A, et al. Subtype distribution of lymphomas in northwestern Iran: a retrospective analysis of 659 cases according to World Health Organization classification. BMC Cancer. 2022;22:1059. doi: 10.1186/s12885-022-10132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen A, Christensen SJ, Scheie D, Højgaard JL, Kondziella D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology. 2019;92:663–74. doi: 10.1212/WNL.0000000000007235. [DOI] [PubMed] [Google Scholar]
- Jung H, Kim HS, Han J, et al. Clinicopathological Characteristics of Primary Pulmonary Hodgkin Lymphoma (PPHL): Two Institutional Experiences with Comprehensive Literature Review of 115 PPHL Cases. J Clin Med. 2022;12:126. doi: 10.3390/jcm12010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li C, Zhi C, et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J Transl Med. 2019;17:1–10. doi: 10.1186/s12967-019-2101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter T, Bodmer-Haecki A, Dirnhofer S, Tzankov A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. 2016;54:17–24. doi: 10.1016/j.humpath.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Monabati A, Safaei A, Mokhtari M, et al. Histopathological and clinical features of nodular lymphocyte-predominant Hodgkin lymphoma and their impact on prognosis: first report from Iran. J Hematop. 2020;13:143–52. [Google Scholar]
- Nakatsuka Si, Aozasa K. Epidemiology and pathologic features of Hodgkin lymphoma. Int J Hematol. 2006;83:391–7. doi: 10.1532/IJH97.05184. [DOI] [PubMed] [Google Scholar]
- Othman T, Herrera A, Mei M. Emerging Therapies in Relapsed and Refractory Hodgkin Lymphoma: What Comes Next After Brentuximab Vedotin and PD-1 Inhibition? Curr Hematol Malig Rep. 2021;16:1–7. doi: 10.1007/s11899-020-00603-3. [DOI] [PubMed] [Google Scholar]
- Paydas S, Bağır E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94:1545–52. doi: 10.1007/s00277-015-2403-2. [DOI] [PubMed] [Google Scholar]
- Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamloo N, Ghannadan A, Jafari M, et al. Head and neck lymphoma in an Iranian population. Iran J Otorhinolaryngol. 2017;29:261. [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Matsubayashi J, Kudo Y, et al. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci Rep. 2021;11:7830. doi: 10.1038/s41598-021-87575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulos TP, Asimakopoulos JV, Konstantopoulos K, Angelopoulou MK. Optimizing outcomes in relapsed/refractory Hodgkin lymphoma: a review of current and forthcoming therapeutic strategies. Ther Adv Hematol. 2020;11:2040620720902911. doi: 10.1177/2040620720902911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Keudell G, Younes A. Novel therapeutic agents for relapsed classical Hodgkin lymphoma. Br J Haematol. 2019;184:105–12. doi: 10.1111/bjh.15695. [DOI] [PubMed] [Google Scholar]
- Weniger MA, Küppers R. Molecular biology of Hodgkin lymphoma. Leukemia. 2021;35:968–81. doi: 10.1038/s41375-021-01204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Zheng X, Niu M, et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:1–27. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Gu C, Huang L, et al. The third-generation anti-CD30 CAR T-cells specifically homing to the tumor and mediating powerful antitumor activity. Sci Rep. 2022;12:10488. doi: 10.1038/s41598-022-14523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.