Abstract
Gastroesophageal reflux (GER) occurs in most cystic fibrosis (CF) patients and is the primary source of bile aspiration in the airway tract of CF individuals. Aspirated bile is associated with the severity of lung diseases and chronic inflammation caused by Pseudomonas aeruginosa as the most common pathogen of CF respiratory tract infections. P. aeruginosa is equipped with several mechanisms to facilitate the infection process, including but not limited to the expression of virulence factors, biofilm formation, and antimicrobial resistance, all of which are under the strong regulation of quorum sensing (QS) mechanism. By increasing the expression of lasI, rhlI, and pqsA-E, bile exposure directly impacts the QS network. An increase in psl expression and pyocyanin production can promote biofilm formation. Along with the loss of flagella and reduced swarming motility, GER-derived bile can repress the expression of genes involved in creating an acute infection, such as expression of Type Three Secretion (T3SS), hydrogen cyanide (hcnABC), amidase (amiR), and phenazine (phzA-E). Inversely, to cause persistent infection, bile exposure can increase the Type Six Secretion System (T6SS) and efflux pump expression, which can trigger resistance to antibiotics such as colistin, polymyxin B, and erythromycin. This review will discuss the influence of aspirated bile on the pathogenesis, resistance, and persistence of P. aeruginosa in CF patients.
Keywords: Bile, Cystic fibrosis, Gastro-esophagus reflux, Quorum sensing, Pathogenesis, Pseudomonas aeruginosa
1. introduction
Cystic fibrosis (CF) is an autosomal recessive genetic disease [[1], [2], [3]] derived from a mutation in the Cystic fibrosis Transmembrane Conductance Regulator (CFTR) gene [4,5]. CF affects multiple organs such as the liver, pancreas, small intestine, sweat glands, reproductive glands, and airways [6,7]. Due to their compromised immune system, the lungs of CF patients are a welcoming environment for dreadful pathogens such as Pseudomonas aeruginosa [[8], [9], [10], [11], [12]]. P. aeruginosa is an opportunistic pathogen that employs the master regulatory mechanism of quorum sensing (QS) to control the biofilm formation and expression of several virulence genes [[13], [14], [15], [16], [17]]. QS is a way of communicating Gram-negative and Gram-positive bacteria based on producing small diffusible signaling molecules known as autoinducers (AI) [[18], [19], [20], [21], [22]].
Through chronic infection of the respiratory tract, P. aeruginosa is the leading cause of morbidity and mortality in CF patients [23]. These patients commonly (35–80 %) [24] suffer from gastroesophageal reflux (GER), which could be a primary source of bile presence in their lungs and result in reduced lung function [14,[25], [26], [27], [28], [29], [30]]. The effect of bile on adapting to a chronic lifestyle in respiratory infections caused by pathogens has been reported by different studies [7,26]. P. aeruginosa can tolerate bile and bile acids and replicate in the presence of these cholesterol-derived factors with a bactericidal nature to other bacteria [7]. Bile directly affects QS and QS-regulated factors involved in the persistence of P. aeruginosa. In addition, bile is responsible for the positive regulation of QS systems and, in this way, can induce biofilm formation, increase promoter activity of T6SS, efflux pump expression, and resistance to some antibiotics such as colistin, polymyxin B, and erythromycin. Meanwhile, bile has a negative influence on swarming motility and type three secretion [7,27,31]. Therefore, bile as a host factor has a critical role in triggering the virulence and antimicrobial resistance of P, aeruginosa. Considering this important fact, a comprehensive understanding of the underlying mechanism of bile effect on the pathogenesis of P. aeruginosa is required for clearance of these drug-resistant infections and decreasing the mortality rate. In this review, we will discuss the role of bile in inducing and regulating QS gene expression, virulence factor secretion, and antibiotic resistance of P. aeruginosa as a successful pathogen in CF patients.
2. Cystic fibrosis
CF is a life-threatening, inherited disease that affects several organs [6,7]. Mutation in Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene in CF patients disrupts the bicarbonate and chloride conducting channel, which regulates water and ion transportation, along with hydration maintenance of epithelial cells. Since mucin unfolding and defense against bacteria in airways is dependent on this bicarbonate secretion, viscid mucus, and respiratory infections are the clinical symptoms of patients with CF, and they can lead to chronic bronchial diseases [5,6,32]. Therefore, from early childhood, the lower airways of these patients are a suitable environment for opportunistic pathogens [9,33] such as P. aeruginosa, Staphylococcus aureus, Hemophilus influenzae, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and Burkholderia species (Table-1) [23,33,34]. P. aeruginosa, an adaptive pathogen that can survive and cause chronic respiratory infections, is responsible for lung function failures and mortality in people suffering from CF [8,35]. Switching to a biofilm mode of growth which is one of the various mechanisms implicated in resistance to multiple antimicrobial agents make the infection caused by this pathogen extremely hard to eradicate [6,36]. Therefore, airway infections, especially at early ages of CF patients, are life-threatening, and understanding the underlying mechanism participating in host-pathogen interaction is helpful in decreasing treatment burden, increase the quality of life, and to impede lung disease progression [37,38].
Table 1.
Microbiology of respiratory tract infections in CF patients.
| Country | Year | Study population | Mean age (Year) | Method of detection | Microbiology | Ref. |
|---|---|---|---|---|---|---|
| United States | 2020 | 31,411 | 23.3 | Culture from throat swap |
Pseudomonas aeruginosa (32.0 %) Burkholderia cepacia (1.6 %) Staphylococcus aureus (63.35 %) Methicillin-sensitive Staphylococcus aureus (48.9 %) Methicillin-resistant Staphylococcus aureus (19.6 %) Stenotrophomonas maltophilia (6.3 %) Mycobacterial species (10.0 %) |
[39] |
| Australia | 2020 | 3538 | 22.6 | Culture/bronchoalveolar lavage (BAL) |
P. aeruginosa (47.8 %) S. aureus (51.5 %) Aspergillus spp (22.9 %) NTM (5.9 %) |
[40] |
| Canada | 2020 | 4332 | Culture/sputum |
S. aureus (49 %) P. aeruginosa (32 %) A. fumigatus (13 %) S. maltophilia (11 %) H. influenza (7 %) MRSA (5 %) Achromobacter species (5 %) B. cepacia complex (3 %) Atypical mycobacteria (5 %) |
[41] | |
| European Cystic Fibrosis Society (ECFS) | 2020 | 52,246 | 21.8 | Culture/sputum |
P. aeruginosa (23.3 %) B. cepacia (2.3 %) H. influenzae (9.8 %) S. aureus (31.7 %) MRSA (4.4 %) NTM (2.1 %) S. maltophilia (7.0 %) Achromobacter Spp. (4.4 %) |
[42] |
| South Africa | 2018 | 449 | 18.0 | Culture/sputum, cough swabs, BAL |
P. aeruginosa (26 %) B. cepacia (3 %) H. influenza (1 %) MRSA (3 %) Aspergillus (6 %) |
[43] |
MRSA: Methicillin-resistant Staphylococcus aureus, MSSA: Methicillin-susceptible Staphylococcus aureus, NTM: Nontuberculous Mycobacteria.
2.1. Gastro-esophageal reflux (GER), the primary source of bile presence in CF lung
The outflow of stomach contents into the esophageal tract is the cause of GER and is common in adolescents and adult CF patients [[44], [45], [46]]. Frequent coughing, delay in stomach evacuation, ketogenic diet, and hyperalimentation are several GER prompting factors [47]. CF patients with GER show lower FEV1 (forced expiratory volume in 1 s) than patients without GER [47]. This condition is followed by pulmonary aspiration, a surge of respiratory diseases, infection, and reduced lung function. Moreover, as a significant co-morbidity in CF patients, both acid and non-acid components of GER can aggravate chronic bronchopulmonary diseases [27,47]. Respiratory failure and acute lung disease result from GER in CF patients and mostly require a lung transplant [48,49] which can lead to more complications [27]. This retrogradation from the gastric to the esophagus happens several times per day in patients and could be a primary source of bile aspiration in the lungs of patients [[25], [26], [27], [28], [29],50]. Bile combines cholesterol, fatty acids, pepsin, bile acids, and salts [50]. As an amphipathic steroid molecule, it is a remarkable host factor derived from cholesterol in the liver. Bile plays a crucial role in digestion, vitamin absorption, bacteriostasis, and the solubilization of cholesterol [51]. By activating receptors, for instance, the nuclear receptor and Farnesoid X Receptor (FXR), bile acts as a signaling molecule [26,52]. The Nr1h4 gene encodes bile acid receptor FXR, which can be a potential therapeutic target for some respiratory diseases [53].
Bile aspiration caused by GER has a direct effect on lung microbiota (Figure-1) [54,55] as well as host response in CF patients. Moreover, poor respiratory outcomes are related to bile aggregation [56]. Bile presence in the lung enviroment of CF patients is associated with induced colonization and forms a microbial community [57]. Through reducing innate immunity and pulmonary surfactants [58], the accumulation of bile can cause airway inflammation and lung damage [26,57,[59], [60], [61], [62], [63], [64], [65]]. Damage to bronchial epithelial cells can be forced by the toxic acidity of gastric fluids and the activity of digestive enzymes like pepsin and bile salts [66]. Bile influences biofilm formation, virulence, pathogenesis, and antibiotic tolerance of respiratory pathogens such as Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Enterobacter, and P. aeruginosa as the dominant pathogen in the respiratory tract of CF patients [7,26]. Notably, the early acquisition of lung pathogens is associated with the presence of bile [67]. Besides, bile acids serve as inflammatory molecules on lung cells, and bile aspiration could lead to chronic lung damage [68]. Therefore, more exploration is required to provide insights into how bile aspiration triggers airway inflammation and persistent infections.
Figure-1.
Schematic photo of Gastro-esophagus reflux as the primary source of bile presence in lungs. Bile aspiration caused by GER has a direct impact on the respiratory pathogen Pseudomonas aeruginosa. The presence of bile is associated with increased antibiotic resistance, biofilm formation, and virulence regulation to cause a chronic infection.
3. Pseudomonas aeruginosa role in CF lung
P. aeruginosa can survive diverse conditions and live in various environments [[69], [70], [71], [72], [73]]. With the ability of adhesion and colonization, this pathogen can switch to the biofilm form of growth. Also, by producing various virulence factors, this pathogen can cause significant tissue damage. Therefore, P. aeruginosa can successfully evade innate and acquired immune defense and cause acute or chronic infection in immunocompromised patients, such as people with CF (29,30). Consequently, better control of P. aeruginosa infections can increase the life expectancy of CF individuals [74]. Infections caused by Multidrug resistance (MDR) P. aeruginosa are tremendously challenging to eliminate due to its rapid mutations and adaptation to gain antibiotic tolerance [30,75]. Several studies in previous decades cleared that P. aeruginosa is becoming more and more resistant to all effective antibiotics (such as carbapenem, quinolones, third-generation cephalosporins, etc.) (Table 2) [[76], [77], [78], [79]]. Excellent recent reviews on antibiotic resistance and effective antibiotics against Pseudomonas infections have been gathered [[80], [81], [82], [83], [84], [85]]. Despite all the efforts, the driving mechanisms that alter the lung environment landscape during the patient's lifetime that prime a chronic infection established by P. aeruginosa within the airway tract is not entirely understood. Therefore, besides the importance of more explorations, preventing P. aeruginosa infection, especially in at an early age, is necessary to avoid the reoccurrence of infection and increase life expectancy [86].
Table 2.
Effective mechanism of common antibiotics in the treatment of Pseudomonas infection and mechanism of resistance.
| Antibiotic class | Mechanism of action | Mechanism of resistance | Ref. |
|---|---|---|---|
| Aminoglycosides | Inhibition of protein synthesis (through binding to 16srRNA within the 30S ribosomal subunit) |
|
[80,82,87] |
| Beta-lactams | Inhibition of cell wall peptidoglycan synthesis |
|
[80,82,87] |
| Polymyxin | cell lysis and death through binding to outer membrane lipopolysaccharide (LPS) |
|
[87,88,89,90] |
| Fluoroquinolones | DNA synthesis blocking (binding to topoisomerases II and IV) |
|
[80,82,87] |
| Cephalosporins | Inhibition of cell wall synthesis |
|
[80,82] |
4. Quorum sensing in P. aeruginosa
The ability to communicate and interact with the host and respond to surrounding tensions is possible through QS. This communication mechanism plays an essential role in regulating behaviors for the adaptation and survival of the whole population [91,92]. Therefore, QS is a pivotal role in colonization, biofilm formation, and virulence factor secretion [[93], [94], [95]]. QS in P. aeruginosa consists of four known hierarchically organized systems, Las, Rhl, Pqs, and Iqs (Fig. 2) [[96], [97], [98]]. Each of these systems contains a synthase to produce a signaling molecule known as AI and a receptor which is a transcriptional regulator. After releasing the AI in a cell density-dependent manner and reaching a critical threshold concentration, they are recognized by their cognate receptors [17,19]. Consequently, after pairing with their specific AI, these receptors can regulate the expression of multiple genes, such as those involved in responding to environmental pressures and creating a successful infection [99,100]. Las, the first system in the QS network, consists of the LasI, encoded by the lasI gene, which is the responsible synthase for producing -(3-oxo-dodecanoyl)- l-homoserine lactone (3OC12-HSL), as an autoinducer. LasR is the cognate receptor for 3OC12-HSL and is a transcriptional regulator for the Las system in P. aeruginosa [101]. At the top of the QS hierarchy network, the Las system can affect host response during infection [102]. Rhl, the second system of the QS network, consists of a transcriptional regulator, RhlR encoded by rhlABR (rhamnolipid synthase gene cluster) [94]. The cognate signaling molecule for this receptor is N-butanoyl-l-homoserine lactone (C4-HSL), produced by the product of the rhlI gene, known as rhlI [103]. The LasR- 3OC12-HSL complex can trigger the transcription of lasI, rhlI, and rhlR [30,94]. Even though the Las system is considered an apex of the QS mechanism, in CF P. aeruginosa mutants with a deactivated LasR, RhlR can still be activated [104]. Rsal expression (Transcriptional repressor of lasI) is induced by lasR in a complex with 3OC12-HSL [99,105]. Therefore, lasR can perform autoregulation and influence Rhl and Pqs systems. 2-heptyl-4(1H)-quinolone (HHQ) and 2-heptyl-3-hydroxy-4(1H)-quinolone (known as Pseudomonas Quinolone Signal or PQS) are AI in the Pqs system. Unlike the previously discussed systems (Las and Rhl), signaling molecules of the third QS pathway in P. aeruginosa are dependent on 2-alkyl-4 quinolones and not homoserine lactone molecules. pqsABCD is responsible for HHQ biosynthesis, and pqsH is responsible for converting HHQ to PQS. PqsR (P. aeruginosa QS regulator) or MvfR (Multiple virulence factor Regulator) is the receptor of this system in P. aeruginosa, which can recognize and pair with both PQS and HHQ. Therefore, the complex of receptor/AI can regulate the expression of pqsABCDE and phnAB. pqsE, the final gene in the PQS biosynthesis operon, is required for the pathogenesis of P. aeruginosa, and by triggering pqsA-E transcription, PqsR (MvfR) can perform autoregulation. Produced PQS in the lungs of CF patients can decrease viability and induce apoptosis in the host cells. Also, PQS is vital for Las and Rhl interaction. PQS can promote C4-HSL production along with the expression of rhlI. Additionally, lasR can increase the pqsR transcription, while rhlR has a negative influence on this matter [[106], [107], [108], [109], [110], [111]]. PqsR repression, along with rhlI expression, can be triggered by RhlR in complex with C4-HSL [112]. Integrated QS system (IQS), the fourth and more recently discovered pathway of the QS network in P. aeruginosa, uses 2- (2-hydroxyphenyl)-thiazole-4- carbaldehyde as a signaling molecule with an unknown receptor. Production of this signaling molecule is under the influence of the Las system. IQS is responsible for responding to environmental stress, and it can partially take over the Las system's central functions under phosphate-limited conditions or in LasI/LasR mutants. Therefore, it can increase Pqs and Rhl system expression [56,108]. QS is required for virulence in P. aeruginosa, which can create fatal infections in individuals with pulmonary disorders. Investigating the role of each communication system of QS could lead to the representation of an attractive alternative to combat P. aeruginosa infections.
Figure-2.
Pseudomonas aeruginosa Quorum sensing network. QS in P. aeruginosa is consist of four systems Las, Rhl, Pqs, and, Iqs. LasI is the synthase for the first system, and it is responsible for 3-oxo-C12 HSL production. This signaling molecule can be recognized by LasR once it's secreted outside and reaches the critical concentration. The complex of LasR and 3-oxo-C12 HSL can influence the Rhl, PQS, and IQS systems. Similarly, C4-HSL is produced by RhlI and, in complex with RhlR can inhibit the PQS system. pqsABCD is responsible for PQS production, which can bind to PqsR. The complex of PqsR- PQS can affect the Rhl system. The fourth system of this network consists of IQS which can be sensed by IqsR. The Complex of IqsR-IQS can regulate the Rhl and Pqs system, specifically in phosphate limitation during stress conditions.
5. pathogenesis of P. aeruginosa in CF
The opportunistic P. aeruginosa can create persistent and life-threatening infections in the airway system of CF patients [113]. Pathogenicity and causing an effective infection in P. aeruginosa are led by the secretion of various virulence factors [[114], [115], [116]]. Through promoting bacterial growth and colonization, virulence factors can adapt to adverse environments, causing damage to the host and escaping the immunity mechanisms [117], especially in the airway tract of CF patients. In an inseparable order, four systems of the QS mechanism (Las, Rhl, Pqs, and Iqs) can control several essential virulence factors [105,118], such as the efflux pump expression, swarming motility [30], as well as production of phenazine (PZ), quinolone oxidase (QQ), and toxins in the opportunistic P. aeruginosa [103]. Secretion of these extracellular virulence factors under QS regulation is essential for forming a chronic infection and causing disease [119,120]. Moreover, biofilm formation strongly interacts with QS in this pathogen [91]. As one of the most significant antibiotic resistance factors, the expression of efflux pumps such as MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-oprM with the ability to export antimicrobial agents can increase resistance to antibiotics [103]. In the following sections, we will provide a detailed discussion of critical virulence factors that elicit the pathogenesis of P. aeruginosa in CF patients.
5.1. virulence factors involved in biofilm formation
QS in P. aeruginosa can regulate the sufficient colony size to be undetected by the host immune system or promote biofilm formation to protect the pathogen from host response by secreting the extracellular matrix once they reach the large size [121]. Microorganisms can live and interact with each other and the environment in complex communities known as biofilms [[122], [123], [124], [125]]. In this bacterial society, microorganisms are embedded in self-secreted extracellular polymeric substances (EPSs), mainly polysaccharides, proteins, lipids, and extracellular DNA (eDNA). This matrix varies in chemical (presence of uronic acid or ketal-linked) and physical properties (pH and hydrophobicity), but it is primarily composed of polysaccharides [126]. Biofilm plays a pivotal role in antimicrobial resistance [103,127] and the protection of bacteria, from chemical and environmental stresses, including phagocytose [94,128,129]. These antibiotic-resistant communities of P. aeruginosa are almost impossible to eradicate and can lead to a fatal decline in the lung function of CF patients [27,129]. QS can promote biofilm formation by regulating the production of essential extracellular factors such as pyoverdine, pyocyanin, exopolysaccharides (such as alginate, Psl, and Pel), lectins, along with rhamnolipid, which is critical for biofilm development and scaping phagocytose [91,[130], [131], [132]]. The extracellular matrix also contains necessary enzymes such as β-lactamase and carbapenemases to inactivate antibiotics such as penicillin, imipenem, and ceftazidime [103,133]. eDNA (10 bp- 30 kb), produced by lysis of bacterial subpopulation under QS control, is another essential component of this extracellular matrix [134]. Besides acting as an energy source, eDNA can participate in biofilm development and stability [135]. Furthermore, horizontal gene transfer and antibiotic resistance are two of the essential roles of this component in biofilm [133,136]. Therefore, biofilm provides an ideal environment for the conjugation and spreading of virulence and antibiotic-resistance genes [126,136]. Biofilm adhesion can increase LasR expression and, thus activate other QS systems [103]. Acyl homoserine lactone (AHL) and Pseudomonas quinolone signaling (PQS) are critical in eDNA generation. Alginate, another vital biofilm component of P. aeruginosa, participates in developing persistent bronchopulmonary infections by increasing the adhesion to solid surfaces [137]. Additionally, alginate and eDNA present in the extracellular matrix can cause protection against phagocytose and bind to aminoglycoside antibiotics. Therefore, they can participate in developing a resistance to the aminoglycoside-class of antibiotics in lung mucosa of CF patients by inhibiting the penetration of antibiotics such as tobramycin within the biofilm, and they are engaged in creating a chronic pseudomonal infection [6,133]. From multiple proteins present in the biofilm matrix, we can count amyloid fibers, CdrA adhesins, Cup fimbria, LecAB lectins, along with the secondary messenger cyclic diguanosine-5′-monophosphate (c-di-GMP) as a critical regulator of the biofilm lifecycle of the P. aeruginosa [87]. Pili and flagellum are virulence factors associated with biofilm formation, adhesion, swimming, and swarming motility. Lipopolysaccharide (LPS) as a significant surface structural component, along with outer membrane proteins (OMPs), and secretion systems which are functional at colonization, are other virulence factors related to biofilm formation and antibiotic resistance [30,81]. Also, the biofilm matrix in P. aeruginosa offers extra protection against the host immune response and antibiotic treatment for the bacteria in the lung [69]. 65–80 % of human infections are caused by biofilm-forming bacteria [133,138,139]. Due to difficult eradication, these antimicrobial-resistant communities of P. aeruginosa can cause a fatal decline in CF lung function [27,94]. Despite expanding knowledge regarding P. aeruginosa biofilm, developing new alternative therapeutic approaches in clinical practice is required, for instance, nanoparticles, quorum sensing inhibitors (QSI), anti-biofilm compounds, and CRISPR gene editing [140].
5.2. Psedumonas secretion systems
P. aeruginosa possesses five types of secretion systems under the regulation of QS [141,142]. Type 1 secretion system (T1SS) is responsible for the secretion of alkaline protease AprA and haemophore HasAp, which are essential for adherence, colonization, and proliferation of P. aeruginosa [30]. The expression of T1SS is regulated due to QS-depended patterns [108]. After pairing with 3OC12-HSL, the virulence gene regulator, lasR, can regulate the expression of hemolysins, proteases, and alkaline [94]. The most important virulence factors secreted by the Type 2 secretion system (T2SS) are LasA, LasB, pyocyanin, ToxA, phospholipase C, PrpL protease, lipase A (LipA), and Lipase B (LipB) [30]. QS regulates the production of two vital elastases, LasA and LasB, as lytic enzymes, which can modify other virulence factors [30] and cause damage to the host cells [141]. LasR also has a pivotal role in lasB expression, along with T2SS regulation, through increasing the secretion of elastases LasA, LasB, and exotoxin A (ExoA) [94,108,143]. In LasR mutants, the third intercellular communication signal (Pqs) takes part in the expression of lasB [94]. The Pqs system regulates pyocyanin, rhamnolipid, elastase, and lectin production [144,145]. On the other hand, a transcriptional repressor of lasI (Rsal) can inhibit pyocyanin and cyanide production [99]. RhlR interacts with C4-HSL; consequently, this transcriptional regulator can control the expression of rhamnolipids, elastase, pyocyanin, and cyanides, such as hydrogen cyanide (HCN) [146]. Type 3 secretion system (T3SS) is crucial in injecting toxic effectors, such as ExoS, ExoY, ExoT, and ExoU, directly into the host cytoplasm [30,141]. T3SS is also involved in the translocation of flagellar proteins and PilA. Destroying cell membranes by phospholipase activity allows ExoU to trigger cell death in phagocytes and epithelium. Therefore, by secreting ExoU, T3SS plays a part in the disease severity, acute lung injuries, and mortality. Through activating protein kinases in the host cell, ExoY can have multiple consequences, such as cell necrosis and lung injury [141]. ExoS gives P. aeruginosa the capability of avoiding phagocytosis, and ExoT can inhibit repairs on lung epithelial [30]. Additionally, T3SS can be negatively regulates by QS and, precisely, the RhL system. Therefore, QS can affect ExoS secretion in this pathogen [108]. P. aeruginosa does not contain the type 4 secretion system [142]. By secreting EstA esterase, the Type 5 secretion system (T5SS) plays a part in biofilm formation, cell motility, and rhamnolipid production [30]. Type 6 secretion system (T6SS) is the most common secretion system in Gram-negative bacteria. Like T3SS, T6SS activity is based on the direct injection of exoproteins into the host cytoplasm [142]. T6SS is responsible for releasing Tse1 (amidase), Tse2, and Tse3 (muramidase) to destroy host microbial flora and defeat other organisms [30]. Secretion systems play a critical role in the survival of bacteria in diverse environments, while assisting the pathogen in colonizing and infecting their host. Inhibiting these secretion systems could be promising in treating severe and antibiotic resistance infections caused by one of the most potent nosocomial pathogens in immunosuppressed and CF patients [147].
5.3. Secondary metabolites
P. aeruginosa produces several toxic secondary metabolites to ensure a successful infection and cause damage to the host. Production of these secondary metabolites is controlled by of QS in P. aeruginosa [148]. Bronchial secretions of CF patients indicated an increase in the secretion of some vicious virulence factors, e.g., elastase, alkaline protease, ExoA, and toxic secondary metabolites (such as pyocyanin, rhamnolipids, and hydrogen cyanide), under control of QS [149]. Pyocyanin is a toxic secondary metabolite [150] and virulence factor with varied roles in the pathogenesis of P. aeruginosa, such as causing damage to the human host cells, inducing neutrophil apoptosis, and killing other competing bacteria [[151], [152], [153], [154]]. Due to its small molecular weight, pyocyanin can diffuse through the host cell membrane and cause a redox reaction [139,155]. An increase in pyocyanin levels during lung infection demonstrates that pyocyanin can cause injuries and death of lung epithelial cells [156]. Hence pyocyanin is crucial for the full virulence of P. aeruginosa and can directly affect gene expression in epithelial cells [157]. With free radical and pro-inflammatory effects, pyocyanin can lead to failures of lung function and the severity of diseases. Also, it can promote colonization in the respiratory tract by increasing mucous secretion, epithelial disruption, and decreasing ciliary beating [141]. This blue, redox-active phenazine can induce auto-poisoning cell death. Therefore, as the most important virulence factor of P. aeruginosa [158], it can take part in eDNA release, which is essential for the formation and maturation of biofilm along with antibiotic resistance [10]. Pyocyanin is also increase biofilm formation through second messenger c-di-GMP signaling [31,117]. Along with antibacterial, anticancer, and antioxidant activities, pyocyanin can defeat competing fungi and protozoa in the environment [159]. Production of secondary metabolites such as rhamnolipids and pyocyanin is influenced byRhlR in the dependent, and independent manner of its cognate AI [160,161]. Therefore, an active RhlR is advantageous for P. aeruginosa [104]. Microbial physiology, metabolism and stress responses are profoundly affected by the secretion of secondary metabolites. These small molecules are not only associated with virulence but also can modulate microbial susceptibility to commonly used antibiotics [162].
5.4. Flagellum and swarming
P. aeruginosa has a single polar flagellum responsible for swimming motility and initial binding to the airway epithelium of CF patients through chemotaxis [10,141,163]. Flagella consists of more than 20 different proteins, and it can participate in adhesion and invasion by promoting motility toward the host cells and adhering to them. Biofilm attachment to the biotic (such as mucus, intestine, connective tissue, epithelium, endothelium, cardiac valves, bone marrow, and the skin) and the abiotic surfaces (such as prostheses, stents, implants, and catheters) occurs through the presence of cell appendages such as flagella, pili, and fimbriae [131,133,164]. Therefore, this virulence factor has a significant role in colonization in the lung environment of CF patients [163].
6. antimicrobial resistance in P. aeruginosa and its impact on treatment in CF patients
The expanding edge of antibiotic resistance, as one of the detrimental souvenirs of this millennium, has encountered us with hazards. The extensive use and misuse of antimicrobial agents result in the rapid resistance development to all antibiotics commonly used in the treatment of P. aeruginosa infection [[165], [166], [167], [168], [169], [170]]. The high level of resistance to the wide range of antibiotics is due to the multiple mechanisms such as the outer membrane's limited uptake, efflux pump expression, horizontal transfer of antibiotic resistance gene, the activity of drug-degrading enzymes, and production of modification enzymes (Table-2) [[88], [89], [171], [172]]. Nevertheless, resistance in P. aeruginosa, as one of the main targets for developing novel antimicrobials and alternative therapeutics [138,167,168], is mainly indebted to the efflux system, which was a part of its genome even before the introduction of antibiotics [173]. These pumps are involved in exporting not only antimicrobial agents but even fatty acids, QS signaling molecules, and toxic lipids [174]. The basal level of efflux pump expression contributes to intrinsic antimicrobial resistance, while acquired resistance is due to the overexpression of these pumps due to mutation [171,175]. Moreover, efflux pump expression can be promoted by several factors, such as host compounds, QS signals, and microbial metabolites [176]. This resistance to the wide variety of antimicrobial agents is attributable to the exporting activity of 5 out of at least 12 efflux pumps [177], including MexAB-OprM, MexCD-OprJ, MexEF-OprN, MexJK-OprM, and MexXY-oprM [103,116]. As one of the most critical efflux pump systems, MexAB-OprM consists of an inner membrane protein, MexB, which is responsible for binding to the antibiotics and initiating the transporting process; MexA, a periplasmic protein, anchored to the inner membrane, which is vital for pump assembly; and finally an outer membrane channel, crucial for antibiotic extrusion, known as OprM [177]. This efflux pump is involved in the secretion of homoserine lactone molecules of the QS mechanism, including C4-HSL [178,179]. Also, the production level of this pump is four times higher when P. aeruginosa is engaged within the biofilm [179,180]. However, the essential role of MexAB-OprM is the contribution to antibiotic resistance. MexAB-OprM provides resistance to β-lactam antibiotics, quinolones, fluoroquinolones, macrolides, novobiocin, chloramphenicol, lincomycin, tetracyclines, trimethoprim and sulphonamides in P. aeruginosa [171,176,[181], [182], [183], [184]]. Multidrug resistance in clinical isolates can derive from an increased expression level of MexAB-OprM [185]; for instance, overexpression of this pump is essential for carbapenem resistance in P. aeruginosa [75,186,187]. Of note, a more comprehensive review of the effect of efflux pumps on antibiotic resistance was published by Scoffone and colleagues in 2021 [188]. Concisely, the rapid evolution toward antibiotic resistance promotes the complication of treatment in P. aeruginosa infections.
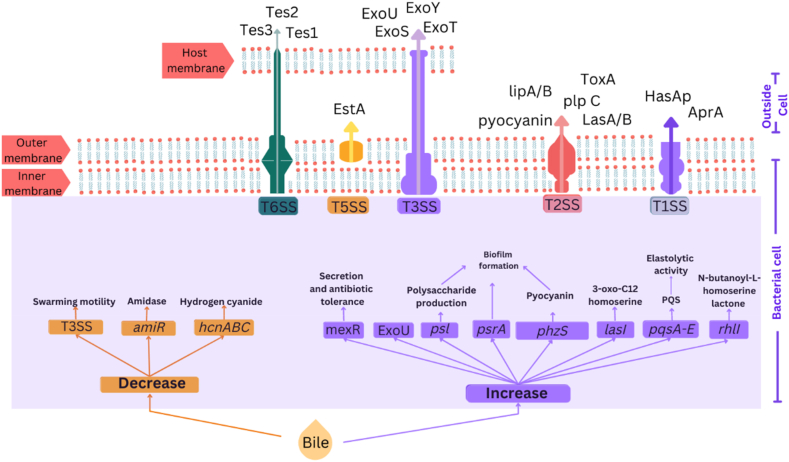
7. 9- Bile affects quorum sensing gene expression and virulence factor production
Aspirated bile has a direct impact on the QS mechanism of P. aeruginosa in the airways of CF patients. Therefore, this host factor can indirectly regulate the expression of multiple genes, such as those involved in creating a dreadful infection (Table 3 and Fig. 3).
Table- 3.
P. aeruginosa gene expression in exposure to bile.
| Gene symbol | Function/presence | Regulation/Expression in response to bile | Technique | Bile/bile salt | Concentration | Strain | Ref. |
|---|---|---|---|---|---|---|---|
| mqoB | Glyoxylate shunt pathway | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| glcB | Glyoxylate shunt pathway | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| acnA | The early stage of the TCA cycle | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| idh | The early stage of the TCA cycle | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| PA0853-54 | The late stages of the TCA cycle | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| aspA | The late stages of the TCA cycle | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| lpdV | glucose metabolism/glycolysis | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| gapA | Glucose metabolism/glycolysis | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| glk | Glucose metabolism/glycolysis | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| aceEF | Glucose metabolism/glycolysis | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| pfm | Proton motive force | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| pqsA | Biofilm formation and virulence | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| pqsA-E | QS/biofilm formation and virulence | Increase | Promoter fusion and thin-layer chromatography | Bile | 0.30 % | PA14, PAO1 and clinical isolates CF242 | [27] |
| rhlI | Acyl-homoserine-lactone synthase | Increase | Promoter fusion and thin-layer chromatography | Bile | 0.30 % | PA14, PAO1 and clinical isolates CF242 | [27] |
| lasI | Acyl-homoserine-lactone synthase | Increase | Promoter fusion and thin-layer chromatography | Bile | 0.30 % | PA14, PAO1 and clinical isolates CF242 | [27] |
| psl | Biofilm formation and virulence | Increase | Congo red binding assay | Bile | 0.30 % | PAO1 | [50] |
| psrA |
Transcriptional regulator associated with biofilm formation |
Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| ppyR | Transcriptional regulator associated with iron-scavenging and exopolysaccharide production | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| ohrR | Transcriptional regulator associated with oxidative stress response | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| mexR | Transcriptional regulator associated with secretion and antibiotic tolerance | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| T3SS | Type III secretion systems (associated with acute infection) | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| T3SS effector exoU | Promoter activity of the T3SS effector exoU | 8.9- fold decrease | Real-time PCR | Bile | 0.30 % | PA14, PAO1 and clinical isolates CF242 | [27] |
| T6SS | Type vI secretion systems | Decrease | Real-time PCR | Bile | 0.30 % | PA14, PAO1 and clinical isolates CF242 | [27] |
| hcnABC | Hydrogen cyanide | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| amiR | Amidase | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| phzA-E | Phenazine | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| gnyR | Transcriptional regulator associated with isoprenoid degrading | Decrease | Real-time PCR | Bile | 0.30 % | UCBPP-PA14, PA14_38430 | [50] |
| sfa2 | Transcriptional regulator associated with T6SS HSI-II expression | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| mexAB-OprM | Efflux pump/Antibiotic tolerance | Increase | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| SiaA-D | Transition to a sessile lifestyle through cyclic-di-GMP synthesis | Upregulated | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
| phzS | PYO biosynthetic genes | Increase | qRT-PCR | Bile | 0.30 % | PA14 | [31] |
| phzH | PYO biosynthetic genes | No significant response | qRT-PCR | Bile | 0.30 % | PA14 | [31] |
| phzM | PYO biosynthetic genes | No significant response | qRT-PCR | Bile | 0.30 % | PA14 | [31] |
| gdbR | Transcriptional regulator associated with choline | Decrease | Real-time PCR | Bile | 0.30 % | PAO1 | [50] |
Figure-3.
Quorum sensing gene expression and virulence regulation of Pseudomonas aeruginosa in exposure to bile. Bile can affect the quorum sensing network by increasing the expression of lasI, rhlI, and pqsA-E. Bile can regulate the expression of various virulence factors in P. aeruginosa. An increase in psl expression and pyocyanin production can influence biofilm formation. Downregulation of Type Three Secretion (T3SS), hydrogen cyanide (hcnABC), amidase (amiR), and phenazine (phzA-E), along with increasing the Type Six Secretion System (T6SS), Exotoxin U, and efflux pump expression are all results of bile exposure.
7.1. Bile effect on QS
GER-derived bile can activate the QS signaling network, and to promote the formation of a persistent infection, bile can upregulate lasI expression and increase pqsA-E and the rhlI promoter activity [27]. A 1.3-fold increase in promoter activity of rhlI, lasI, and pqsA has been reported in exposure to 0.03 % compared to a 2-fold increase in the presence of 0.3 % bile. This enhancement in QS signaling molecules is dependent on the concentration of bile [27]. Induction of QS signaling molecules (PQS and HSLs) by overexpression of pqsA-E, lasI, and rhl in response to bile has a direct impact on the lung microbiome community [26,50]. In addition, bile-induced PQS and HHQ in P. aeruginosa can defeat the biofilm formation of co-colonizing bacteria and fungi such as Aspergillus fumigatus and Candida albicans [26]. Therefore, bile aspiration in the lung of CF patients can modulate the mechanism of QS in P. aeruginosa.
7.2. Bile effect virulence factor secretion
The association of aspirated bile with a decline in CF lung function led to the investigation of virulence factor production in response to GER-derived bile. Several studies have revealed that the interplay between bile and the QS network can manipulate virulence factor secretion in the respiratory pathogen P. aeruginosa. Therefore, it can cause a successful infection, damage the host [26], and lead to respiratory diseases [189]. Aspirated bile represses the expression of acute infection-associated genes but induces the expression of those responsible for creating a persistent one [31]. Bronchial secretions of CF patients showed an increase in the secretion of some vicious virulence factors, such as elastase, alkaline protease, exotoxin A, pyocyanin, rhamnolipids, and hydrogen cyanide, under the control of QS [149]. Therefore, GER-derived bile can promote chronic infection by altering the production of virulence factors that underpin chronic inflammation and eventual lung decline.
7.3. Bile effect on biofilm formation
The presence of bile in the respiratory tract of CF patients can contribute to chronic respiratory diseases by increasing the biofilm formation of P. aeruginosa as the primary pathogen present in the airways of CF patients. A study by Reen et al. showed a significant increase in biofilm formation of P. aeruginosa in response to exogenous bile, which can be associated with the flagellum loss and increase in the surface attachment of this pathogen. This study also showed an increase in the formation of microcolonies in artificial sputum media (ASM). An increase in biofilm formation in response to 0.3 % bile compared to 0.03 % suggests that this biofilm formation in exposure to bile is dose-dependent [27]. Also, by increasing psl, LasI, pqsA expression, and pyocyanin production, bile can promote biofilm formation [31,50]. Therefore, a study by Al-Momani et al. showed switching to the biofilm mode of growth in exposure to a concentration of 0.1–1 mmol/ml bile can be dependent on PQS production [190]. Remarkably another study by Behroozian et al. on CF-derived bronchial epithelial cells with 0.03 % and 3 % (w/v) bovine bile (for 10 min) supported this hypothesis that bovine bile can promote chemoattraction, colonization, and pathogenesis in P. aeruginosa PAO1 strain [7]. Noteworthy, non-salt components of bile, such as cholesterol, pepsin, and fatty acids, cannot induce a meaningful increase in phenotypes related to a chronic lifestyle. In contrast, the physiological concentration (50 μM) of bile salts promotes biofilm formation. Additionally, compared to the mixture of bile salts, only sodium chenodeoxycholate (CDCA) as a single bile salt can facilitate a meaningful response in respiratory pathogens and promote biofilm formation in P. aeruginosa. GER-derived bile as a host factor aspirated in the airway system of CF patients can contribute to chronic infection by influencing chronic phenotypes such as biofilm formation [50,62]. The data mentioned above indicate bile as a trigger factor for the development of chronic phenotypes which are fundamental in the pathophysiology of chronic respiratory disease.
7.4. Bile effect on motility
Subinhibitory concentrations of bile (0.03–0.3 % (w/v) for 30 min) can inhibit flagella production and, therefore, it is followed by reduced swarming motility in P. aeruginosa strains such as PA14 and PAO1. Also, a decrease in swarm distance in the presence of bile is correlated with biofilm formation [27]. Despite the direct effect of bile on QS and biofilm formation, this critical host factor can indirectly pursue biofilm formation through reducing the ability of a pathogen to perform swarming motility. Altogether, this data suggests bile aspiration as an indicator of a persistent infection in the lung of CF individuals.
7.5. Bile effect on the secretion system
To cope with the environment and create an infection, pathogens are relied on secreting a broad range of toxins and hydrolytic enzymes. Bile can influence this secretion system with the aim of creating a persistent infection. GER-derived bile can promote a 3-fold increase in the activity of T6SS promoter tssA but a decrease in T3SS and amidase in P. aeruginosa PAO1 strain [50]. 0.3 % bile can promote T6SS but repress T3SS secretion in P. aeruginosa strains PAO1 and PA14. Therefore, bile influence on the secretion system is less dose-dependent than biofilm formation or signaling molecule production. Consequently, this key host factor can play a part in the pathogenesis of P. aeruginosa [27].
7.6. Bile effect on secondary metabolites (pyocyanin and hydrogen cyanide)
Pyocyanin production is under the influence of bile, and a significant increase in the level of pyocyanin in response to this host factor has been reported by Reen et al. [31]. This study showed a considerable rise in pyocyanin production at several sub-inhibitory concentrations of bovine bile in the PA14 strain. The rise in the level of this toxic secondary metabolite happened at 24 h of incubation. Still, a very noticeable increase occurred at 96 h of incubation in the presence of 3 % (w/v) bile (increase in pyocyanin concentrations from 7.59 μg/mL in the absence of bile to 8.82, 9.85, and 9.01 μg/mL in the presence of 0.03, 0.3, and 3 % (w/v) bile, respectively, after 24h compared to increase pyocyanin concentrations from 3.31 μg/mL in the absence of bile to 4.22, 6.27, and 14.13 μg/mL in the presence of 0.03, 0.3, and 3 % (w/v) bile, respectively, after 96h). Also, qRT-PCR analysis on three genes responsible for pyocyanin biosynthesis (phzS, phzM, and phzH) showed an increase in the expression of phzS, which is the final gene of the biosynthetic pathway of pyocyanin [31]. Therefore, bile significantly increases pyocyanin production in the lung environment of CF patients [31], along with repressing a few other secondary metabolites, such as hydrogen cyanide. This diffusible gas can interfere with the host's cellular respiration. Therefore, the cytotoxic effect of this secondary metabolite can lead to a decline in lung function by increasing colonization and hypoxia-induced cell death [149]. Bile exposure can repress the hydrogen cyanide encoding gene (hcnABC) in the P. aeruginosa PAO1 strain [50]. Virulence factors expression is crucial in the pathogenesis of P. aeruginosa, and alteration of their production under the influence of GER-derived bile is an acritical event in CF patients. Therefore, more investigation is required to provide a comprehensive understanding of the effect of bile on other virulence factors and the underlying mechanism of these effects.
7.7. Bile effect on antibiotic resistance (efflux pumps)
Bile can induce antibiotic resistance in several ways. We pointed out that bile can promote biofilm formation, which is correlated with a higher level of resistance through multiple ways, such as binding antibiotics to components of the biofilm matrix (alginate and eDNA), as well as a difference in the metabolism level of bacteria based on their position within the biofilm. Also, GER-derived bile can increase antibiotic resistance through efflux pump expression. An increase in the expression of efflux pumps such as MexAB-OprM, as the main efflux pump involved in antibiotic resistance [50], is associated with more antibiotic exportation, and so makes the eradication of infections caused by this pathogen almost impossible [133]. Additionally, bile is capable of promoting antibiotic resistance independent of MexAB-OprM. Bile can influence P. aeruginosa to increase tolerance to colistin, polymyxin B, and erythromycin, independent of this efflux pump [26,50]. Instead of clearing the infection, sub-lethal levels antibiotics can promote persistence in pathogens, whereas bile aspiration can accelerate antibiotic resistance progress [50]. Through increasing antibiotic tolerance, the bile aspiration can establish a chronic infection in the CF lung. Therefore, fueling the engine of resistance, bile aspiration has added to the necessity of introducing novel treatments in clinical settings. In the proceeding section, we will count several therapeutic alternatives which could be promising in treating chronic infections caused by P. aeruginosa.
8. non-traditional methods for treatment of P. aeruginosa infections
The inefficiency of conventional antibiotic treatments due to the rapid process of antimicrobial tolerance led to the introduction of alternative therapeutics, such as bacteriophage therapy, which can be an alternative to eradicate infections [[191], [192], [193]]. A study by Cafora et al. demonstrated that phage therapy can reduce lethality, bacterial burden, and the pro-inflammatory response caused by PAO1 infection in a CF zebrafish model [194]. Exposure to phage can modulate different phenotypes, such as virulence and biofilm. There is also a correlation between the QS signaling pathway and resistance to phage [195]. This bacterial communication network, is a crucial regulator of phage adsorption [196] and biofilm formation [197]. In addition, phages may can alter bacterial behavior by manipulating the QS pathway [198]. Aqs1 (anti-QS protein 1) encoded by P. aeruginosa phage DMS3, is a protein with inhibition activity on the LasR and thus, it can to disrupt the QS mechanism [199]. As well, QS and phages are associated with virulence and evolution of P. aeruginosa. For instance, Phage φpa3 can transduce mutations in QS genes in the PAO1 strain [200]. On the other hand, phages can elevate the reduction of P. aeruginosa infections by penetrating the biofilm and destroying its structure, along with facilitating the elimination of bacterial density [120]. Despite the advantages provided by biofilm formation including increased the antibiotic resistance, the increased susceptibility to phage infection and invasion by mobile genetic elements may be the trade-off. Therefore, biofilm formation may be beneficial in low phage loads, or when the threat is attenuated due to high bacterial defenses [197]. P. aeruginosa biofilm in the airways of CF individuals can be targeted by phages. Phages can replicate close to the infection site by infecting bacteria and disrupting the biofilm [201]. The curative effect against P. aeruginosa infections in CF can get elevated by a combination of phages and antibiotic treatment resulting in introducing a novel therapeutic approach with the ability to decrease antibiotic doses and administration time [194]. In addition, using a bacteriophage combination and a combination of phage-antibiotic can elevate the effectiveness of treatment in reducing and dispersing P. aeruginosa biofilm in the airway of CF patients [201].
9. Conclusion
The speedy progress of resistance to antimicrobial agents in P. aeruginosa is alarming; therefore, eradicating infection caused by this pathogen is faced with severe problems. GFR in CF patients, as the primary source of bile aspiration in the lungs, is adding to this complication by triggering QS and chronic lifestyle-associated phenotypes. Exposure to bile is associated with the early acquisition of P. aeruginosa in CF patients, and it can mediate chronic lung inflammation by increasing biofilm formation, virulence regulation, and antibiotic resistance. This will lead to tissue damage and decreased in lung function, while lung transplantation is tailed with more complications. Besides all the efforts made, there are still some shades of a comprehensive understanding of the exact mechanism of Pseudomonal infection response to environmental stress and host-associated factors such as bile.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Data availability statement
No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding: no funding was received.
Contributor Information
Nader Farsad-Akhatr, Email: nader_farsad@tabrizu.ac.ir.
Mohammad Yousef Memar, Email: Y.memar@yahoo.com.
References
- 1.Bhagirath A.Y., Li Y., Somayajula D., Dadashi M., Badr S., Duan K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016;16:174. doi: 10.1186/s12890-016-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellani C., Assael B.M. Cystic fibrosis: a clinical view. Cell. Mol. Life Sci. 2017;74:129–140. doi: 10.1007/s00018-016-2393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutting G.R. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat. Rev. Genet. 2015;16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Future of Cystic Fibrosis Care: a Global persp...: Find it! Options. 2020. [DOI] [Google Scholar]
- 5.kris De Boeck K. De Boeck. Cystic fibrosis in the year 2020: a disease with a new face. Acta Paediatr. 2020;109:893–899. doi: 10.1111/apa.15155. [DOI] [PubMed] [Google Scholar]
- 6.Ciofu O., Tolker-Nielsen T., Jensen P.Ø., Wang H., Høiby N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015;85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Behroozian S., Sampedro I., Dhodary B., Her S., Yu Q., Stanton B.A., Hill J.E. Pseudomonas aeruginosa PAO1 is attracted to bovine bile in a novel, cystic fibrosis-derived bronchial epithelial cell model. Microorganisms. 2022;10:716. doi: 10.3390/microorganisms10040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkins Md W.V., Somayaji R. Epidemiology, biology, and impact of clonal Pseudomonas aeruginosa infections in cystic fibrosis. Clin. Microbiol. Rev. 2018;4:10–1128. doi: 10.1128/CMR.00019-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govan J.R.W., Nelson J.W. Microbiology of lung infection in cystic fibrosis. Br. Med. Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 10.Chadha J., Harjai K., Chhibber S. Revisiting the virulence hallmarks of <scp> Pseudomonas aeruginosa </scp> : a chronicle through the perspective of quorum sensing. Environ. Microbiol. 2022;24:2630–2656. doi: 10.1111/1462-2920.15784. [DOI] [PubMed] [Google Scholar]
- 11.Davies J.C. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr. Respir. Rev. 2002;3:128–134. doi: 10.1016/S1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 12.Oliver A., Cantón R., Campo P., Baquero F., Blázquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–1253. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 13.Mobaraki S., Aghazadeh M., Soroush Barhaghi M.H., Yousef Memar M., Goli H.R., Gholizadeh P., Samadi Kafil H. Prevalence of integrons 1, 2, 3 associated with antibiotic resistance in Pseudomonas aeruginosa isolates from Northwest of Iran. Biomedicine. 2018;8:2. doi: 10.1051/bmdcn/2018080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strateva T., Yordanov D. Pseudomonas aeruginosa – a phenomenon of bacterial resistance. J. Med. Microbiol. 2009;58:1133–1148. doi: 10.1099/jmm.0.009142-0. [DOI] [PubMed] [Google Scholar]
- 15.Breidenstein E.B.M., de la Fuente-Núñez C., Hancock R.E.W. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Davies D.G., Parsek M.R., Pearson J.P., Iglewski B.H., Costerton J.W., Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 17.De Kievit T.R., Gillis R., Marx S., Brown C., Iglewski B.H. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 2001;67:1865–1873. doi: 10.1128/AEM.67.4.1865-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuqua C., Parsek M.R., Greenberg E.P. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 19.Miller M.B., Bassler B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 20.Neil N.J.L.S., Whitehead A., Barnard Anne M.L., Slater Holly, Salmond G.P.C. 2001. Quorum-sensing in Gram-Negative Bacteria. [DOI] [PubMed] [Google Scholar]
- 21.Reading N.C., Sperandio V. Quorum sensing: the many languages of bacteria. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2006;254:1–11. doi: 10.1111/j.1574-6968.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- 22.González J.E., Keshavan N.D. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 2006;70:859–875. doi: 10.1128/MMBR.00002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerd Döring J.S.E., Flume Patrick, Heijerman Harry. 2012. Treatment of Lung Infection in Patients with Cystic Fibrosis Current and Future strategies.Pdf; pp. 461–479. 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Zwenger C. Sam and basu, plant terpenoids: applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008;3 [Google Scholar]
- 25.Pauwels A., Blondeau K., Dupont L.J., Sifrim D. Mechanisms of increased gastroesophageal reflux in patients with cystic fibrosis. Am. J. Gastroenterol. 2012;107:1346–1353. doi: 10.1038/ajg.2012.213. [DOI] [PubMed] [Google Scholar]
- 26.Woods D.F., Flynn S., Caparrós-Martín J.A., Stick S.M., Reen F.J., O'Gara F. Systems biology and bile acid signalling in microbiome-host interactions in the cystic fibrosis lung. Antibiotics. 2021;10:766. doi: 10.3390/antibiotics10070766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reen F.J., Woods D.F., Mooij M.J., Adams C., O'Gara F. Respiratory pathogens adopt a chronic lifestyle in response to bile. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maqbool A., Pauwels A. Cystic Fibrosis and gastroesophageal reflux disease. J. Cyst. Fibros. 2017;16 doi: 10.1016/j.jcf.2017.07.007. S2–S13. [DOI] [PubMed] [Google Scholar]
- 29.Pauwels A., Decraene A., Blondeau K., Mertens V., Farre R., Proesmans M., Van Bleyenbergh P., Sifrim D., Dupont L.J. Bile acids in sputum and increased airway inflammation in patients with cystic fibrosis. Chest. 2012;141:1568–1574. doi: 10.1378/chest.11-1573. [DOI] [PubMed] [Google Scholar]
- 30.Qin S., Xiao W., Zhou C., Pu Q., Deng X., Lan L., Liang H., Song X., Wu M. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Targeted Ther. 2022;7:1–27. doi: 10.1038/s41392-022-01056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn S., Reen F.J., O'Gara F. Exposure to bile leads to the emergence of adaptive signaling variants in the opportunistic pathogen Pseudomonas aeruginosa. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price C.E., O'Toole G.A. The gut-lung Axis in cystic fibrosis. J. Bacteriol. 2021;203 doi: 10.1128/JB.00311-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuthbertson L., Walker A.W., Oliver A.E., Rogers G.B., Rivett D.W., Hampton T.H., Ashare A., Elborn J.S., De Soyza A., Carroll M.P., Hoffman L.R., Lanyon C., Moskowitz S.M., O'Toole G.A., Parkhill J., Planet P.J., Teneback C.C., Tunney M.M., Zuckerman J.B., Bruce K.D., van der Gast C.J. Lung function and microbiota diversity in cystic fibrosis. Microbiome. 2020;8:45. doi: 10.1186/s40168-020-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidd T.J., Canton R., Ekkelenkamp M., Johansen H.K., Gilligan P., LiPuma J.J., Bell S.C., Elborn J.S., Flume P.A., VanDevanter D.R., Waters V.J. Defining antimicrobial resistance in cystic fibrosis. J. Cyst. Fibros. 2018;17:696–704. doi: 10.1016/j.jcf.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Lyczak J.B., Cannon C.L., Pier G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alemayehu D H.C., Casey P.G., McAuliffe O., Guinane C.M., Martin J.G., Shanahan F., Coffey A., Ross R.P. 2012. Bacteriophages φMR299-2 and φNH-4 Can Eliminate Pseudomonas aeruginosa in the Murine Lung and on Cystic Fibrosis Lung Airway Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey C., Weldon S., Elborn S., Downey D.G., Taggart C. The effect of CFTR modulators on airway infection in cystic fibrosis. Int. J. Mol. Sci. 2022;23:3513. doi: 10.3390/ijms23073513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saluzzo F., Riberi L., Messore B., Loré N.I., Esposito I., Bignamini E., De Rose V. CFTR modulator therapies: potential impact on airway infections in cystic fibrosis. Cells. 2022;11:1243. doi: 10.3390/cells11071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cystic Fibrosis Foundation, Cystic Fibrosis Foundation 2020 Annual Data Report. Cystic Fibrosis Foundation Patient Registry; 2021. pp. 1–96. [Google Scholar]
- 40.Ahern S., Dean J., Liman J., Ruseckaite R., Burke N., Gollan M., Keatley L., King S., Kotsimbos T., Middleton P.G., Schultz A., Wainwright C., Wark P., Bell S. Redesign of the Australian cystic fibrosis data registry: a multidisciplinary collaboration. Paediatr. Respir. Rev. 2021;37:37–43. doi: 10.1016/j.prrv.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 41.The Canadian Cystic Fibrosis Registry 2020 Annual Data Report. 2022. https://www.cysticfibrosis.ca/registry/2020AnnualDataReport.pdf [Google Scholar]
- 42.van R.J., et al Orenti A., Zolin A., Jung A. 2022. ECFSPR Annual Report 2020.https://www.ecfs.eu/sites/default/files/ECFSPR_Report_2020_v1.0 %2807Jun2022%29_website.pdf. [Google Scholar]
- 43.et al Zampoli M., Verstraete J., Frauendorf M., Workman L. 2020. South African Cystic Fibrosis Patient Registry Annual Report 2018.https://pulmonology.co.za/wp-content/uploads/2020/11/3474_SACFA_CF_RegistryReport2018.pdf [Google Scholar]
- 44.Brodlie M., Aseeri A., Lordan J.L., Robertson A.G.N., McKean M.C., Corris P.A., Griffin S.M., Manning N.J., Pearson J.P., Ward C. Bile acid aspiration in people with cystic fibrosis before and after lung transplantation. Eur. Respir. J. 2015;46:1820–1823. doi: 10.1183/13993003.00891-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blondeau K., Pauwels A., Dupont L., Mertens V., Proesmans M., Orel R., Brecelj J., López-Alonso M., Moya M., Malfroot A., De Wachter E., Vandenplas Y., Hauser B., Sifrim D. Characteristics of gastroesophageal reflux and potential risk of gastric content aspiration in children with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2010;50:161–166. doi: 10.1097/MPG.0b013e3181acae98. [DOI] [PubMed] [Google Scholar]
- 46.Pauwels A. Reflux Aspiration and Lung Disease. Springer International Publishing; Cham: 2018. Reflux aspiration and cystic fibrosis; pp. 187–194. [DOI] [Google Scholar]
- 47.Robinson N.B., DiMango E. Prevalence of gastroesophageal reflux in cystic fibrosis and implications for lung disease. Annals of the American Thoracic Society. 2014;11:964–968. doi: 10.1513/AnnalsATS.201401-044FR. [DOI] [PubMed] [Google Scholar]
- 48.Reddy C.A., Wakwaya Y.T. Impact of gastroesophageal reflux disease on idiopathic pulmonary fibrosis and lung transplant recipients. Curr. Opin. Gastroenterol. 2022;38:411–416. doi: 10.1097/MOG.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 49.Urso A., D'Ovidio F., Xu D., Emala C.W., Bunnett N.W., Perez-Zoghbi J.F. Bile acids inhibit cholinergic constriction in proximal and peripheral airways from humans and rodents. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L264. doi: 10.1152/ajplung.00242.2019. –L275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reen F.J., Flynn S., Woods D.F., Dunphy N., Chróinín M.N., Mullane D., Stick S., Adams C., O'Gara F. Bile signalling promotes chronic respiratory infections and antibiotic tolerance. Sci. Rep. 2016;6 doi: 10.1038/srep29768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen M.L., Takeda K., Sundrud M.S. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol. 2019;12:851–861. doi: 10.1038/s41385-019-0162-4. [DOI] [PubMed] [Google Scholar]
- 52.Shulpekova Y., Zharkova M., Tkachenko P., Tikhonov I., Stepanov A., Synitsyna A., Izotov A., Butkova T., Shulpekova N., Lapina N., Nechaev V., Kardasheva S., Okhlobystin A., Ivashkin V. The role of bile acids in the human body and in the development of diseases. Molecules. 2022;27:3401. doi: 10.3390/molecules27113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J., Chen J., Chen J. Role of farnesoid X receptor in the pathogenesis of respiratory diseases. Can. Respir. J. J. Can. Thorac. Soc. 2020;2020:1–8. doi: 10.1155/2020/9137251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reen F.J., Woods D.F., Mooij M.J., Chróinín M.N., Mullane D., Zhou L., Quille J., Fitzpatrick D., Glennon J.D., McGlacken G.P., Adams C., O'Gara F. Aspirated bile: a major host trigger modulating respiratory pathogen colonisation in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1763–1771. doi: 10.1007/s10096-014-2133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonnell M.J., Hunt E.B., Ward C., Pearson J.P., O'Toole D., Laffey J.G., Murphy D.M., Rutherford R.M. Current therapies for gastro-oesophageal reflux in the setting of chronic lung disease: state of the art review. ERJ Open Research. 2020;6 doi: 10.1183/23120541.00190-2019. 00190–02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen R., Lurie M., Kane M., DiFilippo C., Cohen A., Freiberger D., Boyer D., Visner G., Narvaez-Rivas M., Liu E., Setchell K. Risk factors for bile aspiration and its impact on clinical outcomes. Clin. Transl. Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn S., Reen F.J., Caparrós-Martín J.A., Woods D.F., Peplies J., Ranganathan S.C., Stick S.M., O'Gara F. Bile acid signal molecules associate temporally with respiratory inflammation and microbiome signatures in clinically stable cystic fibrosis patients. Microorganisms. 2020;8:1741. doi: 10.3390/microorganisms8111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sørli J.B., Balogh Sivars K., Da Silva E., Hougaard K.S., Koponen I.K., Zuo Y.Y., Weydahl I.E.K., Åberg P.M., Fransson R. Bile salt enhancers for inhalation: correlation between in vitro and in vivo lung effects. Int. J. Pharm. 2018;550:114–122. doi: 10.1016/j.ijpharm.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 59.Wang T., Lin S., Liu R., Li H., Liu Z., Xu H., Li Q., Bi K. Acute lung injury therapeutic mechanism exploration for Chinese classic prescription Qingzao Jiufei Decoction by UFLC-MS/MS quantification of bile acids, fatty acids and eicosanoids in rats. J. Pharmaceut. Biomed. Anal. 2020;189 doi: 10.1016/j.jpba.2020.113463. [DOI] [PubMed] [Google Scholar]
- 60.De Luca D., Alonso A., Autilio C. Bile acid-induced lung injury: update of reverse translational biology. Am. J. Physiol. Lung Cell Mol. Physiol. 2022;323 doi: 10.1152/ajplung.00523.2021. 10.1152/ajplung.00523.2021. [DOI] [PubMed] [Google Scholar]
- 61.Urso A., Perez-Zoghbi J., Nandakumar R., Cremers S., Bunnett N., Emala C., D'Ovidio F. Transplantation. European Respiratory Society; 2019. Aspirated bile acids affect lung immunity and function; p. PA3359. [DOI] [Google Scholar]
- 62.Urso A., Leiva-Juárez M.M., Briganti D.F., Aramini B., Benvenuto L., Costa J., Nandakumar R., Gomez E.A., Robbins H.Y., Shah L., Aversa M., Sonnet J.R., Arcasoy S., Cremers S., D'Ovidio F. Aspiration of conjugated bile acids predicts adverse lung transplant outcomes and correlates with airway lipid and cytokine dysregulation. J. Heart Lung Transplant. 2021;40:998–1008. doi: 10.1016/j.healun.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yonker H.M.L., Wood M., Figuereo N.E., Hurley B.P. 2019. Exposure to Bile Salts Alters the Architecture and Host Defense of the Airway Epithelium, American Journal of Respiratory and Critical Care Medicine. 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2583. [Google Scholar]
- 64.Chen B., You W.J., Liu X.Q., Xue S., Qin H., Jiang H.D. Chronic microaspiration of bile acids induces lung fibrosis through multiple mechanisms in rats. Clin. Sci. 2017;131:951–963. doi: 10.1042/CS20160926. [DOI] [PubMed] [Google Scholar]
- 65.Caparrós-Martín J.A., Saladié M., Agudelo-Romero P., Reen J., Ware R.S., Sly P.D., Stick S.M., O'Gara F., Group C.S. Detection of bile acids in bronchoalveolar lavage fluid defines early pathobiological events in infants with cystic fibrosis. SSRN Electron. J. 2022 doi: 10.2139/ssrn.4081064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunt E.B., Sullivan A., Galvin J., MacSharry J., Murphy D.M. Gastric aspiration and its role in airway inflammation, the open respiratory medicine. Journal. 2018;12:1–10. doi: 10.2174/1874306401812010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pittman J.E., Wylie K.M., Akers K., Storch G.A., Hatch J., Quante J., Frayman K.B., Clarke N., Davis M., Stick S.M., Hall G.L., Montgomery G., Ranganathan S., Davis S.D., Ferkol T.W. Association of antibiotics, airway microbiome, and inflammation in infants with cystic fibrosis. Annals of the American Thoracic Society. 2017;14:1548–1555. doi: 10.1513/AnnalsATS.201702-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camillo C., Russum S., Benvenuto L., D'Ovidio F. Bile acids are not only a marker of aspiration as they stimulate fibrosis and derange surfactant homeostasis in human lung cells. J. Heart Lung Transplant. 2023;42:S545. doi: 10.1016/j.healun.2023.02.1487. 1277. [DOI] [Google Scholar]
- 69.Moore N.M., Flaws M.L. Introduction: pseudomonas aeruginosa. Clin. Lab. Sci. 2011;24:41. [PubMed] [Google Scholar]
- 70.Stover C.K., Pham X.Q., Erwin A.L., Mizoguchi S.D., Warrener P., Hickey M.J., Brinkman F.S.L., Hufnagle W.O., Kowalik D.J., Lagrou M., Garber R.L., Goltry L., Tolentino E., Westbrock-Wadman S., Yuan Y., Brody L.L., Coulter S.N., Folger K.R., Kas A., Larbig K., Lim R., Smith K., Spencer D., Wong G.K.-S., Wu Z., Paulsen I.T., Reizer J., Saier M.H., Hancock R.E.W., Lory S., Olson M.V. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 71.van Belkum A., Soriaga L.B., LaFave M.C., Akella S., Veyrieras J.-B., Barbu E.M., Shortridge D., Blanc B., Hannum G., Zambardi G., Miller K., Enright M.C., Mugnier N., Brami D., Schicklin S., Felderman M., Schwartz A.S., Richardson T.H., Peterson T.C., Hubby B., Cady K.C. Phylogenetic distribution of CRISPR-cas systems in antibiotic-resistant Pseudomonas aeruginosa. mBio. 2015;6 doi: 10.1128/mBio.01796-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rossi E., La Rosa R., Bartell J.A., Marvig R.L., Haagensen J.A.J., Sommer L.M., Molin S., Johansen H.K. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2021;19:331–342. doi: 10.1038/s41579-020-00477-5. [DOI] [PubMed] [Google Scholar]
- 73.Mulcahy L.R., Isabella V.M., Lewis K. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol. 2014;68:1–12. doi: 10.1007/s00248-013-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scotet V., L'Hostis C., Férec C. The changing epidemiology of cystic fibrosis: incidence, survival and impact of the CFTR gene discovery. Genes. 2020;11:589. doi: 10.3390/genes11060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khalili Y., Yekani M., Goli H.R., Memar M.Y. Characterization of carbapenem-resistant but cephalosporin-susceptible Pseudomonas aeruginosa. Acta Microbiol. Immunol. Hung. 2019;66:529–540. doi: 10.1556/030.66.2019.036. [DOI] [PubMed] [Google Scholar]
- 76.Bhardwaj S., Bhatia S., Singh S., Franco F., Jr. Growing emergence of drug-resistant Pseudomonas aeruginosa and attenuation of its virulence using quorum sensing inhibitors: a critical review. Iranian Journal of Basic Medical Sciences. 2021;24:699. doi: 10.22038/IJBMS.2021.49151.11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poole K. Pseudomonas aeruginosa: resistance to the max. Front. Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context (US) 2018;7:1–18. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lambert P.A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. Roy. Soc. Med. Suppl. 2002;95:22–26. [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen L., Garcia J., Gruenberg K., MacDougall C. Multidrug-resistant Pseudomonas infections: hard to treat, but hope on the horizon? Curr. Infect. Dis. Rep. 2018;20:23. doi: 10.1007/s11908-018-0629-6. [DOI] [PubMed] [Google Scholar]
- 81.Olivares E B.-B.S., T B., Provot C., Prévost G J.F. Clinical impact of antibiotics for the treatment of Pseudomonas aeruginosa biofilm infections. Front. Microbiol. 2020 doi: 10.3389/fmicb.2019.02894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pachori P., Gothalwal R., Gandhi P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes & Diseases. 2019;6:109–119. doi: 10.1016/j.gendis.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ibrahim D., Jabbour J.-F., Kanj S.S. Current choices of antibiotic treatment for Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2020;33:464–473. doi: 10.1097/QCO.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 84.Soares A., Alexandre K., Etienne M. Tolerance and persistence of Pseudomonas aeruginosa in biofilms exposed to antibiotics: molecular mechanisms, antibiotic strategies and therapeutic perspectives. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rocha A.J., Barsottini M.R. de O., Rocha R.R., Laurindo M.V., de Moraes F.L.L., da Rocha S.L. Pseudomonas aeruginosa: virulence factors and antibiotic resistance genes. Braz. Arch. Biol. Technol. 2019;62 doi: 10.1590/1678-4324-2019180503. [DOI] [Google Scholar]
- 86.Greenwald M.A., Wolfgang M.C. The changing landscape of the cystic fibrosis lung environment: from the perspective of Pseudomonas aeruginosa. Curr. Opin. Pharmacol. 2022;65 doi: 10.1016/j.coph.2022.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ciofu O., Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puja H., Bolard A., Noguès A., Plésiat P., Jeannot K. vol. 64. 2020. (The Efflux Pump MexXY/OprM Contributes to the Tolerance and Acquired Resistance of Pseudomonas aeruginosa to Colistin, Antimicrobial Agents and Chemotherapy). 10.1128/AAC.02033-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee J.-Y., Park Y.K., Chung E.S., Na I.Y., Ko K.S. Evolved resistance to colistin and its loss due to genetic reversion in Pseudomonas aeruginosa. Sci. Rep. 2016;6 doi: 10.1038/srep25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pamp S.J., Gjermansen M., Johansen H.K., Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol. 2008;68:223–240. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 91.Thi M.T.T., Wibowo D., Rehm B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020;21:8671. doi: 10.3390/ijms21228671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waters C.M., Bassler B.L. Quorum SENSING: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 93.Ng W.-L., Bassler B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tuon F.F., Dantas L.R., Suss P.H., Tasca Ribeiro V.S. Pathogenesis of the Pseudomonas aeruginosa biofilm: a review. Pathogens. 2022;11:300. doi: 10.3390/pathogens11030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalaiarasan Ellappan B.N.H., Thirumalaswamy Kottha, Gnanasambandam Vasuki J.J. 2017. Veeresh Kumar Sali, Inhibition of Quorum Sensing-Controlled Biofilm Formation in Pseudomonas aeruginosa by Quorum-Sensing Inhibitors. [DOI] [PubMed] [Google Scholar]
- 96.Lee J., Wu J., Deng Y., Wang J.J., Wang C., Wang J.J., Chang C., Dong Y., Williams P., Zhang L.-H. A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 2013;9:339–343. doi: 10.1038/nchembio.1225. [DOI] [PubMed] [Google Scholar]
- 97.Dela Ahator S., Sagar S., Zhu M., Wang J., Zhang L.-H. Nutrient availability and phage exposure alter the quorum-sensing and CRISPR-cas-controlled population dynamics of Pseudomonas aeruginosa. mSystems. 2022;7 doi: 10.1128/msystems.00092-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng X., Dela Ahator S., Zhang L.-H. Molecular mechanisms of phosphate stress activation of Pseudomonas aeruginosa quorum sensing systems. mSphere. 2020;5 doi: 10.1128/mSphere.00119-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J., Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein & Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whiteley M., Lee K.M., Greenberg E.P. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pesci Ec I.B., Pearson J.P., Seed P.C. 1997. Regulation of Las and Rhl Quorum Sensing in Pseudomonas aeruginosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith R. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003;6:56–60. doi: 10.1016/S1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 103.Patil A., Banerji R., Kanojiya P., Saroj S.D. Foodborne ESKAPE biofilms and antimicrobial resistance: lessons learned from clinical isolates. Pathog. Glob. Health. 2021;115:339–356. doi: 10.1080/20477724.2021.1916158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kostylev M D.A., Kim D.Y., Smalley N.E., Salukhe I., Greenberg E.P. Proc Natl Acad Sci U S A.; 2019. Evolution of the Pseudomonas aeruginosa Quorum-Sensing Hierarchy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venturi V. Regulation of quorum sensing in Pseudomonas. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 2006;30:274–291. doi: 10.1111/j.1574-6976.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 106.Simanek K.A., Taylor I.R., Richael E.K., Lasek-Nesselquist E., Bassler B.L.J.E.P. 2022. The PqsE-RhlR Interaction Regulates RhlR DNA Binding to Control Virulence Factor Production in Pseudomonas aeruginosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wade Ds P.E., Calfee M.W., Rocha E.R., Ling E.A., Engstrom E., Coleman J.P. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2005 doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pena Rt T.M., Blasco L., Ambroa A., González-Pedrajo B., Fernández-García L., López M., Bleriot I., Bou G., García-Contreras R., Wood T.K. Relationship between quorum sensing and secretion systems. Front. Microbiol. 2019 doi: 10.3389/fmicb.2019.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McGrath S., Wade D.S., Pesci E.C. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS) FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2004;230:27–34. doi: 10.1016/S0378-1097(03)00849-8. [DOI] [PubMed] [Google Scholar]
- 110.Xiao G., He J., Rahme L.G., H J., Gaoping Xiao L.G.R. Mutation analysis of the Pseudomonas aeruginosa mvfR and pqsABCDE gene promoters demonstrates complex quorum-sensing circuitry. Microbiology. 2006;152:1679–1686. doi: 10.1099/mic.0.28605-0. [DOI] [PubMed] [Google Scholar]
- 111.Higgins S., Heeb S., Rampioni G., Fletcher M.P., Williams P., Cámara M. Differential regulation of the phenazine biosynthetic operons by quorum sensing in Pseudomonas aeruginosa PAO1-N. Front. Cell. Infect. Microbiol. 2018;8 doi: 10.3389/fcimb.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan S., Wu G. Can biofilm Be reversed through quorum sensing in Pseudomonas aeruginosa? Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hogardt M., Heesemann J. 2011. Microevolution of Pseudomonas aeruginosa to a Chronic Pathogen of the Cystic Fibrosis Lung, in; pp. 91–118. [DOI] [PubMed] [Google Scholar]
- 114.Wu H H.N., Song Z., Givskov M., Doring G., Worlitzsch D., Mathee K., Rygaard J. Pseudomonas aeruginosa mutations in lasI and rhlI quorum sensing systems result in milder chronic lung infection. Microbiology. 2001 doi: 10.1099/00221287-147-5-1105. [DOI] [PubMed] [Google Scholar]
- 115.Blanchard A.C., Waters V.J. Opportunistic pathogens in cystic fibrosis: epidemiology and pathogenesis of lung infection. Journal of the Pediatric Infectious Diseases Society. 2022;11 doi: 10.1093/jpids/piac052. S3–S12. [DOI] [PubMed] [Google Scholar]
- 116.Chatterjee M., Anju C.P., Biswas L., Anil Kumar V., Gopi Mohan C., Biswas R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. International Journal of Medical Microbiology. 2016;306:48–58. doi: 10.1016/j.ijmm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 117.S G., Moradali Mf R.B. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017 doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oluwabusola E.T., Katermeran W.H.G., P N., Poh O., M.B T., Tan L.T., Diyaolu S.A., Tabudravu J., Ebel R., Rice M. Jaspars. 2022. Inhibition of the Quorum Sensing System, Elastase Production and Biofilm Formation in Pseudomonas aeruginosa by Psammaplin A and Bisaprasin, Molecules. 10.3390/molecules27051721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuang Z., Bennett R.C., Lin J., Hao Y., Zhu L., Akinbi H.T., Lau G.W. Surfactant phospholipids act as molecular switches for premature induction of quorum sensing-dependent virulence in Pseudomonas aeruginosa. Virulence. 2020;11:1090–1107. doi: 10.1080/21505594.2020.1809327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chegini Z., Khoshbayan A., Taati Moghadam M., Farahani I., Jazireian P., Shariati A. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: a review. Ann. Clin. Microbiol. Antimicrob. 2020;19:1–17. doi: 10.1186/s12941-020-00389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dockery J. A mathematical model for quorum sensing in Pseudomonas aeruginosa. Bull. Math. Biol. 2001;63:95–116. doi: 10.1006/bulm.2000.0205. [DOI] [PubMed] [Google Scholar]
- 122.Sharma G., Rao S., Bansal A., Dang S., Gupta S., Gabrani R. Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals. 2014;42:1–7. doi: 10.1016/j.biologicals.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 123.Høiby N., Ciofu O., Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- 124.L J., Cheng J. 2019. Quorum Sensing in Pseudomonas aeruginosa and its Relationship to Biofilm Development. [Google Scholar]
- 125.del Mar Cendra M., Torrents E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021;49:107734. doi: 10.1016/j.biotechadv.2021.107734. [DOI] [PubMed] [Google Scholar]
- 126.Donlan R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microb. Infect. 2003;5:1213–1219. doi: 10.1016/j.micinf.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Bjarnsholt T., Jensen P.Ø., Fiandaca M.J., Pedersen J., Hansen C.R., Andersen C.B., Pressler T., Givskov M., Høiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 129.Schulze A., Mitterer F., Pombo J.P., Schild S. Biofilms by bacterial human pathogens: clinical relevance - development, composition and regulation - therapeutical strategies. Microbial Cell. 2021;8:28–56. doi: 10.15698/mic2021.02.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kathirvel Brindhadevi, LewisOscar Felix, Mylonakis Eleftherios, Shanmugam Sabarathinam, Verma Tikendra Nath, Pugazhendhi Arivalagan. Biofilm and Quorum sensing mediated pathogenicity in Pseudomonas aeruginosa. Process Biochemistry. 2020;96:49–57. doi: 10.1016/j.procbio.2020.06.001. [DOI] [Google Scholar]
- 131.Laverty G., Gorman S., Gilmore B. Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathogens. 2014;3:596–632. doi: 10.3390/pathogens3030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alhede M., Bjarnsholt T., Givskov M., Alhede M. 2014. Pseudomonas aeruginosa Biofilms, in; pp. 1–40. 10.1016/B978-0-12-800262-9.00001-9. [DOI] [PubMed] [Google Scholar]
- 133.Sionov R.V., Steinberg D. Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic bacteria. Microorganisms. 2022;10:1239. doi: 10.3390/microorganisms10061239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Panlilio H R.C. The role of extracellular DNA in the formation, architecture, stability, and treatment of bacterial biofilms. Biotechnol. Bioeng. 2021 doi: 10.1002/bit.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.dos Santos A.L.S., Galdino A.C.M., de Mello T.P., Ramos L. de S., Branquinha M.H., Bolognese A.M., Columbano Neto J., Roudbary M. vol. 113. Memórias Do Instituto Oswaldo Cruz; 2018. (What Are the Advantages of Living in a Community? A Microbial Biofilm Perspective!). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Montanaro L., Poggi A., Visai L., Ravaioli S., Campoccia D., Speziale P., Arciola C.R. Extracellular DNA in biofilms. Int. J. Artif. Organs. 2011;34:824–831. doi: 10.5301/ijao.5000051. [DOI] [PubMed] [Google Scholar]
- 137.Boyd A., Chakrabarty A.M. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1994;60:2355–2359. doi: 10.1128/aem.60.7.2355-2359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Santajit S., Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016:2016. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sarkar S. Release mechanisms and molecular interactions of Pseudomonas aeruginosa extracellular DNA. Appl. Microbiol. Biotechnol. 2020;104:6549–6564. doi: 10.1007/s00253-020-10687-9. [DOI] [PubMed] [Google Scholar]
- 140.Fernández-Billón M., Llambías-Cabot A.E., Jordana-Lluch E., Oliver A., Macià M.D. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms. Biofilm. 2023;5 doi: 10.1016/j.bioflm.2023.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jurado-Martín I M.S., Sainz-Mejías M. Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Nt J Mol Sci. 2021 doi: 10.3390/ijms22063128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.B.V.V S., S.G.P R., M.A F., Voulhoux F.R. 2010. Protein Secretion Systems in Pseudomonas aeruginosa: A Wealth of Pathogenic Weapons. [DOI] [PubMed] [Google Scholar]
- 143.B. A J., M. A P., S. A A., F. B C., Frédéric G., Benoit G., Husson M.-O. 2005. Relative Expression of Pseudomonas aeruginosa Virulence Genes Analyzed by a Real Time RT-PCR Method during Lung Infection in Rats. [DOI] [PubMed] [Google Scholar]
- 144.Y W., Lin J S.X., Cheng J. The Pseudomonas quinolone signal (PQS): not just for quorum sensing anymore. Front. Cell. Infect. Microbiol. 2018 doi: 10.3389/fcimb.2018.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soto-Aceves M.P., Cocotl-Yañez M., Merino E., Castillo-Juárez I., Cortés-López H., González-Pedrajo B., Díaz-Guerrero M., Servín-González L., Soberón-Chávez G. Inactivation of the quorum-sensing transcriptional regulators LasR or RhlR does not suppress the expression of virulence factors and the virulence of Pseudomonas aeruginosa PAO1. Microbiology. 2019;165:425–432. doi: 10.1099/mic.0.000778. [DOI] [PubMed] [Google Scholar]
- 146.Diggle Sp W.P., Winzer K., Chhabra S.R., Worrall K.E., Cámara M. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 2003;50(1):29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- 147.Anantharajah A., Mingeot-Leclercq M.-P., Van Bambeke F. Targeting the type three secretion system in Pseudomonas aeruginosa. Trends Pharmacol. Sci. 2016;37:734–749. doi: 10.1016/j.tips.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 148.Winson B.W., K M., Camara M., Latifi A., Foglino M., Chhabra S.R., Daykin M., Bally M., Chapon V., Salmond G.P., Bycroft . 1995. Multiple N-Acyl-L-Homoserine Lactone Signal Molecules Regulate Production of Virulence Determinants and Secondary Metabolites in Pseudomonas aeruginosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Strempel S., Nikola, Neidig Anke, Nusser Michael, Geffers Robert, Vieillard Julien, Lesouhaitier Olivier, Brenner-Weiss Gerald, Overhage Joerg, Fleiszig Human host defense peptide LL-37 stimulates virulence factor production and adaptive resistance in Pseudomonas aeruginosa. PLoS One. 2013 doi: 10.1371/journal.pone.0082240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Essar Dw C.I., Eberly L., Hadero A. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990 doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Usher L.R., Lawson R.A., Geary I., Taylor C.J., Bingle C.D., Taylor G.W., Whyte M.K.B. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J. Immunol. 2002;168:1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- 152.Ran H., Hassett D.J., Lau G.W. Human targets of Pseudomonas aeruginosa pyocyanin. Proc. Natl. Acad. Sci. USA. 2003;100:14315–14320. doi: 10.1073/pnas.2332354100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Froes T.Q., Guido R.V., Metwally K., Castilho M.S. A novel scaffold to fight Pseudomonas aeruginosa pyocyanin production: early steps to novel antivirulence drugs. Future Med. Chem. 2020;12:1489–1503. doi: 10.4155/fmc-2019-0351. [DOI] [PubMed] [Google Scholar]
- 154.Managò A., Becker K.A., Carpinteiro A., Wilker B., Soddemann M., Seitz A.P., Edwards M.J., Grassmé H., Szabò I., Gulbins E. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxidants Redox Signal. 2015;22:1097–1110. doi: 10.1089/ars.2014.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hall S., McDermott C., Anoopkumar-Dukie S., McFarland A., Forbes A., Perkins A., Davey A., Chess-Williams R., Kiefel M., Arora D., Grant G. Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins. 2016;8:236. doi: 10.3390/toxins8080236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lau G.W., Ran H., Kong F., Hassett D.J., Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rada B., Leto T.L. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21:73–81. doi: 10.1016/j.tim.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alatraktchi F.A., Svendsen W.E., Molin S. Electrochemical detection of pyocyanin as a biomarker for Pseudomonas aeruginosa: a focused review. Sensors. 2020;20:5218. doi: 10.3390/s20185218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Am D. Biological activity and applications of pyocyanin produced by Pseudomonas aeruginosa. Open Access Journal of Biomedical Science. 2020;1 doi: 10.38125/OAJBS.000133. [DOI] [Google Scholar]
- 160.Mukherjee S., Moustafa D.A., Stergioula V., Smith C.D., Goldberg J.B., Bassler B.L. The PqsE and RhlR proteins are an autoinducer synthase–receptor pair that control virulence and biofilm development in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2018;115 doi: 10.1073/pnas.1814023115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mukherjee S.S.C., Moustafa D., Goldberg Jb B.B. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS. 2017;13:e1006504. doi: 10.1371/journal.ppat.1006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Perry E.K., Meirelles L.A., Newman D.K. From the soil to the clinic: the impact of microbial secondary metabolites on antibiotic tolerance and resistance. Nat. Rev. Microbiol. 2022;20:129–142. doi: 10.1038/s41579-021-00620-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haiko B. 2013. Johanna; Westerlund-Wikström, the Role of the Bacterial Flagellum in Adhesion and Virulence. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghorbani H., Memar M.Y., Sefidan F.Y., Yekani M., Ghotaslou R. In vitro synergy of antibiotic combinations against planktonic and biofilm Pseudomonas aeruginosa. GMS Hygiene and Infection Control. 2017;12:Doc17. doi: 10.3205/dgkh000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gholizadeh P., Köse Ş., Dao S., Ganbarov K., Tanomand A., Dal T., Aghazadeh M., Ghotaslou R., Ahangarzadeh Rezaee M., Yousefi B., Samadi Kafil H. How CRISPR-cas system could Be used to combat antimicrobial resistance. Infect. Drug Resist. 2020;13:1111–1121. doi: 10.2147/IDR.S247271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sikdar R., Elias M.H. Evidence for complex interplay between quorum sensing and antibiotic resistance in Pseudomonas aeruginosa. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.01269-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rice L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010;31 doi: 10.1086/655995. S7–S10. [DOI] [PubMed] [Google Scholar]
- 168.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fadi Soukarieh M.C., Williams Paul. Michael john stocks, Pseudomonas aeruginosa quorum sensing systems as drug discovery targets: current position and future perspectives. J. Med. Chem. 2018 doi: 10.1021/acs.jmedchem.8b00540. [DOI] [PubMed] [Google Scholar]
- 170.Chadha J., Harjai K., Chhibber S. Repurposing phytochemicals as anti‐virulent agents to attenuate quorum sensing‐regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2022;15:1695–1718. doi: 10.1111/1751-7915.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pesingi P.V., Singh B.R., Pesingi P.K., Bhardwaj M., Singh S.V., Kumawat M., Sinha D.K., Gandham R.K. MexAB-OprM efflux pump of Pseudomonas aeruginosa offers resistance to carvacrol: a herbal antimicrobial agent. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Linden P.K., Kusne S., Coley K., Fontes P., Kramer D.J., Paterson D. Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2003;37 doi: 10.1086/379611. e154–e160. [DOI] [PubMed] [Google Scholar]
- 173.Fujiwara M., Yamasaki S., Morita Y., Nishino K. Evaluation of efflux pump inhibitors of MexAB- or MexXY-OprM in Pseudomonas aeruginosa using nucleic acid dyes. J. Infect. Chemother. 2022;28:595–601. doi: 10.1016/j.jiac.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 174.Housseini B Issa K., Phan G., Broutin I. Functional mechanism of the efflux pumps transcription regulators from Pseudomonas aeruginosa based on 3D structures. Front. Mol. Biosci. 2018;5 doi: 10.3389/fmolb.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fruci M., Poole K. Aminoglycoside-inducible expression of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: involvement of the envelope stress-responsive AmgRS two-component system. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laborda P., Sanz-García F., Hernando-Amado S., Martínez J.L. Pseudomonas aeruginosa: an antibiotic resilient pathogen with environmental origin. Curr. Opin. Microbiol. 2021;64:125–132. doi: 10.1016/j.mib.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 177.Verchère A., Dezi M., Adrien V., Broutin I., Picard M. In vitro transport activity of the fully assembled MexAB-OprM efflux pump from Pseudomonas aeruginosa. Nat. Commun. 2015;6:6890. doi: 10.1038/ncomms7890. [DOI] [PubMed] [Google Scholar]
- 178.Horna G., López M., Guerra H., Saénz Y., Ruiz J. Interplay between MexAB-OprM and MexEF-OprN in clinical isolates of Pseudomonas aeruginosa. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-34694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Aeschlimann J.R. The role of multidrug efflux pumps in the antibiotic resistance of Pseudomonas aeruginosa and other gram-negative bacteria. Pharmacotherapy. 2003;23:916–924. doi: 10.1592/phco.23.7.916.32722. [DOI] [PubMed] [Google Scholar]
- 180.Zahmatkesh H., Mirpour M., Zamani H., Rasti B., Rahmani F.A., Padasht N. Effect of samarium oxide nanoparticles on virulence factors and motility of multi-drug resistant Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2022;38:209. doi: 10.1007/s11274-022-03384-4. [DOI] [PubMed] [Google Scholar]
- 181.Zahedi bialvaei A., Rahbar M., Hamidi-Farahani R., Asgari A., Esmailkhani A., Mardani dashti Y., Soleiman-Meigooni S. Expression of RND efflux pumps mediated antibiotic resistance in Pseudomonas aeruginosa clinical strains. Microb. Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104789. [DOI] [PubMed] [Google Scholar]
- 182.Poole K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 2001;3:255–264. http://www.ncbi.nlm.nih.gov/pubmed/11321581 [PubMed] [Google Scholar]
- 183.Arabestani M.R., Rajabpour M., Yousefi Mashouf R., Alikhani M.Y., Mousavi S.M. Expression of efflux pump MexAB-OprM and OprD of Pseudomonas aeruginosa strains isolated from clinical samples using qRT-PCR. Arch. Iran. Med. 2015;18:102. http://www.ncbi.nlm.nih.gov/pubmed/25644798 –8. [PubMed] [Google Scholar]
- 184.Goli H.R., Nahaei M.R., Rezaee M.A., Hasani A., Samadi Kafil H., Aghazadeh M., Sheikhalizadeh V. Contribution of mexAB-oprM and mexXY (oprA) efflux operons in antibiotic resistance of clinical Pseudomonas aeruginosa isolates in Tabriz, Iran, Infection. Genetics and Evolution. 2016;45:75–82. doi: 10.1016/j.meegid.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 185.Llanes C., Hocquet D., Vogne C., Benali-Baitich D., Neuwirth C., Plésiat P. Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously. Antimicrob. Agents Chemother. 2004;48:1797–1802. doi: 10.1128/AAC.48.5.1797-1802.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pan Y., Xu Y., Wang Z., Fang Y., Shen J. Overexpression of MexAB-OprM efflux pump in carbapenem-resistant Pseudomonas aeruginosa. Arch. Microbiol. 2016;198:565–571. doi: 10.1007/s00203-016-1215-7. [DOI] [PubMed] [Google Scholar]
- 187.Xu Y., Niu H., Hu T., Zhang L., Su S., He H., Wang H., Zhang D. High expression of metallo-β-lactamase contributed to the resistance to carbapenem in clinical isolates of Pseudomonas aeruginosa from baotou, China. Infect. Drug Resist. 2020;13:35–43. doi: 10.2147/IDR.S233987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Scoffone V.C., Trespidi G., Barbieri G., Irudal S., Perrin E., Buroni S. Role of RND efflux pumps in drug resistance of cystic fibrosis pathogens. Antibiotics. 2021;10:863. doi: 10.3390/antibiotics10070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Caparrós-Martín J.A., Flynn S., Reen F.J., Woods D.F., Agudelo-Romero P., Ranganathan S.C., Stick S.M., O'Gara F. The detection of bile acids in the lungs of paediatric cystic fibrosis patients is associated with altered inflammatory patterns. Diagnostics. 2020;10:282. doi: 10.3390/diagnostics10050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Al-Momani H., Perry A., Nelson A., Stewart C.J., Jones R., Krishnan A., Robertson A., Bourke S., Doe S., Cummings S., Anderson A., Forrest T., Forrest I., Griffin M., Wilcox M., Brodlie M., Pearson J., Ward C. Exposure to bile and gastric juice can impact the aerodigestive microbiome in people with cystic fibrosis. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-15375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Forti F., Roach D.R., Cafora M., Pasini M.E., Horner D.S., Fiscarelli E.V., Rossitto M., Cariani L., Briani F., Debarbieux L., Ghisotti D. Design of a broad-range bacteriophage cocktail that reduces Pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02573-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cesta N.A.M., Di Luca M., Corbellino M., Tavio M., Galli M. Bacteriophage therapy: an overview and the position of Italian society of infectious and tropical diseases. Infez Med. 2020;28(3):322–331. [PubMed] [Google Scholar]
- 193.Samir S. Bacteriophages as therapeutic agents: alternatives to antibiotics. Recent Pat. Biotechnol. 2021;15:25–33. doi: 10.2174/1872208315666210121094311. [DOI] [PubMed] [Google Scholar]
- 194.Cafora M., Deflorian G., Forti F., Ferrari L., Binelli G., Briani F., Ghisotti D., Pistocchi A. Phage therapy against Pseudomonas aeruginosa infections in a cystic fibrosis zebrafish model. Sci. Rep. 2019;9:1527. doi: 10.1038/s41598-018-37636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fernández L., Rodríguez A., García P. Phage or foe: an insight into the impact of viral predation on microbial communities. ISME J. 2018;12:1171–1179. doi: 10.1038/s41396-018-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Høyland-Kroghsbo N.M., Paczkowski J., Mukherjee S., Broniewski J., Westra E., Bondy-Denomy J., Bassler B.L. Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system. Proc. Natl. Acad. Sci. USA. 2017;114:131–135. doi: 10.1073/pnas.1617415113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Patterson A.G., Jackson S.A., Taylor C., Evans G.B., Salmond G.P.C., Przybilski R., Staals R.H.J., Fineran P.C. Quorum sensing controls adaptive immunity through the regulation of multiple CRISPR-cas systems. Mol. Cell. 2016;64:1102–1108. doi: 10.1016/j.molcel.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hargreaves K.R., Kropinski A.M., Clokie M.R. Bacteriophage behavioral ecology. Bacteriophage. 2014;4 doi: 10.4161/bact.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shah M., Taylor V., Bona D., Tsao Y., Stanley S.Y., Elardo S., McCallum M., Bondy-Denomy J., Howell P.L., Nodwell J., Davidson A.R., Moraes T., Maxwell K.L. A phage-encoded anti-activator inhibits quorum sensing in Pseudomonas aeruginosa. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3544401. [DOI] [PubMed] [Google Scholar]
- 200.Ambroa A., Blasco L., López-Causapé C., Trastoy R., Fernandez-García L., Bleriot I., Ponce-Alonso M., Pacios O., López M., Cantón R., Kidd T.J., Bou G., Oliver A., Tomás M. Temperate bacteriophages (prophages) in Pseudomonas aeruginosa isolates belonging to the international cystic fibrosis clone (CC274) Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.556706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Al-Wrafy F., Brzozowska E., Górska S., Gamian A. Pathogenic factors of Pseudomonas aeruginosa – the role of biofilm in pathogenicity and as a target for phage therapy. Postępy Higieny Medycyny Doświadczalnej. 2017;71:78–91. doi: 10.5604/01.3001.0010.3792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.