Graphical abstract
Keywords: Parkinson’s disease, Resting-state functional connectivity (RsFC), Motor networks, Non-motor networks, MDS-UPDRS III, Predictive modeling
Highlights
-
•
Motor impairments in people with PD (PPD) are linked with nonmotor cortical areas.
-
•
Visual and attention networks predict clinical motor assessment in PPD.
-
•
Nonmotor cortical areas may compensate for reduced motor automaticity in PPD.
Abstract
Objective
Investigate the brain functional networks associated with motor impairment in people with Parkinson’s disease (PD).
Background
PD is primarily characterized by motor dysfunction. Resting-state functional connectivity (RsFC) offers a unique opportunity to non-invasively characterize brain function. In this study, we hypothesized that the motor dysfunction observed in people with PD involves atypical connectivity not only in motor but also in higher-level attention networks. Understanding the interaction between motor and non-motor RsFC that are related to the motor signs could provide insights into PD pathophysiology.
Methods
We used data from 88 people with PD (mean age: 68.2(SD:10), 55 M/33F) coming from 2 cohorts. Motor severity was assessed in practical OFF-medication state, using MDS‐UPDRS Part‐III motor scores (mean: 49 (SD:10)). RsFC was characterized using an atlas of 384 regions that were grouped into 13 functional networks. Associations between RsFC and motor severity were assessed independently for each RsFC using predictive modeling.
Results
The top 5 % models that predicted the MDS-UPDRS-III motor scores with effect size >0.5 were the connectivity between (1) the somatomotor and Subcortical-Basal-ganglia, (2) somatomotor and Visual and (3) CinguloOpercular (CiO) and language/Ventral attention (Lan/VeA) network pairs.
Discussion
Our findings suggest that, along with motor networks, visual- and attention-related cortical networks are also associated with the motor symptoms of PD. Non-motor networks may be involved indirectly in motor-coordination. When people with PD have deficits in motor networks, more attention may be needed to carry out formerly automatic motor functions, consistent with compensatory mechanisms in parkinsonian movement disorders.
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by a set of cardinal motor symptoms namely, bradykinesia, tremor, rigidity, and postural instability (Braak and Del Tredici, 2008). Although the primary brain pathology in PD is the loss of dopaminergic neurons in the basal ganglia, the basal ganglia are highly interconnected to motor and non-motor cortical areas via corticostriatal loops (Braak and Del Tredici, 2008). While it is known that the functional connectivity of the basal ganglia with sensorimotor cortical regions is altered in people with PD (Tessitore et al., 2014), the impact of PD on other functional networks across the whole brain is not well understood.
Resting-state functional MRI (rs-fMRI) noninvasively characterizes co-activation patterns across brain areas and between brain networks (Power et al., 2011). Rs-fMRI offers a unique opportunity to examine the interplay between the changes in brain function and the observed motor impairments in people with PD. Previous functional imaging studies have shown that motor signs of PD are associated with reduced blood flow in sensorimotor cortical regions, putamen, and globus pallidus and increased cerebral blood flow to premotor areas (Wu et al., 2009, Turner et al., 2003). These studies have been fundamental to understanding which brain regions are affected by PD (Wu et al., 2009, Turner et al., 2003, Filippi et al., 2019). While there are many seed-based imaging studies that examine connectivity patterns from prior hypotheses, it is also important to study whole-brain network reorganization to identify lesser-studied brain regions/functions that might be affected by PD (Hacker et al., 2012, Fling et al., 2014, Luo et al., 2014). Understanding whole-brain network connectivity is important even for treatment options since, RsFC that includes non-motor regions (after DBS) are shown to improve the optimal DBS parameters compared to using RsFC with motor-only regions (Boutet et al., 2021). Elucidating the functional network changes across the whole brain in a range of different severity in people with PD could help the understanding of overall pathophysiology of the disease.
The few whole brain RsFC studies in the literature have looked at people with PD in the ON-levodopa medication state (Tahmasian et al., 2015). However, levodopa is thought to partially normalize brain network changes due to PD so the impact of PD on brain network connectivity can best be understood when we examine the RsFC in the OFF-medication state (Tahmasian et al., 2015, Zhong et al., 2019). Two studies looked at whole brain RsFC OFF-medication and found MDS-UPDRS III motor scores to be associated with default mode network regions, subcortical, somatomotor, cerebellar, pre-frontal lobe, visual (cuneus, lingual and calcarine), medial occipital lobe and frontal and medial parietal lobe (Hou et al., 2016, Wang et al., 2022). Another study identified that cingulate-hippocampal, hippocampal-insular and insular-orbital gyrus connectivities predicted ON-medication MDS-UPDRS III motor scores using OFF-medication rs-fMRI dynamic connectivity (Li et al., 2019). These studies, however, were performed using volume-based registration, which could be up to 3 times less efficient for registration and for the identification of significant areas (Coalson et al., 2018). Another caveat was the inclusion of high head movement data, an important cofound that, if not controlled, can lead to spurious associations (Power et al., 2012, Satterthwaite et al., 2019).
Sample size is becoming an increasingly important factor in replication of the results in neuroimaging studies (Marek et al., 2022). Most of the RsFC studies in people with PD have focused on specific brain regions on very small sample sizes that may not be reproducible (Badea et al., 2017). We used two samples whose size is considered large for this type of studies, a real between samples cross-validation, and instead of using correlations to establish associations of RsFC and MDS-UPDRS III scores, we used a predictive modeling framework that incorporates regularization to minimize the risk of over-fitting (McIntosh and Lobaugh, 2004, Miranda-Domínguez et al., 2020, Silva-Batista et al., 2021). We performed a partial least square regression (PLSR) model with a training dataset and an independent test dataset to see if we could predict disease severity, based on the motor impairments in both datasets. We have also employed stringent head motion corrections, especially since the people with PD were OFF-medication (Tahmasian et al., 2015). While we know that the somatomotor and the basal ganglia networks are impacted in people with PD, involvement of extra-motor networks are still under active investigation (Wang et al., 2022, Sang et al., 2015, Baggio et al., 2015, Göttlich et al., 2013, Li et al., 2016).
The motor impairments in people with PD are likely progressing over time and they are related not only to motor, but to non-motor networks as well. For instance, akinetic-rigidity symptoms are shown to be associated with parietal and limbic systems which are non-motor (Wu et al., 2023a). Altered low-frequency fluctuations in non-motor regions, such as anterior cingulate cortex, inferior parietal lobule, superior frontal gyrus have been shown in people with PD (Mi et al., 2017). The Default Mode Network (DMN) has been a frequent subject of investigation in Parkinson's disease (PD), revealing changes in functional connectivity. However, it remains inconclusive whether these changes are also associated with cognitive alterations (Baggio et al., 2015, Tessitore et al., 2012, Chen et al., 2022, Hou et al., 2021). Non-motor cortical areas may also provide compensatory mechanisms as suggested by studies of mobile imaging that shows larger than normal activation of prefrontal cortex when people with PD are walking, consistent with a loss of automaticity of well-learned motor tasks (Wu et al., 2015). Normally, the sensorimotor network becomes disassociated from the attention network while healthy people perform automated tasks like walking (Bassett et al., 2015). However, people with PD continue to activate their attention networks even for automated tasks like natural walking (Stuart et al., 2019).
The objective of this study was to identify the brain networks that are associated with disease severity in people with PD, as measured by MDS-UPDRS III motor scores. We hypothesized that parkinsonian motor signs, as quantified as a whole by the MDS-UPDRS III motor scores, would be associated with higher-order cognitive networks, as well as sensorimotor and basal ganglia networks, when measured in the OFF-medication state.
2. Methods
2.1. Participants
Data were collected from 96 people who had idiopathic PD clinically diagnosed by a movement disorders specialist using UK Brain Bank Criteria. Out of a total of 96 subjects, the data from 8 subjects were removed because of poor quality MR imaging data. Our final sample size consisted of 88 people with PD. Inclusion criteria for subjects were: (1) between 50 and 90 years old, (2) no major musculoskeletal or peripheral disorders (other than PD) that could significantly affect their balance and gait, (3) ability to stand and walk unassisted for 20 feet, and (4) without claustrophobia, severe tremor, or any health history such as metal implants that would interfere or put the subject at risk near the powerful magnetic field of the MRI scanner (implanted devices including deep brain stimulators).
2.2. Standard protocol approvals, registrations, and patient consents
All participants gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Oregon Health & Science University, OHSU (#4131) and the OHSU/VAPORHCS joint IRB (#8979). These participants were part of a larger interventional study (Clinical Trials NCT02231073 and NCT02236286).
2.3. Clinical data
People with PD were assessed in the practical OFF-medication state (12 hours overnight withdrawal of antiparkinsonian medication). Motor severity was assessed by a certified researcher using Movement Disorders Society Unified Parkinson's Disease Rating Scale motor subscale scores (MDS‐UPDRS III). Disease duration, medication dosage levels, freezing status – defined by the New Freezing of gait questionnaire, and cognitive assessment (MoCA) were also collected. For details, refer Table 1.
Table 1.
Sample Characteristics.
| Feature | Training-Test data |
Training vs Test Kstest2-, p-values | Range | ||
|---|---|---|---|---|---|
| Training dataset | Test dataset | ||||
| Original sample (n = 96) | 71 | 25 | |||
| High quality imaging data (n = 88) | |||||
| Count | 64 | 24 | |||
| Sex (F/M) | 19/45 | 14/10 | 4.9, 0.02 | ||
| Freezing status (F/nF) | 28/36 | 10/14 | <0.01, 0.99 | ||
| Age | 69.0 (10) | 67.5 (17.75) | 0.27, 0.31 | 50 – 88 | |
| Disease duration | 5.9 (7.6) | 3.0 (3.0) | 0.34, 0.09 | 0.3 – 24.6 | |
| H&Y | 2 (0) | 2 (0.25) | 0.05, 0.9 | 1 – 4 | |
| LEDD | 678.5 | 450 | 0.37, 0.09 | 0 – 8680 | |
| MoCA | 25.1 (3.8) | 25.9 (3.6) | 0.15, 0.9 | 14 – 30 | |
| MDS-UPDRS III | 39.7 (14.2) | 39.5 (9.4) | 0.19, 0.77 | 13 – 72 | |
| MDS-UPDRS total | 67.5 (22.3) | 63.1 (17.7) | 0.18, 0.86 | 29 – 123 | |
Training dataset comes from Siemens Trio scanner. Test dataset comes from Siemens Prisma scanner. For differences in training and test set two sample Kolmogorov–Smirnov test was run for continuous variables and Chi squared test for sex and freezing status.
2.4. MRI data and processing
Neuroimaging data were obtained in the practical OFF-medication state within a week of the motor assessment. Due to a scanner upgrade at the Imaging Center during the phase of the study, MR images were acquired using two scanners and used as two samples: a 3 T Siemens Trio scanner (considered as training dataset, N = 64) on initial subset of participants and a 3 T Siemens Prisma scanner for the reminder of participants (considered as test dataset, N = 24).
Data were processed using modified version of Human Connectome Project (HCP) (Glasser et al., 2013) (available in github at https://github.com/DCAN-Labs/abcd-hcp-pipeline) which uses FSL, FreeSurfer and ANTs.
2.4.1. Image acquisition parameters
Data were collected by a trained and certified scanning technician and images were reviewed by a neurologist in the team if suspected anatomical abnormalities were found. Subjects were instructed to lie still and keep their eyes open. Head padding was provided to keep their heads still. High resolution structural T1- and T2-weighted images and resting state functional images were obtained using following parameters: Siemens Trio scanner: T1-weighted images were acquired using a sagittal magnetization prepared rapid gradient echo (MPRAGE) sequence (TR = 2300 ms, TE = 3.58 ms, voxel size = 1 mm x 1 mm x 1.1 mm, slices = 160). T2-weighted images were acquired using the following parameters: TR = 3200 ms, TE = 497 ms, resolution = 1 mm isotropic, slices = 160. Resting state functional BOLD images were acquired in 2 scans of 10 min each with the following parameters: TR/TE = 2000/30 ms, resolution = 3.8 mm isotropic, flip angle = 90°, leading to a total of 600 frames. Siemens Prisma scanner: T1‐weighted images were acquired using a sagittal MPRAGE sequence (TR = 2500 ms, TE = 2.88 ms, TI = 1060 ms, resolution = 1 mm isotropic). T2‐weighted images were acquired using the following parameters: TR = 3200 ms, TE = 565 ms, resolution = 1 mm isotropic, slices = 176. Resting state functional BOLD images were acquired in 2 scans of 10 min each with the following parameters: TR/TE = 2500/30 ms, resolution = 3.8x3.8x3.8 mm, flip angle = 90°, leading to a total of 480 frames. Diffusion field maps were also acquired to correct for geometric distortions caused by susceptibility artefact.
2.4.2. Structural image preprocessing
T1 and T2 images were intensity normalized, brain images were skull stripped, then they were aligned to MNI’s AC-PC axis and transformed to MNI atlas space using nonlinear registration. The transformation involved calculation of a single matrix for each individual to facilitate registration both to an intermediary study template (created using high quality MRI images from the age matched healthy controls of the study group) and to the MNI atlas. This original alignment was refined using boundary-based registration. T1-weighted images were then segmented using recon-all from FreeSurfer. Segmentations were improved by using the enhanced white matter-pial surface contrast of the T2-weighted sequence. The final standard space created the CIFTI file format and the associated grayordinate spatial coordinate system (91282 anchor points) which combined cortical surface and subcortical volume analyses.
2.4.3. Resting state functional MRI preprocessing
Neuroimaging data were processed using standard methods (Miranda-Domínguez et al., 2020, Silva-Batista et al., 2021, Rudolph et al., 2018, Ragothaman et al., 2022). Briefly, the resting state functional data processing steps involved regression of (1) 6 degrees of freedom obtained by rigid-body head motion correction, (2) whole brain signal, (3) ventricular signal averaged from ventricular ROIs, (4) white matter signal averaged from white matte ROI, (5) first-order derivative terms and the squares for whole brain, ventricular and white matter signals to account for variance between regressors. Finally, time courses were filtered using a second-order Butterworth band-pass filter with frequency range from 9 to 80 mHz.
BOLD data were examined for head-movement by checking for framewise displacement (Power et al., 2012) (movement of a frame relative to its previous frame) and excluded frames with head-movement greater than 0.5 mm as well as sequences of up to 3 low head-movement frames if flanked by frames with head-movement greater than or equal to 0.5 mm, as previously described (Power et al., 2012). In this study we included participants with minimum of 5 min of surviving BOLD data. Connectivity matrices were calculated using 5 min of randomly selected low head-movement data.
2.4.4. Functional connectivity and subcortical volume atlases
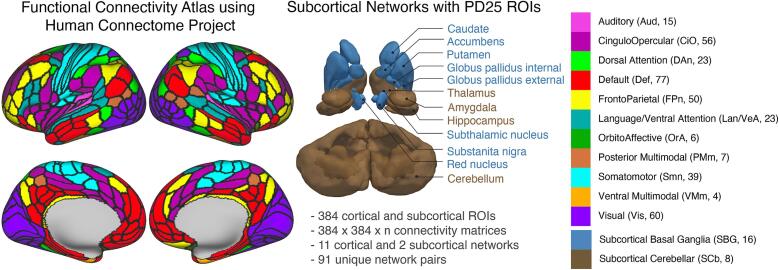
BOLD data were reported from cortical and subcortical ROIs, including subcortical ROIs that are highly relevant in PD (Ragothaman et al., 2022). Cortical areas consisted of 360 ROIs from the HCP (Glasser et al., 2016). Subcortical ROIs consisted of 16 ROIs from the MNI PD25 atlas40 and 8 ROIs from the FreeSurfer ROIs (Table S1) to account for PD-related anatomical/pathological changes in subcortical areas. Together, these 384 cortical and subcortical ROIs were grouped into 11 cortical and 2 subcortical networks (Fig. 1). See Supplementary Table S3 for the number of connections per functional network pair with respective number of connections.
Fig. 1.
Cortical functional parcellation schema with network assignment defined by HCP ColeAnticevic parcellation (Ji et al., 2019) and subcortical parcellation schema defined by combined FreeSurfer (Fischl et al., 2004) and PD25 atlas (Xiao et al., 2017). Networks are color-coded as indicated in the right section of the figure. Number in parenthesis indicates the count of ROIs belonging to each network.
The cortical ROIs were grouped into 11 functional networks (Ji et al., 2019). The included cortical networks are: Auditory, Cingulo-Opercular, Dorsal attention, Default, Frontoparietal, Language, Orbito-Affective, Posterior Multi-modal, Somatomotor, Ventral-Multimodal and Visual. This network definition originally has 12 networks including primary (Visual1) and secondary (Visual2) visual networks. Since we were not focusing on primary versus secondary visual networks, we combined them as one Visual network. So, we had 11 cortical networks. It is worth noting that the so-called language network in the cortical functional atlas is a reorganization of a traditionally used Ventral attention network from previous work (Power et al., 2011) and encompasses regions involved in language processing and attention. For the purpose of this study, the language network would be referred to as Language/Ventral-Attention network. We delineated 24 subcortical ROIs that were grouped into 2 networks, Subcortical-Basal ganglia and Subcortical-Cerebellar to study networks specifically implicated in people with PD.
The subcortical regions consisted of 24 ROIs combined into 2 subcortical networks, Subcortical-Basal ganglia and Subcortical-Cerebellar. It is necessary to study the ROIs and networks specifically implicated in people with PD. The standard FreeSurfer subcortical segmentation does not provide segmentations for substantia nigra, subthalamic nucleus, internal and external pallidum and red nucleus. So, we created a subcortical atlas which has a combination of subcortical ROIs implicated in people with PD and those that are standardly used in FreeSurfer. We used the ROIs from the above mentioned PD25 MNI atlas (Xiao et al., 2017) to segment subthalamic nucleus, substantia nigra, red nucleus, pallidum internal and external, caudate, putamen and thalamus. We used FreeSurfer to segment the rest of subcortical ROIs, namely, accumbens, amygdala, hippocampus and cerebellum. Having the PD25 atlas ROIs along with other standard FreeSurfer ROIs helped in extracting functional information from subcortical regions known to be implicated in people with PD while having access to rest of the regions.
The PD25 MNI ROIs were in 1 mm space. So, we registered PD25 MNI atlas to a 2 mm MNI atlas and brought it to 2 mm space using ANTs. The FreeSurfer ROIs and PD25 ROIs were combined using FSL’s fslmaths command. There was an overlap of caudate and accumbens when the two atlases were combined. So, we performed manual corrections using ITKsnap under the guidance of a radiologist (JW).
2.5. Statistical analysis
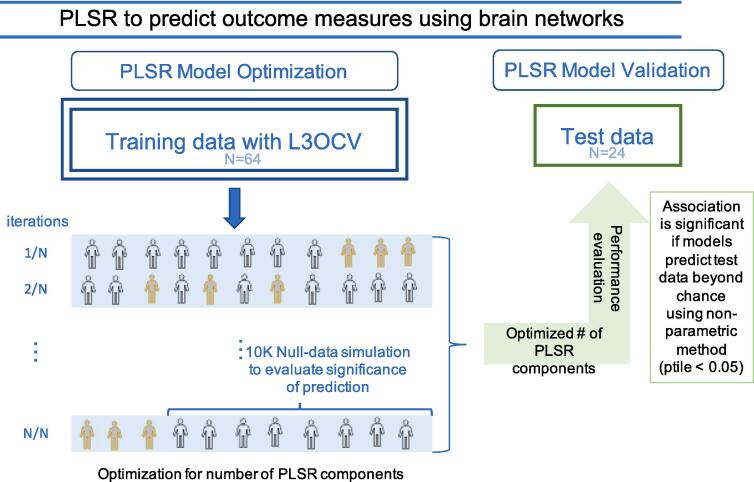
Overview: The purpose of this study was to identify the functional networks linked to motor severity in PD. In order to ensure reliability and reproducibility, we implemented a predictive modeling approach with partial least square regression (PLSR) models using training, validation and independent test samples (McIntosh and Lobaugh, 2004, Miranda-Domínguez et al., 2020, Silva-Batista et al., 2021, Rudolph et al., 2018, Ragothaman et al., 2022). PLSR models predicted the associations between normalized resting state functional connectivity data as the independent variable and standardized MDS-UPDRS III motor scores as the dependent (outcome) variable (Since we did not attempt to determine causality, but rather significant associations between brain activity and motor severity, it is irrelevant which set of measurements are used as dependent or independent variables). Connectivity values were normalized by Fisher z-transform and outcome variables were normalized by z-scores. PLSR transforms the dependent and independent variables into smaller set of components that maximizes the covariance between the independent and dependent variables, hence facilitating outcome prediction. Associations were considered significant if models predicted out-of-sample data beyond random chance. Mean absolute error (MAE) was used to estimate the accuracy of the predictions. Quality of these models were assessed using hold-3-out cross-validation and significance of the findings were determined by comparing them to null data generated through permutations and percentile-values (ptiles) were determined. The optimized models were then applied to predict MDS UPDRS III scores in an independent test dataset. Brief descriptions of each step are provided in the following subsections. The Fig. 2 shows the flowchart of the predictive modeling.
Fig. 2.
Flowchart for the predictive modeling approach employed in this study with a training dataset with leave-3-out-cross-validation (L3OCV) and an independent test dataset to detect significant networks that predict motor severity in people with PD using functional connectivity.
Prediction of normalized MDS UPDRS III scores using functional networks: PLSR models were used to assess the associations between MDS UPDRS III scores and functional connectivity by transforming them into a set of latent variables that maximize outcome prediction. The number of components used in the PLSR models was determined through hold-3-out cross-validation to prevent overfitting.
Optimal Number of Components: For each functional network pair in the training dataset, given the relatively small sample size, 3 subjects were held out for validation and rest were used to train the model. The only tuning parameter was the number of components for the PLSR model. The 3 subjects that were held out were used to test the accuracy of the predictions using MAE (within the training dataset). The modeling data was used to calculate PLSR models with varying numbers of components. We chose the optimal number of components by testing sequentially in the training sample all the possible number of components. Null data, generated by randomly permuting the assignments between connectivity and MDS UPDRS III scores 10,000 times, were used to compare the model's performance against chance. To determine the threshold of the significance, we compared the real data against the cumulative distribution of the MAE predicting the null-hypothesis data and computed Cohen’s d effect size. We selected the optimal number of components that maximized the significance of the prediction in the permutation stage.
Calculating optimal models: Once we determined the optimal number of components, models were recalculated using all the available subjects in the training sample using the optimal number of components. These optimal models were employed to predict scores in the independent test dataset.
Model Evaluation Using Independent Dataset: Finally, we estimated the model performance on the independent test dataset. The optimal models learned from the training dataset were used to predict the MDS-UPDRS III motor scores in the independent test dataset and significant functional connectivity pairs were again selected comparing test data to the permuted null-data. Significance was determined by comparing the predictions of real, unshuffled scores against the distribution of null-hypothesis data.
Criteria for results selection based on training and test dataset performance: The top 5 percentile models with d > 0.5 in training dataset and a ptile < 0.05 in test dataset were considered to predict the outcome beyond chance. This percentile value, calculated using non-parametric methods mentioned above, was not used for network selection. Network selection was based on each model’s ability to predict outcome in both the training and test samples.
PLSR implementations were done using MATLAB. To quantify the potential effect of age, disease duration, levodopa dosage (LEDD) and cognition, Pearson correlations of age, disease duration, LEDD and MoCA score with the residuals of the prediction models were computed. A p-value < 0.05 after FDR correction for multiple comparisons was considered significant for the Pearson’s correlations.
3. Results
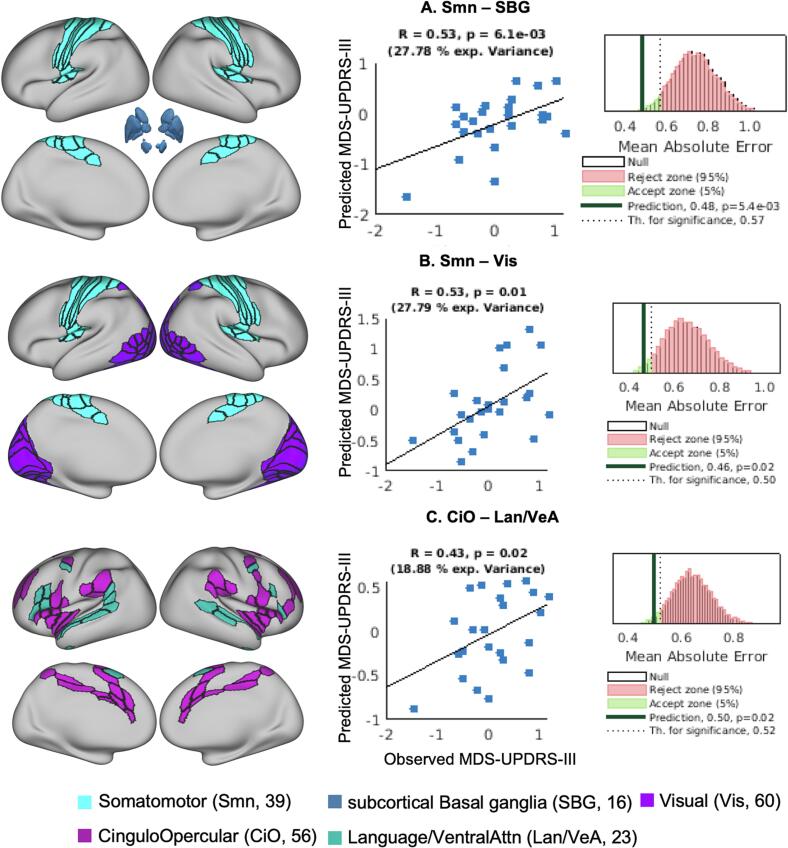
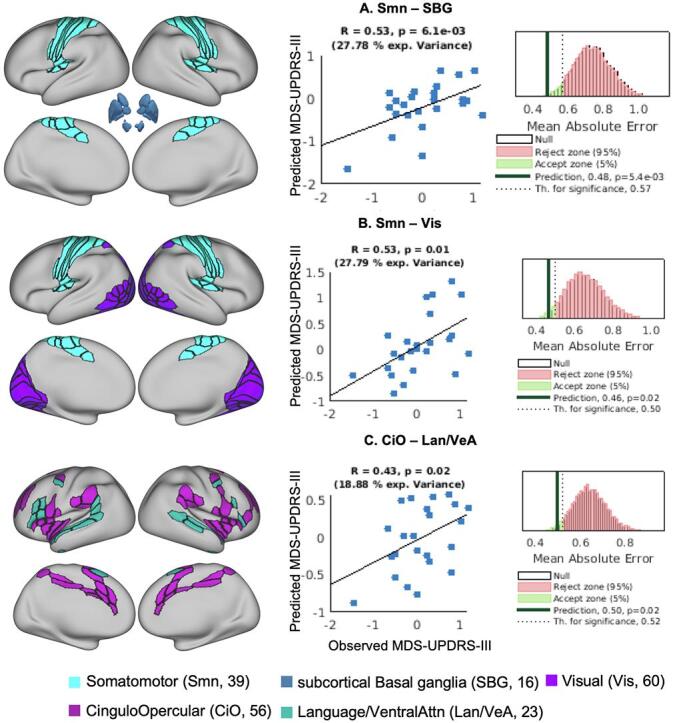
Three functional network pairs predicted the MDS-UPDRS III motor score in both the training dataset and the independent test dataset, representing the top 5 % of the 91 network pairs examined using the partial least square regression (PLSR) model. These 3 functional network pairs had an effect size (d) > 0.5 while predicting motor scores in the training dataset compared to null data. These trained models when predicting the MDS-UPDRS III motor scores in the test dataset had mean absolute error (MAE) < 0.5.
RsFC between the Somatomotor and Subcortical-Basal ganglia network pair had the strongest association between MDS-UPDRS III motor scores (11 components, d = 0.62, MAE = 0.48 (ptile = 0.01); Fig. 2A), followed by RsFC between Somatomotor with Visual network pair (3 components, d = 0.55, MAE = 0.46 (ptile = 0.02); Fig. 2B) and finally, the RsFC between Cingulo-Opercular and Language/Ventral-Attention network pair (1 component, d = 0.49, MAE 0.50 (ptile = 0.02); Fig. 2C). See Table 2 for summary of results. The correlations between the observed and predicted MDS-UPDRS III motor scores were r >0.43. See Fig. 3 for visualization and spatial localization of the network pairs, correlation coefficients and MAE distributions. See Supplementary Table S4 for the ROIs in the significant networks. The beta weights of the trained models for each ROI-ROI connection and distribution of the RsFC values for the training and test datasets for the 3 network pairs are shown in Supplementary Fig. SF1.
Table 2.
Performance metrics of RsFC networks predicting motor severity in people with PD.
| HCP Functional system pair | Within training |
Test data predictions using training dataset |
Optimal # of Components | ||||
|---|---|---|---|---|---|---|---|
| MAE | ES | MAE | ptile | R | ptile | ||
| Smn and SBG | 0.73 | 0.62 | 0.48 | 0.01 | 0.53 | <0.01 | 11 |
| Smn and Vis | 0.71 | 0.55 | 0.46 | 0.02 | 0.53 | 0.01 | 3 |
| CiO and Lan/VeA | 0.72 | 0.49 | 0.50 | 0.02 | 0.43 | 0.02 | 1 |
MAE – Mean absolute error, ES – Cohen’s Effect size (d), ptile – percentile-value computed using non-parametric method, R – correlation between observed and predicted MDS-UPDRS III motor score.
Fig. 3.
Functional connectivity network pairs that predict the MDS-UPDRS III motor scores. The column in the left shows the spatial representation of the functional networks on a very inflated brain; middle column shows the scatter plot of correlation between the observed and predicted MDS-UPDRS III motor scores; column on the right shows the null distribution of the MAE, dotted line represents the threshold for significant prediction and dark black line shows the MAE for the actual prediction made. MAE – mean absolute error.
We also found that associations between RsFC and MDS-UPDRS III motor scores were unlikely to be driven by age, disease duration, LEDD or cognition as accounted by the low and insignificant correlations with the residuals of the predictions of MDS-UPDRS III motor scores. The correlation coefficient and p-values (corrected for multiple comparisons) of age, disease duration, LEDD and MoCA score with network pairs were: Somatomotor and Subcortical-Basal ganglia network pair (r = 0.32, p = 0.13; r = 0.27, p = 0.21; r = 0.19, p = 0.50; r = -0.17, p = 0.43), Somatomotor and Visual network pair (r = 0.19, p = 0.36; r = 0.13, p = 0.55; r = 0.04, p = 0.80; r = -0.22, p = 0.31), and Cingulo-Opercular and Language/Ventral-Attention network pair (r = 0.23, p = 0.29; r = 0.23, p = 0.29; r = 0.46, p = 0.06; r = -0.15, p = 0.47). The scatterplots are shown in supplementary Figs. SF2-SF5.
We repeated the PLSR analysis, while controlling for LEDD and found the results remained significant, with even strong SMN-SBG network connectivity (see Table S2 in Supplement).
4. Discussion
4.1. Summary of results
Whole brain RsFC in people with PD OFF-medication identified higher-order cognitive and visual networks, as well as Somatomotor and basal ganglia networks, that are associated with severity of motor dysfunction in people with PD. Connectivity between Somatomotor and Subcortical-Basal ganglia networks, Somatomotor and Visual networks and Cingulo-Opercular and Language/Ventral-Attention network pairs predicted MDS-UPDRS III motor score. We found that connectivity between motor and non-motor networks is associated with motor severity in people with PD as accounted by predictive modeling and cross-validation using independent samples. Importantly, we also showed that our findings are unlikely to be driven by age, disease duration, Levodopa medication and cognition.
The clinical characteristics of the cohort represents people with PD who were in Hoehn & Yahr stage 2–3 representing mild to medium symptoms. Their MoCA scores were mostly 25–26, with mild cognitive impairments. The disease duration ranged between 5 and 10 years. The participants were scanned during their practical OFF medication state, we checked for effects of Levodopa medication and there was no significant effect of medication on the predictions.
4.2. Somatomotor – Subcortical-basal ganglia network connectivity predicts PD motor severity
The dopaminergic deficit in the striatum is thought to affect the striatal projects (through both direct and indirect pathways) leading to disruption in the cortical regions such as primary motor and pre-motor areas (Braak and Del Tredici, 2008). Consistent with the literature, we showed that the strength of connectivity between the Somatomotor and Subcortical-Basal ganglia networks were predictive of motor dysfunction severity in people with PD (Wu et al., 2009). Alterations within the somatomotor network is among the most identified networks in people with PD across different stages of the disease and different analysis methods as summarized by reviews (Tessitore et al., 2014, Strafella et al., 2018). In addition to alterations within somatomotor network, most ROI based studies have shown reduced connectivity between Somatomotor and striatal networks in people with PD (Sharman et al., 2013, Agosta et al., 2014, Wang et al., 2021). In particular, connectivity between the SMA and putamen has been shown to be negatively correlated with the MDS-UPDRS III motor score (Wu et al., 2009). Another study by the same group showed decreased connectivity between premotor cortex, pre-SMA and putamen in people with PD compared to healthy adults (Wu et al., 2011). The Somatomotor network identified in our study includes not only motor execution ROIs like M1/S1, but also motor planning ROIs like SMA and pre-SMA. Involvement of motor planning regions like SMA, pre-SMA, premotor cortex along with the somatomotor regions like M1/S1 may suggest network level changes at the motor planning level disrupting motor execution.
4.3. Somatomotor – Visual network connectivity predicts PD motor severity
We found that connectivity between Somatomotor and Visual networks predicted motor severity in people with PD. The connectivity between Somatomotor and Visual networks (related to motor severity identified in our study in people with PD) maybe due to (a) reduced connectivity responsible for impairments in spatial orientation or (b) increased connectivity acting as a compensatory network for impaired use of somatosensory inputs in people with PD. In a recent Rs-fMRI study, Somatomotor-Visual network connectivity was reported to be more different than other networks, with PD group showing reduced RsFC (Gratton et al., 2019). Another study showed that motor, visual and subcortical resting state networks predict walking duration (as a function of gait) and the motor impairment attributed to dopaminergic and cholinergic transporters identified using neurotransmitter-receptor/transporter density maps (Wu et al., 2023b). Reduced RsFC between Somatomotor and sensory networks have been attributed to impaired multisensory processing and sensory integration, leading to impairments of kinesthesia, perception of motion, abnormal postural alignment and motor impairments like freezing of gait (Patel et al., 2014). For example, freezing of gait is often triggered by specific visual contexts, such as narrow doorways (Cohen et al., 2011).
Alternatively, the association of PD motor severity with Somatomotor-Visual network connectivity in this study could also represent a compensatory mechanism for the impaired subcortical control of movement and/or impaired kinesthesia. People with PD are known to benefit from visual cueing, particularly for people with freezing (Schlick et al., 2016, Gilat et al., 2021). Anticipatory postural planning for step initiation has been shown to improve with a visual cue, but only if vision of the leg is included (Russo et al., 2022). A recent EEG study on visual cueing in people with PD has shown activation in the visual regions correlated with cueing and gait improvements and attention processing and those with worse attention seem to recruit more visual regions (Stuart et al., 2021).
Excessive connectivity between the visual and somatomotor network could also reflect a propensity for visual hallucinations in people with PD. However, only 16 out of 88 people had reported hallucinations in our cohort in the MDS-UPDRS question 1.2 and most of them had a mild score of one.
4.4. Cingulo-Opercular – Language/ventral attention network connectivity predicts PD motor severity
This is the first study to find that connectivity between the Cingulo-Opercular (CiO) and Language/Ventral attention (Lan/VeA) networks predicted motor severity in people with PD. CiO and Lan/VeA are considered to be part of higher-order attention network. The CiO network is generally attributed to maintaining tonic attention (Sadaghiani and D’Esposito, 2015), whereas Lan/VeA is related to detecting unattended or unexpected stimuli and triggering shifts of attention (Menon and Uddin, 2010). People with PD also exhibit speech impairments and encounter language problems that are caused due to the impaired cognitive functions such as verbal working memory and set switching (for further details see full special issue of Language and Parkinson’s disease (Bastiaanse and Leenders, 2009). While this study did not collect data to evaluate language problems, we indeed were able to identify altered connectivity in networks that later could lead to this problem. Studies have also shown that simple motor performance in people with PD recruits brain networks involved in more complex tasks, such as the anterior cingulate cortex (Carbon et al., 2007) and dorsal anterior insula (Tinaz et al., 2016). Increased connectivity was also observed between the Ventral Attention network and the Cognitive network in people with PD while breaking motor arrest (freezing of gait) in a virtual-reality, motor task (Shine et al., 2013). Whereas healthy older adults show short term activation of the prefrontal cortex when staring to walk, this activity quickly subsides, reflecting automaticity. In contrast, people with PD show persistent activation of attention networks while walking (Stuart et al., 2019).
While the Ventral Attention (VeA) network used in this study contains regions responsible for language processing and attention switching (Ji et al., 2019) interestingly, this network also includes parts of the cortical vestibular network (specifically, the superior temporal sulcus and temporo-parieto-occipital junction) (Frank and Greenlee, 2018). These regions include the parieto-insular vestibular cortex (PIVC) and the posterior insular cortex (PIC), considered to be a core part of the vestibular system, yet they overlap with the CiO and VeA networks. PIVC is known to encode head and body movements and is involved in estimating heading direction while PIC is attributed to coordination of visual and vestibular cues (Frank and Greenlee, 2018). People with PD have difficulty sequentially rotating their eyes, then head, then trunk, then legs while turning and have difficulty balancing in a condition requiring vestibular integration (eyes closed on a compliant surface) (Earhart et al., 2007).
4.5. Strengths and limitations
Our predictive modelling method uses cross-validation, null-hypothesis test and comprises independent training and test datasets to increase reproducibility of our results. We also used whole brain analysis approach that enabled us to identify non-motor networks involved in motor symptoms in people with PD and OFF-medication. We also created a unique, subcortical network consisting of regions specifically implicated in people with PD to identify if other subcortical regions played a role in motor symptoms. Our study is one of the few to include a large cohort of people with PD and OFF-medication. We have implemented stringent correction methods for motion artifacts, particularly important in studying movement disorders like PD (Tahmasian et al., 2015, Gratton et al., 2019). During our registration process, we also registered the MRIs of individuals with PD to an intermediary age-matched atlas to account for structural changes related to aging. Together, these methodological rigors address the potentially confounding imaging factors.
Although our original sample consisted of 96 people with PD, we lost data from a few subjects due to excessive head movement in the scanner, and they predominantly belonged to the training sample. The data lost came from patients with worse motor severity and longer disease duration, compared to the group average. As the disease progresses, neck posture tends to deteriorate, that could be a reason for the increased head motion. Nevertheless, the number of subjects in our study are larger than most imaging studies with people who have PD. Although the training and test dataset had differences in the disease duration and levodopa dosage, they were not statistically significant. The RsFC atlas we used was created from the HCP dataset, which comprised of individuals with an average age of 25. However, during the registration process we registered the MRIs of individuals with PD to an age-matched intermediary atlas created from our age-matched control cohort to control for the age-related anatomical changes. Future studies could use RsFC atlases made from older adults or implement individualized connectivity-based analysis that can be used for personalized prediction. While majority of people with PD experience non-motor symptoms such as depression, apathy and cognitive impairments related to abnormalities in the brain, we focused on motor impairments of PD and not on cognitive or other non-motor impairments. However, a majority of our subjects had a MoCA score of above 25, indicating mild cognitive impairments. Using large samples and methods like brain-wide associations will be more helpful to identify brain association for various non-motor symptoms. Our PD cohort included freezers and non-freezers as well as tremor-dominant and PIGD-dominant groups but was underpowered to look at differences between sub-groups. However, our previous publications from same cohort examined the relationship between PIGD severity (Ragothaman et al., 2022), freezing of gait (Miranda-Domínguez et al., 2020) and functional connectivity. Future studies could look for specific changes caused due to freezing or PIGD impairments.
5. Conclusions
We demonstrate that along with motor networks, connectivity of the Cingulo-Opercular and Visual networks are also associated with motor severity of PD. Involvement of higher-order cognitive networks may explain the attention deficits experienced by people with PD and involvement of visual network may explain why some people with PD benefit from visual cueing. Characterization of these functional connectivity network changes associated with parkinsonian motor signs could help us understand the compensatory role played by non-motor regions in people with PD.
CRediT authorship contribution statement
Anjanibhargavi Ragothaman: Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. Martina Mancini: Data curation, Writing – review & editing. John G. Nutt: Writing – review & editing. Junping Wang: Methodology, Writing – review & editing. Damien A. Fair: Writing – review & editing. Fay B. Horak: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Oscar Miranda-Dominguez: Conceptualization, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Graham R. Harker and Patricia Carlson-Kuhta for helping with data collection. We thank all the participants of this study and the funding agencies, NIH, VA, Tartar Trust and OHSU Parkinson’s Center for Oregon.
Funding
This work was supported by the National Institute of Aging (AG006457) and VA Merit Award (RX001075), the Tartar Trust Fellowship (Miranda-Dominguez), OHSU Parkinson Center Pilot Grant Program (Miranda-Dominguez).
Financial Disclosures of All Authors
AR: Tartar Trust fellowship; MM: Grants – NIH (R01 HD100383, R00 078492, R44 AG056012-02), Kinnie Family Foundation; DAF: Grants – NIH (R01 MH096773, K99/R00 MH091238, R01 MH115357, U01 DA041148, R44 MH122066), the Oregon Clinical and Translational Research Institute, the Bill & Melinda Gates Foundation, the Destafano Innovation Fund; FBH: Employment – OHSU, Clario (ERT-APDM); Grants – NIH (R01 HD100383-01, R44 AG056012, R01CA222605, P50 NS062684), DoD (W81-XWH-18-1-0425, W81XWH-17-1-0424); FBH has a significant financial interest in APDM Wearable Technologies, a Clario company, that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by OHSU. Type: Financial; OMD: Grants – OHSU Fellowship for Diversity and Inclusion in Research Program, Tartar Trust Award, the OHSU Parkinson Center Pilot Grant Program
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103541.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Agosta F., Caso F., Stankovic I., et al. Cortico-striatal-thalamic network functional connectivity in hemiparkinsonism. Neurobiol Aging. 2014;35(11):2592–2602. doi: 10.1016/j.neurobiolaging.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Badea L., Onu M., Wu T., Roceanu A., Bajenaru O. Exploring the reproducibility of functional connectivity alterations in Parkinson’s disease. PLoS One. 2017;12(11):e0188196. doi: 10.1371/journal.pone.0188196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio H.C., Segura B., Junque C. Resting-state functional brain networks in Parkinson’s disease. CNS Neurosci Ther. 2015;21(10):793–801. doi: 10.1111/cns.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Yang M., Wymbs N.F., Grafton S.T. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015;18(5):744–751. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanse R., Leenders K.L. Language and Parkinson’s disease. Cortex. 2009;45(8):912–914. doi: 10.1016/j.cortex.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Boutet A., Madhavan R., Elias G.J.B., et al. Predicting optimal deep brain stimulation parameters for Parkinson’s disease using functional MRI and machine learning. Nat Commun. 2021;12(1):3043. doi: 10.1038/s41467-021-23311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Del Tredici K. Cortico-basal ganglia-cortical circuitry in Parkinson’s disease reconsidered. Exp Neurol. 2008;212(1):226–229. doi: 10.1016/j.expneurol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Carbon M., Felice Ghilardi M., Dhawan V., Eidelberg D. Correlates of movement initiation and velocity in Parkinson’s disease: A longitudinal PET study. Neuroimage. 2007;34(1):361–370. doi: 10.1016/j.neuroimage.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang T., Ma D., Chen Y.C. Altered Default Mode Network Functional Connectivity in Parkinson’s Disease: A Resting-State Functional Magnetic Resonance Imaging Study. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.905121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coalson T.S., Van Essen D.C., Glasser M.F. The impact of traditional neuroimaging methods on the spatial localization of cortical areas. Proc Natl Acad Sci U S a. 2018;115(27):E6356. doi: 10.1073/pnas.1801582115. E6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.G., Chao A., Nutt J.G., Horak F.B. Freezing of gait is associated with a mismatch between motor imagery and motor execution in narrow doorways, not with failure to judge doorway passability. Neuropsychologia. 2011;49(14):3981–3988. doi: 10.1016/j.neuropsychologia.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earhart G.M., Stevens E.S., Perlmutter J.S., Hong M. Perception of active and passive turning in Parkinson disease. Neurorehabil Neural Repair. 2007;21(2):116–122. doi: 10.1177/1545968306290674. [DOI] [PubMed] [Google Scholar]
- Filippi M., Sarasso E., Agosta F. Resting-state Functional MRI in Parkinsonian Syndromes. Mov Disord Clin Pract. 2019;6(2):104–117. doi: 10.1002/mdc3.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J.W., et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fling B.W., Cohen R.G., Mancini M., et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014;9(6):e100291. doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.M., Greenlee M.W. The parieto-insular vestibular cortex in humans: more than a single area? J Neurophysiol. 2018;120(3):1438–1450. doi: 10.1152/jn.00907.2017. [DOI] [PubMed] [Google Scholar]
- Gilat M., Ginis P., Zoetewei D., et al. A systematic review on exercise and training-based interventions for freezing of gait in Parkinson’s disease. NPJ Parkinsons Dis. 2021;7(1):81. doi: 10.1038/s41531-021-00224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlich M., Münte T.F., Heldmann M., Kasten M., Hagenah J., Krämer U.M. Altered resting state brain networks in Parkinson’s disease. PLoS One. 2013;8(10):e77336. doi: 10.1371/journal.pone.0077336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C., Koller J.M., Shannon W., et al. Emergent Functional Network Effects in Parkinson Disease. Cereb Cortex. 2019;29(6):2509–2523. doi: 10.1093/cercor/bhy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker C.D., Perlmutter J.S., Criswell S.R., Ances B.M., Snyder A.Z. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135(Pt 12):3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Luo C., Yang J., et al. Prediction of individual clinical scores in patients with Parkinson’s disease using resting-state functional magnetic resonance imaging. J Neurol Sci. 2016;366:27–32. doi: 10.1016/j.jns.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Hou Y., Wei Q., Ou R., et al. Different resting-state network disruptions in newly diagnosed drug-naïve Parkinson’s disease patients with mild cognitive impairment. BMC Neurol. 2021;21(1):327. doi: 10.1186/s12883-021-02360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J.L., Spronk M., Kulkarni K., Repovš G., Anticevic A., Cole M.W. Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage. 2019;185:35–57. doi: 10.1016/j.neuroimage.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liang P., Jia X., Li K. Abnormal regional homogeneity in Parkinson’s disease: a resting state fMRI study. Clin Radiol. 2016;71(1):e28–e34. doi: 10.1016/j.crad.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Li X., Xiong Y., Liu S., et al. Predicting the Post-therapy Severity Level (UPDRS-III) of Patients With Parkinson’s Disease After Drug Therapy by Using the Dynamic Connectivity Efficiency of fMRI. Front Neurol. 2019;10:668. doi: 10.3389/fneur.2019.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Song W., Chen Q., et al. Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: a resting-state fMRI study. Neurobiol Aging. 2014;35(2):431–441. doi: 10.1016/j.neurobiolaging.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Calabro F.J., et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250. doi: 10.1016/j.neuroimage.2004.07.020. S263. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi T.M., Mei S.S., Liang P.P., et al. Altered resting-state brain activity in Parkinson’s disease patients with freezing of gait. Sci Rep. 2017;7(1):16711. doi: 10.1038/s41598-017-16922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Domínguez Ó., Ragothaman A., Hermosillo R., et al. Lateralized Connectivity between Globus Pallidus and Motor Cortex is Associated with Freezing of Gait in Parkinson’s Disease. Neuroscience. 2020;443:44–58. doi: 10.1016/j.neuroscience.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Jankovic J., Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13(1):100–112. doi: 10.1016/S1474-4422(13)70213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragothaman A., Mancini M., Nutt J.G., Fair D.A., Miranda-Dominguez O., Horak F.B. Resting State Functional Networks Predict Different Aspects of Postural Control in Parkinson’s Disease. Gait Posture. Published Online. 2022 doi: 10.1016/j.gaitpost.2022.07.003. [DOI] [PubMed] [Google Scholar]
- Rudolph M.D., Graham A.M., Feczko E., et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci. 2018;21(5):765–772. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo Y., Stuart S., Silva-Batista C., Brumbach B., Vannozzi G., Mancini M. Does visual cueing improve gait initiation in people with Parkinson’s disease? Hum Mov Sci. 2022;84 doi: 10.1016/j.humov.2022.102970. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., D’Esposito M. Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness. Cereb Cortex. 2015;25(9):2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L., Zhang J., Wang L., et al. Alteration of Brain Functional Networks in Early-Stage Parkinson’s Disease: A Resting-State fMRI Study. PLoS One. 2015;10(10):e0141815. doi: 10.1371/journal.pone.0141815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Ciric R., Roalf D.R., Davatzikos C., Bassett D.S., Wolf D.H. Motion artifact in studies of functional connectivity: Characteristics and mitigation strategies. Hum Brain Mapp. 2019;40(7):2033–2051. doi: 10.1002/hbm.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlick C., Ernst A., Bötzel K., Plate A., Pelykh O., Ilmberger J. Visual cues combined with treadmill training to improve gait performance in Parkinson’s disease: a pilot randomized controlled trial. Clin Rehabil. 2016;30(5):463–471. doi: 10.1177/0269215515588836. [DOI] [PubMed] [Google Scholar]
- Sharman M., Valabregue R., Perlbarg V., et al. Parkinson’s disease patients show reduced cortical-subcortical sensorimotor connectivity. Mov Disord. 2013;28(4):447–454. doi: 10.1002/mds.25255. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Matar E., Ward P.B., et al. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain. 2013;136(Pt 12):3671–3681. doi: 10.1093/brain/awt272. [DOI] [PubMed] [Google Scholar]
- Silva-Batista C., Ragothaman A., Mancini M., et al. Cortical thickness as predictor of response to exercise in people with Parkinson’s disease. Hum Brain Mapp. 2021;42(1):139–153. doi: 10.1002/hbm.25211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella A.P., Bohnen N.I., Pavese N., et al. Imaging Markers of Progression in Parkinson’s Disease. Mov Disord Clin Pract. 2018;5(6):586–596. doi: 10.1002/mdc3.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S., Belluscio V., Quinn J.F., Mancini M. Pre-frontal Cortical Activity During Walking and Turning Is Reliable and Differentiates Across Young, Older Adults and People With Parkinson’s Disease. Front Neurol. 2019;10:536. doi: 10.3389/fneur.2019.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S, Wagner J, Makeig S, Mancini M. Brain Activity Response to Visual Cues for Gait Impairment in Parkinson’s Disease: An EEG Study. Neurorehabil Neural Repair. Published online September 10, 2021:15459683211041316. doi:10.1177/15459683211041317. [DOI] [PMC free article] [PubMed]
- Tahmasian M., Bettray L.M., van Eimeren T., et al. A systematic review on the applications of resting-state fMRI in Parkinson’s disease: Does dopamine replacement therapy play a role? Cortex. 2015;73:80–105. doi: 10.1016/j.cortex.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Esposito F., Vitale C., et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79(23):2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Giordano A., De Micco R., Russo A., Tedeschi G. Sensorimotor connectivity in Parkinson’s disease: the role of functional neuroimaging. Front Neurol. 2014;5:180. doi: 10.3389/fneur.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinaz S., Lauro P., Hallett M., Horovitz S.G. Deficits in task-set maintenance and execution networks in Parkinson’s disease. Brain Struct Funct. 2016;221(3):1413–1425. doi: 10.1007/s00429-014-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.S., Grafton S.T., McIntosh A.R., DeLong M.R., Hoffman J.M. The functional anatomy of parkinsonian bradykinesia. Neuroimage. 2003;19(1):163–179. doi: 10.1016/s1053-8119(03)00059-4. [DOI] [PubMed] [Google Scholar]
- Wang X., Yoo K., Chen H., et al. Antagonistic network signature of motor function in Parkinson’s disease revealed by connectome-based predictive modeling. NPJ Parkinsons Dis. 2022;8(1):49. doi: 10.1038/s41531-022-00315-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang Y., Lei J., Guo S. Investigation of sensorimotor dysfunction in Parkinson disease by resting-state fMRI. Neurosci Lett. 2021;742 doi: 10.1016/j.neulet.2020.135512. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhou C, Guan X, et al. Functional connectomes of akinetic-rigid and tremor within drug-naïve Parkinson’s disease. CNS Neurosci Ther. Published online June 12, 2023. doi:10.1111/cns.14284. [DOI] [PMC free article] [PubMed]
- Wu T., Wang L., Chen Y., Zhao C., Li K., Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson’s disease. Neurosci Lett. 2009;460(1):6–10. doi: 10.1016/j.neulet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Wu T., Long X., Wang L., et al. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp. 2011;32(9):1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Hallett M., Chan P. Motor automaticity in Parkinson’s disease. Neurobiol Dis. 2015;82:226–234. doi: 10.1016/j.nbd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wu C., Qin J., et al. Functional connectome predicting individual gait function and its relationship with molecular architecture in Parkinson’s disease. Neurobiol Dis. 2023;184 doi: 10.1016/j.nbd.2023.106216. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Fonov V., Chakravarty M.M., et al. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinson׳s disease cohort. Data Brief. 2017;12:370–379. doi: 10.1016/j.dib.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Guan X., Zhong X., et al. Levodopa imparts a normalizing effect on default-mode network connectivity in non-demented Parkinson’s disease. Neurosci Lett. 2019;705:159–166. doi: 10.1016/j.neulet.2019.04.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.