Abstract
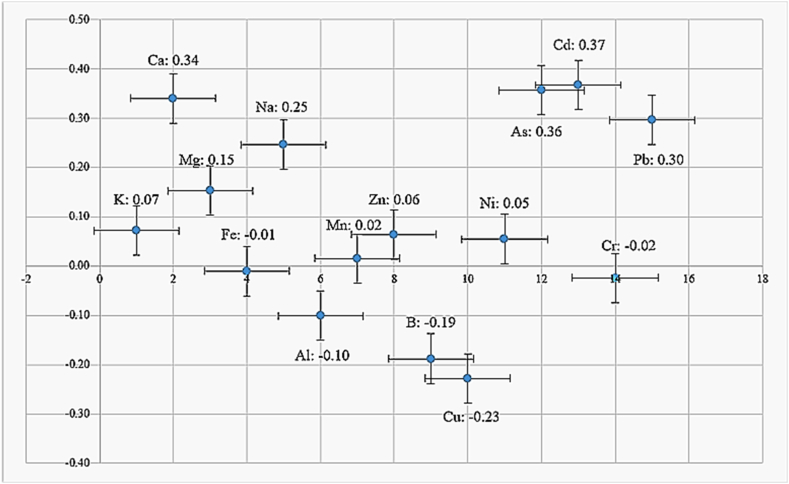
The purpose of this study was to determine the content of twenty-two biogenic elements (BEs) and potentially toxic elements (PTEs) in the soil and fresh Allium ursinum leaves from 43 different locations, in order to examine their bioaccumulation potential. Analyses of soil and plant material were carried out by using Inductively Coupled Plasma coupled with Optical Emission Spectroscopy (ICP-OES), a mercury analyzer (Hg), liquid chromatography (Cr), and AAS hybrid technique (As). The obtained results of the investigated elements were compared with the proposed limit values. The soil contamination factor (CF) as well as plant bioaccumulation factor (BAF) were calculated and the correlation analysis was performed. The results showed that the content of some BEs/PTEs in the soil were above the limit values, with two locations highly contaminated (CF > 6) with five (Cr(VI), Cu, Mn, Ni, V) and four (As, Co, Pb, V) elements. The content of As, Cd, Cr, and Pb in the leaves was higher than the permitted levels at some locations. The BAF was high (K, Ca, Zn, As), medium (Mg, Cu, B, Ni, Na, Pb), and low (Fe, Mn, Cr). The correlation between BEs/PTEs content in the leaves and soil was not significant, except for the following elements: Cd (0.37), Ca (0.34), As (0.36), Pb (0.30), and Na (0.25). The observed medium correlation suggested that the detected elements originated both from the atmosphere and the soil. Although A. ursinum at examined locations seemed to be mostly safe for consumption, a selective mechanism of adoption of certain BEs/PTEs requires continuous monitoring of their content in the future, to avoid quantities that can jeopardize human health through its consumption.
Keywords: PTEs, biogenic elements, Wild garlic, Bioaccumulation factor
Graphical abstract
Highlights
-
•
Detailed survey of A. ursinum across of Serbia was performed.
-
•
Mineral compostion, contamination and bioaccumulation factors (BAF) were determined.
-
•
Two locations were highly contaminted with several potentially toxic element (PTEs).
-
•
BAF values were favorable for potassium, calcium and zinc.
1. Introduction
A large number of wild, non-domesticated plant species that grow in different ecological conditions are used in nutrition and medicine [1]. The plant species Allium ursinum, which has been known for centuries, is used in the diet as an early spring salad and in traditional medicine for the preparation of numerous pharmaceutical products. A. ursinum is a perennial, geophytic plant of the genus Allium, family Alliaceae. It grows at different altitudes, in fresh, moist areas of deciduous and deciduous-coniferous forests on predominantly humus soils [2]. Leaves hold the greatest value of this medicinal plant species. It contains a large number of secondary metabolites, such as sulfuric and phenolic compounds, nutrients, vitamins, chlorophylls, carotenoids, and other organic compounds important for human health [3]. Biologically active metabolites like phenolics that accumulate in the leaf of A. ursinum during its development are of great importance for its antioxidant activity [4].
In addition to organic substances, A. ursinum contains a large number of inorganic components, such as biogenic elements (BEs) like K, Ca, Mg, Na, Ni, and Mn, as well as potentially toxic elements (PTEs) like B, Al, Mn, Ni, Hg, Pb, and Cd [5]. Vuković et al. [6] stated that other species of the genus Allium have a pronounced ability to accumulate both BEs and PTEs. Biogenic elements, such as iron, zinc, and manganese are considered essential and play important roles in physiological cycles. Moreover, copper and zinc have important roles in biological systems but can be toxic if ingested in excessive amounts, while non-essential toxic elements such as lead (Pb), cadmium (Cd), arsenic (As), and mercury (Hg) are toxic even in small amounts [7]. For this reason, the investigation of environmental pollution with PTEs, which is a major problem that directly affects the quality of medicinal plants, is of growing interest today [8].
Contamination with PTE has become a serious threat to food safety and the environment as a result of global economic growth and the rapid development of agriculture and industry [9]. Geochemical and anthropogenic processes lead to the accumulation of toxic elements in the soil. Most soils contain PTEs that harm plants and microorganisms and originate from the parent substrate on which the soil was formed. Therefore, the primary source of toxic elements in the soil is the parent substrate, which mostly consists of basalt and sedimentary rocks. Basaltic rocks are rich in PTEs such as Cd, Cu, Co, and Ni, while sedimentary rocks contain metals such as Pb, Cu, Zn, Mn, and Cd [10]. Industrial activities, such as increased traffic, mining, processing of fossil fuels, disposal of municipal waste, excessive use of pesticides and fertilizers, and irrigation, contribute significantly to the increased concentration of PTEs in the soil [11]. Research by Chizzola [12] confirmed that a large number of medicinal plant species are susceptible to the accumulation of PTEs [12]. Similarly, plant species such as spinach and leafy vegetables have a pronounced bioaccumulation potential, meaning their uptake rates of PTEs are significantly higher than in other plant species [13]. A study by Amin et al. [14] indicated that Allium spp. have a pronounced bioaccumulation potential of PTEs, as was also found by Kowalska [15], where the presence of toxic elements (Cd, Pb, and Hg) was detected in the leaves of A. ursinum. Research by Šamec et al. [16] indicated that the content of PTEs has a direct influence on the content of secondary metabolites in the plant. According to a study by Malizia et al. [17] the content of PTEs in a plant depends on the degree of their bioaccumulation, which is influenced by the location where it grows, the concentration of elements in the soil, the plant's physiological cycle, and other factors [18,19].
The populations of A. ursinum are mainly found in forest ecosystems which are located far from major sources of pollution [2]. The demand for raw materials of A. ursinum is mostly satisfied by collecting it from natural forest ecosystems, which, according to Kadovic et al. [20], have recently worsened sharply due to increased anthropogenic activities, primarily air traffic. Similarly, Vanmechelen et al. [21] noted that forest floor layers and organic surface layers showed the highest concentrations of metals in forest ecosystems. Kadovic et al. [20], reported that forest ecosystems in the Republic of Serbia have specific characteristics that affect the accumulation of certain elements in the soil's surface layers and their movement through the soil profile. The same authors noted that undesirable anthropogenic activities contribute to higher atmospheric deposition, which, together with the layers of the forest mat, has a dominant impact on the concentrations of BEs/PTEs. Therefore, it is important to monitor these elements and understand their impact on food safety and quality since soil serves as a reference point for the introduction of risky elements/compounds that can enter the human food chain [22]. In addition, the most recent research has proved that it is possible to grow A. ursinum under controlled, domesticated conditions by selecting an appropriate soil medium [23].
Therefore, it is essential to know the mineral composition of the A. ursinum leaf in order to prevent adverse effects on human health. Bearing in mind that detailed research on the content and bioaccumulation of BEs and PTEs (K, Ca, Mg, Fe, Mn, Cu, Zn, B, Mo, Ni, Na, Se, Co, As, Cd, Cr, Cr(VI), Hg, Pb, Al, V, Sn, Sb, Tl) in A. ursinum, across the Republic of Serbia had not been conducted, the current study aimed to examine their content (by ICP-OES, HPLC, mercury analyzer and AAS hybrid technique methods) and determine the soil contamination and bioaccumulation potential of A. ursinum originating from a large number of locations characterized by different agro-ecological conditions. Besides, by knowing mineral composition useful information will be obtained for the further introduction and A. ursinum cultivation.
2. Material and method
2.1. Research area
Plant and soil materials were sampled across the territory of the Republic of Serbia. For that purpose, 43 distant locations were selected where the wild plant species A. ursinum grows (Fig. 1). The sampling sites of the material for analysis were located predominantly in forest ecosystems, far from a large number of pollution sources, at altitudes ranging from 70 to 1211 m above sea level (m.a.s.l). The sampling program was conducted just before flowering in early spring of 2021. From each selected location (Fig. 1), A. ursinum leaves were sampled from 50 individual plants within a radius of 5–50 m, after which they were mixed to form a composite sample. Immediately after that, soil samples were collected using a metal probe in the same radius in the surface layer, i.e. in the rhizosphere zone (10–15 cm).
Fig. 1.
Geographical descriptions of localities where A. ursinum populations are collected.
Due to the large number of localities (43) distributed across the territory of the R. Serbia, general data for the entire area were used regarding climate parameters.
Study area of the research is located across the territory of the Republic of Serbia, which has a moderate continental climate with steppe features in the north and southeast, while the mountain climate dominates at higher altitudes. According to the data of the Republic Hydro-Meteorological Service of Serbia (RHMS), the average mid-winter temperatures in lowland areas range from 0 to −2 °C and in mountainous areas from −2 to −4 °C; average summer temperatures in the lowlands are over 22 °C, whereas in the mountains they reach the value of 18 °C. In the northeastern parts, about 580 mm of precipitation falls during the year, while in the southern parts, it goes up to about 555 mm.
2.2. Preparation of plant/soil samples for analysis
For the analysis of elements, the sampled fresh leaves were separately ground, then the microwave digestion was performed. From the homogenized plant material, 1 g of plant sample was used, treated with 10 mL of conc. nitric acid (HNO3) and 30 mL of conc. hydrochloric acid (HCl), (the applied ratio of the acids was 1:3) and then with 2 mL of hydrogen peroxide (H2O2; 0.029 %, w/v). The prepared suspension was heated for 1 h in a water bath with constant addition of distilled water up to 50 mL as the final volume. After cooling, it was filtered through a blue filter strip. In the case of soil collection, the soil samples from all locations were left to dry and then crushed and sieved through a 2 mm sieve. Soil samples prepared for destruction were treated with conc. HNO3 with H2O2 addition according to the EPA 3050B [24].
2.3. Analytical methods for plant/soil samples
The contents of essential biogenic (K, Fe, Mn, Zn, Ni Cu, B), useful/beneficial (Na, Co, V, Se), and potentially toxic (Al, Cd, Pb, Cr, Sb, Tl) elements in the leaves and the soil were determined using the method of Induced-Coupled Plasma Optical Emission Spectroscopy (ICP-OES), on the ICPE-9800 apparatus (Shimadzu, Japan). In addition, the following elements were determined via other appropriate analytical methods: Cr(VI) content was determined by High Performance Liquid Chromatography (HPLC), on the 930 Compact IC Flex Oven/Deg, the 947 professional (UV/VIS Vario SW detector), and the 919 IC auto sampler (Metrohm 930 Compact IC Flex, Switzerland); the content of Hg was determined on the automatic mercury analyzer AMA 254, (Leco corporation, Michigan, USA); while the As content was determined using the atomic absorption spectroscopic (AAS) hybrid technique, on the AA-7000 apparatus (Shimadzu, Japan), with a HGV-1 hybrid generator combined with the ASC-6100 auto sampler. The limits of quantification for the analyzed elements are listed in Table 1. Apart from the content of selected elements in the sampled soil material, following agrochemical parameters were determined: the pH value in H2O and KCl, and the humus content according to Tjurin's method [25].
Table 1.
Limit of quantification of analyzed elements in soil/plant.
| Elements in Soil | Elements in Plant |
|---|---|
| As – 0.1 mg/kg | |
| Mn, Cu, Co – 0.2 mg/kg | As, Cd, Tl, Sn, Sb, Mo – 0.1 mg/kg |
| Se, Pb, B, Cd, Mo, Sb, Sn, V, Tl – 0.5 mg/kg | Mn, Cu, Co – 0.2 mg/kg |
| Cr, Ni– 1 mg/kg | Se, Pb, B – 0.5 mg/kg |
| Fe, Al – 5 mg/kg | Cr, Ni, Na – 1 mg/kg |
| Na – 10 mg/kg (0,001 %) | Fe, Al – 5 mg/kg |
| Mg, Ca, K – 100 mg/kg (0,01 %) | Mg, Ca, K – 10 mg/kg |
| Hg – 0.01 mg/kg | Hg – 0.01 mg/kg |
| Cr(VI) – 0.75 mg/kg |
2.4. Contamination factor
The contamination factor (CF), as an important parameter of soil quality, was calculated according to following equation (1) [26]:
| (eq. 1) |
where cs is the concentration of the toxic element in the soil and crefs is the reference background content for the selected elements (average worldwide) as follows [27]: As (4.4 mg/kg); B (22 mg/kg); Cd (0.37 mg/kg); Co (5.5 mg/kg); Cr (47 mg/kg); Cu (13 mg/kg); Hg (0.05 mg/kg); Mn (270 mg/kg); Ni (13 mg/kg), Pb (22 mg/kg); V (67 mg/kg), Zn (45 mg/kg). It is considered that soil is slightly (CF < 1), moderately (CF = 1–3) considerably (CF = 3–6) and highly (CF > 6) polluted depending on CF values [26].
2.5. Bioaccumulation factor
The bioaccumulation factor (BAF) was calculated according to equation (2) provided by [28] which represents the ratio of the mean value for the concentrations of the elements accumulated in the plant (cleaf), and the concentration of the same elements in the soil (csoil):
| (eq. 2) |
2.6. Data analysis
The obtained results consisted of 24 variables (elements) and 43 locations. Three analyses were performed for each variable, with their arithmetic mean and standard deviation shown in the Tables as the final result. An analysis of variance (ANOVA) followed by Tukey's post hoc test (p < 0.05) was used to compare the variation in the content of the investigated elements at the locations. Then a correlation was performed to determine if there was a quantitative agreement (correlation analysis) between the variations of the observed variables. The data were analyzed with SPSS 26.0 software SPSS, Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Content of biogenic elements (BEs) and potentially toxic elements (PTEs) in soil
The obtained intervals and mean values are shown in Table 2 while all detailed results for soil parameters and elemental composition are given in supplementary files (Tables S1 and S2). One of the parameters that has a great influence on the direction and flow of chemical processes in the soil is the pH of the soil reaction. It directly affects the absorption of elements by reducing the pH value, they become less accessible or even inaccessible [29]. The obtained mean values were in the range from 4.17 to 7.43 (pH in H2O) i.e. 3.65 to 7.69 (pH in KCl). Corresponding to the literature data [30], in 9 locations the soil samples were neutral to slightly alkaline, which is in line with literature [31]. It was calculated that the minimal humus content in the soil of temperate regions was 2 % [32,33]. Since the current research found the content of humus to be in the interval from 2.82 % to 9.32 % soils can be considered as well supplied, which is characteristic of soils originating from forest populations previously sampled from surface layers [34].
Table 2.
Intervals and average values for pH, hummus and biogenic and potential toxic elements content (mg/kg dry weight± SD) in soils where A. ursinum grows.
| Parameters | Imin | Imax | Average |
|---|---|---|---|
| pH-H2O | 4.17 | 7.73 | 6.09 ± 1.09 |
| pH-KCl | 3.65 | 7.43 | 5.40 ± 1.14 |
| % hummus | 2.39 | 9.33 | 5.21 ± 1.92 |
| K | 1002.00 ± 163.00 | 27412 ± 163.30 | 2691.31 ± 393.34 |
| Ca | 250.00 ± 81.65 | 28600.00 ± 1632.99 | 6208.47 ± 775.30 |
| Mg | 1400.00 ± 163.00 | 19166.67 ± 410.96 | 5323.11 ± 429.40 |
| Fe | 11900.00 ± 326.60 | 88900.00 ± 81.65 | 28276.74 ± 128.35 |
| Mn | 270.00 ± 4.08 | 5980.00 ± 8.16 | 1188.95 ± 91.50 |
| Cu | 6.70 ± 0.16 | 416.00 ± 1.63 | 34.56 ± 6.53 |
| Zn | 35.30 ± 1.63 | 262.00 ± 1.73 | 78.91 ± 4.80 |
| B | 0.81 ± 1.63 | 95.4 ± 1.60 | 42.14 ± 2.78 |
| Ni | 9.29 ± 0.07 | 1200.00 ± 0.81 | 94.73 ± 18.41 |
| Na | 60.9 ± 1.63 | 386 ± 1.53 | 125.66 ± 7.53 |
| Co | 3.43 ± 0.02 | 83.5 ± 0.81 | 15.43 ± 2.53 |
| As | 2.15 ± 0.04 | 520.2 ± 0.47 | 21.24 ± 7.16 |
| Cd | 4.92 ± 0.00 | 4.92 ± 0.00 | 4.92 ± 0.00 |
| Cr | 7.70 ± 0.57 | 739.00 ± 0.81 | 67.58 ± 11.13 |
| Cr(VI) | 0.85 ± 0.04 | 7.43 ± 0.02 | 2.49 ± 1.76 |
| Hg | 0.07 ± 0.01 | 0.47 ± 0,05 | 0.17 ± 0.08 |
| Pb | 18.90 ± 1.04 | 984.00 ± 0.81 | 88.67 ± 19.21 |
| Al | 5500.00 ± 116.50 | 30500.00 ± 408.25 | 15641.86 ± 56.18 |
| V | 15.80 ± 0.65 | 1370.00 ± 0.81 | 176.82 ± 26.51 |
| Sb | 54.1 ± 0.00 | 54.1 ± 0.00 | 54.10 ± 0.00 |
*Imin - minimal detected values; Imax - maximal detected values.
For the main biogenic elements, results presented in Table 2 have confirmed the following ranges: 1002–27412 mg/kg (K); 250–28600 mg/kg (Ca); 1400–19166.67 mg/kg (Mg); 11900–88900 mg/kg (Fe). The results in Table 2 show the mean content values for Mn, Cu, Zn, B, Ni, Na, Co, As, Cr, Cr(VI), Hg, Pb, Al, V which were in the ranges: 270–5980 mg/kg (Mn); 6.7–416 mg/kg (Cu); 35.3–262 mg/kg (Zn); 0.81–95.4 mg/kg (B), 9.29–1200.00 mg/kg (Ni); 60.9–386 mg/kg (Na); 3.43–83.5 mg/kg (Co); 2.15–520.2 mg/kg (As), 7.70–739 mg/kg (Cr); 0.81–7.43 mg/kg (Cr(VI)); 0.07–0.47 mg/kg (Hg) 18.9–984 mg/kg (Pb); 5500–30500 mg/kg (Al); 15.8–1370 mg/kg (V), and in two of the locations (L42; L31) Cd i Sb were detected with the values of 4.9 mg/kg (Cd) and 51.0 mg/kg (Sb), whereas Mo, Se, Sn and Tl were not detected in the analyzed soil.
Similar to this, Reimann et al. [35] and Leuschner and Lendzion [36] determined a great variability in element content and stated that climatic factors had a great influence on the soil composition. Taking into account that the soils on which A. ursinum grows were sampled from the surface layer and originated from different locations and different climatic areas, it is possible that it was mostly influenced by these factors. However, according to Vanmechelen et al. [21] anthropogenic aerial deposition of BEs/PTEs plays an important role in changing the chemistry of the organic layers of forest soils, which can increase their concentrations in places far from a large number of pollution sources, as observed in the current study. The given results confirmed some of the findings stated in a similar study [20] in which the content of BEs/PTEs in the surface layer of forest soils was directly conditioned by the intensity of deposition from the atmosphere, habitat conditions, soil type, and vegetation type. It is important to emphasize that the analyzed elements do not have an equal role in the physiological cycles of the plant [37]. Their presence in most of the forest ecosystems is in an altered form compared to the original, and it is not equally important for plant nutrition, with several negative effects on the ecosystem [38]. The concentrations of BEs/PTEs at the locations were compared with the maximum allowable concentration [27] and maximum tolerable value according to Official Gazette of the Republic of Serbia [39].
By comparing the content of PTEs in the surface layer of the tested soils with the limit values (Table 3), it can be concluded that some of the sampled soils were contaminated. The heat map presented in Fig. 2 highlights locations with elevated concentrations of the examined PTEs (indicated by red color). Notably, all sampled locations exhibited heightened levels of aluminum, iron and manganese followed by cobalt, vanadium, nickel and lead. On the other hand, although arsenic was registered at all locations, it was above MAC values at only two locations. Antimony was registered at only one location but above MAC value.
Table 3.
Proposed maximum allowable concentrations (MAC, mg/kg) for selected PTEs in soil given in the literature and concentrations at locations.
| Ea | I | II |
|---|---|---|
| Fe | – | – |
| Mn | – | – |
| Cu | 60–150 | 36 |
| Zn | 100–300 | 140 |
| B | – | 50 |
| Ni | 20–60 | 35 |
| Co | 20–50 | 9 |
| As | 15–20 | 29 |
| Cd | 1–5 | 0.8 |
| Crtotal | 50–200 | 100 |
| Hg | 0.5–5 | 0.3 |
| Pb | 20–300 | 85 |
| Al | – | – |
| V | 150 | 42 |
| Sb | 10 | 3 |
Fig. 2.
Heat map with localities (labeled as red) with content of PTEs above proposed MAC values.
In order to perform more precise assessment of the contamination of the tested soils (based on background referent values worldwide for As; B; Cd; Co; Cr; Cu; Hg; Mn; Ni; Pb; V; Zn) contamination factors (CFs) were calculated and presented in Table 4.
Table 4.
Contamination factor (CF) for PTEs in the tested soils.
| Location | As | B | Cd | Co | Cr | Cu | Hg | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.62 | 0.00 | 0.00 | 0.62 | 0.35 | 1.14 | 2.00 | 1.00 | 1.58 | 0.00 | 2.43 | 1.04 |
| 2 | 1.86 | 0.19 | 0.00 | 2.24 | 2.12 | 1.95 | 4.00 | 1.79 | 9.23 | 0.00 | 0.40 | 1.53 |
| 3 | 1.14 | 4.34 | 0.00 | 1.08 | 0.73 | 1.50 | 5.00 | 2.45 | 2.19 | 1.03 | 3.52 | 1.53 |
| 4 | 2.00 | 3.00 | 0.00 | 0.00 | 0.55 | 1.41 | 1.40 | 1.14 | 2.10 | 0.86 | 3.99 | 1.20 |
| 5 | 1.36 | 0.00 | 0.00 | 1.93 | 0.64 | 1.24 | 2.60 | 3.14 | 2.30 | 1.52 | 4.69 | 1.24 |
| 6 | 1.83 | 1.75 | 0.00 | 1.76 | 1.15 | 2.35 | 2.80 | 3.21 | 7.17 | 0.00 | 0.53 | 1.65 |
| 7 | 1.33 | 0.00 | 0.00 | 1.67 | 0.54 | 0.83 | 2.60 | 2.32 | 3.16 | 0.00 | 0.40 | 1.12 |
| 8 | 1.73 | 2.47 | 0.00 | 1.30 | 0.49 | 1.37 | 2.40 | 2.26 | 1.84 | 0.00 | 4.82 | 1.04 |
| 9 | 2.33 | 0.00 | 0.00 | 1.37 | 0.68 | 1.33 | 1.44 | 1.99 | 3.39 | 0.00 | 0.41 | 1.04 |
| 10 | 1.70 | 3.01 | 0.00 | 2.42 | 0.58 | 1.05 | 3.80 | 3.06 | 1.97 | 1.36 | 4.84 | 1.14 |
| 11 | 2.83 | 0.00 | 0.00 | 2.02 | 0.51 | 1.56 | 3.00 | 6.48 | 2.25 | 1.41 | 0.44 | 1.47 |
| 12 | 4.21 | 0.00 | 0.00 | 1.91 | 0.95 | 1.49 | 2.00 | 2.86 | 7.60 | 0.00 | 0.38 | 1.17 |
| 13 | 1.67 | 0.77 | 0.00 | 2.38 | 1.27 | 1.85 | 4.00 | 2.56 | 6.12 | 0.00 | 0.53 | 1.78 |
| 14 | 2.48 | 2.49 | 0.00 | 2.00 | 0.51 | 1.18 | 2.40 | 3.78 | 2.23 | 0.00 | 0.42 | 1.00 |
| 15 | 2.18 | 0.00 | 0.00 | 1.60 | 0.54 | 1.20 | 1.40 | 22.15 | 2.16 | 1.60 | 4.24 | 1.10 |
| 16 | 1.51 | 0.00 | 0.00 | 2.04 | 0.39 | 0.85 | 2.80 | 4.74 | 1.69 | 0.00 | 0.40 | 2.03 |
| 17 | 1.38 | 0.00 | 0.00 | 2.42 | 0.69 | 1.03 | 1.60 | 2.65 | 2.56 | 0.00 | 0.46 | 1.11 |
| 18 | 1.51 | 1.02 | 0.00 | 4.49 | 4.02 | 32.00 | 3.80 | 3.04 | 30.38 | 2.01 | 0.43 | 5.82 |
| 19 | 3.11 | 0.00 | 0.00 | 3.05 | 1.41 | 3.11 | 3.20 | 4.59 | 5.14 | 0.00 | 1.08 | 1.63 |
| 20 | 0.93 | 0.16 | 0.00 | 0.83 | 0.16 | 0.52 | 1.80 | 2.93 | 0.71 | 1.77 | 0.24 | 1.19 |
| 21 | 1.18 | 4.27 | 0.00 | 5.31 | 2.72 | 6.50 | 4.40 | 5.89 | 5.28 | 0.00 | 2.12 | 1.94 |
| 22 | 2.86 | 0.00 | 0.00 | 2.45 | 0.97 | 1.85 | 4.80 | 4.04 | 3.31 | 0.00 | 0.84 | 1.72 |
| 23 | 1.83 | 0.00 | 0.00 | 3.84 | 2.47 | 3.16 | 3.20 | 3.85 | 12.62 | 0.00 | 0.65 | 1.85 |
| 24 | 1.46 | 0.00 | 0.00 | 1.45 | 0.47 | 1.24 | 2.60 | 2.73 | 1.55 | 1.07 | 4.70 | 0.78 |
| 25 | 1.55 | 2.40 | 0.00 | 2.91 | 1.53 | 1.54 | 2.60 | 3.70 | 4.76 | 3.33 | 6.79 | 1.57 |
| 26 | 3.19 | 0.00 | 0.00 | 2.95 | 1.20 | 2.15 | 2.60 | 4.59 | 6.45 | 2.82 | 0.47 | 1.62 |
| 27 | 5.12 | 0.00 | 0.00 | 5.91 | 2.43 | 2.74 | 4.40 | 8.85 | 22.77 | 2.00 | 1.06 | 1.59 |
| 28 | 2.16 | 0.00 | 0.00 | 5.05 | 2.28 | 3.85 | 4.00 | 5.07 | 16.85 | 4.11 | 0.56 | 3.56 |
| 29 | 2.38 | 0.00 | 0.00 | 1.76 | 0.36 | 1.57 | 4.40 | 4.44 | 1.58 | 0.00 | 0.34 | 2.18 |
| 30 | 0.65 | 2.22 | 0.00 | 5.07 | 2.96 | 1.77 | 1.80 | 3.96 | 11.85 | 0.00 | 1.23 | 0.90 |
| 31 | 0.49 | 0.00 | 0.00 | 15.18 | 15.72 | 1.94 | 8.60 | 10.96 | 92.31 | 4.18 | 20.45 | 1.85 |
| 32 | 6.97 | 0.00 | 0.00 | 2.65 | 0.86 | 2.82 | 3.60 | 7.15 | 3.64 | 0.00 | 0.66 | 1.76 |
| 33 | 1.63 | 0.00 | 0.00 | 2.27 | 0.37 | 2.12 | 9.40 | 2.25 | 1.89 | 1.06 | 3.25 | 1.34 |
| 34 | 2.69 | 1.82 | 0.00 | 2.67 | 0.59 | 2.29 | 3.00 | 5.52 | 3.12 | 3.50 | 0.58 | 2.00 |
| 35 | 2.32 | 0.04 | 0.00 | 2.60 | 1.02 | 2.68 | 4.40 | 5.19 | 5.65 | 3.97 | 4.63 | 2.14 |
| 36 | 2.26 | 0.00 | 0.00 | 2.53 | 0.52 | 2.08 | 4.60 | 4.15 | 3.70 | 0.00 | 0.28 | 2.04 |
| 37 | 2.13 | 1.22 | 0.00 | 1.91 | 0.21 | 0.97 | 4.40 | 5.19 | 0.92 | 2.61 | 1.05 | 1.89 |
| 38 | 3.07 | 0.00 | 0.00 | 2.47 | 0.66 | 0.69 | 2.20 | 6.96 | 1.78 | 1.65 | 0.57 | 1.10 |
| 39 | 0.79 | 0.00 | 0.00 | 3.95 | 1.89 | 2.82 | 1.60 | 2.19 | 4.78 | 1.06 | 0.98 | 1.34 |
| 40 | 2.16 | 0.00 | 0.00 | 1.12 | 0.43 | 0.75 | 3.40 | 2.19 | 2.30 | 3.76 | 0.28 | 0.97 |
| 41 | 2.30 | 0.00 | 0.00 | 2.05 | 0.40 | 3.42 | 2.80 | 5.59 | 2.58 | 1.29 | 4.03 | 3.45 |
| 42 | 11.86 | 1.36 | 13.30 | 2.51 | 1.66 | 3.57 | 4.20 | 2.65 | 3.28 | 44.73 | 8.55 | 5.60 |
| 43 | 2.40 | 0.00 | 0.00 | 2.07 | 1.84 | 2.85 | 2.20 | 4.07 | 4.58 | 0.00 | 14.79 | 1.37 |
The obtained values of CF in the surface layer of the soil were highly variable (Table 4). There were locations 31 and 42 which were highly contaminated (CF > 6) with six (Cr, Cu, Mn, Ni, V) and four (As, Co, Pb, V) elements, respectively. The highest contamination factor was observed for nickel at location 31 (92.31), which was likely caused by either geochemical or anthropogenic factors. This hypothesis is supported by the proximity of the location to an ore deposit, as confirmed by research conducted in the similar area (44°13′36.3″ N 20°39′12.4″E) [40]. The aforementioned study detected moderate contamination levels for As, Mn, Be, Al and Fe, high for Co, Cr and Ni and very high levels for Cd in the surface layers of the soil, which is consistent with the results of the current study. In addition to nickel (at thirteen locations), elevated CF values for manganese (six locations), arsenic, mercury and copper (two locations) were also observed. At one location, CF values above the highly contaminated limit were registered for the following elements: cadmium, cobalt, chromium and lead.
Mazurek et al. [41], presented similar results, where they pointed out that anthropogenic influence is the main cause for the presence of these elements in the forest soils and that the forest mat layer and surface horizon contain the highest concentrations of them. Elements deposited on plant surfaces during their life cycle can transform and be included in the biochemical cycle of element circulation in the soil (recirculation). Similarly, Langenbruch et al. [42] showed that the forest ecosystems, primarily leaves, absorb a vast range of gases from the atmosphere, and after their fall to the ground under the influence of the gravitational force due to their decomposition, many chemical compounds are deposited on the surface of the soil. Talkner et al. [43] stated that the decomposition of the leaves leads to the release of a wide range of chemical compounds, which, combined with precipitation, may directly influence the concentration and availability of BEs/PTEs in the surface levels of the soil. More precisely, according to Weltzin and McPherson [44] atmospheric water (rainfall), including its components such as gases and acids, affect both the decomposition of forest residue and the mobilization of BEs/PTEs in the soil by converting them into different accessible forms. Specifically, when atmospheric water reaches plants and soil, it carries soluble gases (O2, CO2, NH3, SO2) and dissolved acids (HNO2, NHO3). This is primarily due to the composition of atmospheric air which varies greatly across different locations. Therefore, it can be assumed that this variability is the main reason for the observed differences in content in the examined areas.
3.2. Content of biogenic elements (BEs) and potentially toxic elements (PTEs) in the leaf
Considering the physiological and biological properties of A. ursinum as well as the consumption method, it is very important to reflect the risk when ingesting plant material in the presence of BEs/PTEs. Table 5 shows the average content/intervals of BEs/PTEs in A. ursinum leaves. Detailed results for leaf elemental composition are given in supplementary files (Table S3).
Table 5.
The content of biogenic and potential toxic elements in the leaf (mg/kg fresh weight±SD) of A. ursinum.
| Parameters | Imin | Imax | Average |
|---|---|---|---|
| K | 1655 ± 4.08 | 5842 ± 12.47 | 3291.25 ± 87.12 |
| Ca | 110.8 ± 1.63 | 885.9 ± 4.08 | 324.35 ± 16.87 |
| Mg | 165.2 ± 2.05 | 855.6 ± 17.8 | 281.85 ± 11.52 |
| Fe | 11.4 ± 0.4 | 502.0 ± 1.63 | 53.58 ± 8.15 |
| Na | 9.78 ± 0.06 | 53.72 ± 0.01 | 20.94 ± 8.66 |
| Al | 3.98 ± 0.06 | 103.0 ± 2.44 | 25.68 ± 3.71 |
| Mn | 1.84 ± 0.03 | 21.2 ± 0.16 | 4.99 ± 2.98 |
| Zn | 2.54 ± 0.03 | 204.0 ± 3.26 | 10.06 ± 2.57 |
| B | 0.69 ± 0.07 | 3.67 ± 0.05 | 1.47 ± 0.45 |
| Cu | 1.02 ± 0.01 | 2.96 ± 0.04 | 1.53 ± 0.22 |
| Ni | 1.20 ± 0.08 | 10.3 ± 0.31 | 3.07 ± 2.22 |
| As | 0.11 ± 0.01 | 4.39 ± 0.07 | 2.11 ± 1.76 |
| Cd | 2.12 ± 0.00 | 2.12 ± 0.00 | 2.12 ± 0.00 |
| Cr | 1.11 ± 0.00 | 1.11 ± 0.00 | 1.11 ± 0.00 |
| Pb | 15.6 ± 0.16 | 58.1 ± 0.24 | 36.85 ± 1.25 |
*Imin - minimum detected values; Imax - maximum detected values.
Understanding the composition of BEs/PTEs is critical for safe human nutrition and health [45]. The results show that among the analyzed BEs/PTEs, potassium was the most abundant BE in plant material (1695–5842 mg/kg). These results were partially similar to those obtained for A. ursinum from Bosnia and Herzegovina which had a range of 4719.59–4803.48 mg/kg [46]. In a similar study, Piątkowska et al. [47] analyzed the dry leaves of A. ursinum and found that the potassium content was 34.64 g/kg which is partially similar to the results of this study. The potassium content in this study was recalculated on a fresh weight basis, since fresh A. ursinum leaves contain 91 % water [48]. Potassium is a key element for respiration and the regulation of osmotic pressure [49]. In the human body, it plays a major role in all organs affecting the activity of nerve cells, muscle contraction, osmotic regulation, acid-alkaline, and water balance, pH regulation, and the transmembrane potential of osmotic pressure, thereby increasing the concentration of other ions in the cell [50].
A large variability was found in relation to the sodium content: 9.48–53.72 mg/kg, which is partially in accordance with the range (31.07–32.91 mg/kg) for A. ursinum from Bosnia and Herzegovina [46]. Sodium, together with potassium, is involved in enzymatic activity that regulates muscle contraction, mineral transfer, and osmotic potential in the human body [51], while in plants, Na plays an important role in growth regulation and neutralization of the acids generated in plant metabolism [49]. The process of uptake for both ions is similar, as plants take them up through common membrane channels. Plants absorb Na+ ions significantly less compared to K+ ions because a high K/Na ratio is maintained in the cytoplasm due to the fact that the concentration of K in the cytoplasm is from 100 to 200 mM, and Na+ from 1 to 10 mM [37]. A high K/Na ratio is desirable since a high content of sodium in the cytoplasm disturbs the sodium-potassium pump's functioning and water transport. Namely, research by Antonkiewicz et al. [52] indicated that the optimal K/Na ratio should be 10:1 or higher. In the current research, the obtained values for this parameter were above optimal. It makes A. ursinum desirable for consumption as a source of optimal content for both elements.
The concentration range for calcium obtained in this study was from 110.8 to 885.9 mg/kg, which was significantly lower (1532.55–1559.1 mg/kg) than the results of Vučić et al. [46] for the same plant species. The observed differences are mostly related to differences in growing soil and agroecological conditions. In plant organisms, calcium enters into the composition of cell membranes and plays a role in transmitting signals from the external environment to plants [49]. However, its primary function in the human body is the formation of the human skeleton. Its ions participate in blood coagulation and the regulation of osmotic pressure, and they play a major role in cell growth and development [50]. According to literature recommendations, the optimal K/Ca ratio should be 2:1 [52]. In this study, all obtained values for this parameter were favorable, similar to the K/Na ratio.
Accordingly, the concentration range for magnesium was from 165.2 to 855.6 mg/kg, which is a relatively wide range compared to the results of Vučić et al. [46]: 317.16–335.04 mg/kg. In the dry leaf of A. ursinum, Piątkowska et al. [47] determined that the magnesium content (1.72 g/kg) was significantly lower than in this research (Table 6), considering the previous statements of Lukinac and Jukić [48] about leaf water content. The observed variation for magnesium in this study can be explained by the wider geographical area where the samples were collected. In plants, Mg affects the processes of cell and tissue regeneration and organ development by activating a large number of enzymes that are involved in the process of photosynthesis. It is included in the biosynthesis of proteins and the metabolism of nucleic acids, as well as in photosynthesis as a part of chlorophylls [49]. According to the literature, optimal K/Mg ratio should be 6:1 [52], which is consistent with the results obtained in this study. Magnesium plays a major role in the absorption of Ca [50]. A recent study by Costello et al. [53] indicated that an excessive Ca/Mg ratio in the diet (>2.60) can lead to a number of negative chronic conditions such as cardiovascular diseases and metabolic syndrome, as well as some cancers. Comparing the obtained results, it can be observed that the average ratio of Ca/Mg was around 1, which is lower than the suggested upper limit.
Table 6.
Correlation matrix of the ratio of detected elements in the leaf of A. ursinum.
| Variability | K | Ca | Mg | Fe | Na | Al | Mn | Zn | B | Cu | Ni | As | Cd | Cr | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | 1 | ||||||||||||||
| Ca | −0.01 | 1 | |||||||||||||
| Mg | −0.13 | 0.01 | 1 | ||||||||||||
| Fe | 0.11 | 0.10 | 0.07 | 1 | |||||||||||
| Na | 0.17 | −0.28 | 0.17 | 0.03 | 1 | ||||||||||
| Al | 0.09 | 0.00 | 0.12 | 0.73* | −0.01 | 1 | |||||||||
| Mn | 0.01 | 0.11 | −0.04 | 0.79* | −0.19 | 0.62 | 1 | ||||||||
| Zn | 0.08 | 0.13 | 0.06 | 0.87* | 0.08 | 0.31 | 0.68 | 1 | |||||||
| B | 0.09 | 0.10 | 0.08 | 0.04 | 0.20 | −0.10 | 0.01 | 0.09 | 1 | ||||||
| Cu | −0.29 | −0.24 | 0.15 | −0.03 | 0.15 | 0.23 | 0.18 | −0.17 | −0.09 | 1 | |||||
| Ni | −0.21 | −0.02 | 0.28 | 0.15 | 0.14 | 0.06 | 0.08 | 0.18 | 0.08 | 0.11 | 1 | ||||
| As | 0.04 | 0.04 | 0.04 | 0.34 | −0.19 | 0.67 | 0.48 | 0.01 | −0.05 | 0.29 | 0.08 | 1 | |||
| Cd | 0.09 | 0.12 | 0.04 | 0.86* | 0.08 | 0.30 | 0.67 | 0.98* | 0.11 | −0.17 | 0.16 | −0.01 | 1 | ||
| Cr | −0.05 | 0.06 | −0.07 | 0.25 | 0.05 | 0.43 | 0.08 | −0.03 | 0.05 | 0.20 | 0.03 | −0.03 | −0.02 | 1 | |
| Pb | 0.09 | 0.10 | 0.04 | 0.91* | 0.06 | 0.41 | 0.67 | 0.97 | 0.09 | −0.20 | 0.15 | 0.09 | 0.97* | −0.03 | 1 |
*Correlations are significant at p < 0.05.
For all biogenic macroelements (K, Ca, Mg and Na) the results obtained in this research were significantly lower compared to A. ursinum leaf samples collected in Sliven region (Bulgaria)- 16250 mg/kg (for K), 14810 mg/kg (for Ca), 9775 mg/kg (for Mg), 232 mg/kg (for Na) [54], probably due to different geographical origin and agroecological growing conditions.
The detected iron content varied significantly, from 11.4 to 502.0 mg/kg. In a study by Vučić et al. [46] the Fe content at five locations was in the interval from 13.97 to 14.78 mg/kg which was expected since only five locations were included. According to literature, the variability in the iron content of plants is not always consistent [38]. It is strongly influenced by soil composition and climate, as well as the phase in which the plant is harvested. It is also stated that the optimal Fe range should be between 18 and 1000 mg/kg [38], which is consistent with the current study's findings. Iron is a part of many redox enzymes in cellular processes, where it catalyzes respiration. In the plant organism, due to its chemical properties, it affects redox processes during photosynthesis and cellular respiration [49] and is also the main and active element of hemoglobin in blood [55].
The detected content of aluminum ranged from 8.11 to 103.0 mg/kg. Only a few locations had an Al content similar to the content found in literature data (9.48–9.89 mg/kg) [46], whereas in other locations, the content of Al was significantly variable. This was strongly influenced by the significantly wider geographical area encompassed in this study. According to Kabata-Pendias [38] Al is mainly taken up from the soil, and its absorption as well as the available forms are mostly influenced by the pH value of the soil. The same author states that with decreased soil pH (<5.5), the mobility of Al increases significantly, which is probably the reason for the excessive variability in this study, as confirmed by the results (Table 1), which show that 58.1 % of the locations had a pH lower than 5.5. Namely, an excessive concentration of Al negatively affects the absorption of K, Ca, and Mg, by blocking it and increases the absorption of Fe and Mn, which (20) which was also observed. Stahl et al. [56] have emphasized that knowing the Al content is a critical nutritional safety concern. Excessive intake of Al in the diet can cause toxic cellular and neurotoxic effects on human health [57]. High Al content is harmful to the heart, brain, kidneys, and liver. However, pharmacological studies on mice treated with Sparganii rhizome extract that contained high concentrations of Al did not show any symptoms of toxicity or mortality at a dose of up to 2000 mg/kg body weight. This can be explained by the fact that Al can form a complex compound with the N-heterocycle called the Al glycoside complex, thus preventing the manifestation of its toxicity [58].
The content of manganese was from 1.84 to 21.2 mg/kg. Compared to a similar research paper on A. ursinum by Vučić et al. [46] where Mn content was in the range of 13.51–14.4 mg/kg 93 % of the localities in the current research exhibited a lower content of this element. Kabata-Pendias [38] pointed out that the content of Mn depends on its antagonism with Fe (the Fe/Mn ratio should preferably be from 1.5 to 2.5), because they are interconnected in the metabolic functions. If this ratio is not favorable, symptoms of Mn toxicity and Fe deficiency may occur. However, this was not the case in this study. Specifically in humans and animals, Mn is necessary for bone formation, carbohydrates and lipid metabolism, calcium absorption, blood sugar regulation for the functioning of glial and neuronal cells, as well as enzymes involved in the synthesis and metabolism of neurotransmitters [59]. However, excessive uptake of Mn through the respiratory route negatively affects two vital organs, the brain and the lungs [59], and can provoke the development of a neurodegenerative disease known as manganism with symptoms very similar to Parkinson's disease [60].
Zinc content was from 2.54 to 204.0 mg/kg, while significantly lower and less variable content was observed (2.31–2.61 mg/kg) in the literature [46]. The variability of Zn concentration is mostly influenced by weather conditions [38]. Specifically, during heavy rainfall, zinc compounds are easily precipitated by reaction with carbonates or absorbed with minerals and organic compounds and they become less available to plants, which may be one of the reasons for the variability of the obtained results. According to Papoyan et al. [61], Zn exhibits a synergistic effect on the absorption of Cd, Cu while Zn has a synergistic effect on the intake of Fe and As [38]. The suggested synergisms were also observed in this study. Zn plays an important role in DNA and RNA metabolism, protein synthesis, cell division, and the process of signal transmission within the cell [62]. Also, it plays an important role in the functioning of a large number of enzymes in metabolic processes, so it is necessary for all living organisms, including plants, animals, and humans. Increased Zn concentrations pose no health risk, whereas deficiency of this element can result in a variety of serious disorders and diseases [63]. The detected content of boron (0.69–3.69 mg/kg) was significantly less than the optimal content in onions (10 mg/kg) [64]. The low amount of this element can be related to the high Fe content and low Zn content observed in this research [65]. According to Bakirdere et al. [66] boron exhibits negative clinical effects such as gastrointestinal disturbances, mucosal peeling, inflammation, congestion, exfoliative dermatitis, edema, and granular degeneration of renal tubular cells.
Unlike previous elements, copper wasn't found in all locations. The concentration range for Cu was 1.02–2.96 mg/kg at 25 locations. The average Cu content (1.52 mg/kg) was fully in line with the content of this element (1.51 mg/kg) in the samples obtained from Bulgaria [54], as well as for the content in the samples obtained from Bosnia and Herzegovina (1.56–1.93 mg/kg) [46]. The absence of Cu in some locations can be explained by its antagonism and a similar absorption mechanism to Zn, Fe, Cd, Mn, Ni, Cr ions, which can be observed through their concentration in some locations [38]. Copper is an important micronutrient in human metabolism, because it carries out enzyme biocatalysis and electron transfer and participates in the formation of the genetic code [67]. Copper has a distinct ability to change oxidation state, and its most important function is participation in redox reactions in plant metabolism [49].
Nickel content was detected in 19 locations with a concentration range of 1.20–10.9 mg/kg. This was higher than the literature data for bound onions (0.4–0.59 mg/kg) [38]. In a study by Vučić et al. [46] Ni content (0.358–0.388 mg/kg) was several times lower compared to the current research. The presence of Ni as well as its concentration in plant material mostly depends on the external environment [38], while due to Fe deficiencies, an antagonistic effect occurs in the soil, increasing the Ni content in plants [68]. Nickel can be considered a biogenic trace element only in low doses. Otherwise, it can have a carcinogenic effect.
Interestingly, a small number of plant samples contained several toxic elements (As, Cd, Cr and Pb) in different concentrations: 0.11–4.39; 2.2; 11.1; 15.6–58.1 mg/kg respectively. All of these listed elements can have a negative impact on human health [69], and the WHO permissible limits for these elements are quite low: 0.015; 0.007; 1.4; 0.025 mg/kg, indicating PTEs' negative impact on human health. From the obtained results, it can be observed that the content of the analyzed elements varied significantly depending on the habitat where this plant species grows. As indicated by the research [70], the ecotypes have a great influence on the content of some elements in A. ursinum. However, similar research [46] showed that there are no significant differences in the content of these elements. This can be explained by the small number of locations in the mentioned research at a short distance from each other.
Paunović et al. [71] pointed out the fact that altitude plays a big role in the content of elements in the plant species Morus nigra. On the other hand, Macek et al. [72] claim that the altitude does not have a great influence on their accumulation, which is consistent with the results obtained in this study. However, the main reason for the pronounced variability in the content of BEs/PTEs in plants and soil is explained by the research of Blake and Goulding [73] which reported that atmospheric deposition has a major impact on reducing soil pH, potentially affecting the accumulation of BEs/PTEs by plants, with most of them originating from atmospheric sediment. Similar observation was confirmed by Kabata-Pendias [38] who indicated that elemental content primarily depends on environmental and biological factors.
However, in order to determine the arrangement of BEs/PTEs in the plant in a simpler way, meaning to confirm their mutual effect, the correlation matrix of the analyzed elements in the leaf was determined and presented (Table 6).
The correlation matrix shows that the following BEs/PTEs are in a highly positive correlation (p < 0.05): Al–Fe, Mn–Fe, Zn–Fe, Cd–Fe, Pb–Fe, and Pb–Cd. The highest correlative coefficient was confirmed between Zn and Cd (r2 = 0.98), which is in line with literature data [38]. A similar relationship was observed in a study [74] stating that the positive correlation depends on the degree of BEs/PTEs accumulation in the plant, which was also observed for some elements.
The degree of accumulation of the detected elements in this research is shown in Table 7 using the bioaccumulation factor.
Table 7.
The bioaccumulation potential of A. ursinum leaves expressed as bioaccumulation factor (BAF).
| Location | K | Ca | Mg | Fe | Mn | Cu | Zn | B | Ni | Na | As | Cr | Pb | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.507 | 0.024 | 0.027 | 0.003 | 0.010 | nd | 0.119 | nd | 0.156 | 0.125 | nd | nd | nd | 0.003 |
| 2 | 2.106 | 0.082 | 0.034 | 0.002 | 0.006 | nd | 0.046 | 0.260 | nd | 0.200 | nd | nd | nd | 0.002 |
| 3 | 1.553 | 1.228 | 0.066 | 0.002 | 0.003 | 0.064 | 0.063 | 0.011 | nd | 0.243 | nd | nd | nd | 0.002 |
| 4 | 1.284 | 0.027 | 0.209 | 0.003 | 0.007 | 0.084 | 0.204 | 0.020 | 0.051 | 0.409 | nd | nd | nd | 0.003 |
| 5 | 2.701 | 0.195 | 0.095 | 0.002 | 0.005 | 0.084 | 0.091 | nd | nd | 0.209 | nd | nd | nd | 0.002 |
| 6 | 2.118 | 0.012 | 0.053 | 0.002 | 0.005 | 0.053 | 0.060 | 0.030 | nd | 0.130 | nd | nd | nd | 0.002 |
| 7 | 0.551 | 0.218 | 0.118 | 0.001 | 0.012 | 0.130 | 0.114 | nd | 0.088 | 0.081 | nd | nd | nd | 0.001 |
| 8 | 0.805 | 0.011 | 0.043 | 0.001 | 0.005 | 0.001 | 0.062 | 0.021 | nd | 0.245 | nd | nd | nd | 0.001 |
| 9 | 1.586 | 0.034 | 0.104 | 0.002 | 0.005 | 0.156 | 0.111 | nd | 0.234 | 0.348 | nd | nd | nd | 0.002 |
| 10 | 1.947 | 0.526 | 0.097 | 0.003 | 0.017 | 0.099 | 0.075 | 0.010 | nd | 0.167 | nd | nd | nd | 0.004 |
| 11 | 1.623 | 0.108 | 0.059 | 0.000 | 0.002 | 0.082 | 0.071 | nd | nd | 0.051 | nd | nd | nd | 0.001 |
| 12 | 0.942 | 0.016 | 0.048 | 0.001 | 0.005 | 0.078 | 0.073 | nd | nd | 0.118 | nd | nd | nd | 0.001 |
| 13 | 0.122 | 0.262 | 0.070 | 0.003 | 0.004 | 0.057 | 0.058 | 0.050 | nd | 0.155 | nd | nd | nd | 0.003 |
| 14 | 0.814 | 0.027 | 0.115 | 0.002 | 0.008 | 0.097 | 0.080 | 0.031 | nd | 0.267 | nd | nd | nd | 0.002 |
| 15 | 3.087 | 0.291 | 0.131 | 0.002 | 0.001 | nd | 0.084 | nd | 0.089 | 0.505 | nd | nd | nd | 0.002 |
| 16 | 1.919 | 0.411 | 0.114 | 0.003 | 0.005 | 0.103 | 0.028 | nd | nd | 0.196 | nd | nd | nd | 0.004 |
| 17 | 2.100 | 0.112 | 0.097 | 0.001 | 0.005 | 0.101 | 0.148 | nd | 0.230 | 0.418 | nd | nd | nd | 0.002 |
| 18 | 1.984 | 0.029 | 0.024 | 0.001 | 0.004 | nd | 0.024 | 0.054 | 0.011 | 0.136 | nd | nd | nd | 0.001 |
| 19 | 1.425 | 0.020 | 0.052 | 0.001 | 0.003 | nd | 0.061 | nd | nd | 0.152 | nd | nd | nd | 0.000 |
| 20 | 2.038 | 1.547 | 0.173 | 0.015 | 0.021 | 0.442 | 0.146 | 0.387 | 0.274 | 0.094 | 1.079 | nd | nd | 0.010 |
| 21 | 1.191 | 0.016 | 0.016 | 0.000 | 0.002 | nd | 0.045 | 0.012 | nd | 0.089 | nd | nd | nd | 0.000 |
| 22 | 1.457 | 0.034 | 0.060 | 0.001 | 0.004 | nd | 0.095 | nd | 0.052 | 0.168 | nd | nd | nd | 0.000 |
| 23 | 0.935 | 0.409 | 0.050 | 0.005 | 0.007 | 0.048 | 0.056 | nd | 0.011 | 0.195 | nd | 0.011 | nd | 0.007 |
| 24 | 1.770 | 0.573 | 0.135 | 0.001 | 0.005 | nd | 0.169 | nd | 0.115 | 0.103 | nd | nd | nd | 0.001 |
| 25 | 1.156 | 0.015 | 0.067 | 0.001 | 0.003 | nd | 0.072 | 0.028 | nd | 0.187 | nd | nd | nd | 0.001 |
| 26 | 2.321 | 0.133 | 0.067 | 0.001 | 0.004 | 0.049 | 0.084 | nd | 0.017 | 0.274 | nd | nd | nd | 0.001 |
| 27 | 1.307 | 0.235 | 0.070 | 0.001 | 0.002 | 0.029 | 0.083 | nd | nd | 0.330 | nd | nd | nd | 0.001 |
| 28 | 0.948 | 0.528 | 0.033 | 0.000 | 0.003 | nd | 0.019 | nd | nd | 0.208 | nd | nd | nd | 0.000 |
| 29 | 0.938 | 0.155 | 0.131 | 0.011 | 0.003 | nd | nd | nd | 0.113 | 0.202 | nd | nd | nd | 0.002 |
| 30 | 1.634 | 0.251 | 0.020 | 0.001 | 0.003 | 0.048 | 0.120 | 0.054 | nd | 0.078 | nd | nd | nd | 0.001 |
| 31 | 1.932 | 0.402 | 0.052 | 0.000 | 0.001 | nd | 0.048 | nd | 0.002 | 0.153 | nd | nd | nd | 0.000 |
| 32 | 2.702 | 0.179 | 0.058 | 0.001 | 0.002 | 0.031 | 0.065 | nd | 0.044 | 0.228 | nd | nd | nd | 0.001 |
| 33 | 2.431 | 0.584 | 0.160 | 0.001 | 0.012 | 0.073 | 0.094 | nd | nd | 0.343 | nd | nd | nd | 0.002 |
| 34 | 1.585 | 0.115 | 0.052 | 0.000 | 0.002 | 0.050 | 0.061 | 0.030 | nd | 0.109 | nd | nd | nd | 0.000 |
| 35 | 2.122 | 0.456 | 0.061 | 0.015 | 0.015 | nd | 2.123 | nd | 0.048 | 0.181 | 0.011 | nd | 0.665 | 0.003 |
| 36 | 1.851 | 0.034 | 0.089 | 0.000 | 0.002 | nd | 0.087 | nd | 0.057 | 0.185 | nd | nd | nd | 0.000 |
| 37 | 1.721 | 0.364 | 0.068 | 0.001 | 0.003 | 0.094 | 0.077 | 0.065 | nd | 0.146 | nd | nd | nd | 0.001 |
| 38 | 1.014 | 0.698 | 0.163 | 0.001 | 0.003 | 0.159 | 0.126 | nd | nd | 0.122 | nd | nd | nd | 0.001 |
| 39 | 1.285 | 0.023 | 0.023 | 0.001 | 0.003 | 0.041 | 0.071 | nd | nd | 0.194 | nd | nd | nd | 0.000 |
| 40 | 3.225 | 0.031 | 0.079 | 0.002 | 0.006 | nd | 0.242 | nd | 0.042 | 0.248 | nd | nd | nd | 0.002 |
| 41 | 2.311 | 0.058 | 0.082 | 0.002 | 0.003 | nd | 0.082 | nd | nd | 0.149 | nd | nd | nd | 0.001 |
| 42 | 2.531 | 0.273 | 0.065 | 0.007 | 0.009 | nd | 0.036 | 0.042 | 0.028 | 0.218 | 0.004 | nd | 0.016 | 0.006 |
| 43 | 1.281 | 0.012 | 0.015 | 0.001 | 0.003 | 0.042 | 0.068 | nd | nd | 0.064 | nd | nd | nd | 0.001 |
*nd -not detected.
Bioaccumulation represents the ability of a plant to accumulate chemical elements during its life cycle from the soil when the element is necessary for some physiological processes. Understanding bioaccumulation factors is of great importance for the definition of uptake of BEs/PTEs, because the plant indiscriminately adopts elements from the soil [39,75]. As a basis for assessing the degree of bioaccumulation [76,77] a four-point scale was used (Table 8). The obtained bioaccumulation values for BEs/PTEs were as follows (min-max): 0.122–3.225 (K); 0.011–1.547 (Ca); 0.016–0.209 (Mg); 0.001–0.015 (Fe); 0.001–0.021 (Mn); 0.011–0.387 (Cu); 0.019–2.123 (Zn); 0.01–0.387 (B) 0.002–0.274 (Ni); 0.002–0.274 (Na); 1.079 (As); 0.011 (Cr) and 0.016–0.665 (Pb).
Table 8.
Plant accumulation of BEs/PTEs.
| Bioaccumulation factor | Accumulation degree |
|---|---|
| 0.001–0.01 | None |
| 0.01–0.1 | Low |
| 0.1–1.0 | Medium |
| 1.0–10.0 | High |
By comparing the calculated values of the bioaccumulation potential (Table 6) with the degree of bioaccumulation (Table 7) in some of the locations, the bioaccumulation potential of the leaves was high (K, Ca, Zn, As), medium (Mg, Cu, B, Ni, Na, Pb) and low (Fe, Mn, Cr). In a previous study [78] a comparable potential for bioaccumulation was observed. However, for certain elements such as Mg, Cu, B, Na, Pb, a higher degree of bioaccumulation was noted compared to the findings of this current study. According to Kabata-Pendias [38] this disparity can be attributed to various mutually antagonistic and synergistic relationships among environmental factors, which play a dominant role. Similarly, Sipter et al. [75] investigated the degree of bioaccumulation of some PTEs (As, Pb, Cu, Hg, Zn, Hg and Cd) in the species Rumex acetosa and Allium schoenoprasum from the genus Allium and reported similar effects. In a recent study by Vuković et al. [6] similar bioaccumulation in A. odorum and A. schoenoprasum was found for K (1.07–1.74 and 0.75–0.94); Ca (0.28–0.56 and 0.05–0.07); Mg (0.02–0.03 and 0.01–0.02); Fe (0.0002–0.0004 and 0.0004–0.0007); Mn (0.001–0.002 and 0.001–0.003); Cu (0.011–0.014 and 0.006–0.01); Zn (0.017–0.023 and 0.01–0.025); B (0.07–0.27 and 0.04–0.24); Ni (0.0004–0.002 and 0.0005–0.006); Na (not detected −0.058 and not detected); As (not detected - 0.058 and not detected); Cr (0.001–0.003 and 0.002–0.005); Pb (not detected-0.003 and not detected - 0.002); Al (0.0001–0.0004 and 0.0003–0.001) where the accumulation of other potential toxic elements was not significantly pronounced. The relatively low bioaccumulation abilities of A. ursinum for certain elements may be attributed to local agroecological conditions. In wet soils with high concentrations of iron, Fe-hydroxides can form at the top of the roots, blocking the uptake of other elements and potentially contributing to the low uptake of BES/PTE/s [38]. Additionally, the accumulation of some trace elements decreases by half at lower temperatures [38]. Considering the high concentration of Fe in the soil at all locations (Table 3), and the duration of A. ursinum ‘s physiological cycle, it is presumed that these factors are the primary causes of its low bioaccumulation potential for most PTEs. Nonetheless, the fact that this species does not bioaccumulate PTEs is advantageous in terms of its nutritional value, as it is safe for human consumption.
With the aim of determining the possible source of BEs/PTE/s in the leaf of A. ursinum, a correlation between their content in the soil and the leaf has been established (Fig. 3).
Fig. 3.
Correlation of elemental content in soil and leaf.
Based on the results, it can be concluded that all positive correlations between soil and leaf element content (Cd, As, Ca, Pb, Na, Mg, K, Zn, Ni, and Mn) were quite low, while a negative correlation was determined for the following elements: Cu, B, Al, Cr, Fe. The most satisfactory correlations were observed for the content of Ca, Cd, As and Pb, while significantly lower correlations were observed for other elements. Except for Ca, Cd, As and Pb which were detected only in certain locations, indicating that their origin may be influenced by anthropogenic factors, as explained by the research [79] while Ca is likely taken up from the soil. The negative correlation for these elements is explained by their antagonistic and synergistic ways of adoption and the fact that some elements originate not from the soil, but from the atmosphere. Leaves absorb some of these elements through precipitation with the help of stomata [80]. Based on this, it can be assumed that for most PTEs, the main source is air deposition rather than soil uptake.
4. Conclusion
Research has shown that A. ursinum can grow on soils with a wide range of pH values, from highly acidic to neutral, that are well supplied with organic matter. The content of analyzed biogenic and PTEs in the soils (Fe > Al > Ca > Mg > K > Mn > V > Na > Ni > Zn > Cr > Pb > Cu > As > B > Co > Cr(VI) > Sb > Hg > Cd) varied across the different locations, with some soil samples containing levels of these elements above permitted limits.
It has been found that the leaves of A. ursinum are a good source of mineral elements, with the content of these elements varying depending on the habitat in which they grow. The content of the detected elements in descending order was K > Ca > Mg > Fe > Al > Na > Zn > Mn > Pb > B > Ni > Cu > As > Cd > Cr. In some locations, the content of As, Pb, Cd, and Cr was above the limit values. Additionally, research has shown that A. ursinum has a pronounced bioaccumulation potential for the uptake of K, Ca, Mg, Fe, Mn, Cu, Zn, B, Ni, Na, As, Cr, and Pb.
The correlation analysis did not establish a strong relationship between the soil and the plant, which suggests that the plant adopts some of them from the atmosphere. This result is concerning because consuming this medicinal plant without knowing its origin and elemental composition could pose a direct risk to people's health.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Stefan V. Gordanić: Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Aleksandar Ž. Kostić: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Investigation, Funding acquisition, Formal analysis, Conceptualization. Đurđa Krstić: Visualization, Validation, Software, Methodology, Formal analysis. Sandra Vuković: Methodology, Investigation, Formal analysis. Sofija Kilibarda: Methodology, Investigation, Formal analysis. Tatjana Marković: Supervision, Resources, Project administration, Investigation, Conceptualization. Đorđe Moravčević: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Corresponding author is serving as Associate editor in Heliyon journal. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grants: 451-03-47/2023-01/200003 451-03-47/2023-01/200116.
The authors express their gratitude to the Elixir Zorka-Šabac, Internal Laboratory for providing analytical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22134.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Towns A.M., Van Andel T. Wild plants, pregnancy, and the food-medicine continuum in the southern regions of Ghana and Benin. J. Ethnopharmacol. 2016;179:375–382. doi: 10.1016/j.jep.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Rola K. Taxonomy and distribution of Allium ursinum (Liliaceae) in Poland and adjacent countries. Biologia (Bratisl.) 2012;67:1080–1087. doi: 10.2478/s11756-012-0101-2. [DOI] [Google Scholar]
- 3.Sobolewska D., Podolak I., Makowska-Wąs J. Allium ursinum: botanical, phytochemical and pharmacological overview. Phytochemistry Rev. 2015;14:81–97. doi: 10.1007/s11101-013-9334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lachowicz S., Kolniak-Ostek J., Oszmiański J., Wiśniewski R. Comparison of phenolic content and antioxidant capacity of bear garlic (Allium ursinum L.) in different maturity stages. J. Food Process. Preserv. 2017;41 doi: 10.1111/jfpp.12921. [DOI] [Google Scholar]
- 5.Pantelić N.Đ., Jaćimović S., Štrbački J., Milovanović D.B., Dojčinović B.P., Kostić A.Ž. Assessment of spa mineral water quality from Vrnjačka Banja, Serbia: geochemical, bacteriological, and health risk aspects. Environ. Monit. Assess. 2019;191:1–16. doi: 10.1007/s10661-019-7848-7. [DOI] [PubMed] [Google Scholar]
- 6.Vuković S., Moravčević Đ., Gvozdanović-Varga J., Dojčinović B., Vujošević A., Pećinar I., Kilibarda S., Kostić A.Ž. Elemental profile, general phytochemical composition and bioaccumulation abilities of selected Allium species biofortified with selenium under open field conditions. Plants. 2015;12(2):349. doi: 10.3390/plants12020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gençcelep H., Uzun Y., Tunçtürk Y., Demirel K. Determination of mineral contents of wild-grown edible mushrooms. Food Chem. 2009;113:1033–1036. doi: 10.1016/j.foodchem.2008.08.058. [DOI] [Google Scholar]
- 8.Annan K., Dickson R.A., Amponsah I.K., Nooni I.K. The heavy metal contents of some selected medicinal plants sampled from different geographical locations. Pharmacogn. Res. 2013;5:103. doi: 10.4103/0974-8490.110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarwar N., Imran M., Shaheen M.R., Ishaque W., Kamran M.A., Matloob A., Hussain S. Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere. 2017;171:710–721. doi: 10.1016/j.chemosphere.2016.12.116. [DOI] [PubMed] [Google Scholar]
- 10.Alloway B.J. second ed. Blackie; London: 1995. Heavy Metals in Soils. [Google Scholar]
- 11.Li C., Zhou K., Qin W., Tian C., Qi M., Yan X., Han W. A review on heavy metals contamination in soil: effects, sources, and remediation techniques. Soil Sediment Contam. 2019;28:380–394. doi: 10.1080/15320383.2019.1592108. [DOI] [Google Scholar]
- 12.Chizzola R. Metallic mineral elements and heavy metals in medicinal plants. Med. Aromat. Plant Sci. Biotechnol. 2012;61(1):39–53. [Google Scholar]
- 13.Eissa M.A., Negim O.E. Heavy metals uptake and translocation by lettuce and spinach grown on a metal-contaminated soil. J. Soil Sci. Plant Nutr. 2018;18:1097–1107. doi: 10.4067/S0718-95162018005003101. [DOI] [Google Scholar]
- 14.Amin N., Hussain A., Alamzeb S., Begum S. Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, district Mardan, Pakistan. Food Chem. 2013;136:1515–1523. doi: 10.1016/j.foodchem.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 15.Kowalska G. The safety assessment of toxic metals in commonly used herbs, spices, tea, and coffee in Poland. Int. J. Environ. Res. Publ. Health. 2021;18:5779. doi: 10.3390/ijerph18115779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Šamec D., Karalija E., Šola I., Vujčić Bok V., Salopek-Sondi B. The role of polyphenols in abiotic stress response: the influence of molecular structure. Plants. 2021;10:118. doi: 10.3390/plants10010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malizia D., Giuliano A., Ortaggi G., Masotti A. Common plants as alternative analytical tools to monitor heavy metals in soil. Chem. Cent. J. 2012;6:1–10. doi: 10.1186/1752-153X-6-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q.R., Cui Y.S., Liu X.M., Dong Y.T., Christie P. Soil contamination and plant uptake of heavy metals at polluted sites in China. J. Environ. Sci. Health. 2003;38:823–838. doi: 10.1081/ESE-120018594. [DOI] [PubMed] [Google Scholar]
- 19.Kumar Sharma R., Agrawal M., Marshall F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India, Ecotoxicol. Environ. Saf. 2007;66:258–266. doi: 10.1016/j.ecoenv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Kadović R., Košanin O., Belanović S., Knežević M. Heavy metals in the organic soil layer of beech forests in Serbia. Glas. Šumarskog Fak. 2005;92:55–67. doi: 10.2298/GSF0592055K. [DOI] [Google Scholar]
- 21.Vanmechelen L., Groenemans R., Van Ranst E. Forest Soil Coordinating Centre; Bruxel: 1997. Forest Soil Condition, Results of A Large-Scale Soil Survey. [Google Scholar]
- 22.He Z., Shentu J., Yang X., Baligar V.C., Zhang T., Stoffella P.J. Heavy metal contamination of soils: sources, indicators and assessment. Ecol. Indicat. 2015;9:17–18. [Google Scholar]
- 23.Gordanić S., Radanović D., Vuković S., Kolašinac S., Kilibarda S., Marković T., Moravčević Đ., Kostić A.Ž. Phytochemical characterization and antioxidant potential of Allium ursinum L. cultivated on different soil types-a preliminary study. Emir. J. Food Agric. 2022;34:904–914. doi: 10.9755/ejfa.2022.v34.i11.2958. [DOI] [Google Scholar]
- 24.Kimbrough D.E., Wakakuwa J.R. Acid digestion for sediments, sludges, soils and solid wastes: a proposed alternative to EPA SW 8466 method 3050. Environ. Sci. Technol. 1989;23:898–900. [Google Scholar]
- 25.Jakšić S., Vasin J., Ninkov J., Živanov M., Banjac D., Grahovac N., Dozet G. Effect of soil type and forage crops on manganese content in roughage. Field Vegetable Crops Res. 2017;54(1):31–35. doi: 10.5937/ratpov54-12592. 2017. [DOI] [Google Scholar]
- 26.Shaheen S.M., Antoniadis V., Kwon E., Song H., Wang S.L., Hseu Z.Y., Rinklebe J. Soil contamination by potentially toxic elements and the associated human health risk in geo-and anthropogenic contaminated soils: a case study from the temperate region (Germany) and the arid region (Egypt) Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114312. [DOI] [PubMed] [Google Scholar]
- 27.Kabata-Pendias A., Mukherjee A.B. Springer; Berlin: 2007. Trace Elements of Group 10 (Previously Part of Group VIII), Trace Elem. From Soil to Hum; pp. 237–247. [DOI] [Google Scholar]
- 28.Yoon J., Cao X., Zhou Q., Ma L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006;368:456–464. doi: 10.1016/j.scitotenv.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Ruffell A. Forensic pedology, forensic geology, forensic geoscience, geoforensics and soil forensics. Forensic Sci. Int. 2010;202:9–12. doi: 10.1016/j.forsciint.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Falkengren-Grerup U., Tyler G. Soil chemical properties excluding field-layer species from beech forest mor. Plant Soil. 1993;148:185–191. doi: 10.1007/BF00012856. [DOI] [Google Scholar]
- 31.Ernst W.H.O. Population biology of Allium ursinum in northern Germany. J. Ecol. 1979;67:347–362. doi: 10.2307/2259355. [DOI] [Google Scholar]
- 32.Loveland P., Webb J. Is there a critical level of organic matter in the agricultural soils of temperate regions: a review. Soil Tillage Res. 2003;70:1–18. doi: 10.1016/S0167-1987(02)00139-3. [DOI] [Google Scholar]
- 33.Spychalski W., Grzebisz W., Diatta J., Kostarev D. Humus stock degradation and its impact on phosphorus forms in arable soils–a case of the Ukrainian Forest-Steppe Zone, Chem. Speciation Bioavailability. 2018;30:33–46. doi: 10.1080/09542299.2018.1457985. [DOI] [Google Scholar]
- 34.Kögel I., Hempfling R., Zech W., Hatcher P.G., Schulten H.R. Chemical composition of the organic matter in forest soils: 1. Forest litter. Soil Sci. 1988;146(2):124–136. doi: 10.1097/00010694-198808000-00011. [DOI] [Google Scholar]
- 35.Reimann C., Arnoldussen A., Englmaier P., Filzmoser P., Finne T.E., Garrett R.G., Koller F., Nordgulen O. Element concentrations and variations along a 120-km transect in southern Norway–Anthropogenic vs. geogenic vs. biogenic element sources and cycles. Appl. Geochem. 2007;22:851–871. doi: 10.1016/j.apgeochem.2006.12.019. [DOI] [Google Scholar]
- 36.Leuschner C., Lendzion J. Air humidity, soil moisture and soil chemistry as determinants of the herb layer composition in European beech forests. J. Veg. Sci. 2007;20:288–298. doi: 10.1111/j.1654-1103.2009.05641.x. [DOI] [Google Scholar]
- 37.Lambers H., Chapin F.S., Pons T.L. vol. 2. Springer Verlag; New York: 2008. pp. 11–99. (Plant Physiological Ecology). [Google Scholar]
- 38.Kabata-Pendias A. CRC Taylor and Francis Group; Boca Raton: 2011. Trace Elements in Soils and Plants/fourth Editions. [Google Scholar]
- 39.Official gazette of the republic of Serbia . 2011. no. 30/2018 and 64/2019, regulation on limit values of polluting, harmful and dangerous substances in soil.https://www.ekologija.gov.rs/sites/default/files/old-documents/Zemljiste/Uredbe/Uredba-o-granicnim-vrednostima.pdf [Google Scholar]
- 40.Milićević T., Urošević M.A., Relić D., Vuković G., Škrivanj S., Popović A. Bioavailability of potentially toxic elements in soil–grapevine (leaf, skin, pulp and seed) system and environmental and health risk assessment. Sci. Total Environ. 2018;626:528–545. doi: 10.1016/j.scitotenv.2018.01.094. [DOI] [PubMed] [Google Scholar]
- 41.Mazurek R., Kowalska J., Gąsiorek M., Zadrożny P., Józefowska A., Zaleski T. Assessment of heavy metals contamination in surface layers of Roztocze National Park forest soils (SE Poland) by indices of pollution. Chemosphere. 2017;168:839–850. doi: 10.1016/j.chemosphere.2016.10.126. [DOI] [PubMed] [Google Scholar]
- 42.Langenbruch C., Helfrich M., Flessa H. Effects of beech (Fagus sylvatica), ash (Fraxinus excelsior) and lime (Tilia spec.) on soil chemical properties in a mixed deciduous forest. Plant Soil. 2018;352:389–403. doi: 10.1007/s11104-011-1004-7. [DOI] [Google Scholar]
- 43.Talkner U., Krämer I., Hölscher D., Beese F.O. Deposition and canopy exchange processes in central-German beech forests differing in tree species diversity. Plant Soil. 2010;336:405–420. doi: 10.1007/s11104-010-0491-2. [DOI] [Google Scholar]
- 44.Weltzin J.F., McPherson G.R. University of Arizona Press; 2003. Changing Precipitation Regimes and Terrestrial Ecosystems: a North American Perspective. [Google Scholar]
- 45.Hooda P.S. In: Trace Elements in Soils. Hooda P.S., editor. John Wiley & Sons, Inorganic Methods, Springer; Hoboken: 2010. Assessing bioavailability of soil trace elements; pp. 229–265. [DOI] [Google Scholar]
- 46.Vučić G., Vasilisin L., Samelak I., Kukric Z., Kukric N. In: 23rd International Symposium on Biotechnology. Miletić N., Marković D., editors. Faculty of Agronomy; Čačak, Serbia: 2018. Contents of minerals in sremus (Allium ursinum) from different locations of the republic of srpska; pp. 530–535. [Google Scholar]
- 47.Piątkowska E., Kopec A., Leszczynska T. Basic chemical composition, content of micro-and macroelements and antioxidant activity of different varieties of garlic's leaves Polish origin. Żywność Nauka Technologia Jakość. 2018;22(1) doi: 10.15193/zntj/2015/98/014. [DOI] [Google Scholar]
- 48.Lukinac J., Jukić M. Influence of drying temperature on the organoleptic properties, antioxidant activity and polyphenol content in dried leaves of Allium ursinum L. subsp. ucrainicum. Ukr. Food J. 2018;11:2–26. doi: 10.24263/2304-974X-2022-11-1-4. [DOI] [Google Scholar]
- 49.Wiedenhoeft A.C. Chelsea House Publishers; US: 2006. Plant Nutrition. [Google Scholar]
- 50.Gins M., Gins V., Mотyleva S., Kulikov I., Mеdvedev S., Kononkov P., Pivovarov V. Mineral composition of amaranth (Amaranthus L.) seeds of vegetable and grain usage by ARHIVBSP selection. Potravinarstvo. 2018;12:330–336. doi: 10.5219/863. [DOI] [Google Scholar]
- 51.Arslanian S., Austin A. Impaired insulin mediated potassium uptake in adolescents with IDDM. Biochem. Med. Metab. Biol. 1991;46:364–372. doi: 10.1016/0885-4505(91)90084-X. [DOI] [PubMed] [Google Scholar]
- 52.Antonkiewicz J., Popławska A., Kołodziej B., Ciarkowska K., Gambuś F., Bryk M., Babula J. Application of ash and municipal sewage sludge as macronutrient sources in sustainable plant biomass production. J. Environ. Manag. 2020;264 doi: 10.1016/j.jenvman.2020.110450. [DOI] [PubMed] [Google Scholar]
- 53.Costello R.B., Rosanoff A., Dai Q., Saldanha L.G., Potischman N.A. Perspective: characterization of dietary supplements containing calcium and magnesium and their respective ratio—is a rising ratio a cause for concern? Adv. Nutr. 2021;12:291–297. doi: 10.1093/advances/nmaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popova A., Mihaylova D., Alexieva I. GC-MS chemical composition of volatile oil and mineral element content of Allium ursinum and Nectaroscordum siculum. Pakistan J. Bot. 2018;50:2351–2354. [Google Scholar]
- 55.Abbaspour N., Hurrell R., Kelishadi R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014;19(2):164. [PMC free article] [PubMed] [Google Scholar]
- 56.Stahl T., Falk S., Rohrbeck A., Georgii S., Herzog C., Wiegand A., Hotz S., Boschek B., Zorn H., Brunn H. Migration of aluminum from food contact materials to food—a health risk for consumers? Part III of III: migration of aluminum to food from camping dishes and utensils made of aluminum. Environ. Sci. Eur. 2017;29:1–8. doi: 10.1186/s12302-017-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar V., Gill K.D. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch. Toxicol. 2009;83:965–978. doi: 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 58.Sun J., Wei Y.H. A new alkaloid-aluminum glycoside isolated from rhizoma sparganii (Sparganium stolonifera buch. Ham.) J. Med. Plants Res. 2011;14:3128–3131. [Google Scholar]
- 59.Bowman A.B., Kwakye G.F., Hernández E.H., Aschner M. Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellingsen D.G., Shvartsman G., Bast-Pettersen R., Chashchin M., Thomassen Y., Chashchin V. Neurobehavioral performance of patients diagnosed with manganism and idiopathic Parkinson disease. Int. Arch. Occup. Environ. Health. 2019;92:383–394. doi: 10.1007/s00420-019-01415-6. [DOI] [PubMed] [Google Scholar]
- 61.Papoyan A., Piñeros M., Kochian L.V. Plant Cd2+ and Zn2+ status effects on root and shoot heavy metal accumulation in Thlaspi caerulescens. New Phytol. 2007;175:51–58. doi: 10.1111/j.1469-8137.2007.02073.x. [DOI] [PubMed] [Google Scholar]
- 62.Zeitlinger J., Stark A., Kellis M., Hong J.W., Nechaev S., Adelman K., Levine M., Young R.A. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat. Genet. 2007;39:1512–1516. doi: 10.1038/ng.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chasapis C.T., Ntoupa P.S.A., Spiliopoulou C.A., Stefanidou M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020;94:1443–1460. doi: 10.1007/s00204-020-02702-9. [DOI] [PubMed] [Google Scholar]
- 64.Kabata-Pendias A., Mukherjee A.B. Springer; Berlin: 2007. Trace Elements of Group 10 (Previously Part of Group VIII), Trace Elem. From Soil to Hum; pp. 237–247. [DOI] [Google Scholar]
- 65.Graham R.D., Welch R.M., Grunes D.L., Cary E.E., Norvell W.A. Effect of zinc deficiency on the accumulation of boron and other mineral nutrients in barley. Soil Sci. Soc. Am. J. 1987;51:652–657. [Google Scholar]
- 66.Bakirdere S., Orenay S., Korkmaz M. Effect of boron on human health. Open Miner. Process. J. 2010;3(1):54–59. doi: 10.2174/1874841401003010054. [DOI] [Google Scholar]
- 67.Araya M., Pizarro F., Olivares M., Arredondo M., Gonzalez M., Méndez M. Understanding copper homeostasis in humans and copper effects on health. Biol. Res. 2006;39:183–187. doi: 10.4067/S0716-97602006000100020. [DOI] [PubMed] [Google Scholar]
- 68.Khan W.U.D., Shaukat R., Farooq M.A., Ashraf M.N., Nadeem F., Tanveer M., Hamid Y., Sun N. Iron-doped biochar regulated soil nickel adsorption, wheat growth, its physiology and elemental concentration under contrasting abiotic stresses. Sustainability. 2022;14:7852. doi: 10.1007/s11356-015-4881-0. [DOI] [Google Scholar]
- 69.Khan A., Khan S., Khan M.A., Qamar Z., Waqas M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ. Sci. Pollut. Res. 2015;22:13772–13799. doi: 10.1007/s11356-015-4881-0. [DOI] [PubMed] [Google Scholar]
- 70.Blazewicz-Wozniak M., Michowska A. The growth, flowering and chemical composition of leaves of three ecotypes of Allium ursinum L. Acta Agrobot. 2011;64:171–180. doi: 10.5586/aa.2011.058. [DOI] [Google Scholar]
- 71.Paunović S.M., Mašković P., Milinković M. Determination of primary metabolites, vitamins and minerals in black mulberry (Morus nigra) berries depending on altitude. Erwerbsobstbau. 2020;62:355–360. doi: 10.1007/s10341-020-00509-7. [DOI] [Google Scholar]
- 72.Macek P., Klimeš L., Adamec L., Doležal J., Chlumská Z., de Bello F., Dvorský M., Řeháková K. Plant nutrient content does not simply increase with elevation under the extreme environmental conditions of Ladakh, NW Himalaya. Arctic Antarct. Alpine Res. 2012;44:62–66. doi: 10.1657/1938-4246-44.1.62. [DOI] [Google Scholar]
- 73.Blake L., Goulding K.W.T. Effects of atmospheric deposition, soil pH and acidification on heavy metal contents in soils and vegetation of semi-natural ecosystems at Rothamsted Experimental Station, UK. Plant Soil. 2002;240:235–251. doi: 10.1023/A:1015731530498. [DOI] [Google Scholar]
- 74.Singh R., Singh D.P., Kumar N., Bhargava S.K., Barman S.C. Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J. Environ. Biol. 2010;31:421–430. [PubMed] [Google Scholar]
- 75.Sipter E., Auerbach R., Gruiz K., Mathe‐Gaspar G. Change of bioaccumulation of toxic metals in vegetables. Commun. Soil Sci. Plant Anal. 2009;40:285–293. doi: 10.1080/00103620802647165. [DOI] [Google Scholar]
- 76.Michalowski M., Golas J. Zawartosc wybranych metali ciezkich w organach wierzby jako wskaznik wykorzystania jej w utylizacji osadow sciekowych. Zesz. Probl. Postepow Nauk. Rol. 2001;477:411–419. [Google Scholar]
- 77.Ociepa-Kubicka A., Pachura P., Kacprzak M. Effect of unconventional fertilization on heavy metal content in the biomass of giant miscanthus. Desalination Water Treat. 2016;57:1230–1236. doi: 10.1080/19443994.2015.1033132. [DOI] [Google Scholar]
- 78.Stojković M. Faculty of Science; Niš: 2014. Antioksidativna Aktivnost, Fenolni I Mineralni Sastav Biljnih Vrsta: Geranium Macrorrhizum L., Allium Ursinum L., Stachys Germanica L. I Primula Veris L, Niš (Doctoral Dissertation. in Serbian) [Google Scholar]
- 79.Doğanlar Z.B., Atmaca M. Influence of airborne pollution on Cd, Zn, Pb, Cu, and Al accumulation and physiological parameters of plant leaves in Antakya (Turkey) Water, Air, Soil Pollut. 2011;214:509–523. doi: 10.1007/s11270-010-0442-9. [DOI] [Google Scholar]
- 80.Zwolak A., Sarzyńska M., Szpyrka E., Stawarczyk K. Sources of soil pollution by heavy metals and their accumulation in vegetables: a review, Wat. Air Soil Poll. 2019;230:1–9. doi: 10.1007/s11270-019-4221-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.