Abstract
Lactococcin G is a bacteriocin whose activity depends on the complementary action of two peptides, termed α and β. Biologically active, synthetic lactococcin G was used to study the mode of action on sensitive cells of Lactococcus lactis. The α and β peptides can bind independently to the target cell surface, but activity requires the complementary peptide. Once bound to the cell surface, the peptides cannot be displaced to the surfaces of other cells. A complex of α and β peptides forms a transmembrane pore that conducts monovalent cations but not protons. Efflux of potassium ions is observed only above pH 5.0, and the rate of efflux increases steeply with the pH. The consequences of cation fluxes for the viability of the target cells are discussed.
Bacteriocins produced by lactic acid bacteria are peptides displaying bactericidal activity against closely related gram-positive bacteria. In terms of applications, bacteriocins are interesting as preservatives of food products. Lactococcin G activity depends on two peptides, termed α and β, that consist of 39 and 35 amino acids, respectively (14). Bactericidal activity is observed only in the presence of both peptides, and optimal activity is observed when the peptides are present in a 1-to-1 ratio (10). Biologically active lactococcin G can also be obtained by solid-phase peptide synthesis (10). Addition of lactococcin G to sensitive cells results in a collapse of the transmembrane electrical potential but not in dissipation of the transmembrane pH gradient (10). Such cells show a rapid depletion of cellular ATP and release intracellular potassium, as monitored through the use of 86Rb+. These studies have led to the suggestion that the loss of cell viability results from futile cycling of potassium ions via a lactococcin G-induced potassium-specific pore and the ATP-dependent uptake of potassium ions. We now show that lactococcin G not only causes the release of potassium ions but that it has a broader specificity for monovalent cations.
MATERIALS AND METHODS
Materials.
86Rubidium (86Rb+) (1 mCi/1.1 mg), 22Na (1 mCi/3.7 mg), 32Pi (1 mCi/0.11 nmol), and [14C]choline (7.2 mCi/mmol) were obtained from Amersham UK. Gramicidin A′ was obtained from Sigma (St. Louis, Mo.). The bacteriocin peptides were dissolved in 55% (vol/vol) isopropanol and 0.1% (vol/vol) trifluoroacetic acid and mixed in a 1-to-1 ratio.
Peptide synthesis, purification, and analysis of the polypeptides.
The lactococcin G α and β peptides were prepared by solid-phase synthesis, purified, and analyzed as described previously (10).
Strains and culture conditions.
Lactococcus lactis LMG 2081 (10) and L. lactis IL 1403 (3) were used as nonsensitive and sensitive strains, respectively. Both strains were grown at 30°C in M17 broth (Oxoid) without lactose but supplemented with 0.5% (wt/vol) glucose or, alternatively, with both 0.25% (wt/vol) glucose and 0.25% (wt/vol) l-malate. Cells were harvested in the logarithmic growth phase.
Bacteriocin assay.
Bacteriocin activity was measured as previously described (14). Briefly, 200 μl of culture medium (supplemented with 0.1% [vol/vol] Tween 80), bacteriocin fractions at twofold dilutions, and the indicator strain (A600 = 0.1) were added to each well of a microtiter plate. After 3 h of incubation at 30°C, growth inhibition of the indicator strain was measured spectrophotometrically at 600 nm with a microplate reader (Titertek Multiskan MCC/340 MKII, type 347).
Cation flux assays.
Rubidium transport and lactococcin G-mediated rubidium efflux were measured as described previously (10). Cells (0.25 mg of protein/ml) suspended in an appropriate buffer were preenergized in a solution containing 50 mM Na-PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] (pH 7.0) and 0.5% glucose for 2 min at 30°C, and subsequently 86Rb+ (0.45 μCi/ml) was added. Lactococcin G was added after 20 min and, at intervals, samples were filtered over 0.45-μm-pore-size cellulose nitrate filters (Millipore Corp.) and washed twice with 2 ml of ice-cold 0.1 M LiCl as described previously (4). 22Na and [14C]choline were loaded into the cells by overnight incubation of a 30-μl suspension of cells (140 μg of protein) in medium without glucose containing either 4 μCi of 22Na or 2 μCi of [14C]choline. Ion-loaded cells were diluted in 800 μl of 50 mM KPi (pH 7.0), and 100-μl samples were filtered and washed as above. The lactococcin G-mediated cation efflux rate depended on the initial intracellular concentration of radiolabelled cation.
Phosphate flux assays.
Efflux of unlabelled phosphate was measured as follows. Cells in 50 mM Na-PIPES, pH 7.0, were incubated at 30°C (5.3 mg of protein/ml). At time points up to 10 min, samples of 340 μl were subjected to silicon oil centrifugation (6), separating the cells from the buffer. The amount of released phosphate in the supernatant fraction was determined as described by Rouser et al. (18). For phosphate (32Pi) transport, cells were suspended in 50 mM MES (morpholineethanesulfonic acid) buffer, pH 7.0 (300 μg of protein/ml), and supplemented with 32Pi (4.7 μCi/ml). In order to avoid glucose-(bis)phosphate formation, cells were energized with 0.5% (wt/vol) malate (17). After a 5-min uptake, lactococcin G (40 nM) was added either directly or after the addition of valinomycin and nigericin (0.2 μM each), which blocked further uptake of 32Pi. Samples were filtered and washed with LiCl as described above. Radioactivity was measured by liquid scintillation counting in a Packard Tri-Carb 460 CD counter (Packard Instruments Corp.).
Measurements of proton motive force.
Generation of a transmembrane electrical potential (ΔΨ) was measured with the fluorescent probe 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5)]. Cells were used only directly after isolation. The transmembrane pH gradient (ΔpH) was measured by loading the cells with the fluorescent pH indicator 2′,7′-bis-(2-carboxyethyl)-5(and-6)-carboxyfluorescein (BCECF) as described previously (9).
Miscellaneous methods.
Protein measurements were performed according to the method of Lowry et al. (7). ATP depletion was induced by incubation for 30 min at 30°C with freshly prepared 20 mM deoxyglucose.
RESULTS
Lactococcin G elicits cellular release of monovalent cations.
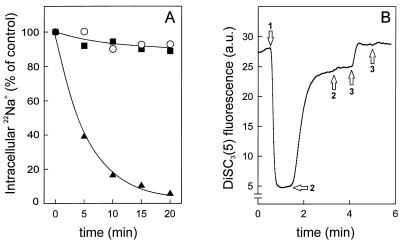
Since lactococcin G induces the release of potassium ions from sensitive cells, it has been suggested that it acts as a potassium-conducting pore (10). To determine the ion specificity of lactococcin G, cells were loaded overnight with radioactive sodium ions (22Na+). When diluted into buffer, the cells only slowly released the 22Na+ (Fig. 1A). 22Na+ efflux was not induced by the addition of solvent or valinomycin. In contrast, lactococcin G addition resulted in rapid and complete release of sodium ions (Fig. 1A). Parallel control experiments with cells loaded overnight by diffusion with 86Rb+ showed that lactococcin G induced 86Rb+ efflux rates comparable to those of 22Na+ (data not shown). This implies that lactococcin G is not only specific for potassium ions (10) but conducts sodium ion movements as well.
FIG. 1.
Lactococcin G causes passage of sodium ions. (A) L. lactis cells were loaded with 22Na+ as described in Materials and Methods. At time zero, cells were diluted in 50 mM KPi to 174 μg of protein/ml, and either 50 nM lactococcin G (▴), 0.25 μM valinomycin (▪), or solvent (○) was added. (B) In L. lactis cells (28 μg of protein/ml) suspended in 50 mM Na-PIPES, pH 7.0, a ΔΨ was generated by 0.25 μM valinomycin (arrow 1), after which 20 nM lactococcin G (arrows 2) and 6.7 μM nisin (arrows 3) were added.
Lactococcin G also dissipates the valinomycin-induced ΔΨ of cells suspended in 50 mM Na-PIPES buffer as monitored by the use of the cyanine dye DiSC3(5) (Fig. 1B). This implies that the valinomycin-mediated potassium ion efflux is counteracted by a lactococcin G-mediated sodium ion influx. Dissipation, however, is not complete. The intracellular potassium concentration can be as high as 800 mM (15), leaving the possibility open that the ion concentration on the outside is too low to counterbalance the valinomycin-induced potassium flux. Indeed, lactococcin G virtually completely dissipated the ΔΨ generated in 300 mM sodium or potassium (data not shown).
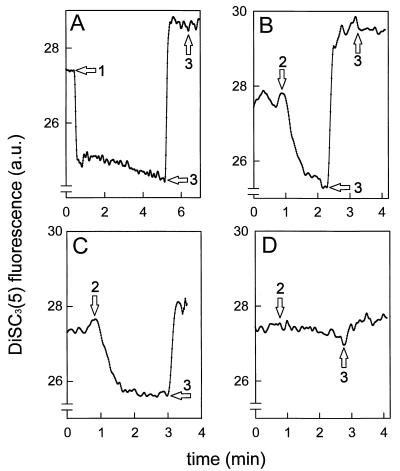
When cells were diluted in 50 mM KPi (Fig. 2B) or in 50 mM Na-PIPES (Fig. 2C), addition of lactococcin G resulted in generation of ΔΨ akin to the valinomycin-induced ΔΨ when cells were diluted into 50 mM KPi (Fig. 2A). When the sodium ion concentration on the outside was raised from 50 to 300 mM, addition of lactococcin G did not result in the generation of a ΔΨ (Fig. 2D). Similar results were obtained when sodium was replaced by potassium ion (data not shown). The ΔΨ was not induced when only α peptide or β peptide was added to cells, nor when prior to lactococcin G addition an excess of the nonspecific pore-forming bacteriocin nisin (6.7 μM) was added. Addition of valinomycin after ΔΨ induction by lactococcin G did not affect the ΔΨ (data not shown).
FIG. 2.
Lactococcin G can induce a ΔΨ. L. lactis cells were suspended at pH 7.0 either in 50 mM KPi (A and B), in 50 mM Na-PIPES (C), or in 300 mM Na-PIPES (D). The final cell protein concentration was 28 μg/ml. Valinomycin at 0.2 μM (arrow 1) and 20 nM lactococcin G (arrows 2) generated a ΔΨ, whereas excess (6.7 μM) nisin (arrows 3) dissipated the ΔΨ.
Lactococcin G induces choline flux.
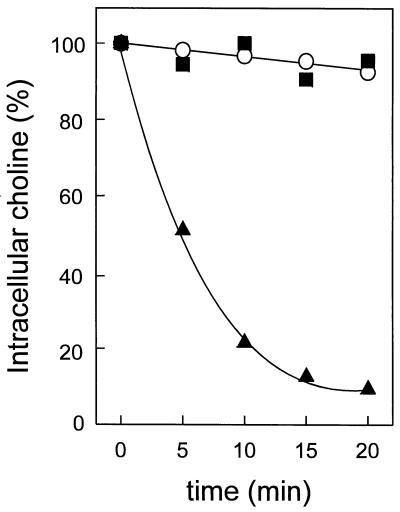
In order to define more precisely the specificity of lactococcin G pores, experiments similar to those above were performed with the choline ion. Lactococcin G causes efflux of [14C]choline from cells which have been loaded overnight by diffusion (Fig. 3). Likewise it dissipates—although not completely—the ΔΨ induced by valinomycin in cells suspended in 50 mM choline chloride (data not shown). Similar results were obtained with lithium, cesium, tetramethyl-ammonium, or Tris as the counteracting cation (data not shown), which demonstrates that lactococcin G permeabilizes the membrane for a broad range of monovalent cations. The rate of lactococcin G-mediated dissipation of the ΔΨ indicates that the conductivity of potassium, sodium, cesium, or lithium is slightly higher than that of choline or tetramethylammonium and much higher than of Tris (data not shown). In contrast, lactococcin G does not at all dissipate the ΔΨ induced by valinomycin in cells suspended either in 50 mM MgCl2, 50 mM MgSO4, or 50 mM bis(Tris)-propane (data not shown). This clearly indicates that lactococcin G has conductance for neither the anions tested here nor the divalent cations.
FIG. 3.
Lactococcin G causes choline flux. L. lactis cells, loaded with [14C]choline, were diluted in 50 mM KPi (174 μg protein/ml) at time zero, and either 50 nM lactococcin G (▴), 30 μM carbonyl cyanide m-chlorophenylhydrazone (▪), or solvent (○) was added.
Lactococcin G does not mediate phosphate efflux.
To investigate whether lactococcin G’s inability to conduct anions also extends to phosphate, release of cellular phosphate was analyzed. Phosphate transport in L. lactis is unidirectional and is likely ATP dependent (16). Intracellular phosphate concentrations can be as high as 140 mM (16). Lactococcin G was unable to induce the release of cellular phosphate, nor did it effect the release of accumulated 32Pi from malate-energized cells (data not shown). This demonstrates that lactococcin G does not conduct the transmembrane movement of one of the major cytosolic constituents.
Lactococcin G causes an increase in the ΔpH.
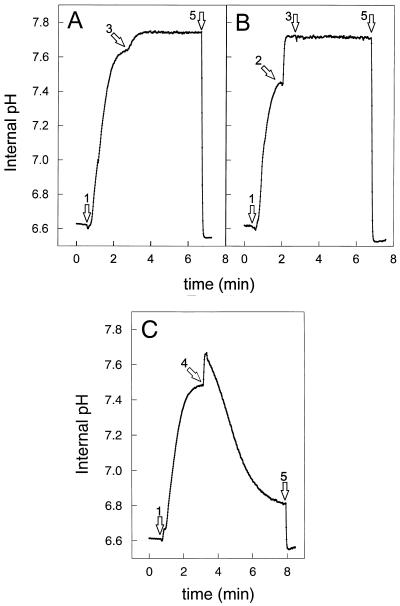
Previous experiments with cells loaded with the fluorescent pH indicator BCECF showed that after addition of valinomycin, lactococcin G is unable to dissipate the ΔpH (10). These experiments were performed in the presence of valinomycin to assure that the ΔpH is the sole component of the proton motive force. As shown in Fig. 4A, addition of lactococcin G to glucose-energized cells results in an elevation of the intracellular pH, as monitored by the fluorescence of cells with entrapped BCECF. This effect is likely due to the dissipation of the ΔΨ and enhanced proton extrusion by the F0F1 ATPase and was not observed when cells were pretreated with valinomycin (Fig. 4B). The lactococcin G action may be comparable to the effect of gramicidin A′ on the ΔpH. Gramicidin A dimers form water-filled channels specific for small cations and—in contrast to lactococcin G—the conductivity for protons can be up to 150 times higher than for sodium ions (13). Initially, gramicidin A′ causes a rapid increase of the ΔpH in L. lactis cells, but this process is immediately followed by a steep decrease in ΔpH (Fig. 4C). This clearly shows that gramicidin channels functionally differ from lactococcin G pores and further suggests that lactococcin G does not conduct proton movements at a significant rate. In the above experiments, the K+/H3O+ ratio was 1.6 × 105. In order to investigate possible competition of K+ and H3O+, cells were suspended in 20 mM bis(Tris)-propane, pH 6.5, instead of 50 mM KPi. A control experiment showed that the presence of bis(Tris)-propane does not affect lactococcin G activity in terms of permeation of small monovalent cations. No change in ΔpH was observed after addition of lactococcin G, which confirms lactococcin G’s inability to conduct protons.
FIG. 4.
Lactococcin G causes an increase in the ΔpH. Cells (28 μg of protein/ml) loaded with BCECF were energized in 50 mM KPi, pH 6.5, with 0.5% glucose (arrows 1). (A) Addition of 20 nM lactococcin G (arrow 3) and 0.2 μM nigericin (arrow 5). (B) Addition of 0.2 μM valinomycin (arrow 2) and 20 nM lactococcin G (arrow 3), followed by 0.2 μM nigericin (arrow 5). (C) Addition of 12 nM gramicidin A′ (arrow 4) followed by addition of 0.2 μM nigericin (arrow 5).
The lactococcin G α and β peptides can interact independently with intact cells.
Lactococcin G is active against cells only when both the α and β peptides are present, preferentially in stoichiometric amounts (10; see also Table 1). However, growth inhibition is also observed when the cells are first pretreated with one peptide, followed by extensive washing, and subsequently supplemented with the complementary peptide (Table 1). This suggests that the α peptide alone, as well as the β peptide, can interact stably with the target cell surface, without losing its potential bactericidal activity in this idle state. In agreement with the data presented above, cells treated with one peptide can accumulate rubidium ions, and only when the complementary peptide is added does rubidium ion efflux occur (Table 1). In contrast, no lactococcin G activity is observed when cells treated with one peptide are mixed with cells treated with the complementary peptide. This demonstrates that the lactococcin G peptide is unable to diffuse to another cell once it is bound to the cell surface.
TABLE 1.
α and β peptides can interact independently with cellsa
| Treatment | IC50 (nM) | 86Rb+ efflux |
|---|---|---|
| α | >29.0 | − |
| β | >29.0 | − |
| α + β | 0.05 ± 0.01 | + |
| β Cells + α | 0.06 ± 0.01 | + |
| α Cells + β | 0.05 ± 0 | + |
| α Cells + β cells | NAb | − |
β cells and α cells, cells pretreated with β and α peptides, respectively. Peptide-treated cells were obtained by treating cells in medium of pH 6.8 for 3 min with 29 nM peptide at 220 μg of cellular protein/ml followed by four washes. IC50, 50% inhibitory concentration. IC50 and 86Rb+ efflux were determined as described in Materials and Methods.
NA, no activity.
Lactococcin G action strongly depends on the extracellular pH.
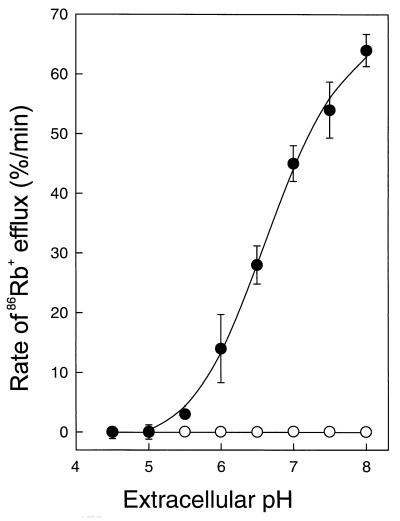
We noted that a premix of both α and β peptides at pH 5.3 caused efflux of 86Rb+ from cells. On the other hand, pretreatment of the cells with one peptide at pH 5.3 followed by the addition of the complementary peptide caused an attenuation of 86Rb+ uptake, rather than efflux (data not shown). This difference between premixing and no premixing of the peptides was not observed at pH 6.8 or when cells were both pretreated with one peptide and washed at pH 5.3 and then subsequently supplemented with the complementary peptide at pH 6.8. These data are indicative of the effect of pH on the interaction of the two peptides. Figure 5 shows the pH dependence of lactococcin G-mediated 86Rb+ efflux. The rate of 86Rb+ efflux increased with pH, whereas in the absence of lactococcin G no efflux occurred. Curve fitting of the pH dependence data suggests an apparent pK of 6.69 ± 0.07 (mean ± standard deviation). When cells loaded with 86Rb+ were depleted of ATP by incubation in the presence of deoxyglucose prior to lactococcin G addition, the same pH dependence of lactococcin G-induced efflux of 86Rb+ was observed (data not shown). These results suggest that lactococcin G is pH dependent and that deprotonation of an amino acid chain with a pK of 6.7 is critical for the mode of action.
FIG. 5.
pH dependence of lactococcin G. L. lactis cells (0.25 μg/ml) that had taken up 86Rb+, as described in Materials and Methods, were subsequently subjected at various pHs to lactococcin G (58 nM) (•) or to solvent (○), and initial 86Rb+ efflux was measured. The apparent pK was 6.69 ± 0.07.
DISCUSSION
Most bacteriocins are one-peptide systems. The present work provides studies on the mechanism of action of a two-component bacteriocin, lactococcin G. Both the α and β peptides are predicted to form an α-helical amphipathic structure (14). The premixing-dependent activity at low pH observed in our complementation studies indicates that the peptides interact. It seems therefore that a complex of α and β peptides forms a transmembrane pore. In L. lactis cells, potassium, sodium, and phosphate are the most abundant inorganic ions. Here we demonstrate that lactococcin G not only induces potassium ion efflux (10) but also sodium and other monovalent cation (in)flux. On the other hand, neither phosphate, other anion, nor divalent cation conductance by lactococcin G was observed. The highest conductivity was measured for potassium and sodium ions. Studies in the present work support previous data (10) indicating that lactococcin G does not conduct protons.
Lactococcin G causes an influx of sodium ions into the cells (Fig. 1B). Intracellular sodium is known to be cytotoxic, although the mechanism of this toxicity is incompletely understood (2). The extensive cation fluxes into and out of the cell cause an osmotic imbalance of the cell’s turgor pressure, a collapse of both the transmembrane Na+ gradient and ΔΨ (10), and—indirectly—ATP depletion (10). Consequently, Na+-coupled transport and ΔΨ- and ATP-requiring (transport) processes are arrested. The above effects together explain the bactericidal activity.
Lactococcin G differs from some other two-component systems by neither dissipating ΔpH, causing Pi efflux, nor being active on liposomes. Acidocin J1132 (19) and thermophilin 13 (8) dissipate both ΔpH and ΔΨ. Lactacin F (1) seems to cause efflux of both potassium and inorganic phosphate.
Lactococcin G exhibits a prominent pH dependence. The activity increases with the pH, with an apparent pK of about 6.7. Histidine is the only amino acid with a pKa in that range. The very C-terminal residue of the α peptide is the only histidine in lactococcin G. Therefore, involvement of this histidine in pH dependence seems very likely. The pH dependence strongly suggests that this histidine needs to be deprotonated before functional membrane interaction is possible or before a functional complex can be formed between the α and β peptides. Strikingly, in the case of lactococcin A (5) and lactocin S (11), the last C-terminal residues are two histidines. Lactocin S is active only below pH 6.0 (12), suggesting that in this case the two C-terminal histidines of lactocin S both need to be protonated for interaction with the target cell surface. It will be of interest to establish, by mutagenesis studies on the α peptide of lactococcin G, whether the pH range can be extended and the target specificity can be modified.
ACKNOWLEDGMENTS
This work was supported by the European Community with the Biotech program, contract no. BIOT-CT94-3055, and by the Norwegian Research Council.
REFERENCES
- 1.Abee T, Klaenhammer T R, Letellier L. Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl Environ Microbiol. 1994;60:1006–1013. doi: 10.1128/aem.60.3.1006-1013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng J, Guffanti A A, Krulwich T A. A two-gene ABC-type transport system that extrudes Na+ in Bacillus subtilis is induced by ethanol or protonophore. Mol Microbiol. 1997;23:1107–1120. doi: 10.1046/j.1365-2958.1997.2951656.x. [DOI] [PubMed] [Google Scholar]
- 3.Chopin A, Chopin M-C, Moillo-Bat A, Langella P. Two plasmid determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984;11:260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- 4.Driessen A J M, Kodde J, de Jong S, Konings W N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987;169:2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holo H, Nilssen Ø, Nes I F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991;173:3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashket E R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- 7.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 8.Marciset O, Jeronimus-Stratingh M C, Mollet B, Poolman B. Thermophilin 13, a nontypical antilisterial poration complex bacteriocin that functions without a receptor. J Biol Chem. 1997;272:14277–14284. doi: 10.1074/jbc.272.22.14277. [DOI] [PubMed] [Google Scholar]
- 9.Molenaar D, Abee T, Konings W N. Measurement of intracellular pH in bacteria with a fluorescent probe. Biochim Biophys Acta. 1991;1115:75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- 10.Moll G N, Ubbink-Kok T, Hildeng-Hauge H, Nissen-Meyer J, Nes I F, Konings W N, Driessen A J M. Lactococcin G is a potassium-ion-conducting two-component bacteriocin. J Bacteriol. 1996;178:600–605. doi: 10.1128/jb.178.3.600-605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mørtvedt C I, Nissen-Meyer J, Sletten K, Nes I F. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991;57:1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mørtvedt-Abildgaard C I, Nissen-Meyer J, Jelle B, Grenov B, Skaugen M, Nes I F. Production and pH-dependent bactericidal activity of lactocin S, a lantibiotic from Lactobacillus sake L45. Appl Environ Microbiol. 1995;61:175–179. doi: 10.1128/aem.61.1.175-179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers V B, Haydon D A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- 14.Nissen-Meyer J, Holo H, Håvarstein L S, Sletten K, Nes I F. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J Bacteriol. 1992;174:5686–5692. doi: 10.1128/jb.174.17.5686-5692.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poolman B, Hellingwerf K J, Konings W N. Regulation of glutamate-glutamine transport systems by intracellular pH in Streptococcus lactis. J Bacteriol. 1987;169:2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poolman B, Nijssen R M, Konings W N. Dependence of Streptococcus lactis phosphate transport on internal phosphate concentration and internal pH. J Bacteriol. 1987;169:5373–5378. doi: 10.1128/jb.169.12.5373-5378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poolman B, Molenaar D, Smid E J, Ubbink T, Abee T, Renault P P, Konings W N. Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J Bacteriol. 1991;173:6030–6037. doi: 10.1128/jb.173.19.6030-6037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 19.Tahara T, Oshimura M, Umezawa C, Kanatani K. Isolation, partial characterization, and mode of action of acidocin J1132, a two-component bacteriocin produced by Lactobacillus acidophilus JCM 1132. Appl Environ Microbiol. 1996;62:892–897. doi: 10.1128/aem.62.3.892-897.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]