Abstract
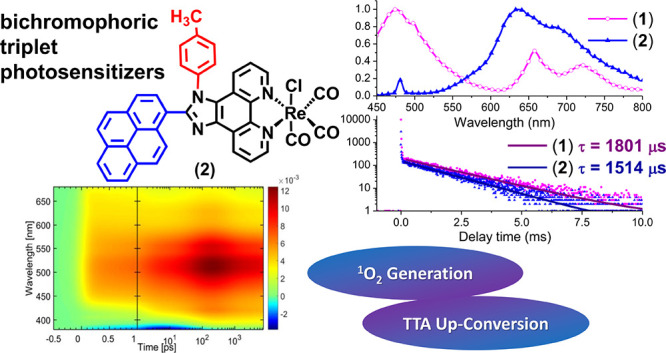
Photochemical applications based on intermolecular photoinduced energy triplet state transfer require photosensitizers with strong visible absorptivity and extended triplet excited-state lifetimes. Using a bichromophore approach, two Re(I) tricarbonyl complexes with 2-(1-pyrenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (pyr-imphen) and 1-(4-(methyl)phenyl)-2-(1-pyrenyl)-imidazo[4,5-f][1,10]phenanthroline (pyr-tol-imphen) showing extraordinary long triplet excited states at room temperature (>1000 μs) were obtained, and their ground- and excited-state properties were thoroughly investigated by a wide range of spectroscopic methods, including femtosecond transient absorption (fs-TA). It is worth noting that the designed [ReCl(CO)3(pyr-imphen)] (1) and [ReCl(CO)3(pyr-tol-imphen)] (2) complexes form a unique pair differing in the mutual chromophore arrangement due to introduction of a 4-(methyl)phenyl substituent into the imidazole ring at the H1-position, imposing an increase in the dihedral angle between the pyrene and {ReCl(CO)3(imphen)} chromophores. The magnitude of the electronic coupling between the pyrene and {ReCl(CO)3(imphen)} chromophores was found to be an efficient tool to tune the photophysical properties of 1 and 2. The usefulness of designed Re(I) compounds as triplet photosensitizers was successfully verified by examination of their abilities for 1O2 generation and triplet–triplet annihilation upconversion. The phosphorescence lifetimes, ∼1800 μs for 1 and ∼1500 μs for 2, are the longest lifetimes reported for Re(I) diimine carbonyl complexes in solution at room temperature.
Short abstract
Two Re(I)-based emitters with extraordinary long triplet excited states at room temperature were obtained and thoroughly explored with the aid of static and time-resolved spectroscopic methods, including transient absorption. Photoinduced processes in the bichromophore Re(I) complexes with imidazophenanthroline-based ligands were additionally modified by the substituent steric hindrance in the imidazole ring at the H1-position. The usefulness of Re(I) compounds as triplet photosensitizers was successfully verified by examination of their abilities for 1O2 generation and triplet−triplet annihilation upconversion.
Introduction
Precise control of electronic excited states in transition metal complexes, and thus their photophysical and photochemical properties, is crucial for rational design of functional materials with predefined photophysical behavior, appropriate for applications in photocatalysis,1−4 organic light-emitting diodes,5−9 solar energy conversion,10−12 phosphorescence molecular sensing,13−15 and chemotherapy and photodynamic therapy. Some of these photochemical applications involve intermolecular photoinduced energy triplet state transfer, with the efficiency being strongly dependent on the visible absorptivity and triplet excited-state lifetime of the photosensitizer. To increase the visible light harvesting of transition metal complexes, ligands with strong visible absorptivity are introduced into the coordination sphere of the metal ion. In turn, to achieve triplet emitters with long excited states, both radiative and nonradiative decay rate constants must be small, which means that the triplet excited state should have a predominant ligand character.13,16−26 An efficient strategy for accessing long-lived phosphorescent dyes is to use an organic ligand with a non-emissive long-lived triplet state (3IL) close in energy to an emissive metal-to-ligand charge transfer triplet excited state (3MLCT). The establishment of thermal equilibration between these triplet states results in an extension of the lifetime of 3MLCT, as the ligand-based triplet excited state becomes the energy reservoir. Extended emission lifetimes are also expected when the 3IL excited state is noticeably lower than 3MLCT, and strong spin–orbit coupling results in the ligand-based phosphorescence at room temperature. Since when Ford and Rodgers first demonstrated a bichromophoric approach,27 it has been successfully used for the synthesis of rhenium(I) carbonyl complexes with naphthalimide-subsitiuted ligands28−30 and ruthenium(II) complexes bearing polypyridyl ligands functionalized with pyrenyl and anthryl groups,19,31−36 giving promising photosensitizers. Recently, our group has explored Re(I) carbonyl complexes with aryl-substituted 2,2′:6′,2″-terpyridines and reported extended room-temperature photoluminescence lifetime for [ReCl(CO)3(4′-pyrenyl-terpy-κ2N)].37
In the current contribution, we present photophysical properties of bichromophore Re(I) carbonyl complexes with 2-(1-pyrenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (pyr-imphen) and 1-(4-(methyl)phenyl)-2-(1-pyrenyl)-imidazo[4,5-f][1,10]phenanthroline (pyr-tol-imphen). The great advantages of imidazo[4,5-f][1,10]phenanthrolines are their prominent antitumor and luminescence properties, as well as the convenient synthesis along with the possibility of introduction of all types of substituents into the imidazole ring at H1- and C2-positions, directly (via the covalent bond) or through an aryl/heterocycle bridge.38,39 Although imidazo[4,5-f][1,10]phenanthrolines have been combined with various transition metal ions,38,39 [ReCl(CO)3(R-imphen)] systems have been rarely investigated, and their photophysics is unfathomable.40−53
Furthermore, the designed complexes [ReCl(CO)3(pyr-imphen)] (1) and [ReCl(CO)3(pyr-tol-imphen)] (2) form a unique pair, making it possible to determine the impact of ligand conformation restriction on the mutual chromophore arrangement and, thus, modification of photoinduced processes and triplet–triplet energy processes in such systems. The 4-(methyl)phenyl substituent, introduced into the imidazole ring at the H1-position, imposes an increase in the dihedral angle between the pyrene and [ReCl(CO)3(imphen)] chromophores and thus induces a weaker electronic coupling between the organic and metal-based chromophores. To the best of our knowledge, this type of a ligand conformation restriction has been first used to tune photophysical achievements of bichromophore transition metal complexes with imidazophenanthroline-based ligands. Taking into consideration a wide range of possible structural modifications of imphen-based ligands, it can be assumed that this approach will be successfully utilized in the design of other multichromophore transition-metal compounds. An important advantage of the attachment of aryl and alkyl groups concerns the increase in the solubility of resulting transition metal complexes, which is beneficial regarding the applications of these systems.
To get a better understanding of the impact of the pyrene chromophore and molecular ligand conformation, ground- and excited-state properties of [ReCl(CO)3(pyr-imphen)] (1) and [ReCl(CO)3(pyr-tol-imphen)] (2) were compared with those for parent complexes [ReCl(CO)3(imphen)] (3) and [ReCl(CO)3(tol-imphen)] (4) (Chart 1).
Chart 1. Rhenium(I) Complexes Employed in the Study.
A complete picture of excited-state processes in the designed systems has been achieved with the aid of static and time-resolved spectroscopic methods, including transient absorption (TA). In addition, both pyrenyl-substituted Re(I) complexes, which showed extraordinary long triplet excited states at room temperature (>1000 μs), were successfully tested as photosensitizers for 1O2 sensitizing and triplet–triplet annihilation for energy upconversion (TTA UC) to verify their potential applications.
Results and Discussion
Synthesis and Structure Confirmation
Complexes [ReCl(CO)3(pyr-imphen)] (1), [ReCl(CO)3(pyr-tol-imphen)] (2), [ReCl(CO)3(imphen)] (3), and [ReCl(CO)3(tol-imphen)] (4) were synthesized by a direct reaction of [Re(CO)5Cl] with the appropriate imidazo[4,5-f][1,10]phenanthroline-based ligand in refluxing acetonitrile-toluene mixture under argon protection. Regarding our previous results,37,53 compounds 1 and 2 have been designed as bichromophore systems with metal-to-ligand charge transfer (3MLCT) and pyrene-localized (3ILpyrene) triplet excited states in energetic proximity. Complexes 3 and 4 were used as reference systems for a better understanding of photoinduced processes in pyrenyl-substituted Re(I) systems.
Successful coordination of the imidazo[4,5-f][1,10]phenanthroline-based ligand and formation of Re(I) carbonyls 1–4 were evidenced with the aid of elemental analyses and spectroscopic tools (see Figures S2–S6 in the Supporting Information). FT-IR spectra of 1–4 show a set of intense ν(C≡O) bands in the region of 2030–1875 cm–1 with a pattern typical of fac-{Re(CO)3}+ moiety accompanied by less intensive stretching and bending vibrations of the coordinated imidazo[4,5-f][1,10]phenanthroline-based ligand (Figure S2). The 1H and 13C NMR signals of model chromophores were fully assigned using one- and two-dimensional 1H–1H COSY, 1H–13C HMQC, and 1H–13C HMBC techniques. For the pyrenyl-substituted Re(I) systems (1 and 2), however, the full assignment of the signals in 1H and 13C NMR spectra was precluded due to the significant overlapping of signals (Figures S3 and S4).
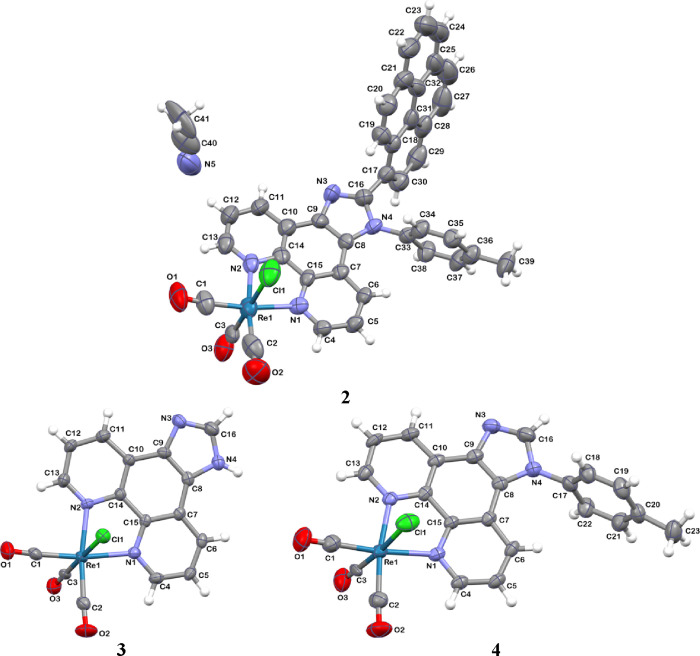
Also, single-crystal X-ray structures of complexes 2–4 were determined (Tables S1–S5, Figure 1, and Figures S1, S7, and S8). Typically, of this class of compounds, the Re(I) ion of 2–4 shows a distorted octahedral coordination environment, and three carbonyl ligands are arranged in fac geometry (Figure 1). The introduction of aryl substituents (pyrenyl and 4-(methyl)phenyl) into the imidazole ring at the C2- and H1-positions of the imphen core does not generate noticeable changes in bond lengths and angles around the Re(I) center (Table S2). Most remarkably, due to the steric hindrance induced by the 4-(methyl)phenyl substituent attached to the imidazole ring at the H1-position, there is a large torsional twist (∼70°) between the planes of imphen and pyrene in the molecular structure 2, which may lead to weak electronic communications between 3MLCT and 3ILpyrene excited states.
Figure 1.
Perspective view showing the asymmetric units of Re(I) complexes along with the atom numbering. Displacement ellipsoids are drawn at the 50% probability level.
The packing and stabilization of the crystal structures of 2–4 are contributed by weak hydrogen bonds as well as π•••π and X–Y•••π intermolecular contacts (Figures S7 and S8 and Tables S3–S5).
Theoretical Insight on Ground- and Excited-State Properties of 1–4
To better understand the effect of the pyrenyl substituent and its inclination toward the imphen core on optical features of resulting Re(I) complexes, DFT and TD-DFT calculations were performed for 1–4 at the PBE0/def2-TZVPD/def2-TZVP level of theory (Figures S9–S18 and Tables S6–S10).
The calculated bond lengths and angles of 1–4 in ground-state geometries (S0) are in satisfactory agreement with X-ray analysis results, and general trends observed in the experimental data are well theoretically reproduced, as shown in Figure S9 and Table S6. Upon going from S0 to T1, noticeable variations in bond distances were found only in the case of the parent complexes. In the triplet-optimized geometry (T1) of 3 and 4, Re–N and Re–Cl distances undergo shortening, and Re–C becomes elongated. The noticeable structural difference between the 1 and 2 complexes is only the pyrene-imphen torsion angle, which was estimated to be ∼39° and ∼61° for ground state (S0)-optimized geometries of 1 and 2, respectively. In the triplet-optimized geometries, the pyrene-imphen dihedral angle decreases to ∼20° for 1 and ∼52° for 2 (Table S6). The calculated torsion angle values clearly indicate that the twist of the aryl group toward the imphen core, and thus the magnitude of their electronic coupling, can be effectively controlled by substituents of suitable steric hindrance introduced into the imidazole ring at the H1-position. It may be an efficient way of tuning the photophysical properties of these systems.
As shown in Figure 2, the pyrenyl-substituted systems show a reduced HOMO–LUMO gap relative to the reference complexes. The attachment of pyrene to the imphen core leads to a ∼0.5 eV destabilization of the HOMO of complexes 1 and 2 in relation to the parent systems, leaving the LUMO energy virtually unperturbed. In all complexes, the lowest unoccupied molecular orbital (LUMO) is predominately constituted of π* imphen ligand orbitals. The highest occupied molecular orbital (HOMO) of 1 and 2 resides almost exclusively on the pyrenyl substituent, contrary to the parent complexes with HOMO spreading over the {Re(CO)3Cl} moiety. Rhenium d orbitals combined with π*CO and pCl orbitals participate in H-1 and H-2 of 1–2 and H-1 and H-3 of 3–4. A noticeable stabilization of L + 2 and L + 3 of complexes 1 and 2 in relation to model systems is attributed to the large contribution of pyrene orbitals.
Figure 2.
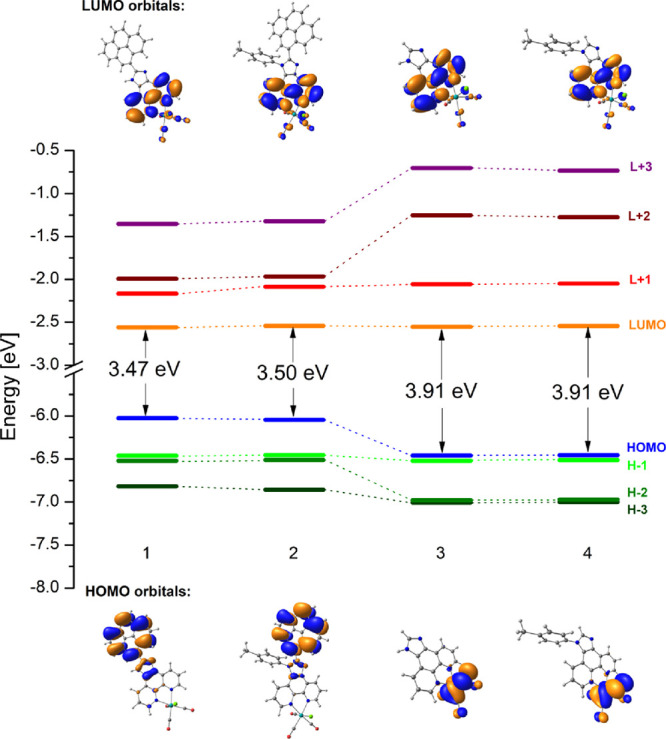
Partial molecular orbital energy level diagrams with the electron density plots of the highest occupied and lowest unoccupied MOs for complexes 1–4.
According to TD-DFT calculations and NBO analysis (Figures S15 and S18 and Tables S7 and S10), the striking difference between 1 and 2 concerns the lowest energy singlet transition. For complex 2, the transition S0 → S1 (exclusively contributed by H-1 → L) is MLCT in nature with a low oscillator strength. The hole is localized on rhenium dπ orbitals, and the particle is constituted by π* imphen ligand orbitals. Conversely, for the complex with a significantly smaller pyrene-imphen torsion angle (1), the lowest energy singlet transition displays mixed character: 1MLCT (dπ(Re) → π*imphen) and 1ILCT (πpyrene → π*imphen). No pure 1MLCT transitions was evidenced theoretically for 1. For model chromophores, all three lowest energy singlet transitions, attributed to the long-wavelength absorption band, are MLCT character.
To achieve the characteristics of the lowest energy triplet excited state of 1–4 complexes, the spin density surfaces were generated from the lowest energy optimized triplet states (Figure S10). For both pyrenyl-substituted Re(I) complexes, the spin density surfaces are predominantly distributed on the pyrenyl substituent but they differ in the contribution of imphen orbitals. A larger imphen participation for complex 1 indicates a greater degree of intraligand charge transfer in the triplet state, assigned as 3ILpyrene /3ILCT for 1 and 3ILpyrene for 2. The spin density of model chromophores, distributed among the {Re(CO)3Cl} unit and π* orbitals of the phen moiety, confirms the 3MLCT character of their triplet states.
UV–Vis Absorption and Emission Spectra
UV–vis spectra of Re(I) complexes (1–4) in DMSO are shown in Figure 3 and Figure S19–S22, and the absorption maxima along with corresponding molar extinction coefficients are summarized in Table S11.
Figure 3.
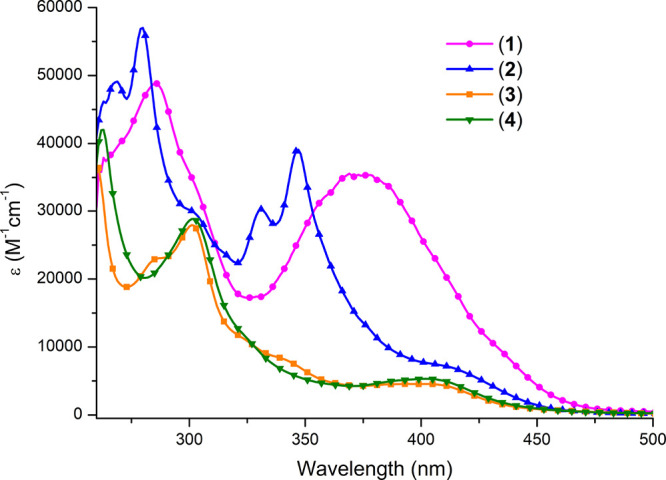
Comparison of UV–vis spectra of complexes 1–4 in DMSO. Concentration of compounds: 25 μM.
The absorption bands of 1–4 in the high-energy region of 220–325 nm are ascribed to intraligand 1π → 1π* transitions of the corresponding imphen-based ligand. In the range of 325–475 nm, complex 1 shows broad and strong absorption with a maximum at ∼370 nm and a weak shoulder at ∼430 nm. By comparing with the spectra of pyr-imphen, imphen, and parent Re(I) complex (3), this band can be assigned to metal-to-ligand charge transfer (1MLCT, dπ(Re) → π*imphen) and intraligand (1IL, πpyrene → π*pyrene) in combination with intraligand charge transfer transitions (1ILCT, πpyrene → π*imphen). The red-shift and significant absorptivity enhancement of the lowest energy absorption in relation to 3, along with the lack of characteristic pyrene vibronic progression between 300 and 350 nm, support the strong electronic coupling between 1-pyrenyl and imphen moieties (see Figure S19). Complex 2 shows a drastically different absorption profile in this region. In this case, the absorption with characteristic vibronic progression corresponding to πpyrene–πpyrene* transitions (325–400 nm) is well resolved from the 1MLCT band, indicating the weak electronic coupling between 1-pyrenyl and imphen units. The observed spectral features of 1 and 2 well correlate with TD-DFT calculations, which showed that the incorporation of 4-(methyl)phenyl at the H1-position of 2-(1-pyrenyl)-1H-imidazo[4,5-f][1,10]phenanthroline leads to a significant increase in the dihedral angle between the appended pyrenyl group and the imphen framework and, thus, better separation of 1MLCT and 1IL states.
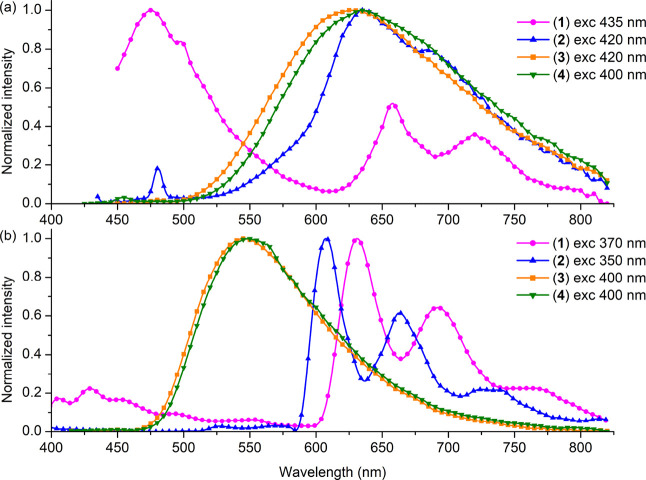
Regarding the emission behavior, parent complexes show an excitation-independent broad and featureless emission band at room temperature (Figure 4 and Figures S23 and S24), which remains unstructured and undergoes a significant blue-shift upon cooling to 77 K, consistent with the emission being predominantly of triplet-state metal-to-ligand charge-transfer (3MLCT) character54−56 (Figure 4).
Figure 4.
Emission spectra of 1–4 in DMSO at RT (a) and in an EtOH:MeOH glass matrix at 77 K (b).
Distinctly from chromophores 3 and 4, pyrenyl-substituted Re(I) complexes (1 and 2) display excitation-dependent dual fluorescence-phosphorescence emission (Figures S23 and S24). With the lower energy excitation, the relative phosphorescence–fluorescence increases. A short-lived higher energy component of 1 and 2 is superimposed with the ligand-centered fluorescence (1IL), but its intensity is significantly quenched relative to that of the corresponding free ligand (Figure S25). Such a phenomenon is well recognized in bichromophoric systems and indicates incomplete energy transfer from the 1IL to 1MLCT excited state via the Förster resonance energy transfer (FRET) mechanism.29,30,57−59 The values of EnT according to eq 1:
| 1 |
where FC and FL are the integrated fluorescence of the complex and free ligand,60,61 equal to 98.76% for 1 and 98.92% for 2. The stability and photostability of 1 and 2 in DMSO exclude the fact that the observed higher energy band is the emission of the ligand that has dissociated from the complex (Figures S21 and S22). The contribution of fluorescence and phosphorescence in emission spectra of 1 and 2 was also evidenced by time-resolved emission spectra (TRES) recorded at room temperature (Figure 5 and Figures S26 and S27).
Figure 5.
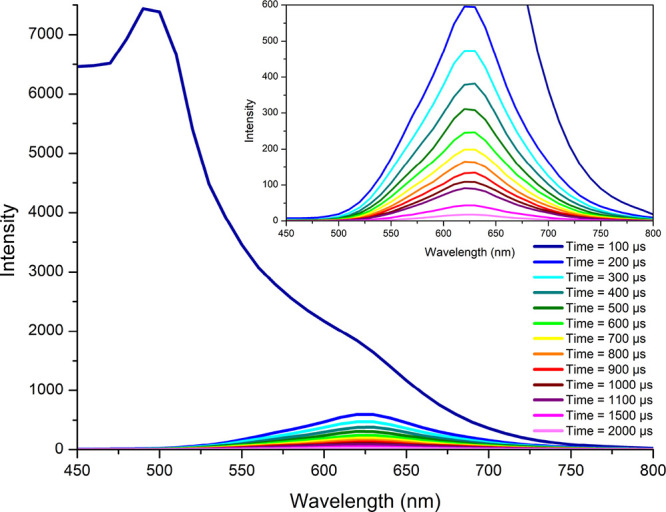
Time-resolved emission spectra (TRES) of 2 in DMSO. Concentration: 50 μM; excitation wavelength: 435 nm. Inset: close-up of low intensity spectra. For TRES spectra of 1 and excitation wavelength of 355 nm, see Figures S26 and S27 in the Supporting Information.
A striking difference between 1 and 2 can be noticed at longer wavelengths (>500 nm). The phosphorescence band of 2 is almost structureless and falls in the range of 3MLCT for the parent chromophores. In turn, complex 1 exhibits a vibronically structured emission band with maxima at 657, 720, and 790 nm, attributable to the pyrene-based phosphorescence. The observation of the room-temperature phosphorescence of pyrene is very limited. Despite a few Ru(II) and Pt(II) examples showing pyrene-based phosphorescence at RT, pyrenyl-substituted transition complexes are generally characterized by a structureless 3MLCT emission band, likewise complex 2, or they are non-emissive at RT.62−69
For both complexes 1 and 2, phosphorescence lifetimes at RT, determined using the monoexponential decay model (∼1800 μs for 1 and ∼1500 μs for 2), are much longer than those of reference chromophores (223 ns for 3 and 179 ns for 4, see Table 1 and Figure 6). It is worth noting that these values are the longest lifetimes reported for Re(I) diimine carbonyl complexes.28 Concerning the extreme sensitivity of triplet excited states of 1 and 2 toward oxygen, it is highly probable that their lifetimes are even longer.
Table 1. Summary of Emission Properties of 1–4.
| complex | medium | λexc (nm) | λem (nm) | lifetime | χ2 | QY (%) |
|---|---|---|---|---|---|---|
| 1 | DMSO | 375 | 460 | τ1 = 1.77 ns | 1.054 | 0.52 |
| 435 | 657, 720, 790 | τ1 = 1800.90 μs | 1.039 | |||
| 77 Ka | 375 | 405, 430, 450, 494 | τavrb = 9.49 ns | 0.995 | ||
| 370 | 631, 691, 762 | τ1 = 169.67 ms | 1.005 | |||
| 2 | DMSO | 375 | 450 | τavrb = 2.12 ns | 0.995 | 1.06 |
| 420 | 636 | τ1 = 1513.84 μs | 1.016 | |||
| 77 Ka | 420 | 608, 663, 730 | τ1 = 372.38 ms | 1.078 | ||
| 3 | DMSO | 405 | 625 | τ1 = 223.27 ns | 0.993 | 3.11 |
| 77 Ka | 400 | 546 | τavrb = 7.76 μs | 0.999 | ||
| 4 | DMSO | 405 | 634 | τ1 = 179.47 ns | 0.956 | 3.82 |
| 77 Ka | 400 | 549 | τavrb = 11.85 μs | 0.973 |
Ethanol:methanol (4:1 v/v) glassy matrix at 77 K.
Average emission lifetime. For more details, see Figure S24 in the Supporting Information.
Figure 6.
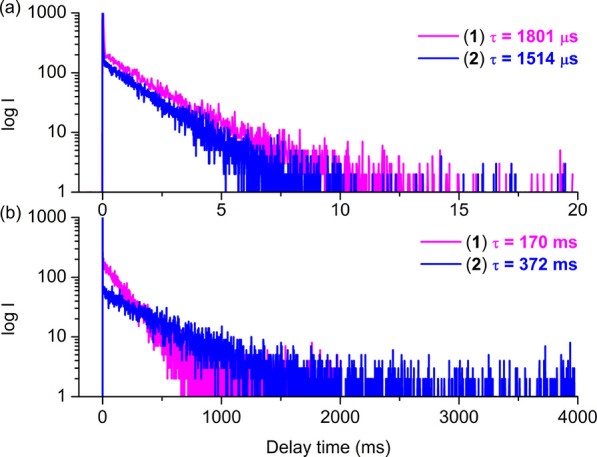
Comparison of decay time curves for 1 and 2 in DMSO at RT (a) and EtOH:MeOH glass matrix at 77 K (b).
To gain further insight, photoluminescence properties of the title complexes were investigated at low temperature (77 K). These studies also revealed noticeable differences between 1 and 2. While complex 2 presents only a vibronically structured emission band attributed to the 3πpyrene, the frozen-state emission spectrum of 1 includes both ligand-based fluorescence (1IL) and phosphorescence (3πpyrene) components. The bathochromic shift of the phosphorescence for complex 1 in comparison to 2 indicates significantly larger participation of 3ILCT (3πpyrene → 3π*(imphen)) in the case of 1.70 The triplet excited-state lifetimes of pyrenyl-substituted Re(I) complexes (∼170 ms for 1 and ∼370 ms 2) are shorter than that for free pyrene (700 ms)71,72 but a few orders of magnitude longer than those for parent chromophores at 77 K (average on 7.76 μs for 3 and 11.85 μs for 4).
3MLCT–3ILpyrene Energy Gap
Extended triplet emission lifetimes and reduced photoluminescence quantum yields of pyrenyl-substituted Re(I) complexes at RT in relation to reference chromophores are supportive of the energetic proximity of metal-to-ligand charge transfer and pyrene-localized triplet excited states.
To estimate the energy gap between the 3MLCT and 3ILpyrene triplet excited states in the pyrene-substituted Re(I) carbonyls (1 and 2), two different methodologies were used. In the first approach, the energies of excited states 3MLCT were determined from the room-temperature emission band of the appropriate model chromophore (3 and 4) by taking the tangent line on the high-energy side of the emission band and its intersection with the wavelength axis. The triplet energies 3ILpyrene were estimated by drawing the tangent line on the high-energy side of the frozen-state phosphorescence band of the corresponding free ligands (L1 and L2) and its intersection with the wavelength axis (Figure S28). The triplet emissions of the free ligands were sensitized by the addition of 10% ethyl iodide. The difference of obtained energy values of 3MLCT and 3ILpyrene states gives energy gaps of 2850 cm–1 for 1 and 3372 cm–1 for 2 (Figure 7).
Figure 7.
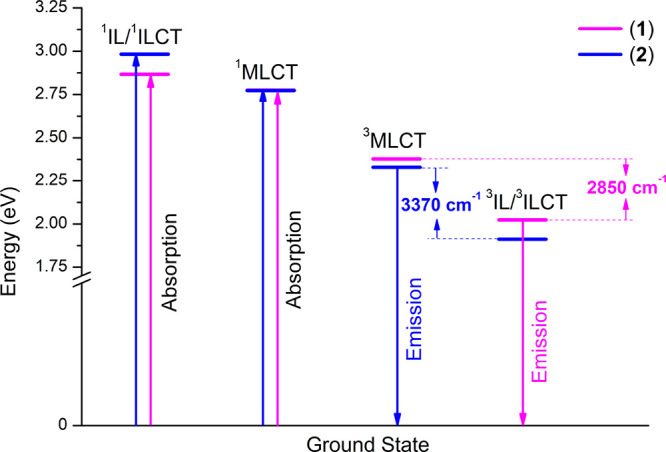
Calculated energies of singlet and triplet excited states of 1 and 2 and the 3MLCT–3IL energy gaps. The energies of the 1MLCT and 1IL/1ILCT excited states were obtained by the tangent line on the lowest-energy side of the longest wavelength absorption band of the appropriate model chromophores (3 and 4) and free ligands (L1 and L2), respectively.
In the second method, the triplet energy gaps between 3MLCT and 3ILpyrene excited states of 1 and 2 were calculated using the following eqs 2–5:
| 2 |
| 3 |
| 4 |
| 5 |
where τ, τpyr, and τMLCT are the phosphorescence lifetimes of pyrene-substituted Re(I) carbonyl (1 or 2), pure pyrene (700 ms), and corresponding model complex, respectively. The parameter α represents the fraction of the 3ILpyrene excited state, while 1 – α corresponds to the fraction of the 3ILpyrene excited state in the established equilibrium. Also, in this method, the calculated energy gap between the 3MLCT and 3ILpyrene triplet is larger for complex 2, but the obtained values are expectedly smaller, 1845 cm–1 for 1 and 1854 cm–1 for 2.
As shown in Figure 7, a weaker electronic communication between the metal-to-ligand charge transfer and pyrene localized triplet excited states, achieved by the increase in the dihedral angle between imphen and pyrenyl moieties due to the introduction of the 4-(methyl)phenyl substituent into the imidazole ring at the H1-position, leads to stabilization of both 3MLCT and 3ILpyrene, simultaneously resulting in the increase in the energy gap between them.
Femtosecond Transient Absorption
To get further insight into photophysical processes and examine their excited-state dynamics, femtosecond transient absorption (fs-TA) studies were performed for 1–4 in DMSO, and obtained fs-TA data were analyzed by global analysis. For reference complexes, fs-TA experiments were performed with the excitation at 355 nm, while pyrenyl-substituted Re(I) carbonyls were excited at the blue and red edges of the lowest energy absorption, which are at 355 and 420 nm. For complexes 1 and 2, two different pump wavelengths were used because the lowest energy absorption band of these systems was found to be contributed by both 1pyrene and 1MLCT excited states, and both Re(I) carbonyls displayed excitation-dependent dual fluorescence–phosphorescence emission. The results of fs-TA studies are summarized in Figures 8 and 9 and Figures S29–S32.
Figure 8.
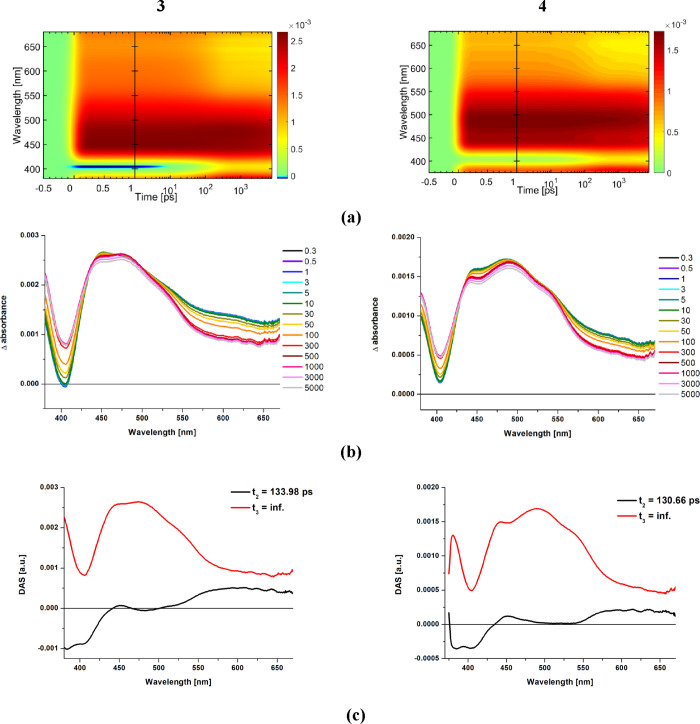
Summary of fs-TA studies for model complexes 3 and 4 at the 355 nm pump: (a) 2D time–wavelength plots; (b) fs-TA spectra at selected time delays; (c) decay-associated spectra (DAS; see also complete data in Figure S31).
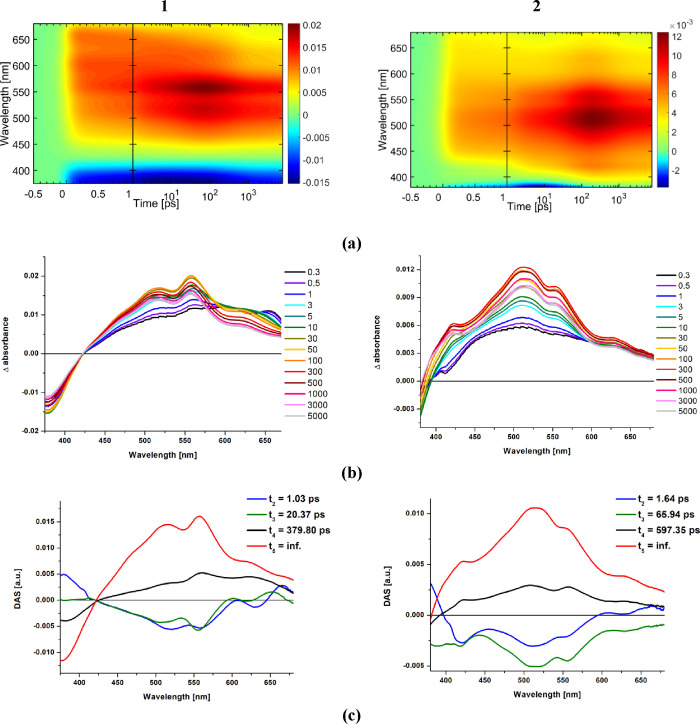
Figure 9.
Summary of fs-TA studies for complexes 1 and 2 at the 355 nm pump. (a) 2D time–wavelength plots; (b) fs-TA spectra at selected time delays; (c) decay-associated spectra (DAS; see also complete data in Figures S29 and S30).
The fs-TA spectra of model complexes are dominated by broad intense excited-state absorption (ESA) in the range of 420–590 nm (Figure 8 and Figure S31), present up to the end of the delay stage (7 ns). This spectral profile is attributable to the 3MLCT excited state, populated due to 1MLCT → 3MLCT transition.73,53 The intersystem crossing process (t1) occurs in the time range shorter than the instrument response, while the lifetimes t2 and t3 determined from global fit analysis (Figure 8) can be assigned to vibrational/structural relaxation of the lowest triplet state 3MLCT and ground-state recovery, respectively.
Following photoexcitation of 2 at 355 nm (Figure 9), an instant spectrally unstructured ESA band in the visible region is observed. With reference to the model complex 4, it can be assigned to the hot 3MLCT excited state. Within 390–675 ps, this component undergoes vibrational cooling, which is manifested by a blue-shift of the signal. With increasing delay time, the ESA evolves into the band with three discernible maxima at 420, 516, and 556 nm. To a small extent, the ESA spectral shape may be slightly distorted at 400–520 nm due to the possible overlapping with the stimulated fluorescence occurring as a result of incomplete energy transfer from 1IL to 1MLCT (Figure S30). Undoubtedly, however, the TA spectral features of 2 are supportive of the 3MLCT–3ILpyrene equilibrium. The intense absorption with maxima at 516 and 556 nm is attributable to the 3ILpyrene excited state, while the higher energy maximum at 420 nm represents 3MLCT.72,74,75 The triplet excited state on the pyrene chromophore is populated due to the triplet–triplet energy transfer (TTET) from the initially formed 3MLCT state. Both 3MLCT and 3ILpyrene excited states are present up to the end of the delay stage. Regarding the spectral features, the photophysical processes occurring upon photoexcitation of 2 may be represented by the following scheme 1MLCT → 3MLCT ↔ 3ILpyrene, followed by the ground-state recovery.
With the use of the global analysis, the fs-data of 2 were the best fit with four decay associated species (DASi) characterized by time constants ti. The ultrafast intersystem crossing 1MLCT → 3MLCT (t1) was not resolved. It occurs in a time range shorter than the instrument response. The component with time constant of 1.64 ps (DAS2) was assigned to the vibrational cooling of the 3MLCT excited state. The decay-associated species with a time constant of 65.94 ps (DAS3) represents the formation of the 3ILpyrene excited state, which is followed by vibrational/structural relaxation of this state (DAS4 with t4 = 597.35 ps). With reference to previous reports,72,74,75 structural changes in the molecular geometry of 2 in the electronically triplet excited state mainly concern the pyrene-imphen torsion angle. Its decrease in the triplet excited state facilitates the population of the 3ILpyrene excited state via the TTET mechanism.
For complex 1 excited at 355 nm, the spectral features at longer time delays are also indicative of the presence of both 3MLCT and 3ILpyrene. In relation to 2, however, the higher energy shoulder corresponding to the 3MLCT excited state shows a noticeable red-shift, and is largely overlapped with components which represent the T1 → Tn transitions of the pyrene unit (maxima at 515 and 558 nm). A bathochromic shift is also visible for partly observed ground-state bleaching (GSB) of 1 in relation to 2, which correlates well with UV–vis results. The stimulated fluorescence may slightly distort the ESA spectral shape in the range of 405–550 nm (Figures S29 and S30).
The most striking difference between 1 and 2 is noticeable in the region >600 nm, where complex 1 shows a more intense absorption contrary to 2. With reference to TA spectra of the free ligands (Figure S32), this component most likely represents the population of the ILCT (pyrene → imphen) excited state. Therefore, taking into consideration the temporal evolution of transient absorption spectra and spectral profiles of decay-associated species of 1, it can be assumed that the more planar geometry of pyr-imphen facilitates the population of the 3ILpyrene excited state and leads to the formation of 3ILpyrene via two paths 1MLCT → 3MLCT → 3ILpyrene and 1ILCT → 3ILpyrene/3ILCT. The lifetime t2 is attributed to the formation of the 3ILpyrene excited state via channel 1MLCT → 3MLCT → 3ILpyrene, while 1ILCT → 3ILpyrene/3ILCT is represented by DAS3 (t2). In analogy to 2 and parent complexes, ISC (1MLCT → 3MLCT) occurs in the time range shorter than the instrument response. The decay-associated species with a time constant of 379.8 ps (DAS4) represents the vibrational/structural relaxation of 3ILpyrene, while the component with infinite lifetime (DAS5) corresponds to the ground-state recovery.
Upon excitation at 420 nm, complexes 1 and 2 show fs-TA spectra, which are qualitatively similar to those for photoexcitation at 355 nm, and only negligible differences can be noticed in estimated time constants ti. The complete fs-TA data for pyrenyl-substituted Re(I) complexes are demonstrated in Figures S29 and S30.
Singlet Oxygen Generation
The efficiency of Re(I) complexes in the generation of singlet oxygen was evaluated by an indirect method using [Ru(bipy)3](PF6)2 as the reference standard and 1,3-diphenylisobenzofuran (DPBF) as the singlet oxygen scavenger. The latter one is highly reactive toward 1O2, forming endoperoxide, which spontaneously decomposes to 1,2-dibenzoylbenzene.76−79 The progress of photo-oxidation of DPBF in the presence of Re(I) complexes and [Ru(bipy)3](PF6)2 was monitored by the gradual decrease in the absorption of DPBF at 417 nm (Figure S33) and quantitatively compared by plotting A/A0 against the irradiation time (Figure 10).
Figure 10.
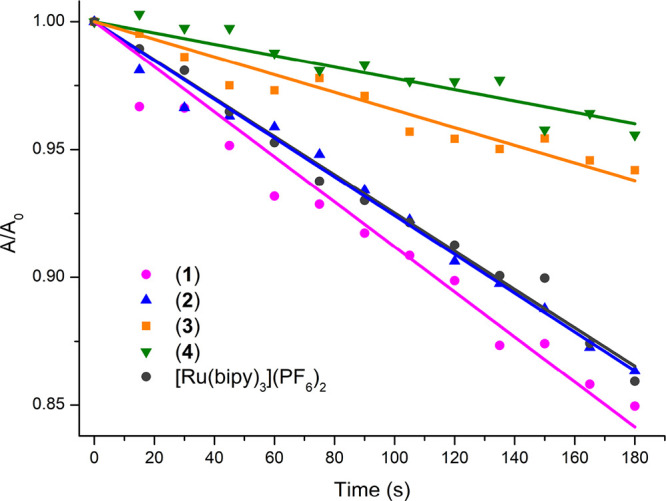
Relative changes in the absorbance of DPBF at 417 nm (A/A0) against the irradiation time in the presence of the studied metal complex, where A0 stands for absorbance at t = 0 s, and A stands for absorbance after consecutive irradiation times.
The obtained 1O2 quantum yield ΦΔ values for these complexes follow the order 1 (82.6%) > 2 (68.8.1%) > 3 (30.7%) > 4 (18.9%), which is consistent with the trend observed for triplet excited-state lifetimes and visible absorptivity for these systems. Both pyrenyl-substituted Re(I) complexes (1 and 2) show remarkably enhanced singlet oxygen-sensitizing abilities compared to model chromophores (3 and 4). With the singlet oxygen quantum yield of ∼80%, complex 1 belongs to a family of the most efficient 1O2 photosensitizers.80
Triplet–Triplet Annihilation Upconversion (TTA UC)
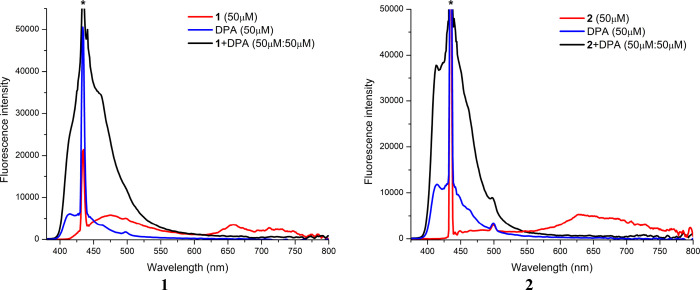
The TTA UC studies were performed upon photoexcitation at 435 nm, with the use of 1,10-diphenylanthracene (DPA) as the triplet acceptor (Figure 11 and Figures S34 and S35). The energy of the lowest triplet excited state of DPA is 1.77 eV. In the presence of DPA, due to the triplet–triplet energy transfer (TTET) between the photosensitizer and the annihilator, the phosphorescence of pyrenyl-substituted complexes was quenched, and lifetimes of the unconverted emission were found to be extremely longer (146 μs for 1 and 172 μs for 2) than the prompt fluorescence lifetime of DPA (6.50 ns), supporting the delayed fluorescence sensitized by pyrenyl-substituted complexes (Figure S35). For reference complexes, in agreement with their short phosphorescence lifetimes, no upconverted emission was observed (Figure S34).
Figure 11.
Emission spectra displaying TTA upconversion of 9,10-diphenylanthracene (DPA) in the presence of complexes 1 and 2. The asterisk denotes an excitation wavelength of 435 nm.
To estimate the efficiency of TTET processes in pyrenyl-substituted complexes, the Stern–Volmer photoluminescence intensity quenching experiments were performed for 1 and 2 with DPA in deaerated DMSO. The obtained quenching constants (KSV), 1.12 × 107 for 1 and 9.88 × 105 M–1 for 2, support a higher efficiency of TTEF for 1, in agreement with its stronger visible absorptivity and more extended triplet excited-state lifetime in relation to complex 2.
Conclusions
Using the bichromophore strategy, two long-lived Re(I)-based triplet emitters able to perform triplet–triplet energy transfer were obtained and thoroughly explored with the aid of static and time-resolved spectroscopic methods, including transient absorption. We demonstrated that coordination of 2-(1-pyrenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (pyr-imphen) and 1-(4-(methyl)phenyl)-2(1-pyrenyl)-imidazo[4,5-f][1,10]phenanthroline (pyr-tol-imphen) to {ReCl(CO)3} resulted in formation of Re(I) tricarbonyl complexes with record-high triplet excited-state lifetimes in solution at room temperature. Additionally, we evidenced the possibility of tuning photoinduced processes and triplet–triplet energy processes in the bichromophore metal complexes with imidazophenanthroline-based ligands by the steric hindrance of substituents introduced into the imidazole ring at the H1-position. On the basis of steady-state and time-resolved spectroscopic data, the differences in the photophysical behavior of 1 and 2 were rationalized by the contribution of intraligand charge-transfer (ILCT) transitions originating from charge delocalization from the pyrenyl group to the imphen acceptor framework (1ILCT and 3ILCT), dominant in the case of the complex with stronger electronic coupling between the pyrenyl and imphen moieties (1) but almost absent for 2. Although slightly better functional parameters were achieved for [ReCl(CO)3(pyr-imphen)] (1), an important advantage of the introduction of the 4-(methyl)phenyl substituent into the imidazole ring at the H1-position was a significant increase in the solubility of the resulting Re(I) complex [ReCl(CO)3(pyr-tol-imphen)] (2), beneficial regarding potential photochemical applications.
The usefulness of designed compounds as triplet photosensitizers was verified by an examination of their abilities for 1O2 generation and triplet–triplet annihilation upconversion. The results presented herein provide an in-depth understanding of the impact of the mutual chromophore orientation on photoinduced processes in bichromophore systems. It can be expected that they will be crucial in view of the development of new efficient photosensitizers for modern technologies, especially that imidazophenanthroline-based ligands are known to be excellent versatile ligands to control the photophysical and antiproliferative behavior of metal complexes.
Experimental Section
The ligands81−84 and Re(I) complexes53 were prepared employing methods reported previously. The analytical, structural, and spectroscopic data for obtained compounds are included in the Supporting Information.
Elemental analyses were performed with the use of a Vario EL Cube (Elementar) for C, H, and N contents. IR spectra were recorded using a Nicolet iS5 FTIR spectrophotometer (4000–400 cm–1) with the use of the KBr pellet method. NMR spectra were registered on a Bruker Avance 500 NMR spectrometer in DMSO-d6 or CDCl3. X-ray diffraction data were collected at room temperature using a Gemini A Ultra diffractometer (Oxford Diffraction) with MoKα radiation (λ = 0.71073 Å). Crystallographic data for 2–4 were deposited with the Cambridge Crystallographic Data Center, CCDC 2279356–2279359. Theoretical calculations were performed using the GAUSSIAN-16 program package85 at the DFT or TD-DFT level with the PBE086,87 functional, def2-TZVPD basis set for rhenium and def2-TZVP basis set for other elements,88−90 and use of the polarizable continuum model (PCM).91−93 UV–vis absorption spectra were measured using an Evolution 220 (ThermoScientific) UV–vis spectrometer in DMSO solutions. Emission spectra as well as the time-resolved measurements of argon-saturated samples in DMSO solutions or ethanol:methanol 77 K frozen matrix (4:1 v/v) were measured on a FLS-980 fluorescence spectrophotometer (Edinburgh Instruments). The fs-TA spectra were measured using a pump–probe transient absorption spectroscopy system (Ultrafast Systems, Helios) described previously.37,70 Singlet oxygen generation (1O2) efficiency was determined using the Evolution 220 UV–vis spectrometer in DMSO with 1,3-diphenylisobenzofuran (DPBF) as a sensitizer. Triplet–triplet annihilation upconversion experiments were performed on the FLS-980 fluorescence spectrophotometer with 9,10-diphenylanthracene (DPA) as a triplet acceptor. Detailed synthesis, characterization methods, and extended experimental description are provided in the Supporting Information.
Acknowledgments
This work was cofinanced by the funds granted under the Research Excellence Initiative of the University of Silesia in Katowice. The calculations were carried out in Wroclaw Centre for Networking and Supercomputing (http://www.wcss.wroc.pl).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c02662.
Additional experimental details, synthetic and experimental data, IR, NMR, single-crystal X-ray analysis, DFT calculations, UV–vis, emission spectroscopy, femtosecond transient absorption, singlet oxygen generation, and triplet–triplet annihilation upconversion data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. B.M., K.C., and E.M. conceptualized, designed, and supervised the research. B.M. and K.C. performed the synthesis of Re(I) complexes and X-ray single-crystal analysis. M.P. carried out the synthesis of organic ligands and NMR analysis. K.C. performed the steady-state spectroscopic measurements, singlet oxygen generation measurements, and theoretical calculations. K.C. and J.P.-G. performed the time-resolved spectroscopic measurements, TTA upconversion measurements, and prepared the Supporting Information file. B.M. and K.C. wrote the manuscript with contributions from the other authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Glaser F.; Wenger O. S. Recent Progress in the Development of Transition-Metal Based Photoredox Catalysts. Coord. Chem. Rev. 2020, 405, 213129 10.1016/j.ccr.2019.213129. [DOI] [Google Scholar]
- Zhao X.; Hou Y.; Liu L.; Zhao J. Triplet Photosensitizers Showing Strong Absorption of Visible Light and Long-Lived Triplet Excited States and Application in Photocatalysis: A Mini Review. Energy Fuels 2021, 35 (23), 18942–18956. 10.1021/acs.energyfuels.1c02130. [DOI] [Google Scholar]
- Nandal N.; Jain S. L. A Review on Progress and Perspective of Molecular Catalysis in Photoelectrochemical Reduction of CO2. Coord. Chem. Rev. 2022, 451, 214271 10.1016/j.ccr.2021.214271. [DOI] [Google Scholar]
- Mawrie L.; Rahman F.; Ali Md. A.; Gazi S. Recent Progress in Homogeneous Molecular Photoredox Catalysis towards Hydrogen Evolution Reaction and Future Perspective. Applied Catalysis A: General 2023, 651, 119010 10.1016/j.apcata.2022.119010. [DOI] [Google Scholar]
- Qin Y.; She P.; Huang X.; Huang W.; Zhao Q. Luminescent Manganese(II) Complexes: Synthesis, Properties and Optoelectronic Applications. Coord. Chem. Rev. 2020, 416, 213331 10.1016/j.ccr.2020.213331. [DOI] [Google Scholar]
- Mao H.-T.; Li G.-F.; Shan G.-G.; Wang X.-L.; Su Z.-M. Recent Progress in Phosphorescent Ir(III) Complexes for Nondoped Organic Light-Emitting Diodes. Coord. Chem. Rev. 2020, 413, 213283 10.1016/j.ccr.2020.213283. [DOI] [Google Scholar]
- Yam V. W.-W.; Law A. S.-Y. Luminescent D8Metal Complexes of Platinum(II) and Gold(III): From Photophysics to Photofunctional Materials and Probes. Coord. Chem. Rev. 2020, 414, 213298 10.1016/j.ccr.2020.213298. [DOI] [Google Scholar]
- Tang M.-C.; Chan M.-Y.; Yam V. W.-W. Molecular Design of Luminescent Gold(III) Emitters as Thermally Evaporable and Solution-Processable Organic Light-Emitting Device (OLED) Materials. Chem. Rev. 2021, 121 (13), 7249–7279. 10.1021/acs.chemrev.0c00936. [DOI] [PubMed] [Google Scholar]
- Jayabharathi J.; Thanikachalam V.; Thilagavathy S. Phosphorescent Organic Light-Emitting Devices: Iridium Based Emitter Materials – An Overview. Coord. Chem. Rev. 2023, 483, 215100 10.1016/j.ccr.2023.215100. [DOI] [Google Scholar]
- Mara M. W.; Fransted K. A.; Chen L. X. Interplays of Excited State Structures and Dynamics in Copper(I) Diimine Complexes: Implications and Perspectives. Coord. Chem. Rev. 2015, 282–283, 2–18. 10.1016/j.ccr.2014.06.013. [DOI] [Google Scholar]
- Liu Y.; Yiu S.-C.; Ho C.-L.; Wong W.-Y. Recent Advances in Copper Complexes for Electrical/Light Energy Conversion. Coord. Chem. Rev. 2018, 375, 514–557. 10.1016/j.ccr.2018.05.010. [DOI] [Google Scholar]
- Kumar A.; Kumar Vashistha V.; Kumar Das D. Recent Development on Metal Phthalocyanines Based Materials for Energy Conversion and Storage Applications. Coord. Chem. Rev. 2021, 431, 213678 10.1016/j.ccr.2020.213678. [DOI] [Google Scholar]
- Nguyen T. N.; Ebrahim F. M.; Stylianou K. C. Photoluminescent, Upconversion Luminescent and Nonlinear Optical Metal-Organic Frameworks: From Fundamental Photophysics to Potential Applications. Coord. Chem. Rev. 2018, 377, 259–306. 10.1016/j.ccr.2018.08.024. [DOI] [Google Scholar]
- Shaikh S.; Wang Y.; ur Rehman F.; Jiang H.; Wang X. Phosphorescent Ir (III) Complexes as Cellular Staining Agents for Biomedical Molecular Imaging. Coord. Chem. Rev. 2020, 416, 213344 10.1016/j.ccr.2020.213344. [DOI] [Google Scholar]
- Zhou J.; Li J.; Zhang K. Y.; Liu S.; Zhao Q. Phosphorescent Iridium(III) Complexes as Lifetime-Based Biological Sensors for Photoluminescence Lifetime Imaging Microscopy. Coord. Chem. Rev. 2022, 453, 214334 10.1016/j.ccr.2021.214334. [DOI] [Google Scholar]
- Kar B.; Das U.; Roy N.; Paira P. Recent Advances on Organelle Specific Ru(II)/Ir(III)/Re(I) Based Complexes for Photodynamic Therapy. Coord. Chem. Rev. 2023, 474, 214860 10.1016/j.ccr.2022.214860. [DOI] [Google Scholar]
- Zhao J.; Wu W.; Sun J.; Guo S. Triplet Photosensitizers: From Molecular Design to Applications. Chem. Soc. Rev. 2013, 42 (12), 5323–5351. 10.1039/c3cs35531d. [DOI] [PubMed] [Google Scholar]
- Zhang K. Y.; Yu Q.; Wei H.; Liu S.; Zhao Q.; Huang W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118 (4), 1770–1839. 10.1021/acs.chemrev.7b00425. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Hou Y.; Xiao X.; Chen X.; Hu M.; Geng X.; Wang Z.; Zhao J. Recent Development of the Transition Metal Complexes Showing Strong Absorption of Visible Light and Long-Lived Triplet Excited State: From Molecular Structure Design to Photophysical Properties and Applications. Coord. Chem. Rev. 2020, 417, 213371 10.1016/j.ccr.2020.213371. [DOI] [Google Scholar]
- Ankathatti Munegowda M.; Manalac A.; Weersink M.; McFarland S. A.; Lilge L. Ru(II) Containing Photosensitizers for Photodynamic Therapy: A Critique on Reporting and an Attempt to Compare Efficacy. Coord. Chem. Rev. 2022, 470, 214712 10.1016/j.ccr.2022.214712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T. C.; Nguyen V.-N.; Choi Y.; Lee S.; Yoon J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121 (21), 13454–13619. 10.1021/acs.chemrev.1c00381. [DOI] [PubMed] [Google Scholar]
- McKenzie L. K.; Bryant H. E.; Weinstein J. A. Transition Metal Complexes as Photosensitisers in One- and Two-Photon Photodynamic Therapy. Coord. Chem. Rev. 2019, 379, 2–29. 10.1016/j.ccr.2018.03.020. [DOI] [Google Scholar]
- Brabec V.; Kasparkova J. Ruthenium Coordination Compounds of Biological and Biomedical Significance. DNA Binding Agents. Coord. Chem. Rev. 2018, 376, 75–94. 10.1016/j.ccr.2018.07.012. [DOI] [Google Scholar]
- Peng K.; Liang B.-B.; Liu W.; Mao Z.-W. What Blocks More Anticancer Platinum Complexes from Experiment to Clinic: Major Problems and Potential Strategies from Drug Design Perspectives. Coord. Chem. Rev. 2021, 449, 214210 10.1016/j.ccr.2021.214210. [DOI] [Google Scholar]
- Yousuf I.; Bashir M.; Arjmand F.; Tabassum S. Advancement of Metal Compounds as Therapeutic and Diagnostic Metallodrugs: Current Frontiers and Future Perspectives. Coord. Chem. Rev. 2021, 445, 214104 10.1016/j.ccr.2021.214104. [DOI] [Google Scholar]
- Pete S.; Roy N.; Kar B.; Paira P. Construction of Homo and Heteronuclear Ru(II), Ir(III) and Re(I) Complexes for Target Specific Cancer Therapy. Coord. Chem. Rev. 2022, 460, 214462 10.1016/j.ccr.2022.214462. [DOI] [Google Scholar]
- Ford W. E.; Rodgers M. A. J. Reversible Triplet-Triplet Energy Transfer within a Covalently Linked Bichromophoric Molecule. J. Phys. Chem. 1992, 96 (7), 2917–2920. 10.1021/j100186a026. [DOI] [Google Scholar]
- Yarnell J. E.; Deaton J. C.; McCusker C. E.; Castellano F. N. Bidirectional “Ping-Pong” Energy Transfer and 3000-Fold Lifetime Enhancement in a Re(I) Charge Transfer Complex. Inorg. Chem. 2011, 50 (16), 7820–7830. 10.1021/ic200974h. [DOI] [PubMed] [Google Scholar]
- Yarnell J. E.; Wells K. A.; Palmer J. R.; Breaux J. M.; Castellano F. N. Excited-State Triplet Equilibria in a Series of Re(I)-Naphthalimide Bichromophores. J. Phys. Chem. B 2019, 123 (35), 7611–7627. 10.1021/acs.jpcb.9b05688. [DOI] [PubMed] [Google Scholar]
- Wells K. A.; Yarnell J. E.; Palmer J. R.; Lee T. S.; Papa C. M.; Castellano F. N. Energy Migration Processes in Re(I) MLCT Complexes Featuring a Chromophoric Ancillary Ligand. Inorg. Chem. 2020, 59 (12), 8259–8271. 10.1021/acs.inorgchem.0c00644. [DOI] [PubMed] [Google Scholar]
- Medlycott E. A.; Hanan G. S. Designing Tridentate Ligands for Ruthenium(II) Complexes with Prolonged Room Temperature Luminescence Lifetimes. Chem. Soc. Rev. 2005, 34 (2), 133–142. 10.1039/b316486c. [DOI] [PubMed] [Google Scholar]
- Wang J.; Fang Y.; Bourget-Merle L.; Polson M. I. J.; Hanan G. S.; Juris A.; Loiseau F.; Campagna S. The Multichromophore Approach: Prolonged Room-Temperature Luminescence Lifetimes in RuII Complexes Based on Tridentate Polypyridine Ligands. Chem. - Eur. J. 2006, 12 (33), 8539–8548. 10.1002/chem.200600245. [DOI] [PubMed] [Google Scholar]
- Lincoln R.; Kohler L.; Monro S.; Yin H.; Stephenson M.; Zong R.; Chouai A.; Dorsey C.; Hennigar R.; Thummel R. P.; McFarland S. A. Exploitation of Long-Lived 3IL Excited States for Metal–Organic Photodynamic Therapy: Verification in a Metastatic Melanoma Model. J. Am. Chem. Soc. 2013, 135 (45), 17161–17175. 10.1021/ja408426z. [DOI] [PubMed] [Google Scholar]
- Deng F.; Lazorski M. S.; Castellano F. N. Photon Upconversion Sensitized by a Ru(II)-Pyrenyl Chromophore. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2015, 373 (2044), 20140322. 10.1098/rsta.2014.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X.; Zhao J.; Mohmood Z.; Zhang C. Accessing the Long-Lived Triplet Excited States in Transition-Metal Complexes: Molecular Design Rationales and Applications. Chem. Rec. 2016, 16 (1), 173–188. 10.1002/tcr.201500237. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Wei M.; Wang X.; Jiang L.; Xiong Y.; Cheng J.; Tan Y.; Liao X.; Wang J. Synthesis and Antibacterial against Staphylococcus Aureus of New Ruthenium (II) Polypyridine Complexes Containing Pyrene Groups. Appl. Organomet. Chem. 2022, 36 (11), e6858 10.1002/aoc.6858. [DOI] [Google Scholar]
- Szlapa-Kula A.; Małecka M.; Maroń A. M.; Janeczek H.; Siwy M.; Schab-Balcerzak E.; Szalkowski M.; Maćkowski S.; Pedzinski T.; Erfurt K.; Machura B. In-Depth Studies of Ground- and Excited-State Properties of Re(I) Carbonyl Complexes Bearing 2,2′:6′,2″-Terpyridine and 2,6-Bis(Pyrazin-2-Yl)Pyridine Coupled with π-Conjugated Aryl Chromophores. Inorg. Chem. 2021, 60 (24), 18726–18738. 10.1021/acs.inorgchem.1c02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Singh S.; Kumar A.; Murthy K. S. R.; Kumar Singh A. PH-Responsive Luminescence Sensing, Photoredox Catalysis and Photodynamic Applications of Ruthenium(II) Photosensitizers Bearing Imidazo[4,5-f][1,10]Phenanthroline Scaffolds. Coord. Chem. Rev. 2022, 452, 214272 10.1016/j.ccr.2021.214272. [DOI] [Google Scholar]
- Naithani S.; Goswami T.; Thetiot F.; Kumar S. Imidazo[4,5-f][1,10]Phenanthroline Based Luminescent Probes for Anion Recognition: Recent Achievements and Challenges. Coord. Chem. Rev. 2023, 475, 214894 10.1016/j.ccr.2022.214894. [DOI] [Google Scholar]
- Liu C.; Li J.; Li B.; Hong Z.; Zhao F.; Liu S.; Li W. Triphenylamine-Functionalized Rhenium (I) Complex as a Highly Efficient Yellow-Green Emitter in Electrophosphorescent Devices. Appl. Phys. Lett. 2006, 89 (24), 243511. 10.1063/1.2408638. [DOI] [Google Scholar]
- Liu C. B.; Li J.; Li B.; Hong Z. R.; Zhao F. F.; Liu S. Y.; Li W. L. A Multicomponent Rhenium-Based Triplet Emitter for Organic Electroluminescence. Chem. Phys. Lett. 2007, 435 (1), 54–58. 10.1016/j.cplett.2006.12.044. [DOI] [Google Scholar]
- Liu Y.-J.; Wang K.-Z. Visible-Light-Excited Singlet-Oxygen Luminescence Probe Based on Re(CO)3Cl(Aeip). Eur. J. Inorg. Chem. 2008, 2008 (33), 5214–5219. 10.1002/ejic.200800699. [DOI] [Google Scholar]
- Si Z.; Li X.; Li X.; Xu G.; Pan C.; Guo Z.; Zhang H.; Zhou L. Novel Re(I) Dendrimers: Synthesis, Characterization and Theoretical Studies. Dalton Trans. 2009, 47, 10592–10600. 10.1039/b912939a. [DOI] [PubMed] [Google Scholar]
- Yi X.; Zhao J.; Wu W.; Huang D.; Ji S.; Sun J. Rhenium(I) Tricarbonyl Polypyridine Complexes Showing Strong Absorption of Visible Light and Long-Lived Triplet Excited States as a Triplet Photosensitizer for Triplet–Triplet Annihilation Upconversion. Dalton Trans. 2012, 41 (29), 8931–8940. 10.1039/c2dt30804e. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Wang J.; Wang Y. A Rhenium(I) Complex with Indolyl-Containing Ligand: Synthesis, Photophysical Properties and Theoretical Studies. Inorg. Chim. Acta 2012, 387, 100–105. 10.1016/j.ica.2012.01.001. [DOI] [Google Scholar]
- Zheng Z.-B.; Wu Y.-Q.; Wang K.-Z.; Li F. PH Luminescence Switching, Dihydrogen Phosphate Sensing, and Cellular Uptake of a Heterobimetallic Ruthenium(II)–Rhenium(I) Complex. Dalton Trans. 2014, 43 (8), 3273–3284. 10.1039/C3DT52568F. [DOI] [PubMed] [Google Scholar]
- Chen C.; Xu Y.; Wan Y.; Fan W.; Si Z. Aggregation-Induced Phosphorescent Emission from ReI Complexes: Synthesis and Property Studies. Eur. J. Inorg. Chem. 2016, 2016 (9), 1340–1347. 10.1002/ejic.201600005. [DOI] [Google Scholar]
- Bonello R. O.; Pitak M. B.; Coles S. J.; Hallett A. J.; Fallis I. A.; Pope S. J. A. Synthesis and Characterisation of Phosphorescent Rhenium(I) Complexes of Hydroxy- and Methoxy-Substituted Imidazo[4,5-f]-1,10-Phenanthroline Ligands. J. Organomet. Chem. 2017, 841, 39–47. 10.1016/j.jorganchem.2017.04.021. [DOI] [Google Scholar]
- Li X.; Zhao G.-W.; Hu Y.-X.; Zhao J.-H.; Dong Y.; Zhou L.; Lv Y.-L.; Chi H.-J.; Su Z. Rational Design and Characterization of Novel Phosphorescent Rhenium(I) Complexes for Extremely High-Efficiency Organic Light-Emitting Diodes. J. Mater. Chem. C 2017, 5 (30), 7629–7636. 10.1039/C7TC02466E. [DOI] [Google Scholar]
- Li X.; Wang Z.; Wei Q.; Wang Z.; Si Z.; Duan Q. Phosphorescent Self-Healing Composites Containing Re(I) Complexes: Preparation and Properties. J. Coord. Chem. 2019, 72 (22–24), 3645–3656. 10.1080/00958972.2019.1700502. [DOI] [Google Scholar]
- Slikboer S. R.; Pitchumony T. S.; Banevicius L.; Mercanti N.; Edem P. E.; Valliant J. F. Imidazole Fused Phenanthroline (PIP) Ligands for the Preparation of Multimodal Re(I) and 99mTc(I) Probes. Dalton Trans. 2020, 49 (42), 14826–14836. 10.1039/D0DT02829K. [DOI] [PubMed] [Google Scholar]
- He S.-F.; Pan N.-L.; Chen B.-B.; Liao J.-X.; Huang M.; Qiu H.-J.; Jiang D.-C.; Wang J.-J.; Chen J.-X.; Sun J. Mitochondria-Targeted Re(I) Complexes Bearing Guanidinium as Ligands and Their Anticancer Activity. J. Biol. Inorg. Chem. 2020, 25 (8), 1107–1116. 10.1007/s00775-020-01827-7. [DOI] [PubMed] [Google Scholar]
- Szłapa-Kula A.; Palion-Gazda J.; Ledwon P.; Erfurt K.; Machura B. A Fundamental Role of the Solvent Polarity and Remote Substitution of the 2-(4-R-Phenyl)-1H-Imidazo[4,5-f][1,10]Phenanthroline Framework in Controlling the Ground- and Excited-State Properties of Re(I) Chromophores [ReCl(CO)3(R-C6H4-Imphen)]. Dalton Trans. 2022, 51 (38), 14466–14481. 10.1039/D2DT02439J. [DOI] [PubMed] [Google Scholar]
- Wrighton M.; Morse D. L. Nature of the Lowest Excited State in Tricarbonylchloro-1,10-Phenanthrolinerhenium(I) and Related Complexes. J. Am. Chem. Soc. 1974, 96 (4), 998–1003. 10.1021/ja00811a008. [DOI] [Google Scholar]
- Lees A. J. Luminescence Properties of Organometallic Complexes. Chem. Rev. 1987, 87 (4), 711–743. 10.1021/cr00080a003. [DOI] [Google Scholar]
- Lees A. J. The Luminescence Rigidochromic Effect Exhibited by Organometallic Complexes: Rationale and Applications. Comments Inorg. Chem. 1995, 17 (6), 319–346. 10.1080/02603599508032711. [DOI] [Google Scholar]
- Tyson D. S.; Luman C. R.; Zhou X.; Castellano F. N. New Ru(II) Chromophores with Extended Excited-State Lifetimes. Inorg. Chem. 2001, 40 (16), 4063–4071. 10.1021/ic010287g. [DOI] [PubMed] [Google Scholar]
- Yarnell J. E.; McCusker C. E.; Leeds A. J.; Breaux J. M.; Castellano F. N. Exposing the Excited-State Equilibrium in an IrIII Bichromophore: A Combined Time Resolved Spectroscopy and Computational Study. Eur. J. Inorg. Chem. 2016, 2016 (12), 1808–1818. 10.1002/ejic.201600194. [DOI] [Google Scholar]
- Yarnell J. E.; Chakraborty A.; Myahkostupov M.; Wright K. M.; Castellano F. N. Long-Lived Triplet Excited State in a Platinum(II) Perylene Monoimide Complex. Dalton Trans. 2018, 47 (42), 15071–15081. 10.1039/C8DT02496K. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R.Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: US, 2006. 10.1007/978-0-387-46312-4. [DOI] [Google Scholar]
- Jalihal A.; Le T.; Macchi S.; Krehbiel H.; Bashiru M.; Forson M.; Siraj N. Understanding of Förster Resonance Energy Transfer (FRET) in Ionic Materials. Sustainable Chemistry 2021, 2 (4), 564–575. 10.3390/suschem2040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.; Wu W.; Wu W.; Song P.; Han K.; Wang Z.; Liu S.; Guo H.; Zhao J. Tuning the Luminescence Lifetimes of Ruthenium(II) Polypyridine Complexes and Its Application in Luminescent Oxygen Sensing. J. Mater. Chem. 2010, 20 (10), 1953–1963. 10.1039/b916468e. [DOI] [Google Scholar]
- Guo H.; Ji S.; Wu W.; Wu W.; Shao J.; Zhao J. Long-Lived Emissive Intra-Ligand Triplet Excited States (3 IL): Next Generation Luminescent Oxygen Sensing Scheme and a Case Study with Red Phosphorescent Diimine Pt(Ii) Bis(Acetylide) Complexes Containing Ethynylated Naphthalimide or Pyrene Subunits. Analyst 2010, 135 (11), 2832–2840. 10.1039/c0an00404a. [DOI] [PubMed] [Google Scholar]
- Wu W.; Sun J.; Ji S.; Wu W.; Zhao J.; Guo H. Tuning the Emissive Triplet Excited States of Platinum(II) Schiff Base Complexes with Pyrene, and Application for Luminescent Oxygen Sensing and Triplet–Triplet-Annihilation Based Upconversions. Dalton Trans. 2011, 40 (43), 11550–11561. 10.1039/c1dt11001b. [DOI] [PubMed] [Google Scholar]
- Li Q.; Guo H.; Ma L.; Wu W.; Liu Y.; Zhao J. Tuning the Photophysical Properties of N ^ N Pt(Ii) Bisacetylide Complexes with Fluorene Moiety and Its Applications for Triplet–Triplet-Annihilation Based Upconversion. J. Mater. Chem. 2012, 22 (12), 5319–5329. 10.1039/c2jm15678d. [DOI] [Google Scholar]
- Wu W.; Wu W.; Ji S.; Guo H.; Zhao J. Observation of Room-Temperature Deep-Red/Near-IR Phosphorescence of Pyrene with Cycloplatinated Complexes: An Experimental and Theoretical Study. Eur. J. Inorg. Chem. 2010, 2010 (28), 4470–4482. 10.1002/ejic.201000488. [DOI] [Google Scholar]
- Pomestchenko I. E.; Luman C. R.; Hissler M.; Ziessel R.; Castellano F. N. Room Temperature Phosphorescence from a Platinum(II) Diimine Bis(Pyrenylacetylide) Complex. Inorg. Chem. 2003, 42 (5), 1394–1396. 10.1021/ic0261631. [DOI] [PubMed] [Google Scholar]
- Kozlov D. V.; Tyson D. S.; Goze C.; Ziessel R.; Castellano F. N. Room Temperature Phosphorescence from Ruthenium(II) Complexes Bearing Conjugated Pyrenylethynylene Subunits. Inorg. Chem. 2004, 43 (19), 6083–6092. 10.1021/ic049288+. [DOI] [PubMed] [Google Scholar]
- Edkins R. M.; Fucke K.; Peach M. J. G.; Crawford A. G.; Marder T. B.; Beeby A. Syntheses, Structures, and Comparison of the Photophysical Properties of Cyclometalated Iridium Complexes Containing the Isomeric 1- and 2-(2′-Pyridyl)Pyrene Ligands. Inorg. Chem. 2013, 52 (17), 9842–9860. 10.1021/ic400819f. [DOI] [PubMed] [Google Scholar]
- Małecka M.; Szlapa-Kula A.; Maroń A. M.; Ledwon P.; Siwy M.; Schab-Balcerzak E.; Sulowska K.; Maćkowski S.; Erfurt K.; Machura B. Impact of the Anthryl Linking Mode on the Photophysics and Excited-State Dynamics of Re(I) Complexes [ReCl(CO)3(4′-An-Terpy-Κ2N)]. Inorg. Chem. 2022, 61 (38), 15070–15084. 10.1021/acs.inorgchem.2c02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. A.; Kassab R. M.; Haneline M. R.; Elbjeirami O.; Gabbaï F. P. Enhancement of the Phosphorescence of Organic Luminophores upon Interaction with a Mercury Trifunctional Lewis Acid. Inorg. Chem. 2003, 42 (7), 2176–2178. 10.1021/ic034066h. [DOI] [PubMed] [Google Scholar]
- Reichardt C.; Pinto M.; Wächtler M.; Stephenson M.; Kupfer S.; Sainuddin T.; Guthmuller J.; McFarland S. A.; Dietzek B. Photophysics of Ru(II) Dyads Derived from Pyrenyl-Substitued Imidazo[4,5-f][1,10]Phenanthroline Ligands. J. Phys. Chem. A 2015, 119 (17), 3986–3994. 10.1021/acs.jpca.5b01737. [DOI] [PubMed] [Google Scholar]
- El Nahhas A.; Consani C.; Blanco-Rodríguez A. M.; Lancaster K. M.; Braem O.; Cannizzo A.; Towrie M.; Clark I. P.; Záliš S.; Chergui M.; Vlček A. Ultrafast Excited-State Dynamics of Rhenium(I) Photosensitizers [Re(Cl)(CO)3(N,N)] and [Re(Imidazole)(CO)3(N,N)]+: Diimine Effects. Inorg. Chem. 2011, 50 (7), 2932–2943. 10.1021/ic102324p. [DOI] [PubMed] [Google Scholar]
- Reichardt C.; Schneider K. R. A.; Sainuddin T.; Wächtler M.; McFarland S. A.; Dietzek B. Excited State Dynamics of a Photobiologically Active Ru(II) Dyad Are Altered in Biologically Relevant Environments. J. Phys. Chem. A 2017, 121 (30), 5635–5644. 10.1021/acs.jpca.7b04670. [DOI] [PubMed] [Google Scholar]
- Reichardt C.; Sainuddin T.; Wächtler M.; Monro S.; Kupfer S.; Guthmuller J.; Gräfe S.; McFarland S.; Dietzek B. Influence of Protonation State on the Excited State Dynamics of a Photobiologically Active Ru(II) Dyad. J. Phys. Chem. A 2016, 120 (32), 6379–6388. 10.1021/acs.jpca.6b05957. [DOI] [PubMed] [Google Scholar]
- Sanasam B.; Raza M. K.; Musib D.; Pal M.; Pal M.; Roy M. Photodynamic Applications of New Imidazo[4,5-f][1,10]Phenanthroline Oxidovanadium(IV) Complexes: Synthesis, Photochemical, and Cytotoxic Evaluation. ChemistrySelect 2020, 5 (44), 13824–13830. 10.1002/slct.202003334. [DOI] [Google Scholar]
- Seth S. K.; Purkayastha P. Unusually Large Singlet Oxygen (1O2) Production by Very Weakly Emissive Pyrene-Functionalized Iridium(III) Complex: Interplay between Excited 3ILCT/3IL and 3MLCT States. Eur. J. Inorg. Chem. 2020, 2020 (31), 2990–2997. 10.1002/ejic.202000442. [DOI] [Google Scholar]
- Sanasam B.; Raza M. K.; Musib D.; Roy M. Photochemical and Photocytotoxic Evaluation of New Oxovanadium (IV) Complexes in Photodynamic Application. J. Chem. Sci. 2021, 133 (2), 42. 10.1007/s12039-021-01896-4. [DOI] [Google Scholar]
- Yuan Z.; He J.; Mahmood Z.; Xing L.; Ji S.; Huo Y.; Zhang H.-L. Deciphering the Ligand’s Geometric Effect on the Photophysical Properties of Osmium Complex and Its Application in Triplet-Triplet Annihilation Upconversion. Dyes Pigm. 2022, 199, 110049 10.1016/j.dyepig.2021.110049. [DOI] [Google Scholar]
- Wolcan E. Photosensitized Generation of Singlet Oxygen from Rhenium(I) Complexes: A Review. Inorg. Chim. Acta 2020, 509, 119650 10.1016/j.ica.2020.119650. [DOI] [Google Scholar]
- Mariappan M.; Maiya B. G. Effects of Anthracene and Pyrene Units on the Interactions of Novel Polypyridylruthenium(II) Mixed-Ligand Complexes with DNA. Eur. J. Inorg. Chem. 2005, 2005 (11), 2164–2173. 10.1002/ejic.200400952. [DOI] [Google Scholar]
- Peuntinger K.; Pilz T. D.; Staehle R.; Schaub M.; Kaufhold S.; Petermann L.; Wunderlin M.; Görls H.; Heinemann F. W.; Li J.; Drewello T.; Vos J. G.; Guldi D. M.; Rau S. Carbene Based Photochemical Molecular Assemblies for Solar Driven Hydrogen Generation. Dalton Trans. 2014, 43 (36), 13683–13695. 10.1039/C4DT01546K. [DOI] [PubMed] [Google Scholar]
- Singh V.; Mondal P. C.; Kumar A.; Jeyachandran Y. L.; Awasthi S. K.; Gupta R. D.; Zharnikov M. Surface Confined Heteroleptic Copper(II)–Polypyridyl Complexes for Photonuclease Activity. Chem. Commun. 2014, 50 (78), 11484–11487. 10.1039/C4CC05063K. [DOI] [PubMed] [Google Scholar]
- Jin C.; Liu J.; Chen Y.; Li G.; Guan R.; Zhang P.; Ji L.; Chao H. Cyclometalated Iridium(III) Complexes with Imidazo[4,5-f][1,10]Phenanthroline Derivatives for Mitochondrial Imaging in Living Cells. Dalton Trans. 2015, 44 (16), 7538–7547. 10.1039/C5DT00467E. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16 Rev. C.01, 2016.
- Adamo C.; Barone V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0Model. J. Chem. Phys. 1999, 110 (13), 6158–6170. 10.1063/1.478522. [DOI] [Google Scholar]
- Ernzerhof M.; Scuseria G. E. Assessment of the Perdew–Burke–Ernzerhof Exchange-Correlation Functional. J. Chem. Phys. 1999, 110 (11), 5029–5036. 10.1063/1.478401. [DOI] [PubMed] [Google Scholar]
- Andrae D.; Häußermann U.; Dolg M.; Stoll H.; Preuß H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theoret. Chim. Acta 1990, 77 (2), 123–141. 10.1007/BF01114537. [DOI] [Google Scholar]
- Weigend F.; Ahlrichs R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7 (18), 3297–3305. 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- Rappoport D.; Furche F. Property-Optimized Gaussian Basis Sets for Molecular Response Calculations. J. Chem. Phys. 2010, 133 (13), 134105. 10.1063/1.3484283. [DOI] [PubMed] [Google Scholar]
- Cancès E.; Mennucci B.; Tomasi J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107 (8), 3032–3041. 10.1063/1.474659. [DOI] [Google Scholar]
- Mennucci B.; Tomasi J. Continuum Solvation Models: A New Approach to the Problem of Solute’s Charge Distribution and Cavity Boundaries. J. Chem. Phys. 1997, 106 (12), 5151–5158. 10.1063/1.473558. [DOI] [Google Scholar]
- Cossi M.; Barone V.; Mennucci B.; Tomasi J. Ab Initio Study of Ionic Solutions by a Polarizable Continuum Dielectric Model. Chem. Phys. Lett. 1998, 286 (3–4), 253–260. 10.1016/S0009-2614(98)00106-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.