Abstract
Background
It is unclear how often survival benefits observed in single-center randomized controlled trials (sRCTs) involving critically ill patients are confirmed by subsequent multicenter randomized controlled trials (mRCTs). We aimed to perform a systemic literature review of sRCTs with a statistically significant mortality reduction and to evaluate whether subsequent mRCTs confirmed such reduction.
Methods
We searched PubMed for sRCTs published in the New England Journal of Medicine, JAMA, or Lancet, from inception until December 31, 2016. We selected studies reporting a statistically significant mortality decrease using any intervention (drug, technique, or strategy) in adult critically ill patients. We then searched for subsequent mRCTs addressing the same research question tested by the sRCT. We compared the concordance of results between sRCTs and mRCTs when any mRCT was available. We registered this systematic review in the PROSPERO International Prospective Register of Systematic Reviews (CRD42023455362).
Results
We identified 19 sRCTs reporting a significant mortality reduction in adult critically ill patients. For 16 sRCTs, we identified at least one subsequent mRCT (24 trials in total), while the interventions from three sRCTs have not yet been addressed in a subsequent mRCT. Only one out of 16 sRCTs (6%) was followed by a mRCT replicating a significant mortality reduction; 14 (88%) were followed by mRCTs with no mortality difference. The positive finding of one sRCT (6%) on intensive glycemic control was contradicted by a subsequent mRCT showing a significant mortality increase. Of the 14 sRCTs referenced at least once in international guidelines, six (43%) have since been either removed or suggested against in the most recent versions of relevant guidelines.
Conclusion
Mortality reduction shown by sRCTs is typically not replicated by mRCTs. The findings of sRCTs should be considered hypothesis-generating and should not contribute to guidelines.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04755-5.
Keywords: Intensive care units, Critical illness, Mortality, Randomized controlled trial, Guideline, Systematic review
Background
Randomized controlled trials (RCTs) are widely accepted as the best available tools to provide scientific evidence, are considered integral to informed clinical decision-making [1] and have been and remain the gold standard for assessing the efficacy of therapeutic agents. However, despite their potential to generate robust evidence, the positive results of single-center randomized controlled trials (sRCTs) may not be replicated when subjected to large multicenter randomized controlled trials (mRCTs), particularly within the context of intensive care settings [2, 3]. The discrepancies in results are often attributed to the inherent limitations of sRCTs. These limitations typically include biases due to local effects, minimal heterogeneity among the enrolled patients, inadequate blinding of personnel and data analysis, and the temporal gap between enrollment completion and publication. In addition, many sRCTs conducted in intensive care settings are often characterized by a low fragility index, indicating that the positive findings of the study depend on a small number of events [4]. Therefore, clinicians should interpret the positive evidence from sRCTs with caution, as clinical practice based on such evidence carries a high risk of bias [2].
Despite the above considerations, no study has systematically evaluated the discrepancy between positive sRCTs and subsequent mRCTs in the intensive care setting to provide a detailed perspective on the reproducibly of sRCTs. Therefore, we conducted a systematic review to identify sRCTs showing a mortality increase or decrease with a statistically significant difference and to evaluate whether following mRCTs confirmed or refuted the positive findings of these sRCTs. The primary objective of this systematic review was to report if significant mortality reduction observed in sRCTs was replicated in subsequent mRCTs. The secondary objective was to observe how clinical guidelines have dealt with these positive sRCTs in their recommendations.
Methods
We performed a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [5] (see PRISMA checklist in Additional file 1). This systematic review was registered in PROSPERO International Prospective Register of Systematic Reviews (CRD42023455362).
Search strategy and selection criteria
Two investigators independently searched PubMed for all RCTs of any non-surgical intervention influencing unadjusted landmark mortality in critically ill patients (> 48 h after randomization), published in three medical journals (i.e., New England Journal of Medicine, JAMA, and Lancet) from inception to December 31st, 2016. We did not consider sRCTs published after 2017 considering the time lag between the publication of sRCTs and their corresponding mRCTs.
We considered a difference in mortality as statistically significant when present at a specific point (> 48 h after randomization) with simple statistical tests and without adjustment for baseline characteristics. We selected articles published in NEJM, JAMA, or the Lancet, with a randomized controlled trial design in a single-center setting, presenting a statistically significant reduction or increase in unadjusted landmark mortality in critically ill patients. A quasi-randomized or non-randomized methodology, multicentric trials, pediatric populations, and absence of data on mortality were considered exclusion criteria. The full PubMed search strategy is available in Additional file 1.
After identification of eligible sRCTs, two investigators independently searched for mRCTs addressing the same PICO (population, intervention, control, outcome) frameworks, which were published from inception to December 31st, 2022.
The risk of bias of each included sRCT was assessed using the Cochrane risk-of-bias tool for randomized trials version 2 (RoB 2) [6].
Data extraction
Two investigators extracted the following variables in a standardized data collection form: PubMed unique identifier, journal, first author, year of publication, study population, number of patients enrolled, intervention, control, mortality data with statistical significance, and timepoint of mortality assessment. If a subsequent mRCT was identified, we evaluated whether the mortality findings of the mRCT were consistent with those of the sRCT. Furthermore, we explored whether sRCTs were incorporated into international clinical practice guidelines. We further assessed whether and when guidelines stopped citing such RCTs or issued recommendations modified by the mRCTs findings.
Statistical analysis
First, positive sRCTs with at least one subsequent mRCT were classified into three groups based on the results of mRCTs: significant mortality reduction (positive mRCTs), no significant difference in mortality (neutral mRCTs), and significant mortality increase (negative mRCTs). The proportion of sRCTs within each group was reported accordingly.
Second, we categorized included sRCTs that were cited at least once in international clinical guidelines based on the current guideline recommendations: supporting the intervention shown to have survival benefits in the sRCT, withholding recommendation due to insufficient evidence, and opposing the intervention of interest or excluding the sRCT cited in the preceding version of the guidelines.
To confirm the robustness of our findings, we performed a sensitivity analysis including only recent positive sRCTs published after 2001 to describe the mortality results of subsequent mRCTs and the guideline recommendations regarding the intervention assessed in the included sRCTs.
Furthermore, the following data were summarized: the number of randomized patients (sRCTs and mRCTs), the number of participating centers (mRCTs), the duration between publications of the sRCT and subsequent mRCT, and the duration from the initial citation of the sRCTs in the guidelines to an alteration in recommendation against its use or removal from guidelines. Missing data were not imputed throughout this study. Continuous variables were described as median and interquartile range (IQR). Categorical variables were expressed as number (percentage). We used RStudio Version 2023.06.0+421 (RStudio Team, Boston, United States).
Results
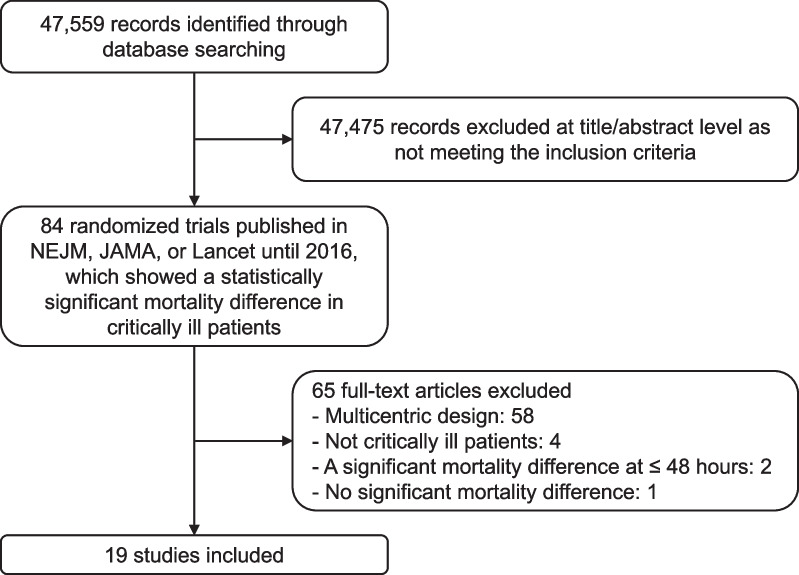
We identified 19 sRCTs published in the three high impact factor journals (7 in New England Journal of Medicine, 7 in JAMA, or 5 in Lancet), which showed a statistically significant mortality difference in critically ill patients [7–25] (Fig. 1). Major exclusions and reasons for exclusion are detailed in Additional file 1: Table S1. These trials were published from 1984 to 2016. The median number of enrolled patients was 231 (IQR 90–430). Acute kidney injury was the most representative condition of interest (4 studies [18, 20, 22, 25]), followed by cardiac arrest (3 studies [11, 17, 21]) and sepsis (3 studies [14, 16, 19]). Standard care or conventional therapy was used as control in 7 studies [8, 9, 14, 16, 19, 21, 23]. The most common timing of significant mortality differences was hospital discharge (8 studies [9–11, 13, 17, 18, 21, 23]). The characteristics of the included sRCTs are described in Table 1. The vast majority of the sRCTs included in this study (18 out of 19) were assessed as having a low risk of bias, while the remaining trial was judged as having some concerns (see Additional file 1: Table S2).
Fig. 1.
Flow chart of study selection. NEJM New England Journal of Medicine
Table 1.
Single-center randomized trials with statistically significant survival benefits
| References | Number of patients | Population | Intervention | Control | Mortality timepoint |
|---|---|---|---|---|---|
| Lachman et al. [14] | 33 | Septic shock | Anti-lipopolysaccharide immunoglobulin G | Conventional treatment |
7.1% versus 47% Hospital discharge |
| Sack et al. [21] | 103 | In-hospital cardiac arrest | Interposed abdominal counterpulsation | Standard care |
75% versus 93% Hospital discharge |
| Boyd et al. [8] | 107 | High-risk surgical patients | Supranormal oxygen delivery | Conventional therapy |
5.7% versus 22% 28 days |
| Levacher et al. [15] | 76 | Upper gastrointestinal bleeding | Terlipressin plus glyceryl trinitrate | Placebo |
20% versus 42% 15 days |
| Antonelli et al. [7] | 40 | Acute respiratory failure after solid organ transplantation | Non-invasive ventilation | Supplemental oxygen |
20% versus 50% ICU discharge |
| Ronco et al. [20] | 425 | Acute renal failure in ICU | Higher intensity renal replacement therapy | Low or intermediate volume ultrafiltration |
41% versus 57% versus 58% 15 days |
| Rivers et al. [19] | 263 | Sepsis and septic shock | Early goal-directed therapy | Standard care |
44% versus 57% 60 days |
| Hilbert et al. [13] | 52 | Acute respiratory failure in immunosuppressed patients | Non-invasive ventilation | Supplemental oxygen |
50% versus 81% Hospital discharge |
| van der Berghe et al. [23] | 1548 | ICU patients | Intensive insulin therapy | Conventional therapy |
4.6% versus 8.0% Hospital discharge |
| Dorian et al. [11] | 347 | Out-of-hospital ventricular fibrillation | Amiodarone | Lidocaine |
77% versus 88% Hospital discharge |
| Schiffl et al. [22] | 160 | Acute renal failure | Daily intermittent hemodialysis | Alternate-day hemodialysis |
28% versus 46% 14 days |
| Phu et al. [18] | 70 | Acute renal failure with urgent renal replacement therapy indication | Venovenous dialysis | Peritoneal dialysis |
15% versus 47% Hospital discharge |
| de Jonge et al. [9] | 934 | Patients on mechanical ventilation | Selective decontamination digestive tract | Standard care |
24% versus 31% Hospital discharge |
| de Silva et al. [10] | 401 | Yellow-oleander poisoning | Charcoal | Placebo |
2.5% versus 8.0% Hospital discharge |
| Olasveengen et al. [17] | 1183 | Out-of-hospital nontraumatic cardiac arrest | CPR with epinephrine administration | CPR without epinephrine administration |
68% versus 79% Hospital admission |
| Morelli et al. [16] | 154 | Septic shock | Esmolol infusion | Standard care |
49% versus 81% 28 days |
| Villanueva et al. [24] | 921 | Upper gastrointestinal bleeding | Restrictive transfusion strategy | Liberal transfusion strategy |
5.0% versus 8.9% 45 days |
| Girardis et al. [12] | 434 | ICU patients | Conservative oxygen supplementation | Conventional oxygen supplementation |
12% versus 20% ICU discharge |
| Zarbock et al. [25] | 231 | Acute kidney injury stage 2 | Early renal replacement therapy | Delayed renal replacement therapy |
39% versus 55% 90 days |
CPR cardiopulmonary resuscitation, ICU intensive care unit
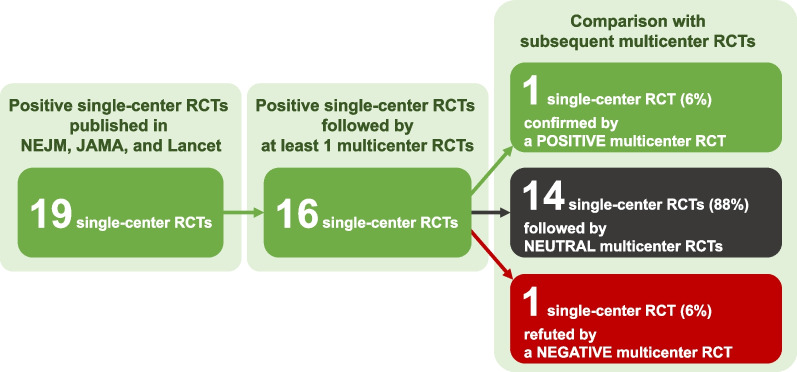
Most of the included sRCTs (16/19, 84%) [7, 9–17, 19, 20, 22–25] were followed by at least one subsequent mRCT (in total 24 studies [26–49]), while no mRCT was available for the remaining three studies [8, 18, 21]. The mRCTs enrolled more patients (median, 1192 [IQR 488–3021] vs. 231 [IQR 90–430] in sRCTs). The median number of participating centers was 29 (IQR 8–37) and one-third of the studies involved multiple countries [27, 29, 32, 34, 40, 41, 45, 48]. The median interval between the publications of a sRCT and its relevant subsequent mRCT was 8 years (IQR 5–13 years). Survival or mortality was the primary outcome in the 10 sRCTs and 17 mRCTs (as listed in Additional file 1: Table S3).
The survival benefits of one intervention (epinephrine during out-of-hospital cardiac arrest [17]) were confirmed by a subsequent mRCT [46]. Fourteen studies [9–13, 16, 19, 22, 24, 25] were followed by neutral mRCTs (no statistically significant mortality difference between groups) [26–33, 35–45, 47–49]. One sRCT reporting survival benefit of intensive insulin therapy [23] was contradicted by a large mRCT documenting a statistically significant mortality increase in patients randomized to the intensive insulin therapy arm [34]. Figure 2 and Table 2 describes the mortality findings of these mRCTs.
Fig. 2.
Mortality findings in multicenter randomized trials following positive single-center trials. RCT randomized controlled trials, NEJM New England Journal of Medicine
Table 2.
Subsequent multicenter randomized trials and their mortality findings
| sRCT | References of mRCT | Number of patients | Number of centers | Number of countries | Mortality |
|---|---|---|---|---|---|
| Anti-lipopolysaccharide immunotherapy in sepsis, 1984 [14] | Greenman et al. [37] | 486 | 33 | 1 | No statistically significant difference |
| Angus et al. [26] | 1102 | 136 | 1 | No statistically significant difference | |
| Interpose abdominal counterpulsation during cardiac arrest, 1992 [21] | Not available | ||||
| Supranormal oxygen delivery, 1993 [8] | Not available | ||||
| Terlipressin for upper gastrointestinal bleeding in cirrhotic patients, 1995 [15] | Feu et al. [33] | 161 | 4 | 1 | No statistically significant difference |
| Escorsell et al. [32] | 219 | 9 | 2 | No statistically significant difference | |
| Non-invasive ventilation in immunosuppressed patients, 2000 [7, 13] | Lemiale et al. [41] | 374 | 28 | 2 | No statistically significant difference |
| High-intensity renal replacement therapy in acute renal failure, 2000 [20] | Palevsky et al. [44] | 1124 | 27 | 1 | No statistically significant difference |
| Bellomo et al. [29] | 1508 | 35 | 2 | No statistically significant difference | |
| Early goal-directed therapy in sepsis, 2001 [19] | Peake et al. [45] | 1600 | 51 | 5 | No statistically significant difference |
| Yealy et al. [49] | 1341 | 31 | 1 | No statistically significant difference | |
| Mouncey et al. [42] | 1260 | 56 | 1 | No statistically significant difference | |
| Intensive insulin therapy, 2001 [23] | Finfer et al. [34] | 6104 | 42 | 4 | Statistically significant mortality increase at 90 days (28% vs. 25%) |
| Amiodarone in refractory ventricular fibrillation, 2002 [11] | Kudenchuk et al. [40] | 3026 | 10a | 2 | No statistically significant difference |
| Daily hemodialysis in acute renal failure, 2002 [22] | Ponce et al. [47] | 407 | 2 | 1 | No statistically significant difference |
| Hemofiltration versus peritoneal dialysis in acute renal failure, 2002 [18] | Not available | ||||
| Selective decontamination of digestive tract, 2003 [9] | de Smet et al. [30] | 5939 | 13 | 1 | No statistically significant difference |
| Myburgh et al. [43] | 5982 | 19 | 1 | No statistically significant difference | |
| Charcoal in poisoning, 2003 [10] | Eddleston et al. [31] | 4632 | 3 | 1 | No statistically significant difference |
| Epinephrine during cardiopulmonary resuscitation, 2009 [17] | Perkins et al. [46] | 8014 | 5b | 1 | Statistically significant mortality reduction at 30 days (3.2% vs 2.4%) |
| Beta blocker in sepsis, 2013 [16] | Kakihana et al. [39] | 151 | 54 | 1 | No statistically significant difference |
| Transfusion thresholds in upper gastrointestinal bleeding, 2013 [24] | Jairath et al. [38] | 936 | 6 | 1 | No statistically significant difference |
| Oxygen targets, 2016 [12] | Schjørring et al. [48] | 2928 | 35 | 7 | No statistically significant difference |
| Gelissen et al. [36] | 574 | 4 | 1 | No statistically significant difference | |
| Timing of renal replacement therapy in acute kidney injury, 2016 [25] | Gaudry et al. [35] | 620 | 31 | 1 | No statistically significant difference |
| Barbar et al. [28] | 488 | 29 | 1 | No statistically significant difference | |
| Bagshaw et al. [27] | 3019 | 168 | 15 | No statistically significant difference |
CPR cardiopulmonary resuscitation, sRCT single-center randomized controlled trial, mRCT multicenter randomized controlled trial
aNumber of clinical centers consisting of the Resuscitation Outcomes Consortium
bNumber of National Health Service ambulance services
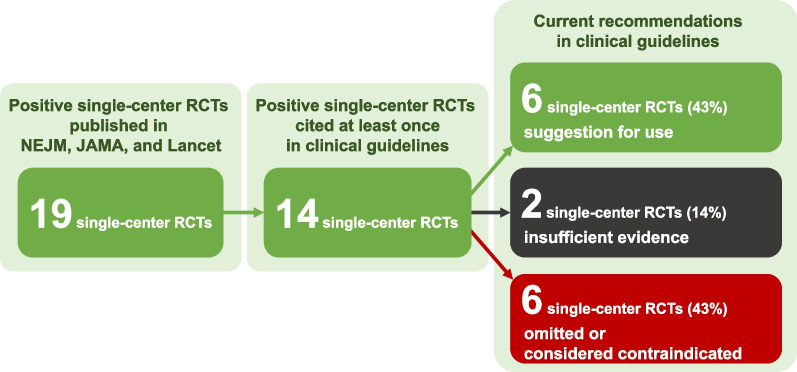
Figure 3 and Table 3 summarizes how clinical guidelines have considered survival benefits shown in sRCTs. Among the included 19 sRCTs, 14 were cited in clinical guidelines at least once (13 sRCTs with subsequent mRCTs and one without) [7, 9, 11–13, 15, 17, 19–25]. Among the 13 sRCTs followed by mRCTs, the guidelines initially provided recommendations or suggestions based on the positive results of seven sRCTs [7, 11, 13, 15, 19, 23, 24]. However, the current guidelines do not support applying two of these interventions anymore [19, 23]. Treatments assessed in the remaining five sRCTs [7, 11, 13, 15, 24] remained as suggestions for use in clinical guidelines. Such suggestions remained even after the publication of mRCTs, which reported neutral mortality findings.
Fig. 3.
Current guideline recommendations of positive single-center trials
Table 3.
Citation of single-center randomized trials in international clinical guidelines
| Research topic | The first guideline citating the sRCT | Recommendation in the first guideline citing the sRCT | Guideline removing or suggesting against the sRCT | Current recommendation in guidelines |
|---|---|---|---|---|
| 16 sRCTs with subsequent mRCTs | ||||
| Anti-lipopolysaccharide immunotherapy in sepsis, 1984 [14] | Not available | Not applicable | Not applicable | Not applicable |
| Terlipressin for upper gastrointestinal bleeding in cirrhotic patients, 1995 [15] | 2018 [50] | Suggested | Not applicable | Suggested [50] |
| Non-invasive ventilation in immunosuppressed patients, 2000 [7] | 2015 [51] | Some evidence of benefit in certain patients with immunosuppression | 2020 [52] | Suggested [52] |
| High-volume ultrafiltration in acute renal failure, 2000 [20] | 2008 [53] | Insufficient evidence | 2021 [54] | Insufficient evidence [54] |
| Non-invasive ventilation in immunosuppressed patients, 2001 [13] | 2015 [51] | Some evidence of benefit in certain patients with immunosuppression | 2023 [55] | Suggested [55] |
| Early goal-directed therapy in sepsis, 2001 [19] | 2004 [56] | Recommended | 2017 [57] | Not mentioned [54] |
| Intensive insulin therapy, 2001 [23] | 2004 [56] | Recommended with a glucose goal of < 150 mg/dL | 2013 [58] | Suggested against [54] |
| Amiodarone in refractory ventricular fibrillation, 2002 [11] | 2006 [59] | Recommended | 2019 [60] | Suggested [61] |
| Daily hemodialysis in acute renal failure, 2002 [22] | 2008 [53] | Insufficient evidence | 2013 [58] | Not mentioned [54] |
| Selective decontamination of digestive tract, 2003 [9] | 2008 [53] | No recommendation | 2016 [57] | Not mentioned [54] |
| Charcoal in poisoning, 2003 [10] | Not available | Not applicable | Not applicable | Not applicable |
| Epinephrine during cardiopulmonary resuscitation, 2009 [17] | 2010 [62] | Insufficient evidence | 2015 [63] | Recommended [64] |
| Beta blocker in sepsis, 2013 [16] | Not available | Not applicable | Not applicable | Not applicable |
| Restrictive transfusion threshold in upper gastrointestinal bleeding, 2013 [24] | 2016 [65] | Recommended | Still cited in the current guideline [66] | Suggested [66] |
| Oxygen targets, 2016 [12] | 2021 [54] | Insufficient evidence | Not available | Insufficient evidence [54] |
| Early initiation of renal replacement therapy in acute kidney injury, 2016 [25] | 2021 [54] | Suggested against | Not available | Suggested against [54] |
| 3 sRCTs without subsequent mRCTs | ||||
| Interpose abdominal counterpulsation during cardiac arrest, 1992 [21] | 2005 [67] | No clear statement | 2015 [63] | Not mentioned [64] |
| Supranormal oxygen delivery, 1993 [8] | Not available | Not applicable | Not available | Not applicable |
| Hemofiltration vs peritoneal dialysis in acute renal failure, 2002 [18] | Not available | Not applicable | Not applicable | Not applicable |
CPR cardiopulmonary resuscitation, sRCT single-center randomized controlled trial
Of the remaining six sRCTs for which guidelines did not support the intervention investigated, five [9, 12, 17, 20, 22] were cited in the guidelines without a clear recommendation, primarily due to inadequate evidence. However, one intervention—epinephrine for cardiac arrest—that exhibited survival benefits in the sRCT [17] and subsequent mRCT [46] is currently recommended in guidelines. The remaining sRCT [25] was not endorsed by the initial relevant guideline as a result of other mRCTs that showed no significant mortality reduction.
Finally, among three sRCTs which has not had a subsequent mRCT, only one study [21] was cited in guidelines without any recommendation but is not referenced in the current guidelines.
Consequently, among 14 sRCTs originally referenced to in clinical guidelines, six (43%) are still cited to suggest for the intervention in current international guidelines [7, 11, 13, 15, 17, 24]. Conversely, six other sRCTs (43%) were either omitted or considered contraindicated in subsequent guideline versions [9, 19, 22, 23, 25]. Among these six studies, the median duration from the initial citation in the guidelines to an alteration in recommendation against its use or removal from guidelines was 9 years (IQR 6–12 years). Regarding the remaining two studies [12, 20], no recommendation was made due to insufficient evidence.
A sensitivity analysis restricted to recent sRCTs confirmed the overall results: survival benefits were infrequently replicated in subsequent mRCTs; half of the positive sRCTs were omitted or considered contraindicated in the current guidelines (detailed in Table S4 in the Additional file 1).
Discussion
Key findings
Our systematic review found 19 sRCTs with a statistically significant mortality decrease in critically ill adult patients. Most of these were followed by at least one subsequent mRCT. Survival benefits observed in sRCTs were rarely corroborated by mRCTs, with most mRCTs reporting neutral results on mortality, and one mRCT finding a significant mortality increase with intensive glucose control. Treatment recommendations based on the initial citation of sRCTs with survival benefits were included in international guidelines and typically remained unchanged for a decade before any revisions were made based on subsequent relevant mRCTs.
Relationship with previous literature
RCTs in intensive care medicine tend to deliver neutral results in terms of mortality for several reasons including heterogeneity of patient characteristics, underlying practice variation, insufficient power, and likely small treatment effects [3]. This fact poses an important challenge for clinicians because they must perform clinical practice without robust evidence supporting their decisions. As a result, positive trials, namely RCTs reporting statistically significant reductions in mortality attributable to the intervention of interest, look attractive and are often taken up by physicians to change their routine management. Unfortunately, however, such positive RCTs frequently suffer from methodological problems, which can limit the applicability of their findings. Furthermore, single-centric design itself carries many other limitations [2, 3].
One of the major challenges of sRCTs is that, to achieve an effect on mortality in the presence of a small sample size, they must achieve an implausibly large effect size. Single-center trials are typically conducted by advocates of the intervention under investigation [2]. The delivery of such interventions generally requires specialized expertise and dedication, which may not be readily transferable to other centers involved in subsequent large mRCTs. Such discrepancies may limit the feasibility of the interventions, potentially diminishing the magnitude of the treatment effects observed in mRCTs compared to sRCTs. In fact, a meta-epidemiological study evaluated the differences in treatment effects between sRCTs and multicenter RCTs and found that single-center trials showed a statistically significant larger treatment effects than multicenter trials (ratio of odds ratios, 0.73; 95% confidence interval 0.64–0.83) [68]. This finding was confirmed by a systematic review assessing treatment effects on mortality in critically ill settings [69]. By pooling 82 eligible RCTs, this systematic review found that a single-center design resulted in larger treatment effects than a multicenter design (ratio of odds ratios, 0.64; 95% confidence interval 0.47–0.87) [69]. Our selection criterion of sRCTs with significant mortality differences is a unique approach; nonetheless, the present systematic review was consistent with previous work showing that survival benefits in sRCTs were rarely replicated in mRCTs. Moreover, we identified one mRCT demonstrating a significant mortality increase by an intervention (intensive glucose control strategy) [34], which reduced mortality within the context of a previous single-center trial [23].
Nearly half of clinical guidelines that cite sRCTs, recommend the relevant intervention based on their positive results, despite some of these endorsements being subsequently refuted in light of accumulated evidence. In addition, a decade was typically required to amend such recommendations from clinical guidelines. Given the pervasive application of interventions examined in RCTs, these initial recommendations might have played a substantial role in the potential consequences on patient outcomes, healthcare resources, and economic costs. For example, early-goal directed therapy was recommended in the surviving sepsis campaign guidelines in 2004 [56]. However, later, three mRCTs found no benefits in clinically relevant outcomes [42, 45, 49]. Furthermore, economic evaluation using one of the mRCTs revealed that early-goal directed therapy was associated with increased health care costs without improving outcomes [70].
Despite these methodological challenges, positive sRCTs have made changes in clinical practice. The early goal-directed therapy for septic shock is a typical example. The initial sRCT [19] showed survival benefits of this protocolized management, which was not replicated in subsequent mRCTs [42, 45, 49]. However, given the difference in patient severity between the sRCT and subsequent mRCTs (e.g., reduced vs. normal central venous oxygen saturation [ScvO2]), clinicians now pay more attention to ScvO2 values than before the sRCT [71]. Furthermore, the lack of multicentric confirmation of survival benefits implies a restricted external validity of sRCTs rather than an indication of them producing false positive results.
As the intensive care community advances the methodology of randomized trial design and execution, there remains a notable lack of evidence demonstrating improved mortality from interventions. These disappointing results have been obtained by using frequentist statistics, where the conclusion is dichotomized to yes or no based on confidence intervals and p values. In contrast, Bayesian analysis provides a probabilistic assessment of the magnitude and direction of true treatment effects, which allows clinicians to augment the interpretation of the trial results. Interestingly, there are several intensive care trials where frequentist statistics denied significant mortality reduction, followed by a Bayesian reanalysis revealing a high probability of survival benefits [72, 73]. Therefore, the integration of Bayesian analysis in intensive care trials may offer a solution to the limitations commonly encountered with frequentist approaches.
Implications
This systematic review found mortality reduction was rarely replicated in mRCTs despite the existence of previous positive sRCTs. This implies that there are potential risks when incorporating novel interventions into routine practice based on positive sRCTs without mRCTs confirmation. Importantly, no intervention is free from complications. In addition, new interventions tested in randomized trials often consume more human resources and economic costs. Therefore, management change will inevitably result in complications, increased workload, and costs, all of which did not exist with previous usual care. Given the high likelihood of no mortality difference or even mortality increase in subsequent mRCTs, our findings imply that clinicians should wait for a large-scale trial prior to changing practice or at least be very careful in interpreting the results of positive sRCTs.
Strengths and limitations
This systematic review is the first study to comprehensively identify sRCTs reporting statistically significant reductions in mortality and their corresponding subsequent mRCTs in the field of intensive care medicine. The infrequent replicability of survival benefits in mRCTs corroborated the limited generalizability of sRCTs’ findings. Evaluating the impact of sRCTs on clinical guidelines may be a novel approach, but it yields important insights into the development and interpretation of guidelines.
We acknowledge several limitations. First, given our focus was solely on intensive care sRCTs, our findings may not translate to other medical disciplines. Nevertheless, the generic limitations of sRCTs are universal, regardless of the targeted population or intervention type. As such, sRCTs need to be perceived as hypothesis-generating and clinicians ought to assess the results of sRCTs with a balanced consideration of their strengths and weaknesses. Second, our study included only sRCTs published until 2016, thereby excluding more recent sRCTs. Despite this limitation, our primary objective was to compare the mortality findings of sRCTs with those of subsequent mRCTs, which necessitated an intervening period between them. In addition, the median duration between sRCT and mRCT publication was 8 years, providing justification for our inclusion criteria. Third, we included positive sRCTs published in the three renowned general medical journals, excluding those in intensive care specialty journals (as listed in Table S5: Additional file 1). As a result, the number of eligible studies was relatively small; nonetheless, we employed this strategy to evaluate whether subsequent mRCTs could replicate the survival benefits observed in sRCTs with rigorous methodologies. Given the high standards of the included studies and the concordance of the results with previous literature, it is plausible that our findings could be extrapolated to positive sRCTs reported in specialty journals. Finally, our search was confined to international guidelines to explore sRCTs’ citations. This approach was chosen to ensure the quality of evidence synthesis and generalized perspective.
Conclusions
Our systematic review found that the statistically significant survival improvement shown in sRCTs was rarely confirmed by multicenter randomized evidence in intensive care settings. Clinicians should be cautious in altering routine clinical practices until well-conducted multicenter randomized trials are available. Given their substantial implications for global clinical practice, international guidelines should refrain from issuing a clear recommendation based solely on the positive results of sRCTs.
Supplementary Information
Additional file 1. Search strategy, PRISMA checklist, Supplementary tables, and Supplementary references.
Acknowledgements
The authors wish to appreciate all the patients and investigators of the included trials.
The authors also wish to thank the following collaborators for their extensive data collection: Rosario Losiggio, Gaetano Lombardi, and Nicolò Maimeri.
Abbreviations
- ICU
Intensive care unit
- IQR
Interquartile range
- mRCT
Multicenter randomized controlled trial
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- sRCT
Single-center randomized controlled trial
- RCT
Randomized controlled trial
Author contributions
YK, ST, AO, MBR, CM, GL, and RB conceived the study. YK, ST, AO, MBR, CM, and GL designed the search strategy and did the literature search. YK, ST, AO, MBR, CM, GL, and RB did the statistical analysis. YK, ST, and GL wrote the initial protocol. YK, ST, AO, MBR, CM, GL, and RB wrote the manuscript. All authors shared the study data, gave a critical appraisal of the protocol, provided crucial revisions, and approved the final manuscript.
Funding
No funding was received concerning this manuscript.
Availability of data and materials
We collected the summary data from published randomized trials. This published article and its supplementary files include all the data generated or analyzed for this study. Further information is available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.A Medical Research Council Investigation Streptomycin treatment of pulmonary tuberculosis. Br Med J. 1948;2:769–782. doi: 10.1136/bmj.2.4582.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. 2009;37:3114–3119. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- 3.Landoni G, Pieri M, Young PJ, Bellomo R. Why do multicenter randomized controlled trials not confirm the positive findings of single center randomized controlled trials in acute care? Minerva Anestesiol. 2019;85:194–200. doi: 10.23736/s0375-9393.18.13070-7. [DOI] [PubMed] [Google Scholar]
- 4.Ridgeon EE, Young PJ, Bellomo R, Mucchetti M, Lembo R, Landoni G. The fragility index in multicenter randomized controlled critical care trials. Crit Care Med. 2016;44:1278–1284. doi: 10.1097/ccm.0000000000001670. [DOI] [PubMed] [Google Scholar]
- 5.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 7.Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283:235–241. doi: 10.1001/jama.283.2.235. [DOI] [PubMed] [Google Scholar]
- 8.Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270:2699–2707. doi: 10.1001/jama.1993.03510220055034. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011–1016. doi: 10.1016/s0140-6736(03)14409-1. [DOI] [PubMed] [Google Scholar]
- 10.de Silva HA, Fonseka MM, Pathmeswaran A, Alahakone DG, Ratnatilake GA, Gunatilake SB, et al. Multiple-dose activated charcoal for treatment of yellow oleander poisoning: a single-blind, randomised, placebo-controlled trial. Lancet. 2003;361:1935–1938. doi: 10.1016/s0140-6736(03)13581-7. [DOI] [PubMed] [Google Scholar]
- 11.Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002;346:884–890. doi: 10.1056/NEJMoa013029. [DOI] [PubMed] [Google Scholar]
- 12.Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 13.Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487. doi: 10.1056/nejm200102153440703. [DOI] [PubMed] [Google Scholar]
- 14.Lachman E, Pitsoe SB, Gaffin SL. Anti-lipopolysaccharide immunotherapy in management of septic shock of obstetric and gynaecological origin. Lancet. 1984;1:981–983. doi: 10.1016/s0140-6736(84)92324-9. [DOI] [PubMed] [Google Scholar]
- 15.Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet. 1995;346:865–868. doi: 10.1016/s0140-6736(95)92708-5. [DOI] [PubMed] [Google Scholar]
- 16.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–1691. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
- 17.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 18.Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895–902. doi: 10.1056/NEJMoa020074. [DOI] [PubMed] [Google Scholar]
- 19.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 20.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/s0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 21.Sack JB, Kesselbrenner MB, Bregman D. Survival from in-hospital cardiac arrest with interposed abdominal counterpulsation during cardiopulmonary resuscitation. JAMA. 1992;267:379–385. doi: 10.1001/jama.1992.03480030057037. [DOI] [PubMed] [Google Scholar]
- 22.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 23.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 25.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 26.Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA. 2000;283:1723–1730. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- 27.The STARRT-AKI Investigators, & for the Canadian Critical Care Trials Group, the Australian and New Zealand Intensive Care Society Clinical Trials Group, the United Kingdom Critical Care Research Group, the Canadian Nephrology Trials Network, and the Irish Critical Care Trials Group Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240–251. doi: 10.1056/NEJMoa2000741. [DOI] [PubMed] [Google Scholar]
- 28.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 29.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 30.de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 31.Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, et al. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371:579–587. doi: 10.1016/s0140-6736(08)60270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escorsell A, Ruiz del Arbol L, Planas R, Albillos A, Bañares R, Calès P, et al. Multicenter randomized controlled trial of terlipressin versus sclerotherapy in the treatment of acute variceal bleeding: the TEST study. Hepatology. 2000;32:471–476. doi: 10.1053/jhep.2000.16601. [DOI] [PubMed] [Google Scholar]
- 33.Feu F, Ruiz del Arbol L, Bañares R, Planas R, Bosch J. Double-blind randomized controlled trial comparing terlipressin and somatostatin for acute variceal hemorrhage. Variceal Bleeding Study Group. Gastroenterology. 1996;111:1291–1299. doi: 10.1053/gast.1996.v111.pm8898643. [DOI] [PubMed] [Google Scholar]
- 34.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 35.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 36.Gelissen H, de Grooth HJ, Smulders Y, Wils EJ, de Ruijter W, Vink R, et al. Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA. 2021;326:940–948. doi: 10.1001/jama.2021.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenman RL, Schein RM, Martin MA, Wenzel RP, MacIntyre NR, Emmanuel G, et al. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA. 1991;266:1097–1102. doi: 10.1001/jama.1991.03470080067031. [DOI] [PubMed] [Google Scholar]
- 38.Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137–144. doi: 10.1016/s0140-6736(14)61999-1. [DOI] [PubMed] [Google Scholar]
- 39.Kakihana Y, Nishida O, Taniguchi T, Okajima M, Morimatsu H, Ogura H, et al. Efficacy and safety of landiolol, an ultra-short-acting β1-selective antagonist, for treatment of sepsis-related tachyarrhythmia (J-Land 3S): a multicentre, open-label, randomised controlled trial. Lancet Respir Med. 2020;8:863–872. doi: 10.1016/s2213-2600(20)30037-0. [DOI] [PubMed] [Google Scholar]
- 40.Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711–1722. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- 41.Lemiale V, Mokart D, Resche-Rigon M, Pène F, Mayaux J, Faucher E, et al. Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA. 2015;314:1711–1719. doi: 10.1001/jama.2015.12402. [DOI] [PubMed] [Google Scholar]
- 42.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 43.Myburgh JA, Seppelt IM, Goodman F, Billot L, Correa M, Davis JS, et al. Effect of selective decontamination of the digestive tract on hospital mortality in critically ill patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2022;328:1911–1921. doi: 10.1001/jama.2022.17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ARISE Investigators and the ANZICS Clinical Trials Group Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 46.Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379:711–721. doi: 10.1056/NEJMoa1806842. [DOI] [PubMed] [Google Scholar]
- 47.Ponce D, Berbel MN, Abrão JM, Goes CR, Balbi AL. A randomized clinical trial of high volume peritoneal dialysis versus extended daily hemodialysis for acute kidney injury patients. Int Urol Nephrol. 2013;45:869–878. doi: 10.1007/s11255-012-0301-2. [DOI] [PubMed] [Google Scholar]
- 48.Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384:1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 49.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Association for the Study of the Liver. European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 51.Beasley R, Chien J, Douglas J, Eastlake L, Farah C, King G, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: “Swimming between the flags”. Respirology. 2015;20:1182–1191. doi: 10.1111/resp.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leone M, Einav S, Chiumello D, Constantin J-M, De Robertis E, De Abreu MG, et al. Noninvasive respiratory support in the hypoxaemic peri-operative/periprocedural patient: a joint ESA/ESICM guideline. Intensive Care Med. 2020;46:697–713. doi: 10.1007/s00134-020-05948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin-Loeches I, Torres A, Nagavci B, Aliberti S, Antonelli M, Bassetti M, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Eur Respir J. 2023 doi: 10.1183/13993003.00735-2022. [DOI] [PubMed] [Google Scholar]
- 56.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 57.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6.pdf. [DOI] [PubMed] [Google Scholar]
- 58.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Intensive Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 59.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 60.Soar J, Maconochie I, Wyckoff MH, Olasveengen TM, Singletary EM, Greif R, et al. 2019 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2019;145:95–150. doi: 10.1016/j.resuscitation.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Soar J, Donnino MW, Maconochie I, Aickin R, Atkins DL, Andersen LW, et al. 2018 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations summary. Resuscitation. 2018;133:194–206. doi: 10.1016/j.resuscitation.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 62.Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council guidelines for resuscitation 2010 @@@. Adult advanced life support. Resuscitation. 2010;81:1305–1352. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Monsieurs KG, Nolan JP, Bossaert LL, Greif R, Maconochie IK, Nikolaou NI, et al. European Resuscitation Council guidelines for resuscitation 2015: @@@. Executive summary. Resuscitation. 2015;95:1–80. doi: 10.1016/j.resuscitation.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 64.Soar J, Böttiger BW, Carli P, Couper K, Deakin CD, Djärv T, et al. European Resuscitation Council guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vlaar APJ, Dionne JC, de Bruin S, Wijnberge M, Raasveld SJ, van Baarle FEHP, et al. Transfusion strategies in bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2021;47:1368–1392. doi: 10.1007/s00134-021-06531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G. European Resuscitation Council guidelines for resuscitation 2005. @@@. Adult advanced life support. Resuscitation. 2005;67(Suppl 1):S39–86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39–51. doi: 10.7326/0003-4819-155-1-201107050-00006. [DOI] [PubMed] [Google Scholar]
- 69.Unverzagt S, Prondzinsky R, Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol. 2013;66:1271–1280. doi: 10.1016/j.jclinepi.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Higgins AM, Peake SL, Ao RB, Ao DJC, Delaney A, Howe BD, et al. The cost-effectiveness of early goal-directed therapy: an economic evaluation alongside the ARISE trial. Crit Care Resusc. 2021;23:329–336. doi: 10.51893/2021.3.OA10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Backer D, Vincent J-L. Early goal-directed therapy: Do we have a definitive answer? Intensive Care Med. 2016;42:1048–1050. doi: 10.1007/s00134-016-4295-6. [DOI] [PubMed] [Google Scholar]
- 72.Zampieri FG, Damiani LP, Bakker J, Ospina-Tascón GA, Castro R, Cavalcanti AB, et al. Effects of a resuscitation strategy targeting peripheral perfusion status versus serum lactate levels among patients with septic shock. A Bayesian reanalysis of the ANDROMEDA-SHOCK Trial. Am J Respir Crit Care Med. 2020;201:423–429. doi: 10.1164/rccm.201905-0968OC. [DOI] [PubMed] [Google Scholar]
- 73.Granholm A, Munch MW, Myatra SN, Vijayaraghavan BKT, Cronhjort M, Wahlin RR, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022;48:45–55. doi: 10.1007/s00134-021-06573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Search strategy, PRISMA checklist, Supplementary tables, and Supplementary references.
Data Availability Statement
We collected the summary data from published randomized trials. This published article and its supplementary files include all the data generated or analyzed for this study. Further information is available from the corresponding authors upon reasonable request.