Abstract
As a frequent complication of inflammatory bowel disease (IBD), Clostridium difficile infection (CDI) was confirmed to not only aggravate the symptoms of IBD but also result in unexpected outcomes, including death. With the increasing prevalence rate of IBD and the updating of CDI diagnosis, the incidence of CDI in IBD patients is also seen rising. Although a detection method consisting of glutamate dehydrogenase immunoassay or nucleic acid amplification test and then toxin A/B enzyme immunoassay was recommended and widely adopted, the diagnosis of CDI in IBD is still a challenge because of the overlap between the symptoms of CDI in IBD and CDI itself. Vancomycin and fidaxomicin are the first-line therapy for CDI in IBD; however, the treatment has different effects due to the complexity of IBD patients’ conditions and the choice of different treatment schemes. Although the use of fecal microbial transplantation is now in the ascendant for IBD management, the prospects are still uncertain and the prevention and treatment of the recurrence of CDI in IBD remain a clinical challenge. In this paper, the epidemiology, pathophysiology, clinical manifestation, prevention, and therapy of CDI in IBD were summarized and presented.
Keywords: Clostridium difficile, Crohn’s disease, treatment, ulcerative colitis
Introduction
Clostridium difficile belongs to gram-positive spore anaerobic bacteria which is one of the most common causes of infectious diarrhea in hospitalized patients. 1 It was first isolated from the feces of breastfed infants in 1935. 2 C. difficile infection (CDI) was first reported as the cause of pseudomembranous enteritis in 1978. 3 Among the general population, the major risk factors of CDI infection include prolonged proton pump inhibitors (PPI) and antibiotics, low immune, elderly, multiple underlying diseases, hypoalbuminemia, renal insufficiency, indwelling nasogastric tube, and gastrointestinal operation. 4 Several studies indicated that inflammatory bowel disease (IBD) was an independent risk factor of CDI for adults and children. 5 According to a meta-analysis that involved 461 patients admitted for the IBD outbreak, 35 (7.6%) had CDI and 10 (2.2%) had nontoxic C. difficile. 6 Studies even showed that the risk of CDI was eight times higher in IBD than in non-IBD patients, and the lifetime infection rate was found around 10%. 7 However, compared with the general population, the prevalence of CDI in IBD was 3–6%, and the infection was seen in younger patients [median 38.5 years, interquartile range (IQR) 30.0–63.7 years], and mostly served as outpatient or community-acquired infections. 1 This may be related to the earlier use of immunosuppressants, antibiotics, and steroids in IBD patients.4,8 It should be noted that suffering from IBD significantly increased the risk of CDI, while CDI not only worsened the symptoms of IBD but also resulted in adverse outcomes, including escalation of therapy, treatment failure, hospitalization, surgical procedures, and increased mortality. 9 Compared with non-IBD controls, the incidence of CDI recurrence in IBD patients was 4.5-fold higher and the incidence of C. difficile carrier status was eight times higher after initial anti-CDI therapy. 7 A 5-year study of patients with IBD in the United Kingdom found that emergency colectomy was twice as frequently performed in IBD patients with CDI as in non-IBD patients.3,10 Studies have shown that CDI is not associated with the short-term risk of colectomy but more than doubled the probability of long-term colectomy. 11 A retrospective study reported that the mortality directly caused by CDI was approximately 5%, and mortality associated with complications was 15–25%. 7
Pathophysiology
Susceptibility factors for CDI in IBD include biodiversity reduction and microbial dysfunction, immunosuppression and antimicrobial therapy, frequent diarrhea, and malnutrition.12–14 C. difficile is ubiquitous in the natural environment and is transmitted through the fecal–oral route. C. difficile spores can survive in the acidic environment of the stomach and reach the intestine. Under appropriate temperatures and the environment of gut microbiota disorder as well as rich primary bile acid, the growth of C. difficile is not inhibited in the intestine and is expected to convert to vegetative forms. The vegetative forms contain a locus that includes regulatory genes, pore protein genes, and TcdA and TcdB genes. Toxin A and toxin B, which are encoded by TcdA and TcdB genes, respectively, are the two main virulence factors that can damage the actin cytoskeleton and destroy the tight junction of the intestinal epithelium, thereby causing fluid accumulation, cell apoptosis, and death. Ultimately, diarrhea occurs due to the disruption of colon integrity.15–20 Thus, a good gut microbial environment is crucial for the prevention of CDI 3 The underlying colitis in IBD patients leads to dysregulation of colonic flora and decreased colonization resistance, which makes IBD an independent risk factor for CDI. 3 In IBD, the dysfunction of T cells and antigen-presenting cells may reduce the body’s tolerance to microbial antigens. Therefore, the risk of CDI is higher in patients with severe IBD.1,21 Persistent disturbance of the intestinal microenvironment increases the recurrence of CDI in patients with IBD. 9
Risk factors
Individual features, such as age >65 years, low immune status, severe CDI, and BI/NAP1/027 infection, significantly increased the risk of recurrent Clostridium difficile infection (rCDI) and/or CDI. It should be noted that, as the number of risk factors increases, the risk of rCDI correspondingly increases.22–24 In univariate analysis, the predictor of a CDI test in IBD was body mass index <18.5 or >25. 25 In addition, sexual distinction was also reported as a risk factor because a higher prevalence of CDI was seen in females than in males. 26
A meta-analysis performed by Balram et al. 11 exhibited that the involvement of IBD in colon disease is an important risk factor for CDI in IBD. According to the National Hospital Discharge Survey, the incidence of CDI in ulcerative colitis (UC) patients was significantly higher than that in Crohn’s disease (CD) patients.9,27 The severity of symptoms of UC patients may be a risk factor for CDI. In a prospective cohort study of 319 UC patients, extensive colitis was identified as a risk factor for CDI (or =2.52, 95% CI: 1.03–6.17). Although there was no risk of C. difficile colitis in patients with UC after colectomy, CDI in patients with ileum bag reconstruction has been recognized in clinical practice. In a prospective cohort study of 319 patients with UC, a genetic risk model was established and extensive colitis was found to be a risk factor for CDI. 26
In addition, in the meta-analysis performed by Balram et al., 11 it was confirmed that the recent use of biological agents and antibiotics is an important risk factor for CDI in IBD. A study found that biotherapy with tumor necrosis factor-alpha (TNF-α) inhibitor-based biotherapy doubled the likelihood of CDI in patients with IBD. In particular, infliximab was associated with opportunistic bacterial infections, including CDI. 11 Stratified studies have shown that rCDI is associated with infliximab but not with adalimumab or immunomodulators.18,28 Furthermore, the use of antibiotics is thought to be a risk factor for CDI and may cause changes in the composition of bile acid in feces. The increase in primary bile acid and the reduction in secondary bile acid contributed to CDI. 29 In early univariate studies, CDI may be increased with the administration of hormones and immunosuppressive agents in IBD.4,30 The continued use of immunosuppressive agents is an independent risk factor in patients with CDI in IBD. 9 A retrospective cohort study of 999 patients with IBD (737 CD and 262 UC) demonstrated a twofold increase in the risk of CDI in IBD with continued immunosuppressive therapy. In addition, a large retrospective cohort study of 10,662 patients admitted to IBD showed an increase in the risk of CDI by more than three times during the 90 days following initiation of corticosteroid treatment [relative risk (RR) = 3.4; 95% CI: 1.9–6.1]. Similarly, in a large sample cohort of IBD, treatment of glucocorticoids had a threefold increase in the risk of CDI compared with immunomodulators or biotherapy, irrespective of the dose and duration of therapy.31,32 A retrospective study by Helene et al. on CDI risk factors in IBD showed that the recent use of nonsteroidal anti-inflammatory drugs (NSAIDs) was a risk factor for CDI related to IBD. In a multivariate analysis, the independent risk factor for CDI among IBD patients was the use of NSAID during 2 months prior to admission (or 3.8, 95% CI: 1.2–12.3, p = 0.02). Furthermore, a number of studies suggest that NSAIDs may also cause or worsen IBD. 25
Clinical presentation
CDI in IBD with young onset has a long course of disease and high mortality. The clinical features of IBD patients with CDI are often atypical and may be characterized by watery stools, bloody stools, fever, leukocytosis, and even toxic colitis. Furthermore, the incidence of extraintestinal manifestations was higher, including arthritis, osteoporosis, osteomalacia, gangrenous pyoderma, psoriatic arthritis, psoriasis, and chronic pancreatitis. 1 But, the classic endoscopic manifestation of pseudomembranous colitis is uncommon, it is difficult to distinguish CDI with active IBD. Currently, the guidelines recommend routine screening for CDI in IBD patients with worsening diarrhea.
Diagnosis
Endoscopic manifestation of pseudomembranous colitis is atypical. 33 An endoscopic examination of 93 hospitalized IBD patients with C. difficile found that only 12 cases, with a percentage around 13%, had endoscopic evidence of pseudomembranous.34,35 As C. difficile colonization is more prevalent in IBD patients, it is a challenge to distinguish CDI in IBD from active IBD. Therefore, CDI is mainly diagnosed by laboratory examinations.36,37
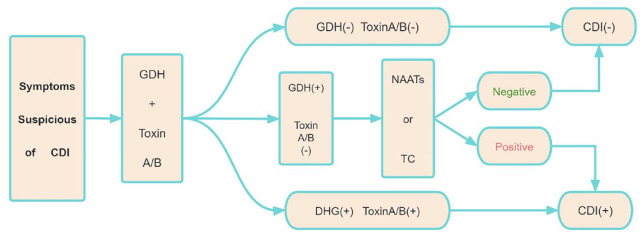
Nowadays, laboratory testing methods for the diagnosis of CDI include glutamate dehydrogenase (GDH), nucleic acid amplification test (NAAT), toxin A/B by enzyme immunoassay (EIA), and toxigenic culture (TC). GDH is a highly expressed metabolic enzyme. Normally, GDH antigens in stool samples can be detected directly by EIAs, but GDH cannot distinguish whether the strain produces toxins or not. Almost 20% of GDH-positive patients have negative virulence tests. The United States Food and Drug Administration (FDA) approved the first NAAT (CDI) in 2009. The DNA of C. difficile was amplified by polymerase chain reaction. The toxin A gene encodes TcdA, and the toxin B gene encodes TcdB. However, it is not sufficiently specific to differentiate the active infection due to the colonization of C. difficile and the production of toxins. Therefore, CDI can be over-diagnosed with NAAT because of the existence of asymptomatic C. difficile. 38 EIAs are often used for detecting the virulence of strain; however, it may lead to misdiagnose for lack of high sensitivity. 9 The European Society for Clinical Microbiology recommends a two-step diagnostic method for detecting CDI in patients with IBD, which is shown in Figure 1, using either an immunoassay or NAAT, followed by a highly specific toxin A/B EIA test. 39 Treatment should be considered only when the patient has a positive toxin EIA result indicating clinical CDI but not C. difficile colonization. 38 TC involves the cultivation of C. difficile from feces, and then testing of the isolated bacteria to determine whether they are toxic or not. Although TC has a sensitivity as high as 95% for the detection of C. difficile, its practicality is restricted by 3–5 days, thus is unsuitable for routine diagnosis.38,40
Figure 1.
The two-step approach for diagnosing CDI. EIA for GDH and toxin A/B.
CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification test; TC, toxigenic culture.
Management
It is recommended to discontinue the antibiotics that cause CDI. The main therapeutic options for CDI are drug therapy, fecal microbial transplantation (FMT), and surgical treatment. Vancomycin and fidaxomicin (FDX) are the first-line therapies for CDI in patients with IBD, and metronidazole is not recommended. 9 rCDI is common in IBD patients. In the most severe cases, only FMT can break the cycle of recurrence. 2 There is a significant increase in risk for each recurrence. The second recurrence rate rose to 40% after the first relapse, followed by more than 60%. Up to 50% of IBD patients with CDI require hospitalization, and 20% ultimately require colon surgery. 33
Prevention and control of hospital infection
Effective isolation measures should be taken for patients suspected of being infected with CDI to prevent community transmission. Patients who are suspected should be kept in independent rooms with at least a dedicated commode. In case of need, CDI patients should be grouped, instead of with those who are colonized or infected with other drug-resistant micro-organisms (such as methicillin-resistant staphylococci). In addition, it is recommended that the isolation should be continued for at least 48 h after the stool has solidified. 41 Gloves and other protective clothing should be worn by medical personnel before coming into contact with a CDI patient. Since alcohol hand sanitizer does not work for C. difficile spores while soap water hand washing has been confirmed the ability of to remove spores effectively (and is the first sanitation measure to prevent CDI transmission), each person who comes into contact with the patient should use soapy water to wash their hand. In addition, chlorine-containing products or other bactericides should be used to clean the ward.41,42 IBD patients were mainly infected with CDI through community and outpatient clinics. Therefore, we should carry out health education for patients, formulate prevention manuals, and emphasize hand hygiene to reduce the spread of C. difficile.
How to manage medications for IBD
Public Health England noted in the 2019 British Society for Gastroenterology guidelines for adult IBD that stopping corticosteroid therapy during CDI therapy is not necessary for patients with acute severe UC. 43 However, a study conducted by Solanky et al. showed that there was a higher risk of colectomy when escalated corticosteroid for IBD with CDI. 41 During acute CDI, clinicians may delay upgrades of steroids until CDI is controlled.
It is confirmed that whether to stop the use of immunosuppressants or not in IBD patients with CDI has not been clarified in the current consensus of guidelines after a systematic review of the current publicly available literature. When being asked to comment on CDI management in IBD, 46% of 169 gastroenterologists (25% of respondents were IBD specialists) opted for a combination of immunosuppression and antibiotics, while the remaining 54% preferred antibiotics alone to treat the flare.32,44 In addition, Beniwal-Patel et al. suggested that new immunosuppressants should not be started and scheduled infusions should be suspended until the symptoms of C. difficile are resolved in the case of new CDI. 10 While Krishna et al. pointed out that IBD patients with CDI should be treated with appropriate antibiotics immediately. If symptoms are not relieved in 48 h, starting/updating immunotherapy should be considered at the same time. 20 The guidelines of the American Gastroenterology Association recommend that steroid or immunosuppressive therapy should be started 72–96 h after the use of antibiotics. With the escalation of immunosuppression, patients should be closely monitored for worsening symptoms and impending complications. 32 The decision on whether or not to continue immunomodulator treatment should be made on an individual basis (including discussed surgery). 43 Similarly, the 2021 European Crohn’s and Colitis Organisation (ECCO) guidelines on the management of IBD stated that the effect of immunosuppressive agents on CDI progression in IBD patients is not clear. For IBD patients with CDI, whether to maintain immunosuppressants should be carefully considered after risk–benefit assessment and clinical judgment. 45
At present, the relationship between biological agents and CDI is still unclear. A retrospective study on the influence of infection type on the persistence and switching of biological agents conducted by Chen et al. found that sepsis and C. difficile were the major causes of drug withdrawal. 44 A cohort study of 10,662 patients with IBD found no meaningful association between infliximab and serious bacterial infections, including CDI. 46 While another prospective cohort study with 120 IBD patients and 40 non-IBD controls revealed that infliximab was independently associated with CDI. 47 Although an increase in opportunistic infections of vedolizumab has not been reported, CDI may also occur because of its intestinal selectivity.46,48 Colombel et al. analyzed safety data from six trials of vedolizumab and found that the exposure-adjusted incidence rate of CDI in patients who had been treated with vedolizumab was 0.7 (0.5 − 1.0)/100 patient-year compared with 0.0 (0.0 − 1.4)/100 patient-year in patients which were treated with placebo. 46 Sands et al. conducted the first head-to-head trial comparing biological agents for IBD. As expected, the rate of CDI was higher with vedolizumab than with adalimumab.47,49 Thus, experts recommend suspending vedolizumab during CDI treatment and restarting treatment after CDI remission. 50 As for ustekinumab, the pooled safety analysis of results from phase II/III studies exhibited a lower CDI risk compared with placebo (0.92 versus 2.18, event/100 patient-year) after 1-year follow-up. 51 In 2019, The British Society of Gastroenterology noted that IBD patients with active tuberculosis, sepsis, or opportunistic infections (including intestinal infections such as CDI) should not receive either vedolizumab or ustekinumab. 43 If a patient receiving anti-TNF, ustekinumab, or tofacitinib is diagnosed with CDI and biologics dosing expires, it is recommended to initiate treatment for CDI and delay (or hold for tofacitinib) biological agents for 5–7 days. After symptom relief and clinical stability are ensured, the biologic treatment can be restarted and the CDI therapy should be completed. This approach balances the risk of recurrent IBD with the risk of concurrent infection. 52
In a multicenter retrospective study analysis, escalation of corticosteroids or biological agents/immunosuppressive therapy was not associated with adverse events in IBD and CDI patient cohorts. For patients with active IBD who require treatment, delayed treatment may have adverse outcomes, including sepsis, colectomy, and/or death. 53 Some patients who still have significant IBD activity after treatment with antibiotics for CDI may benefit from intensive treatment with corticosteroids or biological agents/immunosuppressants. However, in some patients, such as those with sepsis, other infections, or end-organ involvement, the use of escalation therapy is contraindicated. These data from Dana et al. are consistent with current guidelines from the American Academy of Gastroenterology, which suggests that early treatment of IBD is appropriate once CDI-related antibiotics are initiated. 50
With regard to the treatment upgrading of corticosteroids or biological agents/immunosuppressants in the process of controlling CDI in patients with active IBD, there are no clear guidelines so far, and the literature is contradictory. More prospective data are needed to support clinical decisions.
Antibiotic treatments for CDI
Vancomycin
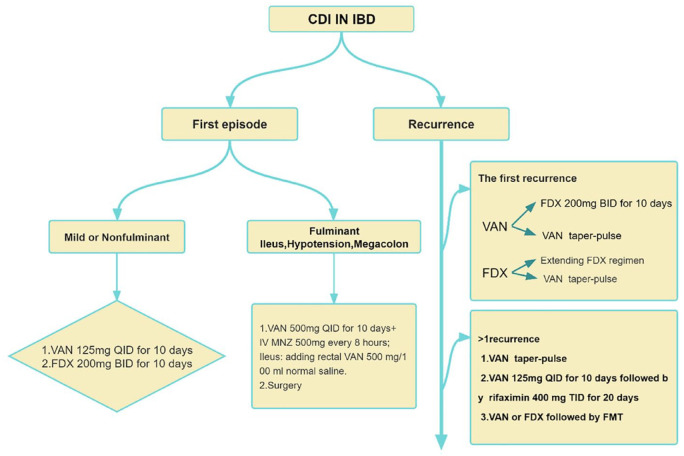
Vancomycin is a glycopeptide antibiotic that can inhibit C. difficile by oral administration. Vancomycin may be used in patients with CDI for the first time (extension may be considered), while metronidazole is not recommended in CDI-infected patients with IBD. 9 Compared with metronidazole, using vancomycin can decrease the rate of colectomy in adults with IBD. 33 The latest guidelines of the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) suggest that vancomycin is the standard therapy for the treatment of mild or non-fulminant CDI, with a dose of 125 mg, taken orally four times a day for 10 consecutive days.54,55 High-dose vancomycin (500 mg orally, four times a day) combined with metronidazole (500 mg intravenously, once every 8 h) is the preferred treatment for fulminant CDI. Rectal vancomycin (500 mg/100 ml normal saline) may be added to patients with severe intestinal obstruction. The IDSA guidelines recommend the use of an oral vancomycin pulsed-tapered regimen (125 mg orally four times/day, 10–14 days, then 125 mg orally two times/day for 7 days,125 mg orally one time/day for 7 days, and 125 mg orally one time/2–3 days for 2–8 weeks) in patients with the first relapse,54,55 shown in Figure 2. About 25% of patients using vancomycin will relapse. 56 Studies showed that the relapse rate of CDI was significantly reduced (1.8% versus 11.7%; p = 0.043) with a long course of vancomycin (21–42 days) compared with a short course of vancomycin (10–14 days) (Figure 2).9,57
Figure 2.
Antimicrobial treatment suggested for Clostridoides difficile infection (CDI) in Inflammatory bowel disease (IBD).VAN taper-pulse: 125 mg oral 4 times/day, 10–14 days, then 125 mg orally 2 times/day for 7 days, 125 mg oral 1 time/day for 7 days, and 125 mg oral 1 time/2–3 days for 2–8 weeks; Extending FDX regimen: FDX 200 mg orally, twice daily on days 1–5, and then once every other on days 7–25.
FDX, fidaxomich; FMT, fecal microbial transplantation; VAN, vancomycin.
Fidaxomicin
FDX, a narrow-spectrum macrocyclic antibiotic, was approved by the FDA in 2011 to treat CDI. The standard usage is 200 mg twice daily (q12h) for 10 days. Compared with the bacteriostatic effect of vancomycin on C. difficile, FDX can kill bacteria and has better in vitro inhibition efficacy on BI/NAP1/027 and other strains. 58 Furthermore, FDX does not have any other therapeutic indications and has little effect on intestinal flora. 59 In 2021, IDSA and SHEA jointly recommended FDX to substitute the standard regimen of vancomycin in patients with initial CDI, but implementation is subject to availability. 59
In clinical trials, FDX was shown to have sustained clinical improvement (initial clinical response, no recurrent symptoms) in CDI patients than vancomycin. The initial clinical response of the two drugs was similar, while the recurrence rate of CDI using FDX was lower. Two phase III registration trials have shown that FDX is quite equivalent to vancomycin in effectiveness in terms of initial treatment of CDI, and FDX significantly reduces the risk of recurrence by 10–15% at 28 days after finished treatment.60,61 In a pooled analysis of four trials, FDX had a longer sustained CDI remission than standard vancomycin [hazard ratio (RR): 1.16; 95% CI: 1.09–1.24]. 59 Similarly, a recent multicenter randomized single-blind clinical trial also has shown that FDX has higher rates of global cure in children and adolescents in CDI than vancomycin. 62 For elderly patients over 60 years old, extended pulse FDX (EPFDX) (200 mg twice daily for 1–5 days and once every other day for 7–25 days) was more effective than the standard dose of vancomycin in the long-term clinical treatment of CDI and exhibited significantly lower relapse rates. 63 For rCDI, if FDX is used in the first episode, the EPFDX regimen may be continued (FDX 200 mg orally, twice daily on days 1–5, and then once every other day on days 7–25), 64 shown in Figure 2. The use of FDX is currently recommended with caution in patients with IBD CDI because it is still unknown if FDX uptake will increase intestinal inflammation associated with IBD. However, in an open-label, single-arm, phase IIIB/IV trial performed by Christoph et al. on FDX pharmacokinetics in CDI patients, the maximal FDX concentration in IBD patients with CDI was found to remain within the range of concentration measured in non-IBD patients with CDI. Absorption of FDX did not increase in IBD. However, the concentration of FDX in feces was found to consistently reach or exceed therapeutic levels for C. difficile. 65 According to current pricing, the estimated annual costs of treating all patients in the United States and Canada with FDX are $2.06 billion and $60 million, respectively. If the cost of FDX could be reduced to below $1140 billion US dollars and $860 million Canadian dollars, respectively, it would promote prescribing practices. 66 Cost-effectiveness analysis also showed the priority of FDX to vancomycin in patients with initial onset CDI. In a recent economic model in Spain, EPFDX is more cost-effective as a first-line therapy than vancomycin in elderly patients over 60 years old for CDI. 63
Fecal microbial transplantation
As a therapeutic intervention to transfer fecal samples from healthy donors to patients, FMT was first performed in 1958, by an American surgeon named Eisman, on four patients with pseudomembranous enteritis. 67 The principle of this treatment is to correct the imbalance of the patient’s flora by transplanting the flora of healthy donors and weakening the pathogenic process. 68 According to the recommendations of the 2018 guidelines of The British Society of Gastroenterology and Healthcare Infection Society, FMT can be provided to patients with rCDI who have at least two recurrences, or patients who have one recurrence and are at risk of further attacks, including patients with severe and complex CDI. 69 Recently, the European Consensus Conference held in Rome highlighted that FMT was recommended as an alternative treatment for rCDI and should be considered for refractory CDI. 70 Furthermore, massive data suggest that FMT may be used in mild to moderate UC patients. 15 A review of FMT also indicated that the extent of IBD disease would be alleviated in IBD patients with C. difficile eradicated by FMT, and the treatment effectiveness of IBD drugs was expected to be improved. 71
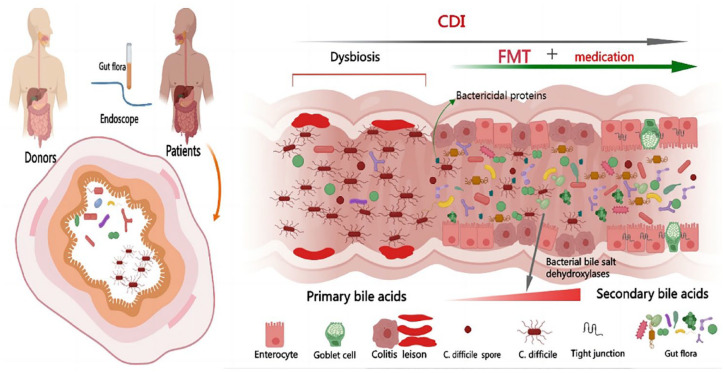
Pathologic stages in the development of CDI include intestinal dysbiosis and toxigenic strains. FMT is not only an effective treatment for rCDI but also a promising therapy for IBD.72,73 Dysbiosis of intestinal flora in IBD patients is a predisposing factor for CDI and may worsen the adverse consequences caused by CDI, including mucosal injury, intestinal inflammation, and increased mortality. 74 The destruction of intestinal flora leads to the imbalance of the proportion of primary bile acids and secondary bile acids in the intestine, thus increasing the susceptibility to CDI. Studies showed that the concentration of primary bile salt in rCDI patients was higher than that in the first episode and the control group. 75 Since the normal gut flora converts primary bile acids into secondary bile acids by bacterial bile salt dehydroxylases FMT can rapidly restore the bile acid metabolism flora, thereby reducing the risk of CDI recurrence. 76 In addition, FMT promotes the production of bactericidal proteins that inhibit the proliferation of C. difficile by reconstituting a rich flora 77 and reduces intestinal permeability by increasing the production of short-chain fatty acids, especially butyric acid, which is shown in Figure 3. Since butyric acid can maintain epithelial cell integrity, disease severity will be reduced. In addition, FMT can inhibit T-cell activity, leukocyte adhesion, and the production of inflammatory factors to correct immune dysregulation. 78 The methods of FMT transplantation include capsule, nasal gastroscope, nasal jejunoscopy, gastroscope, colonoscopy, sigmoidoscopy, and enema. 79 At present, the best transplantation method has not been determined. In a systematic review, the cure rate of CDI by colonoscopy FMT was higher than in other ways. 72 It is recommended that patients with FMT through the upper digestive tract should keep standing for at least 4 h after transplantation to avoid aspiration. 39 In theory, it is recommended to transplant the microbiota of unrelated donors to avoid potential shared genetic and environmental determinants of the gastrointestinal microbiota of relatives. 80
Figure 3.
Transferring fecal samples from healthy donors to patients; The gut flora converts primary bile acids into secondary bile acids by bacterial bile salt dehydroxylases, promoting the production of bactericidal proteins that inhibit the proliferations of Clostridium difficile.
A randomized, double-blind, placebo-controlled trial (Early FMT) has demonstrated that, compared with the standard of vancomycin alone, first-line FMT is more effective and superior in sustained resolution from CDI. 81 At the same time, an increasing body of evidence suggests that FMT is safe and effective for IBD patients with rCDI. It can reduce disease activity and avoid the risk of IBD treatment escalation. In a prospective cohort study of IBD patients treated with FMT, each patient received at least one fecal transplant using a colonoscopy. It was found that the cure rate (94%) of FMT for rCDI was similar to that of non-IBD patients. Among the patients who received a single fecal infusion, 38% of the patients relapsed CDI, and 83% of the patients had an effective second infusion. Therefore, repeated fecal infusion can improve the effective rate of FMT, and a sequential scheme should be considered. 82 A study by Tariq et al. showed that the total cure rate of CDI in IBD was 80% after FMT and no recurrence was found after a median of 9.3 (range, 0.1–51) months of follow-up. 80 In a multicenter cohort study that had 113 IBD patients with rCDI treated by FMT after vancomycin treatment, the cure rate of CDI reached 71%. During the long-term follow-up of 90 patients, 39% of patients had a decrease in IBD activity, and the exacerbation of IBD after FMT is uncommon. 83 At present, a great deal of research has proved that the cure rate of rCDI with FMT is 90%. 78 However, for fulminant CDI, a meta-analysis has demonstrated that low-quality data support FMT which has major adverse events and a low cure rate. 84 On the contrary, a retrospective analysis of 430 severe or fulminant CDI patients who accepted FMT showed that CDI-related mortality was 43.2% before the FMT treatment versus 12.1% after (p < 0.001), which has a great impact on reducing the CDI-related mortality. 85 Current studies have shown that FMT is equally effective and has the same side effects in patients using immunosuppression as in immunocompetent patients. 86 A lot of studies have demonstrateds that common complications of FMT in IBD patients include abdominal distension, diarrhea, abdominal pain, or fever, which can usually be relieved spontaneously. 39 Regarding colectomy and death due to toxic megacolon after FMT, it is considered to be more relevant to the underlying IBD. Currently, no definitive endoscopic data indicate that the outbreak of IBD is associated with FMT. There are currently no long-term safety data on FMT for CDI in IBD patients. 80
Cost-effectiveness analysis shows that FMT is the preferred treatment for rCDI, and the effect of early colonoscopy transplantation is the best. 70 However, there are few studies on the effectiveness of FMT in treating the first CDI infection in IBD. Furthermore, it is necessary to evaluate the efficacy of FMT in the primary prevention of CDI in patients with IBD. More randomized controlled trials (RCTs) will be required to assess the long-term efficacy and safety of FMT, and the issue of a flare-up of autoimmune disease needs further investigation, as well as to design an international standard donor screening program.
Rifaximin
Rifaximin is an oral rifamycin antibiotic that is not absorbable, so it is well tolerated by the human body. It is very suitable for the treatment of C. difficile and is a promising drug for the treatment of rCDI.87–89 Theoretically, the solubility of rifaximin in bile is increased by 70- to 120-fold so that the effective concentration of rifaximin should be in the distal small intestine and proximal colon where C. difficile germinates. Rifaximin inhibits the proliferation of C. difficile, promotes the recovery of intestinal commensal bacteria, and restores the intestinal barrier. An RCT by Major et al. showed that the use of rifaximin could reduce the risk of relapse by following standard therapy (experimental group: 400 mg rifaximin, TID for 2 weeks, followed by 200 mg TID for 2 weeks). 87 In addition, in a prospective single-blind trial, IBD children with CDI were randomly divided into a control group with oral metronidazole and an experimental group with rifaximin treatment. No statistical difference was seen in the cure rate and the recurrence rate. The results indicated that rifaximin was as effective as metronidazole in the treatment of children with CDI and IBD. 90 However, for rCDI, besides the previously recommended vancomycin taper/pulse regimen, IDSA guidelines in 2017 recommended rifaximin 400 mg tid for 20 days after 10 days of vancomycin. 91 In addition, rifaximin ‘tracer’ has been studied as an option to prevent recurrence with a promising result, and it has been listed in the latest guidelines of IDSA for the treatment of second or subsequent CDI recurrence. 92
Bezlotoxumab
Bezlotoxumab (BEZ) was approved by the FDA in October 2016 and is the first fully humanized monoclonal antibody directed against C. difficile toxin B. As a relatively new method to prevent rCDI, BEZ can prevent the damage of C. difficile to colon cells by neutralizing the toxin by combining with toxin B. 63 In vitro research has shown that BEZ can reduce toxicity-induced chemical reactions in tissue inflammation, including inhibition of monocyte, TNF-α, IL-1β (mRNA and/or protein) expression, as well as the reduction in colon epithelial injury. 93 Currently, BEZ has been approved for the prevention of high-risk patients with rCDI. 63 While the FDA has even approved BEZ for secondary prevention of rCDI. Two double-blind, randomized, placebo-controlled, phase III trials conducted by Wilcox et al. demonstrated that the rate of rCDI with BEZ was apparently lower than with that of placebo [MODIFY I: 17% (67 of 386) versus 28% (109 of 395); MODIFY II: 16% (62 of 395) versus 26% (97 of 378)]. 91 Another randomized trial which had 2559 participants showed that the rate of rCDI for BI strains of the control group (no BEZ) was higher than that of the experimental group (treated with BEZ) (43.9% versus 23.6%), and was also higher than that of non-BI strains (36.1% versus 21.4%). 92
BEZ is a one-time infusion for more than 60 min with the recommended dose of 10 mg/kg. The elimination half-life is about 18 days. After at least 3 months since the one-time injection, BEZ will convert into measurable antibody concentrations. No dose adjustment is required with renal or hepatic impairment. There are no publicly available literature about drug–drug interactions relating to BEZ. 59 In patients with primary and rCDI, a single dose of 10 mg/kg BEZ could significantly reduce CDI relapse from 25% to 15% under Standard of Care (SoC) antibiotics. 75 In the modified trial, a single injection of BEZ combined with SoC antibacterial therapy was used to treat adult CDI, and the recurrence rate of CDI within 12 weeks was found to be 40% lower than that of SoC antibacterial therapy alone. 93 The benefit of BEZ is limited to patients with rCDI risk factors, such as age >65 years, history of CDI, immunodeficiency, severe CDI, and infections caused by specific highly toxic C. difficile epidemic strains. In addition, with the increase in the number of risk factors, the benefit of BEZ will also be enhanced. Otherwise, if no recurrence risk factor exists, the patient cannot benefit from BEZ. 55 A study of recurrence risk factors showed that the reduction in rCDI was the largest in participants with ⩾3 risk factors, and participants with 1 or 2 risk factors might also benefit. BEZ is generally well tolerated and its safety is similar to that of placebo. Infusion-related reactions are the most frequent (10%) side effects. 63 Model-based cost-effectiveness analysis showed that the combination of BEZ and SoC antibiotics was superior to SoC alone in the prevention of rCDI. 93 Since BEZ does not affect the efficacy of antibiotics for CDI, and may reduce the use of subsequent antibiotics, the disruption of the intestinal flora could be thereby minimized. In addition, BEZ can shorten hospital stay time and reduce retreatment. BEZ can also be perfused to the 14th day of SoC antibacterial treatment, and be injected in outpatient, which will reduce hospitalization cost. 93 It should be noted that the contraindication of BEZ is heart failure. 64
Conclusion
CDI not only increases the medical cost of patients but also reduces the quality of life of IBD patients. In addition, high recurrence rate and increases in the risk of disease deterioration in IBD patients are caused by CDI. The risk assessment model of IBD-CDI should be established to assess high-risk patients. Whether to stop the use of immunosuppressants or not in IBD patients with CDI has reached consensus because of the contrasting data on the influence of biologics on CDI risk and the lack of RCT trials. However, most experts recommended initiating treatment for CDI and delaying (or holding for tofacitinib) the biologic for 5–7 days in IBD patients with CDI, which seems to balance the risk of recurrent IBD with the risk of concurrent infection. At present, vancomycin and FDX are the first-line drugs for CDI in IBD patients. However, with the wide use of drugs and the change in virulence of C. difficile strains, the antimicrobial activity of drugs is affected so new treatment methods need to be studied. The primary prevention value of FMT and rifaximin in IBD patients needs to be thoroughly analyzed by further RCT and cost-effectiveness evaluation. Recently, plentiful research was available on the use of FMT and rifaximin for rCDI in IBD patients; unfortunately, the treatment of the first infection was not included. The recurrence rate of CDI in IBD patients is high; however, studies on the relevant prevention and treatment are mainly retrospective. So, more prospective, multicenter randomized trials are needed in the future. With the application of biological agents, immunosuppressants and new small-molecule drugs in IBD patients and the improvement of IBD diagnosis technology, patients can receive timely and more effective treatment. Thus, the epidemiology of CDI in IBD patients is also changing subsequently. Corresponding clinical retrospective studies on the epidemiology of CDI in IBD patients needs to be conducted as soon as possible in the near future.
Acknowledgments
None.
Contributor Information
Mei Bai, Department of Gastroenterology, Chongqing General Hospital, Chongqing, China.
Hong Guo, Department of Gastroenterology, Chongqing General Hospital, 28 Jinshan Avenue, Yubei District, Chongqing 401147, China.
Xiao-Yao Zheng, Department of Pharmaceutics, School of Pharmacy, Fudan University, Shanghai, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: All authors have consented for publication.
Author contributions: Mei Bai: Conceptualization; Project administration; Resources; Writing – original draft.
Hong Guo: Conceptualization; Resources; Supervision; Writing – review & editing.
Xiao-Yao Zheng: Formal analysis; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data is available online.
References
- 1. Gillespie W, Marya N, Fahed J, et al. Clostridium difficile in inflammatory bowel disease: a retrospective study. Gastroenterol Res Pract 2017; 2017: 4803262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez C, Romero E, Garrido-Sanchez L, et al. Microbiota insights in clostridium difficile infection and inflammatory bowel disease. Gut Microbes 2020; 12: 1725220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moens A, Verstockt B, Machiels K, et al. Clostridium difficile infection in inflammatory bowel disease: epidemiology over two decades. Eur J Gastroenterol Hepatol 2019; 31: 668–673. [DOI] [PubMed] [Google Scholar]
- 4. Maharshak N, Barzilay I, Zinger H, et al. Clostridium difficile infection in hospitalized patients with inflammatory bowel disease: prevalence, risk factors, and prognosis. Medicine (Baltimore) 2018; 97: e9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang F, Cui B, He X, et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell 2018; 9: 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ran Z, Wu K, Matsuoka K, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J Gastroenterol Hepatol 2021; 36: 637–645. [DOI] [PubMed] [Google Scholar]
- 7. Razik R, Rumman A, Bahreini Z, et al. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. Am J Gastroenterol 2016; 111: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 8. Fu N, Wong T. Clostridium difficile infection in patients with inflammatory bowel disease. Curr Infect Dis Rep 2016; 18: 19. [DOI] [PubMed] [Google Scholar]
- 9. Sehgal K, Yadav D, Khanna S. The interplay of Clostridioides difficile infection and inflammatory bowel disease. Therap Adv Gastroenterol 2021; 14: 1088203261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beniwal-Patel P, Stein DJ, Munoz-Price LS. The juncture between Clostridioides difficile infection and inflammatory bowel diseases. Clin Infect Dis 2019; 69: 366–372. [DOI] [PubMed] [Google Scholar]
- 11. Balram B, Battat R, Al-Khoury A, et al. Risk factors associated with clostridium difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2019; 13: 27–38. [DOI] [PubMed] [Google Scholar]
- 12. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006; 55: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 2012; 13: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer M, Kao D, Kelly C, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016; 22: 2402–2409. [DOI] [PubMed] [Google Scholar]
- 15. Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in Clostridium difficile-associated disease: past and present perspectives. Gut Microbes 2010; 1: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 2005; 18: 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994; 330: 257–262. [DOI] [PubMed] [Google Scholar]
- 18. Allegretti JR, Kearney S, Li N, et al. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther 2016; 43: 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunt JJ, Ballard JD. Variations in virulence and molecular biology among emerging strains of Clostridium difficile. Microbiol Mol Biol Rev 2013; 77: 567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao K, Higgins PD. Epidemiology, diagnosis, and management of clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016; 22: 1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khanna S, Tosh PK. A clinician’s primer on the role of the microbiome in human health and disease. Mayo Clin Proc 2014; 89: 107–114. [DOI] [PubMed] [Google Scholar]
- 22. Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 2018; 67: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abou CC, Pepin J, Sirard S, et al. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One 2014; 9: e98400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Regnault H, Bourrier A, Lalande V, et al. Prevalence and risk factors of Clostridium difficile infection in patients hospitalized for flare of inflammatory bowel disease: a retrospective assessment. Dig Liver Dis 2014; 46: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 26. Ananthakrishnan AN, Oxford EC, Nguyen DD, et al. Genetic risk factors for Clostridium difficile infection in ulcerative colitis. Aliment Pharmacol Ther 2013; 38: 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saffouri G, Gupta A, Loftus EJ, et al. The incidence and outcomes from Clostridium difficile infection in hospitalized adults with inflammatory bowel disease. Scand J Gastroenterol 2017; 52: 1240–1247. [DOI] [PubMed] [Google Scholar]
- 28. D’Aoust J, Battat R, Bessissow T. Management of inflammatory bowel disease with Clostridium difficile infection. World J Gastroenterol 2017; 23: 4986–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Theriot CM, Koenigsknecht MJ, Carlson PJ, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 2014; 5: 3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Y, Xu H, Xu T, et al. Case-control study of inflammatory bowel disease patients with and without Clostridium difficile infection and poor outcomes in patients coinfected with C. difficile and cytomegalovirus. Dig Dis Sci 2018; 63: 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneeweiss S, Korzenik J, Solomon DH, et al. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther 2009; 30: 253–264. [DOI] [PubMed] [Google Scholar]
- 32. Viazis N, Pontas C, Karmiris K, et al. Prevalence of Clostridium difficile infection among hospitalized inflammatory bowel disease patients in Greece. Eur J Gastroenterol Hepatol 2019; 31: 773–776. [DOI] [PubMed] [Google Scholar]
- 33. Issa M, Vijayapal A, Graham M B, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol 2007; 5: 345–351. [DOI] [PubMed] [Google Scholar]
- 34. Ben-Horin S, Margalit M, Bossuyt P, et al. Prevalence and clinical impact of endoscopic pseudomembranes in patients with inflammatory bowel disease and Clostridium difficile infection. J Crohns Colitis 2010; 4: 194–198. [DOI] [PubMed] [Google Scholar]
- 35. Lee H S, Plechot K, Gohil S, et al. Clostridium difficile: diagnosis and the consequence of over diagnosis. Infect Dis Ther 2021; 10: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levy AN, Allegretti JR. Insights into the role of fecal microbiota transplantation for the treatment of inflammatory bowel disease. Therap Adv Gastroenterol 2019; 12: 321941539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bartlett JG. Detection of Clostridium difficile infection. Infect Control Hosp Epidemiol 2010; 31(Suppl. 1): S35–S37. [DOI] [PubMed] [Google Scholar]
- 38. Rancich M, Roman C. Updated guidelines for diagnosing and managing Clostridium difficile. JAAPA 2019; 32: 48–50. [DOI] [PubMed] [Google Scholar]
- 39. Goyal A, Yeh A, Bush BR, et al. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm Bowel Dis 2018; 24: 410–421. [DOI] [PubMed] [Google Scholar]
- 40. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68(Suppl. 3): s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solanky D, Pardi DS, Loftus EV, et al. Colon surgery risk with corticosteroids versus immunomodulators or biologics in inflammatory bowel disease patients with Clostridium difficile infection. Inflamm Bowel Dis 2019; 25: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yanai H, Nguyen GC, Yun L, et al. Practice of gastroenterologists in treating flaring inflammatory bowel disease patients with Clostridium difficile: antibiotics alone or combined antibiotics/immunomodulators?. Inflamm Bowel Dis 2011; 17: 1540–1546. [DOI] [PubMed] [Google Scholar]
- 43. Khanna S, Shin A, Kelly CP. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the clinical practice updates committee of the AGA institute. Clin Gastroenterol Hepatol 2017; 15: 166–174. [DOI] [PubMed] [Google Scholar]
- 44. Chen C, Hartzema A G, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis 2019; 25: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 45. Garcia PG, Chebli LA, Da RRT, et al. Impact of superimposed Clostridium difficile infection in Crohn’s or ulcerative colitis flares in the outpatient setting. Int J Colorectal Dis 2018; 33: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 46. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017; 66: 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sands BE, Peyrin-Biroulet L, Loftus EJ, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019; 381: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 48. Queiroz N, Regueiro M. Safety considerations with biologics and new inflammatory bowel disease therapies. Curr Opin Gastroenterol 2020; 36: 257–264. [DOI] [PubMed] [Google Scholar]
- 49. Click B, Regueiro M. A practical guide to the safety and monitoring of new IBD therapies. Inflamm Bowel Dis 2019; 25: 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lukin DJ, Lawlor G, Hudesman DP, et al. Escalation of immunosuppressive therapy for inflammatory bowel disease is not associated with adverse outcomes after infection with Clostridium difficile. Inflamm Bowel Dis 2019; 25: 775–781. [DOI] [PubMed] [Google Scholar]
- 51. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108: 478–499. [DOI] [PubMed] [Google Scholar]
- 52. Cammarota G, Gallo A, Ianiro G, et al. Emerging drugs for the treatment of clostridium difficile. Expert Opin Emerg Drugs 2019; 24: 17–28. [DOI] [PubMed] [Google Scholar]
- 53. Abad C, Safdar N. A review of Clostridioides difficile infection and antibiotic-associated diarrhea. Gastroenterol Clin North Am 2021; 50: 323–340. [DOI] [PubMed] [Google Scholar]
- 54. Lei D K, Ollech J E, Andersen M, et al. Long-duration oral vancomycin to treat Clostridioides difficile in patients with inflammatory bowel disease is associated with a low rate of recurrence. Am J Gastroenterol 2019; 114: 1904–1908. [DOI] [PubMed] [Google Scholar]
- 55. Ritter AS, Petri WJ. New developments in chemotherapeutic options for Clostridium difficile colitis. Curr Opin Infect Dis 2013; 26: 461–470. [DOI] [PubMed] [Google Scholar]
- 56. Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73: e1029–e1044. [DOI] [PubMed] [Google Scholar]
- 57. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364: 422–431. [DOI] [PubMed] [Google Scholar]
- 58. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12: 281–289. [DOI] [PubMed] [Google Scholar]
- 59. Wolf J, Kalocsai K, Fortuny C, et al. Safety and efficacy of fidaxomicin and vancomycin in children and adolescents with Clostridioides (Clostridium) difficile infection: a phase 3, multicenter, randomized, single-blind clinical trial (SUNSHINE). Clin Infect Dis 2020; 71: 2581–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Castro I, Tasias M, Calabuig E, et al. Doctor, my patient has CDI and should continue to receive antibiotics. The (unresolved) risk of recurrent CDI. Rev Esp Quimioter 2019; 32(Suppl. 2): 47–54. [PMC free article] [PubMed] [Google Scholar]
- 61. Khanna S. My treatment approach to Clostridioides difficile infection. Mayo Clin Proc 2021; 96: 2192–2204. [DOI] [PubMed] [Google Scholar]
- 62. Hogenauer C, Mahida Y, Stallmach A, et al. Pharmacokinetics and safety of fidaxomicin in patients with inflammatory bowel disease and Clostridium difficile infection: an open-label Phase IIIb/IV study (PROFILE). J Antimicrob Chemother 2018; 73: 3430–3441. [DOI] [PubMed] [Google Scholar]
- 63. Patel D, Senecal J, Spellberg B, et al. Fidaxomicin to prevent recurrent Clostridioides difficile: what will it cost in the USA and Canada?. JAC Antimicrob Resist 2023; 5: c138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44: 854–859. [PubMed] [Google Scholar]
- 65. Patel K, Patel A, Hawes D, et al. Faecal microbiota transplantation: looking beyond Clostridium difficile infection at inflammatory bowel disease. Gastroenterol Hepatol Bed Bench 2018; 11: 1–8. [PMC free article] [PubMed] [Google Scholar]
- 66. Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J Hosp Infect 2018; 100(Suppl. 1): S1–S31. [DOI] [PubMed] [Google Scholar]
- 67. Cammarota G, Gallo A, Bibbo S. Fecal microbiota transplant for C. difficile infection: just say yes. Anaerobe 2019; 60: 102109. [DOI] [PubMed] [Google Scholar]
- 68. Nitzan O, Elias M, Chazan B, et al. Clostridium difficile and inflammatory bowel disease: role in pathogenesis and implications in treatment. World J Gastroenterol 2013; 19: 7577–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu Y, Alnababtah K, Cook S, et al. Healthcare providers’ perception of faecal microbiota transplantation with Clostridium difficile infection and inflammatory bowel disease: a quantitative systematic review. Therap Adv Gastroenterol 2021; 14: 1088225655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Borody TJ, Eslick GD, Clancy RL. Fecal microbiota transplantation as a new therapy: from Clostridioides difficile infection to inflammatory bowel disease, irritable bowel syndrome, and colon cancer. Curr Opin Pharmacol 2019; 49: 43–51. [DOI] [PubMed] [Google Scholar]
- 71. Chen Y, Furuya-Kanamori L, Doi SA, et al. Clostridium difficile infection and risk of colectomy in patients with inflammatory bowel disease: a bias-adjusted meta-analysis. Inflamm Bowel Dis 2017; 23: 200–207. [DOI] [PubMed] [Google Scholar]
- 72. Villafuerte GJ, Kelly CP. Bezlotoxumab: anti-toxin B monoclonal antibody to prevent recurrence of Clostridium difficile infection. Expert Rev Gastroenterol Hepatol 2017; 11: 611–622. [DOI] [PubMed] [Google Scholar]
- 73. Messias BA, Franchi BF, Pontes PH, et al. Fecal microbiota transplantation in the treatment of Clostridium difficile infection: state of the art and literature review. Rev Col Bras Cir 2018; 45: e1609. [DOI] [PubMed] [Google Scholar]
- 74. Chen T, Zhou Q, Zhang D, et al. Effect of faecal microbiota transplantation for treatment of Clostridium difficile infection in patients with inflammatory bowel disease: a systematic review and meta-analysis of cohort studies. J Crohns Colitis 2018; 12: 710–717. [DOI] [PubMed] [Google Scholar]
- 75. Sunkara T, Rawla P, Ofosu A, et al. Fecal microbiota transplant: a new frontier in inflammatory bowel disease. J Inflamm Res 2018; 11: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bouri S, Hart A. Fecal microbial transplantation: an update. Curr Opin Clin Nutr Metab Care 2018; 21: 405–410. [DOI] [PubMed] [Google Scholar]
- 77. Haifer C, Leong RW, Paramsothy S. The role of faecal microbiota transplantation in the treatment of inflammatory bowel disease. Curr Opin Pharmacol 2020; 55: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baunwall S, Andreasen SE, Hansen MM, et al. Faecal microbiota transplantation for first or second Clostridioides difficile infection (EarlyFMT): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol 2022; 7: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 79. Ianiro G, Bibbo S, Porcari S, et al. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center. Gut Microbes 2021; 13: 1994834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tariq R, Disbrow MB, Dibaise JK, et al. Efficacy of fecal microbiota transplantation for recurrent C. difficile infection in inflammatory bowel disease. Inflamm Bowel Dis 2020; 26: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 81. van Lingen EE, Baunwall SSMD, Lieberknecht SSC, et al. Short- and long-term follow-up after fecal microbiota transplantation as treatment for recurrent Clostridioides difficile infection in patients with inflammatory bowel disease. Therap Adv Gastroenterol 2023; 16: 1108339261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tixier EN, Verheyen E, Luo Y, et al. Systematic review with meta-analysis: fecal microbiota transplantation for severe or fulminant Clostridioides difficile. Dig Dis Sci 2022; 67: 978–988. [DOI] [PubMed] [Google Scholar]
- 83. Abu-Sbeih H, Ali FS, Wang Y. Clinical review on the utility of fecal microbiota transplantation in immunocompromised patients. Curr Gastroenterol Rep 2019; 21: 8. [DOI] [PubMed] [Google Scholar]
- 84. Ng QX, Loke W, Foo NX, et al. A systematic review of the use of rifaximin for Clostridium difficile infections. Anaerobe 2019; 55: 35–39. [DOI] [PubMed] [Google Scholar]
- 85. Kokkotou E, Moss AC, Michos A, et al. Comparative efficacies of rifaximin and vancomycin for treatment of Clostridium difficile-associated diarrhea and prevention of disease recurrence in hamsters. Antimicrob Agents Chemother 2008; 52: 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. DuPont HL. Review article: The antimicrobial effects of rifaximin on the gut microbiota. Aliment Pharmacol Ther 2016; 43(Suppl. 1): 3–10. [DOI] [PubMed] [Google Scholar]
- 87. Major G, Bradshaw L, Boota N, et al. Follow-on rifaximin for the prevention of recurrence following standard treatment of infection with Clostridium difficile (RAPID): a randomised placebo controlled trial. Gut 2019; 68: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gawronska A, Banasiuk M, Lachowicz D, et al. Metronidazole or rifaximin for treatment of Clostridium difficile in pediatric patients with inflammatory bowel disease: a randomized clinical trial. Inflamm Bowel Dis 2017; 23: 2209–2214. [DOI] [PubMed] [Google Scholar]
- 89. Rao K, Malani PN. Diagnosis and treatment of Clostridioides (Clostridium) difficile infection in adults in 2020. JAMA 2020; 323: 1403–1404. [DOI] [PubMed] [Google Scholar]
- 90. Deeks ED. and Bezlotoxumab: A review in preventing Clostridium difficile infection recurrence. Drugs 2017; 77: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 91. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376: 305–317. [DOI] [PubMed] [Google Scholar]
- 92. Johnson S, Citron DM, Gerding DN, et al. Efficacy of bezlotoxumab in trial participants infected with Clostridioides difficile strain BI associated with poor outcomes. Clin Infect Dis 2021; 73: e2616–e2624. [DOI] [PubMed] [Google Scholar]
- 93. Prabhu VS, Dubberke ER, Dorr MB, et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent clostridium difficile infection. Clin Infect Dis 2018; 66: 355–362. [DOI] [PubMed] [Google Scholar]