Abstract
BACKGROUND
Intravenous fluids and vasopressor agents are commonly used in early resuscitation of patients with sepsis, but comparative data for prioritizing their delivery are limited.
METHODS
In an unblinded superiority trial conducted at 60 U.S. centers, we randomly assigned patients to either a restrictive fluid strategy (prioritizing vasopressors and lower intravenous fluid volumes) or a liberal fluid strategy (prioritizing higher volumes of intravenous fluids before vasopressor use) for a 24-hour period. Randomization occurred within 4 hours after a patient met the criteria for sepsis-induced hypotension refractory to initial treatment with 1 to 3 liters of intravenous fluid. We hypothesized that all-cause mortality before discharge home by day 90 (primary outcome) would be lower with a restrictive fluid strategy than with a liberal fluid strategy. Safety was also assessed.
RESULTS
A total of 1563 patients were enrolled, with 782 assigned to the restrictive fluid group and 781 to the liberal fluid group. Resuscitation therapies that were administered during the 24-hour protocol period differed between the two groups; less intravenous fluid was administered in the restrictive fluid group than in the liberal fluid group (difference of medians, −2134 ml; 95% confidence interval [CI], −2318 to −1949), whereas the restrictive fluid group had earlier, more prevalent, and longer duration of vasopressor use. Death from any cause before discharge home by day 90 occurred in 109 patients (14.0%) in the restrictive fluid group and in 116 patients (14.9%) in the liberal fluid group (estimated difference, −0.9 percentage points; 95% CI, −4.4 to 2.6; P = 0.61); 5 patients in the restrictive fluid group and 4 patients in the liberal fluid group had their data censored (lost to follow-up). The number of reported serious adverse events was similar in the two groups.
CONCLUSIONS
Among patients with sepsis-induced hypotension, the restrictive fluid strategy that was used in this trial did not result in significantly lower (or higher) mortality before discharge home by day 90 than the liberal fluid strategy. (Funded by the National Heart, Lung, and Blood Institute; CLOVERS ClinicalTrials.gov number, NCT03434028.)
Intravenous fluid resuscitation is a common therapy used in the initial treatment of patients with septic shock and sepsis-induced hypotension. The goal of initial fluid therapy is to increase depleted or functionally reduced intravascular volume that occurs in sepsis owing to a vasodilated vascular network.1 This approach can augment macrovascular perfusion (e.g., stroke volume and cardiac output) and microvascular perfusion (e.g., capillary blood flow) and counter organ hypoperfusion, a factor in the pathophysiology of sepsis that tends to drive resuscitation practices. However, intravenous fluid resuscitation can create dilutional coagulopathy, fluid overload, and pathogenic edema in the lungs and other organs.2 Vasopressor agents are also commonly used to treat hypoperfusion by inducing constriction of arterioles and venules and increasing cardiac contractility. Vasopressor therapy also comes with risks that include vasoconstriction resulting in tissue ischemia, increased cardiac work load, and arrhythmias. For decades, clinicians have used these two therapies, typically in combination, to provide supportive care for patients with sepsis-induced hypoperfusion. There are limited data to guide specific use of intravenous fluids or vasopressors in the early care of patients with sepsis-induced hypotension.
Previous trials have shown that early recognition of sepsis and hypotension or shock allows for the delivery of therapies that improve outcomes, a situation that highlights the key need for prompt action.3,4 Although the administration of large volumes of fluid (a liberal fluid strategy) is a common practice during the initial resuscitative phase of septic shock management, this practice is based on low-quality evidence.1,5 Arguments based on physiological factors and observational data provide a strong rationale for an alternative approach that uses lower volumes of fluid and earlier initiation of vasopressor agents (a restrictive fluid strategy); this approach is of growing interest.5–11 Although observational clinical studies suggest that a restrictive fluid strategy is potentially superior to a liberal fluid strategy,12–14 a recent randomized clinical trial involving patients in the intensive care unit (ICU) showed no difference in 90-day mortality or other outcomes when comparing a restrictive approach to unguided resuscitation.15 The lack of robust data to guide fluid and vasopressor use for early sepsis care contributes to practice variability and controversy around approaches to fluid and vasopressor use, especially in the early phase of resuscitation.
We conducted the Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial to compare the effects of a restrictive fluid strategy (with early use of vasopressors) to a liberal fluid strategy. We hypothesized that a restrictive fluid strategy used during the first 24 hours of resuscitation for sepsis-induced hypotension would lead to lower mortality before discharge home by day 90 than a liberal fluid strategy.
METHODS
TRIAL OVERSIGHT
This multicenter, randomized, unblinded superiority trial was funded by the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) as part of the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. The PETAL Clinical Coordinating Center oversaw data acquisition and handling, and the members of the writing committee created the trial protocol and statistical analysis plan (available with the full text of this article at NEJM.org). A central institutional review board and NHLBI-appointed independent data and safety monitoring board reviewed and approved the trial protocol. All the patients or their legal authorized representatives provided written informed consent for participation in the trial.
PATIENTS
Adult patients (≥18 years of age) with a suspected or confirmed infection (broadly defined as the administration or planned administration of antibiotic agents) and sepsis-induced hypotension (systolic blood pressure, <100 mm Hg after the administration of ≥1000 ml of intravenous fluid) were eligible. Key exclusion criteria were an elapse of more than 4 hours since the meeting of the criteria for hypotension refractory to the intravenous administration of at least 1000 ml of fluid, an elapse of more than 24 hours since presentation at the hospital, previous receipt of more than 3000 ml of intravenous fluid during this episode (including prehospital administration of fluid by emergency medical services), the presence of fluid overload, and severe volume depletion from nonsepsis causes. Patients were enrolled at trial sites when research personnel were available to obtain informed consent from patients or their legal authorized representatives; the hours during which research personnel were available varied across locations but was typically during daytime and evening hours with less coverage on weekends. A complete list of the enrollment criteria is provided in the Supplementary Appendix, available at NEJM.org.
TRIAL PROCEDURES
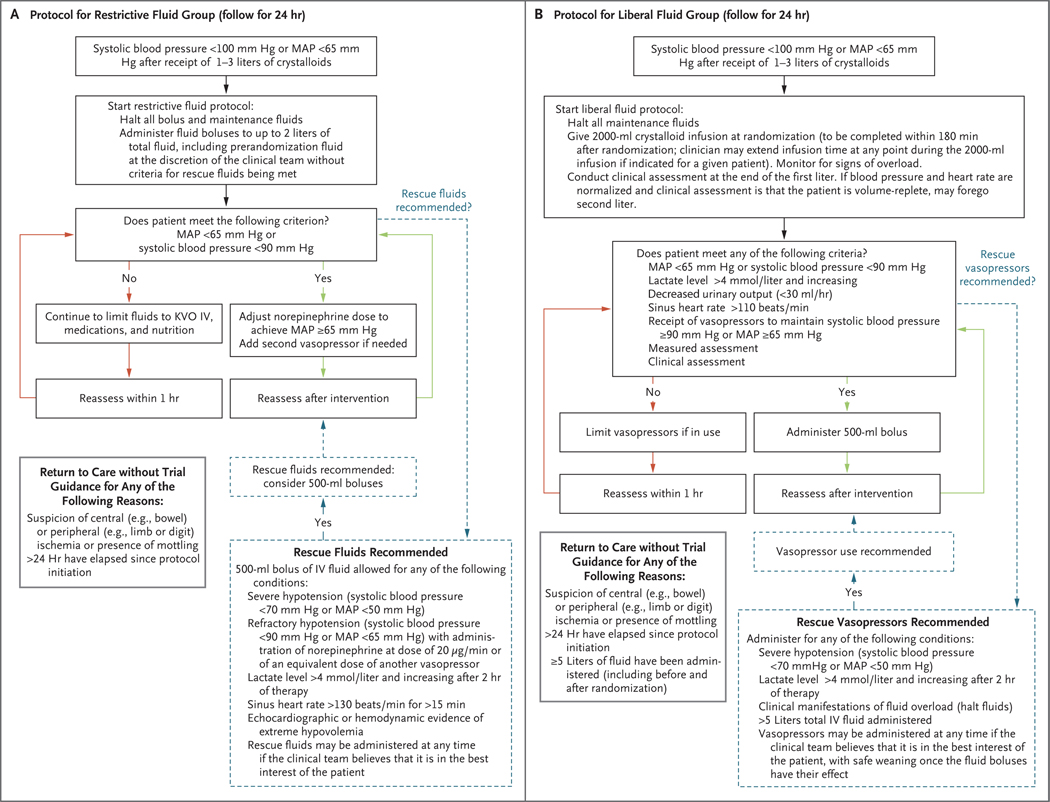
We randomly assigned participants in a 1:1 ratio to either a restrictive fluid strategy (with early vasopressor use) or a liberal fluid strategy; in each group, the assigned protocol was followed for a period of 24 hours. Randomization was conducted with the use of a Web-based centralized system, with stratification according to trial site. The restrictive fluid protocol prioritized vasopressors as the primary treatment for sepsisinduced hypotension, with “rescue fluids” being permitted for prespecified indications that suggested severe intravascular volume depletion (Fig. 1A). The liberal fluid protocol consisted of a recommended initial 2000-ml intravenous infusion of isotonic crystalloid, followed by fluid boluses administered on the basis of clinical triggers (e.g., tachycardia) with “rescue vasopressors” permitted for prespecified indications (Fig. 1B). A protocol amendment implemented in October 2019 allowed for limiting the initial infusion to 1000 ml if the patient’s blood pressure and heart rate had stabilized (systolic blood pressure of ≥110 mm Hg or mean arterial pressure of ≥70 mm Hg and heart rate of <90 beats per minute) and the clinical assessment was that the patient was “volume replete” (i.e., was unlikely to benefit from additional intravenous fluid administration) (see the Supplementary Appendix).
Figure 1. Fluid and Vasopressor Administration Protocols in the Restrictive Fluid Group and the Liberal Fluid Group.
Panel A shows the instructions for intravenous (IV) fluid and vasopressor administration in the restrictive fluid group, and Panel B the instructions in the liberal fluid group. In both trial groups, all protocol assessments, such as frequency of vital-sign monitoring, lactic acid measurements, and echocardiographic interventions, were performed at the discretion of the clinical team. The restrictive fluid protocol suggested norepinephrine as the primary vasopressor and epinephrine as a second vasopressor; neither was required. The restrictive fluid protocol defined “echocardiographic or hemodynamic evidence of extreme hypovolemia” as a maximal diameter of the inferior vena cava (IVC) of less than 5 mm, an empty left ventricle on echocardiography (e.g. left ventricular end diastolic area index, <5.5 cm2 per square meter of body-surface area), or stroke-volume increase of more than 30% in response to a passive leg raise, fluid challenge, or positive-pressure breaths. KVO denotes keep vein open, and MAP mean arterial pressure. The liberal fluid protocol instructed that patients receiving vasopressors should have the dose adjusted down or vasopressors discontinued, as feasible. The protocol included an instruction that care team members could use any available “measured assessment” they chose (e.g. echocardiography, IVC measurement, or central venous pressure measurement) or any type of “clinical assessment” of volume status to trigger use of additional fluids. If a patient had manifestations of fluid overload, fluids were to be halted. The liberal fluid protocol also expressly permitted the use of vasopressors after the administration of 5 liters of total fluid.
A combination of a trial team supporting the protocol (e.g., answering questions and helping to implement the protocol) and the clinical team following the protocol guided the use of vasopressors and fluids for 24 hours. As a protocol-specified option, the clinical team could override the protocol-specified care instructions at any time if it was judged to be in the best interest of the patient. We allowed the initial administration of vasopressor therapy through either a central venous catheter or a peripheral intravenous catheter sized 20 gauge or larger; this practice was outlined in the trial protocol and informed consent form. We monitored protocol adherence in the first 300 patients and in a 10% random sample of patients throughout the rest of the trial (see the Supplementary Appendix).
OUTCOMES
The primary outcome was death from any cause before discharge home by day 90. We defined home as the same setting or a setting similar to the one where the patient resided before becoming ill. Thus, if a patient originated from a private residence and was discharged from the hospital to a rehabilitation setting, we assessed for vital status until return to the private residence.
Secondary outcomes included 28-day measures of the number of days free from ventilator use, days free from renal-replacement therapy, days free from vasopressor use, days out of the ICU, and days out of the hospital. Systematically collected data on safety outcomes included the initiation of mechanical ventilation, new-onset atrial and ventricular arrhythmias, and complications related to peripheral and central venous catheter use.
STATISTICAL ANALYSIS
We sought to detect an absolute between-group difference of 4.5 percentage points in the incidence of death before discharge home by day 90 (the primary outcome), assuming death would occur in 15% of the patients in the liberal fluid group and in 10.5% of those in the restrictive fluid group. Therefore, we estimated that a total sample of 2320 patients would need to be enrolled in order for the trial to have 90% power at an overall two-sided alpha level of 0.05. In addition, the design incorporated prespecified criteria to stop the trial for efficacy in either group or for futility. The data and safety monitoring board could recommend termination of the trial on the basis of data review at one third and two thirds of the total projected enrollment.
Analysis of the primary outcome used Kaplan–Meier 90-day mortality point estimates involving all the patients who were discharged home or were still alive at day 90, with data censored at day 91. Patients who were lost to follow-up had their data censored at the time that they were last known to be alive. We compared the 90-day mortality point estimates in the two treatment groups using a z test with Greenwood’s standard error16 and a 95% Wald confidence interval for the difference in mortality. We assessed the number of adverse events using Poisson regression. For all the other outcomes, we report mean or percentage differences with 95% Wald confidence intervals and median differences using the inverted rank-score test.17 For the primary outcome, we used forest plots to assess treatment heterogeneity for prespecified patient characteristics. The primary outcome was assessed in subgroups defined according to age (≤65 or >65 years); sex; race; ethnic group; location at the time of randomization; presence or absence of chronic heart failure, end-stage renal disease, baseline systolic blood pressure less than 90 mm Hg or vasopressor use, or history of hypertension; total Sequential Organ Failure Assessment score (in quartiles); and primary source of infection (pneumonia or other).
All the analyses used an intention-to-treat approach (including all the patients who had undergone randomization). All the P values are two-sided, and no adjustment to P values or confidence intervals was made for multiple comparisons, such that, except for the primary and safety outcomes, they cannot be used for hypothesis testing. All the statistical analyses were conducted with the use of SAS software, version 9.4 (SAS Institute). Further details are provided in the statistical analysis plan.
RESULTS
CHARACTERISTICS OF THE PATIENTS
From March 7, 2018, to January 31, 2022, we enrolled 1563 patients at 60 U.S. centers. A total of 782 patients were assigned to the restrictive fluid group and 781 to the liberal fluid group (Fig. S1 in the Supplementary Appendix). The data and safety monitoring board recommended the halting of the trial for futility at the second interim analysis owing to a lack of between-group differences in the primary and secondary outcomes (Tables S9 and S10).
Patients in the two groups had similar baseline characteristics and treatment before randomization (Table 1 and Tables S1 and S2). Patients in the restrictive fluid group and the liberal fluid group had received similar volumes of intravenous fluid before randomization (median, 2050 ml [interquartile range, 1500 to 2457] and 2050 ml [interquartile range, 1371 to 2442], respectively). The percentage of patients receiving vasopressors at randomization was similar in the two groups (21% in the restrictive fluid group and 18% in the liberal fluid group). The median time from meeting the trial eligibility criteria to randomization was also similar in the two groups (61 minutes in the restrictive fluid group and 60 minutes in the liberal fluid group).
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Restrictive Fluid Group (N = 782) | Liberal Fluid Group (N = 781) | Total (N = 1563) |
|---|---|---|---|
| Age — yr | 59.1±16.0 | 59.9±15.9 | 59.5±15.9 |
| Female sex — no. (%) | 371 (47.4) | 366 (46.9) | 737 (47.2) |
| Race — no. (%)† | |||
| White | 534 (68.3) | 571 (73.1) | 1105 (70.7) |
| Black | 135 (17.3) | 112 (14.3) | 247 (15.8) |
| Asian | 28 (3.6) | 26 (3.3) | 54 (3.5) |
| Other | 10 (1.3) | 6 (0.8) | 16 (1.0) |
| Not reported | 78 (10.0) | 67 (8.6) | 145 (9.3) |
| Hispanic or Latino ethnic group — no. (%)† | |||
| Yes | 118 (15.1) | 108 (13.8) | 226 (14.5) |
| No | 628 (80.3) | 646 (82.7) | 1274 (81.5) |
| Not reported | 36 (4.6) | 27 (3.5) | 63 (4.0) |
| Coexisting conditions — no./total no. (%) | |||
| Diabetes | 222/777 (28.6) | 224/773 (29.0) | 446/1550 (28.8) |
| Chronic heart failure | 99/777 (12.7) | 79/773 (10.2) | 178/1550 (11.5) |
| End-stage renal disease treated with hemodialysis | 33/777 (4.2) | 40/773 (5.2) | 73/1550 (4.7) |
| SOFA score‡ | 3.4±2.8 | 3.5±2.7 | 3.4±2.7 |
| Systolic blood pressure — mm Hg | 93.2±12.0 | 93.8±12.2 | 93.5±12.1 |
| Median time from meeting trial eligibility criteria to randomization (IQR) — min | 61 (26–116) | 60 (25–117) | 61 (26–116) |
| Location at randomization — no. (%) | |||
| Emergency department | 729 (93.2) | 708 (90.7) | 1437 (91.9) |
| ICU | 44 (5.6) | 62 (7.9) | 106 (6.8) |
| Other | 9 (1.2) | 11 (1.4) | 20 (1.3) |
| Median volume of fluid administered before randomization (IQR) — ml | 2050 (1500–2457) | 2050 (1371–2442) | 2050 (1450–2450) |
Plus–minus values are means ±SD. ICU denotes intensive care unit, and IQR interquartile range.
Race and ethnic group were reported by the patients or their legal representative. More than one race may have been reported per patient. “Other” race included Native American or Pacific Islander.
Sequential Organ Failure Assessment (SOFA) scores range from 0 to 20, with higher scores indicating greater severity.
PROTOCOL-GUIDED RESUSCITATION TREATMENTS
During the first 6 hours after randomization, the volume of administered intravenous fluid differed between the groups, with a median of 500 ml (interquartile range, 130 to 1097) in the restrictive fluid group and 2300 ml (interquartile range, 2000 to 3000) in the liberal fluid group, yielding a difference of −1800 ml (95% confidence interval [CI], −1889 to −1711) (Table 2 and Figs. S2 and S3). The cumulative median volume of fluid administered during the 24 hours after randomization was also lower in the restrictive fluid group (1267 ml; interquartile range, 555 to 2279) than in the liberal fluid group (3400 ml; interquartile range, 2500 to 4495), with a mean difference of −2134 ml (95% CI, −2318 to −1949) (Table 2).
Table 2.
Therapies Administered during the Trial Intervention Period.*
| Therapies | Restrictive Fluid Group (N = 782) | Liberal Fluid Group (N = 781) | Difference (95% CI)† |
|---|---|---|---|
| Median volume of IV fluid administered (IQR) — ml‡ | |||
| Over 6-hr period | 500 (130 to 1097) | 2300 (2000 to 3000) | −1800 (−1889 to −1711) |
| Over 24-hr period | 1267 (555 to 2279) | 3400 (2500 to 4495) | −2134 (−2318 to −1949) |
| Vasopressor administration during first 24-hr period — no./total no. (%) | 460/780 (59.0) | 290/779 (37.2) | 21.7 (16.9 to 26.6) |
| Time from randomization to first vasopressor among patients who had vasopressors administered — hr§ | 1.8±3.4 | 3.2±4.7 | −1.4 (−2.0 to −0.8) |
| Duration of vasopressor use during first 24-hr period among patients who received vasopressor therapy — hr¶ | 9.6±10.0 | 5.4±8.6 | 4.2 (3.3 to 5.2) |
Plus–minus values are means ±SD. Confidence intervals have not been adjusted for multiplicity and may not be used for hypothesis testing.
Differences are medians (for differences in volume), means (for differences in time), or percentage points (for differences between percents).
Estimates of the volume of intravenous (IV) fluid administered were computed on the basis of quantile regression with the use of the proc quantreg statement. The volume of fluid administered over a 6-hour period was assessed in 1550 patients (in 771 in the restrictive fluid group and 779 in the liberal fluid group), and the volume administered over a 24-hour period was assessed in 1555 patients (in 776 and 779, respectively).
Data on the time from randomization to first receipt of vasopressor therapy were available for 743 patients (for 458 in the restrictive fluid group and 285 in the liberal fluid group).
The duration of vasopressor use was assessed in 1542 patients (in 774 in the restrictive fluid group and 768 in the liberal fluid group).
Vasopressors were more commonly used in the restrictive fluid group than the liberal fluid group (in 59% vs. 37% of the patients), initiated earlier (mean difference, −1.4 hours; 95% CI, −2.0 to −0.8), and used for longer during the first 24 hours (mean difference, 4.2 hours; 95% CI, 3.3 to 5.2). The total median cumulative volumes of fluid administered, including the pre-enrollment fluids through 24 hours after randomization, were 3300 ml (interquartile range, 2550 to 4350) in the restrictive fluid group and 5400 ml (interquartile range, 4400 to 6575) in the liberal fluid group. The subsequent administration of intravenous fluids beyond the protocol period was similar up to 7 days after randomization (Table S3). Lactated Ringer’s solution was the most common type of fluid administered (Tables S4 and S5).
Audited protocol adherence was high in both groups, with overall adherence at 97% in the restrictive fluid group and 96% in the liberal fluid group; adherence was sustained over the duration of the trial (Table S6). The October 2019 amendment had minimal effect on treatment delivery (Table S7). Although ICU admission was not part of the treatment protocol, in a post hoc analysis we identified that 525 of 780 patients (67.3%) in the restrictive fluid group and 462 of 780 patients (59.2%) in the liberal fluid group were admitted to the ICU during the protocol period (difference, 8.1 percentage points; 95% CI, 3.3 to 12.8); 2 patients in the restrictive fluid group and 1 patient in the liberal fluid group had indeterminate ICU status (Table S8).
EFFICACY OUTCOMES
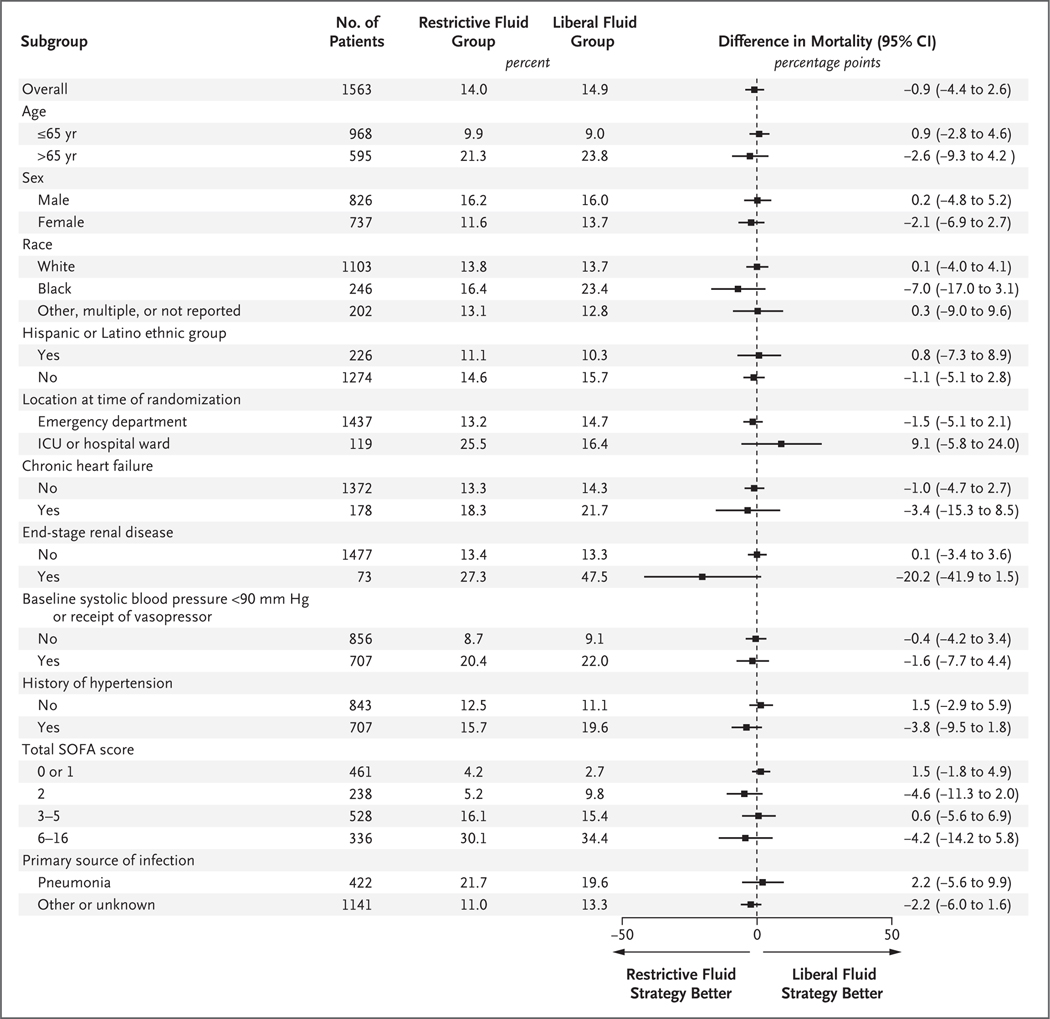
Death before discharge home by day 90 (the primary outcome) occurred in 109 patients (14.0%) in the restrictive fluid group and in 116 patients (14.9%) in the liberal fluid group (estimated difference, −0.9 percentage points; 95% CI, −4.4 to 2.6; P = 0.61) (Table 3, Table S11, and Figs. S4 and S5); 5 patients in the restrictive fluid group and 4 patients in the liberal fluid group had their data censored (lost to follow-up). In prespecified subgroup analyses, treatment effects were not observed in subgroups defined according to systolic blood pressure of less than 90 mm Hg or receipt of vasopressors at randomization (estimated difference, −1.6 percentage points; 95% CI, −7.7 to 4.4), chronic heart failure (estimated difference, −3.4 percentage points; 95% CI, −15.3 to 8.5), end-stage renal disease (estimated difference, −20.2 percentage points; 95% CI, −41.9 to 1.5), and pneumonia as the cause of sepsis (estimated difference, 2.2 percentage points; 95% CI, −5.6 to 9.9) (Fig. 2). The secondary outcomes are reported in Table 3. Post hoc analysis showed no site effects (Fig. S7).
Table 3.
Outcomes.*
| Outcome | Restrictive Fluid Group (N = 782) | Liberal Fluid Group (N = 781) | Difference (95% CI)† | ||
|---|---|---|---|---|---|
| No. of Patients | Mean (95% CI) | No. of Patients | Mean (95% CI) | ||
| Death before discharge home by day 90 — % of patients‡ | 782 | 14.0 (11.6 to 16.4) | 781 | 14.9 (12.4 to 17.4) | −0.9 (−4.4 to 2.6)§ |
| No. of days free from organ-support therapy at 28 days | 778 | 24.0 (23.4 to 24.6) | 778 | 23.6 (23.0 to 24.3) | 0.3 (−0.5 to 1.2) |
| No. of days free from ventilator use at 28 days | 773 | 23.4 (22.7 to 24.1) | 771 | 22.8 (22.0 to 23.5) | 0.6 (−0.4 to 1.6) |
| No. of days free from renal-replacement therapy at 28 days | 737 | 24.1 (23.4 to 24.8) | 738 | 23.9 (23.2 to 24.6) | 0.2 (−0.8 to 1.2) |
| No. of days free from vasopressor use at 28 days¶ | 778 | 22.0 (21.4 to 22.7) | 778 | 21.6 (20.9 to 22.3) | 0.4 (−0.5 to 1.3) |
| No. of days out of the ICU from day 1 to day 28 | 778 | 22.8 (22.2 to 23.4) | 778 | 22.7 (22.0 to 23.3) | 0.1 (−0.8 to 1.0) |
| No of days out of the hospital by day 28 | 778 | 16.2 (15.4 to 17.0) | 778 | 15.4 (14.6 to 16.2) | 0.8 (−0.3 to 1.9) |
| New intubation with invasive mechanical ventilation by 28 days — no. of patients (%) | 701 | 77 (11.0) | 687 | 87 (12.7) | −1.7 (−5.1 to 1.7) |
| Initiation of renal-replacement therapy by 28 days — no. of patients (%) | 738 | 24 (3.3) | 738 | 24 (3.3) | 0.0 (−1.8 to 1.8) |
| KDIGO score on day 3‖ | 585 | 0.35 (0.28 to 0.41) | 604 | 0.34 (0.28 to 0.41) | 0.0 (−0.1 to 0.1) |
| Change in SOFA score from baseline to 72 hr | 619 | −0.7 (−0.9 to −0.4) | 634 | −0.8 (−1.0 to −0.5) | 0.1 (−0.3 to 0.4) |
| Death from any cause at any location by day 90 — no. of patients (%) | 768 | 172 (22.4) | 773 | 169 (21.9) | 0.5 (−3.6 to 4.7) |
| ARDS onset between day 1 and day 7 — no. of patients (%) | 757 | 19 (2.5) | 758 | 20 (2.6) | −0.1 (−1.7 to 1.5) |
| New-onset atrial or ventricular arrhythmia to day 28 — no. of patients (%) | 779 | 59 (7.6) | 778 | 67 (8.6) | −1.0 (−3.7 to 1.7) |
| Severe adverse event — no. of events** | 782 | 21 | 781 | 19 | 2 (−10 to 14) †† |
Percentages and mean values were calculated from nonmissing records. The numbers of patients with data are shown. Confidence intervals have not been adjusted for multiplicity and may not be used for hypothesis testing. ARDS denotes acute respiratory distress syndrome.
Differences are either means (for differences in numbers of days or events or in scores) or percentage points (for differences between percents).
The primary-outcome analysis included all deaths that occurred after randomization in any heath care facility before discharge home until day 90 of the trial. Estimates were from Kaplan–Meier curves. There were 109 deaths and 5 patients with censored data in the restrictive fluid group and 116 deaths and 4 patients with censored data in the liberal fluid group.
P = 0.61.
The analysis excluded vasopressor use during the first 48 hours in order to account for treatment assignment and a washout period.
Kidney International Improving Global Outcomes (KDIGO) scores range from 1 to 3, with a score of 3 indicating the worst renal function.
All the adverse events are listed in Tables S14 and S15. Participants may have had more than one adverse event.
P = 0.75.
Figure 2. Subgroup Analysis for the Primary Outcome.
The primary outcome was death from any cause before discharge home by day 90. Estimates were from Kaplan–Meier curves. Confidence intervals have not been adjusted for multiplicity and may not be used for hypothesis testing. Race and ethnic group were reported by the patients or their legal representative. Sequential Organ Failure Assessment (SOFA) scores range from 0 to 20, with higher scores indicating greater severity. For the purposes of subgroup analysis, subgroups were assessed in quartiles, with quartile 1 including patients with a SOFA score of 0 or 1, quartile 2 those with a score of 2, quartile 3 those with a score of 3 to 5, and quartile 4 those with a score of 6 or higher. (In the trial, the highest SOFA score observed was 16.) ICU denotes intensive care unit.
SAFETY OUTCOMES
The number of reported serious adverse events was similar in the restrictive fluid group (21) and the liberal fluid group (19) (Table 3). There were fewer reported serious adverse events of episodes of fluid overload in the restrictive fluid group than in the liberal fluid group (0 vs. 3) and fewer serious adverse events of pulmonary edema (0 vs. 3) (Tables S12 through S15). The incidence of new invasive ventilation (at 0 to 24 hours; a systematically collected outcome) was 6.2% in the restrictive fluid group and 6.8% in the liberal fluid group (difference, −0.6 percentage points; 95% CI, −3.1 to 1.9) (Table S16). We also systematically collected data regarding use of and adverse outcomes related to vasopressor use through central and peripheral venous catheters (Tables S17, S18, and S19). We found three instances of potential vasopressor extravasation among 500 patients (310 patients in the restrictive fluid group and 190 in the liberal fluid group) who received peripherally administered vasopressors between randomization and 72 hours; these three events resolved without intervention and did not have any residual clinical consequences.
DISCUSSION
We conducted a randomized trial of two different resuscitation strategies for managing the first 24 hours of sepsis-induced hypotension after the initial administration of 1 to 3 liters of intravenous fluid. Despite separation between the two groups with respect to the volume of intravenous fluid administered and the use of vasopressors, we detected no significant difference in mortality before discharge home by day 90 (the primary outcome).
A number of observational studies have assessed the association of fluid volumes with outcomes6,18–25; however, these investigations were limited by biases inherent in the observational study designs used, notably an indication bias in which more severely ill patients tend to receive higher fluid volumes. Previous randomized, controlled trials that have been conducted in resource-limited settings have shown that restrictive fluid approaches yielded better outcomes than liberal fluid approaches, but generalizability to more resource–intensive settings is unclear.13,26 More recently, the Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care (CLASSIC) trial compared a restrictive fluid protocol with a standard fluid approach that resulted in greater volumes of fluid administration among patients who had already been admitted to the ICU after initial resuscitation; this trial showed no difference in 90-day all-cause mortality.15 Our trial almost exclusively enrolled patients with a primary presentation to a hospital emergency department with sepsis, in contrast to the CLASSIC II trial, which enrolled many patients who had received care on a hospital ward (34%) or in the operating room (23%) before ICU admission and trial enrollment.
The results of the CLOVERS trial suggest that for the types of patients enrolled in this trial, the prioritization of either a vasopressor-predominant or fluid-predominant approach resulted in similar patient-centered outcomes. We focused on the larger group of patients with sepsis who had hypotension, in whom the treatment approach is not clearly guided by clinical circumstances. The patients who were enrolled in this trial were representative of the types of patients who present to the hospital with sepsis-induced hypotension (Tables S20 and S21); we expect our findings to be generalizable to these types of patients. Our trial required that clinicians approve their patient’s participation. Patients who were assessed as being not suitable candidates for randomization to either trial group were not enrolled. Therefore, trial results may not be generalizable to patient subgroups not studied, such as patients with extremes of volume overload or volume depletion. We also did not identify any prespecified patient features that delineated patients who were more likely to benefit from one approach or the other. It is possible that subgroups defined according to more sophisticated methods with the use of clinical or biologic measurements (e.g., biomarkers to classify subphenotypes) may exist where there is a preferential treatment effect for one approach or the other. Future initiatives may assess for these types of subgroups and differential treatment effects.27,28
Our trial allowed for the initial administration of vasopressor agents through peripheral intravenous catheters as an alternative to the traditionally preferred central venous catheter. This practice facilitates earlier use of vasopressors.29,30 The presence of only three occurrences of complications (extravasation that resolved without intervention or clinical consequence) among 500 patients who received vasopressors through a peripheral catheter provides data supporting the safety of this practice.
In this trial, the groups used common clinical characteristics and routine assessments to trigger protocol-directed actions for vasopressor and fluid administration. Other studies have used strategies such as the use of noninvasive hemodynamic devices,31 ultrasonographic assessment of the variation in the diameter of the inferior vena cava,32 or cardiac echocardiography33 to assess for volume responsiveness to guide resuscitation. These approaches were neither prioritized nor central to the resuscitation protocols that were tested in this trial. Future studies may consider incorporating these types of assessments to monitor and adjust the resuscitation treatments.
This trial should be interpreted in the context of its limitations. First, despite high adherence to the protocol, some patients who had been randomly assigned to the restrictive fluid group received more fluid than was intended by the protocol, with vasopressors given later than intended by the protocol. Similarly, some patients who had been randomly assigned to the liberal fluid group received lower fluid volumes than were intended, with earlier use of vasopressors. We cannot ensure that the specific variations did not bias observations. Second, there are potentially important subgroups (including patients with specific coexisting conditions for which data were not collected in this trial) that we did not assess that could benefit from one strategy or the other. Third, because this trial was unblinded, group assignment may have influenced the ascertainment and reporting of adverse events (e.g., higher reporting of fluid overload in the liberal fluid group).
Fourth, we did not have a group in this trial in which clinicians received no instructions or guidance on therapy. When designing the trial, we decided that comparing two protocolized groups would be more informative about the relative advantages and disadvantages of different resuscitation strategies than comparing one protocolized strategy to an unstructured care group. Although we can infer that there were no differences in clinical outcomes between the two approaches tested, we cannot infer comparison with an unstructured approach. Fifth, our trial compared two approaches to the use of fluid and vasopressor therapy to achieve common resuscitation targets for mean arterial blood-pressure and lactate levels and does not inform whether outcomes would have differed with different targets, such as permitting lower blood-pressure values. Sixth, we did not assess the safety or effectiveness of the specific resuscitation targets used in the trial. Seventh, the protocol duration was up to 24 hours; thus, it is possible that a longer treatment period may have produced different results. Eighth, enrollment of a trial population with a higher initial severity of illness may have led to a greater effect on outcomes in one of the groups. Finally, we evaluated patients with sepsis-induced hypotension that was recognized early after hospital presentation. These findings may not be generalizable to patients with delayed recognition of sepsis-induced hypotension or who are in the later phases of care.
In this trial involving patients with sepsis-induced hypotension refractory to initial treatment with 1 to 3 liters of intravenous fluid, we found that a restrictive fluid strategy (with earlier vasopressor use) did not result in significantly lower (or higher) mortality before discharge home by day 90 than a liberal fluid strategy.
Supplementary Material
Acknowledgments
Supported by grants (U01 HL122989, U01 HL122998, U01 HL123004, U01 HL123008, U01 HL123009, U01 HL123010, U01 HL123018, U01 HL123020, U01 HL123022, U01 HL123023, U01 HL123027, U01 HL123031, and U01 HL123033) from the National Heart, Lung, and Blood Institute.
We thank the participating patients and their families, the clinical and research staff at the participating trial sites, the staff at the Prevention and Early Treatment of Acute Lung (PETAL) Clinical Coordinating Center, and the members of the PETAL data and safety monitoring board.
Footnotes
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
The lists of the members of the Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) Investigators and the National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury (PETAL) Network are provided in the Supplementary Appendix, available at NEJM.org.
A Quick Take is available at NEJM.org
REFERENCES
- 1.Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49(11):e 1063–e1143. [DOI] [PubMed] [Google Scholar]
- 2.Self WH, Semler MW, Bellomo R, et al. Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med 2018; 72:4 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1 368–77. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015; 41: 1549–60. [DOI] [PubMed] [Google Scholar]
- 5.Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care 2014; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015; 43: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamzaoui O, Georger J-F, Monnet X, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care 2010; 14(4): R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monnet X, Marik P, Teboul J-L. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016;4 2: 193547. [DOI] [PubMed] [Google Scholar]
- 9.Dong Z-Z, Fang Q, Zheng X, Shi H. Passive leg raising as an indicator of fluid responsiveness in patients with severe sepsis. World J Emerg Med 2012; 3: 191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glassford NJ, Eastwood GM, Bellomo R. Physiological changes after fluid bolus therapy in sepsis: a systematic review of contemporary data. Crit Care 2014; 18: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legrand M, Dupuis C, Simon C, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care 2013; 17(6): R278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med 2016; 42: 1695–705. [DOI] [PubMed] [Google Scholar]
- 13.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011; 364: 2483–95. [DOI] [PubMed] [Google Scholar]
- 14.Richard J-C, Bayle F, Bourdin G, et al. Preload dependence indices to titrate volume expansion during septic shock: a randomized controlled trial. Crit Care 2015;1 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyhoff TS, Hjortrup PB, Wetterslev J, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med 2022; 386: 2459–70. [DOI] [PubMed] [Google Scholar]
- 16.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd ed. Hoboken, NJ: John Wiley, 2002. [Google Scholar]
- 17.Chen C An introduction to quantile regression and the QUANTREG procedure. In: Proceedings of the Thirtieth Annual SAS Users Group International Conference. Cary, NC: SAS Institute, 2005: 213–30. [Google Scholar]
- 18.Lee J, de Louw E, Niemi M, et al. Association between fluid balance and survival in critically ill patients. J Intern Med 2015; 277:4 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39: 259–65. [DOI] [PubMed] [Google Scholar]
- 20.Acheampong A, Vincent J-L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care 2015; 19:2 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansoori JN, Linde-Zwirble W, Hou PC, Havranek EP, Douglas IS. Variability in usual care fluid resuscitation and risk-adjusted outcomes for mechanically ventilated patients in shock. Crit Care 2020; 24: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 2008; 12(3): R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg AL, Dechert RE, Park PK, Bartlett RH, NIH NHLBI ARDS Network. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med 2009; 24: 35–46. [DOI] [PubMed] [Google Scholar]
- 24.Shum HP, Lee FMH, Chan KC, Yan WW. Interaction between fluid balance and disease severity on patient outcome in the critically ill. J Crit Care 2011; 26: 613–9. [DOI] [PubMed] [Google Scholar]
- 25.The RENAL Replacement Therapy Study Investigators. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med 2012; 40: 1753–60. [DOI] [PubMed] [Google Scholar]
- 26.Andrews B, Semler MW, Muchemwa L, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA 2017;3 18: 1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gårdlund B, Dmitrieva NO, Pieper CF, Finfer S, Marshall JC, Taylor Thompson B. Six subphenotypes in septic shock: latent class analysis of the PROWESS Shock study. J Crit Care 2018; 47: 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maddali MV, Churpek M, Pham T, et al. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir Med 2022;1 0: 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardenas-Garcia J, Schaub KF, Bel-chikov YG, Narasimhan M, Koenig SJ, Mayo PH. Safety of peripheral intravenous administration of vasoactive medication. J Hosp Med 2015; 10: 581–5. [DOI] [PubMed] [Google Scholar]
- 30.Owen VS, Rosgen BK, Cherak SJ, et al. Adverse events associated with administration of vasopressor medications through a peripheral intravenous catheter: a systematic review and meta-analysis. Crit Care 2021; 25:1 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douglas IS, Alapat PM, Corl KA, et al. Fluid response evaluation in sepsis hypotension and shock: a randomized clinical trial. Chest 2020; 158: 1431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musikatavorn K, Plitawanon P, Lum-lertgul S, et al. Randomized controlled trial of ultrasound-guided fluid resuscitation of sepsis-induced hypoperfusion and septic shock. West J Emerg Med 2021;2 2: 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanspa MJ, Burk RE, Wilson EL, Hirshberg EL, Grissom CK, Brown SM. Echocardiogram-guided resuscitation versus early goal-directed therapy in the treatment of septic shock: a randomized, controlled, feasibility trial. J Intensive Care 2018; 6:5 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.