Abstract
Objectives
Postoperative cognitive decline (POCD) or decreased health-related quality of life (HQL) have been reported after cardiac surgery. A previous investigation showed beneficial effects of postoperative cognitive training on POCD and HQL 3 months after heart surgery. Here, we present the 12-month follow-up results.
Methods
This bicentric, 1:1 randomised and treatment-as-usual controlled trial included elderly patients scheduled for elective heart valve surgery. The training consisted of paper-and-pencil-based exercises practising multiple cognitive functions for 36 min/day 6 days/week over a period of 3 weeks. Neuropsychological tests and questionnaires assessing HQL (36-Item Short Form Health Survey (SF-36)) and cognitive failures in daily living (Cognitive Failures Questionnaire) were performed presurgery and 12 months after training.
Results
Twelve months post training, the training group (n=30) showed improvements in HQL compared with the control group (n=28), especially in role limitations due to physical health (U=−2.447, p=0.015, η2=0.109), role limitations due to emotional problems (U=−2.245, p=0.025, η2=0.092), pain (U=−1.979, p=0.049, η2=0.068), average of all SF-36 factors (U=−3.237, p<0.001, η2=0.181), health change from the past year to the present time (U=−2.091, p=0.037, η2=0.075), physical component summary (U=−2.803, p=0.005, η2=0.138), and mental component summary (U=−2.350, p=0.018, η2=0.095). Furthermore, the training group (n=19) showed an improvement compared with the control group (n=27) in visual recognition memory (U=−2.137, p=0.034, η2=0.099). POCD frequency was 22% (n=6) in the control group and 11% (n=2) in the training group (χ²(1) =1.06, p=0.440; OR=2.43, 95% CI 0.43 to 13.61).
Conclusion
In conclusion, postoperative cognitive training shows enhancing effects on HQL in cardiac surgery patients after 12 months.
Keywords: CARDIAC SURGERY, Cardiac Rehabilitation, Coronary Artery Bypass, Heart Valve Prosthesis Implantation
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Postoperative cognitive training could be a promising approach to prevent postoperative cognitive decline and improve health-related quality of life in patients undergoing cardiac surgery.
WHAT THIS STUDY ADDS
This study presents the findings of a 12-month follow-up, examining the effects of postoperative cognitive training on cognition and health-related quality of life in cardiac surgery patients.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The integration of postoperative cognitive training into inpatient or outpatient rehabilitation programmes could have a significant impact on clinical practice.
Introduction
Postoperative cognitive decline (POCD) after cardiac surgery has a detrimental impact on HQL.1 POCD is commonly defined as a decrease in cognitive functions such as memory, attention and speech from a preoperative to postoperative neuropsychological assessment. It has a prevalence of about 22% between the 6th and 12th postoperative month in patients with coronary artery bypass grafting (CABG)2 and a prevalence of 14% at 3 months and 16% at 3–4 years for aortic valve replacement (AVR).3 Since POCD is often examined psychometrically with objective test procedures, patients’ subjective perceptions in the postoperative course are also important for the estimation of clinical relevance. In this context, subjectively assessed cognitive failures in daily life were reported by the patients themselves as well as by their relatives.4 5
Risk factors involved in the development of POCD have been described for the preoperative (age, diabetes, depression, cognitive impairment), intraoperative (intubation time, duration of surgery), and postoperative (cardiac arrhythmias, delirium) periods.6 7 In particular, POCD in the early postoperative phase is associated with long-term cognitive decline.8 As cognitive functions can potentially be improved by cognitive training,9 we implemented a treatment-as-usual, controlled, paper-and-pencil-based cognitive training programme in the early postoperative period for cardiac surgery patients.10 As a result, we were able to show improved effects on POCD11 and HQL12 at 3 months post training. Here, we report the training effects on POCD, HQL and Cognitive Failures Questionnaire (CFQ) at a 12-month follow-up assessment.
Methods
General conditions
This bicentric, 1:1 randomised and treatment-as-usual controlled trial included elderly patients scheduled for elective aortic or mitral valve replacements/reconstructions with or without CABG. Recruitment took place at the Departments of Cardiac Surgery of the Kerckhoff-Clinic in Bad Nauheim and the University-Hospital in Giessen. Following acute hospitalisation, patients were transferred directly to a rehabilitation centre and received the interventions. In summary, our cognitive training programme consisted of standardised paper-and-pencil-based exercises designed to enhance multidomain cognitive functions (word fluency, working memory, attention and planning), which are particularly vital for quality of life. The daily training programme comprises eight distinct standardised tasks focusing on word processing, categorisation, imagery, mental calculations and planning. Novel word, category, image, mental calculation and planning tasks are presented on each training day. Each daily training session lasted 36 min, and participants were advised to complete these sessions 6 days a week for 3 weeks. Neuropsychological tests and questionnaires assessing HQL (36-Item Short Form Health Survey (SF-36) and cognitive failures in daily living (CFQ) were performed prior to surgery and 12 months after training. Full details on the study procedure, neuropsychological tests, questionnaires and cognitive training have been published in advance.10
Outcomes
The results of the primary outcome of the study have been published in advance.11 Secondary outcomes shown in the present paper are the effect of cognitive training on POCD, HQL, CFQ, depression and anxiety at 12 months after the cognitive training. Since postoperative cognitive improvement (POCI) can likewise occur after cardiac surgery13 and there is a risk of overestimating the incidence of POCD in this context, POCI was also used as an outcome.
Inclusion and exclusion criteria
Inclusion criteria included elective aortic or mitral valve replacement/reconstruction with or without CABG under extracorporeal circulation and sufficient knowledge of German. Exclusion criteria comprised history of stroke, psychiatric or neurological diseases, and health insurance that did not support postoperative rehabilitation at the Kerckhoff-Clinic.
Randomisation and blinding
After performing preoperative neuropsychological examinations on the patients, the study coordinators allocated them to the cognitive training group or the treatment-as-usual group. A computer-generated randomisation list with a 1:1 blocked (sizes varied randomly) allocation ratio was generated, sequentially numbered and concealed by a study coordinator prior to the first enrolment. Neurologists, neuropsychologists and surgeons who were involved in assessing the outcome parameters were blinded to the patients’ randomisation status.
Definitions of POCD/POCI
POCD was defined as a decline and POCI as an enhancement of at least 1 SD in at least 20% of all neuropsychological subdomains from pretests to post tests.2 The difference in SD between the pretests and post tests was calculated using Z-scores (difference between the individual raw values and the mean value of the total baseline data divided by the SD of the total baseline data). The neuropsychological subdomains were defined according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders-5,14 as specified in online supplemental table 1. As we measured several neuropsychological parameters that can be contextually grouped into cognitive subdomains, we summarised them using a mean value. Neuropsychological assessment was performed with a battery of cognitive tests prior to surgery and 12 months after training. Selective attention was assessed using the Trail Making Test A (TMT-A) and the Alterskonzentrationstest (AKT). Verbal memory and learning were evaluated using the Verbaler Lern- und Merkfähigkeitstest (VLMT), a modified German version of the Rey Auditory Verbal Learning Test. Visual memory was measured through the Block-Tapping Test, the Non-Verbal Learning Test (NVLT), and the pictorial memory subtest of the German Syndrom-Kurztest (SKT). Verbal working memory was examined with the Letter Number Test, a subtest of the MATRIX test battery. Cognitive flexibility was assessed using the Trail Making Test B (TMT-B) and a subtest of the SKT, specifically testing inhibition ability. In addition, semantic and phonetic verbal fluency were tested using the Regensburger Wortflüssigkeits-Test (RWT). The Symbolverarbeitungstes’ (SVT) was administered to evaluate symbolic picture processing. Parallel test versions were available for the AKT, VLMT, NVLT, SKT, SVT and RWT. A detailed description of the neuropsychological tests was published in the study protocol.10
openhrt-2023-002411supp001.pdf (139.4KB, pdf)
Questionnaires
HQL was assessed using the SF-36 (Version 1.0),15 which covers eight factors, including physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain and general health. We also determined an average of all eight factors and calculated a two-factor model indicating the physical component and mental component summaries. Additionally, the extent to which health had changed in relation to the past year was evaluated with a single item. The SF-36 was scored using the RAND scoring method.16
The validated German version of the Cognitive Failures Questionnaire for self-assessment (s-CFQ, 25-item)17 was used to evaluate cognitive failures in daily living. The patients’ close relatives responded to the Cognitive Failures Questionnaire to provide external assessment (f-CFQ, 8-item).18 Furthermore, the validated German version of the Memory Complaint Questionnaire (four-item)19 was applied to reveal more information on the cognitive domain of memory. Additionally, we calculated further CFQ factor models that have already been described.20–23
To reveal psychopathological symptoms, we used the validated German version of the Hospital Anxiety and Depression Scale (HADS-D).24
Statistical analyses
We carried out a sample-size calculation for the primary outcome of our study (cognitive training-related effect on objectively assessed cognition), which was published in advance.11 Therefore, we did not perform a sample-size calculation for this report, which refers to the secondary outcomes of our trial, and we analysed the data exploratively.
To analyse the training’s effect on cognition, the frequencies of POCD and POCI were compared with Pearson’s χ2 tests between the groups. The effect size was determined by calculating the OR with a 95% CI. To calculate the training’s effects on each neuropsychological parameter, SF-36, CFQ and HADS, analyses of covariance (ANCOVA) were conducted with the postoperative test values as the dependent variable, groups as the fixed factor, and preoperative test values as the covariate. Confounding continuous variables (demographic data, perioperative details, changes in anxiety and depression) that exhibited statistically significant correlations with the dependent variable were used as additional covariates in the ANCOVAs. The assumptions for the ANCOVAs were assessed using the Levene test to determine variance homogeneity of the dependent variable, visual inspections of QQ and distribution plots of the dependent variable were used to determine normality, and a statistically significant correlation between the dependent variable and covariate (preoperative test value) was calculated with Pearson’s product-moment correlation. When the assumptions for an ANCOVA were violated, the difference values between the pretests and post-tests were calculated in a Mann-Whitney U-test for between-subjects effects. The effect sizes of the ANCOVAs and U-tests are given in η2. The criterion for statistical significance was set at p<0.05 (two sided). Post hoc power (1-ß) was also calculated. Our data set was evaluated using a per-protocol analysis. All statistical analyses were performed with SPSS (V.22), JASP (V.0.17.1) and G*Power (V.3.1.9.2).
Results
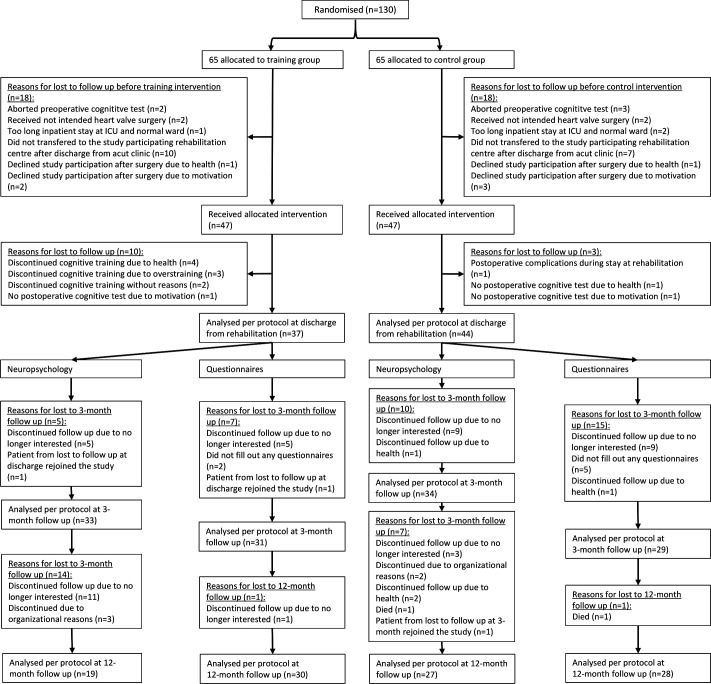
Between 13 July 2016 and 8 January 2020, 130 patients were enrolled, randomised and tested preoperatively. The last patient was tested on 9 March 2021, for the 12-month follow-up. After randomisation of the 130 patients, 36 patients (training group, n=18; control group, n=18) were lost to follow-up before the training or control intervention started. Thus, 94 patients (training group, n=47; control group, n=47) were considered for the baseline sample, which had no statistically significant between-groups differences in baseline demographics, preoperative comorbidities, operative details, postoperative complications (see table 1) or baseline neuropsychological tests, inclusive HADS parameters. After allocating interventions, no clinical strokes were observed. All patients were of white/Caucasian ethnicity. The reasons for losing patients to follow-up are shown in figure 1. The training lasted 14.9 (SD=2.5) days and did not cause adverse events.
Table 1.
Baseline demographics and characteristics of the intention to treat the population
| Training (n=47) | Control (n=47) | |
| Demographics | ||
| Age (years) | 71.2 (4.7) | 73.0 (4.9) |
| Sex | ||
| Women | 8 (17%) | 13 (28%) |
| Men | 39 (83%) | 34 (72%) |
| Education (years) | 13.5 (3.0) | 13.4 (3.0) |
| Medical history | ||
| Body mass index (kg/m²) | 27.7 (3.8) | 26.6 (3.8) |
| Arterial hypertension | 31 (66%) | 31 (66%) |
| Diabetes mellitus | 10 (21%) | 5 (11%) |
| Renal insufficiency | 4 (9%) | 7 (15%) |
| Dyslipidaemia | 39 (83%) | 37 (79%) |
| Atrial fibrillation | 5 (11%) | 11 (23%) |
| LV EF (mildly abnormal) | 5 (11%) | 6 (13%) |
| LV EF (moderately abnormal) | 3 (6%) | 1 (2%) |
| Heart failure | 10 (21.3%) | 9 (19.1%) |
| HADS anxiety | 6.1 (3.7) | 5.7 (3.3) |
| HADS depression | 3.9 (3.2) | 3.8 (2.5) |
| Type of surgery | ||
| AVR | 23 (50%) | 22 (47%) |
| AVR+CABG | 18 (38%) | 19 (40%) |
| MVR | 4 (9%) | 1 (2%) |
| MVR+CABG | 0 (0%) | 3 (6%) |
| AVR+MVR | 2 (4%) | 2 (4%) |
| Perioperative details | ||
| Duration of surgery (minutes) | 190.3 (38.1) | 200.6 (58.9) |
| Duration of extracorporeal circulation (minutes) | 96.5 (26.5) | 105.2 (37.5) |
| Cross-clamp time (minutes) | 69.1 (19.9) | 74.7 (26.7) |
| Ventilation time invasive (minutes) | 616.2 (331.3) | 588.1 (213.6) |
| Postoperative details | ||
| Length of stay ICU (days) | 1.3 (0.5) | 1.5 (0.5) |
| Length of stay normal ward (days) | 9.9 (2.2) | 10.5 (2.8) |
| Delirium | 2 (4%) | 4 (9%) |
| Arrhythmia | 18 (38%) | 21 (45%) |
| Atrial fibrillation | 18 (38%) | 17 (36%) |
| Renal insufficiency | 6 (13%) | 6 (13%) |
| Acute blood loss anaemia | 10 (21%) | 12 (26%) |
| Transient ischaemic attack | 1 (2%) | 0 (0%) |
| Dysathria/aphasia | 0 (0%) | 1 (2%) |
| Medical details at admission to rehabilitation | ||
| Blood pressure (systolic; mm Hg) | 128.1 (12.7) | 127.7 (18.4) |
| Blood pressure (diastolic; mm Hg) | 74.5 (11.1) | 71.4 (10.6) |
Data include means (SD) or number of subjects (%).
Renal insufficiency was defined by a creatinine value above the in-house norms (men: > 1.2 mg/dL, women: > 0.9 mg/dL).
LV EF, left ventricular ejection fraction46
AVR, aortic valve replacement; CABG, coronary artery bypass grafting; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; MVR, mitral valve replacement/reconstruction.
Figure 1.
Consolidated Standards of Reporting Trials flow chart illustrating all steps in the study from randomisation to follow-up and analysis. ICU, intensive care unit.
At 12 months follow-up, the training group (n=19) showed an improvement compared with the control group (n=27) in visual recognition memory (U=−2.137, p=0.034, η2=0.099, 1−ß=0.56). See online supplemental table 2 for details.
POCD frequency was 22% (n=6) in the control group and 11% (n=2) in the training group (χ²(1) =1.06, p=0.440; OR=2.43, 95% CI 0.43 to 13.61). POCI frequency was 47.4% (n=9) in the training group and 29.6% (n=8) in the control group (χ²(1) =1.51, p=0.352; OR=0.47, 95% CI 0.14 to 1.59). No statistical significant training effects on depression or anxiety (HADS) were observed.
As our sample showed an unequal distribution of delirious patients at the 12-month follow-up (control group n=3; training group n=0), and delirium is discussed as a risk factor for POCD,25 we calculated a post hoc explorative analysis without the delirious patients. In this case, the training effect on the POCD/POCI at the 12-month follow-up was about the same as that with the delirious patients (POCD: control group 20.8% (n=5), training group 10.5% (n=2), χ²(1) =0.827, p=0.437, OR=2.24, 95% CI 0.38 to 13.07. POCI: training group 47.4% (n=9), control group 29.2% (n=7), χ²(1) =1.50, p=0.341, OR=0.46, 95% CI 0.13 to 1.61).
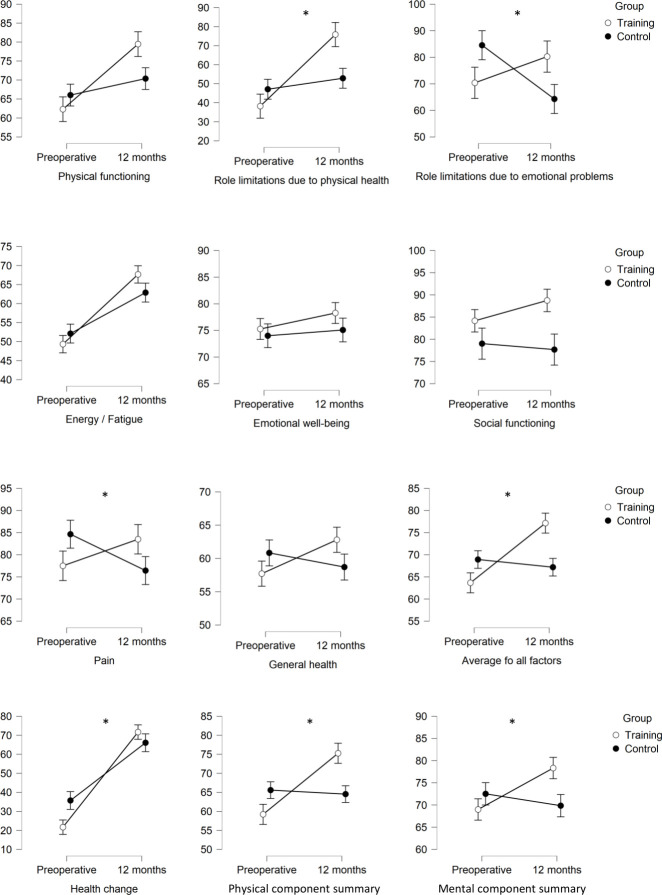
Twelve months after discharge from rehabilitation, the training group (n=30) showed improvement in HQL compared with the control group (n=28), especially in role limitations due to physical health (U=−2.447, p=0.015, η2=0.109, 1−ß=0.7), role limitations due to emotional problems (U=−2.245, p=0.025, η2=0.092, 1−ß=0.62), pain (U=−1.979, p=0.049, η2=0.068, 1−ß=0.51), the average of all SF-36 factors (U=−3.237, p<0.001, η2=0.181, 1−ß=0.93), health change from the past year to the present time (U=−2.091, p=0.037, η2=0.075, 1−ß=0.55), physical component summary (U=−2.803, p=0.005, η2=0.138, 1−ß=0.83), and mental component summary (U=−2.350, p=0.018, η2=0.095, 1−ß=0.66). See figure 2 and online supplemental table 3 for details. No statistically significant differences were found in the patients’ self-reported cognitive impairments in daily life. When close relatives assessed patients’ cognitive abilities in daily living, the control group (n=20) showed a better outcome compared with the training group (n=24) (F(2.41)=5.417, p=0.025, η2=0.052, 1−ß=0.25).
Figure 2.
Interaction effects of all 36-Item Short Form Health Survey factors between the training group and control group. Shown are the mean values (higher scores indicating a better health state), including SE bars for preoperative testing and 12 months after discharge from the rehabilitation clinic. Statistical significant interaction effects with a p value of <0.05 are marked with an asterisk (*).
In an adjusted ANCOVA, no potentially confounding variables made a significant contribution to the results.
Discussion
At the 12-month follow-up assessment, patients in the cognitive training group showed better scores in visual recognition memory. The frequency of POCD and POCI differed numerically between the groups, suggesting a beneficial effect, but the difference did not reach statistical significance. The cognitive training group achieved higher values in several HQL factors, especially in the average of all HQL factors, with a high post hoc power of 0.93. POCD incidence in our control group was higher than in the training group at the time of discharge from the rehabilitation clinic (50% vs 19%, p=0.004) and 3 months after completion of the training (29% vs 6%, p=0.013).11 However, this benefit seemed less pronounced after 12 months (22% vs 11%, p=0.44). On one hand, the training effect in our study might have seemed lower after 12 months due to the reduction of the sample size during follow-up or because the training effect was lost over time. However, this question cannot be answered clearly, because there seem to be no 12-month follow-up data from cognitive training programmes in cardiac surgery patients or older surgical patients after general anaesthesia.26 In older adults comparable in age and duration of training with our study group, inhomogeneous effects have been seen 1 year after cognitive training.27 28 Nevertheless, postoperative cognitive training in cardiac surgical patients exerted promising effects at least 6 months post training in cognitive function and HQL.29 30
Neuroplasticity (changes in brain structure) could be an explanation for the effectiveness of the cognitive training on cognitive functions.9 The improvement of objectively assessed cognitive abilities (ie, working memory or executive functions) through our training may explain enhanced HQL.11 For example, improved working memory could foster more adequate emotion regulation,31 which might explain the enhancement in role limitations due to emotional problems in the training group. Executive functions such as volition, planning, goal-directed action, performance monitoring and inhibition are important for initiating or modifying physical activities.32 33 In this context, it has been shown that elevated levels of executive functions lead to increased physical activity,32 which could account for the heightened physical component of our training group. Patients’ self-rated cognitive failures in daily living did not reveal a difference between the groups. As we assumed, enhanced objective cognition in our training group at 3 months post training11 could lead to increased HQL. The lack of an association with patients’ subjective and objective assessed cognition might indicate that these two perspectives seem to be unrelated.34 35 Unexpectedly, close relatives’ assessments regarding patients’ cognition were better in the control group. However, a post hoc power analysis revealed a clearly underpowered result (1−ß=0.25), so we do not consider this difference meaningful.
Because anxiety and depression could potentially affect cognitive performance,36 we have dedicated more attention to this aspect. As indicated in the baseline table, the average scores on the HADS anxiety scale are approximately 6, and for depression, about 4 in both groups (with no statistically significant between-group differences), falling below the cut-off value of 8 for diagnosing clinically relevant anxiety or depression disorders.37 Therefore, this is interpreted as a minor degree of anxiety or depressive symptoms. However, some patients may exhibit relevant levels of anxiety and depression, which could potentially affect their neuropsychological performance. Nevertheless, when these parameters were included as covariates in an ANCOVA, no significant between-group changes were observed in the parametric training outcomes.
For both groups combined, the proportion of women was 22.3% and for men 77.7%. Because our cohort consists of a mixture of AVR and CABG patients, it is challenging to compare directly these sex frequencies with other research findings. However, when we specifically examined the AVR patients in our study, the proportion of women was 33.3% and for men 66.6%. These are in line with other reports.38 Nevertheless, it is worth noting that the frequency of women in the training group with AVR (26.1%) appears to be slightly lower than the general trend. Given the small sample size and the lack of statistical significance between the groups, we interpret this discrepancy as a random effect.
Findings might be limited by the lack of a healthy control group (to control for time and practice effects), no evaluation of cognition-enhancing activities (playing games, social engagement) during follow-up (for the purpose of control analysis),39 and the small group size. Particularly, an inhomogeneous distribution of patients was observed between the groups at the 12-month follow-up assessment (training: n=19, control: n=27). This discrepancy appears to be largely attributed to a higher dropout rate between the 3-month and 12-month follow-up assessments among the training group patients due to a lack of interest in continuing study participation (n=11), whereas this was not as pronounced in the control group (n=3). Therefore, the described group effects concerning cognitive outcomes could be biased, especially if the lost to follow-up patients in the training group predominantly consisted of patients exhibiting a worse postoperative cognitive trajectory. We performed a sample-size estimation using our primary outcome (neuropsychological effect at discharge from rehabilitation). Therefore, the 12-month follow-up analysis did not consider sample-size calculation. Furthermore, we did not perform a neuropsychological examination after surgery or before the start of the training intervention. This could have shown whether the two groups were homogeneous in terms of neuropsychological conditions. Such a postoperative cognitive examination could have been performed, for example, about 1 week postoperatively, when the patients were admitted to the rehabilitation clinic. However, an extensive and detailed test with a duration of about 90 min, as we used, would have been too overwhelming for patients, who were likely still affected by the side effects of anaesthesia and surgery. An alternative to detailed testing would have been a screening procedure such as the MOCA test,40 which only takes about 10 min to perform. Postoperative delirium was not systematically assessed. The frequency was taken from medical records and may therefore be under-represented, since the hypoactive deliriant type often remains unrecognised due to its intrinsic symptomatology.41 We did not systematically conduct a postoperative evaluation of clinical stroke symptoms using a scoring system such as the National Institutes of Health Stroke Scale. However, our ICU and general ward clinicians are aware of possible postoperative strokes. If a stroke is suspected, a neurologist is consulted for assessment. For organisational reasons, we were unable to enrol the initially planned 144 patients at baseline and had to stop recruitment after reaching 130 patients. As our training concept was at least able to maintain and improve HQL 12 months after cardiac surgery, it could be integrated in postoperative rehabilitative programmes, especially focusing on high-risk patients. Booster sessions should be considered to achieve benevolent effects on cognitive functions up to or beyond 12 months.42 Furthermore, since our training worked well for about 80% of the patients in the early postoperative rehabilitative phase, it could also be implemented in a preoperative setting. Investigations in the preoperative period have already been done related to physical condition, with the aim to improve postoperative outcomes in cardiosurgical patients (prehabilitation).43 Increasing preoperative cognitive reserves with the help of cognitive interventions to protect against potentially postoperative neurocognitive disorders such as delirium or impairments in memory and attention could also be useful.44 45
Conclusion
In conclusion, postoperative cognitive training suggests enhancing effects, especially in HQL, for cardiac surgery patients after 12 months.
Footnotes
Contributors: MB: conceptualisation; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; validation; visualisation; writing-original draft. TG: conceptualisation; funding acquisition; methodology; project administration; supervision; writing-review and editing. GS: conceptualisation; methodology; project administration; supervision; validation; writing-review and editing. JE-S: conceptualisation; funding acquisition; investigation; methodology; project administration; supervision; validation; writing-review and editing. MT, TB, RM, PS, TRD, Y-HC: writing-review and editing. AB: resources; writing-review and editing. TM: conceptualisation; resources; writing-review and editing. MS: conceptualisation; funding acquisition; methodology; project administration; resources; supervision; writing-review and editing. MJ: conceptualisation; funding acquisition; methodology; project administration; supervision; writing-review and editing. MB is the guarantor.
Funding: This work was supported by the German Foundation for Heart Research, Frankfurt am Main, Germany; and the Foundation William G. Kerckhoff Heart and Rheumatism Center, Bad Nauheim, Germany.
Disclaimer: The funding sources are not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Deidentified participants’ data analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Committee of the Justus Liebig University Giessen (Ref.: 28/14). Participants gave informed consent to participate in the study before taking part.
References
- 1.Berger M, Terrando N, Smith SK, et al. Neurocognitive function after cardiac surgeryfrom phenotypes to mechanisms. Anesthesiology 2018;129:829–51. 10.1097/ALN.0000000000002194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greaves D, Psaltis PJ, Ross TJ, et al. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta-analysis of 91,829 patients. Int J Cardiol 2019;289:43–9. 10.1016/j.ijcard.2019.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knipp SC, Weimar C, Schlamann M, et al. Early and long-term cognitive outcome after conventional cardiac valve surgery. Interact Cardiovasc Thorac Surg 2017;24:534–40. 10.1093/icvts/ivw421 [DOI] [PubMed] [Google Scholar]
- 4.Kastaun S, Gerriets T, Schwarz NP, et al. The relevance of postoperative cognitive decline in daily living: results of a 1-year follow-up. J Cardiothorac Vasc Anesth 2016;30:297–303. 10.1053/j.jvca.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Schwarz N, Kastaun S, Schoenburg M, et al. Subjective impairment after cardiac surgeries: the relevance of postoperative cognitive decline in daily living. Eur J Cardiothorac Surg 2013;43:e162–6. 10.1093/ejcts/ezt078 [DOI] [PubMed] [Google Scholar]
- 6.Greaves D, Psaltis PJ, Davis DHJ, et al. Risk factors for delirium and cognitive decline following coronary artery bypass grafting surgery: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e017275. 10.1161/JAHA.120.017275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowden T, Hurt CS, Sanders J, et al. Predictors of cognitive dysfunction after cardiac surgery: a systematic review. Eur J Cardiovasc Nurs 2022;21:192–204. 10.1093/eurjcn/zvab086 [DOI] [PubMed] [Google Scholar]
- 8.Gerriets T, Schwarz N, Bachmann G, et al. Evaluation of methods to predict early long-term neurobehavioral outcome after coronary artery bypass grafting. Am J Cardiol 2010;105:1095–101. 10.1016/j.amjcard.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen L, Murphy K, Andrews G. Cognitive and neural plasticity in old age: a systematic review of evidence from executive functions cognitive training. Ageing Res Rev 2019;53:100912. 10.1016/j.arr.2019.100912 [DOI] [PubMed] [Google Scholar]
- 10.Butz M, El Shazly J, Sammer G, et al. Decreasing postoperative cognitive deficits after heart surgery: protocol for a randomized controlled trial on cognitive training. Trials 2019;20:733. 10.1186/s13063-019-3799-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butz M, Gerriets T, Sammer G, et al. Effects of postoperative cognitive training on neurocognitive decline after heart surgery: a randomized clinical trial. Eur J Cardiothorac Surg 2022;62:ezac251. 10.1093/ejcts/ezac251 [DOI] [PubMed] [Google Scholar]
- 12.Butz M, Gerriets T, Sammer G, et al. The impact of postoperative cognitive training on health-related quality of life and cognitive failures in daily living after heart valve surgery: a randomized clinical trial. Brain Behav 2023;13:e2915. 10.1002/brb3.2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen LS, Siersma VD, ISPOCD GROUP . Postoperative cognitive dysfunction: true deterioration versus random variation. Acta Anaesthesiol Scand 2004;48:1137–43. 10.1111/j.1399-6576.2004.00502.x [DOI] [PubMed] [Google Scholar]
- 14.Edition F. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing, 2013. [Google Scholar]
- 15.Bullinger M, Kirchberger I. SF-36: Fragebogen zum Gesundheitszustand; Handanweisung. Hogrefe, Verlag für Psychologie, 1998. [Google Scholar]
- 16.Hays RD, Sherbourne CD, Mazel RM. The rand 36‐Item health survey 1.0. Health Econ 1993;2:217–27. 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 17.Klumb PL. Cognitive failures and performance differences: validation studies of a German version of the cognitive failures questionnaire. Ergonomics 1995;38:1456–67. 10.1080/00140139508925202 [DOI] [PubMed] [Google Scholar]
- 18.Broadbent DE, Cooper PF, FitzGerald P, et al. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982;21:1–16. 10.1111/j.2044-8260.1982.tb01421.x [DOI] [PubMed] [Google Scholar]
- 19.Heß KJH. Germany: Ruprecht-Karls Universität, Fakultät Für Verhaltens-und Empirische Kulturwissenschaften, the influence of repressive coping behavior on the self-assessment of memory in patients with multiple sclerosis [Doctoral thesis]. 2005 [Google Scholar]
- 20.Wallace JC, Kass SJ, Stanny CJ. The cognitive failures questionnaire Revisited: dimensions and correlates. J Gen Psychol 2002;129:238–56. 10.1080/00221300209602098 [DOI] [PubMed] [Google Scholar]
- 21.Pollina LK, Greene AL, Tunick RH, et al. Dimensions of everyday memory in young adulthood. Br J Psychol 1992;83:305–21. 10.1111/j.2044-8295.1992.tb02443.x [DOI] [PubMed] [Google Scholar]
- 22.Larson GE, Alderton DL, Neideffer M, et al. Further evidence on dimensionality and correlates of the cognitive failures questionnaire. Br J Psychol 1997;88:29–38. 10.1111/j.2044-8295.1997.tb02618.x [DOI] [Google Scholar]
- 23.Rast P, Zimprich D, Van Boxtel M, et al. Factor structure and measurement invariance of the cognitive failures questionnaire across the adult life span. Assessment 2009;16:145–58. 10.1177/1073191108324440 [DOI] [PubMed] [Google Scholar]
- 24.Herrmann-Lingen C, Buss U, Snaith RP. HADS-D: Manual: deutsche adaptation der hospital anxiety and depression scale (HADS) von RP Snaith und AS Zigmond. 2011: Huber, [Google Scholar]
- 25.Brown CH, Probert J, Healy R, et al. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology 2018;129:406–16. 10.1097/ALN.0000000000002253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowden T, Hurt CS, Sanders J, et al. Effectiveness of cognitive interventions for adult surgical patients after general anaesthesia to improve cognitive functioning: a systematic review. J Clin Nurs 2023;32:3117–29. 10.1111/jocn.16423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vance D, Dawson J, Wadley V, et al. The accelerate study: the longitudinal effect of speed of processing training on cognitive performance of older adults. Rehabilitation Psychology 2007;52:89–96. 10.1037/0090-5550.52.1.89 [DOI] [Google Scholar]
- 28.Grönholm-Nyman P, Soveri A, Rinne JO, et al. Limited effects of set shifting training in healthy older adults. Front Aging Neurosci 2017;9:69. 10.3389/fnagi.2017.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajtahed SS, Rezapour T, Etemadi S, et al. Efficacy of Neurocognitive rehabilitation after coronary artery bypass graft surgery in improving quality of life: an Interventional trial. Front Psychol 2019;10:1759. 10.3389/fpsyg.2019.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Tournay-Jetté E, Dupuis G, Denault A, et al. The benefits of cognitive training after a coronary artery bypass graft surgery. J Behav Med 2012;35:557–68. 10.1007/s10865-011-9384-y [DOI] [PubMed] [Google Scholar]
- 31.Xiu L, Wu J, Chang L, et al. Working memory training improves emotion regulation ability. Sci Rep 2018;8:15012. 10.1038/s41598-018-31495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly M, McMinn D, Allan JL. A Bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci 2014;8:1044. 10.3389/fnhum.2014.01044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall PA, Fong GT. Temporal self-regulation theory: a neurobiologically informed model for physical activity behavior. Front Hum Neurosci 2015;9:117. 10.3389/fnhum.2015.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brück E, Larsson JW, Lasselin J, et al. Lack of clinically relevant correlation between subjective and objective cognitive function in ICU survivors: a prospective 12-month follow-up study. Crit Care 2019;23:253. 10.1186/s13054-019-2527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrigan N, Barkus E. A systematic review of cognitive failures in daily life: healthy populations. Neurosci Biobehav Rev 2016;63:29–42. 10.1016/j.neubiorev.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 36.Del Brutto OH, Mera RM, Del Brutto VJ, et al. Influence of depression, anxiety and stress on cognitive performance in community‐dwelling older adults living in rural Ecuador: results of the Atahualpa project. Geriatr Gerontol Int 2015;15:508–14. 10.1111/ggi.12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 38.Chaker Z, Badhwar V, Alqahtani F, et al. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc 2017;6:e006370. 10.1161/JAHA.117.006370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H, Yang R, Qi X, et al. Association of LifeSpan cognitive reserve indicator with dementia risk in the presence of brain pathologies. JAMA Neurol 2019;76:1184–91. 10.1001/jamaneurol.2019.2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomann AE, Goettel N, Monsch RJ, et al. The Montreal cognitive assessment: normative data from a German-speaking cohort and comparison with international normative samples. J Alzheimers Dis 2018;64:643–55. 10.3233/JAD-180080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson TN, Raeburn CD, Tran ZV, et al. Motor subtypes of postoperative delirium in older adults. Arch Surg 2011;146:295–300. 10.1001/archsurg.2011.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felix LM, Mansur-Alves M, Teles M, et al. Longitudinal impact and effects of booster sessions in a cognitive training program for healthy older adults. Arch Gerontol Geriatr 2021;94:104337. 10.1016/j.archger.2021.104337 [DOI] [PubMed] [Google Scholar]
- 43.McCann M, Stamp N, Ngui A, et al. Cardiac prehabilitation. J Cardiothorac Vasc Anesth 2019;33:2255–65. 10.1053/j.jvca.2019.01.023 [DOI] [PubMed] [Google Scholar]
- 44.Saleh AJ, Tang G-X, Hadi SM, et al. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med Sci Monit 2015;21:798–805. 10.12659/MSM.893359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butz M, Meyer R, Gerriets T, et al. Increasing preoperative cognitive reserve to prevent postoperative delirium and postoperative cognitive decline in cardiac surgical patients (INCORE): study protocol for a randomized clinical trial on cognitive training. Front Neurol 2022;13:1040733. 10.3389/fneur.2022.1040733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002411supp001.pdf (139.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Deidentified participants’ data analysed during the current study are available from the corresponding author on reasonable request.