Abstract
Background
It is possible to induce immunomodulation in HER2-positive breast cancer (BC) by modifying the route of administration of trastuzumab.
Methods
In this multicenter randomized phase II trial, all enrolled patients (pts) with T2–T4d HER2-positive BC received 3 cycles of neoadjuvant treatment (NAT) with fluorouracil, epirubicin and cyclophosphamide every 3 weeks (q21), followed by docetaxel/pertuzumab plus intravenous trastuzumab (arm A) or, docetaxel/pertuzumab plus subcutaneous (SC) trastuzumab (arm B) q21x4 cycles. After surgical operation, each pt was treated with trastuzumab q21x14 cycles using the same SC or intravenous formulation of NAT. Primary endpoint was the proportion of subjects with high stromal tumor-infiltrating lymphocytes (sTILs) in postneoadjuvant residual disease (RD).
Results
Sixty-three pts (31 (arm A) and 32 (arm B)) were enrolled. Pathological complete response was obtained by 20/31 pts (64.5%; 95% CI 45.4% to 80.1%) in arm A and 19/32 pts (59.4%; 95% CI 40.1% to 76.3%) in arm B. High sTILs were observed in 27% and 46% of postneoadjuvant residual tumors in arms A and B, respectively. CD8+ T cells increased significantly in RDs of both arms (p=0.014 and 0.002 for arm A and B, respectively), whereas a significant decline in the level of CD4+ FoxP3+ regulatory T cells was observed only in arm B (p=0.016). A significant upregulation of PD-1 on sTILs was found in RD of pts enrolled in arm B (p=0.012), while programmed death-ligand 1 (PD-L1) was significantly overexpressed in residual tumors of arm A (p=0.02). A strong negative correlation was reported in arm B between expression of PD-L1 on pretreatment sTILs and CD3 expression on sTILs in RD (τ: −0.73). Grade≥3 AE incidence rates were similar between the two arms.
Conclusions
SC trastuzumab induced relevant sTILs enrichment, with favorable variations of immune parameters in HER2-positive BC pts with RD after NAT. Novel immunotherapy strategies should be tested to achieve SC-specific, antitumor immune response.
Trial registration number
NCT03144947, and EudraCT number: 2016-000435-41.
Keywords: breast neoplasms; antibodies, neoplasm; adaptive Immunity
WHAT IS ALREADY KNOWN ON THIS TOPIC
Subcutaneous (SC) trastuzumab has similar efficacy and safety to intravenous trastuzumab in early-stage HER2-positive breast cancer (BC).
WHAT THIS STUDY ADDS
SC trastuzumab induces stromal tumor-infiltrating lymphocytes (sTILs) enrichment in residual disease after neoadjuvant chemoimmunotherapy. Particularly, CD8+ T cells increase and a decline of CD4+ FoxP3+ T cells is observed.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
SC administration of trastuzumab may favor a shift towards T-cell mediated antitumor response. A more mature follow-up is required to look for distinct prognostic significance of residual diseases bearing different immune profiles of TILs after either SC or intravenous neoadjuvant trastuzumab. Novel immunotherapy strategies should be tested to achieve SC-specific, antitumor immune response.
Introduction
HER2 overexpression, which is is observed in almost 20% of breast cancers (BCs), is associated with poor survival.1 Trastuzumab (anti-HER2 monoclonal antibody (mAb)) combined with chemotherapy is efficacious across all stages of HER2-positive BC.2 Pertuzumab is a mAb-targeting HER2 at an epitope different from that of trastuzumab.3 In combination with trastuzumab and chemotherapy, it has increased pathological complete response (pCR) rate and improved disease-free survival in both neoadjuvant and adjuvant settings.3 4 However, despite anti-HER2 therapy, disease relapses can occur in up to 25% of patients with early-stage HER2-positive BC and correspond with the development of resistance to anti-HER2 mAbs.2 5 6
Innate and adaptive immune evasion is critical for BC growth and progression.2 7 The presence of high stromal tumor-infiltrating lymphocytes (sTILs) levels have been correlated with good prognosis in patients with early stage, HER2-positive BC.8 sTILs correspond to efficient host antitumor immunity, thus indicating that immune response is crucial for improving survival.2 7 There is also evidence suggesting a relevant contribution of innate and adaptive immune systems to activity of anti-HER2 mAbs.2 7–9
Subcutaneous (SC) trastuzumab has similar efficacy and tolerability to intravenous trastuzumab in early-stage HER2-positive BC.10 11 In contrast to the intravenous route, SC administration does not induce a direct absorption of trastuzumab into the intravascular space.12 SC trastuzumab goes through peripheral and central lymphoid organs and only then is released into the blood circulation.12–14 As a result of its direct absorption to lymphoid system, SC trastuzumab may experience an ‘early contact’ with lymph-metastasizing cells13 and with CD8+ and CD4+ T cells in lymph glands.15 16 Therefore, by changing the route of trastuzumab administration, it could be possible to interact with different immune subsystems, and to produce a favorable HER2-positive BC immunomodulation.
In light of these considerations, the primary objective of this trial was to evaluate host immune response variations to either SC or intravenous trastuzumab combined with pertuzumab and chemotherapy as neoadjuvant treatment of patients with T2-4d HER2-positive BC. Clinical efficacy and tolerability of these regimens were also evaluated.
Materials and methods
Study design
In this multicenter, open-label, randomized, non-comparative phase II study patients with HER2-positive BC randomly received either intravenous trastuzumab (arm A) or SC trastuzumab (arm B) in combination with pertuzumab and chemotherapy (ClinicalTrials.gov number NCT03144947, and EUDRACT 2016-000435-41). The proportion of subjects with high sTILs in postneoadjuvant residual disease was the primary endpoint. No comparison of the primary endpoint between the two study groups was planned. The intention of randomization was to reduce patient selection bias into treatment arms. Outcome assessors, data managers and statisticians were blinded to arm allocation. Secondary endpoints included pCR (absence of invasive tumor in breast and axilla) rate, safety, time-to-event outcomes, immune-related tissue biomarkers and blood immune signatures. Survival analysis and blood immune assays are in progress, and they will be presented separately.
Patient selection
Patients with previously untreated, locally advanced, inflammatory, or early-stage (either>2 cm or node positive) HER2-positive BC, with no metastatic disease, were enrolled. Eligibility criteria included age≥18 years, availability of tumor sample for examination before starting neoadjuvant treatment, Performance Status (PS)≤1, baseline left ventricular ejection fraction (LVEF)≥55, and normal organ function. All patients signed informed consent before any study-specific procedure.
Study treatment
Enrolled subjects received in the neoadjuvant setting chemotherapy consisting of intravenous 5-fluorouracil (500 mg/m2), epirubicin (75 mg/m2) and cyclophosphamide (500 mg/m2) (FEC) every 3 weeks (Q3W) for 3 cycles. Then, subjects were randomized to either arm A to receive intravenous trastuzumab (8 mg/kg loading dose, then 6 mg/kg) in combination with intravenous pertuzumab (840 mg intravenous loading dose, then 420 mg) and docetaxel (75 mg/m2, escalating, if tolerated, to 100 mg/m2) Q3W for 4 cycles or arm B to receive SC trastuzumab (600 mg) and intravenous pertuzumab and docetaxel (at same doses of arm A) Q3W for 4 cycles. Surgery was given from 3 to 7 weeks after the last administration of the neoadjuvant treatment. Post surgery, each patient received trastuzumab Q3W for 14 cycles with the same formulation (SC or intravenous) and dosage used preoperatively. Radiotherapy and endocrine therapy for estrogen receptor (ER)-positive tumors were prescribed as per local standards. Dose modifications for trastuzumab (SC or intravenous) and pertuzumab were not permitted. Docetaxel dose reduction to 55 mg/m2 (or 75 mg/m2 if the dose was previously escalated to 100 mg/m2) was allowed; re-escalation was not permitted. FEC dose reductions were allowed as per local prescribing guidelines. Prophylactic use of granulocyte colony-stimulating factor was permitted.
Clinical evaluation
Tumor assessments were carried out at baseline, prior to trastuzumab-containing treatment, and before surgery using clinical breast examination (CBE) and/or mammography or other conventional methods as per local standards, such as ultrasound, CT, or MRI.
LVEF was measured by echocardiography, which was carried out at baseline, prior to trastuzumab-containing treatment, and before surgery. After surgery, LVEF assessments took place every 3 months during adjuvant trastuzumab, and at the final visit or withdrawal. Adverse events (AEs) were prospectively monitored and graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Histopathological assessment of sTILs
Diagnostic biopsies and surgical resection specimens were collected at the reference laboratory: Pathology Unit, Department of Medicine and Surgery, University of Parma, Italy (Responsible: EMS). Central assessments of HER2 status, pCR and sTILs were performed according to current recommendations.17–20 sTILs were quantitatively scored after image digitalization of H&E slides as a continuous variable and were defined as percentage of stromal areas occupied by mononuclear immune cells (ICs) within the tumor boundaries.19–21 The lymphoid infiltrate composition was evaluated using the following immunohistochemical markers: LCA (clones, 2B11 and PD7/26); CD20 (L26); CD3 (2GLV6); CD4 (SP35); CD8 (SP57); FoxP3; CD56 (MRQ-42); CD25 (4C9); programmed cell death 1 (PD-1) (NAT105); programmed death-ligand 1 (PD-L1) on ICs (SP142); PD-L1 combined positive score (CPS; 22C3).
Statistical analysis
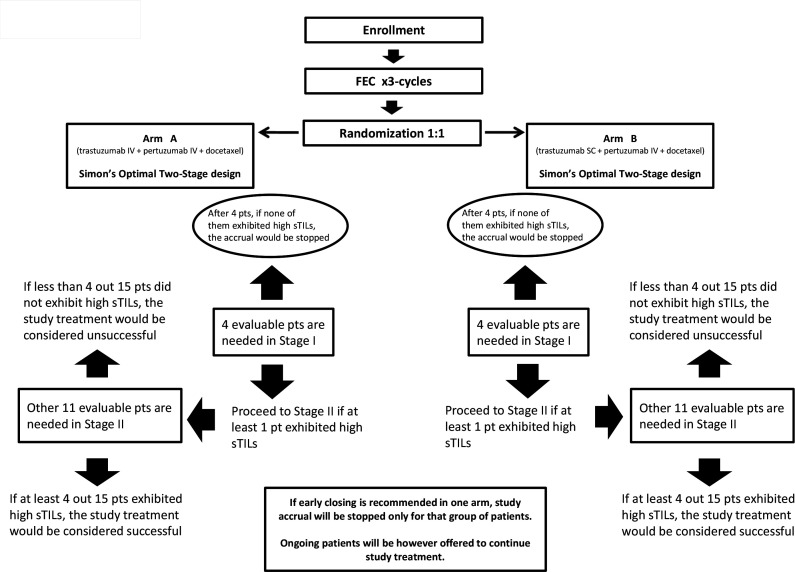
The primary endpoint of this study was the proportion of subjects with high sTILs in postneoadjuvant residual disease. The actual median level of sTILs in pretreatment tumor biopsies was adopted as threshold for classifying subjects with high sTILs versus (vs) low sTILs in post-treatment residual disease. Simon’s optimal two-stage design was adopted for each study arm. Based on this model, the sample size was determined to verify the biological activity of neoadjuvant chemotherapy plus dual anti-HER2 blockade by assuming the alternative hypothesis of an expected proportion of subjects with high sTILs in residual disease of 40%. The null hypothesis was defined under the lowest limit of 10% of subjects with high sTILs, which if verified would have implied the absence of an improvement of interest. Setting α=10% and β=10%, 15 patients needed to be enrolled for each arm. Moreover, considering a pCR rate of 50%, the overall sample size was increased to 63 patients to ensure, under binomial distribution assumption, that, with a probability of 69%, at least 30 patients with residual disease (15 for each arm) would have been evaluable for the primary endpoint analysis. Study design, hypothesis test and predetermined two-stage decision rules are described in figure 1. A simple and balanced (1:1) randomization list was generated by the statistician (GMag) through R Statistical Software to allocate patients to the two treatment arms, and it was centrally stored in the electronic case report form web system to ensure allocation concealment.
Figure 1.
Flowchart for Simon’s two-stage design. SC, subcutaneous; sTILs, stromal tumor-infiltrating lymphocytes.
pCR percentage and its 95% CI were calculated using the Wilson method with continuity correction. Toxicities were graded according to the NCI CTCAE, version 4.0 and reported as cumulative incidence. The Wilcoxon-signed rank test was used to study the modulation of immune-related tissue biomarkers at different timepoints, and the Kendall’s correlation was utilized to measure both strength and direction of the association between biomarkers. The Mann-Whitney U test was used to compare sTILs grades in primary tumors between pCR and non-pCR patients. Exploratory analyses of immune-related tissue biomarkers were not adjusted for multiple testing. The level of significance was p<0.05. Data were analyzed with R Statistical Software V.4.2.2 at the Research and Innovation Unit, University Hospital of Parma, Italy. We used the Consolidated Standards of Reporting Trials checklist when writing our report.22
Results
Between November 2016 and September 2017, 63 patients were recruited from 21 Gruppo Oncologico Italiano di Ricerca Clinica centers in Italy; 31 patients were randomized to arm A and 32 to arm B. Patient and tumor characteristics are reported in table 1; no imbalances were observed across the two arms. Most patients had tumor clinical stage II at diagnosis and presented with ER-positive, histological grade 3 (G3) and high Ki67 (≥ 20%) ductal BCs.
Table 1.
Patient and tumor characteristics
| Chacteristic | Arm A (total, n=31) |
Arm B (total, n=32) |
| N (%) | N (%) | |
| Age, years | ||
| Median (range) | 51 (27–76) | 50 (30–77) |
| Tumor size | ||
| T1 | 2 (7) | 2 (6) |
| T2 | 22 (70) | 23 (72) |
| T3 | 5 (16) | 4 (13) |
| T4 | 2 (7) | 3 (9) |
| Lymph node status | ||
| N0 | 10 (32) | 10 (31) |
| N1 | 17 (55) | 18 (56) |
| N2 | 4 (13) | 4 (13) |
| Histological type | ||
| Ductal | 28 (90) | 27 (84) |
| Lobular | 3 (10) | 5 (16) |
| Tumor grade | ||
| Grade 1 | 0 | 0 |
| Grade 2 | 10 (32) | 9 (28) |
| Grade 3 | 21 (68) | 23 (72) |
| Hormone receptor expression* | ||
| Both ER- and PR-positive | 11 (35) | 11 (34) |
| ER-positive/PR-negative | 8 (26) | 8 (26) |
| ER-negative/PR-positive | 3 (10) | 2 (6) |
| ER-negative/PR-negative | 9 (29) | 11 (34) |
| Ki67 | ||
| ≤20% | 2 (7) | 5 (16) |
| >20% | 29 (93) | 27 (84) |
| HER2 status | ||
| IHC 2+ and FISH-positive | 10 (32) | 12 (38) |
| IHC 3+ | 21 (68) | 20 (62) |
%, percentage; ER, estrogen receptor; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; n, number; PR, progesterone receptor.
Safety profile and clinical activity
At data cut-off in February 2022, all patients had received all scheduled cycles of neoadjuvant treatment, undergone surgery and completed adjuvant trastuzumab therapy. Six patients required epirubicin dose reduction, while no patient needed dose reduction of docetaxel. Fifty-eight (92%) out of 63 patients developed at least one AE. During the neoadjuvant treatment period, most of the AEs were reported as mild (grades 1 and 2) with only 16% of toxicities classified as moderate or serious (grades 3 and 4). Up to 44% of AEs were registered during the administration of anthracyclines, while 23% and 33% of AEs occurred with pertuzumab and docetaxel combined with intravenous trastuzumab (arm A) or SC trastuzumab (arm B), respectively. As expected, AEs of any grade were rare during adjuvant treatment in both study arms. Toxicities worse than grade 1 occurring in ≥10% of patients are summarized in online supplemental table S1. No patients experienced symptomatic left ventricular systolic dysfunction and/or significant declines in LVEF (≥10% points from baseline to<50%) during neoadjuvant and adjuvant treatment.
jitc-2023-007667supp001.pdf (27.1KB, pdf)
A clinical objective response was obtained by 25 (81%) out of 31 patients in arm A, and 25 (78%) out of 32 in arm B. No patients experienced disease progression during neoadjuvant treatment. The rates of breast-conserving surgery (BCS) were 54.8% and 62.5% for patients randomly assigned to arms A and B, respectively. Mastectomy to BCS conversion was achieved in 41.9% and 46.9% of the patients randomly assigned to arms A and B, respectively. Most patients achieved a pCR: 20 (64.5%; 95% CI 45.4% to 80.1%) out of 31 in arm A, and 19 (59.4%; 95% CI 40.1% to 76.3%) out of 32 in arm B.
Analysis of sTILs
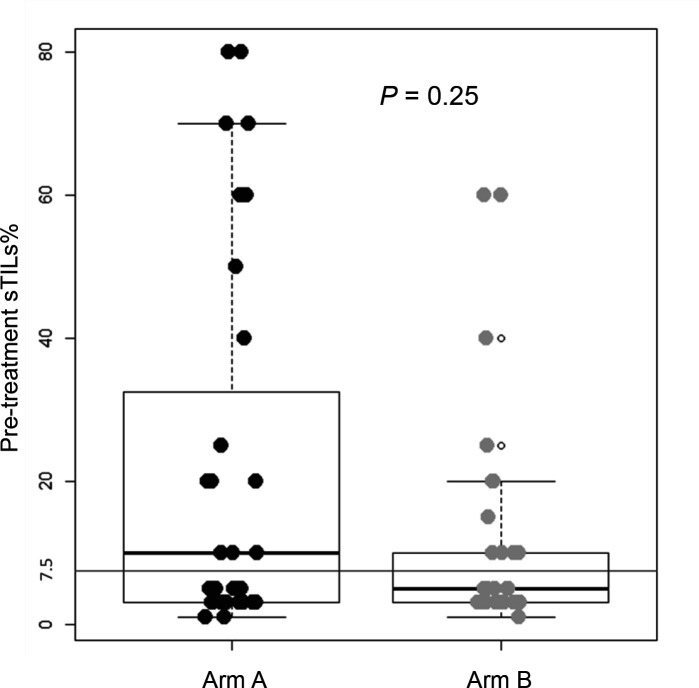
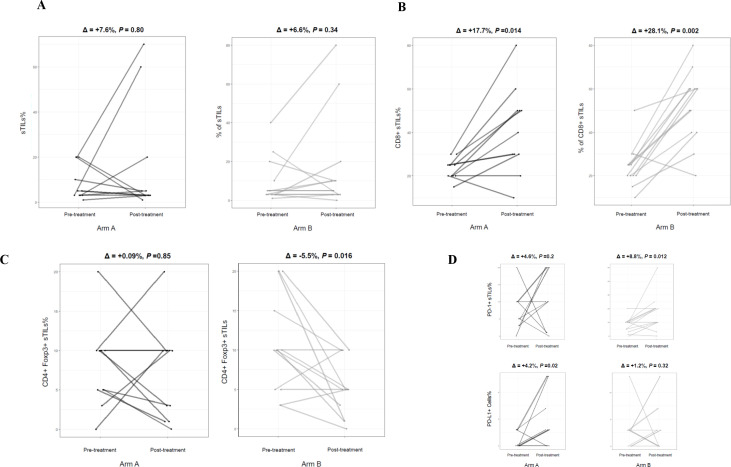
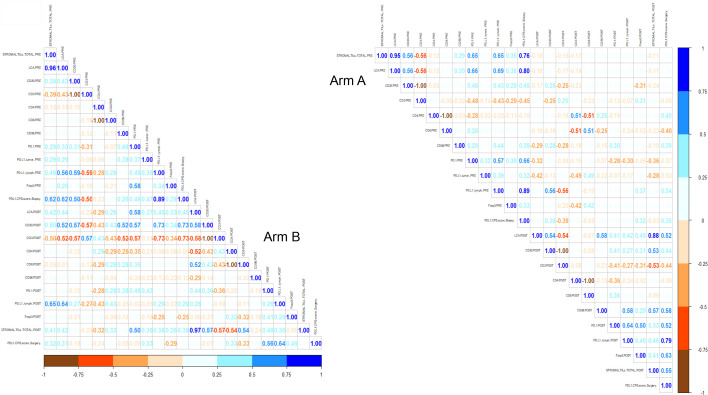
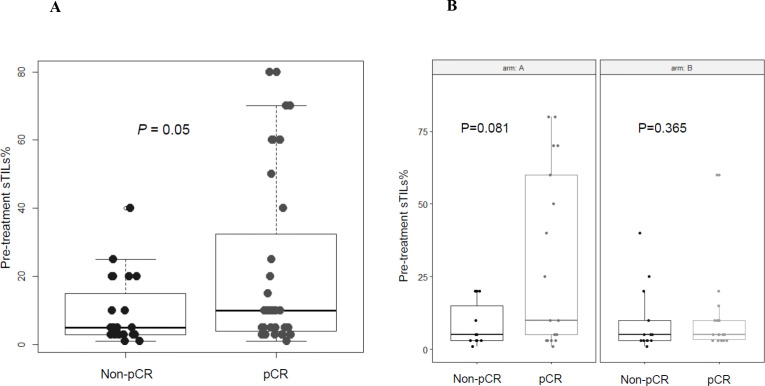
According to the predefined categorization criteria specified in the study protocol, the median level of sTILs of 7.5%, which was observed in pretreatment tumor biopsies of all enrolled patients (figure 2), was adopted as threshold for classifying subjects with high sTILs (greater than the median) versus low sTILs (equal to or lower than the median) in postneoadjuvant residual disease. In the first stage of the study, at least one patient out of four by arm was categorized as high sTILs in postneoadjuvant residual disease, thus allowing to proceed to the second stage of the study according to Simon’s design. To fulfill the statistical hypothesis on the total of 63 patients recruited (31 randomized in arm A and 32 in arm B), at least 4 patients with high sTILs in residual disease were required out of a total of 15 residual diseases, by arm. The total number of patients with residual disease was 11 and 13 in arm A and arm B, respectively. All were evaluable for sTILs analysis. Only three residual tumors (27%) showed high sTILs in arm A, while six ones (46%) expressed high sTILs in arm B. Figure 3 shows changes in sTILs from pretreatment tumor biopsy to postneoadjuvant residual disease in paired samples. Although not statistically significant, total sTILs increased in both arms (average per cent increase of 7.6% and 6.6% in arms A and B, respectively) (figure 3A). We also characterized sTILs by immunohistochemistry; in pretreatment tumor biopsies the lymphocyte infiltrate was mainly composed of CD4+ T cells (online supplemental figure S1). CD8+ T cells significantly increased in post-treatment residual diseases of both arms (p=0.014 and 0.002 for arms A and B, respectively) (figure 3B), whereas a significant decrease in the level of CD4+ FoxP3+ regulatory T cells (Tregs) was observed only in arm B (p=0.016) (figure 3C). A different shift in the tumor immune microenvironment was also observed by analyzing PD-1/PD-L1 expression. A significant upregulation of PD-1 on sTILs was found in postneoadjuvant residual disease of patients enrolled in arm B (p=0.012), while PD-L1 (CPS score≥1%) was significantly overexpressed in residual tumors in arm A (p=0.02) (figure 3D). A strong negative correlation was reported in arm B between PD-L1 expression on pretreatment sTILs and CD3 expression on sTILs in postneoadjuvant residual disease (Kendall’s correlation coefficient (τ): −0.73) (figure 4). A similar correlation, although with lower strength of association, was observed in arm A. The median level of sTILs in pretreatment samples from patients with a pCR was significantly higher as compared with non-pCR patients (figure 5A). Interestingly, this finding seems to be restricted to patients enrolled in arm A, even though there is no significant difference (figure 5B).
Figure 2.
Boxplot showing stromal tumor-infiltrating lymphocytes (sTILs) levels in pretreatment tumor biopsies of patients enrolled in arms A and B. The actual median level of sTILs was 7.5% for all enrolled patients, with no difference between the two study arms.
Figure 3.
Significant changes in stromal tumor-infiltrating lymphocytes (sTILs) from pretreatment tumor biopsy to postneoadjuvant residual disease in matched paired samples (by study arm). (A) Total sTILs. (B) CD8+ T cells. (C) CD4+ FoxP3+ regulatory T cells (Tregs). (D) PD-1+ sTILs and PD-L1+ cells (tumor, lymphocytes and macrophages; combined positive score (CPS) score ≥1%).
Figure 4.
Matrix showing Kendall’s correlation coefficients for all the possible pairs of tumor-infiltrating lymphocyte immunophenotypes in pretreatment tumor biopsies and postneoadjuvant residual diseases, by study arm.
Figure 5.
Boxplots showing pretreatment stromal tumor-infiltrating lymphocyte (sTIL) levels in pathological complete response (pCR) versus non-pCR patients. (A), All enrolled patients. (B), By study arm.
jitc-2023-007667supp002.pdf (222.7KB, pdf)
Discussion
The Immun-HER trial is a multicenter randomized phase II study evaluating host immune response variations to either intravenous (arm A) or SC (arm B) trastuzumab in combination with pertuzumab and chemotherapy as neoadjuvant therapy of patients with HER2-positive BC. The primary endpoint hypothesis of the study was formally not satisfied in arm A, while it was achieved in arm B. To fulfill the statistical hypothesis, at least 4 patients with high sTILs in residual disease would have been required out of a total of 15 residual diseases, by arm. Only three postneoadjuvant residual tumors (27%) showed high sTILs in arm A, while six ones (46%) expressed high sTILs in arm B, thus suggesting that SC trastuzumab may exert a relevant enrichment of sTILs in post-treatment HER2-positive residual disease. However, the interpretation of these findings is limited by the lower-than-expected number of patients with residual disease observed (11 and 13 in arm A and arm B, respectively). A distinct shift in the tumor immune microenvironment and some remarkable correlations between subsets of sTILs was also observed in each study arm. The trial showed relevant pCR rates of 64.5% and 59.4% in arms A and B, respectively. AEs were in line with the known safety profiles of the treatments administered.
The phase III Hannah trial confirmed the comparable efficacy and tolerability of SC and intravenous trastuzumab and highlighted the validity of SC trastuzumab as an alternative administration route for patients with early-stage HER2-positive BC.10 11 In that trial, SC trastuzumab was more immunogenic than intravenous trastuzumab: 6.8% of the patients in the SC group showed non-neutralizing antitrastuzumab antibodies versus the rate of 3.4% observed in the intravenous group.10 Interestingly, the higher presence of antitrastuzumab antibodies with SC trastuzumab did not increase but rather reduced the occurrence of infusion-related AEs.10 12 These findings may be related to the development of specific immune responses, such as IgE-to-IgG class switching, after administration of SC trastuzumab.13 It is also interesting to note that the efficient lymphatic transfer of trastuzumab via the SC route may improve anticancer effect against lymph-metastasizing cells.12 Opsonization of HER2-positive cancer cells with trastuzumab enhances uptake of HER2 by dendritic cells, thus inducing specific, and clinically relevant, T-cell adaptive response.2 23–26 In our study, CD8+ T cells significantly increased in post-treatment residual tumors of both arms. To the contrary, CD4+ FoxP3+ Tregs, which are involved in the control of immune tolerance by limiting immune activation, significantly decreased only after administration of SC trastuzumab.
HER2-positive BCs are significantly associated with the immune surveillance of the host,2 7 and several studies also highlight the significance of the PD-1/PD-L1 immune checkpoint to the disease prognosis and treatment outcome of HER2-positive tumors.2 27 28 Therefore, modulating the PD-1/PD-L1 axis is one of the forthcoming promising strategies to ameliorate anti-HER2 therapy.27 In our study, a significant upregulation of PD-1 on sTILs was found in postneoadjuvant residual disease of patients enrolled in arm B, while PD-L1 was significantly overexpressed in residual tumors in arm A. These findings suggest a different shift in the PD-1/PD-L1 pathway by study arm and, potentially, different ways to enhance immune response by using distinct immune checkpoint inhibitors, each of them associated with alternative routes of administration of anti-HER2 monoclonal antibodies.27 28
Different studies looked at the predictive and prognostic role of sTILs in patients with HER2-positive BC receiving anti-HER2-based neoadjuvant therapy. Higher baseline sTIL levels were associated with increased pCR rates and better survival, independent of any anti-HER2 agent and chemotherapy used.29 30 We observed the same association between baseline sTILs and pCR even if it was less evident for patients treated with SC trastuzumab. In our patients, PD-L1 expression on pretreatment sTILs inversely correlated with CD3 expression on sTILs in residual disease after SC trastuzumab, thus suggesting that SC administration of trastuzumab may favor a shift towards T-cell mediated antitumor response. A more mature follow-up is required to look for distinct prognostic significance of residual diseases bearing different immune profiles of TILs after either SC or intravenous neoadjuvant trastuzumab.
Conclusion
Our study suggests that the SC route of administration is an alternative way to improve immune function of mAbs. Pertuzumab and trastuzumab combination for SC injection (Phesgo) was approved by the Food and Drug Administration on June 29, 2020, for the treatment of HER2-positive early-stage and metastatic BC.31 32 Based on our findings, novel immunotherapy strategies using Phesgo should be tested in clinical trials to achieve SC-specific, antitumor immune response.
jitc-2023-007667supp003.pdf (79KB, pdf)
jitc-2023-007667supp004.pdf (870.7KB, pdf)
Acknowledgments
The authors want to thank the patients enrolled in the trial, their families, and the study hospitals’ staffs.
Footnotes
Twitter: @emilio.bria, @a_musolino
BP and CT contributed equally.
GM and AM contributed equally.
Contributors: AM conceived the presented idea. All authors contributed to enrolling patients in the trial. AM, EMS and GMissale planned the experiments. EMS and GMissale carried out the experiments. GMaglietta performed statistical analysis. BP, CT, and AM took the lead in writing the manuscript. All authors made substantial contributions to acquisition and interpretation of data. All authors revised the article and approved the submitted version. BP (first author) and CT (co-first author) equally contributed to this work. GMaglietta (co-last author) and AM (last author) equally contributed to this work. AM is responsible for the overall content as guarantor.
Funding: The Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC) sponsored the trial. BP was supported by ESMO through a grant from Roche. Roche provided support for study drug supply and trial management. At present, EB is supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (Investigator Grant n: IG20583). EB is supported by academic funds of Università Cattolica (UCSC-project D1), and by funds of Ministero della Salute (Ricerca Corrente). LC is supported by AIRC (My First AIRC Grant (IG) n: MFAG25149).
Competing interests: BP reports research grants from Lilly, Pfizer, Novartis; and personal fees from MSD outside the submitted work. EC reports travel grants from Pfizer and Ipsen outside the submitted work. EB reports research grants from Roche, AstraZeneca; personal fees from MSD, AstraZeneca, BMS, Roche, Novartis, Pfizer, Lilly; and travel grants from Roche and AstraZeneca outside the submitted work. LC reports personal fees from AstraZeneca, Novartis and Lilly outside the submitted work. LG reports personal fees from Daiichi, Seagen; and travel grants from Ipsen, Novartis, Pfizer outside the submitted work. AZ reports personal fees from AstraZeneca, Lilly, Pfizer, Daiichi, MSD, Roche, Seagen, Exact Sciences, Gilead and Istituto Gentili outside the submitted work. FM reports personal fees from Roche, Novartis, AstraZeneca, Daiichi, Seagen, MSD, Lilly, Pfizer, Pierre Fabre; and travel grants from Roche and AstraZeneca outside the submitted work; from May 15th 2023, FM is Roche employee. AM reports research grants from Lilly; personal fees from Seagen, Daiichi, Gilead, Novartis, AstraZeneca; and travel grants from Gilead and Novartis outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Comitato Etico per Parma. Reference number: 36004 - October 18, 2016. Participants gave informed consent to participate in the study before taking part.
References
- 1. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 2. Musolino A, Boggiani D, Pellegrino B, et al. Role of innate and adaptive immunity in the efficacy of anti-HER2 Monoclonal antibodies for Her2-positive breast cancer. Crit Rev Oncol Hematol 2020;149:102927. 10.1016/j.critrevonc.2020.102927 [DOI] [PubMed] [Google Scholar]
- 3. Gianni L, Pienkowski T, Im Y-H, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (Neosphere): a randomised Multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. 10.1016/S1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 4. Piccart M, Procter M, Fumagalli D, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years' follow-up. J Clin Oncol 2021;39:1448–57. 10.1200/JCO.20.01204 [DOI] [PubMed] [Google Scholar]
- 5. Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and Trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2+ early breast cancer. Cancer Res 2016;76:S5–04. 10.1158/1538-7445.SABCS15-S5-04 [DOI] [Google Scholar]
- 6. Lavaud P, Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med 2014;12:132. 10.1186/s12916-014-0132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musolino A, Gradishar WJ, Rugo HS, et al. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J Immunother Cancer 2022;10:e003171. 10.1136/jitc-2021-003171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40–50. 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 9. Norton N, Fox N, McCarl C-A, et al. Generation of HER2-specific antibody immunity during trastuzumab adjuvant therapy Associates with reduced relapse in Resected Her2 breast cancer. Breast Cancer Res 2018;20:52. 10.1186/s13058-018-0989-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (Neo)Adjuvant Trastuzumab in patients with Her2-positive, clinical stage I-III breast cancer (Hannah study): a phase 3, open-label, Multicentre, randomised trial. Lancet Oncol 2012;13:869–78. 10.1016/S1470-2045(12)70329-7 [DOI] [PubMed] [Google Scholar]
- 11. Jackisch C, Stroyakovskiy D, Pivot X, et al. Subcutaneous vs intravenous trastuzumab for patients with Erbb2-positive early breast cancer: final analysis of the Hannah phase 3 randomized clinical trial. JAMA Oncol 2019;5:e190339. 10.1001/jamaoncol.2019.0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dahlberg AM, Kaminskas LM, Smith A, et al. The lymphatic system plays a major role in the intravenous and subcutaneous pharmacokinetics of Trastuzumab in rats. Mol Pharm 2014;11:496–504. 10.1021/mp400464s [DOI] [PubMed] [Google Scholar]
- 13. Fathallah AM, Bankert RB, Balu-Iyer SV. Immunogenicity of subcutaneously administered therapeutic proteins--a mechanistic perspective. AAPS J 2013;15:897–900. 10.1208/s12248-013-9510-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wynne C, Harvey V, Schwabe C, et al. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/IB trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol 2013;53:192–201. 10.1177/0091270012436560 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt ST, Khadke S, Korsholm KS, et al. The administration route is decisive for the ability of the vaccine adjuvant Caf09 to induce antigen-specific CD8(+) T-cell responses: the immunological consequences of the biodistribution profile. Journal of Controlled Release 2016;239:107–17. 10.1016/j.jconrel.2016.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van de Ven K, Borst J. Targeting the T-cell Co-stimulatory CD27/CD70 pathway in cancer immunotherapy: rationale and potential. Immunotherapy 2015;7:655–67. 10.2217/imt.15.32 [DOI] [PubMed] [Google Scholar]
- 17. Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 18. von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. JCO 2012;30:1796–804. 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 19. Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the Neoaltto trial. JAMA Oncol 2015;1:448–54. 10.1001/jamaoncol.2015.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after Neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-oncology biomarker working group on breast cancer. Semin Cancer Biol 2018;52:16–25. 10.1016/j.semcancer.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 21. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860–7. 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu MM, Pu Y, Zhang Y, et al. The role of adaptive immunity in the efficacy of targeted cancer therapies. Trends Immunol 2016;37:141–53. 10.1016/j.it.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Modica M, Tagliabue E, Triulzi T. Predicting the efficacy of HER2-targeted therapies: a look at the host. Dis Markers 2017;2017:7849108. 10.1155/2017/7849108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park S, Jiang Z, Mortenson ED, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010;18:160–70. 10.1016/j.ccr.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gall VA, Philips AV, Qiao N, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res 2017;77:5374–83. 10.1158/0008-5472.CAN-16-2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Padmanabhan R, Kheraldine HS, Meskin N, et al. Crosstalk between HER2 and PD-1/PD-L1 in breast cancer: from clinical applications to mathematical models. Cancers (Basel) 2020;12:636. 10.3390/cancers12030636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan C, Liu Z, Yu Q, et al. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci Rep 2019;9:14356. 10.1038/s41598-019-50898-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the Finher trial. Ann Oncol 2014;25:1544–50. 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 30. Kim RS, Song N, Gavin PG, et al. NRG oncology/NSABP B-31: Stromal tumor infiltrating lymphocytes (sTILs) and outcomes in early-stage HER2-positive breast cancer (BC). JNCI 2019;111:867–71. 10.1093/jnci/djz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan AR, Im S-A, Mattar A, et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (Federica): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol 2021;22:85–97. 10.1016/S1470-2045(20)30536-2 [DOI] [PubMed] [Google Scholar]
- 32. Annex 1: Summary of product characteristics. n.d. Available: https://www.ema.europa.eu/en/documents/product-information/phesgo-epar-product-information_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007667supp001.pdf (27.1KB, pdf)
jitc-2023-007667supp002.pdf (222.7KB, pdf)
jitc-2023-007667supp003.pdf (79KB, pdf)
jitc-2023-007667supp004.pdf (870.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request.