Abstract
As a first approach in understanding the possible efficacy and toxicity of human immunodeficiency virus protease inhibitors during breast feeding, the milk-to-plasma ratio of nelfinavir was determined in lactating rats. The milk-to-plasma ratio of nelfinavir was determined to be 0.56 ± 0.10 (means ± standard deviations). Western blotting indicated that P-glycoprotein is expressed in rat mammary and brain tissue; however, the multidrug-resistant modulator GF120918 showed a significant effect only at the blood-brain barrier and not at the mammary-epithelial tissue barrier.
There is little information concerning the extent of distribution of most xenobiotics into breast milk. Even less is known about the mechanisms (i.e., passive diffusion and active transport) by which a xenobiotic gains access to milk from plasma. Mammary epithelial cells restrict the entry of xenobiotics into milk by forming tight junctions that limit paracellular transport. Therefore, xenobiotics must either passively diffuse through the mammary epithelial barrier or be transported by proteins in order to reach breast milk. Alcorn et al. detected the mRNA for many drug transporters in human lactating epithelial cells, including ABCB1, which is the gene responsible for encoding the drug transporter P-glycoprotein (P-gp) (1).
Human immunodeficiency virus (HIV) protease inhibitors are designed to inhibit the HIV protease, which is responsible for producing a mature infectious virion (3). HIV protease inhibitors have been used in conjunction with reverse transcriptase inhibitors to reduce the HIV plasma load to below detectable levels (10). One of the dilemmas in the treatment of HIV in developing countries is the vertical transmission of HIV from mother to child during breast feeding (4). Unfortunately, there is little information concerning the distribution of HIV protease inhibitors into breast milk. This lack of information makes it difficult to determine if HIV protease inhibitors could be used in conjunction with reverse transcriptase inhibitors to prevent the vertical transmission of HIV via milk. Therefore, the distribution of the HIV protease inhibitor nelfinavir into breast milk was determined in rats.
Nelfinavir is a relatively lipophilic drug (log P = 4.1) (6), and therefore it should readily diffuse through mammary epithelial cells and into breast milk. However, nelfinavir, like other HIV protease inhibitors, is a substrate for the drug transporter P-gp, which is known to limit the distribution of nelfinavir into the central nervous system (CNS) and testes of rodents (2). Preliminary studies in this lab have shown that the mRNA for mdr1b, one of the rodent genes responsible for the production of P-gp, is expressed in lactating rat mammary tissue. These observations suggest that P-gp may play a role in the distribution of P-gp substrates, including HIV protease inhibitors, into breast milk. Unfortunately, the role of P-gp at the mammary epithelial tissue barrier has yet to be elucidated. Therefore, the distribution of nelfinavir into milk and the CNS of lactating rats were also determined in the presence and absence of GF120918, a multidrug-resistant (MDR) modulator (5).
Drugs and reagents.
Nelfinavir free base was extracted from viracept (nelfinavir mesylate) tablets (Agouron Pharmaceuticals, Inc., a Pfizer Company, Ann Arbor, Mich.), yielding a white solid having a purity of 98.6% by microtitration and exhibiting a single peak by high-performance liquid chromatography (HPLC). N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) was a gift from GlaxoSmithKline (Research Triangle Park, N.C.). Sodium acetate, acetic acid, and acetonitrile were obtained from Fisher Scientific (Pittsburg, Pa.). C219 (mouse anti-human P-gp) and JSB1 (mouse anti-hamster P-gp) primary antibodies were obtained from Signet Laboratories (Dedham, Mass.). Alkaline phosphatase-linked immunoglobulin G2a (IgG2a) (rabbit anti-mouse) and IgG1 (rabbit anti-mouse) antibodies were obtained from ZYMED Laboratories, Inc. (San Francisco, Calif.). Nitro blue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate (NBT-BCIP) was obtained from Pierce (Rockford, Ill.).
Animals.
Six adult female lactating Sprague-Dawley rats with pups (250 to 350 g) were purchased from Harlan Laboratories (Indianapolis, Ind.). Animals were maintained under a 12 h/12 h light/dark cycle and given access to food and water ad libitum prior to and during the experiments. All animal research was conducted in accordance with guidelines of the University of Kentucky Institutional Animal Care and Use Committee.
Infusion of nelfinavir.
A cross-over design was used to determine the distribution of nelfinavir into milk. Two cannulas were placed in the femoral and jugular veins of the female lactating rats for drug delivery and plasma sampling, respectively. Approximately 24 h after the surgery, the pups were removed from the mother to allow milk accumulation in the mammary ducts. Nelfinavir was given as a constant infusion (9 mg of free base · h−1 · kg−1 of body weight) in water (1.5 ml · h−1; pH 2) through the femoral vein for 8 h. At the 6th hour of nelfinavir infusion, either dimethyl sulfoxide (DMSO) (0.1 ml) or GF120918 (10 mg in DMSO · kg−1 [0.1 ml]) was given intravenously (1 min) through the jugular vein cannula. Plasma samples were taken at 0, 1, 3, 5, 6, 7, and 8 h. Milk was collected after the 8-h plasma sample was drawn. After a 20-h washout period, the animals were given a second infusion of nelfinavir. Each rat was given the treatment opposite that from the previous day (either DMSO or GF120918) at the 6th hour of nelfinavir infusion. At the end of the infusion the animals were euthanized, and the brain and mammary tissues were excised after the 8th hour. Nelfinavir concentrations were determined in milk, plasma, and brain tissue. The presence of P-gp was detected in both brain and mammary tissues.
HPLC assay for nelfinavir.
A 100-μl plasma or 50-μl milk sample was mixed with 500 μl of acetonitrile and vortexed for 5 min, followed by centrifugation for 10 min. The supernatant was decanted and dried down under nitrogen. The sample was then resuspended in 100 μl of mobile phase, and a 50-μl aliquot was injected onto the HPLC system. Brain samples were treated and analyzed for nelfinavir as described by Savolainen et al. and Morgan et al. (8, 11). Plasma, milk, and brain tissue samples were analyzed for nelfinavir using a previously reported HPLC assay (11). The HPLC system consisted of a Shimadzu LC-10ADvp LC pump, an SCL-10Avp system controller, an SIL-10ADvp auto injector, and an SPD-10Avp UV-VIS detector (254 nm). The mobile phase consisted of acetonitrile and 200 mM acetate buffer at pH 4 (48:52, vol:vol) with a flow rate of 1.5 ml · min−1. A reverse-phase SUPELCOSIL ABZ Plus column (25 cm by 4.6 mm, 5 μm) at 37°C was used to separate nelfinavir. The dynamic range for nelfinavir was 0.05 to 12 μg · ml−1 (r2 > 0.99), with the intra- and interday variability being less than 10%.
Western blot analysis for P-gp.
P-gp expression was determined using the C219 or JSB1 monoclonal antibody. Brain, mammary, and liver tissue samples were homogenized in hypotonic lysis buffer (10 mM Tris-HCl, 10 mM NaCl, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride) and centrifuged at 3,000 × g for 15 min at 4°C to remove nuclear and particulate matter. The supernatants were collected and centrifuged at 40,000 × g for 30 min at 4°C, and the remaining pellet was resuspended in 1 ml of lysis buffer. The amount of total protein in the samples was determined using the Lowry method (7).
Homogenized tissue samples (40 μg of total protein) were incubated at 70°C for 5 min and loaded onto a 4 to 12% NuPage Bis-Tris gel (Invitrogen). The proteins were separated with electrophoresis chromatography for 1 h at 200 V (constant) and 120 mA (initial) and transferred to a polyvinylidene difluoride (PVDF) membrane at 25 V (constant) and 160 mA (initial) for 2 h. The membrane was blocked over night at 4°C with 5% albumin, and then it was incubated with C219 or JSB1 antibody (200 ng · ml−1) for 1 h. The membrane was then incubated with IgG2a or IgG1 alkaline phosphatase-linked (Pierce) secondary antibody (1:25,000 dilution) for 1 h. The membrane was imaged using NBT-BCIP staining according to the manufacturer's instructions.
Statistics.
A Student's t test was used to determine if there was a significant difference in the milk-to-plasma ratio, the brain-to-plasma ratio, and the 8-h plasma concentration of nelfinavir between the control and GF120918-treated animals.
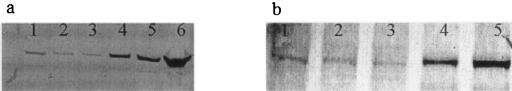
Western blots of rat brain and mammary tissue homogenates showed a distinct band for P-gp with the C219 and JSB-1 antibodies (Fig. 1). The expression of P-gp in rat mammary tissue suggests that P-gp may play a role in the distribution of HIV protease inhibitors into milk. To determine what effects P-gp has on the milk-to-plasma ratio of nelfinavir, a constant intravenous infusion of nelfinavir was given to lactating rats in the presence and absence of the MDR modulator GF120918. The plasma concentrations of nelfinavir in the control rats (Fig. 2a) appeared to plateau at approximately 5 h. However, the plasma concentrations of nelfinavir in the GF120918-treated rats (Fig. 2b) appeared to continuously increase throughout the duration of the nelfinavir infusion. It is difficult to determine if GF120918 altered the systemic clearance of nelfinavir, because the plasma concentrations of nelfinavir appear to continually increase even prior to the administration of GF120918 (administered at the 6th hour of the nelfinavir infusion). There was no statistical difference (P = 0.35) in the plasma concentrations of nelfinavir at 8 h between the control (5.50 ± 1.43 μg · ml−1) and GF120918-treated rats (7.40 ± 4.28 μg · ml−1). The plasma concentrations of nelfinavir were slightly higher than plasma concentrations reported in human clinical trials with nelfinavir (9) and the viracept product sheet (∼3 μg · ml−1; Agouron Pharmaceuticals, Inc.).
FIG. 1.
P-gp expression in rat brain and mammary tissues. The expression of P-gp in rat brain (lanes 1 to 3) and mammary (lanes 4 to 6) tissue homogenates (40 μg of protein) was determined using Western blots with C219 (a) or JSB-1 (b) antibody.
FIG. 2.
Concentration-time profiles of nelfinavir in rat plasma (n = 6) and nelfinavr concentrations in milk (n = 6) and brain tissue (n = 3) at the 8-h time point in control animals (a) and GF120918-treated (10 mg · kg−1) animals (b). All values represent means ± SD.
The mean milk-to-plasma ratio of nelfinavir (Fig. 3) was determined to be 0.56 ± 0.10 (means ± standard deviations [SD]). There was no statistically significant difference (P = 0.18) in the milk-to-plasma ratio of nelfinavir in the presence of GF120918 (0.48 ± 0.08, means ± SD) compared to that of the control group (0.56 ± 0.10, means ± SD) (Fig. 3). There was a significant increase (>19-fold; P = 0.04) in the brain-to-plasma ratio of nelfinavir in the presence of GF120918 (0.69 ± 0.38) compared to that in control rats (0.035 ± 0.01), indicating that the systemic concentrations of GF120918 were high enough to inhibit P-gp in vivo (Fig. 4). This would indicate that P-gp is expressed in mammary tissue but does not appear to play a significant role at the mammary-epithelial tissue barrier, which is consistent with a previous report showing low levels of ABCB1 in human lactating mammary epithelial cells (1).
FIG. 3.

Milk-to-plasma ratio of nelfinavir in the presence and absence of GF120918. All values represent means ± SD (n = 6 for each group).
FIG. 4.

Brain-to-plasma ratio of nelfinavir in the presence and absence of GF120918. All values represent means ± SD (n = 3 for each group).
In conclusion, the concentration of nelfinavir in milk is approximately half the concentration of nelfinavir in plasma when a continuous intravenous infusion of nelfinavir was administered. The accumulation of nelfinavir in milk would suggest that nelfinavir could be a possible treatment in reducing the vertical transmission of wild-type HIV through breast milk, although more conclusive in vivo studies looking at HIV replication and transmission in milk in the presence of nelfinavir are needed to fully qualify the use of nelfinavir to reduce vertical transmission. It was also shown that P-gp is expressed in lactating rat mammary tissue; however, it does not play a significant role in the distribution of nelfinavir, and most likely other P-gp substrates, into rat milk.
Acknowledgments
J.E. was supported by NIEHS training grant ES07266. This work was supported by National Institutes of Health grant HD37463.
REFERENCES
- 1.Alcorn, J., X. Lu, J. A. Moscow, and P. J. McNamara. 2002. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J. Pharmacol. Exp. Ther. 303:487-496. [DOI] [PubMed] [Google Scholar]
- 2.Choo, E. F., B. Leake, C. Wandel, H. Imamura, A. J. Wood, G. R. Wilkinson, and R. B. Kim. 2000. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab. Dispos. 28:655-660. [PubMed] [Google Scholar]
- 3.Flexner, C. 1998. HIV-protease inhibitors. N. Engl. J. Med. 338:1281-1292. [DOI] [PubMed] [Google Scholar]
- 4.Fowler, M. G., and M. L. Newell. 2002. Breast-feeding and HIV-1 transmission in resource-limited settings. J. Acquir. Immune Defic. Syndr. 30:230-239. [DOI] [PubMed] [Google Scholar]
- 5.Hyafil, F., C. Vergely, P. Du Vignaud, and T. Grand-Perret. 1993. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 53:4595-4602. [PubMed] [Google Scholar]
- 6.Longer, M., B. Shetty, I. Zamansky, and P. Tyle. 1995. Preformulation studies of a novel HIV protease inhibitor, AG1343. J. Pharm. Sci. 84:1090-1093. [DOI] [PubMed] [Google Scholar]
- 7.Lowry, O. H., N. J. Rosebrough, and, R. J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 8.Morgan, M. E., S. C. Chi, K. Murakami, H. Mitsuya, and B. D. Anderson. 1992. Central nervous system targeting of 2′,3′-dideoxyinosine via adenosine deaminase-activated 6-halo-dideoxypurine prodrugs. Antimicrob. Agents Chemother. 36:2156-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyle, G. J., M. Youle, C. Higgs, J. Monaghan, W. Prince, S. Chapman, N. Clendeninn, and M. R. Nelson. 1998. Safety, pharmacokinetics, and antiretroviral activity of the potent, specific human immunodeficiency virus protease inhibitor nelfinavir: results of a phase I/II trial and extended follow-up in patients infected with human immunodeficiency virus. J. Clin. Pharmacol. 38:736-743. [DOI] [PubMed] [Google Scholar]
- 10.Rana, K. Z., and M. N. Dudley. 1999. Human immunodeficiency virus protease inhibitors. Pharmacotherapy 19:35-59. [DOI] [PubMed] [Google Scholar]
- 11.Savolainen, J., J. E. Edwards, M. E. Morgan, P. J. McNamara, and B. D. Anderson. 2002. Effects of a P-glycoprotein inhibitor on brain and plasma concentrations of anti-human immunodeficiency virus drugs administered in combination in rats. Drug Metab. Dispos. 30:479-482. [DOI] [PubMed] [Google Scholar]