Abstract
The activities of short synthetic, nonhemolytic peptides derived from the C-terminal region of myotoxin II, a catalytically inactive phospholipase A2 homologue present in the venom of the snake Bothrops asper, have been shown to reproduce the bactericidal activity of the parent protein. They combine cationic and hydrophobic-aromatic amino acids, thus functionally resembling the antimicrobial peptides of innate defenses. This study evaluated the antimicrobial and antiendotoxic properties of a 13-mer derivative peptide of the C-terminal sequence from positions 115 to 129 of myotoxin II, named pEM-2. This peptide (KKWRWWLKALAKK) showed bactericidal activity against both gram-positive and gram-negative bacteria. In comparison to previously described peptide variants derived from myotoxin II, the toxicity of pEM-2 toward eukaryotic cells in culture was significantly reduced, being similar to that of lactoferricin B but lower than that of polymyxin B. The all-d enantiomer of pEM-2 [pEM-2 (d)] retained the same bactericidal potency of its l-enantiomeric counterpart, but it showed an enhanced ability to counteract the lethal activity of an intraperitoneal lipopolysaccharide challenge in mice, which correlated with a significant reduction of the serum tumor necrosis factor alpha levels triggered by this endotoxin. Lethality induced by intraperitoneal infection of mice with Escherichia coli or Salmonella enterica serovar Typhimurium was reduced by the administration of pEM-2 (d). These results demonstrate that phospholipase A2-derived peptides may have the potential to counteract microbial infections and encourage further evaluations of their actions in vivo.
Microbicidal cationic peptides have emerged as promising therapeutic alternatives to cope with the increasing rates of antibiotic resistance encountered worldwide (15). In higher animals, a number of cationic peptides are localized at sites highly exposed to microorganisms, such as the skin; intestine; lungs; and body secretions, such as tears, sweat, and saliva (16, 27). They are now considered key components of the innate immune defenses, with physiologically relevant activities against both gram-negative and gram-positive bacteria, protozoa, and fungi (39). More than 500 cationic peptides have been isolated from mammals, amphibians, arthropods, plants, bacteria, and even viruses (15).
On the other hand, several types of mammalian secreted phospholipases A2 (PLA2s; EC 3.1.1.4) have been reported to exert potent bactericidal actions that are dependent on their enzymatic activities (5, 18, 19, 20, 30, 37). Notably, a group of PLA2 homologues present in snake venoms, known as Lys49 PLA2s (13, 24), have also been shown to be bactericidal, even though they lack enzymatic activity (29, 34). In the case of myotoxin II, a Lys49 PLA2 isolated from the venom of the snake Bothrops asper, this bactericidal effect was reproduced by a synthetic 13-mer peptide corresponding to the sequence from positions 115 to 129 located at its C-terminal loop (p115-129). Thus, an enzymatically independent bactericidal effect of a PLA2 protein was demonstrated for the first time and was mapped to a specific membrane-damaging protein site (21, 29).
Although p115-129, which is derived from a snake venom PLA2, does not have significant sequence homology with any known cationic peptides of the innate defenses, it shows functional similarities with them. Previous studies showed that this peptide interacts with lipopolysaccharide (LPS) and lipid A from different gram-negative bacteria or with lipoteichoic acid from Staphylococcus aureus and relies on a membrane-permeabilizing mechanism to exert its bactericidal effects (29). LPS is a complex molecule composed of a fatty acid (lipid A), an O-polysaccharide chain, and a core sugar, inserted into the outer membrane of gram-negative bacteria. It plays a major role in the pathophysiology of septic shock and promotes a massive activation of macrophages, endothelial cells, and fibroblasts, which release diverse proinflammatory mediators, such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and nitric oxide (1, 14). Severely affected patients present with fever, shock, disseminated intravascular coagulation, multiple-organ failure, and death (1, 11).
Variants of the original p115-129 sequence have recently been tested in vitro with the aim of optimizing its bactericidal activity while minimizing its toxicity toward eukaryotic cells. Such studies identified a peptide variant, named pEM-2, as an interesting candidate for further evaluation of its antimicrobial potential (32). Therefore, in the present investigation, the bactericidal activities and antiendotoxic properties of synthetic pEM-2 in both its l- and d-enantiomeric forms were characterized in vitro and in vivo.
(This work was performed in partial fulfillment of the requirements for the M.Sc. degree of C. Santamaría at the University of Costa Rica.)
MATERIALS AND METHODS
Peptide synthesis.
Peptides were derived from the sequence from positions 115 to 129 (KKYRYYLKPLCKK) of B. asper myotoxin II (29), with some modifications. They were synthesized by N-(9 fluorenyl)-methoxycarbonyl chemistry, as described previously (8). Peptide p115-W3 (KKWRWWLKPLCKK) corresponds to a peptide with a triple tyrosine-to-tryptophan substitution (23), whereas in pEM-2 (KKWRWWLKALAKK) the proline and cysteine residues of p115-W3 were each replaced by an alanine residue. The latter peptide was synthesized in its l- and d-enantiomeric forms. All peptides were purified by reverse-phase high-performance liquid chromatography and were obtained with >95% final purity. Their observed molecular masses corresponded to the expected theoretical values. Peptides were stored dry at −20°C and were dissolved in sterile pyrogen-free saline solution (0.15 M NaCl) immediately before use.
Bacteria, LPS, and antimicrobials.
Bacteria were maintained at −70°C in Trypticase soy broth (TSB) containing 10% (vol/vol) glycerol. Frozen stocks were thawed and cultured for 4 h (enterobacteria) or overnight (Brucella spp.) in TSB. Purified Escherichia coli O111:H4 LPS was kindly provided by A. Weintraub (Karolinska Institute, Stockholm, Sweden). Lyophilized LPS was diluted in endotoxin-free saline and was dispersed by sonication for 30 s, immediately before use. Polymyxin B sulfate was obtained from Sigma (St. Louis, Mo.), whereas lactoferricin B was kindly provided by W. Bellamy (Morinaga Dairy Co., Higashihara, Japan).
Bactericidal assay.
Bactericidal activities were assayed as described previously (29). Briefly, log-phase bacteria were obtained from TSB cultures, and their concentration was adjusted to 4 × 106 CFU/ml in 0.01 M phosphate buffer (pH 7.4) containing 1% peptone (buffer A) by reading the absorbance at 540 nm (enterobacteria) or 420 nm (Brucella spp.). One hundred microliters of these suspensions, which contained 4 × 105 CFU, was incubated with 10 μl of various concentrations of peptides or buffer A alone for 20 min. Then, the remaining viable bacteria were counted by plating appropriate dilutions on Trypticase soy or blood agar after 24 h of growth at 37°C. The minimal microbicidal concentration (MMC) was defined as the lowest peptide concentration that resulted in the complete absence of bacterial growth after the bacteria were plated on agar.
Cytolytic activity.
The cytolytic effects of the peptides were determined with the murine skeletal muscle myoblast cell line C2C12 (ATCC CRL-1772), as described previously (22). C2C12 is the most sensitive target for Lys49 PLA2-derived peptides and their parent proteins. In brief, cells were grown in 96-well plates in Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin-streptomycin solution (100 U of penicillin per ml, 100 μg of streptomycin per ml) and 15% fetal calf serum (FCS) in an atmosphere with 7% CO2 at 37°C. Immediately before the experiment, the growth medium was removed and then various amounts of the peptides (12.5, 25, 50, and 100 μg) were added to the assay medium (DMEM with 1% FCS). After 3 h at 37°C, the supernatants were collected and the activity of lactate dehydrogenase released from the damaged cells was determined (Sigma kit 500). Controls for 0 and 100% toxicity values consisted of assay medium and 0.1% Triton X-100 in assay medium, respectively.
LAL assay.
In vitro neutralization of LPS activity was assessed by the Limulus amoebocyte lysate (LAL) chromogenic assay (BioWhittaker, Walkersville, Md.). In brief, E. coli LPS was solubilized at a concentration of 5 endotoxin units/ml by sonication in endotoxin-free water. Peptides were prepared at concentrations of 12.5, 25, 50, 100, and 200 μg/ml. Fifty microliters of endotoxin was mixed with 50 μl of the different peptide solutions in a 96-well plate, and the plate was incubated at 37°C for 20 min. Then, 100 μl of LAL reagent was added to each well, and the plate was incubated for 10 min at 37°C. Finally, 100 μl of substrate (the colorless peptide acetyl-Ile-Glu-Ala-Arg-para-nitroaniline) was added, and the plate was incubated at 37°C for 6 min. The reactions were stopped with 100 μl of 25% (vol/vol) acetic acid. The final absorbances at 405 nm were determined on a microplate reader (Labsystems). Assays were performed in triplicate wells, and the results were expressed as the percent inhibition compared with the control endotoxin activity values.
LPS stimulation of RAW 264.7 cells.
The murine macrophage cell line RAW 264.7 (ATCC TIB-71) was grown in DMEM, as described above. Twenty-four hours before stimulation, the cells were washed with DMEM containing 15% FCS and detached by gentle scraping. Then, 5 × 105 cells were seeded into 24-well plates and were allowed to adhere overnight. Immediately before stimulation, the growth medium was removed, and the cells were washed three times in serum-free DMEM and then incubated with various concentrations of peptides (0,12.5, 25, 50, 100, and 200 μg/ml) in 500 μl of DMEM. After 10 min, LPS (100 ng/ml) was added to the wells, and the mixture was incubated for 3 h at 37°C. The supernatants were collected and stored at −70°C until they were assayed. TNF-α levels in serum or the cell culture supernatants were determined by an enzyme-linked immunosorbent assay (R&D Systems, Milwaukee, Wis.). Samples were diluted 1:6 and assayed in duplicate wells in at least two independent experiments. All experiments were performed with a minimum of 90% viability, as determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay (28).
Endotoxic shock model.
In order to corroborate the neutralization of LPS by pEM-2 observed in vitro by the LAL assay, experiments were also carried out with CD-1 mice. All in vivo assays were approved by the Committee for Animal Care and Use, University of Costa Rica. Endotoxic shock was induced by intraperitoneal (i.p.) injection of E. coli O111:H4 LPS. Mice were presensitized with an i.p. injection of 15 mg of d-galactosamine 1 h before the LPS challenge (10). Three groups of mice (body weight, 16 to 18 g) received an i.p. injection containing (i) 10 μg of LPS alone, (ii) 50 μg of pEM-2 alone, or (iii) a mixture of LPS (10 μg) and pEM-2 (50 μg) preincubated for 20 min at 37°C. One hour after injection, a blood sample was collected by retroorbital bleeding and serum was obtained in order to quantify TNF-α levels, as described above. Lethality was recorded after 24, 48, and 72 h.
Bacterial peritonitis model.
Three groups of CD-1 mice (body weight, 21 to 26 g) received an i.p. injection of (i) 5 × 108 E. coli or 2 × 106 Salmonella enterica serovar Typhimurium CFU (obtained from log-phase TSB cultures) in 0.25 ml of saline, (ii) the bacteria followed immediately by 100 μg of the all-d enantiomer of pEM-2 [pEM-2 (d)] in 0.25 ml of saline, or (iii) 100 μg of pEM-2 (d) alone. Lethality was recorded daily over an observation period of 5 days.
Statistical analyses.
All endotoxin and TNF-α experiments were performed in triplicate. At least two independent assays were performed for all data collection. Values are expressed as means ± standard deviations (SDs) and were statistically compared by Fisher's exact test, an unpaired Student's t test, or analysis of variance, as appropriate, by using InStat (version 2.04) GraphPad software.
RESULTS
Microbicidal activity of p115-129 variants.
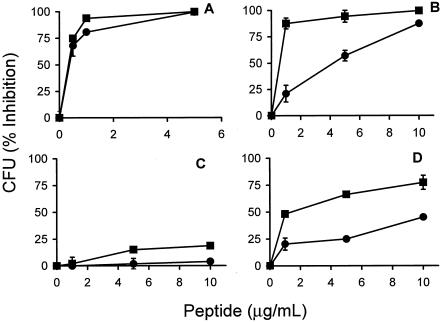
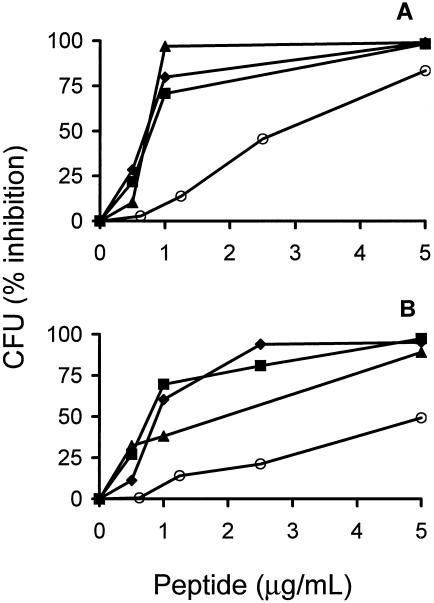
As assessed by the broth microdilution method, a triple Trp→Tyr substitution in p115-129 enhanced its microbicidal action against both gram-negative (S. enterica serovar Typhimurium) and gram-positive (S. aureus) bacteria or some resistant bacteria, such as Brucella abortus (Fig. 1). The greatest enhancement in the activity of p115-W3 was observed against S. enterica serovar Typhimurium, suggesting a stronger interaction with components of the gram-negative bacterial membrane. Two additional changes were introduced in the sequence of p115-W3. Its cysteine and proline were replaced by two alanine residues, respectively, in order to avoid oxidative dimerization (Cys) and to facilitate synthesis (Pro). This peptide, named pEM-2, displayed bactericidal activity against S. aureus and S. enterica serovar Typhimurium (Table 1) similar to that of p115-W3. In addition, pEM-2 showed strong action against other gram-negative bacteria, such as Pseudomonas aeruginosa, Shigella sonnei, and Vibrio cholerae, with MMCs of 1 μg/ml, and a lower level of activity against Klebsiella pneumoniae, with an MMC of 20 μg/ml (Table 1). In comparison, pEM-2 (d) was evaluated for its bactericidal activity. As shown in Fig. 2, pEM-2 (d) displayed microbicidal activity similar to that of its l-enantiomeric counterpart against S. enterica serovar Typhimurium and S. aureus. Both enantiomers of pEM-2 showed higher levels of activity than lactoferricin B (Fig. 2).
FIG. 1.
Bactericidal activities of synthetic myotoxin II peptide p115-129 (•) and modified peptide p115-W3 (▪) against S. aureus ATCC 23923 (A), S. enterica serovar Typhimurium ATCC 14028 (B), B. abortus 2308 (C), and B. abortus 45/20 (D). Each point represents the mean ± SD of triplicate viable counts, as described in Materials and Methods.
TABLE 1.
Microbicidal spectrum of pEM-2 (l) against various bacteria
| Bacterium | MMC (μg/ml)a |
|---|---|
| Pseudomonas aeruginosa ATCC 27853 | 1 |
| Vibrio cholerae Ogawa IMS 124 | 1 |
| Shigella sonnei ATCC 25931 | 1 |
| Escherichia coli ATCC 25922 | 5 |
| Salmonella enterica serovar Typhimurium D984 | 5 |
| Enterococcus faecalis ATCC 29212 | 5 |
| Staphylococcus aureus ATCC 23923 | 5 |
| Klebsiella pneumoniae ATCC 13883 | 20 |
| Brucella abortus 45.20 | >250 |
Assays were performed in triplicate. MMCs refer to the minimum peptide concentrations that completely killed bacteria in liquid medium, as evidenced by the lack of subsequent growth after plating of appropriate dilutions in agar, as described in Materials and Methods.
FIG. 2.
Bactericidal activity of the l enantiomer (•) and the d enantiomer (▪) of synthetic peptide pEM-2 against S. enterica serovar Typhimurium ATCC 14028 (A) and S. aureus ATCC 23923 (B). Polymyxin B (▴) and lactoferricin B (○) were included for comparison. Each point represents the mean ± SD of triplicate viable counts.
Comparative cytolytic activities of p115-W3 and pEM-2.
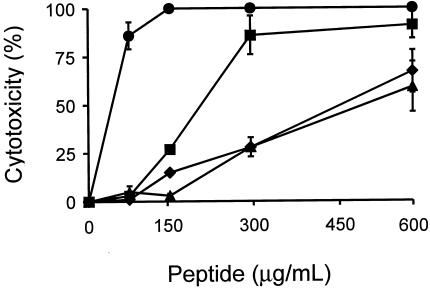
It was previously reported that even though p115-W3 is nonhemolytic, it displays a marked toxic effect toward eukaryotic cells in comparison to that of the original p115-129 of myotoxin II (23). As shown in Fig. 3, 150 μg of p115-W3 per ml caused 100% lysis of murine muscle cells, an effect more potent than that of polymyxin B. In contrast, pEM-2 exerted significantly weaker activity against these cells, reaching only half-maximal toxicity at the highest dose tested (600 μg/ml). The minimal concentration of pEM-2 required to induce complete lysis of the cultures in vitro was estimated to be ∼2.7 mg/ml (data not shown). The toxic activity of pEM-2 in this assay was similar to that of lactoferricin B (Fig. 3), a bactericidal peptide derived from mammalian lactoferrin.
FIG. 3.
Cytotoxic activities of p115-W3 (•) and pEM-2 (▴) on C2C12 myoblasts. Polymyxin B (▪) and lactoferricin B (⧫) were included for comparison. Each point represents the mean ± SD of triplicate cultures.
In vitro endotoxin-neutralizing activities of pEM-2 enantiomers.
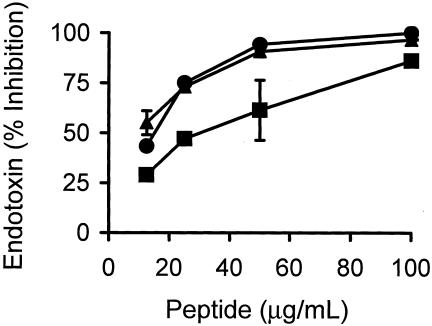
In order to evaluate the endotoxin-neutralizing ability of pEM-2, both of its enantiomers were tested in the LAL assay. pEM-2 (l) showed significant LPS-neutralizing activity, similar to that of polymyxin B, whereas pEM-2 (d) also inhibited endotoxin, although to a lower degree (Fig. 4). Control peptides alone did not induce a response in the LAL assay.
FIG. 4.
Inhibition of the endotoxin activity of LPS in vitro (by the chromogenic LAL assay) by the l enanatiomer (•) and the d enantiomer (▪) of pEM-2. Polymyxin B (▴) was included as a reference. Each point represents the mean ± SD of triplicate assays.
Effects of pEM-2 enantiomers on TNF-α secretion in vitro.
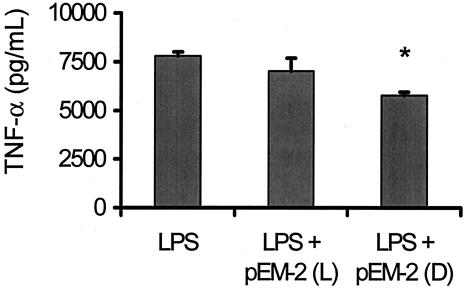
In order to assess the inhibition of LPS-induced TNF-α release by pEM-2, RAW 264.7 macrophages were challenged with 100 ng of LPS per ml, either alone or in combination with different concentrations of peptides. Control experiments indicated that at the concentrations used the peptides alone were not toxic for these cells, since there was no TNF-α release, and the viability (as judged by MTT-reducing activity) was nearly 100% in all wells. Only a weak inhibition of LPS-induced cytokine release was observed with pEM-2 (d) in these in vitro experiments. At the highest dose tested (100 μg/ml), pEM-2 (d) reduced the level of TNF-α secretion triggered by LPS by 25%, whereas virtually no inhibition was recorded for pEM-2 (l) (Fig. 5).
FIG. 5.
Inhibition of endotoxin-induced TNF-α release in RAW 264.7 cells by l and d enantiomers of pEM-2. E. coli O111:H4 LPS (100 ng/ml) was incubated at 37°C for 30 min alone or with the peptides (100 μg/ml) and was then added to the cell cultures, as described in Materials and Methods. *, statistically significant difference (P < 0.05) between LPS alone and pEM-2 (d).
Effects of pEM-2 enantiomers on LPS-induced lethality and serum TNF-α levels.
Injection of 10 μg of E. coli LPS by the i.p. route caused 100% lethality in galactosamine-sensitized CD-1 mice within the first 24 h. As shown in Table 2, no reduction in lethality was observed when the mice were challenged with the same dose of LPS if it was previously incubated with pEM-2 (l). In these animals, the serum TNF-α levels 1 h after challenge were not statistically different from those in the control group receiving LPS alone. On the other hand, pEM-2 (d) prevented the endotoxin-induced lethality by nearly 40% in three independent experiments. Moreover, serum TNF-α levels in pEM-2 (d)-treated mice decreased by more than 40% compared to those in the nontreated control group receiving LPS alone (Table 2).
TABLE 2.
Effects of pEM-2 (l) or pEM-2 (d) on serum TNF-α levels and lethality induced by LPS challenge in mice
| Mouse treatment group | Lethality (no. of deaths/ total no. of mice)a | Serum TNF-α level (pg/ml) |
|---|---|---|
| pEM-2 (l) | 0/6 | <40 |
| LPS control | 10/10 | 10,412 ± 5,286 |
| LPS + pEM-2 (l) | 10/10 | 9,515 ± 3,643 |
| pEM-2 (d) | 0/6 | <40 |
| LPS control | 10/10 | 12,276 ± 5,438 |
| LPS + pEM-2 (d) | 6/10b | 7,241 ± 4,189c |
All deaths occurred within the first 24 h of observation.
Statistically significant difference (P < 0.01) from the results for the control group receiving LPS alone, by Fisher's exact test.
Statistically significant difference (P < 0.01) from the results for the control group receiving LPS alone, by Student's t test.
Effect of pEM-2 (d) on E. coli or S. enterica serovar Typhimurium-induced lethal peritonitis.
Injection of 5 × 108 E. coli cells into the peritoneal cavities of CD-1 mice resulted in 100% lethality, and this always occurred within 24 h (Table 3). In mice that were immediately treated with 100 μg of pEM-2 (d) by the i.p. route, 25% survival was recorded after 72 h of observation. However, this reduction in lethality was not statistically significant by Fisher's extact test (P > 0.05). Similar results were obtained in experiments in which peritonitis was induced with S. enterica serovar Typhimurium, in which treatment of the mice with pEM-2 (d) rescued one of five animals from lethality (P > 0.05). No signs of acute toxicity were observed in the control mice that received the peptide alone (Table 3).
TABLE 3.
Effect of pEM-2 (d) on the lethality induced by peritoneal infection of gram-negative bacteriaa into mice
| Mouse treatment group | Lethality (no. of deaths/total no. of mice)b |
|---|---|
| Saline control | 0/4 |
| pEM-2 (D) + saline | 0/8 |
| E. coli + saline | 12/12 |
| E. coli + pEM-2 (d) | 9/12c |
| S. enterica serovar Typhimurium + saline | 5/5 |
| S. enterica serovar Typhimurium + pEM-2 (d) | 4/5c |
E. coli ATCC 25922 and S. enterica serovar Typhimurium ATCC 14028.
All deaths occurred within the first 24 h.
Nonsignificant (P > 0.05) reduction, by Fisher's exact test.
DISCUSSION
The bactericidal activity of myotoxin II against gram-positive and gram-negative bacteria has been reproduced by a short segment (positions 115 to 129) near its C-terminal loop (29) which has characteristics that functionally resemble those of natural antimicrobial cationic peptides. The original sequence from positions 115 to 129 was initially modified to enhance its hydrophobicity and ability to interact with membranes by replacing its three tyrosine residues by tryptophan. This change (p115-W3) increased the bactericidal potency against E. coli by 1 order of magnitude, but toxicity toward eukaryotic cells was also drastically potentiated (23). Such an enhancement of the bactericidal effect of p115-W3 was confirmed in the present study with a broader range of bacteria, including both gram-positive and gram-negative species, as well as Brucella spp., which are known to be highly resistant to cationic peptides (9, 26). Nevertheless, due to the high degree of toxicity of p115-W3, a series of modifications were examined for their effects on the bactericidal and cytolytic potencies of the peptides (32). From such analyses, pEM-2, a modified synthetic peptide, was selected for further characterization. The present results showed that pEM-2 displays lower cytolytic activity than p115-W3 toward myoblasts, with a toxicity comparable to that of lactoferricin B, a well-characterized cationic peptide with a high degree of bactericidal action and a low-level cytotoxic effect (3, 40). Interestingly, pEM-2 showed lower toxicity toward myoblasts than polymyxin B, a peptide restricted to topical therapeutic use (17).
Despite its lower toxicity, pEM-2 conserved the microbicidal characteristics of p115-W3, suggesting the importance of its tryptophan residues in affecting prokaryotic membranes. In agreement with this, it was previously observed that if the N-terminal hydrophobic segment of p115-W3, which contains the tryptophan cluster, is replaced by the same cationic sequence of its C-terminal half (KKALAKLKALAKK), the peptide loses all bactericidal activity (32).
The microbicidal spectrum of pEM-2 appears to be broad and includes several gram-positive and gram-negative bacteria. Thus, the actions of pEM-2 are similar to those of other cationic peptides such as lactoferricin (25) and P-113, a derivative of histatin 5, which have lengths comparable to that of pEM-2 (31).
Although many investigations on the in vitro actions of cationic peptides have been performed, there are still disadvantages when they are evaluated in vivo with animal models. Their absorption is extremely poor, thus limiting oral administration, and high local concentrations of these compounds are difficult to achieve. Two additional limitations include their relatively high levels of toxicity as well as their rapid degradation by natural proteases (16). The former disadvantage has been approached by attempting to make modifications to their amino acid sequences in order to improve their selectivities toward prokaryotes (7). On the other hand, the introduction of d-amino acids in their sequences circumvents the actions of proteases, while it preserves their bactericidal properties (36). As observed in this study, pEM-2 (d) retained the bactericidal potency of pEM-2 (l) against S. aureus and S. enterica serovar Typhimurium, suggesting that use of the d enantiomer would be an interesting alternative for exploration of the possible antiendotoxic properties of pEM-2 in vivo.
Septic shock is the most common and, unfortunately, the most dangerous complication of bacterial infection. LPS, a complex macromolecule from the outer membranes of gram-negative bacteria, is central to the pathophysiology of sepsis, and lipid A is particularly central to the pathophysiology of sepsis (1, 4, 11, 14, 33). The LAL assay was used to assess the endotoxin-neutralizing potentials of pEM-2 enantiomers. In this test, lipid A triggers a coagulation event based on an enzymatic cascade. Therefore, peptides interacting with lipid A of LPS can inhibit the LAL reaction. The results showed that both pEM-2 (l) and pEM-2 (d) interact with LPS in vitro and inhibit its triggering of the LAL reaction. The inhibition observed with the l enantiomer was very similar to that caused by polymyxin B, suggesting an interesting antiendotoxin potential of the pEM-2 sequence. Nevertheless, pEM-2 (l) was unable to prevent the LPS-triggered TNF-α release from murine macrophages in vitro, whereas pEM-2 (d) presented a significant, although partial, inhibition (∼25%) in comparison to that of control cultures stimulated with LPS alone. These results agree with the observation that pEM-2 (l)- or pEM-2 (d)-treated macrophage cultures showed similar levels of the transcription factors NF-κB and AP-1 compared to those in the control cultures stimulated with LPS alone (unpublished data). Some studies have described similar findings, i.e., a weak inhibition of TNF-α release in vitro, despite the stronger inhibition observed in vivo (35).
The differences in the inhibition of TNF-α release caused by the enantiomers might be explained by the possible degradation of pEM-2 (l) in the macrophage cultures. Experiments with mice confirmed the better performance of pEM-2 (d) in comparison to that of pEM-2 (l). Whereas the former decreased the rate of LPS-induced mortality by 40% (concomitantly with a significant reduction in the levels of TNF-α in serum), no significant differences in lethality or in TNF-α concentrations were observed in pEM-2 (l)-treated mice. These results suggest that pEM-2 (l) might be rapidly eliminated by proteolysis or renal clearance, similar to other small cationic l-peptides (2, 6, 35, 38), whereas the half-life of pEM-2 (d) might be more prolonged in vivo.
In the present work, pEM-2 was preincubated with LPS before i.p. injection in mice in order to assess its antiendotoxic ability independently of pharmacokinetic considerations. Also, the potential of pEM-2 (d) to counteract a lethal bacterial peritonitis process was evaluated immediately after administration of the bacteria. The modest protection from lethality observed in both models, with preincubation and by independent administration, encourages the performance of further studies with delayed administration of peptides to evaluate their actual performance in established cases of endotoxemia or sepsis. It was recently shown that the results derived from septic shock models based on LPS injection are very similar to those derived from other experimental models, such as cecal ligation and puncture, which better reflect the conditions observed in patients with sepsis (12). This suggests that pEM-2 (d) could also be assessed in such models, for example. Further preclinical investigations are needed to evaluate the potential of these peptides for the treatment of bacterial infections.
Acknowledgments
We gratefully acknowledge the financial support for these studies by NeTropica (grant 01-R-2003), the Lindbergh Foundation, the American Society for Microbiology-MIRCEN, the Florida, Ice & Farm of Costa Rica, the CR-USA Foundation, and the University of Costa Rica.
Thanks are also due to P. Fourquet (CIML, Marseille, France) for peptide synthesis, A. Tarkowski and M. Bokarewa (University of Göteborg, Göteborg, Sweden) for transcription factor expression analyses, Y. Angulo for collaboration in cytotoxicity experiments, and E. Chaves-Olarte for critical reading of the manuscript.
An INSERM patent (patent 04165A10) has been submitted.
REFERENCES
- 1.Adrie, C., and M. R. Pinsky. 2000. The inflammatory balance in human sepsis. Intensive Care Med. 26:364-375. [DOI] [PubMed] [Google Scholar]
- 2.Battafarano, R., P. Dahlberg, C. Ratz, J. Johnston, B. Gray, J. Haseman, K. Mayo, and D. Dunn. 1995. Peptide derivatives of three distinct lipopolysaccharide binding proteins inhibit lipopolysaccharide-induced tumor necrosis factor-alpha secretion in vitro. Surgery 118:318-324. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy, W., M. Takase, W. Wakabayashi, K. Kawase, and M. Tomita. 1992. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin J. Appl. Bacteriol. 73:472-479. [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B., and A. Poltorak. 2001. Sepsis and evolution of the innate immune response. Crit. Care Med. 29:S2-S7. [DOI] [PubMed] [Google Scholar]
- 5.Buckland, A., and D. Wilton. 2000. The antibacterial properties of secreted phospholipases A2. Biochem. Biophys. Acta 1488:71-82. [DOI] [PubMed] [Google Scholar]
- 6.Dankesreiter, S., A. Hoess, J. Schneider, H. Wagner, and T. Miethke. 2000. Synthetic endotoxin-binding peptide block endotoxin-triggered TNF-α production by macrophages in vitro and in vivo and prevent endotoxin-mediated toxic shock. J. Immunol. 164:4804-4811. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-López, S., H. Kim, E. Choi, M. Delgado, J. Granja, A. Khasanov, K. Kraehenbuehl, G. Long, D. Weinberger, K. Wilcoxen, and M. R. Ghadiri. 2001. Antibacterial agents based on the cyclic d,l-α-peptide architecture. Nature 412:452-455. [DOI] [PubMed] [Google Scholar]
- 8.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161-214. [DOI] [PubMed] [Google Scholar]
- 9.Freer, E., E. Moreno, I. Moriyón, J. Pizarro-Cerdá, A. Weintraub, and J. P. Gorvel. 1996. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J. Bacteriol. 178:5867-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanos, C., M. A. Freudenberg, and W. Reutter. 1979. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 76:5939-5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gando, S., S. Nanzaki, and S. Sasaki. 1998. Activation of the extrinsic coagulation pathway in patients with severe sepsis and septic shock. Crit. Care Med. 26:2005-2009. [DOI] [PubMed] [Google Scholar]
- 12.Giacometti, A., O. Cirioni, R. Ghiselli, F. Mocchegiani, M. S. Del Prete, C. Viticchi, W. Kamysz, E. Lempicka, V. Sabba, and G. Scalise. 2002. Potential therapeutic role of cationic peptides in three experimental models of septic shock Antimicrob. Agents Chemother. 46:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez, J. M., and B. Lomonte. 1997. Phospholipase A2 myotoxins from Bothrops snake venoms, p. 321-352 In R. M. Kini (ed.), Venom phospholipase A2 enzymes: structure, function, and mechanism. John Wiley & Sons, Chichester, England.
- 14.Hack, C. E., and S. Zeerleder. 2001. The endothelium in sepsis: source of and a target for inflammation. Crit. Care Med. 29:S21-S27. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, R. E., and D. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, R. E., and M. Scott. 2001. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellman, J., and S. H. Warren. 1999. Antiendotoxin strategies. Infect. Dis. Clin. N. Am. 13:371-386. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz, S. S., L. Tan, X. Qu, Y. Cho, P. B. Eisenhawer, and R. I. Lehrer. 1995. Bactericidal properties of murine intestinal PLA2. J. Clin. Investig. 95:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kini, R. M., and H. Evans. 1989. A common cytolytic region in myotoxins, hemolysins, cardiotoxins and antibacterial peptides. Int. J. Protein Res. 34:277-286. [DOI] [PubMed] [Google Scholar]
- 20.Koduri, R. S., J. O. Gronroos, V. J. Laine, C. Le Calvez, G. Lambeau, T. J. Nevalainen, and M. H. Gelb. 2002. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 277:5849-5857. [DOI] [PubMed] [Google Scholar]
- 21.Lomonte, B., E. Moreno, A. Tarkowski, L. Å Hanson, and M. Maccarana. 1994. Neutralizing interaction between heparins and myotoxin II, a Lys49 phospholipase A2 from Bothrops asper snake venom. Identification of a heparin-binding and cytolytic toxin region by the use of synthetic peptides and molecular modeling. J. Biol. Chem. 269:29867-29873. [PubMed] [Google Scholar]
- 22.Lomonte, B., Y. Angulo, S. Rufini, W. Cho, J. R. Giglio, M. Ohno, J. J. Daniele, P. Geoghegan, and J. M. Gutiérrez. 1999. Comparative studies of the cytolytic activity of myotoxic phospholipases A2 on mouse endothelial (tEnd) and skeletal muscle (C2C12) cells in vitro. Toxicon 37:145-158. [DOI] [PubMed] [Google Scholar]
- 23.Lomonte, B., J. Pizarro-Cerdá, Y. Angulo, J. P. Gorvel, and E. Moreno. 1999. Tyr→Trp-substituted peptide 115-129 of a Lys49 phospholipases A2 expresses enhanced membrane-damaging activities and reproduces its in vivo myotoxic effect. Biochim. Biophys. Acta 1461:19-26. [DOI] [PubMed] [Google Scholar]
- 24.Lomonte, B., Y. Angulo, and L. Calderón. 2003. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon 42:885-901. [DOI] [PubMed] [Google Scholar]
- 25.Lupetti, A., K. Paulusma-Annema, M. Welling, S. Senesi, J. van Diessel, and P. Nibbering. 2000. Candidacidal activity of human lactoferrin peptides derived from the N terminus. Antimicrob. Agents Chemother. 44:3257-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez de Tejada, G., J. Pizarro-Cerdá, E. Moreno, and I. Moriyón. 1995. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect. Immun. 63:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyakawa, Y., P. Ratnakar, R. Gurara, M. Costello, O. Mathieu-Costello, R. Lehrer, and A. Catanzaro. 1996. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect. Immun. 64:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55. [DOI] [PubMed] [Google Scholar]
- 29.Páramo, L., B. Lomonte, J. Pizarro-Cerdá, J. A. Bengoechea, J. P. Gorvel, and E. Moreno. 1998. Bactericidal activity of Lys-49 and Asp-49 myotoxic phospholipases A2 from Bothrops asper snake venom: synthetic Lys-49 myotoxin II-(115-129) peptide identifies its bactericidal region. Eur. J. Biochem. 253:452-461. [DOI] [PubMed] [Google Scholar]
- 30.Qu, X. D., and R. I. Lehrer. 1998. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect. Immun. 66:2791-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothtein, D., P. Spacciapoli, L. Tran, T. Xu, D. Roberts, M. Dalla Serra, D. Buxton, F. Oppenheim, and P. Friden. 2001. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob. Agents Chemother. 45:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santamaría, C., S. Larios, Y. Angulo, J. Pizarro, J. P. Gorvel, E. Moreno, and B. Lomonte. Antimicrobial activity of myotoxic phospholipases A2 from crotalid snake venoms and synthetic peptide variants derived from their C-terminal region. Toxicon, in press. [DOI] [PubMed]
- 33.Sasse, K. C., E. Nauenberg, and A. Long. 1995. Long-term survival after intensive care units admission with sepsis. Crit. Care Med. 23:1040-1047. [DOI] [PubMed] [Google Scholar]
- 34.Soares, A. M., R. Guerra-Sá, C. Borja-Oliveira, V. M. Rodrigues, L. Rodrigues-Simioni, V. Rodrigues, M. R. M. Fontes, B. Lomonte, J. M. Gutiérrez, and J. R. Giglio. 2000. Structural and functional characterization of BnSP-7, a lysine-49 myotoxic phospholipase A2 homologue from Bothrops neuwiedi pauloensis venom. Arch. Biochem. Biophys. 378:201-209. [DOI] [PubMed] [Google Scholar]
- 35.Uknis, M., K. Wasiluk, R. Acton, H. Klaerner, P. Dahlberg, E. Ilyina, J. Haseman, B. Gray, K. Mayo, and D. Dunn. 1997. Design of a potent novel endotoxin antagonist. Surgery 122:380-385. [DOI] [PubMed] [Google Scholar]
- 36.Wade, D., A. Boman, B. Wahlin, C. M. Drain, D. Andreu, H. Boman, and B. Merrifield. 1990. All d amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA 87:4761-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinrauch, Y., P. Elsbach, L. M. Madsen, A. Foreman, and J. Weiss. 1996. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14 kD phospholipase A2. J. Clin. Investig. 97:250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss, C., K. Wasiluk, T. Kellogg, and D. Dunn. 2000. Bactericidal and endotoxin neutralizing activity of a peptide derived from Limulus antilipopolysaccharide factor. Surgery 128:339-344. [DOI] [PubMed] [Google Scholar]
- 39.Zasloff, M. 2002. Antimicrobial peptides of multicellular organism. Nature 415:385-395. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, G., D. Mann, and C. Tsai. 1999. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derivated peptide. Infect. Immun. 67:1353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]