Abstract
Lactoferrin-induced cell depolarization and a delayed tobramycin-killing effect on Pseudomonas aeruginosa cells were correlated. This antibiotic tolerance effect (ATE) reflects the ability of a defense protein to modify the activity of an antibiotic as a result of its modulatory effect on bacterial physiology. P. aeruginosa isolates from cystic fibrosis patients showed higher ATE values (≤6-fold) than other clinical strains.
Aminoglycoside antibiotics have been shown to be effective in treating Pseudomonas aeruginosa lung infections of cystic fibrosis (CF) patients (8). However, medical experience shows that in vitro aminoglycoside-susceptible P. aeruginosa may persist in an infected lung despite appropriate antibiotic treatment, indicating a dissociation between in vitro and in vivo antibiotic susceptibility (13). This paradoxical phenomenon reflects the influence of environmental, bacterial, and host factors that are not present in in vitro susceptibility tests. For example, antibiotic susceptibility is modified in vitro and in vivo by bacterial factors, including biofilm formation (10), or by some physiological changes, such as membrane depolarization of anaerobically grown bacteria that results in aminoglycoside resistance (4). Some host defense compounds (e.g., lactoferrin) have also been reported as modifying the in vitro antibiotic susceptibility of bacteria and yeasts (1, 3, 9). Despite the fact that some of these defense compounds increase during the infection, there are few studies exploring their influence on antibiotic activity.
Human lactoferrin (hLf) is an innate defense protein, mainly present in mucosal secretions, that is found at high concentrations (∼0.9 mg/ml) in the sputa of CF patients infected by P. aeruginosa (6). We have recently reported that this protein induces a decrease in the transmembrane electrical potential of bacteria and yeasts (14, 15). Since aminoglycoside uptake is dependent on electrical potential of the bacterial membrane, we attempted to determine whether membrane depolarization induced by lactoferrin may decrease susceptibility to tobramycin. If this scenario were so, that effect could partly explain the apparently different in vivo and in vitro susceptibilities of P. aeruginosa strains isolated from CF patients.
The strains studied included P. aeruginosa ATCC 9027, P. aeruginosa FRD1 (cystic fibrosis isolate mucA22 [Alg+]) and P. aeruginosa FRD1131 (mucA22 algD::Tn501-33 [Alg−]) (a gift from D. E. Ohman, Virginia Commonwealth University). Fourteen clinical isolates of P. aeruginosa from the sputum of different CF patients were provided by F. Baquero (Hospital Ramón y Cajal, Madrid, Spain), C. Bousoño (Central Hospital of Asturias, Oviedo, Spain), and I. Planells (Vall d'Hebron Hospitals, Barcelona, Spain); six isolates from non-CF patients were also included in the study. The clinical isolates were identified by the API 20NE system. Recombinant human lactoferrin (rhLf) was provided by Ventria Bioscience (Sacramento, Calif.). Gentamicin and tobramycin were purchased from Sigma (St. Louis, Mo.). MICs were determined by the NCCLS microdilution method (12) with Mueller-Hinton medium (Difco) and inocula of 5 × 105 CFU/ml. The MIC was defined as the lowest concentration at which there was no visible growth after 24 h of incubation at 37°C.
Time-kill assays were carried out with all P. aeruginosa strains as described previously (14). Briefly, the bacterial suspensions (106 CFU/ml) in 10 mM Tris-HCl buffer (pH 7.4) containing 100 mM NaCl were preincubated with rhLf (0.9 mg/ml) for 10 min before the addition of the antibiotic (at the MIC). Duplicate samples were then removed every 30 min during 3.5 h, and dilutions were plated onto Mueller-Hinton agar to obtain a viable count. Bacterial viability (log10 CFU/ml) was plotted against time for each experiment. The antibiotic tolerance effect (ATE) was defined as the difference in time (in hours) between the rhLf-treated and untreated suspensions for the bacterial counts to decrease 1 log unit below that measured immediately after the addition of antibiotic. This effect was calculated by the following equation: ATE = T − C, where T is the time required for the host factor (rhLf)-exposed cell suspension to decrease 1 log10 below the count observed immediately after the addition of the drug (tobramycin or gentamicin) and C is the time required for the untreated suspension to decrease 1 log10 below the count observed immediately after the addition of the drug.
The membrane potential was determined by using the fluorescent probe bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4; Molecular Probes, Eugene, Oreg.) (3) as described previously (14). Briefly, exponential-phase bacteria were washed and resuspended (105 CFU/ml) in 10 mM Tris-HCl buffer (pH 7.4) containing 100 mM NaCl and then incubated with rhLf (0.9 mg/ml) at 37°C for 15, 30, 60, 90, 120, and 180 min. Samples were then reincubated for an additional 10 min with DiBAC4 (0.4 μM, final concentration) (3) and analyzed by cytofluorometry.
All experiments were performed at least in triplicate. Results were analyzed by the Mann-Whitney U test. A P value of <0.05 was considered significant.
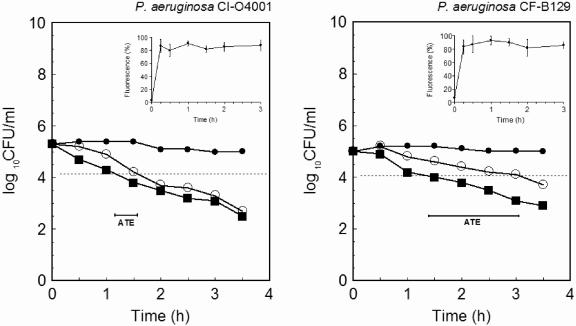
The P. aeruginosa strains studied were sensitive to the aminoglycosides tobramycin and gentamicin in a conventional MIC test (Table 1). Since the in vitro bactericidal activity of lactoferrin was inversely related to the extracellular concentrations of NaCl and divalent cations (14, 15), the evaluation of the MIC of rhLf and combination effects (i.e., fractional inhibitory concentration) of rhLf and the antibiotic was not possible by using standard microbiological media. Consequently, time-kill assays were performed in Tris buffer containing NaCl. Figure 1 shows illustrative results from time-kill assays corresponding to P. aeruginosa clinical isolate O4001 and CF strain B129 isolated from a diabetic foot ulcer and the sputum of a CF patient, respectively. The killing by tobramycin in rhLf-pretreated cells was not immediate. Table 1 summarizes the ATE values of 21 P. aeruginosa strains, including 14 clinical isolates from different CF patients attended in three hospitals located in different geographical areas. The comparison of the results obtained by using individual MICs of tobramycin or gentamicin and rhLf (0.1 to 1 mg/ml) showed that the ATE values of CF isolates were significantly (P < 0.05) higher (≤6-fold) than those calculated for the non-CF isolates. This difference was apparently not related to the controversial affinity of aminoglycosides to the exopolysaccharide alginate, because both P. aeruginosa FRD1 (Alg+) and its nonmucoid derivate P. aeruginosa FRD1131 (Alg−) showed similar ATE values (data not shown). The influence of alginate on this effect, if any, could be more relevant in the cystic fibrotic lung, where alginate-overproducing P. aeruginosa communities are organized in biofilms exhibiting increased tobramycin resistance (7, 10). In this case, the exposure of this pathogen to suboptimal antibiotic concentrations as a consequence of the aminoglycoside binding to the alginate matrix could increase the ATE values induced by hLf with respect to those observed in our assays with planktonic cells.
TABLE 1.
Antibiotic tolerance induced by lactoferrin in P. aeruginosa strains
| P. aeruginosa straina | Parameter for tobramycin (parameter for gentamicin)b
|
|||
|---|---|---|---|---|
| MIC (μg/ml) | T (h) | C (h) | ATE (h) (mean ± SD) | |
| ATCC 9027 | 1 | 1.4 | 0.9 | 0.5 ± 0.1 |
| CI strains | ||||
| O1082 | 1 (2) | 0.9 (1.7) | 0.3 (1) | 0.4 ± 0.1 (0.7 ± 0.1) |
| O1090 | 1 | 1.6 | 0.8 | 0.6 ± 0.1 |
| O1100 | 0.25 | 0.7 | 0.4 | 0.3 ± 0.2 |
| O1160 | 1 | 1.2 | 0.6 | 0.6 ± 0.1 |
| O2785 | 0.5 | 1.4 | 0.7 | 0.4 ± 0.1 |
| O4001 | 1 (2) | 1.6 (2.3) | 1.1 (1.5) | 0.5 ± 0.1 (0.8 ± 0.1) |
| CF strains | ||||
| B129 | 1 | 3 | 1.5 | 1.5 ± 0.2 |
| B191 | 1 | 1.9 | 0.4 | 1.5 ± 0.3 |
| B218 | 1 | 2.4 | 0.8 | 1.6 ± 0.2 |
| B231 | 1 | 2.4 | 0.9 | 1.5 ± 0.1 |
| B529 | 2 | 2.8 | 1.4 | 1.4 ± 0.1 |
| B566 | 0.5 | 1.7 | 0.5 | 1.2 ± 0.2 |
| O50850 | 2 (2) | 2.1 (2.4) | 0.8 (0.9) | 1.3 ± 0.3 (1.5 ± 0.2) |
| O52733 | 2 (2) | 2.4 (2.7) | 1.1 (1.4) | 1.3 ± 0.3 (1.3 ± 0.3) |
| O65711 | 1 | 2.2 | 0.6 | 1.6 ± 0.1 |
| O90401 | 1 | 2.6 | 0.7 | 1.9 ± 0.5 |
| O93814 | 4 | 2.9 | 1.2 | 1.7 ± 0.5 |
| M19915 | 1 | 1.8 | 0.7 | 1.1 ± 0.1 |
| M65323 | 1 | 2.3 | 1 | 1.3 ± 0.2 |
| M99130 | 1 | 1.7 | 0.3 | 1.4 ± 0.1 |
CI strains, clinical isolates from different sources. CF strains, clinical isolates from sputum of CF patients.
T, time required for the host factor-exposed cell suspension to decrease 1 log10 below the count observed immediately after the addition of the drug; C, time required for the untreated suspension to decrease 1 log10 below the count observed immediately after the addition of the drug. Also determined were the ATEs of the MICs of tobramycin and gentamicin in the presence of lactoferrin (0.9 mg/ml).
FIG. 1.
Time-kill assay curves for tobramycin in the presence of lactoferrin. P. aeruginosa cells were incubated with 0.9 mg of lactoferrin (•)/ml, 1 μg of tobramycin (▪)/ml, or 0.9 mg of rhLf/ml and 1 μg of tobramycin/ml (○). Results are the means from duplicates of at least three independent assays. The antibiotic tolerance effects are indicated (bars). The effect of lactoferrin on the membrane potential of P. aeruginosa was determined by flow cytometry using the fluorescent probe DiBAC4 (3) (insets). Percentages of fluorescence (fluorescent cells) correspond to bacteria with membrane depolarization. CI, clinical isolate. CF, clinical isolate from sputum of a CF patient.
After rapid electrostatic binding, the aminoglycoside uptake in P. aeruginosa has been reported to occur in two phases: a slow-uptake phase that depends on the magnitude of the bacterial transmembrane electrical potential and a subsequent rapid-uptake phase due mainly to the membrane permeabilization caused by the antibiotic (2, 4). Although the accumulation of tobramycin in P. aeruginosa cells was not verified in the present study, it is well known that the depolarization of the membrane potential implies less uptake of the aminoglycosides and consequently a decreased bacterial susceptibility (4). Representative data from experiments performed with P. aeruginosa clinical isolate O4001 and CF strain B129 exposed to rhLf at previously indicated times are shown in Fig. 1. A high percentage (>80%) of fluorescent cells, indicative of rapid membrane depolarization, was observed. Similar data were obtained with all P. aeruginosa isolates exposed to rhLf, as well as in control assays when the cells were treated with carbonyl cyanide m-chlorophenylhydrazone (10 μM) (data not shown). Since we have recently reported that hLf decreases the bacterial membrane potential without a permeabilizing effect (14), we speculate that the depolarization inhibited the antibiotic uptake during the slow-uptake phase, as the inability of tobramycin to decrease the number of P. aeruginosa cells seems to reflect. This delayed phase was followed by a rapid loss of cell viability that apparently corresponded to the later rapid-uptake phase (2), since it was partially inhibited by the presence of 1 mM MgCl2 (data not shown) as described previously (5).
Aminoglycoside activity against P. aeruginosa in the sputum of CF patients is significantly decreased (10- to 25-fold) by host and bacterial factors that are still not fully understood (11). The identification of these factors may therefore contribute to improving antibiotic dosing regimens to eradicate P. aeruginosa from these patients. Based on our results, we hypothesize that lactoferrin could be a host factor that decreases the efficacy of tobramycin in vivo. Since the ATE value was tobramycin concentration dependent (data not shown), it is possible that this effect might be partially overcome when high drug concentrations are achieved at the site of the infection (e.g., in inhalatory therapy).
In conclusion, this study shows that lactoferrin induces a transitory tolerance to tobramycin on P. aeruginosa significantly in CF clinical isolates. Although it is known that environmental parameters and bacterial and host factors may modulate antibiotic activity, our results show for the first time that a host defense protein is able to modify antibiotic susceptibility as a consequence of its modulatory effect on bacterial physiology.
Acknowledgments
This work was supported by Fundación Sira Carrasco para la Ayuda a la Fibrosis Quística (SV-03-FSCARRASCO/Universidad de Oviedo).
REFERENCES
- 1.Alkawash, M., M. Head, I. Alshami, and J. S. Soothill. 1999. The effect of human lactoferrin on the MICs of doxycycline and rifampicin for Burkholderia cepacia and Pseudomonas aeruginosa strains. J. Antimicrob. Chemother. 44:385-387. [DOI] [PubMed] [Google Scholar]
- 2.Bryan, L. E., and S. Kwan. 1983. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob. Agents Chemother. 23:835-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler, C. E., J. S. Soothill, and L. Oakes. 1997. MICs of rifampicin and chloramphenicol for mucoid Pseudomonas aeruginosa strains are lower when human lactoferrin is present. J. Antimicrob. Chemother. 40:877-879. [DOI] [PubMed] [Google Scholar]
- 4.Fraimow, H. S., J. B. Greenman, I. M. Leviton, T. J. Dougherty, and M. H. Miller. 1991. Tobramycin uptake in Escherichia coli is driven by either electrical potential or ATP. J. Bacteriol. 173:2800-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancock, R. E. W., V. J. Raffle, and T. I. Nicas. 1981. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 19:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbitz, O., A. O. Jenssen, and O. Smidsrod. 1984. Lysozyme and lactoferrin in sputum from patients with chronic obstructive lung disease. Eur. J. Respir. Dis. 65:512-520. [PubMed] [Google Scholar]
- 7.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Høiby, N. 2002. New antimicrobials in the management of cystic fibrosis. J. Antimicrob. Chemother. 49:235-238. [DOI] [PubMed] [Google Scholar]
- 9.Kuipers, M. E., H. G. de Vries, M. C. Eikelboom, D. K. Meijer, and P. J. Swart. 1999. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 43:2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O′Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 11.Mendelman, P. M., A. L. Smith, J. Levy, A. Weber, B. Ramsey, and R. L. Davis. 1985. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am. Rev. Respir. Dis. 132:761-765. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M07-A6. NCCLS, Wayne, Pa.
- 13.Smith, A. L., S. B. Fiel, N. Mayer-Hamblett, B. Ramsey, and J. L. Burns. 2003. Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration: lack of association in cystic fibrosis. Chest 123:1495-1502. [DOI] [PubMed] [Google Scholar]
- 14.Viejo-Diaz, M., M. T. Andrés, J. Pérez-Gil, M. Sánchez, and J. F. Fierro. 2003. Potassium efflux induced by a new lactoferrin-derived peptide mimicking the effect of native human lactoferrin on the bacterial cytoplasmic membrane. Biochemistry (Moscow) 68:217-227. [DOI] [PubMed] [Google Scholar]
- 15.Viejo-Diaz, M., M. T. Andrés, and J. F. Fierro. 2004. Modulation of in vitro fungicidal activity of human lactoferrin against Candida albicans by extracellular cation concentration and target cell metabolic activity. Antimicrob. Agents Chemother. 48:1242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]