Abstract
We describe the emergence of a new ganciclovir resistance mutation in the UL97 gene of human cytomegalovirus, deletion of codon 601, after valaciclovir and short-term ganciclovir therapy following kidney transplantation. Its role in ganciclovir resistance was supported by decreased ganciclovir phosphorylation in a recombinant vaccinia virus system.
Human cytomegalovirus (CMV) disease is a major complication of organ transplantation. Prophylactic treatment with valaciclovir, an acyclovir prodrug, significantly reduces the incidence of laboratory-confirmed CMV disease (12). Ganciclovir, a nucleoside analogue, remains the most widely used antiviral drug for the treatment of systemic CMV disease. Ganciclovir and acyclovir must be phosphorylated to exert their antiviral activity as inhibitors of viral DNA polymerase UL54. The UL97 kinase, a virus-encoded product, activates both drugs by monophosphorylation. CMV resistance to ganciclovir is favored by prolonged therapy and is mainly associated with the presence of mutations within the UL97 gene. Amino acid substitutions and deletions shown to induce CMV resistance have been mapped to the UL97 region encompassing positions 460 to 607 (3, 4, 6, 7, 8). Ganciclovir-resistant CMV strains can be selected by acyclovir as effectively as by ganciclovir in vitro as reported by Michel et al. (17). However, the clinical relevance of selection because of acyclovir has to be evaluated in vivo. Whether valacyclovir prophylaxis may favor the rapid emergence of resistance is questionable. We describe herein the emergence of a ganciclovir-resistant isolate harboring a new ganciclovir resistance-related mutation in a renal transplant recipient previously treated by valacyclovir prophylaxis.
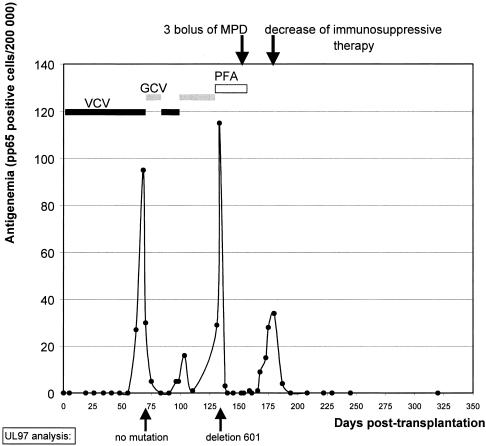
A 17-year-old CMV-seronegative patient underwent a first kidney allograft transplantation from a CMV-seropositive donor in November 2001. Neither the patient nor the donor had ever received ganciclovir or acyclovir before. The immunosuppressive regimen included basiliximab (anti-interleukin-2 antibody) at induction, cyclosporin (4.7 mg/kg twice a day), mycophenolate mofetil (600 mg/m2 twice a day), and corticosteroids. Valacyclovir prophylaxis for CMV disease was started on day 2 after grafting at a daily dose progressively adapted to renal function. CMV infection was monitored by pp65 antigenemia testing (Argène Biosoft, Varilhes, France). Asymptomatic CMV infection occurred on day 64. Prophylaxis was then shifted to intravenous curative ganciclovir treatment (5 mg/kg/day for 3 weeks, according to creatinine clearance), which resulted in a rapid decrease in the CMV load, as shown in Fig. 1. On day 83, antigenemia testing became negative. Ganciclovir administration was then stopped and valacyclovir administration was resumed. After 13 days of valacyclovir therapy (day 96), the patient presented with fever. The level of pp65 antigenemia was 5 nuclei per 2 × 105 leukocytes. Ganciclovir treatment (5 mg/kg/day) was resumed. However, antigenemia levels increased again and the fever continued. Ganciclovir was then shifted to intravenous foscarnet therapy (130 mg/kg/day from day 133 to day 156). Antigenemia became undetectable on day 140. In order to treat acute graft rejection, proven by biopsy, immunosuppressive treatment was reinforced (500 mg of methylprednisolone administered intravenously on days 151, 153, and 155). On day 168, antigenemia was detected again. Cyclosporin was tapered off, and mycophenolate mofetil was switched to azathioprine. Antigenemia was definitely undetectable on day 194. To date, this patient has had no other viral complication and his creatinine clearance is stable at 56 ml/min.
FIG. 1.
Virological follow-up of the patient. Abbreviations: VCV, valacyclovir; GCV, ganciclovir; PFA, foscarnet; MDP, methylprednisolone.
Two CMV isolates (G1 and G2, isolated on days 68 and 133, respectively) were recovered from blood samples by conventional culture on human embryonic fibroblasts (MRC-5; Biomerieux, Lyon, France). Ganciclovir and acyclovir resistance phenotype assays were performed by the AC11 CMV group consensus method (9). Briefly, a plaque reduction assay was used to measure the drug concentration required to reduce the number of plaques by 50% (50% inhibitory dose [ID50]) compared to that of controls. Human fibroblasts grown as monolayers in 24-well plates were inoculated with infected cells (50 to 100 PFU per well) and fed with medium containing serial dilutions of drug. Each drug concentration was tested in quadruplicate. The cells were incubated for 4 days and then fixed in a cold mixture of 90% acetone and 10% distilled water. Revelation was done by immunostaining with monoclonal antibody E13 directed against human CMV immediate-early antigens (Argène Biosoft). The plaques were counted, and the ID50 was calculated by graphic extrapolation. The sensitivity index (SI50 = ID50 of the isolate/ID50 of simultaneously tested reference strain AD169-ATCC VR-538) was calculated. Isolates with an SI50 of >3 were considered resistant. Isolate G1 was found to be sensitive to both antiviral drugs (SI50 of <3), whereas isolate G2, obtained after 58 days of ganciclovir therapy, was 16-fold less sensitive to ganciclovir than AD169 was and 8-fold less sensitive than G1 was (the ID50s of G1 and G2 were 2 and 16.5 μM, respectively). Isolate G2 was twofold less sensitive to acyclovir than isolate G1 was (the ID50s of G1 and G2 for acyclovir were 60 and 123 μM, respectively).
Genotypic assays were performed by amplification and sequencing of the full-length UL97 and UL54 genes from both isolates and from the corresponding whole-blood samples. A nested in-house PCR assay previously described (1) was used. Sequences were performed with the ABI Prism Big Dye Terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.). After comparison with AD169 and Towne sequences, a deletion of three nucleotides leading to the deletion of threonine at position 601 (named del601), was observed in the UL97 gene of G2, while the G1 UL97 sequence was wild type. To verify the absence of a mutated minority subpopulation in G1, the UL97 PCR product of G1 was cloned in vector pGEM T-Easy (Promega, Lyon, France) and 20 clones were sequenced. In addition, a discriminating PCR assay with forward primer 5′TGGAGAACGGCAAGCTCCA3′, which harbored a deletion of nucleotides 1801 to 1803, and reverse primer 5′TAAATACAGCCCGTCGCTCG3′ was designed to specifically amplify del601-mutated strains. This assay was able to detect 3% of the mutated strain within a 97% wild-type population (verified with a panel of mixtures of wild-type and del601 UL97 sequences). Neither clone sequencing nor a discriminative PCR assay detected a minority mutated subpopulation within G1. None of the mutations known to confer resistance to ganciclovir, cidofovir, or foscarnet was detected in gene UL54 encoding the viral DNA polymerase.
The role of del601 in ganciclovir resistance was assessed with the recombinant vaccinia virus (rVV) system as previously described (14). The UL97 coding region (with del601 or wild type) was introduced into naturally ganciclovir-resistant vaccinia virus by homologous recombination as described elsewhere (14). The expression of UL97 proteins in cells infected either with the UL97 wild type or rVV carrying the mutated UL97 gene was confirmed by Western blot analysis with a polyclonal antiserum specific for UL97 (15). The levels of UL97 protein expression were quantitatively similar. However, after infection of thymidine kinase-deficient 143B cells and high-performance liquid chromatography analysis of the cell extracts, the two viruses (wild-type or mutated pUL97) exhibited different levels of ganciclovir phosphorylation activity. Thereby, the ganciclovir phosphorylation in cells infected with rVV-UL97del 601 was decreased to 10% of the phosphorylation in cells infected with the rVV-UL97 wild type.
The capacity of pUL97del601 to autophosphorylate was also assessed as described by Michel et al. (16), with protein kinase assays. Phosphorylated UL97 proteins were detected by autoradiography after separation by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. The autophosphorylation of pUL97del601 was found to be equivalent to that of wild-type pUL97.
We present here the first description of a UL97 codon 601 deletion in a ganciclovir-resistant CMV isolate. This deletion emerged in a renal transplant recipient after valacyclovir prophylaxis and ganciclovir treatment for CMV infection. A T601M mutation has been previously described in an AIDS patient in association with clinical resistance to ganciclovir (5), but its responsibility for the occurrence of resistance has not been demonstrated. In our study, threonine 601, located close to amino acids 598 and 607 involved in ganciclovir phosphorylation (4), was shown to be essential for ganciclovir phosphorylation by the rVV system assay. The susceptible and resistant isolates from the patient differed only in the threonine 601 deletion in UL97. Therefore, deletion of threonine 601 was likely to confer ganciclovir resistance because of a decreased ability to activate ganciclovir.
CMV ganciclovir resistance is an emerging clinical problem in solid-organ recipients. Resistance is a late posttransplantation complication and is observed predominantly among CMV-seronegative recipients of organs from seropositive donors. Prolonged exposure to antiviral treatment favors the emergence of resistance. Durations of ganciclovir therapy before CMV resistance onset ranged from 51 to 438 days in kidney recipients (11), but resistance was detected after 3 months of treatment for most patients (11, 13). A striking feature of our case report is the short duration of exposure to ganciclovir before the occurrence of resistance, associated with the failure of valacyclovir to prevent early CMV reactivation, and its ineffectiveness as maintenance therapy. Therefore, the role of valacyclovir prophylaxis in the emergence of ganciclovir resistance is questionable. Michel et al. (17) demonstrated in vitro selection of CMV resistant to ganciclovir from clinical isolates under selection pressure from acyclovir. In vivo, two recent studies have described the emergence of ganciclovir-resistant CMV after acyclovir or valacyclovir prophylaxis in transplant recipients (1, 10), but the susceptibility of CMV isolates to acyclovir has not been assessed. In our case report, isolate G1 recovered after valacyclovir administration was susceptible to ganciclovir and acyclovir and antigenemia levels rapidly decreased under ganciclovir induction treatment, whereas G2 was eightfold less susceptible to ganciclovir and twofold less susceptible to acyclovir than G1 was. This slight decrease in acyclovir susceptibility might not be in favor of its role in the emergence of resistance. However, the decreases in acyclovir sensitivity induced in vitro by either acyclovir or ganciclovir ranged from 2.1- to 12-fold and were never as pronounced as those detected for the ganciclovir sensitivity, in agreement with the lower capacity of UL97 to phosphorylate acyclovir in the rVV system (17). This suggests that the acyclovir selective pressure may favor ganciclovir resistance. In addition, acyclovir is not a potent inhibitor of CMV replication and valacyclovir has failed to control CMV reactivation in our patient. On the whole, we can hypothesize that valacyclovir and ganciclovir administration might have exerted continuous selective pressure, associated with immunosuppression, and resistance occurred after 133 days of cumulative antiviral treatment.
The increase in immunosuppressive therapy for acute graft rejection favored the development of the new active infection detected after foscarnet withdrawal. As previously suggested by Anglicheau et al. (2), the decrease in immunosuppressive therapy was the best way to cure the active CMV infection. On the other hand, because of the increasing use of ganciclovir, cidofovir, and foscarnet, the incidence of resistant CMV strains will progressively rise.
This case confirms the benefit of virological follow-up of immunocompromised patients under antiviral prophylaxis and/or antiviral treatment in order to detect the early emergence of resistant strains carrying new or already known mutations. It shows that accumulating low-dose treatments may favor the emergence of resistance and that a less potent immunosuppressive regimen should be recommended first to stop active mild infections.
Nucleotide sequence accession number.
The GenBank accession number for UL97 del601 is AY681345.
Acknowledgments
We gratefully acknowledge Reina Watanabe for reviewing the English language in the manuscript.
D.M. and T.M. were supported by the Landesstiftung Kompetenznetzwerk Baden-Württemberg (Resistenzentwicklung humanpathogener Erreger, TP12). S.H. and S.A. were supported by grants from the Fondation pour la Recherche Médicale, the Région Limousin, the University of Limoges (EA 3175), and the CPAM de Haute-Vienne.
REFERENCES
- 1.Alain, S., S. Hantz, C. Scieux, A. Karras, M. C. Mazeron, J. C. Szelag, B. M. Imbert, A. M. Fillet, S. Gouarin, C. Mengelle, A. de Wilde, N. Cogne, G. Champier, S. Rogez, C. Legendre, and F. Denis. 2004. Detection of ganciclovir resistance after valacyclovir-prophylaxis in renal transplant recipients with active cytomegalovirus infection. J. Med. Virol. 73:566-573. [DOI] [PubMed] [Google Scholar]
- 2.Anglicheau, D., A. Lautrette, C. Scieux, M. Flamant, F. Morinet, and C. Legendre. 2003. Efficacy and safety of lowering immunosuppression to treat CMV infection in renal transplant recipients on valaciclovir prophylaxis: a pilot study. Nephrol. Dial. Transplant. 8:1654-1656. [DOI] [PubMed] [Google Scholar]
- 3.Baldanti, F., E. Silini, A. Sarasini, C. L. Talarico, S. C. Stanat, K. K. Biron, M. Furione, F. Bono, G. Palu, and G. Gerna. 1995. A three-nucleotide deletion in the UL97 open reading frame is responsible for the ganciclovir resistance of a human cytomegalovirus clinical isolate. J. Virol. 69:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldanti, F., D. Michel, L. Simoncini, M. Heuschmid, A. Zimmermann, R. Minisini, P. Schaarschmidt, T. Schmid, G. Gerna, and T. Mertens. 2002. Mutations in the UL97 ORF of ganciclovir-resistant clinical cytomegalovirus isolates differentially affect ganciclovir phosphorylation as determined in a recombinant vaccinia virus system. Antiviral Res. 54:59-67. [DOI] [PubMed] [Google Scholar]
- 5.Baldanti, F., S. Paolucci, A. Parisi, L. Meroni, and G. Gerna. 2002. Emergence of multiple drug resistant human cytomegalovirus variants with human immunodeficiency virus infection unresponsive to highly active antiretroviral therapy. Clin. Infect. Dis. 34:1146-1149. [DOI] [PubMed] [Google Scholar]
- 6.Baldanti, F., N. Lurain, and G. Gerna. 2004. Clinical and biologic aspects of human cytomegalovirus resistance to antiviral drugs. Hum. Immunol. 65:403-409. [DOI] [PubMed] [Google Scholar]
- 7.Chou, S., and C. L. Meichsner. 2000. A nine-codon deletion mutation in the cytomegalovirus UL97 phosphotransferase gene confers resistance to ganciclovir. Antimicrob. Agents Chemother. 44:183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou, S., R. H. Waldemer, A. E. Senters, K. E. Michels, G. W. Kemble, R. G. Miner, and W. L. Drew. 2002. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J. Infect. Dis. 185:162-169. [DOI] [PubMed] [Google Scholar]
- 9.Ducancelle, A., S. Belloc, S. Alain, C. Scieux, M. Malphettes, F. Petit, J. C. Brouet, M. J. Sanson Le Pors, and M. C. Mazeron. 2004. Comparison of sequential cytomegalovirus isolates in a patient with lymphoma and failing antiviral therapy. J. Clin. Virol. 29:241-247. [DOI] [PubMed] [Google Scholar]
- 10.Erice, A., N. Borrell, W. Li, and W. J. Miller. 1998. Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J. Infect. Dis. 178:531-534. [DOI] [PubMed] [Google Scholar]
- 11.Limaye, A. P. 2002. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin. Infect. Dis. 35:866-872. [DOI] [PubMed] [Google Scholar]
- 12.Lowance, D., H. H. Neumayer, C. M. Legendre, J. P. Squifflet, J. Kovarik, P. J. Brennan, D. Norman, R. Mendez, M. R. Keating, G. L. Coggon, A. Crisp, and I. C. Lee. 1999. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N. Engl. J. Med. 13:1462-1470. [DOI] [PubMed] [Google Scholar]
- 13.Lurain, N. S., S. M. Bhorade, K. J. Pursell, R. K. Avery, V. V. Yeldandi, C. M. Isada, E. S. Robert, D. J. Kohn, M. Q. Arens, E. R. Garrity, A. J. Taege, M. G. Mullen, K. M. Todd, J. W. Bremer, and B. Yen-Lieberman. 2002. Analysis and characterization of antiviral drug-resistant cytomegalovirus isolates from solid organ transplant recipients. J. Infect. Dis. 186:760-768. [DOI] [PubMed] [Google Scholar]
- 14.Metzger, C., D. Michel, K. Schneider, A. Luske, H. J. Schlicht, and T. Mertens. 1994. Human cytomegalovirus UL97 kinase confers ganciclovir susceptibility to recombinant vaccinia virus. J. Virol. 68:8423-8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel, D., I. Pavic, A. Zimmermann, E. Haupt, K. Wunderlich, M. Heuschmid, and T. Mertens. 1996. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J. Virol. 70:6340-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel, D., P. Schaarschmidt, K. Wunderlich, M. Heuschmid, L. Simoncini, D. Muhlberger, A. Zimmermann, I. Pavic, and T. Mertens. 1998. Functional regions of the human cytomegalovirus protein pUL97 involved in nuclear localization and phosphorylation of ganciclovir and pUL97 itself. J. Gen. Virol. 79:2105-2112. [DOI] [PubMed] [Google Scholar]
- 17.Michel, D., S. Höhn, T. Haller, D. Ju, and T. Mertens. 2001. Aciclovir selects for ganciclovir-cross resistance of human cytomegalovirus in vitro that is only in part explained by known mutations in the UL97 protein. J. Med. Virol. 65:70-76. [PubMed] [Google Scholar]