Abstract
The daptomycin biosynthetic gene cluster of Streptomyces roseosporus was analyzed by Tn5099 mutagenesis, molecular cloning, partial DNA sequencing, and insertional mutagenesis with cloned segments of DNA. The daptomycin biosynthetic gene cluster spans at least 50 kb and is located about 400 to 500 kb from one end of the ∼7,100-kb linear chromosome. We identified two peptide synthetase coding regions interrupted by a 10- to 20-kb region that may encode other functions in lipopeptide biosynthesis.
Streptomyces roseosporus NRRL 11379 produces A21978C, a complex of acidic lipopeptide antibiotics (9). The cyclic depsi-peptide portion contains 13 amino acids linked by an ester bond between the carboxyl group of kynurenine (Kyn) and the hydroxyl group of Thr. The A21978C factors contain C10, C11, or C12 fatty acids attached to the terminal amino group of Trp (9). The fatty acid side chains are readily removed by incubation with Actinoplanes utahensis (6), and the cyclic peptide can be reacylated at the N terminus of Trp to produce fatty acyl, aroyl, and extended peptide derivatives (8). The n-decanoyl analog of A21978C, daptomycin or LY146032, is a potent antibiotic active against gram-positive bacteria, including methicillin-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis, vancomycin-resistant enterococci, and penicillin-resistant Streptococcus pneumoniae (4). Daptomycin has a novel mechanism of action, the inhibition of lipoteichoic acid biosynthesis (4, 7).
Many linear and cyclic peptides are produced by actinomycetes, bacilli, and fungi (14, 15, 35). The peptide assembly is generally nonribosomal and requires very large multifunctional peptide synthetases containing one or more subunits (15, 29, 30, 33–35). Each peptide synthetase enzyme or subunit is composed of domains or modules dedicated to the processing of individual amino acids. Domains contain sites for binding amino acids and ATP and enzyme activities for amino acid adenylate formation, thioester formation, transthiolation to phosphopantetheine, transpeptidation, and sometimes other activities (15, 30, 33). The structural organization and DNA sequence of the genes encoding several different peptide synthetases have been determined (21, 33). The linear sequence of DNA modules encoding the functional domains in the peptide synthetases generally corresponds to the linear sequence of amino acids in the peptide. The average coding region for an individual module is about 3.2 kb. Since the individual modules encode similar functions, they contain regions of partial homology displayed as highly conserved motifs (1, 21, 30, 33, 35).
We are interested in understanding the structural organization and regulation of the daptomycin biosynthetic genes in S. roseosporus. Since daptomycin contains 13 amino acids, its coding region should contain 13 segments containing conserved peptide synthetase motifs, spanning about 42 kb or more. We have developed methods to introduce DNA into S. roseosporus and have identified mutants blocked in daptomycin production by Tn5099 transposition mutagenesis (20). We report here the localization of the daptomycin biosynthetic gene cluster by Tn5099 transposition mutagenesis, cloning, insertional mutagenesis, physical mapping, and partial DNA sequence analysis. The daptomycin biosynthetic genes map to a region about 400 to 500 kb from one end of the linear chromosome of S. roseosporus.
MATERIALS AND METHODS
Bacterial strains, plasmids, and transposons.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| S17-1 | Contains plasmid RP4 integrated in the chromosome | 25 |
| XL1-Blue MRF′ | Δ(mcrA)183 Δ(mcrcB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac[F′ proAB lacIqZΔM15Tn10(Tetr)] | Stratagene |
| DH5α | F−recA1 gyrA96 thi-1 hsdR17 supE44 relA1 deoR (lacZYA-argF)U196 φ80 dlacZΔM15 | Bethesda Research Laboratories |
| DH10B | F− ΔmcrA Δ(mrr-hsdRMS-mcrBD) φ80dlacZφM15 ΔlacX74 deoR recA1 Δara-139 Δ(ara leu)7697 galU galK λ− rpsL endA1 nupG | Gibco BRL |
| S. roseosporus | ||
| A21978.6 | Produces daptomycin | ATCC 31568 |
| A21978.65 | Produces elevated levels of daptomycin | Lilly Culture Collection |
| MM91 | A21978.6Ω91 (draI–E::Tn5099) | This work |
| MM92 | A21978.6Ω92 (draI–E::Tn5099) | This work |
| MM93 | A21978.6Ω93 (draI–C::Tn5099) | This work |
| MM94 | A21978.6Ω94 (draI–C::Tn5099) | This work |
| MM95 | A21978.6Ω95 (draI–C::Tn5099) | This work |
| MM114 | A21978.65::pRHB152 | This work |
| MM123 | A21978.65::pRHB153 | This work |
| MM138 | A21978.6::pRHB154 | This work |
| MM139 | A21978.6::pRHB155 | This work |
| MM140 | A21978.65::pRHB157 | This work |
| MM131 | A21978.6::pRHB166 | This work |
| MM132 | A21978.6::pRHB168 | This work |
| MM133 | A21978.6::pRHB169 | This work |
| MM134 | A21978.6::pRHB170 | This work |
| MM135 | A21978.6::pRHB172 | This work |
| MM136 | A21978.6::pRHB173 | This work |
| MM137 | A21978.6::pRHB174 | This work |
| Plasmids | ||
| pOJ260 | 3.5-kb plasmid containing AmrreppUC MCS lacZα oriT | 5 |
| pKC1471 | 3.0-kb cosmid containing Sprrepp15A MCS | 16 |
| pBluescript II KS− | Apr ColE1 rep | Stratagene |
| pCRII | 3.9-kb TA cloning vector | Invitrogen |
| pCZA213 | 11.2-kb plasmid containing Tn5099 | 27 |
| pRHB146 | 3.4-kb pUCBM21 derivative containing oriT | 20 |
| pRHB152 | pRHB146 containing Tn5099 and 7.1 kb of S. roseosporus flanking DNA from MM91 | This work |
| pRHB153 | pRHB146 containing Tn5099 and 6.0 kb of S. roseosporus flanking DNA from MM93 | This work |
| pRHB154 | pRHB146 containing Tn5099 and 5.0 kb of S. roseosporus flanking DNA from MM92 | This work |
| pRHB155 | pRHB146 containing Tn5099 and 5.0 kb of S. roseosporus flanking DNA from MM94 | This work |
| pRHB157 | pRHB146 containing Tn5099 and 7.0 kb of S. roseosporus flanking DNA from MM95 | This work |
| pRHB159 | pKC1471 containing 55-kb insert of S. roseosporus DNA | This work |
| pRHB160 | pKC1471 containing 45-kb insert of S. roseosporus DNA | This work |
| pRHB161 | pKC1471 containing 46-kb insert of S. roseosporus DNA | This work |
| pRHB162 | pKC1471 containing 45-kb insert of S. roseosporus DNA | This work |
| pRHB166 | pOJ260 containing 14-kb EcoRV fragment from pRHB160 | This work |
| pRHB168 | pOJ260 containing 5.2-kb EcoRI left-end fragment from pRHB159 | This work |
| pRHB169 | pOJ260 containing 2.3-kb EcoRI left-end fragment from pRHB161 | This work |
| pRHB170 | pOJ260 containing 3.9-kb EcoRI left-end fragment from pRHB162 | This work |
| pRHB172 | pOJ260 containing 7-kb EcoRI fragment from pRHB160 | This work |
| pRHB173 | pOJ260 containing 4.5-kb KpnI-ScaI fragment from pRHB161 inserted in KpnI-DraI site | This work |
| pRHB174 | pOJ260 containing 10-kb ScaI fragment from pRHB161 inserted in EcoRV site | This work |
| pUCBM21 | pUC19 with expanded MCS | Boehringer Mannheim Biochemicals |
| pRHB599 | pBluescript II KS− containing 2.7-kb KpnI-EcoRI fragment from pRHB614 | This work |
| pRHB602 | pBluescript II KS− containing 2.5-kb EcoRI fragment from pRHB614 | This work |
| pRHB603 | pBluescript II KS− containing 0.5-kb EcoRI fragment from pRHB614 | This work |
| pRHB680 | pBluescript II KS− containing 0.6-kb ScaI fragment from pRHB613 | This work |
| pRHB678 | pCRII containing 362-bp internal fragment of S. roseosporus masC homolog | This work |
| pRHB588 | pK1139 containing 0.36-kb EcoRI fragment from pRHB678 containing internal masC sequence | This work |
| pIF175 | Mini-γδ plasmid for deletions and inversions | 36 |
| M13mp19 | Vector for DNA sequencing | 38 |
Amr, apramycin resistance; reppUC, pUC replicon for E. coli; oriT, RK2 origin of transfer; MCS, multiple cloning site; Spr, spectinomycin resistance.
Media and growth conditions.
The streptomycete strains were grown in TS broth and fragmented into individual colony-forming units by ultrasonic vibration as described previously (3). Fermentation and high-pressure liquid chromatography (HPLC) analysis of daptomycin production were carried out as described previously (20).
DNA techniques and plasmid constructions.
DNA cloning procedures were carried out generally as described previously (24). Restriction endonucleases and other enzymes were used according to the recommendations of the manufacturers. Pulsed-field gel electrophoresis (PFGE) analysis of S. roseosporus DNA was carried out as described previously (20, 26) with a CHEF Mapper system (Bio-Rad Laboratories). Southern blot hybridizations were carried out as described previously (26) with Genius system (Boehringer Mannheim Biochemicals) nonradioactive labeling probes. The DNA sequence was determined with a Taq Dye Deoxy terminator cycle sequencing kit and a model 373A DNA sequencing system (Applied Biosystems). PCR amplification was performed with a Perkin-Elmer Gene Amp 9600 apparatus and standard conditions.
Plasmids were constructed with Escherichia coli XL1-Blue MRF′, DH10B, or DH5α as follows. DNA from S. roseosporus MM91 was cleaved with MluI, separated by gel electrophoresis, and probed with Tn5099. An 11.5-kb fragment that hybridized to Tn5099 was size selected, ligated with MluI-cleaved pRHB146, and introduced into E. coli XL1-Blue MRF′, yielding pRHB152. DNA from S. roseosporus MM93 was cleaved with BglII, separated by gel electrophoresis, and probed with Tn5099. A 10.4-kb fragment that hybridized to Tn5099 was size selected, ligated with BglII-cleaved pRHB146, and introduced into E. coli XL1-Blue MRF′ by transformation to hygromycin resistance (Hmr), yielding pRHB153. DNA from S. roseosporus MM95 was cleaved with ApaI, separated by gel electrophoresis, and probed with Tn5099. An 11.4-kb fragment that hybridized to Tn5099 was size selected, ligated with ApaI-cleaved pRHB146, and introduced into E. coli DH5α by transformation to Hmr, yielding pRHB157. DNA from S. roseosporus MM94 was cleaved with MluI, separated by gel electrophoresis, and probed with Tn5099. A 9.4-kb fragment that hybridized to Tn5099 was size selected, ligated with MluI-cleaved pRHB146, and introduced into E. coli by transformation to Hmr, yielding pRHB155. DNA from S. roseosporus MM92 was cleaved with MluI, separated by gel electrophoresis, and probed with Tn5099. A 9.4-kb fragment that hybridized to Tn5099 was size selected, ligated with MluI-cleaved pRHB146, and introduced into E. coli by transformation to Hmr, yielding pRHB154. S. roseosporus inserts in pRHB153 and pRHB157 were purified and used as probes to identify cosmids containing daptomycin biosynthetic genes. Fragments of cosmids with large S. roseosporus DNA inserts (12) were subcloned in pOJ260 in E. coli for insertion mutagenesis as follows. Plasmid pRHB160 was cleaved with EcoRV, and a 14-kb fragment was ligated with EcoRV-cleaved pOJ260, yielding pRHB166. Plasmid pRHB159 was cleaved with EcoRI, and a 5.2-kb fragment was ligated with EcoRI-cleaved pOJ260, yielding pRHB168. Plasmid pRHB161 was cleaved with EcoRI, and a 2.3-kb fragment was ligated with EcoRI-cleaved pOJ260, yielding pRHB169. Plasmid pRHB162 was cleaved with EcoRI and ligated with EcoRI-cleaved pOJ260, yielding pRHB170. Plasmid pRHB160 was cleaved with EcoRI, and a 7.0-kb fragment was ligated with EcoRI-cleaved pOJ260, yielding pRHB172. Plasmid pRHB161 was cleaved with KpnI and ScaI, and a 4.5-kb KpnI-ScaI fragment was ligated with pOJ260 cleaved with KpnI plus DraI, yielding pRHB173. Plasmid pRHB161 was cleaved with ScaI, and a 10-kb fragment was ligated with EcoRV-cleaved pOJ260, yielding pRHB174. DNA from strain MM132 containing pRHB168 inserted into the chromosome was digested with KpnI and self-ligated. Transformants were selected for Amr, yielding pRHB613. DNA from strain MM135 containing pRHB172 inserted into the chromosome was digested with KpnI and self-ligated. Transformants were selected for Amr, yielding pRHB614. A 2.7-kb KpnI-EcoRI fragment from pRHB614 was ligated to pBluescript II KS− digested with KpnI and EcoRI, yielding pRHB599. A 2.5-kb EcoRI fragment from pRHB614 was ligated with pBluescript II KS− digested with EcoRI, yielding pRHB602. A 0.5-kb EcoRI fragment from pRHB614 was ligated to pBluescriptII KS− digested with EcoRI, yielding pRHB603. A 0.6-kb SacI fragment from pRHB613 was ligated to pBluescriptII KS− digested with SacI, yielding pRHB680. A 362-bp fragment containing an internal segment of S. roseosporus masC was PCR amplified with primers PR182 (5′GGTGCGGCCCTTTGATGAAT3′) and PR183 (5′CCACGACTGGCTGACCGAGA3′) and ligated with pCRII to yield pRHB678. A 0.36-kb EcoRI fragment from pRHB678 was ligated to EcoRI-digested pKC1139 to yield pRHB588. All plasmid constructions were confirmed by restriction analysis.
Transposition, transformation, electroporation, and conjugation.
Transposition of Tn5099 (11) in S. roseosporus and conjugation of plasmid DNA from E. coli S17-1 to S. roseosporus were carried out as described previously (20). Plasmid DNA was introduced into E. coli strains by transformation (24) or electroporation (28).
Nucleotide sequence and data analysis.
Derived amino acid sequences were analyzed with the Genetics Computer Group software package (version 8) (10). Amino acid sequence homology searches were performed by use of the BLAST server at the National Center for Biotechnology Information (Bethesda, Md.) and nonredundant protein sequence databases (2). Protein sequence databases PIR (release 35.0) and Swiss-Prot (release 24.0) were searched by use of the EMBL FASTA file server facility with default parameter values.
Nucleotide sequence accession numbers.
The nucleotide sequences for cpsA and cpsB have been assigned GenBank accession no. AF021262 and AF021263, respectively.
RESULTS AND DISCUSSION
Physical mapping of Tn5099 insertions.
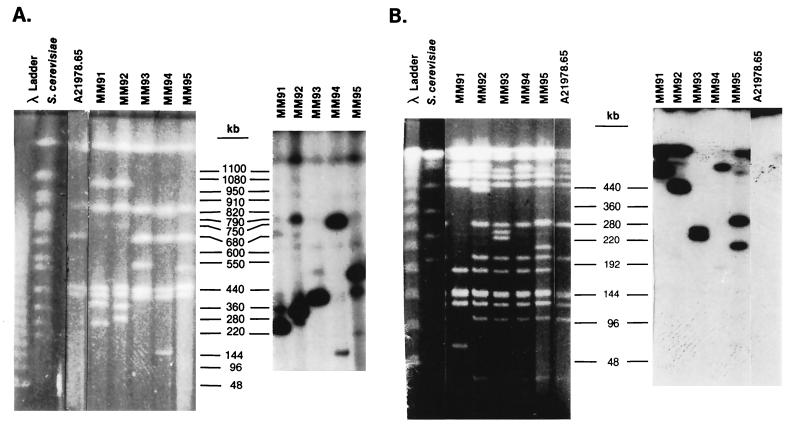
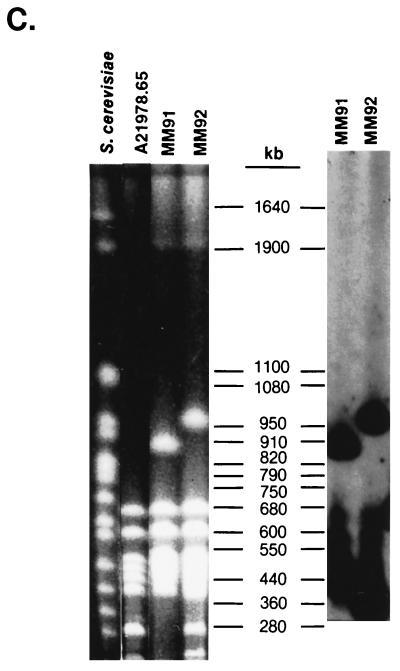
In a previous study (20), we identified five mutants that were induced by Tn5099 insertions and that produced little or no daptomycin. Since Tn5099 contains DraI and AseI (AsnI) sites, the DraI and AsnI fragments containing the transposon are split into two new fragments upon restriction endonuclease cleavage, and the distances of the DraI and AsnI sites from the ends of the fragments can be determined. Tn5099 insertions in S. roseosporus MM91 and MM92 mapped to the DraI-E fragment and to the AsnI-B fragment (Fig. 1). MM91 had a deletion of about 900 kb of DNA, including AsnI fragments I, J, K, O, P, and Q, and contained a new 50-kb fragment (O′), suggesting that the lack of daptomycin production in MM91 may have been due to deletion rather than to transposition. This suggestion is consistent with the results of the gene disruption experiment described below. MM92 was not completely blocked in daptomycin biosynthesis (20), so the insertion in this strain may have had a pleiotropic effect on daptomycin production.
FIG. 1.
PFGE and Southern hybridization analysis of S. roseosporus mutants containing Tn5099 insertions. Southern hybridizations were carried out with a gel-purified 4-kb HindIII fragment from pCZA213 containing Tn5099 as the probe. (A) Contour-clamped homogeneous electric field (CHEF) gel and Southern blot of S. roseosporus DNA cleaved with DraI. A 1% FastLane agarose gel in 0.25× Tris-borate-EDTA buffer (TBE) was run at 6 V/cm and 14°C at an angle of 120°C with a 30- to 90-s pulse (linear over 16 h). (B) CHEF gel and Southern blot of S. roseosporus DNA cleaved with AsnI. A 1% FastLane agarose gel in 0.1× TBE was run at 6 V/cm and 16°C at an angle of 120°C with a 2- to 30-s pulse (linear over 15 h). Note that strain A21978.65 lacks the 180-kb AsnI-K2 fragment present in the other strains, which are derived from A21978.6 (20). (C) CHEF gel and Southern blot of S. roseosporus DNA cleaved with AsnI. A 1% FastLane agarose gel in 0.25× TBE was run at 6 V/cm and 14°C at an angle of 120°C with a 60- to 110-s pulse (linear over 24 h).
The Tn5099 transpositions in MM93, MM94, and MM95 mapped to the DraI-C fragment and to the AsnI-E fragment (20) (Fig. 1). Tn5099 split the DraI-C fragment in MM93 into fragments of 440 and 560 kb, the DraI-C fragment in MM95 into fragments of 460 and 540 kb, and the DraI-C fragment in MM94 into fragments of 180 and 820 kb. The AsnI-E fragment was split into fragments of 260 and 270 kb in MM93, 240 and 290 kb in MM95, and about 520 and 20 kb in MM94. Southern hybridization analysis confirmed these fragment assignments (Fig. 1). Southern hybridizations to SpeI-digested DNA demonstrated that Tn5099 was inserted into the 330-kb SpeI fragment in MM93 and MM95 and into the 190-kb SpeI fragment in MM94 (data not shown).
Since the DraI site is located close to one end of Tn5099 (11), the direction of transcription of the xylE gene, the Hmr gene, and open reading frames A and B, which are all transcribed in the same direction, can be deduced from the relative intensities of hybridization in Southern blots (Fig. 1). This information was used to align the transposon inserts on the DraI-C fragment (Fig. 2). The insert in MM93 reads from left to right, and the inserts in MM94 and MM95 read from right to left. Since the xylE gene in Tn5099 lacks a promoter, it can be used to determine the direction of transcription if it is inserted in a structural gene. Only MM93 expressed catechol dioxygenase; thus, Tn5099 appears to have been inserted into a structural gene in MM93, with the direction of transcription being from left to right (Fig. 2).
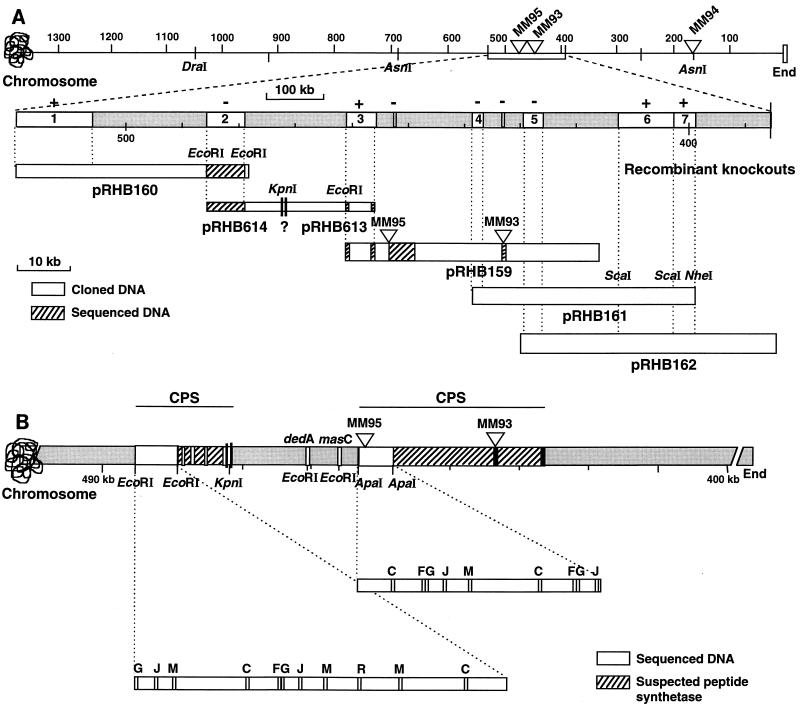
FIG. 2.
Partial physical map of the daptomycin gene cluster. (A) The top line shows the locations of the Tn5099 insertions (triangles) within the 1,050-kb DraI-C fragment and the 490-kb AsnI-E fragment relative to the end of the chromosome in strains MM95, MM93, and MM94. The region containing the insertions in MM95 and MM93 is expanded below to show the overlapping cosmid inserts (pRHB159, pRHB160, pRHB161, and pRHB162) and plasmids (pRHB614 and pRHB613) that span the peptide synthetase coding region. The segments in white marked 1 through 7 represent subcloned fragments used for gene disruption analysis. Recombinants were analyzed for daptomycin production (+ or − above the segments). The cross-hatched segments were sequenced. (B) Summary of the partial DNA sequencing of the daptomycin cluster. The 7.9-kb EcoRI fragment contains motifs present on peptide synthetase modules that activate and epimerize amino acids (33). The 5.3-kb ApaI fragment contains motifs found in modules that activate l-amino acids (33). The lines above the map summarize the locations of two regions encoding cyclic peptide synthetase (CPS) subunits that are separated by a region containing dedA and masC homologs.
We also cleaved DNA from MM93, MM94, and MM95 with SspI, separated the DNA by PFGE, and probed it with a HindIII fragment that contained the xylE gene and the Hmr gene from Tn5099. This probe should hybridize to DNA on the right side of the Tn5099 insertions in MM94 and MM95, since Tn5099 contains an SspI site just to the right of the Hmr gene and open reading frame A (11). SspI cleavage yielded hybridizing fragments of about 190 and 460 kb for MM94 and MM95, respectively. Thus, the apparent SspI and DraI sites on the right side of the Tn5099 insertions mapped to the same location, about 180 kb from the Tn5099 insertion in MM94. This location is just to the right of an SpeI site that yielded a fragment of 180 kb that mapped to the same right-end location as DraI and SspI. Since Tn5099 mapped to an extreme end of the AsnI-E fragment in MM94 (Fig. 2A), it was of interest to know what fragment flanked the right end of the AsnI-E fragment. A partial AsnI digest of MM94 was separated by PFGE and probed with the 4-kb HindIII fragment from pCZA213 containing Tn5099. The 10-kb fragment at the left end of the AsnI-E fragment was linked to the 180-kb AsnI-K2 fragment (20). Thus, the left end of the AsnI-K2 fragment mapped to the same site as the end defined by DraI, SspI, and SpeI. The congruence of these four apparent restriction sites strongly suggests that Tn5099 is inserted approximately 180 kb from one end of a linear chromosome in S. roseosporus MM94. The linked insertions in MM93 and MM95 are located about 440 and 460 kb, respectively, from the same end of the chromosome (Fig. 2). These data are consistent with other data indicating that genes involved in red pigment production map to the other end of the linear chromosome of S. roseosporus (20) and with the general observation that streptomycete chromosomes are linear (17–19, 22, 23).
Insertional mutagenesis and chromosome probing with DNA flanking Tn5099 insertions.
To determine if the Tn5099 insertions in strains MM91 through MM95 were directly associated with the loss of daptomycin production, we cloned DNA flanking the insertions into pRHB146, a plasmid capable of conjugation from E. coli S17-1 to S. roseosporus but lacking replication functions for streptomycetes (Table 1). Thus, transconjugants can be formed only by insertion of plasmid DNA into the chromosome. DNA from each of the strains was cleaved with several different restriction endonucleases, and Southern hybridizations with a Tn5099 probe were carried out to identify fragments containing Tn5099 plus flanking DNA for subsequent insertional mutagenesis studies. Internal recombinational insertions into the peptide synthetase coding region(s) should disrupt daptomycin biosynthesis. Plasmids pRHB152, pRHB153, pRHB154, pRHB155, and pRHB157, containing S. roseosporus DNA flanking the Tn5099 insertions in strains MM91, MM92, MM93, MM94, and MM95, respectively, were introduced into S. roseosporus A21978.6 or A21978.65, and Hmr transconjugants were obtained. The transconjugants were grown under fermentation conditions, and daptomycin production was determined by HPLC analysis. Table 2 shows that recombinants containing plasmids pRHB152 and pRHB155 produced essentially control yields of daptomycin. Thus, the Tn5099 insertions in strains MM91 and MM94 probably did not cause the disruption of daptomycin biosynthesis by insertion into the peptide synthetase coding region. Recombinants containing plasmid pRHB154 produced a reduced amount of daptomycin (∼50% that in the control), so the original Tn5099 insertion in MM92 was also not likely to be in a daptomycin peptide synthetase gene. However, recombinants harboring plasmid pRHB153 or pRHB157 produced no daptomycin. This result suggests that the Tn5099 insertions in strains MM93 and MM95 might be located in the peptide synthetase coding region.
TABLE 2.
Daptomycin production by S. roseosporus strains containing plasmid insertions
| Strain | Insert | Daptomycin production, in μg/ml (% of control) |
|---|---|---|
| A21978.6 | 197 (100) | |
| A21978.65 | 732 (100) | |
| MM114 | A21978.65::pRHB152 | 769 (105) |
| MM123 | A21978.65::pRHB153 | 7 (<2) |
| MM138 | A21978.6::pRHB154 | 116 (59) |
| MM139 | A21978.6::pRHB155 | 188 (95) |
| MM140 | A21978.65::pRHB157 | 0 (0) |
Plasmids pRHB152, pRHB153, pRHB154, pRHB155, and pRHB157 were also used to probe S. roseosporus DNA cleaved with different restriction endonucleases. Plasmid pRHB153 hybridized to multiple BalII bands ranging from 3 to 6 kb and totaling over 21 kb. Plasmid pRHB153 also hybridized to a single high-molecular-weight HindIII band, suggesting that it contains a conserved sequence reiterated over a relatively short segment of the chromosome. This suggestion is consistent with the presence of a closely linked conserved peptide synthetase motif(s). Plasmids pRHB154 and pRHB155 each hybridized to a single MluI band, and plasmid pRHB157 hybridized strongly to a 6.1-kb ApaI band and weakly to a 3.5-kb band.
Sequence sampling of DNA flanking Tn5099 insertions in strains MM93 and MM95.
Since the insertions in MM93 and MM95 appeared to disrupt daptomycin biosynthesis and since the DNA flanking the insertion in MM93 contained a sequence reiterated over a relatively small region of the chromosome, we attempted to gain additional information on the sites of insertion by DNA sequence sampling. DNA from plasmid pRHB153 was cloned into pIF275 containing mini-γδ (36), and several random deletion clones were sequenced and analyzed for open reading frames. The corresponding amino acid sequences were compared to other sequences by BLAST analysis (2). Plasmid pRHB153 contained an open reading frame that encoded a predicted polypeptide that showed high sequence similarity to motif C. The sequence SGSTGQPKG differed from the consensus motif C sequence, SGTTGXPKG (33), in only one position, Ser for Thr. This sequence has been proposed to participate in ATP and AMP binding and is observed in virtually all peptide synthetase modules.
The DNA flanking the Tn5099 insertion in plasmid pRHB157 was subcloned in M13mp19, and random inserts were sequenced. BLAST analysis of several segments of nucleotide sequence identified two regions of DNA that encoded amino acid sequences highly conserved in peptide synthetases. One segment encoded the sequence QVKILGFRIE, which differed from the consensus motif G sequence, QVKIRGXRIE, by only one amino acid. This sequence has been proposed to play a significant role in the formation of aminoacyl adenylate (33). Another polypeptide contained the LGGHS sequence, which corresponded to the highly conserved motif J sequence, LGGXS, which participates in the covalent binding of 4′-phosphopantetheine to Ser. Analysis of the nucleotide sequences of these three regions also showed very high similarities to the corresponding regions in the Nocardia lactamdurans A, C and V modules and high homology to peptide synthetase modules from Bacillus brevis and Cephalosporium acremonium. These data support the conclusion that the Tn5099 insertions in strains MM93 and MM95 are located in genes encoding peptide synthetases.
Cosmid cloning and physical mapping of daptomycin biosynthetic genes.
Cosmids from a library of S. roseosporus DNA cloned in pKC1471 were combined in pools of 12 and screened by hybridization to a 2.1-kb SphI fragment isolated from pRHB153 and to a 5.2-kb DraI-KpnI fragment isolated from pRHB157. Individual cosmids from the hybridizing pools were identified by hybridization to the same probes.
Several methods were used to physically map the cosmids that hybridized to peptide synthetase sequences. These included comparison of restriction endonuclease cleavage patterns, hybridization of partial digests of cosmids to the pCK1471 vector sequences flanking the cosmid inserts, restriction endonuclease cleavage patterns of hybridization to the 5.2-kb DraI-KpnI peptide synthetase probe isolated from pRHB157, and chromosomal orientation of the two Tn5099 insertions in strains MM93 and MM95. These approaches were used to align cosmids pRHB159, pRHB161, and pRHB162 to each other and to one end of the S. roseosporus chromosome (Fig. 2).
Attempts to link pRHB160 to the other three cosmids by finding a linking cosmid from the S. roseosporus library were unsuccessful. In an attempt to link pRHB160 to pRHB159, DNA adjacent to pRHB160 and pRHB159 was obtained by a recombinational cloning strategy with strains MM135 and MM133, which contained insertions of pRHB172 and pRHB168 into the chromosome (sections 2 and 3 in Fig. 2A), respectively. Since pRHB172 and pRHB168 each contain a KpnI site adjacent to the S. roseosporus DNA insert in the multiple cloning site, cleavage of DNA from MM135 and MM133 with KpnI should give fragments containing the complete plasmid pKC1471, the original S. roseosporus DNA insert, plus additional contiguous S. roseosporus DNA up to the next KpnI site in the chromosome. Therefore, depending on the orientation of the original inserts in pRHB172 and pRHB168, cleavage of DNA from strains MM135 and M133 and self-ligation should give plasmids either extending the linkage or containing segments internal to cosmids pRHB160 and pRHB159 (Fig. 2A). In both cases, the plasmids excised from the chromosome extended the linkage to an apparent linking KpnI site (Fig. 2A). Plasmid pRHB614 extended cosmid pRHB160 to the right about 5.8 kb, whereas plasmid pRHB613 extended cosmid pRHB159 about 9.0 kb to the left. We cannot rule out the possibility that a small KpnI fragment is located between the S. roseosporus DNAs cloned in pRHB614 and pRHB613. End sequence analysis of pRHB599, pRHB602, and pRHB603, which contained fragments derived from pRHB614, identified segments of DNA containing peptide synthetase genes. Thus, it is likely that most or all of the S. roseosporus DNA in pRHB614 encodes peptide synthetase (Fig. 2).
Functional mapping of the daptomycin biosynthetic gene cluster in S. roseosporus.
The physical map and DNA hybridization data of the four cosmids digested with various restriction enzymes and probed with the 5.2-kb DraI-KpnI fragment from pRHB157 were used to select fragments to be subcloned for gene disruption analysis. Restriction fragments (sections 1 to 7 in Fig. 2A) were cloned into pOJ260 and introduced into S. roseosporus A21978.6 by conjugation from E. coli S17-1. Transconjugants expressing Amr were tested for antibiotic production. A summary of the antibiotic production is shown in Fig. 2A. The gene disruption analysis indicated that sections 2, 4, and 5 probably contain insertions within the peptide synthetase coding regions. Sections 1, 6, and 7 may be outside the peptide synthetase coding regions. Interestingly, DNA inserted in section 3, which is internal to two apparent peptide synthetase coding regions, did not disrupt daptomycin biosynthesis. As shown below, this region of DNA encodes other functions.
DNA sequence analysis of two regions of the cyclic peptide gene cluster.
A 7.9-kb EcoRI fragment containing module cspB (segment 2 in Fig. 2A) and a 5.3-kb ApaI fragment containing a Tn5099 insertion in cpsA in strain MM95 (Fig. 2) were subcloned and sequenced. Figure 3A shows the deduced amino acid sequences of CpsA and CpsB compared to those of other peptide synthetase domains. A high degree of homology was observed between CpsA and CpsB and other peptide synthetase domains, particularly within conserved core motifs B to G (or 1 to 5), associated with ATP binding and aminoacyl adenylation, and motif J (or section 6), associated with covalent linkage of 4′-phosphopantetheine to Ser (15, 29, 33).
FIG. 3.
Comparison of the deduced amino acid sequences of the peptide synthetase modules. (A) Modules located on the 7.9-kb EcoRI fragment (SrcpsB) and the 5.3-kb ApaI fragment (SrcpsA) (Fig. 2B) are compared to peptide synthetase modules from Streptomyces fradiae A54145 (SfcpsA), N. lactamdurans (NlpcbA), Penicillium chrysogenum (PcacvA), B. subtilis (BssrfA), and Pseudomonas aeruginosa (PafpvA). The conserved motifs (A through J) (33) are shown as dashes. The consensus (Cons) line contains letters for amino acids showing 100% conservation and asterisks for conservative amino acid substitutions. (B) The segment of DNA (Srepim) from the 7.9-kb EcoRI fragment containing epimerase conserved motifs (Fig. 2B) is compared to peptide synthetase segments encoding epimerase activity from Streptomyces pristinaespiralis (Spepim), N. lactamdurans (Nlepim), B. brevis (Bbepim), B. subtilis (Bsepim), and P. crysogenum (Pcepim). The conserved motifs and consensus sequences are depicted as in panel A.
CpsB contains an additional ∼500-amino-acid region toward the carboxy end of the module that corresponds to the racemase domain (motifs N, O, P, and R), observed only in modules that epimerize l-amino acids to d-amino acids (15, 34). The putative CpsB racemization region is compared to five other racemization regions in other peptide synthetases in Fig. 3B. A high degree of homology to motifs K to R was observed. Epimerase regions have a separate set of motifs K to R which differ from motifs K to R of elongation domains (15, 33). Thus, the CpsB module contains regions for amino acid activation, thioester formation, and epimerization. The CpsB module may be responsible for the incorporation of either d-Ala or d-Ser into daptomycin (4).
We also obtained partial DNA sequences from the ends of the EcoRI fragment in pRHB159 (segment 3 in Fig. 2A). These open reading frames showed high sequence similarities to dedA in E. coli and masC in Mycobacterium leprae. Disruption of the masC gene blocked daptomycin production (13), suggesting that it encodes some function in daptomycin biosynthesis other than peptide formation. We also obtained end sequences from three EcoRI fragments located between the right end of segment 2 and the KpnI site (about 483 to 490 kb from the chromosome end; Fig. 2), and each had homology to peptide synthetase sequences (data not shown). Therefore, the daptomycin gene cluster appears to have at least two discrete peptide synthetase coding regions separated by a region of 10 to 20 kb containing dedA and masC homology. Wessels et al. (37) have shown that the daptomycin peptide synthetase has three subunits of about 670, 670, and 240 kDa. Our DNA sequencing data suggest that cpsA and cpsB are located in the coding regions for the two 670-kDa proteins (Fig. 2B). The 240-kDa protein activates Kyn and is likely to contain modules for the incorporation of the last two amino acids (methyl-Glu and Kyn) (37). It is not clear where the coding region for the 240-kDa protein is located, but it may lie to the right of the Tn5099 insertion in strains MM93 (Fig. 2B).
We inserted an Hmr gene into the cpsA module (13) and developed a positive selection system for double crossovers in S. roseosporus (12). It should now be possible to exchange heterologous peptide synthetase modules into the coding region to produce novel derivatives of daptomycin. Module exchanges have been carried out with the Bacillus subtilis surfactin peptide synthetase cluster to produce novel active derivatives of surfactin (30, 31), thus demonstrating the feasibility of this approach.
ACKNOWLEDGMENTS
We thank S. Kuhstoss for plasmid pKC1471, L. Boeck, M. Favret, and J. Mynderse for fermentation and HPLC analysis, I. Jenkins for assistance with DNA sequencing, and J. Wiley for typing the manuscript.
We thank Lilly Research Laboratories for supporting the work. T. Hosted was a Lilly postdoctoral fellow.
REFERENCES
- 1.Aharonowitz Y, Bergmeyer J, Cantoral J M, Cohen G, Demain A L, Fink U, Kinghorn J, Kleinkauf H, MacCabe A, Palissa H, Pfeifer E, Schwecke T, Van Liempt H, von Döhren H, Wolfe S, Zhang J. δ-(l-α-Aminoadipyl)-l-cysteinyl-d-valine synthetase, the multienzyme integrating the four primary reactions in β-lactam biosynthesis, as a model peptide synthetase. Bio/Technology. 1992;11:807–810. doi: 10.1038/nbt0793-807. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment research tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Baltz R H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978;107:93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- 4.Baltz R H. Lipopeptide antibiotics produced by Streptomyces roseosporus and Streptomyces fradiae. In: Strohl W R, editor. Biotechnology of industrial antibiotics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. p. 415. [Google Scholar]
- 5.Bierman M, Logan R, O’Brien K, Seno E T, Rao R N, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 6.Boeck L, Fukuda D S, Abbott B J, Debono M. Deacylation of A21978C, an acidic lipopeptide antibiotic complex, by Actinoplanes utahensis. J Antibiot. 1988;41:1085–1092. doi: 10.7164/antibiotics.41.1085. [DOI] [PubMed] [Google Scholar]
- 7.Canepari P, Boaretti M. Lipoteichoic acid as a target for antimicrobial action. Microb Drug Resist. 1996;2:85–89. doi: 10.1089/mdr.1996.2.85. [DOI] [PubMed] [Google Scholar]
- 8.Debono M, Abbott B J, Malloy R M, Fukuda D S, Hunt A H, Daupert V M, Counter F T, Ott J L, Carrell C B, Howard L C, Boeck L D, Hamill R L. Enzymatic and chemical modifications of lipopeptide antibiotic A21978C: the synthesis and evaluation of daptomycin ( LY146032) J Antibiot. 1988;41:1093–1105. doi: 10.7164/antibiotics.41.1093. [DOI] [PubMed] [Google Scholar]
- 9.Debono M, Barnhart M, Carrell C B, Hoffman J A, Occolowitz J L, Abbott B J, Fukuda D S, Hamill R L. A21978C, a complex of new acidic peptide antibiotics: isolation, chemistry, and mass spectral structure elucidation. J Antibiot. 1987;40:761–777. doi: 10.7164/antibiotics.40.761. [DOI] [PubMed] [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn D R, Solenberg P J, Baltz R H. Tn5099, a xylE promoter probe transposon for Streptomyces spp. J Bacteriol. 1991;173:5573–5577. doi: 10.1128/jb.173.17.5573-5577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosted T J, Baltz R H. Use of rpsL for dominance selection and gene replacement in Streptomyces roseosporus. J Bacteriol. 1997;179:180–186. doi: 10.1128/jb.179.1.180-186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosted, T. J., and R. H. Baltz. Unpublished data.
- 14.Kleinkauf H, von Döhren H. Bioactive peptides—recent advances and trends. In: Kleinkauf H, von Dohren H, editors. Biochemistry of peptide antibiotics. New York, N.Y: Walter de Gruyter & Co.; 1990. pp. 1–31. [Google Scholar]
- 15.Kleinkauf H, von Döhren H. A ribosomal system of peptide biosynthesis. Eur J Biochem. 1996;236:335–351. doi: 10.1111/j.1432-1033.1996.00335.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuhstoss, S. Unpublished data.
- 17.Leblond P, Fischer G, Francon F-X, Berger F, Guerineau M, Decaris B. The unstable region of Streptomyces ambofaciens includes 210 kb terminal inverted repeats flanking the extremities of the linear chromosomal DNA. Mol Microbiol. 1996;19:261–271. doi: 10.1046/j.1365-2958.1996.366894.x. [DOI] [PubMed] [Google Scholar]
- 18.Leblond P, Redenbach M, Cullum J. Physical map of the Streptomyces lividans 66 genome and comparison with that of the related strain Streptomyces coelicolor A3(2) J Bacteriol. 1993;175:3422–3429. doi: 10.1128/jb.175.11.3422-3429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y-S, Kieser H M, Hopwood D A, Chen C W. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993;10:923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 20.McHenney M A, Baltz R H. Gene transfer and transposition mutagenesis in Streptomyces roseosporus: mapping of insertions that influence daptomycin or pigment production. Microbiology. 1996;142:2363–2373. doi: 10.1099/00221287-142-9-2363. [DOI] [PubMed] [Google Scholar]
- 21.Pavela-Vrancic M, Van Liempt H, Pfeifer E, Freist W, von Döhren H. Nucleotide binding by multienzyme peptide synthetases. Eur J Biochem. 1994;220:535–542. doi: 10.1111/j.1432-1033.1994.tb18653.x. [DOI] [PubMed] [Google Scholar]
- 22.Redenbach M, Flett F, Piendl W, Glocker I, Rauland U, Wafzig O, Kliem R, Leblond P, Cullum J. The Streptomyces lividans 66 chromosome contains 1 MB deletogenic region flanked by amplifiable regions. Mol Gen Genet. 1993;241:255–262. doi: 10.1007/BF00284676. [DOI] [PubMed] [Google Scholar]
- 23.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 25.Simon R, Preifer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 26.Solenberg P J, Baltz R H. Transposition of Tn5096 and other IS493 derivatives in Streptomyces griseofuscus. J Bacteriol. 1991;173:1096–1104. doi: 10.1128/jb.173.3.1096-1104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solenberg P J, Baltz R H. Hypertransposing derivatives of streptomycete insertion sequence IS493. Gene. 1994;147:47–54. doi: 10.1016/0378-1119(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 28.Speyer J F. A simple and effective electroporation apparatus. BioTechniques. 1990;8:28–30. [PubMed] [Google Scholar]
- 29.Stachelhaus T, Huser A, Marahiel M A. Biochemical characterization of peptidyl carrier protein (PCP), the thiolation domain of multifunctional peptide synthetases. Chem Biol. 1996;3:913–921. doi: 10.1016/s1074-5521(96)90180-5. [DOI] [PubMed] [Google Scholar]
- 30.Stachelhaus T, Marahiel M A. Modular structure of genes encoding multifunctional peptide synthetases required for non-ribosomal peptide synthesis. FEMS Microbiol Lett. 1995;125:3–14. doi: 10.1111/j.1574-6968.1995.tb07328.x. [DOI] [PubMed] [Google Scholar]
- 31.Stachelhaus T, Schneider A, Marahiel M A. Rational design of peptide antibiotics by targeted replacement of bacterial and fungal domains. Nature. 1995;269:69–72. doi: 10.1126/science.7604280. [DOI] [PubMed] [Google Scholar]
- 32.Stachelhaus T, Schneider H, Marahiel M A. Engineered biosynthesis of peptide antibiotics. Biochem Pharmacol. 1996;52:177–186. doi: 10.1016/0006-2952(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 33.Stein T, Vater J. Amino acid activation and polymerization at modular multienzymes in nonribosomal peptide biosynthesis. Amino Acids. 1996;10:210–227. doi: 10.1007/BF00807324. [DOI] [PubMed] [Google Scholar]
- 34.Stein T, Vater J V, Kruft A, Otto B, Wittman-Liebold P, Franke M, Panico M, McDowell R, Morris H R. The multiple carrier model of nonribosomal peptide biosynthesis at modular multienzymatic templates. J Biol Chem. 1996;271:15428–15435. doi: 10.1074/jbc.271.26.15428. [DOI] [PubMed] [Google Scholar]
- 35.Von Döhren H, Pfeifer E, van Liempt H, Lee Y-O, Pavela-Vrancic M, Schwecke T. The nonribosomal system: what we learn from the genes encoding protein templates. In: Baltz R H, Hegeman G D, Skatrud P L, editors. Industrial microorganisms: basic and applied molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 159–167. [Google Scholar]
- 36.Wang G, Xu X, Chen J-M, Berg D E, Berg C M. Inversions and deletions generated by a mini-γδ (Tn1000) transposon. J Bacteriol. 1994;176:1332–1338. doi: 10.1128/jb.176.5.1332-1338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessels P, von Döhren H, Kleinkauf H. Biosynthesis of acyl peptidolactones of the daptomycin type. A comparative analysis of peptide synthetases forming A21978C and A54145. Eur J Biochem. 1996;242:665–673. doi: 10.1111/j.1432-1033.1996.0665r.x. [DOI] [PubMed] [Google Scholar]
- 38.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]