Abstract
The recent shortage of the brand name drug Fungizone has necessitated a change to generic formulations of amphotericin B deoxycholate. Clinical trials cannot be conducted in a timely manner to provide data on the safety and efficacy of these formulations. We therefore compared generic amphotericin B and Fungizone for activity and safety in the treatment of experimental invasive pulmonary aspergillosis (IPA) in persistently neutropenic rabbits. Fungizone and generic amphotericin B are similar in efficacy, pharmacokinetics, and safety in the treatment of experimental IPA.
Invasive pulmonary aspergillosis is an important cause of morbidity and mortality in patients with cancer, transplant recipients, and those with other immunodeficiencies (2, 4, 9, 12, 15, 16). Although new antifungal agents have been developed, amphotericin B deoxycholate remains a frequently used drug for the treatment of pulmonary aspergillosis. Conventional amphotericin B has been available in the United States under the brand name Fungizone. This brand of amphotericin B deoxycholate has been manufactured by Bristol-Meyers Squibb (Princeton, N.J.) and distributed by Geneva Pharmaceuticals (Geneva Pharmaceuticals, Inc., Dayton, N.J.). The first generic amphotericin B (GenericAmB) formulation was approved in the United States in 1992. Two GenericAmB formulations are available in the United States. One is provided by X-Gen (formerly Pharma Tek); the other is available from Pfizer under the trade name Amphocin. These two generic products are identical in content and are, in fact, produced by the same contract manufacturer (Cardinal, Albuquerque, N.Mex.) under X-Gen's license.
The recent shortage of Fungizone has necessitated a change in many institutions to GenericAmB formulations. As there are no data showing whether the generic formulation is equivalent in safety and efficacy, we saw no recourse except to perform studies to evaluate the safety and efficacy of GenericAmB and directly address this public health issue. Clinical trials cannot be conducted with humans in a timely manner in order to provide clinicians information about the safety and efficacy of these generic formulations. We therefore compared GenericAmB (Pharma-Tek, Pharma-Tek, Inc., Huntington, N.Y.) and Fungizone for antifungal activity and safety in the treatment of experimental pulmonary aspergillosis in persistently neutropenic rabbits.
Aspergillus fumigatus National Institutes of Health isolate 4215 (ATCC MYA-1163) was used in all experiments. MICs against A. fumigatus were determined in accordance with NCCLS standard M38-A microdilution method (6, 13). The MICs of Fungizone and GenericAmB for A. fumigatus were each 1.0 μg/ml in RPMI 1640 medium (BioWhittaker, Walkersville, Md.).
Healthy female New Zealand White rabbits (Hazleton Research Products, Inc., Denver, Pa.) weighing 2.6 to 3.5 kg were used in all experiments. All rabbits were monitored under humane care and use in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and in accordance with National Institutes of Health guidelines for animal care in fulfillment of the guidelines of the National Research Council (14) and under approval by the Animal Care and Use Committee of the National Cancer Institute. Vascular access was established in each rabbit as previously described (17). Serial serum samples for antigen and plasma pharmacokinetic studies were drawn from all rabbits at the initiation of immunosuppression and throughout the course of pulmonary aspergillosis. Rabbits were euthanized by intravenous administration of pentobarbital (65 mg of pentobarbital sodium per kg of body weight; pentobarbital sodium was in the form of 0.5 ml of beuthanasia-D special [euthanasia solution; Schering-Plough Animal Health Corp., Union, N.J.]) at the end of each experiment, 24 h after the administration of the last dose of study drug.
Pulmonary aspergillosis and profound persistent neutropenia were established and maintained as previously described (7). Rabbits were grouped to receive GenericAmB at 0.5 mg/kg (GenericAmB0.5) or 1 mg/kg (GenericAmB1) or amphotericin B (Fungizone) at 0.5 mg/kg (Fungizone0.5) or 1 mg/kg (Fungizone1) for treatment of established invasive pulmonary aspergillosis or no drug (untreated controls [UC]). Amphotericin B (concentration of 1 mg/ml) was administered intravenously after dilution with D5W solution (Abbott Laboratories, North Chicago, Ill.) for 12 days starting 24 h after endotracheal inoculation of A. fumigatus conidia.
The following panel of outcome variables was used to assess antifungal efficacy: pulmonary infarct score, lung weight, residual fungal burden (log CFU per gram), survival (7), and galactomannan antigenemia. Serum galactomannan concentrations were determined by the Platelia Aspergillus EIA (Bio-Rad Laboratories, Redmond, Wash.) one-stage immunoenzymatic sandwich microplate assay method, which was performed in accordance with the manufacturer's (Bio-Rad, Verriéres-le-Buisson, France) directions. Serum chemistry panels were prepared with the penultimate sample drawn from each rabbit.
The pharmacokinetics of amphotericin B in plasma were investigated with three to eight infected rabbits per treatment group by optimal plasma sampling. Concentrations of amphotericin B in plasma were determined after protein precipitation with methanol (1:1.5 [vol/vol]) by a reverse-phase high-performance liquid chromatography-UV method (10). The following pharmacokinetic parameters for amphotericin B were determined by model-independent analysis: maximum concentration in plasma (Cmax), concentration at 24 h after dosing, the area under the plasma concentration-time curve from 0 to 24 h (AUC0-24), dose linearity, drug clearance from plasma (CL), apparent volume of distribution at steady state (Vss), and elimination half-life (t1/2) (8).
Comparisons between groups were performed by the Kruskal-Wallis test (nonparametric analysis of variance or the Mann-Whitney U test, as appropriate. A two-tailed P value of <0.05 was considered to be statistically significant. Survival was plotted by Kaplan-Meier analysis. Differences in survival between treatment groups and UC were analyzed by log rank test. Values are expressed as means and standard errors of the means.
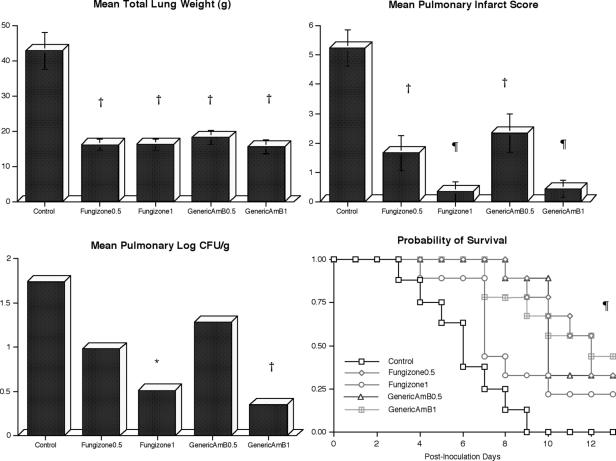
Rabbits treated with either drug showed dose-dependent decreases in organism-mediated pulmonary injury similar to those of UC, as measured by total lung weights and pulmonary infarct scores (Fig. 1). Rabbits treated with Fungizone1 or GenericAmB1 also showed a significant decrease in the residual fungal burden of A. fumigatus in lung tissue in comparison with that of UC (P < 0.05 and P < 0.01, respectively). Even though there was a trend toward reduction of CFU in Fungizone0.5- and GenericAmB0.5-treated rabbits, these differences were not significant in comparison with UC. Also, there were no significant differences in reduction of CFU between Fungizone0.5- and GenericAmB0.5-treated rabbits. Survival was significantly improved to similar degrees in all treatment groups in comparison with that of UC (P < 0.01).
FIG. 1.
Responses of primary pulmonary aspergillosis in persistently neutropenic rabbits to antifungal therapy as measured by the following panel of outcome parameters: lung weight, pulmonary infarct score, mean concentration of residual organisms (log CFU per gram) in pulmonary tissue (n = 8; 95% confidence interval in UC, 1.07 to 2.41) (GenericAmB0.5 [n = 9] 95% confidence interval, 0.28 to 1.69; GenericAmB1 [n = 9] 95% confidence interval, −0.08 to 1.10; Fungizone0.5 [n = 9] 95% confidence interval, 0.62 to 1.94; Fungizone1 [n = 9] 95% confidence interval, −0.14 to 0.83), and survival. Lung weight and pulmonary infarct scores are expressed as the mean ± the standard error of the mean. In comparison with UC, P values are indicated as follows: *, P < 0.05; †, P < 0.01; ¶, P < 0.001. For the survival measurements, the values on the y axis are probability of survival, P ≤ 0.001 (log rank test) for all treatment groups in comparison with UC.
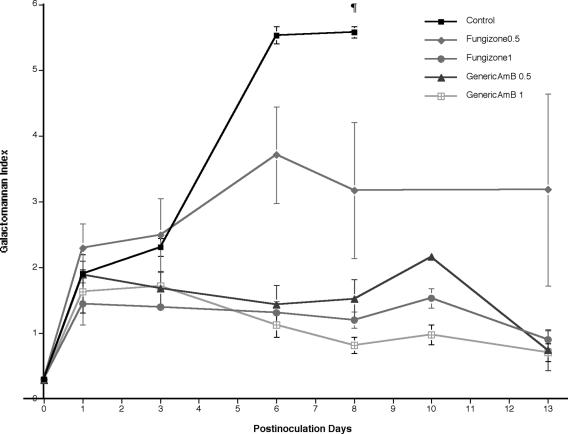
Serial serum samples from UC showed progressive galactomannan antigenemia through day 8 of treatment (Fig. 2). Rabbits treated with Fungizone0.5 showed increased but stable galactomannan antigenemia through day 13. On the other hand, rabbits treated with Fungizone1, GenericAmB0.5, or GenericAmB1 showed lower galactomannan antigenemia throughout treatment. However, the levels of galactomannan were significantly lower on day 8 in all treatment groups in comparison with those in UC (P ≤ 0.01).
FIG. 2.
Expression of galactomannan antigenemia in persistently neutropenic rabbits with pulmonary aspergillosis treated with GenericAmB0.5, GenericAmB1, Fungizone0.5, or Fungizone1. Galactomannan antigenemia levels were significantly lower on day 8 in all treatment groups in comparison with that of UC (¶, P ≤ 0.01). All values represent means ± the standard errors of the means.
There was a significant increase in serum creatinine in rabbits treated with Fungizone1 (1.76 ± 0.27 mg/dl) or GenericAmB1 (3.08 ± 0.78 mg/dl) in comparison with that in UC (0.91 ± 0.15 mg/dl; P < 0.05) There also was a significant increase in serum urea nitrogen in rabbits treated with Fungizone1 (66.4 ± 13.0 mg/dl) or GenericAmB1 (106.1 ± 18.3 mg/dl) in comparison with that of UC (18.5 ± 1.89 mg/dl; P < 0.01). On the other hand, the differences in serum creatinine and urea nitrogen levels between Fungizone1- and GenericAmB1-treated rabbits were not significant.
Pharmacokinetic parameters are presented in Table 1. With increasing dosage, there were similar increases in Cmax, AUC0-24, and Vss. There were no significant differences in pharmacokinetics parameters between Fungizone0.5 andGenericAmB0.5 or between Fungizone1and GenericAmB1 in terms of Cmax, concentration at 24 h after dosing, AUC0-24, AUC0-∞/dose, Vss, CL, and t1/2 (Table 1).
TABLE 1.
Pharmacokinetic parameters of Fungizone and GenericAmB in rabbits with invasive pulmonary aspergillosisa
| Parameter | Fungizone0.5 | GenericAmB0.5 | P value | Fungizone1.0 | GenericAmB1.0 | P value |
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | 14.18 ± 1.71 | 14.62 ± 1.15 | 0.5358 | 21.06 ± 3.06 | 32.55 ± 6.69 | 0.2667 |
| Cmin (μg/ml) | 1.52 ± 0.16 | 1.49 ± 0.06 | 0.9551 | 1.84 ± 0.30 | 2.48 ± 0.16 | 0.0667 |
| AUC0-24 (μg/ml · h) | 56.10 ± 4.39 | 59.34 ± 1.39 | 0.1893 | 73.08 ± 2.38 | 94.36 ± 4.99 | 0.0667 |
| AUC0-∞/dose | 0.09 ± 0.01 | 0.09 ± 0.004 | 0.9551 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.3833 |
| Vrss (ml/kg) | 150.20 ± 9.25 | 139.90 ± 6.40 | 0.4634 | 229.64 ± 13.02 | 180.56 ± 8.53 | 0.0667 |
| CL (ml/h/kg) | 5.18 ± 0.56 | 4.90 ± 0.19 | 0.5358 | 7.89 ± 1.43 | 6.06 ± 0.49 | 0.1167 |
| t1/2 (h) | 21.77 ± 2.62 | 20.10 ± 1.62 | 0.8665 | 21.70 ± 4.22 | 21.08 ± 1.18 | 0.5167 |
All values are expressed as the mean ± the standard error of the mean. Cmin, concentration at 24 h after dosing; AUC0-∞/dose, dose-normalized area under the plasma concentration-versus-time curve from time zero to infinity.
Fermentation and production of amphotericin B deoxycholate is complicated, and the drug may vary in purity and potency. Although they are uncommon for most antibiotics, variations between generic and proprietary formulations have been noted for vancomycin (1, 5, 11). Little is known about this potential for altered toxicity or efficacy among amphotericin B formulations.
Although reliable usage estimates are difficult to obtain, the national demand for amphotericin B is projected at approximately 800,000 to 1,000,000 50-mg vials per annum (Jeff Granger, X-Gen, personal communication, 23 February 2004), thus confirming that conventional amphotericin B deoxycholate remains in demand.
As measured by a panel of five outcome variables (survival, residual fungal burden, pulmonary infarct score, lung weight, and galactomannan resolution), Fungizone and GenericAmB were similar in efficacy. These formulations also demonstrated similar safety profiles. Previously, Cleary and colleagues described differences among amphotericin B deoxycholate formulations manufactured by different companies in expression of interleukin-1β in in vitro models (3). However, these differences were not reflected in vivo in our animal model by efficacy, safety, or pharmacokinetics. The results of these experiments provide reassurance that this generic formulation of amphotericin B is comparable to Fungizone in efficacy, safety, and pharmacokinetics.
REFERENCES
- 1.Alexander, M. R. 1974. Review of vancomycin after 15 years of use. Drug Intell. Clin. Pharm. 8:520. [Google Scholar]
- 2.Anaissie, E. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14(Suppl. 1):S43-S53. [DOI] [PubMed] [Google Scholar]
- 3.Cleary, J. D., P. D. Rogers, and S. W. Chapman. 2003. Variability in polyene content and cellular toxicity among deoxycholate amphotericin B formulations. Pharmacotherapy 23:572-578. [DOI] [PubMed] [Google Scholar]
- 4.Denning, D. W. 2001. Chronic forms of pulmonary aspergillosis. Clin. Microbiol. Infect. 7(Suppl. 2):25-31. [DOI] [PubMed] [Google Scholar]
- 5.Eng, R. H., L. Wynn, S. M. Smith, and F. Tecson-Tumang. 1989. Effect of intravenous vancomycin on renal function. Chemotherapy 35:320-325. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., M. Bartlett, R. Bowden, N. X. Chin, C. Cooper, Jr., A. Fothergill, M. R. McGinnis, P. Menezes, S. A. Messer, P. W. Nelson, F. C. Odds, L. Pasarell, J. Peter, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, G. S. Shankland, T. J. Walsh, and I. Weitzman. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed., p. 55-459. Marcel Dekker, Inc., New York, N.Y.
- 9.Groll, A. H., P. M. Shah, C. Mentzel, M. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 10.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 11.Ingerman, M. J., and J. Santoro. 1989. Vancomycin. A new old agent. Infect. Dis. Clin. N. Am. 3:641-651. [PubMed] [Google Scholar]
- 12.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.National Research Council Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 15.Pannuti, C., R. Gingrich, M. A. Pfaller, C. Kao, and R. P. Wenzel. 1992. Nosocomial pneumonia in patients having bone marrow transplant. Attributable mortality and risk factors. Cancer 69:2653-2662. [DOI] [PubMed] [Google Scholar]
- 16.Wald, A., W. Leisenring, J. A. van Burik, and R. A. Bowden. 1997. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J. Infect. Dis. 175:1459-1466. [DOI] [PubMed] [Google Scholar]
- 17.Walsh, T. J., J. Bacher, and P. A. Pizzo. 1988. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab. Anim. Med. 38:467-470. [PubMed] [Google Scholar]