Abstract
Orthodontic treatment has been associated with chronic extraoral pain that is often resistant to common treatments such as drugs or physiotherapy, adversely affecting patients’ quality of life. In this case series, we discuss the potential impact of orthodontics on chronic cervical spine pain or gonalgia and explore the long-term effect of local anesthetic injections as a possible therapeutic intervention. Six orthodontic patients with chronic cervical spine pain or gonalgia that substantially affected their quality of life were treated with injections of 0.5% procaine into individual lesions and at palpable points of tissue tension in the oral mucosa and extraoral myofascial areas. All patients in this case series reported significant improvement in their chronic pain, with no residual pain recorded at the 6-month follow-up. Injecting local anesthetic at stress points in the oral mucosal and extraoral myofascial regions may be an effective treatment for post-orthodontic neck and knee pain. Further research is required to better understand the potential benefits of this intervention for patients experiencing orthodontic-related musculoskeletal pain.
Keywords: Chronic pain, local anesthetic, procaine, orthodontic, neural therapy, musculoskeletal pain
Introduction
Chronic pain, defined as pain that persists for longer than 3 months, 1 has an immense impact on health. 2 It results in cognitive, sensory, and affective components such as anxiety and depression. 3 A substantial proportion of chronic musculoskeletal pain involves the cervical spine or knees, 4 leading to functional limitations, disability, and decreased quality of life. 2 Unfortunately, few therapeutic options are available for such patients. 4
More than 90% of orthodontic patients develop “orthodontic pain” 5 caused by the nociceptive stimulus generated by brace activation, resulting in hyperalgesia. 6 Orthodontic mobilization is achieved by prolonged force on the teeth and resultant bone remodeling and can induce mild to moderate pain.7,8 Pain signals arising in the jaw are thought to be transmitted to the limbic system, hippocampus, and somatosensory cortex via the trigeminal ganglion, trigeminal caudate nucleus, and routes ascending from the thalamus. 5 The tissues of the oral cavity are closely interrelated, with the entire region affected by pathology originating from any single structure. The cervical region is associated with the dental region through the spinal nucleus of the trigeminal nerve. 9
Patients with temporomandibular dysfunction experience more pain, functional limitations, and sensitivity in the cervical spine than those without such dysfunction.10,11 Our clinical experience includes patients whose onset of cervical or knee pain coincided with orthodontic implementation.
Therapy with local anesthetics (LAs), known as neural therapy, involves short-acting and low-concentration (0.5%–1.0%) injections of an LA at specific points for therapeutic, not anesthetic, purposes.12,13 Their effect is produced by regulatory mechanisms of the nervous system that interrupt the positive feedback actions of pain.12–14 The generation of a guided stimulus (via the needle) and selective extinction of other stimuli (via the LA) impact nervous system function and tissue perfusion.12–14 Injections are performed at individual lesions and palpable points of tissue tension in accordance with the patient’s clinical symptoms and medical history. 15
A chronic asymptomatic disorder involving one structure of the body (e.g., chronic inflammation or scarring) may cause a symptomatic disorder in another area of the body.12,13, 15 Several authors have reported relief or elimination of distant pain after injecting an LA into a scar or an area of distal chronic inflammation.14–18 Additionally, improvement of refractory chronic cervical pain after infiltration of the periodontal tissue of an impacted third molar with an LA was recently reported. 19
The present study showed favorable results after LA injection in six orthodontic patients with chronic cervical or knee pain refractory to different treatments. Our study is reported with the aim of motivating future research of the efficacy of LA injections for post-orthodontic musculoskeletal pain.
Methods
Pain measurement
Pain was self-reported by the patients at their baseline visit and monitoring visits using a visual analogue scale (VAS).
Examination
A detailed anamnesis was obtained during the first visit. Tension was palpated at various points in the patient’s body, particularly in the temporomandibular, upper cervical, and trapezius regions. Palpation of the oral cavity was performed by sliding the index finger along the upper and lower vestibular fundus from anterior to posterior. Tension was defined as the manual perception of an area exhibiting hypertonicity, hardness, or a change in texture compared with adjacent areas, often accompanied by a painful sensation.
Intervention
In each session, 0.5 mL of 0.5% procaine was injected at oral (submucosal) tension points (TPs), and an additional 1.0 mL was administered at extraoral (myofascial) TPs. The infraorbital and mental nerves were intraorally injected. The average number of sessions required for long-term remission was 3.2 (range, 1–7) per patient.
Monitoring
Patients returned to our center if the pain persisted or reappeared. Telephone monitoring was performed at 3 and 6 months.
Compliance with ethical guidelines
Data were collected retrospectively, and all information was anonymized. Therefore, institutional review board approval was not required for this study. All patients provided verbal and written informed consent for the use of this minimally invasive treatment modality and for the publication of this study. The reporting of this study conforms to the CARE guidelines. 20
Results
The six cases in this study are reported below, and their characteristics are summarized in Table 1. All patients were asymptomatic at the 3- and 6-month telephone recalls. No complications or side effects were reported.
Table 1.
(a) Patients’ demographic and clinical characteristics. (b) Patients’ treatment and clinical evolution.
| Table 1(a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sex | Age (years) | Reason for consultation | Time since pain onset | Inability to lead a normal life | Dental device (age) in v1 | Time since orthodontics and pain onset | Comorbidities | Exploration visit 1 |
|
| Oral | Non-oral | |||||||||
| 1 | Female | 25 | Cervical pain that sometimes radiated to low back pain | 4 years | Yes | Removable and fixed orthodontics (10–14 years of age), fixed retainers (current) | 11 years | Progressive scoliosis | Tension points in oral vestibules | Suboccipital, cervical, and lumbar tension |
| 2 | Female | 47 | Cervical pain and tension at Pfannenstiel scar | 5 years | Yes | Fixed orthodontics with braces (coinciding with onset of pain), fixed retainers until current presentation | 0 years | Anxiety, vulvar pruritus | Tension points in oral vestibules | TMJ, suboccipital, trapezius, and suprapubic area tension |
| 3 | Female | 48 | Cervical pain with dizziness following whiplash from car accident | 6 years | Yes | Braces 6 years previously (started 2 months after the accident, retained for 18 months), retainers from that time to current presentation | 0 years | Paresthesia in both hands since pregnancy (2 years earlier), left foot paresthesia since caesarean labor (13 months earlier), carpal tunnel syndrome | Exodontia of left upper third molar 10 years previously, remaining third molar retained; tension points in upper vestibule | Suboccipital, trapezius, lumbar, and left soleus muscle tension |
| 4 | Female | 15 | Gonalgia | 6 months | Yes | Removable in palate (8–9 years of age), fixed orthodontics (12–14 years of age), nighttime retainers until current presentation | 6 years | Allergic bronchitis and atopic skin | Tension points in upper vestibule | TMJ, suboccipital, and lumbar tension |
| 5 | Male | 16 | Gonalgia | 1 year | Yes | Palatal disjunctor (11 years of age), fixed orthodontics (12 years of age to present), extraction of second premolars (12 years of age) | 4 years | Patellofemoral syndrome and tendinitis that did not improve with osteopathy or physiotherapy, anxiety with epigastric pain | Tension points in oral vestibules | Suboccipital, pectorals, and epigastrium tension |
| 6 | Female | 15 | Gonalgia | 4 years | Yes (had to abandon skating) | 0 years | Tension points in oral vestibules | TMJ, epigastrium, and iliac fossa tension | ||
TMJ, temporomandibular joint; VAS, visual analogue scale (0–10); v, visit in which intervention took place.
| Table 1(b) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention visit 1 |
Baseline VAS score | Immediate VAS score, visit 1 | Days without pain | Number of re-interventions | Days between v1 and last re-intervention | VAS score after last re-intervention | Days between v1 and last assessment | VAS score at latest assessment | Other interventions | ||
| Case | Oral* | Non-oral** | |||||||||
| 1 | Upper, infraorbital, and mental vestibules | Areas of highest pre-sternal, trapezius, and lumbar tension | 7–9 | 0 | 15 (reappeared with VAS score f 4) | 1 | 15 | 0 | 90 | 0 | |
| 2 | Upper, infraorbital, and mental vestibules | Areas of highest TMJ, suboccipital, trapezius, and Pfannenstiel scar tension | 7–10 | 3 | 0 | 2 | 42 | 0 | 130 | 0 | |
| 3 | Upper, infraorbital, and mental vestibules and third molar area | Areas of highest trapezius, suboccipital, and left soleus tension; lumbar fascia tension; and Pfannenstiel scar tension | 10 | 5 | 0 | 4 | 338 | 0 | 518 | 0 | Retainer was removed between v4 and v5 |
| 4 | Upper, infraorbital, and mental vestibules | None | 8 | 0 | >90 | 0 | No further interventions | – | 90 | 0 | Night retainer was removed after v1 |
| 5 | Upper, infraorbital, and mental vestibules | None | 6–8 | 0 | 2 | 6 | 171 | 0 | 351 | 0 | Fixed retainer was replaced with one removable night retainer (between v2 and v3) |
| 6 | Upper, infraorbital, and mental vestibules | Areas of highest TMJ, epigastrium, and iliac fossa tension | 7.5 | 0 | 15 | 1 | 25 | 0 | 205 | 0 | |
TMJ, temporomandibular joint; VAS, visual analogue scale (0–10) for pain; v, visit in which intervention took place.
The oral intervention consisted of a 0.5-mL injection of 0.5% procaine into the submucosa at the described points.
The intervention of other non-oral points consisted of 1.0-mL injection of 0.5% procaine at the myofascial level of the described points.
Case 1
A 25-year-old woman presented with back pain that had been present since adolescence and had worsened in the cervical spine region 4 years previously (VAS score of 7–9). The pain radiated to the lower back and was accompanied by functional impairments.
Medical history: The patient had had progressive scoliosis since childhood and had undergone both pharmacological and physical therapy. Dental history: She wore removable appliances and had undergone placement of fixed upper and lower orthodontics between the ages of 10 and 14 years. She had fixed upper and lower retainers at the time of presentation. Physical examination: TPs were present in the oral vestibules in the cervical-occipital and lumbar areas. Treatment and evolution: Immediately after the oral TP injections, the patient experienced relief of the cervical and lumbar pain. Procaine was subsequently applied to TPs in the parasternal, trapezius, and thoracolumbar muscles. Two weeks later, the pain reappeared with less intensity and disappeared immediately after injection of the oral vestibular TP.
Case 2
A 47-year-old woman presented with a 10-year history of cervical pain (VAS score of 7–10) that had worsened when she underwent orthodontic treatment 5 years previously. She had worn fixed retainers since.
Medical history: The patient had undergone both pharmacological and physiotherapy treatment. She had also undergone caesarean sections 13 and 21 years previously and hysterectomy 2 years previously. Dental history: She had worn fixed retainers for 5 years during clinical treatment. She had agenesis of teeth 1.8, 3.8, and 4.8 with current eruption of tooth 2.8. Physical examination: The patient showed limited cervical range of motion, which intensified with lateral rotation. She reported anxiety coinciding with the onset of braces application. She felt tension and dysesthesia in the region of her Pfannenstiel scar. Treatment and evolution: After injection of the oral TP, the patient reported relief of cervical pain (VAS score of 3) and increased range of motion in the neck. Two additional interventions were performed by injection into the myofascial TP of the temporomandibular joint (TMJ), suboccipital and trapezius regions, and Pfannenstiel scar, with improvement in the nearby areas of tension.
Case 3
A 48-year-old woman presented with cervical pain (VAS score of 10) and dizziness following whiplash after a car accident 6 years prior. The pain affected her daily life.
Medical history: The patient had experienced paresthesia in both hands since pregnancy and paresthesia in the left foot since a caesarean section, for which she underwent three sessions of epidural anesthesia. Dental history: The patient began orthodontic treatment 2 months after the traffic accident while still experiencing pain due to whiplash, and with upper and lower retainers having been placed after wearing braces for 18 months. The left maxillary third molar had been extracted 10 years previously. Physical examination: The patient felt substantial tension in her mouth. Treatment and evolution: The patient was nearly pain-free for several weeks after each monitoring intervention (five sessions total), in which the TPs of the oral, TMJ, trapezius, suboccipital, and left soleus muscles; lumbar fascia; and caesarean section scar were injected. Her dizziness disappeared and cervical rotation mobility increased after the initial visit. Her paresthesia in the hands and left foot improved. The patient reported great relief in her overall tension and had a tearful emotional release. She returned to the dancing activity that she had been forced to discontinue after the whiplash injury.
Case 4
A 15-year-old girl presented because of a 6-month history of knee pain (VAS score of 8). The pain appeared in conjunction with physical activity and persisted despite physical therapy.
Medical history: The patient had a history of asthma, bronchitis, allergies, and atopy. Dental history: At the age of 8 years, she underwent placement of removable appliances on the palate. From the age of 12 to 14 years, she wore fixed orthodontics with a nighttime retainer. Physical examination: TPs were present in the upper third molars, infraorbital region, and mental vestibules. Extraorally, she exhibited tension in the TMJ, suboccipital, and lumbar areas. Treatment and evolution: Immediate relief of the knee pain and TMJ tension was observed after treatment of the oral vestibules, suboccipital, and lumbar areas. The retainer was thereafter removed.
Case 5
A 16-year-old boy presented with a 1-year history of knee pain (VAS score of 6–8) (patellofemoral syndrome and tendonitis). No improvement had been observed with conventional treatment.
Medical history: The patient exhibited pain in the epigastrium with anxiety upon examination. Dental history: A palatine circuit breaker appliance was worn at the age of 11 years, followed by fixed orthodontics with extraction of the maxillary second premolar. He was wearing a fixed retainer upon presentation. Physical examination: TPs were present in the mouth, pectoral region, occipital region, and epigastrium. Treatment and evolution: Injections into the oral vestibule led to immediate disappearance of the knee pain and functional limitations. The pain returned 48 hours later; after re-injecting the same points, however, the patient remained pain-free for 48 hours. The fixed retainer was replaced with a removable nighttime retainer after consultation with the dental department. Progressive improvement was observed in each of the subsequent four monthly sessions until complete pain elimination, including resolution of the patient’s anxiety and epigastric tension.
Case 6
A 15-year-old girl presented with a 4-year history of knee pain (VAS score of 7.5) coinciding with menarche and fixed orthodontic treatment. The pain did not respond to pharmacological or physical therapy. She had been diagnosed with growing pain based on radiography and magnetic resonance imaging. At the age of 13 years, the pain had caused her to discontinue figure skating (which she had performed since 6 years of age). The pain was exacerbated with both physical activity and menstruation.
Medical history: The patient had a history of headache, otitis at 4 years of age, mild constipation, and episodes of anxiety with palpitations and painful tension in the epigastrium and iliac fossa. Dental history: She had worn a fixed orthodontic device from 11 to 13 years of age. Her third molars showed a tilted orientation. Physical examination: Tension was present in the upper, infraorbital, and mental vestibules; TMJ; epigastric region; and iliac fossa. Treatment and evolution: After injection of the TPs in the oral vestibules and TMJ, the patient experienced immediate relief of the pain in the knees and abdomen. In addition, the TPs in the epigastric and iliac fossa, third molar vestibule, and small knee scar were injected. The patient reported an absence of pain and headache as well as improved anxiety.
Discussion
We have herein described six orthodontic patients with long-term (6 months–6 years), severe (VAS score of 6–10) cervical and knee pain that was refractory to treatment. Concomitant pathologies had been ruled out, and the patients were diagnosed with chronic musculoskeletal pain. Procaine injections into the oral or myofascial TP resulted in immediate reduction (two patients) or disappearance (four patients) of the pain. In half of the patients, two to six re-interventions were required for sustained improvement. At the last assessment (3–17 months after the first visit), the pain had disappeared (VAS score of 0). In half of the patients, the orthodontic devices were removed.
Procaine was chosen as the LA because of its rapid metabolism (metabolized by plasma cholinesterases), high safety profile, low neurotoxicity, sympatholytic effects, favorable effects on the microcirculation, and short half-life. 21 The modulating effects of LAs on the immune system have been described in the medical literature. The main mechanisms of action that have been proposed are leukocyte inhibition, decreased inflammatory mediators, and decreased vascular hyperpermeability and edema formation.13,22,23
Chronic pain can be a secondary effect of inflammation, structural changes affecting the musculoskeletal system, or diseases of the motor nervous system (e.g., spasticity after nerve injury or stiffness due to disease). 1 We hypothesize that one physiological mechanism of the improvement observed in this case series was associated with the mechanism of mechanotransduction, which is a complex, dynamic process that converts biomechanical stimuli into intracellular biochemical signals that induce tissue responses. Disruption of mechanotransduction can lead to a variety of diseases and disorders, such as chronic pain. 24 The compression, traction, and deformation forces exerted by orthodontics can generate large mechanical load patterns that translate into biological responses in the tissues surrounding the teeth. 25
Myofascial trigger points or articular dysfunction can arise from nociceptive stimuli from the skin, internal organs, or musculoskeletal system via spinal reflexes. The musculature reacts as a polysegmentally interconnected cross-segmental functional chain. 12 The identification of trigger points is based on palpation. 26
The role of oral tension in musculoskeletal pain in the back and lower extremities has been previously described. 10 Several studies have investigated the paravertebral musculature in patients with chronic low back or neck pain. 27 The paraspinal muscles support neck movements and provide stability to the cervical spine. Weakness of these muscles can cause neck pain. 27 In a study of rats, the responsiveness of lumbar neurons increased in patients with multifidus muscle pathology, such as chronic inflammation, and this may be relevant to some cases of chronic spinal pain. 28 The multifidus muscle is a polysegmented transversospinal muscle extending from the sacrum to the axis, and it is innervated by the posterior branches of adjacent spinal nerves; it contributes to spinal posture and stabilizes the movements of each vertebra. In a randomized double-blind controlled trial of patients with chronic low back pain, neurostimulation of the dorsal branch nerve (multifidus nerve supply) resulted in significant clinical improvement. 29
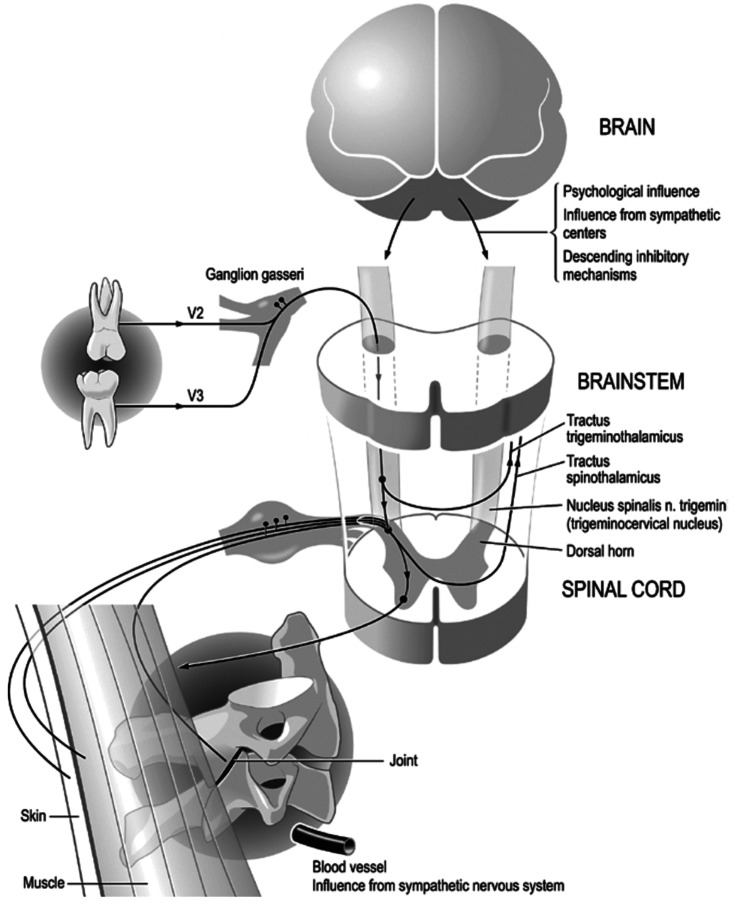
Stimulatory and inhibitory responses have been described in the cervical muscles following trigeminal nerve stimulation. Percutaneous stimulation of the infraorbital nerve with a single stimulus evokes responses of the splenius and sternocleidomastoid muscles, which are innervated by the posterior branches of the cervical nerves and by the accessory nerve (sternocleidomastoid). This response is significantly enhanced by double or repetitive stimuli. 30 The structures of the oral cavity are mostly innervated by the trigeminal nerve but also by the facial, accessory, hypoglossal, glossopharyngeal, and vagus nerves. The first four of these also innervate the neck muscles. A recent description of the trigeminal-cervical complex provides further clarification of these connections. 12 Tusita and Fischer 19 described a patient with chronic neck pain that did not respond to conventional treatment. The patient had apparently asymptomatic wisdom teeth with spacing problems. The neck pain significantly improved after removal of the wisdom teeth and subsequent injections of procaine into the extraction sites. Figure 1 shows the neuroanatomic relationship between teeth, trigeminal structures, and the cervical area. 19
Figure 1.
Illustration of the neuroanatomy relevant to the cases reported. Because of the convergence of the afferents from different cranial nerves (V, VII, IX, and X) as well as from the upper cervical nerves, it is evident that in the upper cervical medulla, there is a correspondence or fusion between the spinal trigeminal nuclei and the column of the head of the posterior horn. The diagram illustrates how conditions of the teeth and related structures of the trigeminal area can influence the muscular and articular structures of the cervical spine (pain, tension, and blockage). These processes are amplified by feedback mechanisms via the brainstem and brain. In parallel, the sympathetic nervous system can also affect pain, inflammation, and microcirculation. Figure obtained from article by Tusita and Fischer 18 and presented with permission from the authors.
Previous research has indicated a significant reduction in the sensitivity of the trapezius region in patients with chronic neck pain, trapezius tension, and chronic pharyngeal irritation after receiving bilateral submucosal injections of 0.5 mL of 1% procaine in the pharyngeal region. 31 We identified several case reports on the therapeutic effects of LA injections in patients with chronic pain, in addition to their anesthetic effects.13–15,17,32 Nociceptive processes cause a reflex response that is largely mediated by sympathetic nerves and involves changes in blood flow, increased skin turgor and muscle tone, hyperalgesia in localized areas of the skin, and organ dysregulation at corresponding dermatome levels. 14 Sympathetic–afferent coupling and neuroplastic changes within the spinal cord and brain create several other potential mechanisms for creating or exacerbating pain. The sympathetic nervous system can induce neurogenic inflammation through vasodilation, plasma extravasation, and the release of proinflammatory neuropeptides from its own nerve fibers. Inflammation reduces the nociceptor response threshold and simultaneously recruits inactive or “silent” nociceptors. Thus, peripheral sensitization reinforces this pain cycle.13,14
LA injection can alleviate pain through the needle stimulation of somatic nerve fibers, inducing a presynaptic brake. Additionally, the sympatholytic effect of Las contributes to closure of the pain gate as proposed by Egli et al. 14 This helps disrupt the cycle of nociceptor activation, sympathetic excitation, vascular dysregulation, neurogenic inflammation, and muscle tension at different sites simultaneously.12,13,15 Repeated use of LAs can also directly reduce neurogenic inflammation.13,14 Injections into multiple specific points appear to reduce pain more effectively than an injection into a single site. LA injections can reduce pain intensity for several weeks.13,15
In three of our patients, after pain recurred following LA injection, dental adjustments were made on the advice of the dentist (removal of a fixed retainer in one patient, discontinuation of a nighttime retainer in another, and transition from a fixed appliance to a nighttime retainer in the third). Although combined dental adjustments and LA injections resulted in prolonged relief, we cannot confirm a direct relationship between retainer adjustments and musculoskeletal pain reduction. A dedicated clinical trial without interventions such as procaine injections would be necessary to establish this. Central sensitization caused by persistent and high-intensity pain can lead to anxiety, headaches, other pain disorders, and reduced quality of life. In these cases, the observed reduction in concomitant symptoms such as headaches, paresthesia, and anxiety may be attributed to the sustained minimization of pain.
As with any case series, this study has limitations. The participants were patients who came to our clinic after receiving previous treatments, such as physiotherapy, osteopathy, or prescription drugs. Longer follow-up would allow assessment of long-term effects or recurrence of symptoms, although treatment lasted 6 months and all six patients with a history of chronic pain lasting 6 months to 10 years experienced pain relief during this period. Improved diagnostic and longitudinal protocols would further strengthen our evaluations. Although our patients showed significant improvements and the potential underlying mechanisms have been elucidated, a case series alone cannot confirm a causal relationship between orthodontic treatment and cervical or knee pain. These findings emphasize the need for clinical trials to further explore this relationship and assess the efficacy of the intervention.
Conclusion
We have reported the long-term remission of pain in six orthodontic wearers with chronic neck pain or knee pain after injection of an LA (procaine) into sites of injury or points of palpable tissue tension according to the patient’s medical history (neural therapy). Within an average of three treatment sessions, their pain disappeared for a minimum of 6 months with no observed side effects. Future randomized clinical trials could help elucidate the role of this intervention in orthodontic-wearing patients with chronic musculoskeletal pain.
Acknowledgements
We appreciate the patients’ participation. We thank Richard Nahas, MD for his critical review of the manuscript and valuable suggestions regarding language. We also thank Lucy Naomi Shiratori Tusita, DDS and Lorenz Fischer, PhD for permission to use the figure in this article.
Author contributions: All authors contributed to the literature review, interpreted the data, and drafted and critically revised the manuscript. David Vinyes performed the care and follow-up of the patients. All authors read and approved the submitted version of the manuscript.
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement
Data are available on request from the authors.
References
- 1.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019; 160: 19–27. [DOI] [PubMed] [Google Scholar]
- 2.Araya EI, Baggio DF, Koren LO, et al. Acute orofacial pain leads to prolonged changes in behavioral and affective pain components. Pain 2020; 161: 2830–2840. [DOI] [PubMed] [Google Scholar]
- 3.Miller VE, Chen DG, Barrett D, et al. Understanding the relationship between features associated with pain-related disability in people with painful temporomandibular disorder: an exploratory structural equation modeling approach. Pain 2020; 161: 2710–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn DM. Chronic musculoskeletal pain: nonpharmacologic, noninvasive treatments. Am Fam Physician 2020; 102: 465–477. [PubMed] [Google Scholar]
- 5.Zhang F, Li F, Yang H, et al. Effect of experimental orthodontic pain on gray and white matter functional connectivity. CNS Neurosci Ther 2021; 27: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos LA, Santos-Pinto A, Marôco J, et al. Pain perception in orthodontic patients: a model considering psychosocial and behavioural aspects. Orthod Craniofacial Res 2019; 22: 213–221. [DOI] [PubMed] [Google Scholar]
- 7.Ayoub LJ, McAndrews MP, Barnett AJ, et al. Baseline resting-state functional connectivity determines subsequent pain ratings to a tonic ecologically valid experimental model of orofacial pain. Pain 2021; 162: 2397–2404. [DOI] [PubMed] [Google Scholar]
- 8.Totolini P, Fernández-Bondereau E. Orthodontics and periodontics. Av Odontoestomatol 2011; 27: 197–206. [Google Scholar]
- 9.Torisu T, Tanaka M, Murata H, et al. Modulation of neck muscle activity induced by intraoral stimulation in humans. Clin Neurophysiol 2014; 125: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 10.Kraus S. Temporomandibular disorders, head and orofacial pain: cervical spine considerations. Dent Clin North Am 2007; 51: 161–193. [DOI] [PubMed] [Google Scholar]
- 11.Pedroni CR, De Oliveira AS, Bérzin F. Pain characteristics of temporomandibular disorder - a pilot study in patients with cervical spine dysfunction. J Appl Oral Sci 2006; 14: 388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel R, Barop H, Giebel J, et al. The influence of modern neurophysiology on the previous definitions of “segment” and “interference field” in neural therapy. Complement Med Res 2022; 29: 257–267. [DOI] [PubMed] [Google Scholar]
- 13.Vinyes D, Muñoz-Sellart M, Fischer L. Therapeutic Use of Low-Dose Local Anesthetics in Pain, Inflammation, and Other Clinical Conditions: A Systematic Scoping Review. J Clin Med 2023; 12: 7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egli S, Pfister M, Ludin SM, et al. Long-term results of therapeutic local anesthesia (neural therapy) in 280 referred refractory chronic pain patients. BMC Complement Altern Med 2015; 15: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinyes D, Muñoz-Sellart M, García-Caballero T. Local anesthetics as a therapeutic tool for post COVID-19 patients. Medicine (Baltimore) 2022; 101: E29358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer L, Ludin SM, Puente de la Vega K, et al. Neuralgia of the glossopharyngeal nerve in a patient with posttonsillectomy scarring: recovery after local infiltration of procaine—case report and pathophysiologic discussion. Case Rep Neurol Med 2015; 2015: 560546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lóriz Peralta O, Raya Rejón A, Pérez Morales D, et al. Intervention study on subacute and chronic pain in primary care: an approach to the effectiveness of neural therapy. Aten Primaria 2011; 43: 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinschenk S, Hollmann MW, Göllner R, et al. Injections of local anesthetics into the pharyngeal region reduce trapezius muscle tenderness. Forsch Komplementmed 2016; 23: 111–116. [DOI] [PubMed] [Google Scholar]
- 19.Tusita LNS, Fischer L. Chronic therapy-resistant neck pain in a fifty-year-old man. The role of partially impacted third molars. Case report and new pathophysiological insights. Complement Med Res 2023; 30: 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagnier JJ, Kienle G, Altman DG, et al. ; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 21.Weinschenk S, Weiss C, Benrath J, et al. Anti-inflammatory characteristics of local anesthetics: inhibition of TNF-α secretion of lipopolysaccharide-stimulated leucocytes in human blood samples. Int J Mol Sci 2022; 23: 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand 2006; 50: 265–282. [DOI] [PubMed] [Google Scholar]
- 23.Hollmann MW, Durieux ME, Fisher DM. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 2000; 93: 858–875. [DOI] [PubMed] [Google Scholar]
- 24.Delmas P, Coste B. Mechano-gated ion channels in sensory systems. Cell 2013; 155: 278–284. [DOI] [PubMed] [Google Scholar]
- 25.Feller L, Khammissa RA, Schechter I, et al. Periodontal biological events associated with orthodontic tooth movement: the biomechanics of the cytoskeleton and the extracellular matrix. Sci World J 2015; 2015: 894123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah JP, Thaker N, Heimur J, et al. Myofascial trigger points then and now: a historical and scientific perspective. PM R 2015; 7: 746–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun Y, Lee EJ, Kim Y, et al. Asymmetric atrophy of cervical multifidus muscles in patients with chronic unilateral cervical radiculopathy. Medicine (Baltimore) 2019; 98: E16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taguchi T, Hoheisel U, Mense S. Dorsal horn neurons having input from low back structures in rats. Pain 2008; 138: 119–129. [DOI] [PubMed] [Google Scholar]
- 29.Gilligan C, Volschenk W, Russo M, et al. An implantable restorative-neurostimulator for refractory mechanical chronic low back pain: a randomized sham-controlled clinical trial. Pain 2021; 162: 2486–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leandri M, Gottlieb A, Cruccu G. Head extensor reflex evoked by trigeminal stimulation in humans. Clin Neurophysiol 2001; 112: 1828–1832. [DOI] [PubMed] [Google Scholar]
- 31.Weinschenk S, Göllner R, Hollmann MW, et al. Inter-rater reliability of neck reflex points in women with chronic neck pain. Forsch Komplementmed 2016; 23: 223–229. [DOI] [PubMed] [Google Scholar]
- 32.Rey Novoa M, Muñoz-Sellart M, Catalán Soriano M, et al. Treatment of localized vulvar pain with neural therapy: a case series and literature review. Complement Med Res 2021; 28: 571–577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.