Abstract
Risks to health are the prime consideration in all human situations of ionizing radiation exposure and therefore of relevance to radiation protection in all occupational, medical, and public exposure situations. Over the past few decades, advances in therapeutic strategies have led to significant improvements in cancer survival rates. However, a wide range of long-term complications have been reported in cancer survivors, in particular circulatory diseases and their major risk factors, metabolic diseases. However, at lower levels of exposure, the evidence is less clear. Under real-life exposure scenarios, including radiotherapy, radiation effects in the whole organism will be determined mainly by the response of normal tissues receiving relatively low doses, and will be mediated and moderated by systemic effects. Therefore, there is an urgent need for further research on the impact of low-dose radiation. In this article, we review radiation-associated risks of circulatory and metabolic diseases, addressing epidemiological, biological, risk modelling, and systems biology aspects, highlight the gaps in knowledge and discuss future directions to address these gaps.
Keywords: ionizing radiation, circulatory disease, heart, endothelial, metabolic syndrome, radiotherapy, systems biology
1. Introduction
The general goal of this review is to have a comprehensive quantitative and mechanistic understanding of all radiogenic health effects, especially at low doses. Dose limits in radiation protection are based on knowledge of radiation cancer risk derived from epidemiological studies and assumed risk of heritable effects in humans (ICRP 2007). Epidemiologically-derived health risk estimates are limited in power below 100 mSv; depending on the cancer type, the applied models for risk inference can be linear or linear quadratic. However, for risk management purposes, it is a linear non-threshold (LNT) model that is applied, justified on the basis of a biologically plausible argument that relates direct damage to nuclear DNA to mutations in specific genes that drive carcinogenesis.
In addition to cancer risks, there is increasing evidence for risk of non-cancer conditions, notably circulatory disease, cataracts and neurological effects in response to low and moderate radiation doses. Defining the risks of non-cancer diseases at low and moderate dose levels would be important for protection of people and the system of radiation protection. The identification of such risks at low doses could lead to reconsideration of radiation detriment calculations, tissue weighting factors, dose limits, and reference levels. Furthermore, there might also be a need to consider additional protection measures.
In the context of the Multidisciplinary European Low Dose Initiative (MELODI), low doses and/or low dose rates refer to a range of acute and/or protracted exposures of ionizing radiation (IR) that are typical of those encountered in the workplace, the environment and in diagnostic and therapeutic medicine. Doses below 100 mSv (100 mGy for X-rays and gamma rays) are considered low. Moderate doses, typically in the order of 100–1000 mSv, refer to doses that may be encountered by normal tissues in interventional radiology, radiotherapy (RT) or in nuclear or radiological accidents. Doses above 1000 mSv are considered high and may cause symptoms of acute radiation sickness when received as a total body exposure during a short period of time. Low dose rates were defined by the United Nations Scientific Committee on Effects of Atomic Radiation (UNSCEAR) as dose rates smaller than 0.1 mGy/min averaged over an hour or smaller than or equal to 6 mGy/h averaged over a few hours, consistent with the BEIR VII report (BEIR 2006) and others, as reviewed recently (Ruhm et al. 2015). In the radiation protection context, annual dose rates below 100 mSv may be considered low.
The aim of the current paper is to review the state-of-the-art of radiation-induced circulatory and metabolic diseases, present research gaps and recommend research strategies. The review is based on presentations and discussions during the workshop on “Non-cancer effects of ionizing radiation” which took place in Sitges, Spain, in April 2019. The workshop was organized by MELODI together with CONCERT, European Joint Programme for the Integration of Radiation Protection Research. The participants discussed clinical and epidemiological studies, experimental models, mechanistic approaches, and dosimetric aspects, judging the quality and quantity of information available. A wide range of long-term complications have been reported among cancer survivors treated by RT, in particular cardiovascular diseases (CVD) and metabolic diseases that are major risk factors for circulatory diseases. Here, we focus on the question of low-dose effects and consider potential mechanisms of radiation action. The objective of the Sitges workshop was to provide input for the European roadmap for research in these areas, summarizing the research needs, giving recommendations on research needed, required resources anticipated and the expected timespan for studies that could be expected to yield considerable progress in this area. Furthermore, potential implications for the system of radiation protection were discussed. As for the resources required and timespan needed, a joint roadmap was developed by MELODI in collaboration with other European radiation protection research associations after the Sitges workshop (CONCERT et al. 2020).
2. Radiation-associated circulatory effects, overview of the epidemiology and the role of lifestyle factors
The risks of radiation-associated circulatory disease have been observed in therapeutically- or diagnostically-exposed cohorts even at low doses. Similarly, occupationally- and environmentally-exposed groups and the Life Span Study (LSS) and related Adult Health Study (AHS) of Japanese atomic bomb survivors show increased circulatory risk. Ischaemic heart disease and cerebrovascular disease are mostly caused by atherosclerosis and may lead to acute myocardial infarction and stroke. In the following section, the attention will be concentrated on studies with high quality individual organ dosimetry, based on the previous systematic reviews (Little et al. 2012a; Little et al. 2008), which have been updated for the present paper, based in part on updates, but also on previously reported (non-systematic) reviews of the moderate/low-dose literature (Little 2013; Little and Lipshultz 2015); similar to a recent review (Little 2016) the organ or tissue dose range that is to be considered is not constrained.
2.1. Therapeutically exposed groups
Recent studies have suggested radiation-associated excess morbidity and mortality in groups of childhood cancer survivors (Table S1) (El-Fayech et al. 2017; Haddy et al. 2016; Mulrooney et al. 2009; Tukenova et al. 2010a). The RT organ dosimetry is of variable quality, but higher in the French childhood cancer cohort (El-Fayech et al. 2017; Haddy et al. 2016; Tukenova et al. 2010b), in that it is fully individualized, based on Monte Carlo reconstructions derived from individual treatment records (Diallo et al. 1996; Shamsaldin et al. 1998). The US study adjusted for tobacco use (Mulrooney et al. 2009), and although data on smoking and body mass index was available in some studies (El-Fayech et al. 2017; Haddy et al. 2016), adjustment for these did not materially alter results. In addition, none of these four studies corrected for known risk factors for circulatory disease. There have been a number of studies of circulatory disease in Hodgkin lymphoma (HL) patients, (Cutter et al. 2015; Hahn et al. 2017; Maraldo et al. 2015; van Nimwegen et al. 2017; van Nimwegen et al. 2016), as well as studies focusing on circulatory disease in lung cancer (Dess et al. 2017; Wang et al. 2017a; Wang et al. 2017b) and breast cancer patients (Darby et al. 2013; Jacobse et al. 2019), doses in both of which tend to be lower than in the HL patients. Most of these studies suggest modest but generally highly significant radiation-associated excess risk (Table S1).
Doses in the US peptic ulcer patients (Little et al. 2012b) were generally lower than in any of the cancer studies and documented significant excess mortality risks for all circulatory disease and ischaemic heart disease (IHD) and indications of excess risk for cerebrovascular disease (CeVD). Doses to a number of different target tissues, specifically heart, thyroid, kidney, pancreas, and brain, were used to assess radiation effects. Using thyroid dose (a surrogate for dose to the carotid artery) for CeVD and heart dose for other circulatory disease endpoints resulted in significant heterogeneity of risk between endpoints, which was not the case when heart dose was used throughout (Little et al. 2012b). Using brain or thyroid dose resulted in somewhat higher risks for CeVD, the risk being particularly high for brain dose. As noted by Little et al., “one limitation of the study is that the radiation dosimetry, although of high quality in many respects, fails to account for variability in patient anatomy, e.g., the heart size/shape/position and its relation to the diaphragm and stomach” (Little et al. 2012b).
Many of these studies have a rich set of lifestyle and environmental risk factors, including many of the known risk factors for circulatory disease, in particular the childhood cancer studies (El-Fayech et al. 2017; Haddy et al. 2016; Mulrooney et al. 2009), the Netherlands HL studies (Cutter et al. 2015; van Nimwegen et al. 2017; van Nimwegen et al. 2016), the Nordic breast cancer study (Darby et al. 2013) and the US peptic ulcer study (Little et al. 2012b), and adjustment for these did not materially alter results.
The risks suggested by these studies are generally consistent with each other, and with those in the diagnostically and other, lower-dose, studies; a possible exception is the French-UK study (Tukenova et al. 2010b), where risk is much higher than for many of the other studies considered. The discrepancy with other studies (e.g., of adult exposure) may reflect the younger exposure age or younger age at follow-up in this group, although this would not explain the discrepancy with risks in the US childhood-cancer survivor study (Mulrooney et al. 2009). Excess relative risk (ERR) of circulatory disease in the Japanese LSS cohort is significantly modified by attained age (Little et al. 2012a; Shimizu et al. 2010). The fact that the ERR in relation to cumulative heart dose or in relation to equivalent doses delivered in 2-Gy fractions (EQD2) in the Nordic breast cancer study (Darby et al. 2013) agrees well with those in many other radiation-exposed groups (Little 2016), suggests that either of these measures (cumulative heart dose, EQD2 heart dose) may be relevant for this outcome (IHD) (Little et al. 2013). The fact that risks evaluated using brain dose for CeVD in the US peptic ulcer study yielded much higher risks than those observed using heart or thyroid dose, or in the LSS (Little et al. 2012a; Shimizu et al. 2010), in which doses are more or less uniform whole body, suggests that, assuming the risks in the two cohorts are to be consistent, brain may not be the most relevant organ for CeVD.
2.2. Diagnostically exposed groups
The two major studies of circulatory disease mortality in relation to medical diagnostic exposure are both of groups that received repeated fluoroscopic doses as part of the lung collapse treatment for tuberculosis (TB), in Canada (Zablotska et al. 2014) and in Massachusetts (Little et al. 2016). A pooled analysis of the two datasets has recently appeared (Table A1) (Tran et al. 2017). Although there is no dose-response overall in the pooled data, if analysis is restricted to persons with < 0.5 Gy, the dose response trends (ERR/Gy) for all circulatory disease and IHD become much steeper, and statistically significant. This observation is consistent with the findings from a recent meta-analysis (Little 2016) and also from findings in the LSS discussed below. In both cohorts there is limited medical and lifestyle information. This is more extensive in the Massachusetts data, and includes smoking and alcohol consumption, thoracoplasty, and pneumolobectomy; some of these variables were included in baseline models for certain disease endpoints (Little et al. 2016).
2.3. Non-medically exposed groups
2.3.1. Japanese atomic bomb survivors
Excess radiation-associated mortality from heart disease and stroke has been observed in the LSS cohort (Shimizu et al. 2010; Takahashi et al. 2017), also for stroke among those exposed in early childhood (Tatsukawa et al. 2008). In the latest follow-up of the Adult Health Study (AHS), a subset of the LSS cohort subject to biennial clinical examinations showed no radiation-associated excess risks of hypertension or myocardial infarction morbidity (Table S2) (Yamada et al. 2004). Analysis within the AHS of those exposed in early childhood showed a significantly increased incidence of non-fatal stroke or myocardial infarction, although there was no excess risk among those exposed in utero for whom the average exposures were much lower (Tatsukawa et al. 2008). Recent analysis of the Japanese LSS data taking account of dose measurement error suggested downward curvature in the dose response for all circulatory disease, so that the ERR/Gy is higher at lower doses (Little et al. 2020).
2.3.2. Occupationally exposed groups
The International Agency for Research on Cancer 15-country study of radiation workers found increasing dose-related trends for mortality from all circulatory diseases and CeVD, and decreasing trends for IHD, heart failure, deep vein thrombosis, and pulmonary embolism (Vrijheid et al. 2007), although none of these trends was statistically significant. Further follow-up of the UK, US and French cohorts from this dataset has been recently published. This study suggests significant radiation-associated increases in risk for all circulatory disease, IHD and CeVD, with significant downward curvature in the dose response for CeVD, but no curvature for the other endpoints (Table S2) (Gillies et al. 2017). It would appear from the results of the component workforces that make up this study that the significant trends are largely driven by those in the UK (Azizova et al. 2018; McGeoghegan et al. 2008; Muirhead et al. 2009), the trends in the US and French data being generally null (Leuraud et al. 2017; Schubauer-Berigan et al. 2015); however, there are only borderline significant indications of variation by country (Gillies et al. 2017).
Radiation-associated excess circulatory disease, IHD and CeVD morbidity were observed in Chernobyl recovery workers, although morbidity from hypertensive heart disease and other heart disease was not increased (Ivanov et al. 2006; Kashcheev et al. 2017; Kashcheev et al. 2016). The analysis of circulatory disease mortality in this cohort was based only on comparison with external circulatory disease rates, via use of standardized mortality ratios (Ivanov et al. 2001). As such, this analysis almost certainly yields biased estimates of risk, as the general Russian population is very likely not representative of the Chernobyl recovery workers, because of generally observed healthy-worker selection effects (Bell and Coleman 1987; Doll et al. 1965).
For IHD and CeVD in the Mayak workers there was a highly statistically significant trend with dose for morbidity, but the trend of IHD and CeVD mortality was less clear, and generally not statistically significant. There have been a number of analyses of the Mayak worker cohort in the last few years (Azizova et al. 2018; Azizova et al. 2015a; Azizova et al. 2015b; Azizova et al. 2014; Moseeva et al. 2014; Simonetto et al. 2014; Simonetto et al. 2015), based on a similar underlying dataset, but with slight variations in disease endpoints, year of follow-up and dosimetry system. The study is unusual in that doses to certain internal organs, especially the lung and liver, were dominated by doses from internally deposited radionuclides, especially plutonium. Doses in this study are among the highest for the occupationally-exposed groups considered in this section, and arguably more comparable with at least the medical-diagnostic or even the RT-exposed groups considered above: average total body doses for external γ rays were 0.5 to 0.6 Gy. However, unlike the partial-body doses received from RT, or even those in the tuberculosis fluoroscopy cohorts, the external whole-body doses received by the Mayak workers generally accumulated over a long time with an average dose rate of <5 mGy/hour, so must it be considered a low dose-rate exposure (Wakeford and Tawn 2010).
2.3.3. Environmentally exposed groups
A study of a cohort of environmentally exposed individuals in the Southern Ural Mountains reported a statistically significant or borderline significant increase (depending on the latent period used) of both all circulatory disease mortality and IHD mortality (Table S2) (Krestinina et al. 2013).
Circulatory disease mortality was studied in a Kazakhstan group exposed to fallout from nuclear weapons tests at the Semipalatinsk site (Grosche et al. 2011). No excess circulatory disease risk was reported in the group of exposed settlements, although if exposed and unexposed settlements were analysed together, the excess risks were highly statistically significant and implausibly large. The dosimetry in this cohort is problematic because it is based on assessments of residence, estimates of time spent outdoors, and diet, all of which were collected by interviews more than 30 years after the bomb tests. As such, the results of this study may be less informative than others considered here.
2.4. Lifestyle factors as targets for intervention
The lifestyle factors are major contributors to circulatory diseases illustrated by an example from North Karelia, eastern province in Finland. The extremely high cardiovascular mortality caused great concern among the local population. In response, the North Karelia project was launched in 1972 to carry out a comprehensive community-based prevention program, as reviewed recently (Vartiainen 2018). The main aim to reduce the high serum cholesterol, blood pressure and smoking levels with lifestyle changes and improved drug treatment, especially for hypertension, was successful. Coronary mortality reduced in the middle-age population by 84% from 1972 to 2014. About 2/3 of the mortality decline was explained by risk factor changes and 1/3 by improvement of new treatments developed since 1980s. This example illustrates how important it is to collect information on lifestyle factors in any radiation epidemiological studies addressing risk of circulatory diseases.
2.5. Genetic susceptibility to circulatory diseases
Identifying genetic predisposition to circulatory disease could help understanding the disease process and thereby feed into the research strategies investigating the role of radiation in the pathogenesis. Genome-wide association studies (GWAS) have identified over 200 genome-wide significant and suggestive risk loci that modulate the risk of coronary artery disease (CAD) (Nikpay et al. 2015). Several of the genes implicated to date in large-scale analyses of CAD susceptibility encode proteins with a known role in the biology of circulating risk factors for CAD, notably lipid levels and the metabolism of lipoproteins; others relate to additional atherosclerosis risk factors, such as genes implicated in systemic inflammation and in hypertension. Interestingly, genes involved in DNA damage response pathways do not show up in these GWAS studies. Almost all risk variants identified by GWAS are commonly found in the European population, and average European carries tens of these risk alleles (Kessler et al. 2016). However, most CAD-associated single nucleotide polymorphisms (SNPs) are located in non-coding regions of the genome and do not have known biological roles (Kovacic and Bakran 2012). This suggests that these variants are more likely to affect gene regulation rather than protein structure.
So far, there is lack of GWAS studies looking for loci predisposing to circulatory disease among radiation-exposed populations. Circulatory disease is considered as a late-appearing tissue reaction, having a latency of up to decades after the radiation exposure (ICRP 2012). GWAS studies among patients treated with RT have looked for loci associated with the development of toxicity in normal tissues in the months or few years following RT, focusing on tissue toxicity endpoints that are typical for the affected area and causing functional impairment. As reviewed recently, RT for breast or lung cancer inevitably leads to the exposure of the heart and major blood vessels in the chest and neck area (Rosenstein 2017). In the radiogenomics studies on breast cancer, associations with adverse toxic outcomes have been reported for SNPs related to the Tgfb1 gene (a multipotent growth factor affecting cell differentiation, proliferation, apoptosis and matrix production) or the Tnfa (a cell signalling cytokine involved in systemic inflammation), as well as genes whose products are involved with responses to oxidative stress. Furthermore, a SNP in Xrcc1 that plays a role in base excision repair of oxidative damage produced by radiation was found to be associated with risk for skin toxicities. Toxicities related to the RT of the lung have revealed associations with heat shock proteins, e.g. HSP27, that increase cellular resistance to heat shock, oxidative stress and inflammatory mediators. Inflammation and oxidative stress are among the mechanisms implicated in the development of circulatory disease after radiation exposure, with inflammation emerging from GWAS studies as a common factor associated with circulatory diseases and tissue toxicities with or without radiation exposure.
There is increasing but still limited experimental evidence for genetic susceptibility to radiation-induced circulatory disease in mouse models (Foray et al. 2016). Irradiation accelerated the development of atherosclerotic lesions in apolipoprotein E deficient (ApoE−/−) mice (Stewart et al. 2006) which may have implications for atherosclerosis-prone individuals. Accumulation of p53 was required for the transition from cardiac hypertrophy to heart failure (Sano et al. 2007) and p53 deficiency in endothelial cells caused myocardial injury and heart failure after irradiation (Lee et al. 2012). ApoE−/− p53+/− mice showed the accelerated progression of spontaneous and radiation-induced atherosclerosis compared with ApoE−/− p53+/+ (Mitchel et al. 2013). Mice lacking p21 were predisposed to radiation-induced myocardial injury (Lee et al. 2012). Deficiency of Wip1 phosphatase, a negative regulator of the protein kinase ataxia-telangiectasia mutated (ATM) signalling, prevented atherosclerosis (Le Guezennec et al. 2012) but deficiency of ATM accelerated atherosclerosis in ApoE−/− mice (Schneider et al. 2006). These suggest that ATM, p53, and p21 may play a protective role in atherogenesis.
3. Radiation-associated circulatory effects, overview of the biology
High doses of IR increase the risk of CVD, with damage to coronary arteries (atherosclerosis) and microvascular insufficiency frequently observed after high-dose irradiation (Adams et al. 2003; Demirci et al. 2009; Tapio 2016). Endothelial cells (EC), a monolayer forming the inner coating of all vasculature, play an important role in these pathologies. EC form a barrier between blood and the sub-endothelial matrix and modulate blood clotting, vascular tone, and immune and inflammatory response (Luscher et al. 1990; Marsden et al. 1991). High-dose IR induces endothelial dysfunction that is characterized by pro-inflammatory and pro-fibrotic state with premature endothelial senescence, increased oxidative stress, reduced levels of nitric oxide (NO), and enhanced adhesiveness based on high expression of selectins, integrins, and other cellular adhesion molecules such as VCAM, ICAM, and PECAM (Azimzadeh et al. 2015; Philipp et al. 2017; Sievert et al. 2015; Yentrapalli et al. 2013a; Yentrapalli et al. 2013b). Although much less is known about the possible adverse effects of low-dose IR, some recent data suggest that biological mechanisms may differ depending on the dose in a nonlinear manner (Averbeck et al. 2018).
3.1. Cellular studies
Increased adhesion and leukocyte migration through the endothelium is the first step in atherosclerosis. Using human coronary artery endothelial cell line (HCAEC) as a model, increased adhesion of monocytes and release of pro-inflammatory cytokines (Interleukin-6 and IL-8) was observed at the dose of 2 Gy X-rays (Baselet et al. 2017a). This effect became significant seven days after the exposure. However, when cells were exposed to iron ions (2 Gy, 170 keV/μm) a decreased adhesion of monocytes and no effect on the release of pro-inflammatory cytokines was observed (Baselet et al. 2017a). Both X-rays and iron ions resulted in a marked decline of cell numbers in the confluent EC monolayer.
The radiation exposure of human umbilical vein endothelial cells (HUVEC) to single doses of X-rays (≥2 Gy) and carbon ions (≥0.1 Gy) resulted in significantly increased expression of ICAM-1 protein, which was partially mediated by TGF-beta1 (Kiyohara et al. 2011). Even lower X-ray doses have shown similar effects, and exposure to single X-ray doses of 0.125, 0.25 and 0.5 Gy or fractionated doses of 2 x 0.125 Gy and 2 x 0.25 Gy, with 24-hour interfraction interval, resulted in significantly increased ICAM-1 protein expression and enhanced adhesion of peripheral blood mononuclear cells (PBMC) 18 hours post-irradiation (Cervelli et al. 2014). A simultaneous enhancement of intracellular reactive oxygen species (ROS) generation and activation of NF-κB via p65 phosphorylation was observed. These doses neither triggered apoptosis nor had any effect on viability. This study suggests that even fractionated low-dose radiation may accelerate chronic vascular inflammation preceding the atherosclerotic process.
However, if the EC are in an inflammatory state at the time of radiation exposure, low-dose irradiation is known to have an anti-inflammatory effect. This low-dose effect is being used in the X-irradiation therapy that has been clinically applied for several decades especially in Germany in the treatment of non-malignant inflammatory and degenerative diseases (Ott et al. 2014; Seegenschmiedt et al. 2004). To further clarify this mechanism, the endothelial cell line EA.hy926 was grown to confluence, stimulated using TNF-α to induce a pro-inflammatory state and irradiated 4 hours later using X-ray doses between 0 and 1 Gy (Large et al. 2015; Large et al. 2014). The adhesion of PBMC showed a nonlinear significant reduction at 0.5 Gy, returning to elevated levels at higher doses. This reduction coincided with a decreased expression and activity of oxidative stress proteins glutathione peroxidase, superoxide dismutase 1, and nuclear factor erythroid 2–related factor 2 (Nrf2) suggesting an enhanced ROS production at 0.5 Gy. Indeed, scavenging of ROS by N-acetyl-L-cysteine or activation of Nrf2 by its activator molecule AI-1 significantly restored adhesion of PBMC to EC at the dose of 0.5 Gy. A similar effect was seen with primary human dermal microvascular EC (Large et al. 2015), implying an anti-inflammatory effect at doses around 0.5 Gy.
In addition to increased adhesion and production of ROS in EC, the reduction of NO, responsible for the maintenance of basal vasodilator tone, is a hallmark of EC dysfunction (Sandoo et al. 2010). An immediate (1 day) radiation-induced decline in the activity of endothelial NO synthase was followed by a late (14 days) reduction on the level of NO for a dose as low as 0.5 Gy (X-rays) in HCAEC (Azimzadeh et al. 2017b). The late reduction of NO coincided with the reduced expression of the antioxidant protein glutathione S-transferase omega-1 and increased levels of oxidized proteins, suggesting that increased production of ROS was coupled to reduced levels of NO. Downregulation of proteasome activity, increased protein ubiquitination and enhanced expression of senescence markers p16INK4a and p21WAF1 was also observed 14 days post-irradiation (Azimzadeh et al. 2017b). Furthermore, the phosphorylation of RhoGDI, a modulator of rho family G proteins, was reduced 24 hours after irradiation, thus leading to the induction of RhoA signalling.
A rapid activation of the RhoA pathway was also seen in EA.hy926 cells for doses as low as 0.2 Gy (Co-60 gamma rays). Activated RhoA signalling is known to result in increased cell adhesion and permeability (Beckers et al. 2010; Wojciak-Stothard and Ridley 2002). Previous data further suggest that RhoA activation by inflammatory cytokines and agonists of G-protein-coupled receptors triggers vascular disease (Seasholtz and Brown 2004). Indeed, Rho kinase inhibitors have been shown to have therapeutic potential in prevention and treatment of CVD (Shimokawa and Takeshita 2005).
Transcriptomic profiling of HCAEC revealed an immediate induction of pro-inflammatory genes 24 hours after exposing the cells to 0.5 Gy or 2.0 Gy X-rays (Baselet et al. 2017b). Furthermore, these cells showed increased secretion of pro-inflammatory cytokines IL-6 and chemokine CCL2 between days 2 and 7 post-irradiation. This pro-inflammatory phenotype was not observed at 0.05 Gy or 0.1 Gy. In contrast, senescence-associated β-galactosidase activity was enhanced at all doses implying that premature senescence may occur even at low doses.
In agreement with these data, non-irradiated HCAEC treated with the secretome of irradiated cells using 10 Gy X-rays showed significantly increased expression of p21Waf1 (Philipp et al. 2017). In addition, these non-irradiated, but secretome-treated bystander cells showed enhanced levels of ICAM-1, STAT1, and p38 suggesting a pro-inflammatory phenotype.
Chronic low-dose-rate radiation of HUVEC also triggered premature endothelial senescence observed by increased senescence-associated β-galactosidase staining (Yentrapalli et al. 2013a; Yentrapalli et al. 2013b). Senescent cells expressed low levels of RhoGDI, high levels of p21Waf1 and showed inhibition of the P13K/Akt pathway.
3.2. Animal studies
Data from animal models using high-dose IR are in agreement with some of the human data from cancer therapy patients highlighting acceleration of atherosclerotic plaque formation and predisposition to an inflammatory, thrombotic plaque phenotype (Cottin et al. 2001; Hoving et al. 2008; Pakala et al. 2003; Raghunathan et al. 2017; Stewart et al. 2006; Stewart et al. 2010). Some human studies such as those in Tinea capitis cohorts with relatively low cardiac radiation doses also show increased CVD risk (Boaventura et al. 2018; Shai et al. 2009). Many animal studies have used mice that are genetically predisposed to atherosclerosis such as the ApoE−/− mouse.
The few low-dose studies existing at the moment emphasize the role of dose rate in the atherosclerotic plaque formation. Mitchel et al. exposed ApoE−/− mice to total body Co-60 gamma doses of 0.025, 0.05. 0.10 or 0.50 Gy, either at low dose rate (1.0 mGy/min) or at high dose rate (about 0.15 Gy/min) at an early (2 months of age) or late (8 months of age) stage of atherogenesis (Mitchel et al. 2011). If the irradiation was given early at a low dose rate and at the lowest doses examined (25 or 50 mGy), the lesion frequency was reduced in animals 3 months after the exposure, and this effect persisted until 6 months after the exposure. Similarly at the high dose rate, the lowest doses used (25–50 mGy) tended to be the most effective at reducing both lesion frequency and size. However, the lesion severity and pro-inflammatory status as well as the total serum cholesterol levels were increased at all doses. If the irradiation was given late at a low dose rate, a small inhibitory effect on the progression of lesion severity was seen but the deceleration was only significant at 0.025 Gy. Furthermore, a dose-dependent decrease in total serum cholesterol was observed. No persistent significant changes were seen at the high dose rate.
A similar study comparing the effect of low and high dose rates on the atherosclerotic progress in ApoE−/− mice with cumulative doses of 0.3 Gy or 6.0 Gy showed that at high-dose-rate irradiation the number of plaques was significantly increased at 6.0 Gy but not at 0.3 Gy (Mancuso et al. 2015). The plaques developed principally in the aortic arch. Using the low-dose-rate irradiation (1 or 20 mGy/day), both doses increased markedly the plaque area although the number of plaques decreased. Plaques were observed both in the aortic arch and in the descending thoracic aorta.
Since the dose rate seems to play an important role in the atherosclerotic outcome, Ebrahimian et al. investigated the effect of very low-dose-rate radiation (12 or 28 μGy/h) on the plaque formation in ApoE−/− mice that were exposed chronically for 8 months (Ebrahimian et al. 2018). The cumulative doses were around 67 mGy and 157 mGy, respectively. Exposure at 12 μGy/h had no significant effect on the parameters tested, but that at 28 μGy/h resulted in an increase in anti-inflammatory cytokine production (IL-4, IL-10, IL-13, IL-18) and in antioxidative gene expression (Cat, Ctat1, Sod1, Sod2). Furthermore, it reduced the plaque size and increased the plaque stability.
Although anti-inflammatory and hormetic effects of low doses have been observed in cellular and animal studies their role in the context of radiation protection is not yet known. The fact that low-dose RT has been successfully used for benign degenerative inflammatory disorders for decades (Ott et al. 2019) is out of the scope of this review but emphasizes the need for more research on the low-dose area.
4. Biologically-based models for the radiation risk of cardiovascular events
IHD and CeVD are mostly caused by atherosclerosis and may lead to acute myocardial infarction and stroke. Classical risk factors of atherosclerosis comprise smoking, high blood pressure, dyslipidemia, obesity and diabetes. The pathobiological mechanisms of radiation on the circulatory system have been reviewed recently (Baselet et al. 2016; Tapio 2016). Hughson et al. have presented an overview of the pathological processes targeted by radiation at different levels of biological organization from the molecular and cellular to the organ level (Hughson et al. 2018). In general, radiation exposure can initiate or accelerate many pathogenic processes that may increase incidence of myocardial infarction and stroke. RT significantly enhances the risk for these events by about 7% per Gy in breast cancer patients (Darby et al. 2013). These data were later confirmed results with the suggestion that the volume of the left ventricle receiving 5 Gy was the most important prognostic dose-volume parameter (van den Bogaard et al. 2017).
Atherosclerotic changes of the vasculature may be the main drivers for the risk of myocardial infarction and stroke in response to RT. These changes and the impact of radiation on the vascular system can be measured with imaging procedures such as computed tomography (CT) scans and ultrasonography. Thickness of the carotid intima media (CIMT) increased by about 0.1 mm in astronauts after long space flights and in head and neck cancer patients after RT (Arbeille et al. 2016; Wilbers et al. 2014). CIMT is a moderate predictor of risk for myocardial infarction and stroke using a linear response in Cox regression models of proportional hazards (Lorenz et al. 2007). Myocardial infarction and stroke are caused by ruptures of arterial walls which have become unstable under the influence of atherosclerotic plaques (Libby and Theroux 2005). Wall rupture is a critical phenomenon characterised by a sudden transition brought about by continuously deteriorating stability. Simonetto et al. (2020) suggested that an age-adequate CIMT in a healthy person does not pose an additional risk for myocardial infarction or stroke and observed a strong nonlinear risk response for a positive deviation from the age-adequate value (Simonetto et al. 2020a). As a consequence, radiation risk cannot be characterized by the same coefficient for all exposed individuals, but rather strongly depends on the stage of atherosclerotic progression. For healthy individuals, radiation exposure might not pose an additional risk for myocardial infarction or stoke, whereas exposure for individuals with vasculature showing an advanced atherosclerotic state carry a high risk. In view of these findings the risk coefficient of 7% per Gy should be considered as an average value.
Biologically-based models for the risk of myocardial infarction and stroke must address the emergence of atherosclerosis in the vasculature in space and time. However, such risk models are currently not available. In the EU-funded MEDIRAD project a mechanistic prediction model for the risk of myocardial infarction and stroke in breast cancer patients receiving RT is under development (http://www.medirad-project.eu/). Since wall rupture is the key event in the causal chain which builds up the risk for myocardial infarction or stroke, a good conceptual model should address this chain in an adequate form. The chain involves the development of atherosclerosis from fatty streaks via fibrous plaques to complicated lesions that eventually cause a rupture.
To model the dynamics of atherosclerosis, Simonetto et al. have constructed a Markov Chain Monte Carlo code with continuous state space representing the coronary artery intimal surface area that is burdened with atherosclerotic lesions of increasing severity (Figure 1) (Simonetto et al. 2020b). Myocardial infarction rates are assumed to be proportional to the area of most complicated lesions which appear at the end of the chain. The model is fitted simultaneously to infarction incidence rates observed in the German KORA registry (Kuch et al. 2008), and to the age-dependent prevalence and spatial extent of atherosclerotic lesions in the PDAY study (PDAY 1993). To allow for nonlinear transition rates and to consider at the same time randomness and inter-individual heterogeneity, the model applies extensive simulations of the state space. The model provides biological explanation for pertinent features of the incidence curve. For women, the significant age-dependence of model parameters around the age of menopause qualitatively reproduces the known vascular effects of female sex hormones. For men, the incidence curve flattens at old age. According to the model, flattening is explained by a) saturation of the atherogenic process and b) removal from individuals at high risk from the cohort (frailty). Importantly, the model offers an interface to derive its parameters from imaging data that is routinely taken in the form of CT scans before and after RT. Hence, the individual atherosclerotic state of a patient can be projected into a biologically-based model. Compared to the one-size-fits-all approach of applying a single risk coefficient to every patient, this approach would offer a step forward to a more personalized projection of CVD risks after RT.
Figure 1. A model of early plaque development.
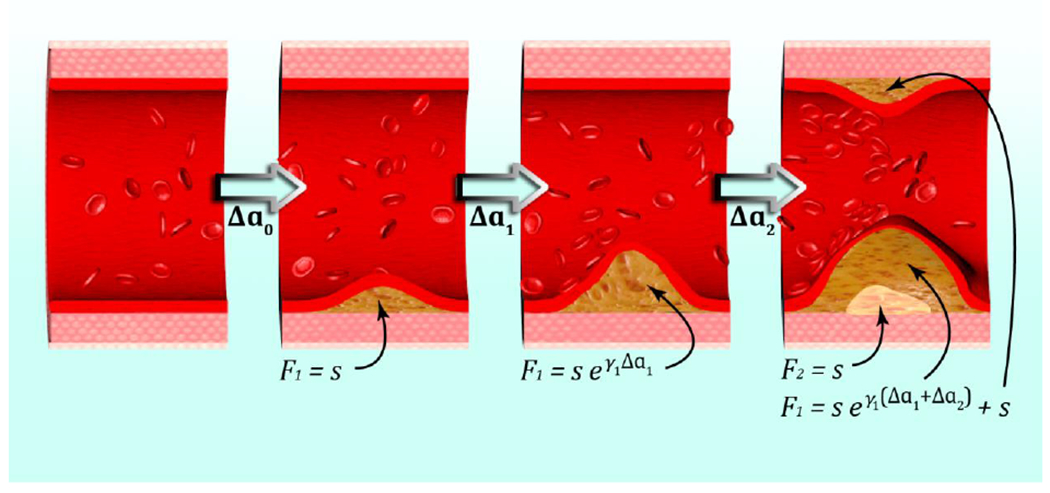
A first fatty streak appears after time Δa0 with area F1 = s. Subsequently it expands with growth rate γ1. At the last depicted time point, part of the fatty streak has become a fibrous plaque, with area F2 = s. The intimal surface area involved with fatty streaks (and more advanced lesions), F1, results from further growth of the first fatty streak and from the origin of a second fatty streak (Simonetto et al. 2020b).
5. Radiation-associated metabolic effects
Metabolic syndrome (MS) is a pathologic condition characterised by abdominal obesity, insulin resistance, hypertension, and hyperlipidemia (Alberti et al. 2009). It is one of the leading causes of death (Saklayen 2018) and strongly associated with increased risk for CVD, type 2 diabetes, and other disabilities (Tune et al. 2017). MS shares many features with the known consequences of high-dose IR, both showing pro-inflammatory, pro-thrombotic phenotype and elevated levels of oxidative stress (Azzam et al. 2012b; Kajimoto and Kaneto 2004; Pickup 2004; Spahis et al. 2017). Discrimination between MS-related and radiation-related atherogenicity or the causal relation between radiation exposure and development of MS represent therefore major challenges.
High concentrations of triglycerides in combination with low concentrations of HDL-C are important factors for MS-related atherogenicity. Atherogenic index of plasma (AIP), defined as the logarithm of the ratio between the triglyceride concentration and the high-density lipoprotein cholesterol (HDL-C) (Dobiásová and Frohlich 2001) is a promising tool to estimate the level of MS-related atherogenicity. AIP has been successfully used in cohorts of children, healthy adults, pre- and postmenopausal women, patients with hypertension, type 2 diabetes, dyslipidemia and patients with positive or negative angiography findings (Dobiásová 2004; Guo et al. 2020). MS-related risk factors include large waist circumference, and increased levels of HDL-C, triglycerides, fasting glucose and blood pressure (Alberti et al. 2009; Assmann et al. 2007). Other factors related to MS are age, sex, family history, physical inactivity, atherogenic diet, and also the traditional plasma lipid ratios (Manninen et al. 1992).
Recently, data have been accruing suggesting high-dose IR as a causal factor for MS. In particular, a wide range of long-term complications including CVD and MS have been reported in cancer survivors (Diller et al. 2009; Oeffinger et al. 2006; Phillips et al. 2015), with cancer treatments playing a pivotal role in this context (Carver et al. 2007; Oeffinger et al. 2006; Phillips et al. 2015). In particular, RT that is received by over 40% of cancer patients, in the form of targeted or total body irradiation (TBI) (Baskar et al. 2012), significantly increases the risk for endocrine and metabolic diseases up to decades after exposure (Baker et al. 2013; Geenen et al. 2010; Oeffinger et al. 2009). Such metabolic complications, ranging from obesity to insulin resistance, further predispose exposed survivors to CVD and dramatically affect their health trajectory (Baker et al. 2013; Geenen et al. 2010; Oeffinger et al. 2009). In light of the constant increase in the number cancer survivors, there is an urgent need to better understand the link between the use of RT and the development of MS to identify individuals at risk of CVD and guide preventive and therapeutic strategies. As there is paucity of information available regarding the impact of low dose irradiation in the context of cancer, the currently available knowledge obtained from high dose studies will be reviewed here.
5.1. Cranial RT, endocrine disturbances and body composition
Although cranial RT (CRT) is not as commonly used as it once was in the treatment of childhood malignancies, the increase in survival rates for childhood cancer has led to the emergence of a constantly growing population at risk of metabolic diseases (Darzy et al. 2009; Follin et al. 2014). Some of the complications reported in that population include growth hormone (GH) and adrenal insufficiency (Follin et al. 2014), impaired leptin metabolism (Brennan et al. 1999), altered body composition or increased obesity (Baker et al. 2013; Garmey et al. 2008), and insulin resistance or diabetes (Baker et al. 2013; Oeffinger et al. 2009). Several reports have identified an increase in body mass index (BMI) in cancer survivors treated with CRT (Garmey et al. 2008; Meacham et al. 2010; Oeffinger et al. 2009). Body composition, and in particular adiposity, fall under the influence of hormonal factors, including GH and the appetite-regulating hormone leptin. GH deficiency (GHD) has been frequently reported following CRT for childhood cancer (Brennan et al. 1999), leading to increased adiposity, with GH supplementation normalizing adiposity (Bulow et al. 2004). Such hormonal or body composition changes have long been established as risk factors of insulin resistance. Thus, several large cohorts of cancer survivors have identified CRT as a contributor to the development of insulin resistance (Baker et al. 2013; Oeffinger et al. 2009). However, conflicting observations have also been reported with some studies failing to link GHD or leptin disturbances with increased BMI or insulin resistance (Papadia et al. 2007). Such discrepancy could stem from the various radiation doses used in the course of treatment, with reports observing that only higher doses of radiation (usually above 20 Gy) resulted in increased BMI (Craig et al. 1999; Meacham et al. 2005; Oeffinger et al. 2003). The risk of CRT-induced metabolic complications is also dependent on the age at treatment, as children receiving CRT at a younger age are at a higher risk of obesity (Garmey et al. 2008) or gender-dependent, with female survivors showing higher risks of obesity than males (Craig et al. 1999; Garmey et al. 2008; Oeffinger et al. 2009).
5.2. Metabolic complications following total body or abdominal irradiation
Similarly to CRT, TBI and abdominal irradiation have been widely associated with changes in body composition, altered lipid profile (Amorim et al. 2020; Neville et al. 2006; Oeffinger et al. 2009; van Waas et al. 2012) and the development of insulin resistance in childhood cancer survivors (Daniel et al. 2018; Meacham et al. 2009; Neville et al. 2006; Oeffinger et al. 2009), with some studies identifying TBI as exerting a stronger effect than CRT or abdominal irradiation (Meacham et al. 2010). Similar to CRT, GH deficiency (Galletto et al. 2014), altered body composition, including fat distribution, and impaired pancreatic function have been observed in response to TBI (Amorim et al. 2020; Wei et al. 2015a; Wei et al. 2015b) or abdominal irradiation (Cicognani et al. 1997; de Vathaire et al. 2012; Neville et al. 2006; Oeffinger and Sklar 2012; Teinturier et al. 1995; van Waas et al. 2012). Increased systemic levels of triglycerides, low density lipoprotein cholesterol and free fatty acids up to decades post-irradiation (Daniel et al. 2018; Amorim et al. 2020), suggestive of impaired lipid metabolism, could indicate long-term damage to the liver, but also potentially to the adipose tissue which has shown a particular sensitivity to radiation exposure (Nylander et al. 2016; Poglio et al. 2009). In addition, the persistence of elevated glucose or insulin levels (Daniel et al. 2018; Meacham et al. 2009; Neville et al. 2006; Amorim et al. 2020) is suggestive of long-lasting alterations affecting the pancreas (van Waas et al. 2012), the liver, the adipose tissue or the skeletal muscle considering their anatomical position and their critical role in the regulation of glucose metabolism (Saltiel and Kahn 2001), although clinical data is still lacking to clearly establish the presence of such damage.
Thus, mouse models have been used to investigate the molecular mechanisms underlying radiation-induced metabolic changes. Mice exposed to 6 Gy TBI developed long-lasting perturbations in skeletal muscles metabolism, adipocytes and pancreatic functions, mediated by epigenetic changes, resulting in altered whole body metabolism and insulin resistance (Amorim et al. 2020; Nylander et al. 2016). Such perturbations led to a dramatic remodelling of the systemic lipidomic signature in mice, consistent with the signature observed in long-term childhood cancer survivors (CCS) more than 10 years after TBI (Amorim et al. 2020). In the same population, a remodelling of the T-cell epigenome was associated with increased T-cell activation and increased production of pro-inflammatory cytokines (Daniel et al. 2018). These immune changes were associated with systemic inflammation and insulin resistance, mimicking what has been previously described in the context of diabetes (Shoelson et al. 2006). These observations suggest that skeletal muscle and the immune system could play a role in the development of radiation-induced metabolic complications in exposed populations.
In BALB/c mice, low-dose TBI (0.1 Gy X-rays) induced a metabolic shift from oxidative phosphorylation to aerobic glycolysis in the small intestine, resulting in increased radiation resistance (Lall et al. 2014). This metabolic shift, also observed in human lymphocytes and fibroblasts, highlighted the upregulation of genes encoding glucose transporters and enzymes of glycolysis and the oxidative pentose phosphate pathway, concomitant with downregulation of mitochondrial genes, with corresponding alterations in metabolic flux through these pathways (Lall et al. 2014). This phenomenon was ROS-dependent and occurred in vitro only under physiological oxygen concentration (5% O2).
5.3. Radiation-induced changes in cardiac metabolism
Heart muscle needs continuously high levels of energy due to its lifelong contracting function. The energy is mainly produced as ATP in the respiratory chain of cardiac mitochondria (Lopaschuk 2017). Fatty acids are the heart’s main source of fuel, although it can also derive energy from glucose, lactate, pyruvate or ketone bodies (Grynberg and Demaison 1996). In the normal heart, there is a great flexibility to switch from one source to another depending on the energy demand. However, the cardiac ATP store is limited and can support only a few cardiac cycles (Grynberg and Demaison 1996).
The first indications that IR may affect the cardiac energy metabolism came from studies using mice locally irradiated to the heart (Azimzadeh et al. 2013; Barjaktarovic et al. 2011; Barjaktarovic et al. 2013). A high dose of 16 Gy (X-rays) resulted in a decline in cardiac mitochondrial number, destruction of the cristae structure and decreased expression of the mitochondrial transcription factor TFAM (Azimzadeh et al. 2013). Furthermore, a deactivation (phosphorylation) of peroxisome proliferator-activated receptor alpha (PPAR alpha), the main regulator of the lipid metabolism in the heart, and a slight but significant increase of serum glucose and oxidised LDL was shown 16 weeks after local heart irradiation (Azimzadeh et al. 2015; Azimzadeh et al. 2013). The X-ray dose of 2 Gy, but not 0.2 Gy, resulted in a rapid (4 weeks) but persistent (40 weeks) reduction in the succinate-driven respiration in mitochondria isolated from irradiated mouse hearts (Barjaktarovic et al. 2011; Barjaktarovic et al. 2013).
Using the Gottingen minipig model, Kenchegowda et al. showed radiation-associated insulin like growth factor-1 (IGF-1)-dependent inhibition of the anti-inflammatory, anti-oxidant insulin signal transduction pathway (IRS/PI3K/Akt) and induction of the pro-inflammatory mitogen-activated protein kinase (MAPK) pathway (Kenchegowda et al. 2018). Selective IGF-1 resistance was associated with an amplification of reactive oxygen species-induced damage, inflammation and endothelial dysfunction.
A dose-dependent increase in IHD incidence and mortality has been shown in the Mayak workers chronically exposed to external gamma rays (Azizova et al. 2010; Azizova et al. 2012). A post mortem proteomics analysis was carried out using cardiac left ventricle samples from three dose groups (<100 mGy, 100–500 mGy, and >500 mGy) compared to non-exposed control group (Azimzadeh et al. 2017a). All workers and the participants of the control group were diagnosed with IHD, which was the cause of death. A dose-dependent down-regulation of glycolytic and mitochondrial proteins and inactivation of PPAR-alpha was shown in the two highest dose groups (Azimzadeh et al. 2017a; Papiez et al. 2018) suggesting an impairment in both lipid and carbohydrate utilization. This observation may represent an exceptional pathological scenario not similar to that of a failing heart (Ingwall 2009; Neubauer 2007). It suggests little flexibility in metabolic switching between lipids and carbohydrates for energy production and indicates a slow but progressive depletion of ATP, possibly leading to impairment of the contractile function (Azimzadeh and Tapio 2017).
6. Systems biology in the present and future circulatory and metabolic studies: an outlook
According to the current understanding, one of the major determinants of biological response to IR is the induction of damage to DNA, proteins, and lipids which initiates further responses of the cells or tissues and to a great extent determines the systemic nature of radiation effects (Formenti and Demaria 2009; Ji et al. 2019; Mavragani et al. 2019). The magnitude of the initial damage is very important not only in the area of radiation protection, but is of major concern in different areas of biology, with implications to human pathophysiology (Ermolaeva and Schumacher 2014; Martin et al. 2011).
For the biological response, communications of DNA damage response (DDR) with the immune system play an important role at multiple levels. DDR, induced especially in the case of complex DNA damage in healthy tissue, may be instrumental in the initiation of various chronic and late effects involving inflammatory and immune responses and usually through the mediation of oxidative stress (Pouget et al. 2018). In other words, the initial cellular damage usually results in a cascade of events involving the release of ROS (Azzam et al. 2012a) and damage-associated molecular patterns (DAMPs) which are recognized by Toll-like receptors (TLRs), even at low doses (0.05 and 0.1 Gy) (El-Saghire et al. 2013). Bioinformatics work has shown a close association and crosstalk between radiation response and immune system components (Georgakilas et al. 2015), as well as those of DDR and innate immune system (Pateras et al. 2015).
Exposure conditions (e.g., partial body/total body, dose and dose rate, fractionation, acute or chronic, radiation quality) might affect the immune system to different degrees. The idea of the pivotal, but also complicated involvement of the immune system in the organism’s reaction to whole-body or partial-body irradiation is also supported by different studies as reviewed recently (Diegeler and Hellweg 2017). Nevertheless, complex DNA damage is currently accepted as the main instigator of unrepaired or misrepaired lesions. The low dose effects can be partially attributed to these complex DNA lesions that may arise from one double strand break (DSB) if several other non-DSB lesions or even single strand breaks are expected in the vicinity. As shown in some studies, these complex non-DSB DNA lesions can be converted into DSBs during the repair process (Mavragani et al. 2019). Furthermore, DNA damage after low radiation doses may escape cellular surveillance and repair and progress to a mutagenic catastrophic event at later stages especially when replication or mitosis is initiated (Lobrich et al. 2005).
Radiation exposure can affect different levels of cellular organization and communications between DNA and proteins or between proteins and lipids, a process similar to that observed in the induction of oxidative stress and oxidative damage. This suggests the use of more integrative biology approaches like omics: transcriptomics, proteomics (Fedorova et al. 2015).
Although many radiation studies on the plethora of biological and especially non-cancer effects refer to moderate-to-high doses like those applied in RT (Rodriguez-Ruiz et al. 2018) or space travel (Beheshti et al. 2019), low doses are always implicated in the real-life scenarios (Hammi et al. 2020). In RT, doses at surrounding normal tissues can be below 0.1 Gy. Therefore, radiation effects in the whole organism after an acute or chronic radiation exposure will be determined mainly by the relatively low doses at normal tissues and systemic effects mediated by the immune system (Mavragani et al. 2016).
As mentioned earlier, cardiac and metabolic effects of low-dose IR have been studied under various biological models from cells to mice and humans (Azzam et al. 2011; Baselet et al. 2016; Beheshti et al. 2019; Hughson et al. 2017; Yan et al. 2014) and particularly highlighted in the recent NASA Twins study (Garrett-Bakelman et al. 2019). Most epidemiological studies agree that inflammatory and immune response networks play a central role in the biological response (Baselet et al. 2016). Recent studies suggest that the nucleotide-binding domain and leucine-rich-repeat-containing family pyrin 3 (NLRP3) inflammasome plays a pivotal role in radiation-induced cardiovascular injury (Huang et al. 2020). Omics studies performing transcriptome profiling in the left ventricular murine cardiomyocytes isolated from mice exposed to 0.9 Gy, 1 GeV proton (1H) and 0.15 Gy, 1 GeV/nucleon iron (56Fe) over 28 days after exposure have categorized some major differentially expressed genes (DEGs) belonging to cell cycle, oxidative responses, inflammation and transcriptional regulation functional groups (Coleman et al. 2015). Recent metabolomics analysis in female mice receiving local heart irradiation with 6 MV photons at 0.2 and 2 Gy induced changes in energy metabolism, fatty acid beta-oxidation, oxidative stress and cellular damage. This study suggests that metabolomics may be an applicable and sensitive tool even in future human studies investigating cardiac response to radiation (Gramatyka et al. 2020).
7. Concluding remarks
This review provides strong evidence in support of a causal association between acute high-dose and chronic low-dose radiation exposure and most types of circulatory disease, in particular IHD and CeVD. These findings confirm the results of previous systematic reviews and meta-analysis of moderate and low-dose groups (Little 2016; Little et al. 2012a). The association is less certain for other circulatory diseases given the only marginal level of statistical significance and the highly significant inter-study heterogeneity of risks in studies of this endpoint (Little 2016). A previous meta-analysis suggested that if the association between low-level radiation exposure and the risk of circulatory disease reflects an underlying causal relationship, linear in dose, then the overall excess risk of mortality after exposure to low doses or low dose-rates of radiation may be about twice that currently assumed (Little et al. 2012a). Since the risks that are derived here using a somewhat larger body of data are consistent with those, the implications for low-dose radiation risk are unaltered. Nevertheless, the possible mechanisms for risk at low doses and low dose rates are, in contrast to the situation at higher doses and dose rates, relatively little understood. There is an urgent need for further research in this area (Kreuzer et al. 2015).
The biological data are supporting the epidemiological data indicating that even low-dose IR, if given to EC or vasculature in a normal non-inflammatory state, results in a pro-atherogenic outcome in the form of increased adhesiveness, inflammation, and premature senescence. Furthermore, adverse metabolic consequences can be seen in the heart muscle even below 0.5 Gy. In contrast, if the cells or vasculature are in inflammatory state low doses of IR may function in an anti-inflammatory manner.
As in the epidemiology, the knowledge of possible cellular and molecular mechanisms and relationships between radiation exposure and consequent circulatory and metabolic effects is still scarce. Especially, the years of latency between radiation exposure and the appearance of clinically relevant symptoms and the complexity of the clinically relevant endpoints make it difficult to identify those early cellular and molecular changes that are involved in the development of relevant late effects. This is partly due to the lack of good animal models for the human situation. At the moment, almost all animal data of the low-dose IR come from the ApoE−/− mouse studies. Furthermore, radiation exposure in connection with other risk factors is of additional importance. Other risk factors such as MS, associated with obesity or diabetes, can either precede or occur during or after the radiation exposure. Since recent data indicate that especially high-dose IR can be a causal factor for both CVD and MS it is relevant to investigate the molecular mechanisms that play a role in the aetiology of these different scenarios.
Recent data strongly suggest an association between radiation and the development of metabolic complications in cancer survivors exposed to high localized RT dose or TBI. Although the leading mechanisms are yet to be fully uncovered, persisting endocrine changes, functional alterations affecting the pancreas, the adipose tissue, the liver or skeletal muscles, and long-lasting immune impairments could all play a role. If total body irradiation seems to exert the strongest effect on the development of metabolic diseases, CRT and abdominal irradiation can also affect metabolic functions. There is currently no evidence on causal association between low doses and metabolic changes in human, only in animal models, but in occupational settings moderate doses (100 – 500 mGy) at low dose rate are able to alter the energy preferences in the cardiac muscle.
In the face of a constantly growing population diagnostically, environmentally or occupationally exposed to low-dose radiation, there is an urgent need to better identify and monitor individuals at risk of circulatory or metabolic complications. Especially low doses may contribute to the systemic normal tissue damage in all these populations. Therefore, low-dose studies are the basis for prevention and amelioration of adverse late effects providing a deeper understanding of the long-lasting impact of radiation on circulatory and metabolic functions and the underlying mechanisms.
8. Recommendations for future research
MELODI developed several recommendations for the way forward in research on radiation-induced circulatory and metabolic diseases. The two main recommendations for future research were (i) to continue to follow large adult cohort studies (Japanese atomic bomb survivors, worker and clinical studies) as well as paediatric cohorts (e.g. Tinea capitis, CT) and (ii) and to collect more biological samples in order to better understand the radiation impact on the disease process. Furthermore, identifying new existing suitable cohorts with a good statistical power will be important in the future. A precise dosimetric assessment in epidemiological studies is necessary and should include information on dose and dose rate, fractionation, and part of body or organ exposed, in particular in the case of sensitive structures such as heart, liver or carotid arteries. In the follow-up studies, information about morbidity and mortality, a clear diagnosis and definition of heart disease and related diseases such as MS or diabetes, but also heart function and echocardiology are relevant. In particular, patient and occupationally exposed cohorts may be ideal due to reliable information on dosimetry and diagnoses, but also presenting a possibility to access biosamples. In the short term, the highest potential to advance understanding in radiation-induced circulatory and metabolic diseases is expected from studies at near-field and/or out-of-field therapeutic doses. In the long term, basic and clinical research addressing CVD and metabolic diseases at progressively lower doses is warranted.
A general weakness of the current low-dose studies involving circulatory and metabolic disease is the lack of information on lifestyle factors, in particular those having an impact on the pathogenesis such as smoking, alcohol consumption, diet, obesity, physical activity, and genetic background. A range of biological samples (blood, urine, saliva, fecal samples) can be informative to fill this gap, elucidating radiation-associated changes in the metabolic (fasting glucose, cholesterol, AIP or other plasma lipid ratios), inflammatory (acute phase proteins, cytokines, chemokines), or cardiovascular status (e.g. cardiac troponin, C-reactive protein, B-type natriuretic peptide). For that matter, imaging data, biopsies and even autopsies represent good sources of information.
It is obvious that also mechanisms other than the classical DDR pathways are involved in the radiation-induced circulatory and metabolic responses. This calls for a new insight on potential mechanisms and effect of modulating factors, such as inflammatory, stress and immune responses, and lifestyle factors (Table 1). Disease relevant cell types, such as EC, macrophages and smooth muscle cells of the vessel wall, hepatocytes and adipocytes should be used for hypothesis-driven studies addressing possible mechanisms, looking at, but not limited to, epigenetics, accelerated aging, mitochondrial dysfunction, oxidative stress, and (other) links between metabolic and circulatory diseases.
Table 1.
Adverse Outcome Pathways (AOP) leading to long-term health consequences.
| Level of organisation | AOP | ||||
|---|---|---|---|---|---|
| Macromolecular (seconds, hours, days) | Deposition of energy to tissue | ||||
| DNA Damage | Damage to other cellular components (RNA, proteins and lipids) | ||||
| DNA mutation | Misrepair | Apoptosis | Biochemical response to damage | ||
| Mutations in critical genes | Chromosomal aberrations Complex damage |
Damage reponse | Induction of oxidative stress | Cytokines reponding to damage | |
| Cell/tissue (days, weeks) | Oncogene activation | Altered signalling, bystander effects | Senescence | Epigenetic changes | |
| Tumour suppressor gene inactivation | Increased proliferation | Genomic instability | Oxidative damage | Inflammation | |
| Organ/Organ system (months, years) | Clonal expansion Hyperproliferation Genomic instability |
Altered physiology | Systemic effects | Immunological effects | Endothelial dysfunction |
| Tumour | Tumour microenvironment | Dyslipidemia Insulin resistance |
Circulatory disease | Atherosclerosis | |
| Health outcome (years, decades) | Cancer Metastasis | Metabolic syndrome | Mortality | Heart attack | |
Adverse outcome pathways of low-dose-related CVD and MS should be further investigated (Figure 2) (OECD 2013). Advances in medical imaging and automatic image analysis will significantly improve the quantitative assessment of progressing atherosclerosis in the vascular system. Integrating these imaging data with epidemiological cohorts of incidence for CVD provides opportunities for the development and testing of biologically-based risk models that make use of the actual pathologic state for more accurate personalized risk projection.
Figure 2.
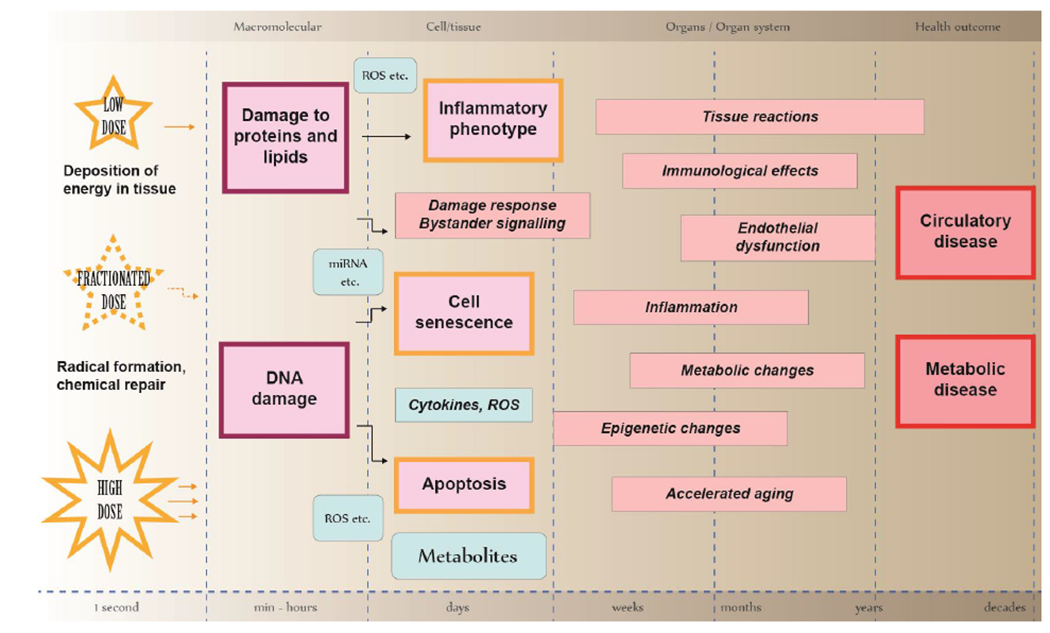
Schematic representation of the components, interrelations and timing of a network of adverse outcome pathways and key events contributing to the development of adverse health outcome: circulatory and metabolic diseases.
Macromolecular interactions:
The left column illustrates the initiating events, the deposition of energy in tissue, whereby highly reactive radicals are formed and most of them are quickly removed by chemical repair. Some radicals react with cellular macromolecules, either directly with DNA (4% of energy deposition), proteins, and lipids (16% of energy deposition) or indirectly via radiolysis of water (80% of energy deposition). The physical attributes of the radiation (radiation quality) and dose delivery (e.g. high or low single dose or fractionated/protracted exposure) modulate the responses.
Cell and tissue level effects:
Unrepaired or misrepaired damage to cellular macromolecules leads to damage response signalling and bystander effects mediated by reactive oxygen species (ROS), miRNAs, cytokines and other small molecules. At the cellular level, damage manifests as apoptosis, cellular senescence, and inflammatory phenotype creating continuous oxidative stress. Damage response signalling and bystander effects expand the physiological change from single cells to a population of cells and tissues. Note that mutations caused by misrepaired DNA damage are not shown in this scheme, although they are major drivers for stochastic effects (cancer, germline instability). Such clonal expansion of cells carrying specific mutations is not known to take place in the development of circulatory or metabolic diseases.
Organ and organ system level:
The cellular and tissue level effects can lead to a variety of tissue reactions impairing organ functions within weeks (early toxicity/tissue reactions), months or years (late toxicity/tissue reactions). Both the dose and the dose rate impact the presentation and severity of the tissue reaction or toxicity. Typical phenomena and mechanisms underlying tissue reactions include inflammation, oxidative stress, immunological effects, epigenetic changes, and accelerated aging of cells and tissues. Such adverse pathways contribute to endothelial dysfunction and metabolic changes that lead to adverse circulatory and metabolic outcomes.
Adverse health outcome:
Circulatory disease and metabolic disease represent the main complications appearing after a long latency period of years or even decades. The length of the latency period depends on the dose, high doses leading to an earlier onset of the tissue reaction. Note that circulatory and metabolic diseases are interrelated, metabolic disease being a major risk factor for circulatory disease.
Supplementary Material
Highlights.
Ionizing radiation causes adverse circulatory and metabolic health effects at high doses
Strong epidemiological evidence supports of a causal association between chronic low-dose radiation exposure and most types of circulatory disease
The impact of low-dose radiation may be modulated by lifestyle factors and inflammatory, stress and immune responses but more research is needed
Acknowledgements
This project has received funding from the EURATOM research and training programme 2014-2018 under grant agreement No 662287 (CONCERT) and under grant agreement No 755523 (MEDIRAD). The work of MPL was supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics.
This publication reflects only the author’s view. Responsibility for the information and views expressed therein lies entirely with the authors. The European Commission is not responsible for any use that may be made of the information it contains.
We thank Stefanie Winkler for the help with the figures.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams MJ; Hardenbergh PH; Constine LS; Lipshultz SE Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol 2003;45:55–75 [DOI] [PubMed] [Google Scholar]
- Alberti KG; Eckel RH; Grundy SM; Zimmet PZ; Cleeman JI; Donato KA; Fruchart JC; James WP; Loria CM; Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- Amorim NML; Kee A; Coster ACF; Lucas C; Bould S; Daniel S; Weir JM; Mellett NA; Barbour J; Meikle PJ; Cohn RJ; Turner N; Hardeman EC; Simar D Irradiation impairs mitochondrial function and skeletal muscle oxidative capacity: significance for metabolic complications in cancer survivors. Metabolism 2020;103:154025. [DOI] [PubMed] [Google Scholar]
- Arbeille P; Provost R; Zuj K Carotid and Femoral Artery Intima-Media Thickness During 6 Months of Spaceflight. Aerosp Med Hum Perform 2016;87:449–453. doi: 410.3357/AMHP.4493.2016. [DOI] [PubMed] [Google Scholar]
- Assmann G; Guerra R; Fox G; Cullen P; Schulte H; Willett D; Grundy SM Harmonizing the definition of the metabolic syndrome: comparison of the criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European populations. Am J Cardiol 2007;99:541–548 [DOI] [PubMed] [Google Scholar]
- Averbeck D; Salomaa S; Bouffler S; Ottolenghi A; Smyth V; Sabatier L Progress in low dose health risk research: Novel effects and new concepts in low dose radiobiology. Mutat Res 2018;776:46–69.: 10.1016/j.mrrev.2018.1004.1001. Epub 2018 Apr 1017. [DOI] [PubMed] [Google Scholar]
- Azimzadeh O; Azizova T; Merl-Pham J; Subramanian V; Bakshi MV; Moseeva M; Zubkova O; Hauck SM; Anastasov N; Atkinson MJ; Tapio S A dose-dependent perturbation in cardiac energy metabolism is linked to radiation-induced ischemic heart disease in Mayak nuclear workers. Oncotarget 2017a;8:9067–9078. doi: 9010.18632/oncotarget.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh O; Sievert W; Sarioglu H; Merl-Pham J; Yentrapalli R; Bakshi MV; Janik D; Ueffing M; Atkinson MJ; Multhoff G; Tapio S Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res 2015;14:1203–1219. doi: 1210.1021/pr501141b. Epub 502015 Jan 501129. [DOI] [PubMed] [Google Scholar]
- Azimzadeh O; Sievert W; Sarioglu H; Yentrapalli R; Barjaktarovic Z; Sriharshan A; Ueffing M; Janik D; Aichler M; Atkinson MJ; Multhoff G; Tapio S PPAR alpha: a novel radiation target in locally exposed Mus musculus heart revealed by quantitative proteomics. J Proteome Res 2013;12:2700–2714. doi: 2710.1021/pr400071g. Epub 402013 Apr 400029. [DOI] [PubMed] [Google Scholar]
- Azimzadeh O; Subramanian V; Stander S; Merl-Pham J; Lowe D; Barjaktarovic Z; Moertl S; Raj K; Atkinson MJ; Tapio S Proteome analysis of irradiated endothelial cells reveals persistent alteration in protein degradation and the RhoGDI and NO signalling pathways. Int J Radiat Biol 2017b;93:920–928. doi: 910.1080/09553002.09552017.01339332. Epub 09552017 Jul 09553011. [DOI] [PubMed] [Google Scholar]
- Azimzadeh O; Tapio S Proteomics landscape of radiation-induced cardiovascular disease: somewhere over the paradigm. Expert Rev Proteomics 2017;14:987–996. doi: 910.1080/14789450.14782017.11388743. Epub 14782017 Oct 14789410. [DOI] [PubMed] [Google Scholar]
- Azizova TV; Bannikova MV; Grigorieva ES; Bagaeva YP; Azizova EV Risk of lower extremity arterial disease in a cohort of workers occupationally exposed to ionizing radiation over a prolonged period. Radiat Environ Biophys 2016;55:147–159 [DOI] [PubMed] [Google Scholar]
- Azizova TV; Batistatou E; Grigorieva ES; McNamee R; Wakeford R; Liu H; de Vocht F; Agius RM An Assessment of Radiation-Associated Risks of Mortality from Circulatory Disease in the Cohorts of Mayak and Sellafield Nuclear Workers. Radiat Res 2018;189:371–388 [DOI] [PubMed] [Google Scholar]
- Azizova TV; Grigorieva ES; Hunter N; Pikulina MV; Moseeva MB Risk of mortality from circulatory diseases in Mayak workers cohort following occupational radiation exposure. Journal of radiological protection : official journal of the Society for Radiological Protection 2015a;35:517–538 [DOI] [PubMed] [Google Scholar]
- Azizova TV; Grigoryeva ES; Haylock RGE; Pikulina MV; Moseeva MB Ischaemic heart disease incidence and mortality in an extended cohort of Mayak workers first employed in 1948-1982. Br J Radiol 2015b;88:20150169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizova TV; Haylock RGE; Moseeva MB; Bannikova MV; Grigoryeva ES Cerebrovascular diseases incidence and mortality in an extended Mayak Worker Cohort 1948-1982. Radiat Res 2014;182:529–544 [DOI] [PubMed] [Google Scholar]
- Azizova TV; Muirhead CR; Druzhinina MB; Grigoryeva ES; Vlasenko EV; Sumina MV; O’Hagan JA; Zhang W; Haylock RG; Hunter N Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948-1958. Radiat Res 2010;174:155–168 [DOI] [PubMed] [Google Scholar]
- Azizova TV; Muirhead CR; Moseeva MB; Grigoryeva ES; Vlasenko EV; Hunter N; Haylock RG; O’Hagan JA Ischemic heart disease in nuclear workers first employed at the Mayak PA in 1948-1972. Health Phys 2012;103:3–14 [DOI] [PubMed] [Google Scholar]
- Azzam EI; Jay-Gerin J-P; Pain D Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer letters 2012a;327:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI; Jay-Gerin JP; Pain D Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI; Jay-Gerin JP; Pain D Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012b;327:48–60. doi: 10.1016/j.canlet.2011.1012.1012. Epub 2011 Dec 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KS; Chow EJ; Goodman PJ; Leisenring WM; Dietz AC; Perkins JL; Chow L; Sinaiko A; Moran A; Petryk A; Steinberger J Impact of treatment exposures on cardiovascular risk and insulin resistance in childhood cancer survivors. Cancer Epidemiol Biomarkers Prev 2013;22:1954–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjaktarovic Z; Schmaltz D; Shyla A; Azimzadeh O; Schulz S; Haagen J; Dorr W; Sarioglu H; Schafer A; Atkinson MJ; Zischka H; Tapio S Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS One 2011;6:e27811. doi: 27810.21371/journal.pone.0027811. Epub 0022011 Dec 0027818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjaktarovic Z; Shyla A; Azimzadeh O; Schulz S; Haagen J; Dorr W; Sarioglu H; Atkinson MJ; Zischka H; Tapio S Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiother Oncol 2013;106:404–410. doi: 410.1016/j.radonc.2013.1001.1017. Epub 2013 Mar 1020. [DOI] [PubMed] [Google Scholar]
- Baselet B; Azimzadeh O; Erbeldinger N; Bakshi MV; Dettmering T; Janssen A; Ktitareva S; Lowe DJ; Michaux A; Quintens R; Raj K; Durante M; Fournier C; Benotmane MA; Baatout S; Sonveaux P; Tapio S; Aerts A Differential Impact of Single-Dose Fe Ion and X-Ray Irradiation on Endothelial Cell Transcriptomic and Proteomic Responses. Front Pharmacol 2017a;8:570.: 10.3389/fphar.2017.00570. eCollection 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselet B; Belmans N; Coninx E; Lowe D; Janssen A; Michaux A; Tabury K; Raj K; Quintens R; Benotmane MA; Baatout S; Sonveaux P; Aerts A Functional Gene Analysis Reveals Cell Cycle Changes and Inflammation in Endothelial Cells Irradiated with a Single X-ray Dose. Front Pharmacol 2017b;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselet B; Rombouts C; Benotmane AM; Baatout S; Aerts A Cardiovascular diseases related to ionizing radiation: The risk of low-dose exposure (Review). International journal of molecular medicine 2016;38:1623–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar R; Lee KA; Yeo R; Yeoh KW Cancer and radiation therapy: current advances and future directions. Int J Med Sci 2012;9:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers CM; van Hinsbergh VW; van Nieuw Amerongen GP Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 2010;103:40–55. doi: 10.1160/TH1109-1106-0403. Epub 2009 Sep 1130. [DOI] [PubMed] [Google Scholar]
- Beheshti A; McDonald JT; Miller J; Grabham P; Costes SV GeneLab Database Analyses Suggest Long-Term Impact of Space Radiation on the Cardiovascular System by the Activation of FYN Through Reactive Oxygen Species. International journal of molecular sciences 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEIR. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2 edêds. Washington, DC: The National Academies Press; 2006 [PubMed] [Google Scholar]
- Bell CM; Coleman DA Models of the healthy worker effect in industrial cohorts. Stat Med 1987;6:901–909 [DOI] [PubMed] [Google Scholar]
- Boaventura P; Duraes C; Mendes A; Costa NR; Chora I; Ferreira S; Araujo E; Lopes P; Rosa G; Marques P; Tavares S; Chaves V; Bettencourt P; Oliveira I; Costa F; Ramos I; Teles MJ; Guimaraes JT; Sobrinho-Simoes M; Soares P Is Low-Dose Radiation Exposure a Risk Factor for Atherosclerotic Disease? Radiat Res 2018;189:418–424. doi: 410.1667/RR14942.14941. Epub 12018 Feb 14920. [DOI] [PubMed] [Google Scholar]
- Brennan BM; Rahim A; Blum WF; Adams JA; Eden OB; Shalet SM Hyperleptinaemia in young adults following cranial irradiation in childhood: growth hormone deficiency or leptin insensitivity? Clin Endocrinol (Oxf) 1999;50:163–169 [DOI] [PubMed] [Google Scholar]
- Bulow B; Link K; Ahren B; Nilsson AS; Erfurth EM Survivors of childhood acute lymphoblastic leukaemia, with radiation-induced GH deficiency, exhibit hyperleptinaemia and impaired insulin sensitivity, unaffected by 12 months of GH treatment. Clin Endocrinol (Oxf) 2004;61:683–691 [DOI] [PubMed] [Google Scholar]
- Carver JR; Shapiro CL; Ng A; Jacobs L; Schwartz C; Virgo KS; Hagerty KL; Somerfield MR; Vaughn DJ; Panel ACSE American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007;25:3991–4008 [DOI] [PubMed] [Google Scholar]
- Cervelli T; Panetta D; Navarra T; Andreassi MG; Basta G; Galli A; Salvadori PA; Picano E; Del Turco S Effects of single and fractionated low-dose irradiation on vascular endothelial cells. Atherosclerosis 2014;235:510–518. doi: 510.1016/j.atherosclerosis.2014.1005.1932. Epub 2014 Jun 1017. [DOI] [PubMed] [Google Scholar]
- Cicognani A; Cacciari E; Mancini AF; Pasini A; Salardi S; Salmi S; Gualandi S; Paolucci G Abnormal insulin response to glucose following treatment for Wilms’ tumor in childhood. Eur J Pediatr 1997;156:371–375 [DOI] [PubMed] [Google Scholar]
- Coleman MA; Sasi SP; Onufrak J; Natarajan M; Manickam K; Schwab J; Muralidharan S; Peterson LE; Alekseyev YO; Yan X; Goukassian DA Low-dose radiation affects cardiac physiology: gene networks and molecular signaling in cardiomyocytes. American journal of physiology Heart and circulatory physiology 2015;309:H1947–H1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONCERT; Impens N; Salomaa S; Bouffler S; Madas B; Roy L; Gilbin R; Vandenhove H; Real A; Beresford N; Horemans N; Battle JVI; Schneider T; Raskob W; Camps J; Bacher K; Damilakis J; Frija G; Hoeschen C; Paolo G; Perko T; Turcanu C; Molyneux-Hodgson S; Zeleznik N; L. MM; Sabatier L; V. S; Ottolenghi A; Harrison R; Vanhavere F; Ruehm W; López MA; Zorko B. Second joint roadmap for radiation protection research. Deliverable D3.7 of CONCERT, European Joint Programme for the integration of radiation protection research. https://wwwconcert-h2020eu/en/Publications 2020; [Google Scholar]
- Cottin Y; Kollum M; Kolodgie FD; Chan RC; Kim HS; Vodovotz Y; Virmani R; Waksman R; Yazdi H Intravascular radiation accelerates atherosclerotic lesion formation of hypercholesteremic rabbits. Cardiovasc Radiat Med 2001;2:231–240. doi: 210.1016/s1522-1865(1002)00129-00124. [DOI] [PubMed] [Google Scholar]
- Craig F; Leiper AD; Stanhope R; Brain C; Meller ST; Nussey SS Sexually dimorphic and radiation dose dependent effect of cranial irradiation on body mass index. Arch Dis Child 1999;81:500–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter DJ; Schaapveld M; Darby SC; Hauptmann M; van Nimwegen FA; Krol ADG; Janus CPM; van Leeuwen FE; Aleman BMP Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst 2015;107:djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S; Nylander V; Ingerslev LR; Zhong L; Fabre O; Clifford B; Johnston K; Cohn RJ; Barres R; Simar D T cell epigenetic remodeling and accelerated epigenetic aging are linked to long-term immune alterations in childhood cancer survivors. Clin Epigenetics 2018;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby SC; Ewertz M; McGale P; Bennet AM; Blom-Goldman U; Brønnum D; Correa C; Cutter D; Gagliardi G; Gigante B; Jensen M-B; Nisbet A; Peto R; Rahimi K; Taylor C; Hall P Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–998 [DOI] [PubMed] [Google Scholar]
- Darzy KH; Thorner MO; Shalet SM Cranially irradiated adult cancer survivors may have normal spontaneous GH secretion in the presence of discordant peak GH responses to stimulation tests (compensated GH deficiency). Clin Endocrinol (Oxf) 2009;70:287–293 [DOI] [PubMed] [Google Scholar]
- de Vathaire F; El-Fayech C; Ben Ayed FF; Haddy N; Guibout C; Winter D; Thomas-Teinturier C; Veres C; Jackson A; Pacquement H; Schlumberger M; Hawkins M; Diallo I; Oberlin O Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. The Lancet Oncology 2012;13:1002–1010 [DOI] [PubMed] [Google Scholar]
- Demirci S; Nam J; Hubbs JL; Nguyen T; Marks LB Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys 2009;73:980–987 [DOI] [PubMed] [Google Scholar]
- Dess RT; Sun Y; Matuszak MM; Sun G; Soni PD; Bazzi L; Murthy VL; Hearn JWD; Kong FM; Kalemkerian GP; Hayman JA; Ten Haken RK; Lawrence TS; Schipper MJ; Jolly S Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo I; Lamon A; Shamsaldin A; Grimaud E; de Vathaire F; Chavaudra J Estimation of the radiation dose delivered to any point outside the target volume per patient treated with external beam radiotherapy. RadiotherOncol 1996;38:269–271 [DOI] [PubMed] [Google Scholar]
- Diegeler S; Hellweg CE Intercellular Communication of Tumor Cells and Immune Cells after Exposure to Different Ionizing Radiation Qualities. Frontiers in Immunology 2017;8:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller L; Chow EJ; Gurney JG; Hudson MM; Kadin-Lottick NS; Kawashima TI; Leisenring WM; Meacham LR; Mertens AC; Mulrooney DA; Oeffinger KC; Packer RJ; Robison LL; Sklar CA Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol 2009;27:2339–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobiásová M Atherogenic index of plasma (Log(Triglycerides/HDL-Cholesterol)]: theoretical and practical implications. Editorial. Clin Chem 2004;50:1113–1115 [DOI] [PubMed] [Google Scholar]
- Dobiásová M; Frohlich J The plasma parameter Log (TG/HDL-C) as an atherogenic index_corr with LP particle size and esterification rate. Clin Biochem 2001;34:583–588 [DOI] [PubMed] [Google Scholar]
- Doll R; Fisher RE; Gammon EJ; Gunn W; Hughes GO; Tyrer FH; Wilson W Mortality of Gasworkers with Special Reference to Cancers of the Lung and Bladder, Chronic Bronchitis, and Pneumoconiosis. Br J Ind Med 1965;22:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubay D; Caer-Lorho S; Laroche P; Laurier D; Rage E Mortality from Circulatory System Diseases among French Uranium Miners: A Nested Case-Control Study. Radiat Res 2015;183:550–562 [DOI] [PubMed] [Google Scholar]
- Ebrahimian TG; Beugnies L; Surette J; Priest N; Gueguen Y; Gloaguen C; Benderitter M; Jourdain JR; Tack K Chronic Exposure to External Low-Dose Gamma Radiation Induces an Increase in Anti-inflammatory and Anti-oxidative Parameters Resulting in Atherosclerotic Plaque Size Reduction in ApoE(−/−) Mice. Radiat Res 2018;189:187–196. doi: 110.1667/RR14823.14821. Epub 12017 Dec 14811. [DOI] [PubMed] [Google Scholar]
- El-Fayech C; Haddy N; Allodji RS; Veres C; Diop F; Kahlouche A; Llanas D; Jackson A; Rubino C; Guibout C; Pacquement H; Oberlin O; Thomas-Teinturier C; Scarabin PY; Chavaudra J; Lefkopoulos D; Giroud M; Bejot Y; Bernier V; Carrie C; Diallo I; de Vathaire F Cerebrovascular Diseases in Childhood Cancer Survivors: Role of the Radiation Dose to Willis Circle Arteries. International journal of radiation oncology, biology, physics 2017;97:278–286 [DOI] [PubMed] [Google Scholar]
- El-Saghire H; Michaux A; Thierens H; Baatout S Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med 2013;32:1407–1414 [DOI] [PubMed] [Google Scholar]
- Ermolaeva MA; Schumacher B Systemic DNA damage responses: organismal adaptations to genome instability. Trends in Genetics 2014;30:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova M; Ni Z; Griesser E; Vemula V; Weber D; Milic I; Hoffmann R OP44 - Systems biology of oxidative stress: first insights in lipid oxidation and protein modification cross-talks. Free Radical Biology and Medicine 2015;86:S17 [Google Scholar]
- Follin C; Wiebe T; Moell C; Erfurth EM Moderate dose cranial radiotherapy causes central adrenal insufficiency in long-term survivors of childhood leukaemia. Pituitary 2014;17:7–12 [DOI] [PubMed] [Google Scholar]
- Foray N; Bourguignon M; Hamada N Individual response to ionizing radiation. Mutat Res 2016;770:369–386. doi: 310.1016/j.mrrev.2016.1009.1001. Epub 2016 Sep 1014. [DOI] [PubMed] [Google Scholar]
- Formenti SC; Demaria S Systemic effects of local radiotherapy. The Lancet Oncology 2009;10:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletto C; Gliozzi A; Nucera D; Bertorello N; Biasin E; Corrias A; Chiabotto P; Fagioli F; Guiot C Growth impairment after TBI of leukemia survivors children: a model- based investigation. Theor Biol Med Model 2014;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmey EG; Liu Q; Sklar CA; Meacham LR; Mertens AC; Stovall MA; Yasui Y; Robison LL; Oeffinger KC Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26:4639–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Bakelman FE; Darshi M; Green SJ; Gur RC; Lin L; Macias BR; McKenna MJ; Meydan C; Mishra T; Nasrini J; Piening BD; Rizzardi LF; Sharma K; Siamwala JH; Taylor L; Vitaterna MH; Afkarian M; Afshinnekoo E; Ahadi S; Ambati A; Arya M; Bezdan D; Callahan CM; Chen S; Choi AMK; Chlipala GE; Contrepois K; Covington M; Crucian BE; De Vivo I; Dinges DF; Ebert DJ; Feinberg JI; Gandara JA; George KA; Goutsias J; Grills GS; Hargens AR; Heer M; Hillary RP; Hoofnagle AN; Hook VYH; Jenkinson G; Jiang P; Keshavarzian A; Laurie SS; Lee-McMullen B; Lumpkins SB; MacKay M; Maienschein-Cline MG; Melnick AM; Moore TM; Nakahira K; Patel HH; Pietrzyk R; Rao V; Saito R; Salins DN; Schilling JM; Sears DD; Sheridan CK; Stenger MB; Tryggvadottir R; Urban AE; Vaisar T; Van Espen B; Zhang J; Ziegler MG; Zwart SR; Charles JB; Kundrot CE; Scott GBI; Bailey SM; Basner M; Feinberg AP; Lee SMC; Mason CE; Mignot E; Rana BK; Smith SM; Snyder MP; Turek FW The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019;364:eaau8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen MM; Bakker PJ; Kremer LC; Kastelein JJ; van Leeuwen FE Increased prevalence of risk factors for cardiovascular disease in long-term survivors of acute lymphoblastic leukemia and Wilms tumor treated with radiotherapy. Pediatr Blood Cancer 2010;55:690–697 [DOI] [PubMed] [Google Scholar]
- Georgakilas AG; Pavlopoulou A; Louka M; Nikitaki Z; Vorgias CE; Bagos PG; Michalopoulos I Emerging molecular networks common in ionizing radiation, immune and inflammatory responses by employing bioinformatics approaches. Cancer Lett 2015;368:164–172 [DOI] [PubMed] [Google Scholar]
- Gillies M; Richardson DB; Cardis E; Daniels RD; O’Hagan JA; Haylock R; Laurier D; Leuraud K; Moissonnier M; Schubauer-Berigan MK; Thierry-Chef I; Kesminiene A Mortality from Circulatory Diseases and other Non-Cancer Outcomes among Nuclear Workers in France, the United Kingdom and the United States (INWORKS). Radiat Res 2017;188:276–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramatyka M; Boguszewicz; Ciszek M; Gabrys D; Kulik R; Sokol M. Metabolic changes in mice cardiac tissue after low-dose irradiation revealed by 1H NMR spectroscopy. J Radiat Res 2020;61:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche B; Lackland DT; Land CE; Simon SL; Apsalikov KN; Pivina LM; Bauer S; Gusev BI Mortality from cardiovascular diseases in the Semipalatinsk historical cohort, 1960–1999, and its relationship to radiation exposure. Radiat Res 2011;176:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynberg A; Demaison L Fatty acid oxidation in the heart. J Cardiovasc Pharmacol 1996;28:S11–17. doi: 10.1097/00005344-199600003-199600003. [DOI] [PubMed] [Google Scholar]
- Guo Q; Zhou S; Feng X; Yang J; Qiao J; Zhao Y; Shi D; Zhou Y The sensibility of the new blood lipid indicator--atherogenic index of plasma (AIP) in menopausal women with coronary artery disease. Lipids Health Dis 2020;19:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddy N; Diallo S; El-Fayech C; Schwartz B; Pein F; Hawkins M; Veres C; Oberlin O; Guibout C; Pacquement H; Munzer M; N’Guyen TD; Bondiau PY; Berchery D; Laprie A; Scarabin PY; Jouven X; Bridier A; Koscielny S; Deutsch E; Diallo I; de Vathaire F Cardiac Diseases Following Childhood Cancer Treatment: Cohort Study. Circulation 2016;133:31–38 [DOI] [PubMed] [Google Scholar]
- Hahn E; Jiang H; Ng A; Bashir S; Ahmed S; Tsang R; Sun A; Gospodarowicz M; Hodgson D Late Cardiac Toxicity After Mediastinal Radiation Therapy for Hodgkin Lymphoma: Contributions of Coronary Artery and Whole Heart Dose-Volume Variables to Risk Prediction. International journal of radiation oncology, biology, physics 2017;98:1116–1123 [DOI] [PubMed] [Google Scholar]
- Hammi A; Paganetti H; Grassberger C 4D blood flow model for dose calculation to circulating blood and lymphocytes. Physics in Medicine & Biology 2020;65:055008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoving S; Heeneman S; Gijbels MJ; te Poele JA; Russell NS; Daemen MJ; Stewart FA Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(−/−) mice. Int J Radiat Oncol Biol Phys 2008;71:848–857 [DOI] [PubMed] [Google Scholar]
- Huang S; Che J; Chu Q; Zhang P The Role of NLRP3 Inflammasome in Radiation-Induced Cardiovascular Injury. Frontiers in Cell and Developmental Biology 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson RL; Helm A; Durante M Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nature reviews Cardiology 2017; [DOI] [PubMed] [Google Scholar]
- Hughson RL; Helm A; Durante M Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat Rev Cardiol 2018;15:167–180. doi: 110.1038/nrcardio.2017.1157. Epub 2017 Oct 1020. [DOI] [PubMed] [Google Scholar]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1–332. doi: 310.1016/j.icrp.2007.1010.1003. [DOI] [PubMed] [Google Scholar]
- ICRP. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs--threshold doses for tissue reactions in a radiation protection context. Ann ICRP 2012;41:1–322. doi: 310.1016/j.icrp.2012.1002.1001. [DOI] [PubMed] [Google Scholar]
- Ingwall JS Energy metabolism in heart failure and remodelling. Cardiovasc Res 2009;81:412–419. doi: 410.1093/cvr/cvn1301. Epub 2008 Nov 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VK; Gorski AI; Maksioutov MA; Tsyb AF; Souchkevitch GN Mortality among the Chernobyl emergency workers: estimation of radiation risks (preliminary analysis). Health Phys 2001;81:514–521 [DOI] [PubMed] [Google Scholar]
- Ivanov VK; Maksioutov MA; Chekin SY; Petrov AV; Biryukov AP; Kruglova ZG; Matyash VA; Tsyb AF; Manton KG; Kravchenko JS The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys 2006;90:199–207 [DOI] [PubMed] [Google Scholar]
- Jacobse JN; Duane FK; Boekel NB; Schaapveld M; Hauptmann M; Hooning MJ; Seynaeve CM; Baaijens MHA; Gietema JA; Darby SC; van Leeuwen FE; Aleman BMP; Taylor CW Radiation Dose-Response for Risk of Myocardial Infarction in Breast Cancer Survivors. International journal of radiation oncology, biology, physics 2019;103:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K; Wang Y; Du L; Xu C; Liu Y; He N; Wang J; Liu Q Research Progress on the Biological Effects of Low-Dose Radiation in China. Dose Response 2019;17:1559325819833488–1559325819833488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto Y; Kaneto H Role of oxidative stress in pancreatic beta-cell dysfunction. . Ann N Y Acad Sci 2004;1011:168–176 [DOI] [PubMed] [Google Scholar]
- Kashcheev VV; Chekin SY; Karpenko SV; Maksioutov MA; Menyaylo AN; Tumanov KA; Kochergina EV; Kashcheeva PV; Gorsky AI; Shchukina NV; Lovachev SS; Vlasov OK; Ivanov VK Radiation risk of cardiovascular diseases in the cohort of Russian emergency workers of the Chernobyl accident. Health Phys 2017;113:23–29 [DOI] [PubMed] [Google Scholar]
- Kashcheev VV; Chekin SY; Maksioutov MA; Tumanov KA; Menyaylo AN; Kochergina EV; Kashcheeva PV; Gorsky AI; Shchukina NV; Karpenko SV; Ivanov VK Radiation-epidemiological study of cerebrovascular diseases in the cohort of Russian recovery operation workers of the Chernobyl accident. Health Phys 2016;111:192–197 [DOI] [PubMed] [Google Scholar]
- Kenchegowda D; Legesse B; Hritzo B; Olsen C; Aghdam S; Kaur A; Culp W; Derrien-Colemyn A; Severson G; Moroni M Selective Insulin-like Growth Factor Resistance Associated with Heart Hemorrhages and Poor Prognosis in a Novel Preclinical Model of the Hematopoietic Acute Radiation Syndrome. Radiat Res 2018;190:164–175. doi: 110.1667/RR14993.14991. Epub 12018 May 14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler T; Vilne B; Schunkert H The impact of genome-wide association studies on the pathophysiology and therapy of cardiovascular disease. EMBO Mol Med 2016;8:688–701. doi: 610.15252/emmm.201506174. Print 201502016 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara H; Ishizaki Y; Suzuki Y; Katoh H; Hamada N; Ohno T; Takahashi T; Kobayashi Y; Nakano T Radiation-induced ICAM-1 Expression via TGF-β1 Pathway on Human Umbilical Vein Endothelial Cells; Comparison between X-ray and Carbon-ion Beam Irradiation. Journal of Radiation Research 2011;52:287–292 [DOI] [PubMed] [Google Scholar]
- Kovacic S; Bakran M Genetic susceptibility to atherosclerosis. Stroke Res Treat 2012;2012:362941.: 10.1155/2012/362941. Epub 362012 Mar 362926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krestinina LY; Epifanova S; Silkin S; Mikryukova L; Degteva M; Shagina N; Akleyev A Chronic low-dose exposure in the Techa River Cohort: risk of mortality from circulatory diseases. Radiat Environ Biophys 2013;52:47–57 [DOI] [PubMed] [Google Scholar]
- Kreuzer M; Auvinen A; Cardis E; Hall J; Jourdain JR; Laurier D; Little MP; Peters A; Raj K; Russell NS; Tapio S; Zhang W; Gomolka M Low-dose ionising radiation and cardiovascular diseases--Strategies for molecular epidemiological studies in Europe. Mutat Res Rev Mutat Res 2015;764:90–100 [DOI] [PubMed] [Google Scholar]
- Kreuzer M; Dufey F; Sogl M; Schnelzer M; Walsh L External gamma radiation and mortality from cardiovascular diseases in the German WISMUT uranium miners cohort study, 1946–2008. Radiat Environ Biophys 2013;52:37–46 [DOI] [PubMed] [Google Scholar]
- Kuch B; Heier M; von Scheidt W; Kling B; Hoermann A; Meisinger C 20-year trends in clinical characteristics, therapy and short-term prognosis in acute myocardial infarction according to presenting electrocardiogram: the MONICA/KORA AMI Registry (1985–2004). J Intern Med 2008;264:254–264. doi: 210.1111/j.1365-2796.2008.01956.x. Epub 02008 Apr 01954. [DOI] [PubMed] [Google Scholar]
- Lall R; Ganapathy S; Yang M; Xiao S; Xu T; Su H; Shadfan M; Asara JM; Ha CS; Ben-Sahra I; Manning BD; Little JB; Yuan ZM Low-dose radiation exposure induces a HIF-1-mediated adaptive and protective metabolic response. Cell Death Differ 2014;21:836–844. doi: 810.1038/cdd.2014.1024. Epub 2014 Feb 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M; Hehlgans S; Reichert S; Gaipl US; Fournier C; Rodel C; Weiss C; Rodel F Study of the anti-inflammatory effects of low-dose radiation: The contribution of biphasic regulation of the antioxidative system in endothelial cells. Strahlenther Onkol 2015;191:742–749. doi: 710.1007/s00066-00015-00848-00069. Epub 02015 Jun 00068. [DOI] [PubMed] [Google Scholar]
- Large M; Reichert S; Hehlgans S; Fournier C; Rodel C; Rodel F A non-linear detection of phospho-histone H2AX in EA.hy926 endothelial cells following low-dose X-irradiation is modulated by reactive oxygen species. Radiat Oncol 2014;9:80.: 10.1186/1748-1717X-1189-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guezennec X; Brichkina A; Huang YF; Kostromina E; Han W; Bulavin DV Wip1-dependent regulation of autophagy, obesity, and atherosclerosis. Cell Metab 2012;16:68–80. doi: 10.1016/j.cmet.2012.1006.1003. [DOI] [PubMed] [Google Scholar]
- Lee CL; Moding EJ; Cuneo KC; Li Y; Sullivan JM; Mao L; Washington I; Jeffords LB; Rodrigues RC; Ma Y; Das S; Kontos CD; Kim Y; Rockman HA; Kirsch DG p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal 2012;5:ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuraud K; Fournier L; Samson E; Caër-Lorho S; Laurier D Mortality in the French cohort of nuclear workers. Radioprotection 2017;52:199–210 [Google Scholar]
- Libby P; Theroux P Pathophysiology of coronary artery disease. Circulation 2005;111:3481–3488. doi: 3410.1161/CIRCULATIONAHA.3105.537878. [DOI] [PubMed] [Google Scholar]
- Little MP A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys 2013;52:435–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP Radiation and circulatory disease. Mutat Res 2016;770:299–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP; Azizova TV; Bazyka D; Bouffler SD; Cardis E; Chekin S; Chumak VV; Cucinotta FA; de Vathaire F; Hall P; Harrison JD; Hildebrandt G; Ivanov V; Kashcheev VV; Klymenko SV; Kreuzer M; Laurent O; Ozasa K; Schneider T; Tapio S; Taylor AM; Tzoulaki I; Vandoolaeghe WL; Wakeford R; Zablotska LB; Zhang W; Lipshultz SE Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012a;120:1503–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP; Kleinerman RA; Stovall M; Smith SA; Mabuchi K Analysis of dose response for circulatory disease after radiotherapy for benign disease. Int J Radiat Oncol Biol Phys 2012b;84:1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP; Lipshultz SE Low dose radiation and circulatory diseases: a brief narrative review. Cardio-Oncology 2015;1:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP; Pawel D; Misumi M; Hamada N; Cullings HM; Wakeford R; Ozasa K Lifetime mortality risk from cancer and circulatory disease predicted from the Japanese atomic bomb survivor Life Span Study data taking account of dose measurement error. Radiat Res 2020;194:259–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP; Tawn EJ; Tzoulaki I; Wakeford R; Hildebrandt G; Paris F; Tapio S; Elliott P A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res 2008;169:99–109 [DOI] [PubMed] [Google Scholar]
- Little MP; Zablotska LB; Brenner AV; Lipshultz SE Circulatory disease mortality in the Massachusetts tuberculosis fluoroscopy cohort study. Eur J Epidemiol 2016;31:287–309 [DOI] [PubMed] [Google Scholar]
- Little MP; Zablotska LB; Lipshultz SE Ischemic heart disease after breast cancer radiotherapy. N Engl J Med 2013;368:2523–2524 [DOI] [PubMed] [Google Scholar]
- Lobrich M; Rief N; Kuhne M; Heckmann M; Fleckenstein J; Rube C; Uder M In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A 2005;102:8984–8989. doi: 8910.1073/pnas.0501895102. Epub 0501892005 Jun 0501895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk GD Metabolic Modulators in Heart Disease: Past, Present, and Future. Can J Cardiol 2017;33:838–849. doi: 810.1016/j.cjca.2016.1012.1013. Epub 2016 Dec 1021. [DOI] [PubMed] [Google Scholar]
- Lorenz MW; Markus HS; Bots ML; Rosvall M; Sitzer M Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–467. doi: 410.1161/CIRCULATIONAHA.1106.628875. Epub 622007 Jan 628822. [DOI] [PubMed] [Google Scholar]
- Luscher TF; Richard V; Tschudi M; Yang ZH; Boulanger C Endothelial control of vascular tone in large and small coronary arteries. J Am Coll Cardiol 1990;15:519–527 [DOI] [PubMed] [Google Scholar]
- Mancuso M; Pasquali E; Braga-Tanaka I 3rd; Tanaka S; Pannicelli A; Giardullo P; Pazzaglia S; Tapio S; Atkinson MJ; Saran A Acceleration of atherogenesis in ApoE−/− mice exposed to acute or low-dose-rate ionizing radiation. Oncotarget 2015;6:31263–31271. doi: 31210.18632/oncotarget.35075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen V; Tenkanen L; Koskinen P; Huttunen JK; Manttari M; Heinonen OP; Frick MH Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation 1992;85:37–45. doi: 10.1161/1101.cir.1185.1161.1137. [DOI] [PubMed] [Google Scholar]
- Maraldo MV; Giusti F; Vogelius IR; Lundemann M; van der Kaaij MA; Ramadan S; Meulemans B; Henry-Amar M; Aleman BM; Raemaekers J; Meijnders P; Moser EC; Kluin-Nelemans HC; Feugier P; Casasnovas O; Fortpied C; Specht L; European Organisation for R; Treatment of Cancer Lymphoma, G. Cardiovascular disease after treatment for Hodgkin’s lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol 2015;2:e492–502 [DOI] [PubMed] [Google Scholar]
- Marsden PA; Goligorsky MS; Brenner BM Endothelial cell biology in relation to current concepts of vessel wall structure and function. J Am Soc Nephrol 1991;1:931–948 [DOI] [PubMed] [Google Scholar]
- Martin OA; Redon C; Nakamura AJ; Dickey JS; Georgakilas AG; Bonner WM Systemic DNA damage related to cancer. Cancer Res 2011;71:3437–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavragani IV; Laskaratou DA; Frey B; Candeias SM; Gaipl US; Lumniczky K; Georgakilas AG Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicology Research 2016;5:12–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavragani IV; Nikitaki Z; Kalospyros SA; Georgakilas AG Ionizing Radiation and Complex DNA Damage: From Prediction to Detection Challenges and Biological Significance. Cancers 2019;11:1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoghegan D; Binks K; Gillies M; Jones S; Whaley S The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005. Int J Epidemiol 2008;37:506–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham LR; Chow EJ; Ness KK; Kamdar KY; Chen Y; Yasui Y; Oeffinger KC; Sklar CA; Robison LL; Mertens AC Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2010;19:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham LR; Gurney JG; Mertens AC; Ness KK; Sklar CA; Robison LL; Oeffinger KC Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer 2005;103:1730–1739 [DOI] [PubMed] [Google Scholar]
- Meacham LR; Sklar CA; Li S; Liu Q; Gimpel N; Yasui Y; Whitton JA; Stovall M; Robison LL; Oeffinger KC Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med 2009;169:1381–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel RE; Hasu M; Bugden M; Wyatt H; Hildebrandt G; Chen YX; Priest ND; Whitman SC Low-dose radiation exposure and protection against atherosclerosis in ApoE(−/−) mice: the influence of P53 heterozygosity. Radiat Res 2013;179:190–199. doi: 110.1667/RR3140.1661. Epub 2013 Jan 1664. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ; Hasu M; Bugden M; Wyatt H; Little MP; Gola A; Hildebrandt G; Priest ND; Whitman SC Low-dose radiation exposure and atherosclerosis in ApoE−/− mice. Radiat Res 2011;175:665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseeva MB; Azizova TV; Grigoryeva ES; Haylock R Risks of circulatory diseases among Mayak PA workers with radiation doses estimated using the improved Mayak Worker Dosimetry System 2008. Radiat Environ Biophys 2014;53:469–477 [DOI] [PubMed] [Google Scholar]
- Muirhead CR; O’Hagan JA; Haylock RGE; Phillipson MA; Willcock T; Berridge GLC; Zhang W Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009;100:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney DA; Yeazel MW; Kawashima T; Mertens AC; Mitby P; Stovall M; Donaldson SS; Green DM; Sklar CA; Robison LL; Leisenring WM Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer S The failing heart--an engine out of fuel. N Engl J Med 2007;356:1140–1151. doi: 1110.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- Neville KA; Cohn RJ; Steinbeck KS; Johnston K; Walker JL Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab 2006;91:4401–4407 [DOI] [PubMed] [Google Scholar]
- Nikpay M; Goel A; Won HH; Hall LM; Willenborg C; Kanoni S; Saleheen D; Kyriakou T; Nelson CP; Hopewell JC; Webb TR; Zeng L; Dehghan A; Alver M; Armasu SM; Auro K; Bjonnes A; Chasman DI; Chen S; Ford I; Franceschini N; Gieger C; Grace C; Gustafsson S; Huang J; Hwang SJ; Kim YK; Kleber ME; Lau KW; Lu X; Lu Y; Lyytikainen LP; Mihailov E; Morrison AC; Pervjakova N; Qu L; Rose LM; Salfati E; Saxena R; Scholz M; Smith AV; Tikkanen E; Uitterlinden A; Yang X; Zhang W; Zhao W; de Andrade M; de Vries PS; van Zuydam NR; Anand SS; Bertram L; Beutner F; Dedoussis G; Frossard P; Gauguier D; Goodall AH; Gottesman O; Haber M; Han BG; Huang J; Jalilzadeh S; Kessler T; Konig IR; Lannfelt L; Lieb W; Lind L; Lindgren CM; Lokki ML; Magnusson PK; Mallick NH; Mehra N; Meitinger T; Memon FU; Morris AP; Nieminen MS; Pedersen NL; Peters A; Rallidis LS; Rasheed A; Samuel M; Shah SH; Sinisalo J; Stirrups KE; Trompet S; Wang L; Zaman KS; Ardissino D; Boerwinkle E; Borecki IB; Bottinger EP; Buring JE; Chambers JC; Collins R; Cupples LA; Danesh J; Demuth I; Elosua R; Epstein SE; Esko T; Feitosa MF; Franco OH; Franzosi MG; Granger CB; Gu D; Gudnason V; Hall AS; Hamsten A; Harris TB; Hazen SL; Hengstenberg C; Hofman A; Ingelsson E; Iribarren C; Jukema JW; Karhunen PJ; Kim BJ; Kooner JS; Kullo IJ; Lehtimaki T; Loos RJF; Melander O; Metspalu A; Marz W; Palmer CN; Perola M; Quertermous T; Rader DJ; Ridker PM; Ripatti S; Roberts R; Salomaa V; Sanghera DK; Schwartz SM; Seedorf U; Stewart AF; Stott DJ; Thiery J; Zalloua PA; O’Donnell CJ; Reilly MP; Assimes TL; Thompson JR; Erdmann J; Clarke R; Watkins H; Kathiresan S; McPherson R; Deloukas P; Schunkert H; Samani NJ; Farrall M A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. doi: 1110.1038/ng.3396. Epub 2015 Sep 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander V; Ingerslev LR; Andersen E; Fabre O; Garde C; Rasmussen M; Citirikkaya K; Baek J; Christensen GL; Aznar M; Specht L; Simar D; Barres R Ionizing Radiation Potentiates High-Fat Diet-Induced Insulin Resistance and Reprograms Skeletal Muscle and Adipose Progenitor Cells. Diabetes 2016;65:3573–3584 [DOI] [PubMed] [Google Scholar]
- OECD. Guidance document on developing and assessing adverse outcome pathways. Paris: OECD Publishing; 2013;(OECD series on testing and assessment number 184; ENV/JM/MONO(2013)6). [Google Scholar]
- Oeffinger KC; Adams-Huet B; Victor RG; Church TS; Snell PG; Dunn AL; Eshelman-Kent DA; Ross R; Janiszewski PM; Turoff AJ; Brooks S; Vega GL Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 2009;27:3698–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC; Mertens AC; Sklar CA; Kawashima T; Hudson MM; Meadows AT; Friedman DL; Marina N; Hobbie W; Kadan-Lottick NS; Schwartz CL; Leisenring W; Robison LL; Childhood Cancer Survivor S Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–1582 [DOI] [PubMed] [Google Scholar]
- Oeffinger KC; Mertens AC; Sklar CA; Yasui Y; Fears T; Stovall M; Vik TA; Inskip PD; Robison LL; Childhood Cancer Survivor S Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2003;21:1359–1365 [DOI] [PubMed] [Google Scholar]
- Oeffinger KC; Sklar CA Abdominal radiation and diabetes: one more piece in the puzzle. Lancet Oncol 2012;13:961–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott OJ; Hertel S; Gaipl US; Frey B; Schmidt M; Fietkau R The Erlangen Dose Optimization trial for low-dose radiotherapy of benign painful elbow syndrome. Long-term results. Strahlenther Onkol 2014;190:293–297. doi: 210.1007/s00066-00013-00504-00061. Epub 02014 Jan 00016. [DOI] [PubMed] [Google Scholar]
- Ott OJ; Micke O; Mucke R; Niewald M; Rodel F; Schafer U; Seegenschmiedt MH; Arenas M; Frey B; Gaipl US Low-dose radiotherapy: Mayday, mayday. We’ve been hit! Strahlenther Onkol 2019;195:285–288. doi: 210.1007/s00066-00018-01412-00061. Epub 02018 Dec 00018. [DOI] [PubMed] [Google Scholar]
- Pakala R; Leborgne L; Cheneau E; Chan RC; Yazdi H; Fournadjiev J; Weber D; Hellinga D; Kolodgie F; Virmani R; Waksman R Radiation-induced atherosclerotic plaque progression in a hypercholesterolemic rabbit: a prospective vulnerable plaque model? Cardiovasc Radiat Med 2003;4:146–151. doi: 110.1016/S1522-1865(1003)00182-00183. [DOI] [PubMed] [Google Scholar]
- Papadia C; Naves LA; Costa SS; Vaz JA; Domingues L; Casulari LA Incidence of obesity does not appear to be increased after treatment of acute lymphoblastic leukemia in Brazilian children: role of leptin, insulin, and IGF-1. Horm Res 2007;68:164–170 [DOI] [PubMed] [Google Scholar]
- Papiez A; Azimzadeh O; Azizova T; Moseeva M; Anastasov N; Smida J; Tapio S; Polanska J Integrative multiomics study for validation of mechanisms in radiation-induced ischemic heart disease in Mayak workers. PLoS One 2018;13:e0209626. doi: 0209610.0201371/journal.pone.0209626. eCollection 0202018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateras IS; Havaki S; Nikitopoulou X; Vougas K; Townsend PA; Panayiotidis MI; Georgakilas AG; Gorgoulis VG The DNA damage response and immune signaling alliance: Is it good or bad? Nature decides when and where. Pharmacol Ther 2015;154:36–56 [DOI] [PubMed] [Google Scholar]
- PDAY. Natural history of aortic and coronary atherosclerotic lesions in youth. Findings from the PDAY Study. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb 1993;13:1291–1298. doi: 1210.1161/1201.atv.1213.1299.1291. [DOI] [PubMed] [Google Scholar]
- Philipp J; Azimzadeh O; Subramanian V; Merl-Pham J; Lowe D; Hladik D; Erbeldinger N; Ktitareva S; Fournier C; Atkinson MJ; Raj K; Tapio S Radiation-Induced Endothelial Inflammation Is Transferred via the Secretome to Recipient Cells in a STAT-Mediated Process. J Proteome Res 2017;16:3903–3916. doi: 3910.1021/acs.jproteome.3907b00536. Epub 02017 Sep 00514. [DOI] [PubMed] [Google Scholar]
- Phillips SM; Padgett LS; Leisenring WM; Stratton KK; Bishop K; Krull KR; Alfano CM; Gibson TM; de Moor JS; Hartigan DB; Armstrong GT; Robison LL; Rowland JH; Oeffinger KC; Mariotto AB Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev 2015;24:653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup JC Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–823 [DOI] [PubMed] [Google Scholar]
- Poglio S; Galvani S; Bour S; Andre M; Prunet-Marcassus B; Penicaud L; Casteilla L; Cousin B Adipose tissue sensitivity to radiation exposure. Am J Pathol 2009;174:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget J-P; Georgakilas AG; Ravanat J-L Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxidants & redox signaling 2018;29:1447–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan D; Khilji MI; Hassan SA; Yusuf SW Radiation-Induced Cardiovascular Disease. Curr Atheroscler Rep 2017;19:22. doi: 10.1007/s11883-11017-10658-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ruiz ME; Vanpouille-Box C; Melero I; Formenti SC; Demaria S Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends in immunology 2018;39:644–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein BS Radiogenomics: Identification of Genomic Predictors for Radiation Toxicity. Semin Radiat Oncol 2017;27:300–309. doi: 310.1016/j.semradonc.2017.1004.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhm W; Woloschak GE; Shore RE; Azizova TV; Grosche B; Niwa O; Akiba S; Ono T; Suzuki K; Iwasaki T; Ban N; Kai M; Clement CH; Bouffler S; Toma H; Hamada N Dose and dose-rate effects of ionizing radiation: a discussion in the light of radiological protection. Radiat Environ Biophys 2015;54:379–401. doi: 310.1007/s00411-00015-00613-00416. Epub 02015 Sep 00415. [DOI] [PubMed] [Google Scholar]
- Saklayen MG The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR; Kahn CR Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806 [DOI] [PubMed] [Google Scholar]
- Sandoo A; van Zanten JJ; Metsios GS; Carroll D; Kitas GD The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 2010;4:302–12.: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M; Minamino T; Toko H; Miyauchi H; Orimo M; Qin Y; Akazawa H; Tateno K; Kayama Y; Harada M; Shimizu I; Asahara T; Hamada H; Tomita S; Molkentin JD; Zou Y; Komuro I p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 2007;446:444–448. doi: 410.1038/nature05602. Epub 02007 Mar 05604. [DOI] [PubMed] [Google Scholar]
- Schneider JG; Finck BN; Ren J; Standley KN; Takagi M; Maclean KH; Bernal-Mizrachi C; Muslin AJ; Kastan MB; Semenkovich CF ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab 2006;4:377–389. doi: 310.1016/j.cmet.2006.1010.1002. [DOI] [PubMed] [Google Scholar]
- Schubauer-Berigan MK; Daniels RD; Bertke SJ; Tseng CY; Richardson DB Cancer mortality through 2005 among a pooled cohort of U.S. nuclear workers exposed to external ionizing radiation. Radiat Res 2015;183:620–631 [DOI] [PubMed] [Google Scholar]
- Seasholtz TM; Brown JH Rho signaling in vascular diseases. Mol Interv 2004;4:348–357 [DOI] [PubMed] [Google Scholar]
- Seegenschmiedt MH; Micke O; Willich N Radiation therapy for nonmalignant diseases in Germany. Current concepts and future perspectives. Strahlenther Onkol 2004;180:718–730. doi: 710.1007/s00066-00004-09197-00069. [DOI] [PubMed] [Google Scholar]
- Shai E; Siegal S; Michael Z; Itzhak K; Ronen R; Dror M; Sylvia B; Adina BH; Ramzia AH; Marina F; Angela C; Dov Y; Joshua W; Arie B Carotid atherosclerotic disease following childhood scalp irradiation. Atherosclerosis 2009;204:556–560. doi: 510.1016/j.atherosclerosis.2008.1009.1030. Epub 2008 Oct 1018. [DOI] [PubMed] [Google Scholar]
- Shamsaldin A; Grimaud E; Hardiman C; Diallo I; de Vathaire F; Chavaudra J Dose distribution throughout the body from radiotherapy for Hodgkin’s disease in childhood. RadiotherOncol 1998;49:85–90 [DOI] [PubMed] [Google Scholar]
- Shimizu Y; Kodama K; Nishi N; Kasagi F; Suyama A; Soda M; Grant EJ; Sugiyama H; Sakata R; Moriwaki H; Hayashi M; Konda M; Shore RE Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ 2010;340:b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H; Takeshita A Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 2005;25:1767–1775 [DOI] [PubMed] [Google Scholar]
- Shoelson SE; Lee J; Goldfine AB Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert W; Trott KR; Azimzadeh O; Tapio S; Zitzelsberger H; Multhoff G Late proliferating and inflammatory effects on murine microvascular heart and lung endothelial cells after irradiation. Radiother Oncol 2015;117:376–381 [DOI] [PubMed] [Google Scholar]
- Simonetto C; Azizova TV; Grigoryeva ES; Kaiser JC; Schöllnberger H; Eidemüller M Ischemic heart disease in workers at Mayak PA: latency of incidence risk after radiation exposure. PloS one 2014;9:e96309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetto C; Heier M; Rospleszcz S; Meisinger C; Then C; Seissler J; Peters A; Kaiser JC Risk for cardiovascular events responds nonlinearly to carotid intima-media thickness in the KORA F4 study. Atherosclerosis 2020a;296:32–39.: 10.1016/j.atherosclerosis.2020.1001.1013. Epub 2020 Jan 1020. [DOI] [PubMed] [Google Scholar]
- Simonetto C; Rospleszcz S; Heier M; Meisinger C; Peters A; Kaiser JC Simulating the dynamics of atherosclerosis to the incidence of myocardial infarction, applied to the KORA population. Statistics in Medicine 2020b;under review [DOI] [PubMed] [Google Scholar]
- Simonetto C; Schöllnberger H; Azizova TV; Grigoryeva ES; Pikulina MV; Eidemüller M Cerebrovascular Diseases in Workers at Mayak PA: The Difference in Radiation Risk between Incidence and Mortality. PloS one 2015;10:e0125904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahis S; Borys JM; Levy E Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid Redox Signal 2017;26:445–461 [DOI] [PubMed] [Google Scholar]
- Stewart FA; Heeneman S; Te Poele J; Kruse J; Russell NS; Gijbels M; Daemen M Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 2006;168:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FA; Hoving S; Russell NS Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res 2010;174:865–869 [DOI] [PubMed] [Google Scholar]
- Takahashi I; Shimizu Y; Grant EJ; Cologne J; Ozasa K; Kodama K Heart disease mortality in the Life Span Study, 1950–2008. Radiat Res 2017;187:319–332 [DOI] [PubMed] [Google Scholar]
- Tapio S Pathology and biology of radiation-induced cardiac disease. J Radiat Res 2016;57:439–448. doi: 410.1093/jrr/rrw1064. Epub 2016 Jul 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa Y; Nakashima E; Yamada M; Funamoto S; Hida A; Akahoshi M; Sakata R; Ross NP; Kasagi F; Fujiwara S; Shore RE Cardiovascular disease risk among atomic bomb survivors exposed in utero, 1978–2003. RadiatRes 2008;170:269–274 [DOI] [PubMed] [Google Scholar]
- Teinturier C; Tournade MF; Caillat-Zucman S; Boitard C; Amoura Z; Bougneres PF; Timsit J Diabetes mellitus after abdominal radiation therapy. Lancet 1995;346:633–634 [DOI] [PubMed] [Google Scholar]
- Tran V; Zablotska LB; Brenner AV; Little MP Radiation-associated circulatory disease mortality in a pooled analysis of 77,275 patients from the Massachusetts and Canadian tuberculosis fluoroscopy cohorts. Sci Rep 2017;7:44147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukenova M; Guibout C; Oberlin O; Doyon F; Mousannif A; Haddy N; Guerin S; Pacquement H; Aouba A; Hawkins M; Winter D; Bourhis J; Lefkopoulos D; Diallo I; de Vathaire F Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J ClinOncol 2010a;28:1308–1315 [DOI] [PubMed] [Google Scholar]
- Tukenova M; Guibout C; Oberlin O; Doyon F; Mousannif A; Haddy N; Guérin S; Pacquement H; Aouba A; Hawkins M; Winter D; Bourhis J; Lefkopoulos D; Diallo I; de Vathaire F Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 2010b;28:1308–1315 [DOI] [PubMed] [Google Scholar]
- Tune JD; Goodwill AG; Sassoon DJ; Mather KJ Cardiovascular consequences of metabolic syndrome. Transl Res 2017;183:57–70.: 10.1016/j.trsl.2017.1001.1001. Epub 2017 Jan 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard VA; Ta BD; van der Schaaf A; Bouma AB; Middag AM; Bantema-Joppe EJ; van Dijk LV; van Dijk-Peters FB; Marteijn LA; de Bock GH; Burgerhof JG; Gietema JA; Langendijk JA; Maduro JH; Crijns AP Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J Clin Oncol 2017;35:1171–1178. doi: 1110.1200/JCO.2016.1169.8480. Epub 2017 Jan 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen FA; Ntentas G; Darby SC; Schaapveld M; Hauptmann M; Lugtenburg PJ; Janus CPM; Daniels L; van Leeuwen FE; Cutter DJ; Aleman BMP Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 2017;129:2257–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen FA; Schaapveld M; Cutter DJ; Janus CPM; Krol AD; Hauptmann M; Kooijman K; Roesink J; van der Maazen R; Darby SC; Aleman BMP; van Leeuwen FE Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 2016;34:235–243 [DOI] [PubMed] [Google Scholar]
- van Waas M; Neggers SJ; Raat H; van Rij CM; Pieters R; van den Heuvel-Eibrink MM Abdominal radiotherapy: a major determinant of metabolic syndrome in nephroblastoma and neuroblastoma survivors. PLoS One 2012;7:e52237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen E The North Karelia Project: Cardiovascular disease prevention in Finland. Glob Cardiol Sci Pract 2018;2018:13. doi: 10.21542/gcsp.22018.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M; Cardis E; Ashmore P; Auvinen A; Bae JM; Engels H; Gilbert E; Gulis G; Habib R; Howe G; Kurtinaitis J; Malker H; Muirhead C; Richardson D; Rodriguez-Artalejo F; Rogel A; Schubauer-Berigan M; Tardy H; Telle-Lamberton M; Usel M; Veress K Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol 2007;36:1126–1135 [DOI] [PubMed] [Google Scholar]
- Wakeford R; Tawn EJ The meaning of low dose and low dose-rate. J Radiol Prot 2010;30:1–3 [DOI] [PubMed] [Google Scholar]
- Wang K; Eblan MJ; Deal AM; Lipner M; Zagar TM; Wang Y; Mavroidis P; Lee CB; Jensen BC; Rosenman JG; Socinski MA; Stinchcombe TE; Marks LB Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017a;35:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K; Pearlstein KA; Patchett ND; Deal AM; Mavroidis P; Jensen BC; Lipner MB; Zagar TM; Wang Y; Lee CB; Eblan MJ; Rosenman JG; Socinski MA; Stinchcombe TE; Marks LB Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2017b;125:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C; Thyagiarajan M; Hunt L; Cox R; Bradley K; Elson R; Hamilton-Shield J; Stevens M; Crowne E Reduced beta-cell reserve and pancreatic volume in survivors of childhood acute lymphoblastic leukaemia treated with bone marrow transplantation and total body irradiation. Clin Endocrinol (Oxf) 2015a;82:59–67 [DOI] [PubMed] [Google Scholar]
- Wei C; Thyagiarajan MS; Hunt LP; Shield JP; Stevens MC; Crowne EC Reduced insulin sensitivity in childhood survivors of haematopoietic stem cell transplantation is associated with lipodystropic and sarcopenic phenotypes. Pediatr Blood Cancer 2015b;62:1992–1999 [DOI] [PubMed] [Google Scholar]
- Wilbers J; Dorresteijn LD; Haast R; Hoebers FJ; Kaanders JH; Boogerd W; van Werkhoven ED; Nowee ME; Hansen HH; de Korte CL; Kappelle AC; van Dijk EJ Progression of carotid intima media thickness after radiotherapy: a long-term prospective cohort study. Radiother Oncol 2014;113:359–363. doi: 310.1016/j.radonc.2014.1010.1012. Epub 2014 Nov 1014. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B; Ridley AJ Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 2002;39:187–199. doi: 110.1016/s1537-1891(1003)00008-00009. [DOI] [PubMed] [Google Scholar]
- Yamada M; Wong FL; Fujiwara S; Akahoshi M; Suzuki G Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res 2004;161:622–632 [DOI] [PubMed] [Google Scholar]
- Yan X; Sasi SP; Gee H; Lee J; Yang Y; Mehrzad R; Onufrak J; Song J; Enderling H; Agarwal A; Rahimi L; Morgan J; Wilson PF; Carrozza J; Walsh K; Kishore R; Goukassian DA Cardiovascular risks associated with low dose ionizing particle radiation. PLoS One 2014;9:e110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yentrapalli R; Azimzadeh O; Barjaktarovic Z; Sarioglu H; Wojcik A; Harms-Ringdahl M; Atkinson MJ; Haghdoost S; Tapio S Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation. Proteomics 2013a;13:1096–1107 [DOI] [PubMed] [Google Scholar]
- Yentrapalli R; Azimzadeh O; Sriharshan A; Malinowsky K; Merl J; Wojcik A; Harms-Ringdahl M; Atkinson MJ; Becker KF; Haghdoost S; Tapio S The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS One 2013b;8:e70024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotska LB; Little MP; Cornett RJ Potential increased risk of ischemic heart disease mortality with significant dose fractionation in the Canadian fluoroscopy cohort study. Am J Epidemiol 2014;179:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivin S; Guseva Canu I; Davesne E; Blanchardon E; Garsi JP; Samson E; Niogret C; Zablotska LB; Laurier D Circulatory disease in French nuclear fuel cycle workers chronically exposed to uranium: a nested case-control study. Occup Environ Med 2018;75:270–276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


