ABSTRACT
Recent years have seen a lot of interest in mycosporine-like amino acids (MAAs) because of their alleged potential as a natural microbial sunscreen. Since chemical ultraviolet (UV) absorbers are unsafe for long-term usage, the demand for natural UV-absorbing substances has increased. In this situation, MAA is a strong contender for an eco-friendly UV protector. The capacity of MAAs to absorb light in the UV-A (320–400 nm) and UV-B (280–320 nm) range without generating free radicals is potentially relevant in photoprotection. The usage of MAAs for purposes other than photoprotection has now shifted in favor of medicinal applications. Aside from UV absorption, MAAs also have anti-oxidant, anti-inflammatory, wound-healing, anti-photoaging, cell proliferation stimulators, anti-cancer agents, and anti-adipogenic properties. Recently, MAAs application to combat SARS-CoV-2 infection was also investigated. In this review article, we highlight the biomedical applications of MAAs that go beyond photoprotection, which can help in utilizing the MAAs as promising bioactive compounds in both pharmaceutical and cosmetic applications.
KEYWORDS: mycosporine-like amino acids, microbial sunscreen, photo-protection, biomedical application, UV-absorbing compounds
INTRODUCTION
Mycosporines and mycosporine-like amino acids (MAAs) are a large family of natural ultraviolet (UV) absorbing compounds evolved for protection against chronic UV radiation exposure in a wide variety of organisms such as cyanobacteria, microalgae, fungi, seaweeds, corals, and lichens, as well as in freshwater and marine animals (1, 2). The UV radiation reaching the earth’s surface comprises <1% UV-B (280–320 nM) and <7% UV-A (320–400 nM). However, the ozone layer absorbs the remaining UV radiation before reaching the stratosphere. The incident 10% of UV rays are highly energetic and are responsible for several skin diseases such as sunburn, erythema, edema, premature aging, hyperpigmentation, immune suppression, and even DNA damage leading to skin cancer (3 – 5). Therefore, the application of sunscreen has gained much attention to reduce the harmful effects of UV irradiation. The harmful effects produced by chemical UV filters have made researchers look for the replacement of chemical sunscreens with natural UV-absorbing compounds.
In 1993, the evidence for the photo-protectant activity of MAAs was confirmed by their ability to block photons. It was revealed that MAAs can prevent 3 out of 10 photons from hitting the cytoplasm of cyanobacteria. Various literatures have reported the efficiency of MAAs in photo protection (6). This ability has led MAAs to be known as primary sunscreen. Several organisms produce MAAs as natural UV protector. The difference in UV-absorbance lies in the type of core chromophore, which is either cyclohexenone or cyclohexenimine structure depending on oxo-mycosporines or imino-mycosporine like amino acids and having absorption maxima ranging from 310 to 362 nM (7).
To understand the biological roles of MAAs, pure fractions of these molecules must be produced. Various extraction and separation techniques have been developed and evaluated on a wide range of organisms (8). MAAs were extracted using methanol and ethanol in concentrations ranging from 20% to 80%. However, the possibility of using MAAs for cosmetic applications depends on their isolation using green solvents like water and ethanol and the avoidance of some organic solvents like methanol because of their toxicity. On the other hand, various chromatographic methods have been employed to isolate and characterize MAAs. The most common method is reverse-phase high-performance liquid chromatography (HPLC) analysis using retention time and UV spectra; for molecular weight analysis, mass spectrometry (MS), including liquid chromatography (LC)–MS has been used so far. The structural elucidation was established by tandem mass spectrometry (MS/MS) and nuclear magnetic resonance (NMR 1D and 2D) spectroscopy (3). Recently, the ultrahigh-performance liquid chromatography (UHPLC) method with diode-array detection (DAD) was used to characterize 11 MAAs from various algal species. When compared to standard protocols for MAAs analysis, UHPLC-MS shows high separation efficiency with shorter analysis time as well as significant versatility regarding the detection mode. This can be utilized for further studies on MAAs (9).
MAAs have mainly been studied for their application as UV protective molecules. However, they have bioactivities that extend beyond photo-protection. In addition to UV absorption, MAAs also have antioxidant, anti-inflammatory, anti-aging, wound-healing, anti-cancer, anti-adipogenic properties, and protect DNA damage. Recently, it was also discovered that MAAs can act as an anti-viral agent against COVID-19 infection (4, 10). These multiple roles of MAAs make them interesting biomolecules. These characteristics indicate MAAs to be a promising candidate for natural sunscreen along with other pharmaceutical and cosmetic applications. The purpose of this mini-review is to summarize the identified bioactivities of MAAs, which suggest that they can be used as a therapeutic candidate in addition to their well-known function as a sunscreen component. In particular, we focus on the biomedical applications of MAAs other than photo-protectants. The chemical structures and biological functions of commonly found MAAs are summarized in Table 1.
TABLE 1.
Different bioactive properties of MAAs
| Compound | Structure | Bioactive effect | Assay type/Model | Source | Concentration | Reference |
|---|---|---|---|---|---|---|
| Mycosporine-glycine |

|
Antioxidant Property | PC assay | Palythoa tuberculosa | 15 µM | (11) |
| ABTS+ decolorization method | Lichina pygmaea | 3 µM (IC50) | (12) | |||
| Human fibroblast cell line HaCaT, DPPH assay | Chlamydomonas hedleyi | 4.23 ± 0.21 µM (IC50) | (13) | |||
| Anti-inflammatory Activity | HaCaT cell lines, COX-2 mRNA (decrease) | Chlamydomonas hedleyi | 300 µM | (14) | ||
| Anti-aging | HaCaT cell lines, Involucrin (decrease), Elastin (increase) | Chlamydomonas hedleyi | 150 µM | (14) | ||
| Wound Healing Property | HaCaT human keratinocytes | Chlamydomonas hedlyei, Porphyra yezoensis | 0.1 mg/mL | (14) | ||
| Shinorine |

|
Anti-inflammatory Activity | HaCaT cell lines, COX-2 mRNA (decrease) | Chlamydomonas hedleyi | 30 µM | (13) |
| Anti-aging | HaCaT cell lines, Involucrin (decrease), PCOLCE (increase) | Chlamydomonas hedleyi | 150 µM | (13) | ||
| Anti-adipogenic Effect | 3T3-L1 preadipocytes, PPARγ2 (decrease), adiponectin (decrease), C/EBPα (decrease), leptin (decrease) | Porphyra denta | 0.1 µM | (15) | ||
| Wound-healing Property | HaCaT human keratinocytes | Chlamydomonas hedlyei, Porphyra yezoensis | 0.05 mg/mL | (16) | ||
| HaCaT human keratinocytes, scratch assay | Bostrychia scorpiodes | 1.0 and 10 µM | (17) | |||
| Anti-viral | ACE2 receptor protein (molecular docking) | PubChem database | 7.0 kcal/mol | (10) | ||
| Porphyra-334 |

|
Anti-aging | HaCaT cells PCOLCE (increase), elastin (increase) |
Chlamydomonas hedleyi | 150 µM | (13) |
| Anti-adipogenic Effect | 3T3-L1 preadipocytes, PPARγ2 (decrease), C/EBPα (decrease), leptin (decrease), adiponectin (decrease) | Porphyra dentata | 1.0 µM | (15) | ||
| Wound-healing Property | HaCaT human ke ratinocytes | Chlamydomonas hedlyei, Porphyra yezoensis | 0.05 mg/mL | (16) | ||
| HaCaT cell lines, Scratch assay | Bostrychia scorpiodes | 1.0 and 10 µM | (17) | |||
| Usujirene |

|
Antioxidant Property | In-vitro, FTC aasay | Porphyra yezoensis | 2 mg/mL | (18) |
| Asterina-330 |

|
Antioxidant Property | In-vitro, ABTS aasay | Gelidium corneum | 10 µM | (19) |
| Palythine |

|
Antioxidant Property | In-vitro, ABTS aasay | Gelidium corneum | 10 µM | (19) |
| Bostrychine-B |
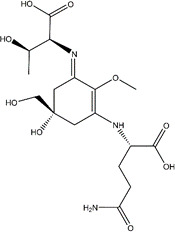
|
Wound-healing Property | Scratch Assay | Bostrychia scorpiodes | 10 µM | (17) |
| Mycosporine glycine-valine |

|
Anti-viral | ACE2 receptor protein (molecular docking) | PubChem database | 7.2 kcal/mol (binding energy) |
(10) |
The review starts its journey from the evolution of MAA to its diverse bioactivities and finally ends with concluding remarks.
Evolution and distribution of MAAs
The MAAs have been isolated from various organisms inhabiting diverse environments; mainly those are exposed to high solar radiation, high temperature, as well as desiccation and other stresses. They are widely distributed in nature. The first mycosporine-like amino acids (mycosporines P310) were discovered in the 1960s from terrestrial fungi Stereum hirsutum associated with light-stimulated sporulation. Cyanobacteria are thought to be the primitive producers of MAAs, but later, the genes responsible for the biosynthesis of MAAs get transferred to other organisms through symbiosis or by the food chain (20). In cyanobacteria, shinorine is the MAA that is most prevalent. The MAAs such as mycosporine-glycine and shinorine were isolated from cyanobacteria exposed to high solar radiation inhabiting paddy fields, rooftops and bark of trees, which aids in photoprotection (21, 22). MAAs such as asterina-330, mycosporine-glycine, and shinorine were also reported in freshwater cyanobacteria inhabiting high-mountain lakes found in Austria’s Central Alps (23). Besides photoprotection, shinorine and porphyra-334 help in absorbing UV-B radiation and maintaining the surface blooms in Microcystis aeruginosa (24). A high level of MAAs was first reported in the halophilic cyanobacterial community inhabiting the gypsum crust formed in the bottom of the hypersaline pond in Eilat, Israel (25). Rastogi et al. (26), isolated different MAAs from the cyanobacterial mat obtained from an old temple located in Bangkok, Thailand. The MAAs from the cyanobacterial mat was found to be porphyra-334, shinorine, palythiol, and mycosporine-glycine. They also found two unknown MAAs having absorption shown at 320 and 329 nm, respectively. The cyanobacterial mat includes Nostoc sp., Gloeocapsa sp., Synechocystis sp., Gloeocapsopsis sp., and Scytonema sp.
Later MAAs were identified from various organisms such as microalgae, macroalgae, lichens, heterotrophic bacteria, corals, sea urchins, dinoflagellates, sponges, and also from vertebrates (fishes) (11, 27 – 36). Several studies concluded that animals could acquire MAAs through algal or bacterial symbiosis or through their food chain (22). Besides the dietary source, gadusol, the precursor compound for the de novo synthesis of MAAs, was found in corals and fish (33, 37, 38). However, MAAs were not reported in higher plants, in which flavonoids help in UV protection. While in higher vertebrates, melanin helps in photoprotection (39).
Previous studies reported that MAAs could influence the cells to tolerate various abiotic stressors such as salinity, desiccation resistance, and thermal stress (40 – 42). Peinado et al. (43) showed that MAAs could also serve as an internal nitrogen storage. It was found that ammonium ions and ultraviolet radiation worked together to boost MAAs level. The accumulation of MAAs as intracellular nitrogen storage molecules would lead to nitrogen mobilization in the absence of other forms of nitrogen. A possible role for MAAs as nitrogen storage molecules has been proposed; however, there is currently insufficient data about the intracellular breakdown of MAAs and the release of nitrogen atoms.
In addition to the functions mentioned above, MAAs can regulate light-stimulated fungal sporulation and germination, mainly among the fungal phylum Basidiomycetes, Ascomycetes, Deuteromycetes, and Zygomycetes (44, 45). The function of MAAs in marine invertebrate reproduction was also investigated (46, 47).
Structural and functional diversity of MAAs
Mycosporines and mycosporine-like amino acids, known as “MAAs” comprise a large family of more than 40 compounds. These are low molecular weight (<400 Da), water-soluble, colorless UV-absorbing compounds that protect the organism against the high intensity of UV radiation. Their absorption maxima range between 309 and 362 nm depending on the type of ring and its substituents, with large molar extinction coefficients ɛ = 28,100–50,000 M−1.cm−1 (11). Most MAAs are stable throughout a wide range of temperatures (up to 60°C) and pH (up to pH-11), however, these may not apply to all MAAs.
MAAs have a cyclohexenone or cyclohexenimine ring as their core chromophore, and in the C1 position, they differ by having an oxo or an imino moiety. The methoxy moiety and amino group are present in both types of mycosporines at the C2 and C3 positions. In the case of mycosporines, the C1 position is occupied by the keto group, commonly known as oxo- mycosporines or mono-substituted mycosporines (Fig. 1) (48). The mono-substituted mycosporines include mycosporine-glycine, mycosporine-alanine, mycosporine-taurine, mycosporine-serine, mycosporine-serinol, mycosporine hydroxylglutamicol, mycosporine-glutaminol, mycosporine glutamine, mycosporine glutamicol, and collemin A, having an absorption maximum at 310 nM which is in UV-B range. Imino or di-substituted MAAs includes palythine, porphyra-334, shinorine, mycosporine-glycine-valine, mycosporine-2-glycine, catenelline, asterina-330, mycosporine-glutamic acid-glycine, aplysiapalythine A, B, C, usujirene, 13-O-β-galactosyl-porphyra-334, and euhalothece-263, having an absorption maximum varies from 320 to 362 nm in the UV-A region (20, 49 – 51).
Fig 1.
(A) Core structure of Mycosporine (Cyclohexenone ring), where R = glycine or taurine. (B) Core structure of Mycosporine-like amino acids (Cyclohexenimine ring), where R1 = amino acid residue, R 2 = H or CH3 or CH2COO−, R3 = H or SO3 −.
Recently Orfanoudaki et al. (52) isolated six novel MAAs named Bostrychines A to F from the red algae Bostrychia scorpioides. Its absorption maximum ranges from 322 to 337 nm. All the novel MAAs had a cyclohexenimine backbone with glutamine, glutamic acid, and threonine as the amino acid side chains.
Biosynthesis of MAAs
Cyanobacteria are the primitive producer of MAAs; subsequently, the genes involved in their biosynthesis are transmitted to other organisms. MAAs biosynthesis takes place through the first branch of the shikimate pathway (53), in which phosphoenolpyruvate (derived from pyruvate or oxaloacetate) and erythrose 4-phosphate (derived from the pentose phosphate pathway) are combined to form 3-deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) by the enzyme deoxy-D-arabino-heptulosonate phosphate synthase. Furthermore, the DAHP gets converted into 3-dehydroquinate (3-DHQ), by the enzyme 3-dehydroquinate synthase. The common precursor for all MAAs is 3-DHQ, having a six-membered carbon ring which further gets converted to cyclohexenones (gadusol) and then to 4-deoxygadusol (4-DG) (54, 55). However, some studies also reported that 4-DG can be derived from the pentose phosphate pathway, utilizing the precursors ribose-5-phosphate (from glucose) and 3-hydroxypyruvate (from serine) which will lead to the formation of an intermediate sedoheptulose-7-phosphate (SH-7-P) (3, 55, 56). Subsequently, the enzyme 2-epi-5-epi-valiolone (EV) synthase catalyzes the transformation of the SH-7-P into EV, which is then turned into 4-DG (3, 56, 57). In both routes, the enzyme O-methyltransferase is involved in the formation of 4-DG, which is the core structure for the synthesis of mycosporines and MAAs. The biosynthetic pathways and types of MAAs produced are summarized in Fig. 2.
Fig 2.
The biosynthetic pathways and types of Mycosporines and Mycosporine-like amino acids produced.
Mycosporine-glycine was the first MAA synthesized by adding the amino acid glycine to the 4-deoxygadusol by the enzyme ATP-grasp amino acid ligase. The mycosporine-glycine served as a precursor for other mono- and bi-substituted mycosporines through chemical or biochemical means, specifically through amino acid substitution. The di-substituted MAAs were synthesized by the addition of single amino acid residues such as serine, threonine, etc., to the simple mono-substituted mycosporines yielding common MAAs such as shinorine and porphyra-334. Other MAAs were synthesized by the modifications in the nitrogen substituents and attached side groups (e.g., dehydration, esterification, decarboxylation, amidation, sulfonation, hydroxylation, and glycosylation). The difference in absorption spectra of MAAs is due to the variation in amino acids side chains (5, 20).
Various environmental factors, such as light, temperature, salt concentration, nutrient availability, and seasonal fluctuations, can alter the MAAs concentration. However, high UV radiation, basic pH, and high nitrate and phosphate concentration can significantly increase the MAAs content (58, 59).
Biomedical potentials of MAAs
MAAs are well-known for their photoprotection and as a potential source of environmental-friendly sunscreen. In addition to photoprotection, other bioactive properties of MAAs studied are summarized in Table 1.
Natural sunscreen
The MAAs are the most studied photoprotective agent or “microbial sunscreen” due to their ability to provide broad UV ray protection and therefore are gaining huge commercial interest. Prolonged UV exposure induces skin photoaging, wrinkles, tanning, and even skin cancer risk. To overcome this, physical/chemical UV filters formulated into sunscreens are marketed. However, it can cause harmful effects on human health as well as on the ecosystem due to bioaccumulation. The application of MAAs as natural sunscreen has been well explored in recent years. MAAs can prevent 3 out of 10 photons hitting on a cytoplasmic target in cyanobacteria (60). MAAs show high absorption of UV radiation ranging from 310 to 365 nm. Various characteristics of MAAs include photostability, the ability to prevent UV-induced thymine dimer formation, high molar extinction coefficients, capable of absorbing the high energy photons without generating reactive oxygen species (ROS), dissipating the energy as heat, and resistance to various abiotic stressors demonstrates that MAAs are a promising photo-absorbing compound (50, 56, 61).
Many studies reported the application of MAAs as sunscreen formulation; however, they are not yet produced on a large scale, and till now, only a few products have been commercialized. Commercially available sunscreen products include Helioguard365 and Helionori. Helioguard365 is a natural sunscreen product derived from the red algae Porphyra umbilicalis that contains porphyra-334 and shinorine. Helioguard365 offers both anti-aging and photoprotective properties against UV-A-induced skin damage and increased cell viability in a dose-dependent manner (62). This commercial product has extensive stability, lasting 3 mo at temperatures ranging from 4˚C to 37˚C (44, 62). Furthermore, Torres et al. (51) measured the SPF value of collemin A and olive oil (1:10) formulation on the inner forearm of a volunteer at a concentration of 6 µg cm−2. The treated region was subjected to four minimal erythema doses (MEDs) 360 mJ cm−2 of UV-B radiation 15 min after application, and erythema was detected 24 h later. They discovered that when compared to the positive control, octinoxate commercial sunscreen, their formulation was similarly effective. However, the studies dependability is limited due to the involvement of only one participant. de la Coba et al. (63) investigated the SPF value of a sunscreen formulation containing 4.1:2.9% porphyra-334 with shinorine as a UV-A filter and mycosporine-serinol as a UV-B filter. They discovered that these MAAs had SPF values ranging from 4 to 6. However, when combined, the value improved to 8.37 ± 2.12, which is very close to the value of 9.54 ± 1.53 for the reference sunscreen formulation containing avobenzone as the UV-A filter and octinoxate as a UV-B filter in 4.5:2.6% ratio, respectively.
Helionori is another commercially available natural sunscreen derived from the red algae Porphyra umbilicalis that contains shinorine, palythine, and porphyra-334. The UV-A protection was demonstrated by this substance in human fibroblast and keratinocyte cell lines. This product has high resistance to light and temperature, and it can also give maximal DNA preservation by retaining membrane lipids (8, 64).
DNA damage/erythema protecting activity
Prolonged exposure to UV radiation leads to skin diseases such as sunburn, erythema, edema, premature aging, hyperpigmentation, immune suppression, and even melanoma and non-melanoma skin cancers. The most common form of DNA damage induced by the UV-B rays involves the dimerization of pyrimidines, which produces cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6–4) pyrimidone photoproducts [(6–4) PPs]. CPDs can also be induced by UV-A but to a lower extent. In addition, both UV-B and UV-A can stimulate the formation of oxidized DNA bases such as 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG) via an indirect mechanism. CPDs are persistent lesions and are substantially mutagenic when they contain cytosine or 5-methyl cytosine. However, oxidized bases contribute to UV mutagenesis only in a minor way since it gets repaired rapidly (65). Misonou et al. (66) analyzed the MAAs to block the thymine photo dimer formation. They showed that MAAs such as porphyra-334, palythine, and shinorine, from Porphyra yezoensis can block UV-induced DNA lesions like CPDs and 6–4 photoproduct.
Studies on the photo-protective activity of mycosporine; Collemin-A found that it can partially prevent UV-B-induced pyrimidine dimer formation. Additionally, Collemin-A can completely prevent UV-B-induced erythema (sunburn) on the human skin when applied prior to the irradiation (51).
MAAs as antioxidants
High-intensity UV radiation produces ROS, leading to the DNA damage (67). Antioxidants can prevent oxidative stress and lessen the negative consequences of ROS. High-energy photons can be absorbed by MAAs, which then release the energy as heat and scavenge ROS like superoxide anions, hydroxyl radicals, hydroperoxyl radicals, and singlet oxygen (42). Yakovleva et al. (19) were the first to report the antioxidant activity of MAA. They analyzed the thermal stress susceptibility of two scleractinian corals, Platygyra ryukyuensis and Stylophora pistillata. High temperature disrupts photosynthetic machinery and increases antioxidant enzymes such as superoxide dismutase and catalase in Stylophora pistillata, while in the case of Platygyra ryukyuensis shows little effect of these enzymes. The difference in susceptibility is due to the high initial concentration of mycosporine-glycine in Platygyra compared to Stylophora. When temperature exposure was prolonged to 12 h, mycosporine-glycine concentration tended to decrease in Platygyra and was completely depleted in Stylophora. The oxidative stress cause reduction in the abundance of intracellular mycosporine-glycine but not of other MAAs. The increase in activity of antioxidant enzymes of Stylophora, which contains a low concentration of mycosporine-glycine while delay in the onset of oxidative stress in Platygyra, having a high concentration of mycosporine-glycine suggests that it provides rapid protection in oxidative stress before the induction of antioxidant enzymes. They concluded that the biological antioxidant mycosporine-glycine plays a crucial role in coral tissue and zooxanthellae in ensuring coral reefs survive under thermal stress (19).
Coba et al. (12) studied the in vitro antioxidative activity and inhibition of lipid peroxidation in the water-soluble medium by MAAs. The hydrosoluble radical scavenging activity of MAAs (shinorine, asterina-330, palythine, and mycosporine-glycine) was found to be dose-dependent and it increased with the alkalinity of the medium from pH 6 to 8.5. Mycosporine-glycine and shinorine exhibited high ROS scavenging activity, although mycosporine-glycine showed eight-fold higher antioxidative activity compared to ascorbic acid at pH 8.5. Studies on the antioxidant activity of porphyra-334 on human skin fibroblasts show a dose-dependent reduction of UV-A-induced ROS based on the modified 2'−7'-dichlorofluorescein diacetate (DCF-DA) fluorescence assay (68).
MAAs antioxidant properties may help scavenge free radicals caused by UV radiation or other environmental stress. These compounds neutralize free radicals by adding an electron to form an electron pair. Studies performed on molecular mechanism led to the hypothesis that the carbonyl group of the cyclohexenone chromophore can be responsible for singlet oxygen quenching activity of mycosporine-glycine. It is also more susceptible to oxidation, making it as superior antioxidant because it has a lower redox potential and can donate an electron to stabilize a free radical. The stronger antioxidant effects of cyclohexenimine-type MAAs show hydrogen ion removal from the cyclohexenimine ring at C-4, C-6, or the methylene group at C-9 of the glycine residue, together with the resonance stabilization of the double bond in the carbon ring structure.
Different abiotic stresses such as salinity, temperature, acidity, and desiccation can increase the antioxidant properties of MAAs (7, 41, 42). Kageyama et al. (7) summarized both in vitro and in vivo radical scavenging activity of mono- and di-substituted MAAs. Tarasuntisuk et al. (69) showed the antioxidant effects of mycosporine-2-glycine in RAW-264.7 macrophages under the stimulation of lipopolysaccharide (LPS) by inhibiting the antioxidant-associated genes, Cu/Zn-superoxide dismutase (Sod1), nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2), and catalase (Cat).
Recent studies conducted by Nishida et al. (13) conclude that the antioxidant activity was increased by the heated MAAs. They isolated palythine and porphyra-334 from the red alga dulse and performed 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) radical scavenging activity and found that at pH 5.8–8.0, the heated MAAs exhibited a high ABTS radical scavenging activity. A possible explanation for the enhanced oxidation of imino-MAAs, is that the heat is required to disperse the absorbed energy. In fact, heat frequently increases the oxidation process because temperature can operate as a form of activation energy.
Anti-inflammatory activity
The role of MAAs as an anti-inflammatory agent has been studied in immortal human keratinocytes (HaCaT cells) and RAW264.7 macrophage models. Suh et al. (14) analyzed the anti-inflammatory effect of porphyra-334, shinorine, and mycosporine-glycine on UV-induced HaCaT cell lines, and results showed that only mycosporine-glycine and shinorine inhibited the COX-2 (inflammation-related marker gene) expression in a concentration-dependent manner, while porphyra-334 did not show any significant response. Likewise, mycosporine-2-glycine shows anti-inflammatory activity in LPS-stimulated RAW264.7 macrophages. Transcriptional analysis showed that the expression of iNOS (inducible NO synthase) and COX-2 were suppressed by mycosporine-2-glycine, and thereby it repressed the nuclear factor kappa B (NF-κB) pathway (69).
The immunomodulatory effect of porphyra-334 and shinorine was studied in human myelomonocytic THP-1 and THP-1-Blue cells under the stimulation of LPS. Results showed that shinorine increases the activation of transcription factor NF-κB in a dose-dependent manner. In contrast, porphyra-334 showed a reduction in NF-κB activity, thereby concluding its anti-inflammatory action (70).
These findings concluded that MAAs such as mycosporine-glycine, mycosporine-2-glycine, and shinorine are anti-inflammatory in nature. Hence, they can be used in antiallergic treatment as well as in oxidative stress.
MAAs as anti-photoaging agents
Prolonged exposure of UV radiation can cause degradation of collagen fiber and reduction in elastin content leading to photoaging (71). UV radiation induces oxidative stress as well as DNA mutations, thereby increasing the rate of skin aging (72 – 74). Like anti-inflammatory activity, Suh et al. (14) also analyzed the expression of genes responsible for skin aging (procollagen C proteinase enhancer (PCOLCE), involucrin and elastin). Results showed that adding MAAs can increase the mRNA expression of UV-suppressed genes. Interestingly, MAAs such as mycosporine-glycine and shinorine downregulated the mRNA expression of involucrin on UV exposure but not by porphyra-334.
Collagen, the primary structural protein of the extracellular matrix (ECM), provides the cell with a supportive framework and is responsible for the tensile strength, elasticity, and hydration of the epidermis. Increased synthesis of dermal enzymes (collagenase and elastase) in inflamed tissue following UV radiation or during wound healing leads to the eventual destruction of ECM. This affects the dermal connective tissues functioning, elasticity, moisture, and thickness negatively. In contrast, the proliferation and differentiation of keratinocytes towards a healthy epidermal barrier, result in skin sagging and development of wrinkles (75). Anti-collagenase activity of MAAs was studied by Hartmann et al. (76). They found that collagenase from Clostridium histolyticum was inhibited by shinorine, porphyra-334, and palythine with IC50 (half-maximal inhibitory concentration) values of 104.0, 105.9, and 158.9 µM, respectively. A dose-dependent moderate inhibition was observed for all the MAAs. Moreover, molecular docking studies suggested that the MAAs binding to the active site of the enzyme via competitive inhibition.
Rui et al. (74) studied the anti-aging properties of ICR-mice (Institute for Cancer Research) and concluded that MAAs could suppress the degradation of collagen and elastin. Recently the inhibitory effects of MAAs on protein glycation were also studied. Glycation of proteins leads to the generation of advanced glycation end-products (AGEs), which are involved in the progression of aging and age-related diseases. They analyzed the glycation-dependent crosslinking of hen egg white lysozyme (HEWL; structural analogue of human lysozyme) with the addition of mycosporine-2-glycine and a mixture of shinorine and porphyra-334 (isolated from Aphanothece halophytica). Among these, mycosporine-2-glycine showed more significant activity than the mixture of porphyra-334 and shinorine. These observations suggested that mycosporine-2-glycine can prevent the formation of AGEs (16). Likewise, various studies confirmed the MAAs as anti-photoaging agents (68, 74, 76).
Wound healing properties of MAAs
Choi et al. (17) explored the wound-healing property of MAAs from Chlamydomonas hedlyei and Porphyra yezoensis on human keratinocytes (HaCaT). They showed that shinorine (0.05 mg/mL), porphyra-334 (0.05 mg/mL), and mycosporine-glycine (0.1 mg/mL) isolated from the C. hedlyei and P. yezoensis promoted wound repair in the HaCaT human keratinocytes. The signaling pathways involved in the MAA-dependent wound healing on skin cells are mitogen-activated protein kinases (MAP kinases) and focal adhesion kinases (FAK). The MAAs stimulate the phosphorylation of FAK at tyrosine 397, which in turn activates the extracellular signal-regulated kinases (ERK) associated with MAP kinases and c-Jun N-terminal kinases (JNK). Therefore, MAAs promote the wound healing through the activation of three kinases mainly FAK, JNK, and ERK.
Similar to this, Orfanoudaki et al. (15) demonstrated that the MAAs extracted from the red algae Bostrychia scorpiodes had wound-healing and anti-aging properties on human keratinocytes cell line HaCaT by carrying out three distinct assays, including the wound healing assay (scratch assay), inhibition of collagenase, and inhibition of advanced glycation end products (AGEs). After 24 h of treatment, they discovered that MAAs like shinorine, porphyra-334, mycosporine-alanine-glycine, and bostrychine-B exhibited increased cellular proliferation and migration in a dose-dependent manner. A marginally better ability to heal wounds was exhibited by bostrychine B.
Anti-adipogenic effect of MAAs
Recent studies found that MAAs such as shinorine and porphyra-334 from Porphyra sp. shows in vitro anti-adipogenic effect in 3T3-L1 mature adipocytes (77). Both compounds suppress the lipid droplet accumulation and adipocyte differentiation during adipogenesis of 3T3-L1 cells in a dose-dependent manner (0.1 and 1.0 µM), without causing a cytotoxic effect (<200 µg/mL). Furthermore, these compounds also reduce the mRNA expression of adipogenesis-related genes such as peroxisome proliferator-activated receptor γ2 (PPARγ2), adiponectin, CCAAT/enhancer-binding protein alpha (C/EBPα), and leptin in 3T3-L1 cells. These findings concluded that MAAs such as shinorine and porphyra-334 have a potential inhibitory effect on adipogenesis in 3T3-L1 preadipocytes. The mechanism of action was shown in Fig. 3. Similar results were also reported by Kim et al. (78). They found that 3T3-L1 adipocytes treated with MAAs-rich methanolic extract from Porphyra yezoensis (laver extract) lowered the lipid accumulation and decreased expressions of adipogenic proteins, such as C/EBPα, fatty acid binding protein 4 (FABP4), and fatty acid synthase (FAS), suggesting that it can down-regulate the transcription of adipogenic genes. The absorption spectrum of the laver extract showed λmax at 334 nM, which is the range of porphyra-334 or shinorine. Additionally, laver extract also reduced preadipocyte proliferation and the removal of mature adipocytes through apoptosis. These findings concluded that MAAs has a potential effect in controlling obesity.
Fig 3.
Graphical representation of anti-adipogenic activity.
MAAs as an anti-cancer agent
MAAs can act as an anti-cancer agent, due to their anti-proliferative nature on neoplastic cells. The study made by Yuan et al. (79) confirmed that MAAs such as shinorine, palythinol, palythine, asterina-330, and porphyra-334 from red algae Palmaria palmata (dulse), could inhibit the proliferation of murine skin melanoma cell line B16-F1 in a dose-dependent manner. After 48 h of incubation, dulse extracts from locations having low (grade 1) and high (grade 2) UV radiation show half maximal effective concentration (EC50) inhibition values of 5.3 and 3.2 mg mL−1, respectively. The increased anti-proliferative activity of grade 2 dulse extract was likely due to the presence of an additional MAA, usujirene; the least polar MAA. The reduced polarity of usujirene may have permitted the passive absorption of this MAA into the murine skin melanoma cell membranes, hence strengthening the antiproliferative actions of this extract.
Similar results were also shown by methanolic extracts from both wild-harvested (Palmaria palmata Chondrus crispus, and Mastocarpus stellatus) and cultivated (C. crispus) red macroalgae, with the same MAAs profile (80). The study found that the concentration between 0.125 and 4 mg mL−1 had an increasing anti-proliferative effect on both HeLa cells from cervical cancer and U-937 cells from histiocytic lymphoma. In addition, the extracts from wild P. palmata and cultivated C. crispus exhibited characteristic apoptotic alterations on the HeLa cells, concluding that the antiproliferative effect is mediated by the induction of apoptosis.
Misonou et al. (66) analyzed that MAAs can block the thymine photo dimer formation. They showed that MAAs from Porphyra yezoensis can block UV-induced DNA lesions such as CPDs and 6–4 photoproduct. It was also discovered that the MAAs from the P. yezoensis extract can trigger apoptosis in UV-damaged HaCaT cells, a potential method for eliminating cancer cells (81).
Bozkurt et al. (18) recently reported that genetically engineered MAAs containing Bifidobacterium sp. can protect against colorectal cancer. They found that MAAs can inhibit the thiobarbituric acid reactive oxygen species (TBAR), which were over-expressed in colorectal cancer. The Bifidobacterium animalis subspecies lactis bacterium, a common member of the human colon microbiota that aids in immune-modulatory and anti-inflammatory effects on host cells; when combined with the gene that produces MAA, the strain exerts even more potent immune-stimulatory properties, and can become an effective strain against colorectal cancer prevention.
MAAs as inhibitors against SARS-CoV-2 infection
SARS-CoV-2 or COVID-19 infection widely spread across the world in recent years. Researches are focused on the natural inhibitors which can combat SARS-CoV-2 infection. Recently molecular docking studies carried out by Sahu et al. (10) showed the inhibitory effect of MAAs on viral adhesion. They used mycosporine–glycine–valine, scytonemin, shinorine, reduced scytonemin, tetramethoxyscytonemin, dimethoxyscytonemin, and scytonemin for the analysis. The computational studies concluded that MAAs such as mycosporine–glycine–valine and shinorine can effectively bind to the active sites of human angiotensin-converting enzyme (ACE2) receptor protein without any cell cytotoxicity. The blocking of the receptor-binding domain of ACE2 can prevent viral establishment on host cells. These in silico studies can be explored further as a drug candidate against COVID-19 infection.
Additional use of MAAs
The non-biomedical applications of MAAs have also been reported in recent years. Fernandes et al. (82) developed chitosan-conjugated MAAs (CS-MAA), which act as a UV-protective green material. The CS-MAA conjugates were created by forming amide bonds between the carboxylic acid groups on the selected MAAs such as mycosporine-glycine, porphyra-334, and shinorine, and the primary amino groups on the chitosan, with EDAC (N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride) acting as the carbodiimide coupling agent. The formed biomaterial can be used for various purposes such as cosmetics, wound healing agent, artificial skin, artificial cornea, contact lenses, etc., as well as on non-living objects in textiles and outdoor materials, food and drug packaging, coatings, etc. In comparison to individual MAAs, the results showed that CS-MAA conjugates exhibit various advantages such as thermoresistant, biodegradable, biocompatible, non-toxic, and more over enhanced efficacy against both UV-A and UV-B radiation (82, 83).
Concluding remarks and future prospects
The immense properties of MAAs make them an appealing substance with a promising future in the pharmaceutical and cosmetic industries. The biological uses of MAAs include natural sunscreens, anti-cancer agents, activators of cell proliferation, anti-photoaging molecules, anti-inflammatory activity, wound healing properties, stimulators of skin renewal, anti-adipogenic agents, and more recent evidence suggests that it can combat COVID-19 infection.
Many studies reported the application of MAAs as a sunscreen formulation. Recently, it has been shown that; they conjugate with biopolymers or nanoparticles improving their stability and efficiency, leading to highly efficient skin healthcare products. Howbeit, the utilization of biological sources for the large-scale production of these economically important molecules has not been thoroughly investigated. Till now, only a few products have been commercialized. Reconstructing the MAAs biosynthetic pathway in microbial cells with the help of a synthetic biology platform paves the way for the efficient production of MAAs. In this regard, many studies have reported the biosynthesis and heterologous production of mycosporine-like amino acids in various organisms such as bacteria, yeasts, and fungi. The in vitro/in vivo screening and expression methods make large-scale manufacturing possible.
From the above studies, it has been concluded that MAAs could be an interesting natural product which can be utilized in the fields of both cosmeceutical and pharmaceutical industries as an environment-friendly bioactive compound. In the future, research on the correlation between MAAs chemical structure and bioactivity could lead to a wider application of these compounds in cosmetics, medicine, and dietary supplements.
ACKNOWLEDGMENTS
This work was supported by the University Grants Commission, India. (UGC, Ref. No. 221610201188) through junior research fellowship scheme.
Contributor Information
Akhil Agrawal, Email: akhilagrawal@curaj.ac.in.
Nicole R. Buan, University of Nebraska-Lincoln, Lincoln, Nebraska, USA
REFERENCES
- 1. Rosic NN. 2019. Mycosporine-like amino acids: making the foundation for organic personalised sunscreens. Mar Drugs 17:638. doi: 10.3390/md17110638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosic NN, Dove S. 2011. Mycosporine-like amino acids from coral dinoflagellates. Appl Environ Microbiol 77:8478–8486. doi: 10.1128/AEM.05870-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geraldes V, Pinto E. 2021. Mycosporine-like amino acids (Maas): biology, chemistry and identification features. Pharmaceuticals (Basel) 14:63. doi: 10.3390/ph14010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh A, Čížková M, Bišová K, Vítová M. 2021. Exploring Mycosporine-like amino acids (Maas) as safe and natural protective agents against UV-induced skin damage. Antioxidants 10:683. doi: 10.3390/antiox10050683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawrence KP, Long PF, Young AR. 2019. Mycosporine-like amino acids for skin photoprotection. CMC 25:5512–5527. doi: 10.2174/0929867324666170529124237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kageyama H, Waditee-Sirisattha R. 2018. Mycosporine-like amino acids as multifunctional secondary metabolites in cyanobacteria: from biochemical to application aspects. In Studies in natural products chemistry. Elsevier, New York. [Google Scholar]
- 7. Kageyama Hakuto, Waditee-Sirisattha R. n.d. Antioxidative, anti-inflammatory, and anti-aging properties of Mycosporine-like amino acids: molecular and cellular mechanisms in the protection of skin-aging. Marine Drugs 17:222. doi: 10.3390/md17040222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vega J, Schneider G, Moreira BR, Herrera C, Bonomi-Barufi J, Figueroa FL. 2021. Mycosporine-like amino acids from red Macroalgae: UV-Photoprotectors with potential Cosmeceutical applications. Applied Sciences 11:5112. doi: 10.3390/app11115112 [DOI] [Google Scholar]
- 9. Zwerger M, Ganzera M. 2022. Fast and efficient separation of eleven mycosporine-like amino acids by UHPLC-DAD and their quantification in diverse red algae. Marine Drugs 20:395. doi: 10.3390/md20060395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahu N, Mishra S, Kesheri M, Kanchan S, Sinha RP. 2023. Identification of cyanobacteria-based natural inhibitors against SARS-CoV-2 druggable target ACE2 using molecular docking study, ADME and toxicity analysis. Indian J Clin Biochem 38:361–373. doi: 10.1007/s12291-022-01056-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunlap WC, Shick JM. 1998. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. J Phycol 34:418–430. doi: 10.1046/j.1529-8817.1998.340418.x [DOI] [Google Scholar]
- 12. de la Coba F, Aguilera J, Figueroa FL, de Gálvez MV, Herrera E. 2009. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine Lichen. J Appl Phycol 21:161–169. doi: 10.1007/s10811-008-9345-1 [DOI] [Google Scholar]
- 13. Nishida Y, Saburi W, Miyabe Y, Kishimura H, Kumagai Y. 2022. Characterization of antioxidant activity of heated mycosporine-like amino acids from red alga dulse palmaria palmata in Japan. Mar Drugs 20:184. doi: 10.3390/md20030184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suh SS, Hwang J, Park M, Seo HH, Kim HS, Lee JH, Moh SH, Lee TK. 2014. Anti-inflammation activities of mycosporine-like amino acids (Maas) in response to UV radiation suggest potential anti-skin aging activity. Mar Drugs 12:5174–5187. doi: 10.3390/md12105174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orfanoudaki M, Hartmann A, Alilou M, Gelbrich T, Planchenault P, Derbré S, Schinkovitz A, Richomme P, Hensel A, Ganzera M. 2020. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar Drugs 18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tarasuntisuk S, Patipong T, Hibino T, Waditee-Sirisattha R, Kageyama H. 2018. Inhibitory effects of mycosporine-2-glycine isolated from a halotolerant cyanobacterium on protein Glycation and collagenase activity. Lett Appl Microbiol 67:314–320. doi: 10.1111/lam.13041 [DOI] [PubMed] [Google Scholar]
- 17. Choi YH, Yang DJ, Kulkarni A, Moh SH, Kim KW. 2015. Mycosporine-like amino acids promote wound healing through focal adhesion kinase (FAK) and mitogen-activated protein Kinases (MAP Kinases) signaling pathway in Keratinocytes. Mar Drugs 13:7055–7066. doi: 10.3390/md13127056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bozkurt HS, Quigley EM, Kara B. 2019. Bifidobacterium Animalis subspecies Lactis engineered to produce mycosporin-like amino acids in colorectal cancer prevention. SAGE Open Med 7:2050312119825784. doi: 10.1177/2050312119825784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yakovleva I, Bhagooli R, Takemura A, Hidaka M. 2004. Differential susceptibility to oxidative stress of two scleractinian corals: antioxidant functioning of mycosporine-glycine. Comp Biochem Physiol B Biochem Mol Biol 139:721–730. doi: 10.1016/j.cbpc.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 20. Wada N, Sakamoto T, Matsugo S. 2015. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants (Basel) 4:603–646. doi: 10.3390/antiox4030603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh A, Tyagi MB, Kumar A. 2017. Cyanobacteria growing on tree barks possess high amount of sunscreen compound Mycosporine-like amino acids (Maas). Plant Physiol Biochem 119:110–120. doi: 10.1016/j.plaphy.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 22. Wada N, Sakamoto T, Matsugo S. 2013. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 3:463–483. doi: 10.3390/metabo3020463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sommaruga R, Garcia-Pichel F. 1999. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain Lake. Fal 144:255–269. doi: 10.1127/archiv-hydrobiol/144/1999/255 [DOI] [Google Scholar]
- 24. Babele PK, Singh G, Singh A, Kumar A, Tyagi MB, Sinha RP. 2017. UV-B radiation and temperature stress-induced alterations in metabolic events and defense mechanisms in a bloom-forming cyanobacterium microcystis aeruginosa. Acta Physiol Plant 39:1–11. doi: 10.1007/s11738-017-2540-4 [DOI] [Google Scholar]
- 25. Oren A. 1997. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiology J 14:231–240. doi: 10.1080/01490459709378046 [DOI] [Google Scholar]
- 26. Rastogi RP, Madamwar D, Incharoensakdi A. 2015. Sun-screening bioactive compounds mycosporine-like amino acids in naturally occurring cyanobacterial biofilms: role in photoprotection. J Appl Microbiol 119:753–762. doi: 10.1111/jam.12879 [DOI] [PubMed] [Google Scholar]
- 27. Shick JM, Dunlap WC. 2002. Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu Rev Physiol 64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802 [DOI] [PubMed] [Google Scholar]
- 28. Sinha RP, Singh SP, Häder DP. 2007. Database on mycosporines and mycosporine-like amino acids (Maas) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J Photochem Photobiol B 89:29–35. doi: 10.1016/j.jphotobiol.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 29. Sinha RP, Hader DP. 2002. Life under solar UV radiation in aquatic organisms. Adv Space Res 30:1547–1556. doi: 10.1016/s0273-1177(02)00370-8 [DOI] [PubMed] [Google Scholar]
- 30. Sinha RP, Häder DP. 2008. UV-protectants in cyanobacteria. Plant Science 174:278–289. doi: 10.1016/j.plantsci.2007.12.004 [DOI] [Google Scholar]
- 31. Rastogi RP, Sinha RP, Moh SH, Lee TK, Kottuparambil S, Kim YJ, Rhee JS, Choi EM, Brown MT, Häder DP, Han T. 2014. Ultraviolet radiation and cyanobacteria. J Photochem Photobiol B 141:154–169. doi: 10.1016/j.jphotobiol.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 32. Helbling EW, Menchi CF, Villafañe VE. 2002. Bioaccumulation and role of UV-absorbing compounds in two marine crustacean species from patagonia, argentina. Photochem Photobiol Sci 1:820–825. doi: 10.1039/b206584c [DOI] [PubMed] [Google Scholar]
- 33. Newman SJ, Dunlap WC, Nicol S, Ritz D. 2000. Antarctic Krill (Euphausia Superba) acquire a UV-absorbing Mycosporine-like amino acid from dietary algae. J Exp Mar Biol Ecol 255:93–110. doi: 10.1016/s0022-0981(00)00293-8 [DOI] [PubMed] [Google Scholar]
- 34. Kicklighter CE, Kamio M, Nguyen L, Germann MW, Derby CD. 2011. Mycosporine-like amino acids are multifunctional molecules in sea hares and their marine community. Proc Natl Acad Sci U S A 108:11494–11499. doi: 10.1073/pnas.1103906108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davidson AT. 1998. The impact of UVB radiation on marine plankton. Mutat Res 422:119–129. doi: 10.1016/s0027-5107(98)00183-3 [DOI] [PubMed] [Google Scholar]
- 36. Holzinger A, Lütz C. 2006. Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron 37:190–207. doi: 10.1016/j.micron.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 37. Osborn AR, Almabruk KH, Holzwarth G, Asamizu S, LaDu J, Kean KM, Karplus PA, Tanguay RL, Bakalinsky AT, Mahmud T. 2015. De novo synthesis of a sunscreen compound in vertebrates. Elife 4:e05919. doi: 10.7554/eLife.05919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, Fujie M, Fujiwara M, Koyanagi R, Ikuta T, Fujiyama A, Miller DJ, Satoh N. 2011. Using the acropora digitifera genome to understand coral responses to environmental change. Nature 476:320–323. doi: 10.1038/nature10249 [DOI] [PubMed] [Google Scholar]
- 39. Cockell CS, Knowland J. 1999. Ultraviolet radiation screening compounds. Biol Rev Camb Philos Soc 74:311–345. doi: 10.1017/s0006323199005356 [DOI] [PubMed] [Google Scholar]
- 40. Jiang H, Gao K, Helbling EW. 2008. UV-absorbing compounds in porphyra haitanensis (Rhodophyta) with special reference to effects of desiccation. J Appl Phycol 20:387–395. doi: 10.1007/s10811-007-9268-2 [DOI] [Google Scholar]
- 41. Kogej T, Gostinčar C, Volkmann M, Gorbushina AA, Gunde-Cimerman N. 2006. Mycosporines in extremophilic fungi - novel complementary osmolytes Environ Chem 3:105. doi: 10.1071/EN06012 [DOI] [Google Scholar]
- 42. Oren A, Gunde-Cimerman N. 2007. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites FEMS Microbiol Lett 269:1–10. doi: 10.1111/j.1574-6968.2007.00650.x [DOI] [PubMed] [Google Scholar]
- 43. Peinado NK, Abdala Díaz RT, Figueroa FL, Helbling EW. 2004. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in porphyra columbina (Rhodophyta) from patagonia, argentina. J Phycol 40:248–259. doi: 10.1046/j.1529-8817.2004.03013.x [DOI] [Google Scholar]
- 44. Chrapusta E, Kaminski A, Duchnik K, Bober B, Adamski M, Bialczyk J. 2017. Mycosporine-like amino acids: potential health and beauty ingredients. Mar Drugs 15:1–29. doi: 10.3390/md15100326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sommaruga R. 2001. The role of solar UV radiation in the ecology of alpine lakes. J Photochem Photobiol B 62:35–42. doi: 10.1016/s1011-1344(01)00154-3 [DOI] [PubMed] [Google Scholar]
- 46. Bandaranayake WM, Bourne DJ, Sim RG. 1997. Chemical composition during maturing and spawning of the sponge Dysidea herbacea (Porifera: Demospongiae). Comp Biochem Physiol B Biochem Mol Biol 118:851–859. doi: 10.1016/S0305-0491(97)00180-6 [DOI] [Google Scholar]
- 47. Bandaranayake WM, Rocher AD. 1999. Role of secondary metabolites and pigments in the epidermal tissues, ripe ovaries, viscera, gut contents and diet of the sea cucumber holothuria ATRA. Marine Biology 133:163–169. doi: 10.1007/s002270050455 [DOI] [Google Scholar]
- 48. Whitehead K, Vernet M. 2000. Influence of mycosporine-like amino acids (Maas) on UV absorption by particulate and dissolved organic matter in La Jolla Bay. Limnol Oceanogr 45:1788–1796. doi: 10.4319/lo.2000.45.8.1788 [DOI] [Google Scholar]
- 49. Ishihara K, Watanabe R, Uchida H, Suzuki T, Yamashita M, Takenaka H, Nazifi E, Matsugo S, Yamaba M, Sakamoto T. 2017. Novel glycosylated mycosporine-like amino acid, 13-O-(Β-Galactosyl)-porphyra-334, from the edible cyanobacterium nostoc sphaericum-protective activity on human keratinocytes from UV light. J Photochem Photobiol B 172:102–108. doi: 10.1016/j.jphotobiol.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 50. Carreto JI, Carignan MO. 2011. Mycosporine-like amino acids: relevant secondary metabolites chemical and ecological aspects. Mar Drugs 9:387–446. doi: 10.3390/md9030387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torres A, Hochberg M, Pergament I, Smoum R, Niddam V, Dembitsky VM, Temina M, Dor I, Lev O, Srebnik M, Enk CD. 2004. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete collema cristatum. Eur J Biochem 271:780–784. doi: 10.1111/j.1432-1033.2004.03981.x [DOI] [PubMed] [Google Scholar]
- 52. Orfanoudaki M, Hartmann A, Miladinovic H, Nguyen Ngoc H, Karsten U, Ganzera M. 2019. Bostrychines A – F, six novel mycosporine-like amino-acids and a novel betaine from the red alga Bostrychia scorpioides Mar Drugs 17:356. doi: 10.3390/md17060356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pope MA, Spence E, Seralvo V, Gacesa R, Heidelberger S, Weston AJ, Dunlap WC, Shick JM, Long PF. 2015. O-methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413. Chembiochem 16:320–327. doi: 10.1002/cbic.201402516 [DOI] [PubMed] [Google Scholar]
- 54. Geraldes V, Medeiros LS de, Lima ST, Alvarenga DO, Gacesa R, Long PF, Fiore MF, Pinto E. 2020. Genetic and biochemical evidence for redundant pathways leading to mycosporine-like amino acid biosynthesis in the cyanobacterium Sphaerospermopsis torques-reginae ITEP-024. ALGAE 35:177–187. doi: 10.4490/algae.2020.35.5.19 [DOI] [Google Scholar]
- 55. Portwich A, Garcia-Pichel F. 2003. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium. Phycologia 42:384–392. doi: 10.2216/i0031-8884-42-4-384.1 [DOI] [Google Scholar]
- 56. Gao Q, Garcia-Pichel F. 2011. Microbial ultraviolet sunscreens. Nat Rev Microbiol 9:791–802. doi: 10.1038/nrmicro2649 [DOI] [PubMed] [Google Scholar]
- 57. Balskus EP, Walsh CT. 2010. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 329:1653–1656. doi: 10.1126/science.1193637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guihéneuf F, Gietl A, Stengel DB. 2018. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from Western Ireland. J Appl Phycol 30:2573–2586. doi: 10.1007/s10811-018-1436-z [DOI] [Google Scholar]
- 59. Jofre J, Celis-Plá PSM, Figueroa FL, Navarro NP. 2020. Seasonal variation of mycosporine-like amino acids in three subantarctic red seaweeds. Mar Drugs 18:1–17. doi: 10.3390/md18020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garcia-Pichel F, Wingard CE, Castenholz RW. 1993. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp . Appl Environ Microbiol 59:170–176. doi: 10.1128/aem.59.1.170-176.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Derikvand P, Llewellyn CA, Purton S. 2017. Cyanobacterial metabolites as a source of sunscreens and moisturizers: a comparison with current synthetic compounds. Europ J Phycol 52:43–56. doi: 10.1080/09670262.2016.1214882 [DOI] [Google Scholar]
- 62. Schmid D, Schürch C, Zülli F. 2006. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet 9:1–4. [Google Scholar]
- 63. de la Coba F, Aguilera J, Korbee N, de Gálvez M, Herrera-Ceballos E, Álvarez-Gómez F, Figueroa F. 2019. UVA and UVB Photoprotective capabilities of topical formulations containing mycosporine-like amino acids (Maas) through different biological effective protection factors (Bepfs). Marine Drugs 17:55. doi: 10.3390/md17010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Colabella F, Moline M, Libkind D. 2014. UV Sunscreens of microbial origin: mycosporines and mycosporine- like aminoacids. Recent Pat Biotechnol 8:179–193. doi: 10.2174/1872208309666150102104520 [DOI] [PubMed] [Google Scholar]
- 65. Pfeifer GP, Besaratinia A. 2012. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci 11:90–97. doi: 10.1039/c1pp05144j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Misonou T, Saitoh J, Oshiba S, Tokitomo Y, Maegawa M, Inoue Y, Hori H, Sakurai T. 2003. UV-absorbing substance in the red alga Porphyra yezoensis (bangiales, rhodophyta) block thymine photodimer production. Mar Biotechnol (NY) 5:194–200. doi: 10.1007/s10126-002-0065-2 [DOI] [PubMed] [Google Scholar]
- 67. Woolley JM, Staniforth M, Horbury MD, Richings GW, Wills M, Stavros VG. 2018. Unravelling the photoprotection properties of mycosporine amino acid motifs. J Phys Chem Lett 9:3043–3048. doi: 10.1021/acs.jpclett.8b00921 [DOI] [PubMed] [Google Scholar]
- 68. Ryu J, Park SJ, Kim IH, Choi YH, Nam TJ. 2014. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int J Mol Med 34:796–803. doi: 10.3892/ijmm.2014.1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tarasuntisuk S, Palaga T, Kageyama H, Waditee-Sirisattha R. 2019. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch Biochem Biophys 662:33–39. doi: 10.1016/j.abb.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 70. Becker K, Hartmann A, Ganzera M, Fuchs D, Gostner JM. 2016. Immunomodulatory effects of the mycosporine-like amino acids shinorine and porphyra-334. Mar Drugs 14:1–12. doi: 10.3390/md14060119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grimbaldeston MA, Simpson A, Finlay-Jones JJ, Hart PH. 2003. The effect of ultraviolet radiation exposure on the prevalence of mast cells in human skin. Br J Dermatol 148:300–306. doi: 10.1046/j.1365-2133.2003.05113.x [DOI] [PubMed] [Google Scholar]
- 72. Bhandari R, Sharma PK. 2007. Effect of UV-B and high visual radiation on photosynthesis in freshwater (Nostoc Spongiaeforme) and marine (Phormidium Corium) cyanobacteria. Indian J Biochem Biophys 44:231–239. [PubMed] [Google Scholar]
- 73. Gao K, Xu J. 2008. Effects of solar UV radiation on diurnal photosynthetic performance and growth of Gracilaria lemaneiformis (Rhodophyta). Europ J Phycol 43:297–307. doi: 10.1080/09670260801986837 [DOI] [Google Scholar]
- 74. Rui Y, Zhaohui Z, Wenshan S, Bafang L, Hu H. 2019. Protective effect of maas extracted from porphyra TENERA against UV irradiation-induced photoaging in mouse skin. J Photochem Photobiol B: Biol 192:26–33. doi: 10.1016/j.jphotobiol.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 75. Tümen İ, Akkol EK, Taştan H, Süntar I, Kurtca M.. 2018. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J Ethnopharmacol 211:235–246. [DOI] [PubMed] [Google Scholar]
- 76. Hartmann A, Gostner J, Fuchs JE, Chaita E, Aligiannis N, Skaltsounis L, Ganzera M. 2015. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Med 81:813–820. doi: 10.1055/s-0035-1546105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Choi S-Y, Lee SY, Kim HG, Jeong JC, Batara DC, Kim S-H, Cho J-Y. 2022. Shinorine and porphyra-334 isolated from laver (Porphyra Dentata) inhibit adipogenesis in 3T3-L1 cells. Food Sci Biotechnol 31:617–625. doi: 10.1007/s10068-022-01055-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim H, Lee Y, Han T, Choi EM. 2015. The Micosporine-like amino acids-rich aqueous methanol extract of laver (Porphyra Yezoensis) inhibits adipogenesis and induces apoptosis in 3T3-L1 Adipocytes. Nutr Res Pract 9:592–598. doi: 10.4162/nrp.2015.9.6.592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yuan YV, Westcott ND, Hu C, Kitts DD. 2009. Mycosporine-like amino acid composition of the edible red alga, palmaria palmata (Dulse) harvested from the West and East coasts of grand Manan Island, new Brunswick. Food Chemistry 112:321–328. doi: 10.1016/j.foodchem.2008.05.066 [DOI] [Google Scholar]
- 80. Athukorala Y, Trang S, Kwok C, Yuan YV. 2016. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red Macroalgae. Molecules 21:E119. doi: 10.3390/molecules21010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim S, You DH, Han T, Choi EM. 2014. Modulation of viability and apoptosis of UVB-exposed human Keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J Photochem Photobiol B 141:301–307. doi: 10.1016/j.jphotobiol.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 82. Fernandes SCM, Alonso-Varona A, Palomares T, Zubillaga V, Labidi J, Bulone V. 2015. Exploiting mycosporines as natural molecular sunscreens for the fabrication of UV-absorbing green materials. ACS Appl Mater Interfaces 7:16558–16564. doi: 10.1021/acsami.5b04064 [DOI] [PubMed] [Google Scholar]
- 83. Dash M, Chiellini F, Ottenbrite RM, Chiellini E. 2011. Chitosan - A versatile semi-synthetic polymer in BIOMEDICAL applications. Progress Polymer Sci 36:981–1014. doi: 10.1016/j.progpolymsci.2011.02.001 [DOI] [Google Scholar]





