ABSTRACT
The use of biological nitrification inhibitors (BNIs) holds a great potential to effectively reduce nitrogen losses from agroecosystems and conforms with the current move toward ecological-intensified agriculture. Knowledge of the activity of BNIs to soil nitrifiers is limited and is generally based on a single Nitrosomonas europaea bioassay. We determined the in vitro activity of multiple plant-derived compounds as BNIs such as (i) root-derived compounds [sakuranetin, methyl 3-(4-hydroxyphenyl)-propionate (MHPP), and zeanone]; (ii) other phytochemicals (caffeic, quinic, chlorogenic, and shikimic acids); and (iii) analogs of statins (simvastatin), triazoles (1-butyl-4-propyl-triazole, 1,4-dibutyltriazole), and zeanone (2-methoxy-1,4-naphthoquinone) on distinct soil-derived ammonia-oxidizing bacteria (AOB) (Nitrosospira multiformis and Nitrosomonas europaea), ammonia-oxidizing archaea (AOA) (Candidatus Nitrosotalea sinensis and Candidatus Nitrosocosmicus franklandianus), and a nitrite-oxidizing bacterium (NOB) (Nitrobacter sp.). Our results indicate that AOA were more sensitive than AOB to BNIs. Sensitivity within the AOA group was BNI dependent, unlike AOB, for which N. multiformis was consistently more sensitive than N. europaea. Several compounds were inhibitory to Nitrobacter sp. with MHPP and caffeic acid being more potent against NOB compared to the ammonia-oxidizing strains, an observation with potential implications for soil quality and productivity. Overall, zeanone was the most potent ΒNI against ammonia oxidizers, while caffeic acid was the most potent BNI against Nitrobacter sp. We provide pioneering evidence for the activity range of multiple BNIs on soil nitrifiers, stress the need for revisiting the biological screening systems currently used for BNI determination, and advocate for a more thorough monitoring of the impact of BNI candidates on a range of target and non-target microorganisms.
IMPORTANCE
Synthetic nitrification inhibitors are routinely used with nitrogen fertilizers to reduce nitrogen losses from agroecosystems, despite having drawbacks like poor efficiency, cost, and entry into the food chain. Plant-derived BNIs constitute a more environmentally conducive alternative. Knowledge on the activity of BNIs to soil nitrifiers is largely based on bioassays with a single Nitrosomonas europaea strain which does not constitute a dominant member of the community of ammonia-oxidizing microorganisms (AOM) in soil. We determined the activity of several plant-derived molecules reported as having activity, including the recently discovered maize-isolated BNI, zeanone, and its natural analog, 2-methoxy-1,4-naphthoquinone, on a range of ecologically relevant AOM and one nitrite-oxidizing bacterial culture, expanding our knowledge on the intrinsic inhibition potential of BNIs toward AOM and highlighting the necessity for a deeper understanding of the effect of BNIs on the overall soil microbiome integrity before their further use in agricultural settings.
KEYWORDS: biological nitrification inhibitors, ammonia-oxidizing bacteria, ammonia-oxidizing archaea, nitrite-oxidizing bacteria, in vitro assays, EC50
INTRODUCTION
Nitrogen (N) is an essential nutrient for plant growth and productivity, and increases in its supply through fertilization are actively exploited in agriculture to enhance crop yields (1). Approximately 2% of the world’s energy is consumed for the production of N fertilizers annually (2) with the amount of reactive N (Nr) introduced into the biosphere by these means reaching 1.5 × 108 t/year (3). Critically, due to the low N fertilizer use efficiency (NUE) in agriculture, only 30%–50% of this amount is assimilated by plants (4), while the remaining, in the forms of ammonia (NH3), nitrate (NO3 −), and N oxides (NxO), enriches Nr pools and contributes to environmental quality degradation, biodiversity loss, and greenhouse gas (GHG)-driven climate change (5, 6). N losses from fertilized soils are linked to the microbially mediated process of nitrification, which generates NO3 –. This reactive N species is mobile in soil and susceptible to groundwater leaching in addition to being a substrate for soil-nitrifying and soil-denitrifying microorganisms that could emit nitrous oxide (N2O) (7), a GHG with a warming potential 298 times greater than carbon dioxide (8).
Currently, the use of chemical-based technologies holds a great promise to increase NUE and reduce the adverse environmental impact of the Nr cascade. Such chemicals are small molecules (≤500 dalton), either synthetic or naturally produced, which can decelerate or inhibit the key steps of the nitrification process, and particularly the first and often rate-limiting step of ammonia oxidation (7, 9, 10). In this step, NH3 is oxidized to hydroxylamine by the enzyme ammonia monooxygenase (AMO) and further to nitrite (NO2 −) by canonical ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and complete ammonia-oxidizing (comammox) bacteria, with nitric oxide being a pivotal intermediate in all ammonia-oxidation pathways (11, 12).
To this end, synthetic nitrification inhibitors (SNIs) like 2-chloro-6-(trichloromethyl) pyridine (nitrapyrin), dicyandiamide (DCD), and 3,4-dimethylpyrazole phosphate (DMPP) are routinely incorporated into fertilizers to stabilize the supply of N available in soil (13). The use of SNIs is recommended by the Intergovernmental Panel on Climate Change as a means of mitigating climate change (14). However, these compounds have been also reported to have drawbacks such as difficulties in application, highly variable efficacy across soils, high cost, pollution of natural resources, bioaccumulation in plants and animals, and potential entry into the food chain (7, 15, 16). In response to the constraints of SNIs and the public demand for novel bio-based solutions in agriculture, biological nitrification inhibitors (BNIs), compounds that are synthesized or secreted by plants and have nitrification inhibition properties, have recently received increased attention as safer and potentially more effective alternatives to SNIs (17).
BNI has been thought of as an evolutionary adaptation of certain plants to retain N in the form of NH4 + in N-limited soils, leading to optimized NUE and a less leaky N cycle (18). Until now, multiple BNI compounds exudated from the roots of several plant species, mostly grasses, have been identified, including sorgoleone, sakuranetin, and methyl 3-(4-hydroxyphenyl) propionate (MHPP) from sorghum (Sorghum bicolor) (19, 20), brachialactone from signal grass (Brachiaria humidicola) (21), 1,9-decanediol and syringic acid from rice (Oryza sativa) (22, 23), 2,7-dimethoxy-1,4-naphthoquinone (zeanone), and 6-methoxy-2(3H)-benzoxazolone from maize (Zea mays) (24, 25). BNIs can be also plant secondary metabolites (e.g., terpenoids, tannins, phenolic acids, flavonoids, and polyphenols) detected and recovered from other plant organs like stems, leaves, seeds, and their litter (7, 17, 26 – 28). BNIs can suppress up to 90% of soil nitrification activity and increase soil N retention, providing plants with a competitive advantage for assimilating the N contained in fertilizers (21). However, relatively little is known about the exact mechanisms by which BNIs act on soil-nitrifying microbes (29), with the vast majority of studies relying on screening tests with a single AOB Nitrosomonas europaea strain (19, 20, 24, 30) and only few recent studies focusing on larger and more representative sets of ammonia-oxidizing microorganism (AOM) (31, 32). However, ammonia oxidation in soil is often dominated by AOA (33), especially in acidic soils (34) where BNI release from plant roots is promoted (35 – 37), and AOB belonging to the genus Nitrosospira sp., rather than Nitrosomonas, in fertilized soils (38, 39).
Hence, inhibition assays with a diverse range of soil-derived nitrifying strains could be a first and necessary step to define the spectrum of activity of nitrification inhibitors (NIs) destined for use in agricultural settings (10). Such assays have been used for determining the activity of SNIs toward AOM (10, 31, 40, 41) and very recently of some BNIs (31, 32). These studies allowed for the estimation of inhibition thresholds based on the percentage reduction of ammonia oxidation (NO2 − accumulation) (10) or growth (μmax) (32). Research on BNIs has been intensified in recent years, and new studies are reporting new plant-derived BNIs whose activity on the full breadth of soil-nitrifying microbial groups remains unknown. This knowledge is required to get a first verification of their potential efficiency in agricultural settings, as well as their inhibition potential toward non-target soil microorganisms that might be expected based on the inherent role of some of these phytochemicals as plant defence agents (42).
We aimed to determine the NI efficiency of multiple plant-derived molecules, including (i) three root-exudated molecules with known BNI activity (MHPP, sakuranetin, and zeanone); (ii) four phytochemicals isolated from other than root, plant organs (caffeic acid, quinic acid, chlorogenic acid, and shikimic acid); and (iii) four analogs of compounds with known inhibitory activity towards AOM such as simvastatin (analogue of statin), 1-butyl-4-propyl-triazole and 1,4-dibutyltriazole (triazole analogues), and 2-methoxy-1,4-naphthoquinone (analogue of zeanone) (Table 1), on liquid cultures of ecophysiologically and phylogenetically distinct soil-derived AOA and AOB strains. Specifically, we used AOB strains Nitrosomonas europaea and Nitrosospira multiformis, belonging to AOB clusters 7 and 3, respectively, and AOA strains Candidatus Nitrosocosmicus franklandianus and Candidatus Nitrosotalea sinensis, occupying contrasting ecological niches and representing widely distributed neutrophilic and acidophilic AOA lineages, respectively (10). We expanded our assays to Nitrobacter nitrite-oxidizing bacterium (NOB) (Nitrobacter sp. NHB1), known as superior competitors than Nitrospira at high resource availability (e.g., fertilized soils) (43 – 45), to gain insights on the impact of these compounds on other microbial players in the nitrification process that are known to be spatially and functionally connected with AOM (46). To standardize and interpret the effects observed, the stability of the selected NIs in the laboratory assays was also determined.
TABLE 1.
Summary of the NIs a used and detailed information about their (i) chemical structures, (ii) molecular weight (Da), (iii) chemical classification, (iv) origin, and (v) presumed mode of action
| NI group | NI | Chemical structure | Molecular weight (Da) |
Classification | NI origin | Presumed mode of action | Reference |
|---|---|---|---|---|---|---|---|
| Root-exudated BNIs | Sakuranetin |

|
286.28 | Flavanone | Sorghum (Sorghum bicolor) | AMO and HAO | (20) |
| Methyl 3-(4-hydroxyphenyl)-propionate |

|
180.20 | Phenolic acid | Sorghum (Sorghum bicolor) | AMO-specific | (19, 27) | |
| 2,7-Dimethoxy-1,4-naphthoquinone (zeanone) |
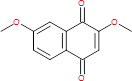
|
218.21 | Naphthoquinone (quinazolines) | Maize (Zea mays) | Unknown | (24) | |
| Other BNI phytochemicals | Caffeic acid |
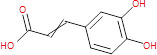
|
180.16 | Hydroxycinnamic acid (phenolic acid) | Various plants, including barley, ferns and eucalyptus bark, coffee beans, propolis, and vegetables (olives, potatoes, and carrots) | NO scavenger | (47, 48) |
| Quinic acid |

|
192.17 | Cyclohexane carboxylic acid (intermediate in phenolic acid biosynthesis) |
Bark of Eucalyptus globulus, cinchona bark, coffee beans, Urtica dioica shoot | Unknown | (49) | |
| Chlorogenic acid |

|
354.31 | Hydroxycinnamic acid (phenolic acid), ester of caffeic acid, and quinic acid |
Leaves of Hibiscus sabdariffa, shoots of Calluna vulgaris, coffee beans, litter of Andropogon scoparius | Cell membrane disruption/QS inhibitor | (26, 27), (50 – 52) | |
| Shikimic acid |
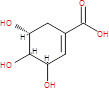
|
174.15 | Cyclohexane carboxylic acid (intermediate in phenolic acid biosynthesis) | Illicium anisatum flower (Japanese shikimi) | Cell membrane disruption/NO- scavenging agent | (53, 54) | |
| ΝΙ analogs | Simvastatin |
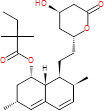
|
418.57 | Statin | Semi-synthetic derivative of lovastatin, which is made from the fungus Aspergillus terreus | Inhibition of HMG-CoA b reductase-AOA membrane biosynthesis | (34, 35) |
| 1-Butyl-4-propyl-triazole |
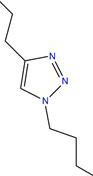
|
167.25 | Triazole | Synthetic compound serving as natural product conjugates | Copper corrosion inhibitor | (55, 56) | |
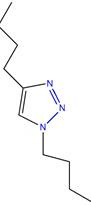
|
(55) | ||||||
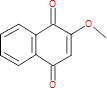
|
(57) |
NI, nitrification inhibitor.
HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA.
RESULTS
The inhibition of root exudated BNIs on the activity of AOM and NOB strains
Sakuranetin induced a complete and persistent inhibition of the activity of N. multiformis at concentrations ≥250 μΜ, but it did not significantly affect (P > 0.05) NO2 − production by N. europaea at the tested concentration range (10–500 μΜ). Conversely, the activity of both AOA strains was fully inhibited by sakuranetin at all tested concentrations (≥10 μΜ). NO2 − consumption by Nitrobacter sp. was fully inhibited at 250 μΜ, while a weaker inhibitory effect was observed at 500 μΜ (Fig. 1).
Fig 1.
The effect of different concentrations of sakuranetin, MHPP, and zeanone on the activity of the ammonia-oxidizing bacteria N. multiformis and N. europaea, the ammonia-oxidizing archaea Ca. N. sinensis and Ca. N. franklandianus, and the nitrite-oxidizing bacterium Nitrobacter sp., determined by monitoring the production or consumption of nitrite. Error bars represent the standard error of the mean of triplicate cultures.
MHPP significantly reduced (P < 0.05) the activity of N. multiformis at levels ≥150 μΜ, and complete inhibition for both AOB strains was evident at ≥500 μΜ. Ammonia oxidation by Ca. N. sinensis and Ca. N. franklandianus was significantly suppressed (P < 0.05) at concentrations of ≥500 μΜ. Finally, nitrite oxidation by Nitrobacter sp. was fully inhibited at all concentrations tested (≥50 μΜ) (Fig. 1).
Zeanone fully inhibited the activity of N. multiformis and of the two AOA strains at concentrations of ≥0.2 and ≥2 μΜ, respectively, while no significant inhibition (P > 0.05) on the activity of N. europaea was observed at the tested concentration range (0.2–46.0 μΜ). Nitrite oxidation by Nitrobacter sp. was significantly (P < 0.05) suppressed at 20 μΜ and completely halted at 40 μΜ of zeanone (Fig. 1).
The inhibition of phytochemicals on the activity of AOM and NOB strains
Caffeic acid induced a complete and persistent inhibition of the activity of N. multiformis and N. europaea at concentrations of ≥300 μΜ, while the same concentration of caffeic acid (300 μΜ) induced a temporal inhibitory effect in the activity of Ca. N. franklandianus (Fig. 2). On the other hand, Ca. N. sinensis was fully inhibited at lower concentrations (≥30 μΜ). Nitrobacter sp. was inhibited at concentrations of ≤30 μΜ, whereas a rapid consumption of NO2 − was observed at the higher concentrations of caffeic acid of ≥300 μΜ.
Fig 2.
The effect of different concentrations of caffeic acid, quinic acid, chlorogenic acid, and shikimic acid on the activity of the ammonia-oxidizing bacteria N. multiformis and N. europaea, the ammonia-oxidizing archaea Ca. N. sinensis and Ca. N. franklandianus, and the nitrite-oxidizing bacterium Nitrobacter sp., determined by monitoring the production or consumption of nitrite. Error bars represent the standard error of the mean of triplicate cultures.
Quinic acid fully inhibited ammonia oxidation by N. multiformis at concentrations of ≥3 μΜ (Fig. 2). A complete and persistent inhibition of the activity of N. europaea and Ca. N. sinensis was observed only at the highest tested concentration of 3000 μΜ. At the same concentration level, quinic acid significantly reduced but not fully inhibited the activity of Ca. N. franklandianus. Finally, Nitrobacter sp. was not significantly affected (P > 0.05) by quinic acid at the tested concentration range (3–3,000 μΜ).
Chlorogenic acid, induced a complete and persistent inhibition of the activity of N. multiformis and N. europaea at concentrations of ≥150 and 1500 μΜ, respectively (Fig. 2). AOA activity was significantly reduced (P < 0.05) only at the concentration of 1,500 μΜ with the inhibitory effect being non-reversible only in the case of Ca. N. sinensis (Fig. 2). Finally, we noted a significant inhibition of the nitrite oxidation activity of the Nitrobacter strain only at the 150 μΜ, while the higher tested level of chlorogenic acid (1,500 μΜ) did not inhibit the activity of the NOB strain.
Shikimic acid significantly reduced the activity of N. multiformis, Ca. N. sinensis, and Nitrobacter sp. at concentrations of ≥500 μΜ. In contrast, a complete and persistent inhibition of the activity of Ca. N. franklandianus was induced only at the highest tested concentration of 5,000 μΜ, while the same concentration had no effect on N. europaea (Fig. 2).
The inhibition of analogs of NIs on the activity of AOM and NOB strains
Simvastatin did not significantly affect (P > 0.05) the activity of AOB and Nitrobacter sp. at the tested concentration range. In contrast, it significantly reduced (P < 0.05) the ammonia oxidation activity of Ca. N. sinensis and Ca. N. franklandianus at concentrations of ≥ 5 and ≥15 μΜ, respectively (Fig. 3).
Fig 3.
The effect of different concentrations of simvastatin, 1-butyl-4-propyl-triazole, 1,4-dibutyltriazole, and 2-methoxy-1,4-naphthoquinone on the activity of the ammonia-oxidizing bacteria N. multiformis and N. europaea, the ammonia-oxidizing archaea Ca. N. sinensis and Ca. N. franklandianus, and the nitrite-oxidizing bacterium Nitrobacter sp., determined by monitoring the production or consumption of nitrite. Error bars represent the standard error of the mean of triplicate cultures.
1-Butyl-4-propyl-triazole significantly inhibited (P < 0.05) ammonia-oxidation by N. multiformis, AOA strains, and Nitrobacter sp. only at the highest concentration tested, 500 μΜ, with the effect on Ca. N. franklandianus being temporal (Fig. 3). No effect on N. europaea was observed.
1,4-Dibutyltriazole did not significantly affect (P > 0.05) ammonia oxidation by AOB at the tested concentration range but induced a significant reduction on the activity of the AOA strains and of the Nitrobacter strain at the highest tested concentration of 500 μΜ (Fig. 3).
2-Methoxy-1,4-naphthoquinone only temporarily inhibited ammonia oxidation by N. multiformis, at the highest concentration tested, 218 μΜ, while at the same concentration level, N. europaea activity was not significantly affected (P > 0.05). Ammonia oxidation by Ca. N. sinensis and Ca. N. franklandianus was significantly reduced at concentrations of ≥5 μΜ (P < 0.05). Nitrite consumption by Nitrobacter sp. was completely inhibited at concentrations of ≥50 µM (Fig. 3).
The inhibition of nitrapyrin on the activity of AOM and NOB strains
Nitrapyrin, used as an internal control in our assays, at a single concentration (5 μΜ), (i) induced a complete and persistent inhibition of the activity of the AOB strains; (ii) imposed a significant inhibition of the activity of the AOA strains, with the effect on Ca. N. sinensis being temporal; and (iii) did not affect nitrite oxidation by the NOB Nitrobacter sp., in agreement with the data of Papadopoulou et al. (10) (Fig. S1).
The effect of selected BNIs on the growth of AOM and NOB strains
During activity testing, we noted a gradual decrease in the concentration of NO2 − in the cultures of Ca. N. sinensis amended with the highest concentrations of caffeic acid (300 μΜ), chlorogenic acid (150 μΜ), and shikimic acid (5,000 μΜ) (Fig. 2). Although this could be attributed to a potential interaction under acidic conditions between these compounds and the produced NO2 −, as reported before (58 – 60), we verified the inhibitory effect of these compounds by quantitative PCR (qPCR) monitoring of their growth. Indeed, the growth inhibition pattern of Ca. N. sinensis was congruent with NO2 − production patterns at concentrations of ≥3 μΜ (caffeic acid), 150 μΜ (chlorogenic acid), and ≥500 μΜ (shikimic acid) (Fig. S2).
Similarly, we monitored the growth of Nitrobacter sp. in the cultures amended with caffeic acid (300 and 2,200 μΜ), chlorogenic acid (1,500 μΜ), shikimic acid (500 and 5,000 μΜ), and sakuranetin (500 μΜ) to verify the activity inhibition of Nitrobacter sp., since the NO2 − consumption patterns of Nitrobacter sp. deviated from a typical dose response pattern (Fig. 1 and 2). In contrast to the nitrite consumption measurements, all compounds imposed a significant reduction in the abundance of the nxrB gene of Nitrobacter sp., unlike sakuranetin, where no significant inhibition of the growth of Nitrobacter sp. at the 500 μΜ level was observed (Fig. S3). We speculate that this inconsistency was due to the limited water solubility of sakuranetin; hence, the relevant data from this concentration level were excluded from the calculation of the respective inhibition thresholds for all nitrifying strains tested.
The persistence of NIs on AOM and NOB cultures
The persistence of sakuranetin, MHPP, and zeanone varied among the different cultures and NI concentration levels (Fig. S4). Caffeic acid and chlorogenic acid showed high degradation levels (ca. 80%–100%) in AOB and Ca. N. franklandianus cultures, all growing in alkaline media, while lower degradation rates were observed in the Ca. N. sinensis and Nitrobacter sp. cultures (Fig. S5), both growing in acidic media. Shikimic acid was generally stable and showed appreciable degradation only at the lower concentration levels of 5 and 50 μΜ (Fig. S5). Simvastatin was less stable under acidic growing conditions (Fig. S6). 1-Βutyl-4-propyl-triazole and 1,4-dibutyltriazole showed significant degradation levels only at the lowest concentration levels, while the persistence of 2-methoxy-1,4-napthoquinone varied in the different nitrifying cultures (Fig. S6). NIs showed similar degradation patterns in the inoculated and non-inoculated (abiotic controls) SW medium of N. multiformis (Fig. S4 through S6). In contrast, MHPP, zeanone, simvastatin, and 2-methoxy-1,4-napthoquinone showed significantly higher degradation levels (P < 0.05) in the cultures inoculated with N. europaea compared to the corresponding non-inoculated SW medium. Similarly, sakuranetin, zeanone, chlorogenic acid, and simvastatin showed significantly higher degradation levels (P < 0.05) in the cultures inoculated with Ca. N. sinensis compared to the respective non-inoculated FW medium cultures (Fig. S4 through S6). A more detailed description of the data regarding the persistence of NIs is provided in the supplemental material.
EC50 values of the tested NIs per strain
AOA (mean EC50 = 220.5 µM) were overly more sensitive than AOB (mean EC50 = 519.1 µM) to the NIs tested (P = 0.017), while Nitrobacter sp. (EC50 = 390.6 µM) showed no significant difference in its sensitivity compared to AOB and AOA (P > 0.05) (Fig. 4).
Fig 4.
The mean EC50 values (μΜ) derived from all tested nitrification inhibitors (NIs) for ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and the nitrite-oxidizing bacterium (NΟΒ). In cases where no EC50 could be deduced from the statistical analysis, the lowest or the highest tested concentrations were used instead. NS, non-significant difference (P > 0.05).
The two tested AOB strains, N. multiformis and N. europaea, varied in their sensitivity to the tested NIs with the former being consistently more sensitive (P < 0.05) than the latter to 8 out of the 11 NIs tested (Fig. 5; Table S1). Zeanone (EC50 <0.2 µM) and quinic acid (EC50 <3 µM) were the most potent inhibitors of N. multiformis, while caffeic acid (EC50 = 203.3 ± 15.8 µM) and MHPP (EC50 = 339.3 ± 32.4 µM) were the most potent inhibitors of N. europaea (Fig. 5). The most potent NIs toward AOA were zeanone, its analog 2-methoxy-1,4-naphthoquinone, caffeic acid, sakuranetin and simvastatin (Fig. 5). Ca. N. sinensis was generally more sensitive than Ca. N. franklandianus to 6 out of the 11 NIs tested. Zeanone and its analog 2-methoxy-1,4-naphthoquinone were significantly more potent to Ca. N. franklandianus compared to Ca. N. sinensis, while 1-butyl-4-propyl-triazole and 1,4-dibutyltriazole were equally potent to both AOA strains (Fig. 5; Table S2).
Fig 5.
Mean EC50 values (μM) of the tested NIs calculated based on their inhibitory activity on the ammonia or nitrite oxidation capacity of the tested ammonia-oxidizing bacteria, ammonia-oxidizing archaea, and the nitrite-oxidizing bacterium. Standard errors of the mean values (denoted by ±) are given in brackets. Upper case letters indicate significant differences (P < 0.05) between microorganisms for each individual NI, and lower-case letters indicate significant differences (P < 0.05) between NIs for each tested microorganism. The asterisks denote that no EC50 could be deduced from the data. One asterisk (*) indicates that EC50 value is lower than the minimum tested concentration, while two asterisks (**) indicate that the EC50 value is higher than the maximum tested concentration. Dendrograms based on the euclidean distances and the complete linkage clustering method using log transformed mean EC50 values are presented for identifying NIs. The table is color-coded by orders of magnitude for EC50 values according to the color legend. NI, nitrification inhibitor.
Regarding the NOB strain Nitrobacter sp., caffeic acid (EC50 <3 µM) was its most potent inhibitor. This was followed by MHPP, zeanone, 2-methoxy-1.4-naphthoquinone, and chlorogenic acid which were equally potent against the NOB strain (P > 0.05). Among them MHPP showed the lowest (EC50 = 7.4 ± 1.3 µM) and chlorogenic acid the highest EC50 value (71.9 ± 8.9 µM) (Fig. 5).
DISCUSSION
We determined the inhibitory activity of a wide range of plant-derived molecules categorized as (i) root-derived BNIs; (ii) phytochemicals with potential BNI activity; and (iii) analogs of statins, triazoles and zeanone, on a range of AOA and AOB strains. The first group was composed of known BNIs such as sakuranetin, MHPP, and zeanone. Sakuranetin exhibited higher inhibitory activity toward AOA isolates as also observed by Kaur-Bhambra et al. (32). Regarding N. europaea and Ca. N. franklandianus, Kaur-Bhambra et al. (32) reported an IC80 of 259 and 173 µM, respectively, compared to our EC50 values of >250 and <10 µM, for the same AOB. The differences in the inhibition thresholds between the two studies could be attributed either to the different N. europaea strains tested (ATCC 19718 instead of ATCC 25978 in our study) or most probably to the different approaches used for the calculation of the inhibition thresholds (concentration of each BNI leading to 80% inhibition of μmax vs concentration leading to 50% inhibition of nitrite production in our study). MHPP was the only tested BNI that showed higher potency toward AOB suppressing their activity at concentrations of ≥150 μΜ compared to ≥500 μΜ for AOA isolates. This is in line with previous studies which showed 80% inhibition of AOB (N. europaea, N. multiformis, Nitrosospira tenuis, and Nitrosospira briensis) and AOA (Ca. N. sinensis, Ca. N. franklandianus, and Nitrososphaera viennensis) growth at levels varying from 80 to 545 μΜ for AOB and from 149 to 1028 μΜ for AOA (32). In accord with our findings for MHPP inhibition on N. europaea (EC50 339.3 ± 32.4), Lu et al. (28), using a different N. europaea strain (NBRC 14298), reported a similar ED50 value of 295 µM. Zeanone, a novel naphthoquinone BNI isolated from maize root exudates (24), was reported with an ED80 of 8 µM, determined using the recombinant N. europaea assay. Based on our study, which provides a broader characterization of its inhibitory activity, zeanone was a strong inhibitor of N. multiformis (EC50 <0.2 µM) and of the two AOA isolates (EC50 = 4.5 ± 0.6 µM and 0.4 ± 0.1 µM for Ca. N. sinensis and Ca. N. franklandianus, respectively) but failed to inhibit N. europaea. The discrepancy in the inhibitory threshold values for N. europaea between our study and the study of Otaka et al. (24) might be the result (i) of the different N. europaea strains tested [wild-type ATCC 25978 in the present study vs genetically modified ATCC 19718 in the study of Otaka et al. (24)] and most probably (ii) of the different approaches used for the calculation of the inhibition thresholds. In our study the calculation of EC50s was based on multiple measurements of NO2 − production throughout the duration of N. europaea growth at optimum conditions (28°C), while in the study of Otaka et al. (24), ED80 estimation was based on a single fluorescence measurement within 30 s, after a 30-min preincubation period of concentrated cells of the AOB at 15°C.
The second group of compounds tested included phytochemicals with potential NI activity like caffeic, quinic, chlorogenic, and shikimic acid. As it was expected by its reported mode of action, as NO scavenger (61), caffeic acid showed a higher inhibition activity on AOA (mean EC50 = 10.7 μΜ) compared to AOB strains (mean EC50 = 171.7 μΜ). AOA are known to be inhibited by NO scavengers like 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO), which has been interpreted as evidence that NO is an intermediate in the AOA pathway but not in AOB (62, 63). This hypothesis was disproved by Caranto and Lancaster (64), who showed that NO is also an intermediate in ammonia oxidation by AOB which are also inhibited by NO scavengers, albeit at higher concentrations than AOA. The NO scavenging activity of caffeic acid has been previously tested against three AOA strains (Nitrosopumilus maritimus SCM1, AOA-6f, and AOA-G6) and the AOB N. europaea strain ATCC 19718 by Sauder et al. (47), who reported an EC50 of <10 µM for N. maritimus and AOA-6f, and an EC50 of 84.3 µM for AOA-G6, in line with our study. However, the same authors did not observe any inhibition of N. europaea by caffeic acid (EC50 of >300 µM) compared to our findings where an EC50 = 203.3 µM was calculated, using a different N. europaea strain. The NI activity of quinic acid was assessed for the first time and showed variable inhibition levels being more inhibitory toward N. multiformis (EC50 <3 μΜ) and Ca. N. sinensis (EC50 84.3 ± 14.0 μΜ) compared to N. europaea (EC50 1596.6 ± 123.1 μΜ), and Ca. N. franklandianus (EC50 >300 μΜ). Chlorogenic acid, an ester of caffeic acid and quinic acid, showed an intermediate inhibitory activity between these two compounds, suggesting a potential influence of chemical structure to their inhibition mechanism. Earlier studies showed that both caffeic acid and chlorogenic acid could inhibit nitrification at a concentration range of 0.1 to 1,000.0 μΜ (65), in line with our study. Shikimic acid completely inhibited the activity of N. multiformis and Ca. N. sinensis at lower concentrations (≥500 μΜ) than those found to suppress Ca. N. franklandianus (complete inhibition at 5,000 μΜ). At the same concentration range (up to 5,000 μΜ), N. europaea was only slightly inhibited, in line with previous studies that reported no inhibitory effect of shikimic acid on N. europaea ATCC 19718 strain at concentrations up to 2,500 µM (22, 23).
The third group of potential NIs included analogs of compounds with known inhibitory activity toward AOM like simvastatin (analog of lovastatin) (66), 1-butyl-4-propyl-triazole and 1,4-dibutyltriazole (triazole analogs) and 2-methoxy-1,4-naphthoquinone (analog of zeanone). Simvastatin selectively inhibited both AOA isolates but did not affect the AOB strains, in accord with Zhao et al. (34). The selectivity of simvastatin toward AOA is due to its known activity as an inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase, a crucial enzyme in the mevalonate pathway in archaeal cell membrane biosynthesis (67, 68), with AOB using an alternative pathway (69). The two triazole derivatives selectively inhibited AOA strains, showing a much weaker effect on AOB. Although there are no data available regarding the effect of these compounds on pure cultures of soil nitrifiers, previous studies demonstrated their high efficacy in inhibiting nitrification in soil (70, 71) and highlighted their potential use as NIs that can be synthesized with good yields and high atom economy from readily available starting materials (72). Finally, 2-methoxy-1,4-naphthoquinone showed a distinct higher inhibition potential for AOA vs AOB, similarly to its chemical analog zeanone, although the latter was clearly more inhibitory to N. multiformis compared to 2-methoxy-1,4-naphthoquinone, which showed no activity to the AOB isolates.
When the data from all 11 tested compounds and AOM strains were assessed comparatively between and across AOM groups, we identified clear trends of selectivity in the inhibition between AOA and AOB as well as within the different groups of AOM tested. As a first general hint, we noted a higher sensitivity of AOA compared to AOB in 10 out of the 11 potential BNI compounds tested with the sole exception of MHPP. Previous culture-based studies on the sensitivity of AOM to SNIs and BNIs have shown a rather selective inhibition of AOB by SNIs (10, 41, 73) and a stronger inhibition of AOA by BNIs (32). The variable sensitivity of soil AOM to different NIs may arise from fundamental differences in their biochemistry and physiology (74, 75) and could be related to the particular mode of action of each NI at cellular level with some of them (e.g., simvastatin) targeting group-specific (AOA) biochemical pathways.
We further observed differences in the sensitivity between the AOA and the AOB strains tested. N. multiformis was consistently more sensitive than N. europaea, while the sensitivity of AOA strains was compound dependent, with Ca. N. franklandianus being less sensitive than Ca. N. sinensis to 6 out of the 11 NIs tested. Variations in the sensitivity of different AOA and AOB strains to SNIs (10, 34, 76) and BNIs (32) have been reported before. Papadopoulou et al. (10) observed a higher sensitivity of N. multiformis over N. europaea to the potent SNI quinone imine and of Ca. N. sinensis over Ca. N. franklandianus to the commercial SNIs DCD and DMPP. Kaur-Bhambra et al. (32) demonstrated the higher sensitivity of Ca. N. sinensis over Ca. N. franklandianus (and Nitrososphaera viennensis) and of N. multiformis over N. europaea to various root- and shoot-derived BNI compounds. The reasons for the different sensitivities of the tested AOB and AOA strains to the potential BNIs studied are most probably driven by differences in their metabolism, ecophysiology, and other cellular characteristics. For example, the membranes of all AOA studied contain crenarchaeol, a glycerol dialkyl glycerol tetraether lipid produced exclusively in archaea of the phylum Thaumarchaeota (77, 78). However, the proportion of crenarchaeol was found to be much higher in all tested neutrophilic AOAs (including Ca. N. franklandianus) compared to the acidophilic Ca. Nitrosotalea strains (79). This could potentially explain the lower sensitivity of Ca. N. frankandianus to NIs like simvastatin, shikimic acid, and chlorogenic acid presumed to interfere with membrane biosynthesis (34) or affect its integrity (27, 53). The higher tolerance of Ca. N. franklandianus to NIs has been also presumed to be associated with its capacity to produce extracellular polymeric substances (EPS) leading to aggregate formation that could potentially prevent NIs, and especially hydrophilic compounds like quinic acid and shikimic acid, from accessing the surface of cells attached to the hydrophobic EPS matrix (10, 80). This production of EPS is a common feature for all Ca. Nitrosocosmicus isolates (81 – 85) and has been also reported as an AOB defense mechanism against NIs (86). Regarding AOB, N. europaea and N. multiformis possess a largely different set of oxidative stress response proteins, alkyl hyperoxide reductase vs superoxide dismutase and rubrerythrin, respectively (87), that might exhibit different efficiencies to oxidative stress potentially imposed by certain BNIs (88). In addition, N. europaea possesses a cytochrome P460 (CytL) that is involved in the oxidation of NH2OH and NO to NO2 −, and the NO-responsive transcriptional regulators NsrR and NnrS known to regulate the expression of multiple genes involved in nitrosative stress responses. In contrast, all this enzymatic arsenal against nitrosative stress, potentially imposed by BNIs, is not present in N. multiformis (89). Alternatively, the wider range of membrane protein transporters being operative in N. europaea has been suggested as a potential mechanism to cope with the inhibitory effects of chemicals like NIs (87).
We expanded our tests to a Nitrobacter sp., as a representative of NOB, whose response to BNIs remains unknown. NOBs are fueled by substrates produced by AOM, and their activity results in the rapid conversion of potentially hazardous (for both plants and microbes) NO2 − to NO3 − that it is consumed by plants and soil microbes (90). In heavily fertilized agricultural soils, the NOB-derived NO3 − contributes to N losses and environmental pollution through NO3 − leaching and subsequent production of nitrogen oxides (e.g., N2O) through the denitrification process (91). We demonstrated that caffeic acid, MHPP, zeanone, 2-methoxy-1.4-naphthoquinone, and chlorogenic acid were suppressive to Nitrobacter sp. at concentrations of <100 μΜ. Among them, caffeic acid and MHPP were the two NIs that showed higher activity to the Nitrobacter strain compared to the AOA and AOB strains tested. The impact of these compounds on the activity of both AOM and NOB could significantly affect the direction and degree of nitrogen transformation during nitrification, and especially in cases where NIs inhibit NOB to a greater extent than AOM. This could potentially lead to nitrite accumulation in soil, raising serious concerns for environmental quality (e.g., increased nitrite driven N2O production) and agricultural production (e.g., nitrite toxicity to plants). We provide pioneering data regarding the impact of BNIs and their synthetic analogs on a pure NOB culture. Further studies extended to other NOB, including the widely distributed and diverse Nitrospira species, are required to determine the full inhibitory potential of BNIs on soil NOB.
To date, the overwhelming majority of BNI activity testing has been performed with a single genetically modified AOB Nitrosomonas europaea strain (19, 20, 24, 30). This approach was initially developed to monitor the nitrification process in wastewater treatment plants (92), where N. europaea represents a key ammonia-oxidizing microbe, and it was then adopted for screening root exudates for BNI activity (19, 20, 24, 30). Kaur-Bhambra et al. (32) assessed the activity of BNIs produced by plant roots and shoots on selected AOB and AOA strains and questioned the relevance of N. europaea as a single indicator of BNI activity based on its highly variable response to BNIs compared to strains that are more representative of natural AOM soil assemblages. Our study verified the high variation in the sensitivity of N. europaea to BNIs, compared to N. multiformis, which constitutes a more representative soil AOB, reinforcing the need for NI assays with diverse strains of soil representative AOM. In addition, inhibition thresholds of BNIs (i.e., sakuranetin, MHPP, zeanone, and caffeic acid) estimated for N. europaea in this study differ significantly from those reported by other researchers using either a wild-type (22, 32, 47) or a genetically modified N. europaea strain (19, 20, 24, 30) and different experimental approaches (e.g., detection of inhibition based on measurements of μmax, NO2 − production or changes in fluorescence, duration of bioassays from 30 min to several days, and determination of IC80, IC70, or EC50 values). All these might imply an inherent variability in the response of N. europaea to in vitro testing conditions further stressing its unsuitability for use as a sole indicator of NI activity in screening assays. In addition, our findings highlight the need for standardization of the existing and under development, high-throughput BNIs screening biological systems to obtain more meaningful and comparable information.
Finally, an important limitation of the currently available BNI screening systems is the lack of data regarding the persistence and stability of these chemicals under the testing conditions. These are often essential for the interpretation of the inhibitory effects observed, directly associated with the duration of the exposure to the tested compounds. We provide first results on the stability and persistence of BNI compounds during laboratory incubation. We noted that some of the tested chemicals showed a pH-dependent stability (simvastatin), while others were overly unstable (caffeic and chlorogenic acid) or persistent (triazole derivatives) in all growth media. It is worth noting the potential involvement of N. europaea and Ca. N. sinensis in the cometabolic biodegradation of certain NIs (e.g., zeanone and simvastatin) serving as non-growth substrates for AOM strains in the presence of NH3/NH4 + (growth substate). The potential catabolism of NIs by AOM is not unexpected, considering earlier evidence that the AMO enzymes of both AOB (e.g., N. europaea) and AOA (e.g., N. gargensis) could transform co-metabolically a wide range of aliphatic and aromatic substrates (93 – 96) including pharmaceuticals/antibiotics (e.g., β-lactams, fluoroquinolones, and sulfonamides) through distinct biodegradation pathways involving deamination, hydroxylation, and co-oxidation (97, 98). Still the involvement of the tested AOM strains in the degradation of the tested NIs is a particularly interesting aspect which warrants further investigation.
Conclusions
We provided a systematic assessment of the inhibition potential of multiple BNI molecules on the activity of soil derived AOB (Nitrosospira multiformis and Nitrosomonas europaea), AOA (Ca. Nitrosotalea sinensis and Ca. Nitrosocosmicus franklandianus), and NOB (Nitrobacter sp.) strains, which represent main nitrifier lineages in soil. Accurate analytical methods for determining the degradation and stability of the NIs in the liquid cultures of the nitrifying isolates were developed and validated, in parallel. Our study (i) offers benchmarking knowledge of the activity of diverse BNIs and analogs to a range of soil AOM and Nitrobacter NOB, whose response to BNIs was unknown; (ii) provides unprecedented data on the inhibition potential of the recently discovered hydrophobic maize isolated BNI, zeanone, and its natural analog, 2-methoxy-1,4-naphthoquinone, on nitrification; (iii) verifies the selective activity of the currently available BNIs on AOA and stresses the necessity for elucidation of the inhibition mechanisms against target AOM; (iv) illustrates the variation in the sensitivity of different AOM isolates to BNIs; this further highlights the limitations of studies relying on a single AOB strain and argues for testing against a broad range of ecophysiologicaly and phylogenetically distinct soil-derived nitrifiers to extrapolate a robust estimate of the NI potency of plant derived compounds; (v) provides first evidence for the stability of BNIs during biological screening allowing for accurate and comparable determination of inhibition thresholds; and (vi) demonstrates the potential inhibitory activity of BNIs on nitrifying microbial groups beyond AOM, modulating downstream processes in N cycling like NOB. The potency of the BNIs tested on Nitrobacter sp. questions the specificity of certain BNI molecules toward AOM that, like other phytochemicals, might have a wider antimicrobial activity, beyond just prokaryotic nitrifiers, that should be assessed before further use in agricultural settings.
MATERIALS AND METHODS
Microbial strains and growth conditions
Our inhibition assays included two AOB strains, Nitrosomonas europaea ATCC 25978 and Nitrosospira multiformis ATCC 25196, two AOA strains, Candidatus Nitrosotalea sinensis Nd2 (99) and Candidatus Nitrosocosmicus franklandianus C13 (82), and one NOB strain, Nitrobacter sp. NHB1 (100). All strains were grown aerobically in the dark without shaking. N. multiformis and N. europaea were grown at 28°C, in Skinner and Walker’s medium (SW) (101) containing 1-mM NH4 + [(NH4)2SO4] and phenol red (0.5 mg/L) as a pH indicator (pH 7.5–8.0). AOAs Ca. N. franklandianus and Ca. N. sinensis were incubated at 35°C in a medium supplemented with 1.0- and 0.5 mM NH4 + (NH4Cl), respectively. The former was cultured in HEPES-buffered modified freshwater medium [pH 7.5 (90)], while the latter was grown in MES-buffered freshwater (FW) medium [pH 5.2 (88)]. Nitrobacter sp. was grown at 28°C in FW medium [pH 5.2 (102)] supplemented with 0.5 mM NO2 − (NaNO2).
BNIs and synthetic analogs
High-purity (>95%) analytical standards of sakuranetin, methyl 3-(4-hydroxyphenyl)-propionate (MHPP), 2,7-dimethoxy-1,4-naphthoquinone (zeanone), caffeic acid, quinic acid, chlorogenic acid, shikimic acid, simvastatin, 1-butyl-4-propyl-triazole, 1,4-dibutyltriazole, and 2-methoxy 1,4-naphthoquinone were provided by Syngenta Crop Protection AG (Basel, Switzerland). Analytical standard of nitrapyrin (≥98%) was purchased from Sigma-Aldrich (Germany) and used as an internal control of the microbial cell response, according to a previous study by Papadopoulou et al. (10). Detailed information on the tested compounds is provided in Table 1.
Liquid culture inhibition assays
To determine the inhibition thresholds per strain and compound, the activity of all NIs was tested in liquid batch cultures over a range of concentrations (see Table S3). Cultures were established in triplicate for each NI × concentration combination in 100-mL Duran bottles containing 30 mL of growth medium and inoculated with a 1% or 2% (vol/vol) transfer of exponentially growing culture of AOB or AOA/NOB respectively. All compounds except quinic acid and shikimic acid were added to the cultures as filter sterilized dimethyl sulfoxide (DMSO) solutions due to their low water solubility. The final concentration of DMSO in all cultures was 0.1% (vol/vol), which did not exert a significant inhibitory effect to any of the isolates tested, in line with previous studies with the same isolates (10, 32). Quinic acid and shikimic acid were dissolved in sterile dH2O before addition in the cultures. All NIs were added to batch cultures at the beginning of the exponential growth phase. Triplicate cultures with the same inoculum amended with DMSO or sterile dH2O (in case of quinic acid and shikimic acid) without NIs were included as control. For each strain, triplicate cultures were amended with a single concentration of nitrapyrin [5 μΜ, with known inhibitory activity to the tested strains based on Papadopoulou et al. (10)] to verify the consistency of our inhibition assays. For each of the tested NIs, triplicate non-inoculated cultures of SW [pH 7.8 (92)] and FW medium [pH 5.2 (102)] were also prepared at one concentration level to determine the contribution of abiotic processes in the degradation of the tested compounds. It should be noted that these two media were selected to demonstrate the abiotic degradation of the NIs under alkaline and acidic conditions.
Upon inoculation, all cultures were sampled at regular time intervals (once or twice daily) to determine the effect of NIs on the activity of the nitrifying microorganisms by measuring changes in nitrite concentrations. Nitrite concentrations were determined colorimetrically at 540 nm in a 96-well plate format assay by diazotizing and coupling with Griess reagent (103). At specific cases (see Fig. S2 and S3), the effects of NIs on the growth of AOM and NOB were determined via qPCR measurement of the abundance of the amoA and nxrB genes at selected time points based on the activity of the control treatment (early logarithmic and/or stationary phase). The degradation of NIs in the growth media was determined in samples collected at two time points (start: early logarithmic phase, end: early stationary phase) via HPLC.
qPCR measurement of microbial growth of AOM and NOB
The abundance of amoA and nxrB genes was determined in a Biorad CFX Real-Time PCR system. DNA was extracted from a cell pellet obtained from 2-mL aliquots of the microbial cultures using the tissue DNA extraction kit (Macherey-Nagel, Germany). The amoA gene of Ca. N. sinensis was amplified with primers Arch-amoAF/Arch-amoAR (104), as described by Rousidou et al. (105), and the nxrB gene of Nitrobacter sp. was quantified with primers nxrB-1F and nxrB-1R (106). The qPCR assays used the following thermal cycling conditions: 95°C for 3 min, followed by 40 cycles of 95°C for 30 s, 57°C for 20 s, 72°C for 30 s, with a final dissociation curve analysis. The abundance of amoA and nxrB was determined via external standard curves as described by Rousidou et al. (105). qPCR amplification efficiency ranged from 87.9% to 90.9% with r 2 values of >0.98.
Analysis of the stability of BNIs in liquid cultures
NIs were extracted from the growth media by mixing liquid culture with acetonitrile (MeCN) or methanol (MeOH) in a 1:2, 1:5, or 1:10 (v/v) ratio. The derived mixtures were vortexed for 1 min and stored at −20°C until HPLC analysis. Details of the extraction methods employed per compound are given in the supplemental material. Recovery tests at three concentration levels (in the range of the tested concentrations) showed recoveries of >70% for all compounds studied (Table S4). The sole exception was sakuranetin, for which the achieved recoveries at the highest tested concentration (500 μΜ) were 28.2% and 33.7% in the SW and FW medium, respectively.
Chromatographic analyses
HPLC analyses was performed in a Shimadzu LC-20ADHPLC system equipped with an UV (Ultraviolet) /VIS (Visible) PDA (Photodiode Array) detector. A Shimadzu GVP-ODs (4.6 mM × 150 mM, 5 mM) precolumn, connected to a RP Shimadzu VPODs (4.6 mM × 150 mM, 5 mM) column, was used for NIs separation. Chromatographic separation of all compounds was achieved at isocratic conditions as summarized in Table S5. Calibration curves obtained by the injection of standard solutions of NIs in MeOH or MeCN (in case of 2-methoxy-1,4-naphthoquinone and zeanone), ranging from 1 to 100 mg/L for caffeic acid and from 0.05 to 20 mg/L for all other compounds, were used for quantification.
Calculation of inhibition threshold levels (EC50)
The concentration of each NI achieving 50% inhibition (EC50) in the activity of the AOB, AOA, and NOB strains was calculated according to Papadopoulou et al. (10). Briefly, dose-response modeling was performed using normalized data whereby nitrite concentration values were divided by the mean value of the matching control. Analyses were carried out using the dose response curves v.3.0–1 package (107) of the R software.
Data analysis
Nitrite data were subjected to one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test (P < 0.05). Variance between the EC50 values of the different NIs for one strain and between different strains for a given NI was analyzed by one-way ANOVA and Tukey’s post hoc test (P < 0.05). Comparison of mean EC50 values of AOB, AOA, and NOB for all NIs was performed using the non-parametric Wilcoxon signed-rank test.
ACKNOWLEDGMENTS
This project has received funding from Syngenta Crop Protection AG, Basel, Switzerland. This article has been released as a pre-print at bioRxiv (reference (108), at doi: https://doi.org/10.1101/2023.07.12.548655).
Contributor Information
Evangelia S. Papadopoulou, Email: evapapadopoulou@uth.gr.
Jennifer B. Glass, Georgia Institute of Technology, Atlanta, Georgia, USA
DATA AVAILABILITY
The data that support the findings of this study are available in the supplemental material.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.01380-23.
Supplemental Results and Material and Methods.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M. 2013. The global nitrogen cycle in the twenty first century. Philos Trans R Soc Lond B Biol Sci 368:20130164. doi: 10.1098/rstb.2013.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wendeborn S. 2020. The chemistry, biology, and modulation of ammonium nitrification in soil. Angew Chem Int Ed 59:2182–2202. doi: 10.1002/anie.201903014 [DOI] [PubMed] [Google Scholar]
- 3. Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. doi: 10.1126/science.1136674 [DOI] [PubMed] [Google Scholar]
- 4. Lassaletta L, Billen G, Grizzetti B, Anglade J, Garnier J. 2014. 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ Res Lett 9:105011. doi: 10.1088/1748-9326/9/10/105011 [DOI] [Google Scholar]
- 5. Cassman KG, Dobermann A, Walters DT. 2002. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31:132–140. doi: 10.1579/0044-7447-31.2.132 [DOI] [PubMed] [Google Scholar]
- 6. Sutton MA, Oenema O, Erisman JW, Leip A, van Grinsven H, Winiwarter W. 2011. Too much of a good thing. Nature 472:159–161. doi: 10.1038/472159a [DOI] [PubMed] [Google Scholar]
- 7. Coskun D, Britto DT, Shi W, Kronzucker HJ. 2017. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat Plants 3:17074. doi: 10.1038/nplants.2017.74 [DOI] [PubMed] [Google Scholar]
- 8. Forster P. 2007. In climate change 2007: the physical science basis. In Solomon S (ed), Cambridge univ. press for the intergovernmental panel on climate change. Cambridge. [Google Scholar]
- 9. Qiao C, Liu L, Hu S, Compton JE, Greaver TL, Li Q. 2015. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob Chang Biol 21:1249–1257. doi: 10.1111/gcb.12802 [DOI] [PubMed] [Google Scholar]
- 10. Papadopoulou ES, Bachtsevani E, Lampronikou E, Adamou E, Katsaouni A, Vasileiadis S, Thion C, Menkissoglu-Spiroudi U, Nicol GW, Karpouzas DG. 2020. Comparison of novel and established nitrification inhibitors relevant to agriculture on soil ammonia- and nitrite-oxidizing isolates. Front Microbiol 11:581283. doi: 10.3389/fmicb.2020.581283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lehtovirta-Morley LE. 2018. Ammonia oxidation: ecology, physiology, biochemistry and why they must all come together. FEMS Microbiol Lett 365:fny058. doi: 10.1093/femsle/fny058 [DOI] [PubMed] [Google Scholar]
- 12. Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, Wagner M. 2015. Complete nitrification by Nitrospira bacteria. Nature 528:504–509. doi: 10.1038/nature16461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, Watanabe T, Suenaga K, Rondon M, Rao IM. 2006. Scope and strategies for regulation of nitrification in agricultural systems—challenges and opportunities. Crit Rev Plant Sci 25:303–335. doi: 10.1080/07352680600794232 [DOI] [Google Scholar]
- 14. Gao Y, Cabrera Serrenho A. 2023. Greenhouse gas emissions from nitrogen fertilizers could be reduced by up to one-fifth of current levels by 2050 with combined interventions. Nat Food 4:170–178. doi: 10.1038/s43016-023-00698-w [DOI] [PubMed] [Google Scholar]
- 15. Akiyama H, Yan X, Yagi K. 2010. Evaluation of effectiveness of enhanced- efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils, meta-analysis. Glob Chang Biol 16:1837–1846. doi: 10.1111/j.1365-2486.2009.02031.x [DOI] [Google Scholar]
- 16. Qiu H, Sun D, Gunatilake SR, She J, Mlsna TE. 2015. Analysis of trace dicyandiamide in stream water using solid phase extraction and liquid chromatography UV Spectrometry. J Environ Sci (China) 35:38–42. doi: 10.1016/j.jes.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Bai J, Xie T, Wang W, Zhang G, Yin S, Wang D. 2021. Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: a review. Ecotoxicol Environ Saf 220:112338. doi: 10.1016/j.ecoenv.2021.112338 [DOI] [PubMed] [Google Scholar]
- 18. Subbarao GV, Wang HY, Ito O, Nakahara K, Berry WL. 2007. NH4 + triggers the synthesis and release of biological nitrification inhibition compounds in Brachiaria humidicola roots. Plant Soil 290:245–257. doi: 10.1007/s11104-006-9156-6 [DOI] [Google Scholar]
- 19. Zakir H, Subbarao GV, Pearse SJ, Gopalakrishnan S, Ito O, Ishikawa T, Kawano N, Nakahara K, Yoshihashi T, Ono H, Yoshida M. 2008. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4- hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol 180:442–451. doi: 10.1111/j.1469-8137.2008.02576.x [DOI] [PubMed] [Google Scholar]
- 20. Subbarao GV, Nakahara K, Ishikawa T, Ono H, Yoshida M, Yoshihashi T, Zhu Y, Zakir H, Deshpande SP, Hash CT, Sahrawat KL. 2013. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 366:243–259. doi: 10.1007/s11104-012-1419-9 [DOI] [Google Scholar]
- 21. Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi-Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O. 2009. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Natl Acad Sci U S A 106:17302–17307. doi: 10.1073/pnas.0903694106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun L, Lu Y, Yu F, Kronzucker HJ, Shi W. 2016. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol 212:646–656. doi: 10.1111/nph.14057 [DOI] [PubMed] [Google Scholar]
- 23. Lu Y, Zhang X, Ma M, Zu W, Kronzucker HJ, Shi W. 2022. Syringic acid from rice as a biological nitrification and urease inhibitor and its synergism with 1,9-decanediol. Biol Fertil Soils 58:277–289. doi: 10.1007/s00374-021-01584-y [DOI] [Google Scholar]
- 24. Otaka J, Subbarao GV, Ono H, Yoshihashi T. 2022. Biological nitrification inhibition in maize—isolation and identification of hydrophobic inhibitors from root exudates. Biol Fertil Soils 58:251–264. doi: 10.1007/s00374-021-01577-x [DOI] [Google Scholar]
- 25. Otaka J, Subbarao GV, MingLi J, Ono H, Yoshihashi T. 2023. Isolation and characterization of the hydrophilic BNI compound, 6-methoxy-2(3h)-benzoxazolone (MBOA), from maize roots. Plant Soil 489:341–359. doi: 10.1007/s11104-023-06021-7 [DOI] [Google Scholar]
- 26. Rice EL, Pancholy SK. 1974. Inhibition of nitrification by climax ecosystems. III. inhibitors other than tannins. American J of Botany 61:1095–1103. doi: 10.1002/j.1537-2197.1974.tb12327.x [DOI] [Google Scholar]
- 27. Nardi P, Laanbroek HJ, Nicol GW, Renella G, Cardinale M, Pietramellara G, Weckwerth W, Trinchera A, Ghatak A, Nannipieri P. 2020. Biological nitrification inhibition in the rhizosphere: determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol Rev 44:874–908. doi: 10.1093/femsre/fuaa037 [DOI] [PubMed] [Google Scholar]
- 28. Lu Y, Zhang X, Ma M, Zu W, Kronzucker HJ, Shi W. 2022. Syringic acid from rice as a biological nitrification and urease inhibitor and its synergism with 1,9-decanediol. Biol Fertil Soils 58:277–289. doi: 10.1007/s00374-021-01584-y [DOI] [Google Scholar]
- 29. Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, Rao IM, Lata J-C, Kishii M, Braun H-J. 2015. Suppression of soil nitrification by plants. Plant Sci. 233:155–164. doi: 10.1016/j.plantsci.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 30. Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL. 2006. A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288:101–112. doi: 10.1007/s11104-006-9094-3 [DOI] [Google Scholar]
- 31. O’Sullivan CA, Duncan EG, Whisson K, Treble K, Ward PR, Roper MM. 2017. A colourimetric microplate assay for simple, high throughput assessment of synthetic and biological nitrification inhibitors. Plant Soil 413:275–287. doi: 10.1007/s11104-016-3100-1 [DOI] [Google Scholar]
- 32. Kaur-Bhambra J, Wardak DLR, Prosser JI, Gubry-Rangin C. 2022. Revisiting plant biological nitrification inhibition efficiency using multiple archaeal and bacterial ammonia-oxidising cultures. Biol Fertil Soils 58:241–249. doi: 10.1007/s00374-020-01533-1 [DOI] [Google Scholar]
- 33. Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. doi: 10.1038/nature04983 [DOI] [PubMed] [Google Scholar]
- 34. Zhao J, Bello MO, Meng Y, Prosser JI, Gubry-Rangin C. 2020. Selective inhibition of ammonia oxidising archaea by simvastatin stimulates growth of ammonia oxidising bacteria. Soil Biol Biochem 141:107673. doi: 10.1016/j.soilbio.2019.107673 [DOI] [Google Scholar]
- 35. Zhu Y, Zeng H, Shen Q, Ishikawa T, Subbarao GV. 2012. Interplay among NH4 + uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots – possible mechanisms and underlying hypothesis. Plant Soil 358:131–141. doi: 10.1007/s11104-012-1151-5 [DOI] [Google Scholar]
- 36. Di T, Afzal MR, Yoshihashi T, Deshpande S, Zhu Y, Subbarao GV. 2018. Further insights into underlying mechanisms for the release of biological nitrification inhibitors from sorghum roots. Plant Soil 423:99–110. doi: 10.1007/s11104-017-3505-5 [DOI] [Google Scholar]
- 37. Zhang X, Lu Y, Yang T, Kronzucker HJ, Shi W. 2019. Factors influencing the release of the biological nitrification inhibitor 1,9-decanediol from rice (Oryza sativa L.) roots. Plant Soil 436:253–265. doi: 10.1007/s11104-019-03933-1 [DOI] [Google Scholar]
- 38. Kowalchuk GA, Stephen JR. 2001. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529. doi: 10.1146/annurev.micro.55.1.485 [DOI] [PubMed] [Google Scholar]
- 39. Norton JM, Klotz MG, Stein LY, Arp DJ, Bottomley PJ, Chain PSG, Hauser LJ, Land ML, Larimer FW, Shin MW, Starkenburg SR. 2008. Complete genome sequence of Nitrosospira multiformis, an ammonia-oxidizing bacterium from the soil environment. Appl Environ Microbiol 74:3559–3572. doi: 10.1128/AEM.02722-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lehtovirta-Morley LE, Verhamme DT, Nicol GW, Prosser JI. 2013. Effect of nitrification inhibitors on the growth and activity of Nitrosotalea devanaterra in culture and soil. Soil Biol Biochem 62:129–133. doi: 10.1016/j.soilbio.2013.01.020 [DOI] [Google Scholar]
- 41. Shen T, Stieglmeier M, Dai J, Urich T, Schleper C. 2013. Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344:121–129. doi: 10.1111/1574-6968.12164 [DOI] [PubMed] [Google Scholar]
- 42. Dayan FE, Rimando AM, Pan Z, Baerson SR, Gimsing AL, Duke SO. 2010. Sorgoleone. Phytochemistry 71:1032–1039. doi: 10.1016/j.phytochem.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 43. Xia W, Zhang C, Zeng X, Feng Y, Weng J, Lin X, Zhu J, Xiong Z, Xu J, Cai Z, Jia Z. 2011. Autotrophic growth of nitrifying community in an agricultural soil. ISME J 5:1226–1236. doi: 10.1038/ismej.2011.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nowka B, Daims H, Spieck E. 2015. Comparison of oxidation kinetics of nitrite-oxidizing bacteria: nitrite availability as a key factor in niche differentiation. Appl Environ Microbiol 81:745–753. doi: 10.1128/AEM.02734-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Papadopoulou ES, Bachtsevani E, Papazlatani CV, Rousidou C, Brouziotis A, Lampronikou E, Tsiknia M, Vasileiadis S, Ipsilantis I, Menkissoglu-Spiroudi U, Ehaliotis C, Philippot L, Nicol GW, Karpouzas DG. 2022. The effects of quinone imine, a new potent nitrification inhibitor, dicyandiamide, and nitrapyrin on target and off-target soil microbiota. Microbiol Spectr 10:e0240321. doi: 10.1128/spectrum.02403-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones CM, Hallin S. 2019. Geospatial variation in co-occurrence networks of nitrifying microbial guilds. Mol Ecol 28:293–306. doi: 10.1111/mec.14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sauder LA, Ross AA, Neufeld JD. 2016. Nitric oxide scavengers differentially inhibit ammonia oxidation in ammonia-oxidizing archaea and bacteria. FEMS Microbiol Lett 363:fnw052. doi: 10.1093/femsle/fnw052 [DOI] [PubMed] [Google Scholar]
- 48. Espíndola KMM, Ferreira RG, Narvaez LEM, Silva Rosario ACR, da Silva AHM, Silva AGB, Vieira APO, Monteiro MC. 2019. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol 9:541. doi: 10.3389/fonc.2019.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Santos SAO, Freire CSR, Domingues MRM, Silvestre AJD, Pascoal Neto C. 2011. Characterization of phenolic components in polar extracts of eucalyptus globulus labill. bark by high-performance liquid chromatography–mass spectrometry. J Agric Food Chem 59:9386–9393. doi: 10.1021/jf201801q [DOI] [PubMed] [Google Scholar]
- 50. Jalal MAF, Read DJ, Haslam E. 1982. Phenolic composition and its seasonal variation in Calluna vulgaris. Phytochemistry 21:1397–1401. doi: 10.1016/0031-9422(82)80150-7 [DOI] [Google Scholar]
- 51. Zhen J, Villani TS, Guo Y, Qi Y, Chin K, Pan M-H, Ho CT, Simon JE, Wu Q. 2016. Phytochemistry, antioxidant capacity, total phenolic content, and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem 190:673–680. doi: 10.1016/j.foodchem.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 52. Tajik N, Tajik M, Mack I, Enck P. 2017. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr 56:2215–2244. doi: 10.1007/s00394-017-1379-1 [DOI] [PubMed] [Google Scholar]
- 53. Bai J, Wu Y, Liu X, Zhong K, Huang Y, Gao H. 2015. Antibacterial activity of shikimic acid from pine needles of Cedrus deodara against Staphylococcus aureus through damage to cell membrane. Int J Mol Sci 16:27145–27155. doi: 10.3390/ijms161126015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eykman JF. 1881. The botanical relations of Illicium religiosum Sieb., Illicium anisatum Lour. Am J Pharm 53 [Google Scholar]
- 55. Guo H-Y, Chen Z-A, Shen Q-K, Quan Z-S. 2021. Application of triazoles in the structural modification of natural products. J Enzyme Inhib Med Chem 36:1115–1144. doi: 10.1080/14756366.2021.1890066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. PubChem . 2023. National Center for Biotechnology Information. PubChem compound summary for CID 68680561, 1-butyl-4-propyltriazole. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/1-Butyl-4-propyltriazole. Retrieved 8 May 2023.
- 57. Chen M, Vial ML, Gee L, Davis RA, St John JA, Ekberg JAK. 2020. The plant natural product 2-methoxy-1,4-naphthoquinone stimulates therapeutic neural repair properties of olfactory ensheathing cells. Sci Rep 10:951. doi: 10.1038/s41598-020-57793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cotelle P, Vezin H. 2001. Reaction of caffeic acid derivatives with acidic nitrite. Tetrahedron Letters 42:3303–3305. doi: 10.1016/S0040-4039(01)00441-5 [DOI] [Google Scholar]
- 59. Napolitano A, d’Ischia M. 2002. New insights into the acid-promoted reaction of caffeic acid and its esters with nitrite: decarboxylation drives chain nitrosation pathways toward novel oxime derivatives and oxidation/fragmentation products thereof. J Org Chem 67:803–810. doi: 10.1021/jo015965v [DOI] [PubMed] [Google Scholar]
- 60. Rabelo TK, Zeidán-Chuliá F, Caregnato FF, Schnorr CE, Gasparotto J, Serafini MR, de Souza Araújo AA, Quintans-Junior LJ, Moreira JCF, Gelain DP. 2015. In vitro neuroprotective effect of shikimic acid against hydrogen peroxide-induced oxidative stress. J Mol Neurosci 56:956–965. doi: 10.1007/s12031-015-0559-9 [DOI] [PubMed] [Google Scholar]
- 61. Sueishi Y, Hori M, Kita M, Kotake Y. 2011. Nitric oxide (NO) scavenging capacity of natural antioxidants. Food Chem 129:866–870. doi: 10.1016/j.foodchem.2011.05.036 [DOI] [PubMed] [Google Scholar]
- 62. Martens-Habbena W, Qin W, Horak REA, Urakawa H, Schauer AJ, Moffett JW, Armbrust EV, Ingalls AE, Devol AH, Stahl DA. 2015. The production of nitric oxide by marine ammonia oxidizing archaea and inhibition of archaeal ammonia oxidation by a nitric oxide scavenger. Environ Microbiol 17:2261–2274. doi: 10.1111/1462-2920.12677 [DOI] [PubMed] [Google Scholar]
- 63. Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. 2016. Pathways and key intermediates required for obligate aerobic ammonia dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J 10:1836–1845. doi: 10.1038/ismej.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caranto JD, Lancaster KM. 2017. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc Natl Acad Sci U S A 114:8217–8222. doi: 10.1073/pnas.1704504114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rice EL, Pancholy SK. 1973. Inhibition of nitrification by climax ecosystems. II. additional evidence and possible role of Tannins. American J of Botany 60:691–702. doi: 10.1002/j.1537-2197.1973.tb05975.x [DOI] [Google Scholar]
- 66. Manzoni M, Rollini M. 2002. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol 58:555–564. doi: 10.1007/s00253-002-0932-9 [DOI] [PubMed] [Google Scholar]
- 67. Lam WL, Doolittle WF. 1992. Mevinolin-resistant mutations identify a promoter and the gene for a eukaryote-like 3-hydroxy-3-methylglutaryl-coenzyme a reductase in the Archaebacterium Haloferax uolcanii. J Biol Chem 267:5829–5834. doi: 10.1016/S0021-9258(18)42628-2 [DOI] [PubMed] [Google Scholar]
- 68. Miller TL, Wolin MJ. 2001. Inhibition of growth of methane-producing bacteria of the ruminant forestomach by hydroxymethylglutaryl~SCoA reductase inhibitors. J Dairy Sci 84:1445–1448. doi: 10.3168/jds.s0022-0302(01)70177-4 [DOI] [PubMed] [Google Scholar]
- 69. Jain S, Caforio A, Driessen AJM. 2014. Biosynthesis of archaeal membrane ether lipids. Front Microbiol 5:641. doi: 10.3389/fmicb.2014.00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McCarty GW, Bremner JM. 1989. Inhibition of nitrification in soil by heterocyclic nitrogen compounds. Biol Fert Soils 8:204–211. doi: 10.1007/BF00266480 [DOI] [Google Scholar]
- 71. Dixit D, Verma PK, Marwaha RK. 2021. “A review on ‘triazoles’: their chemistry, synthesis, and pharmacological potentials”. J Iran Chem Soc 18:2535–2565. doi: 10.1007/s13738-021-02231-x [DOI] [Google Scholar]
- 72. Taggert BI, Walker C, Chen D, Wille U. 2021. Substituted 1,2,3-triazoles: a new class of nitrification inhibitors. Sci Rep 11:14980. doi: 10.1038/s41598-021-94306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Taylor AE, Vajrala N, Giguere AT, Gitelman AI, Arp DJ, Myrold DD, Sayavedra-Soto L, Bottomley PJ. 2013. Use of aliphatic-alkynes to discriminate soil nitrification activities of ammonia-oxidizing Thaumarchaea and bacteria. Appl Environ Microbiol 79:6544–6551. doi: 10.1128/AEM.01928-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schleper C, Nicol GW. 2010. Ammonia-oxidising archaea--physiology, ecology and evolution. Adv Microb Physiol 57:1–41. doi: 10.1016/B978-0-12-381045-8.00001-1 [DOI] [PubMed] [Google Scholar]
- 75. Hodgskiss LH, Melcher M, Kerou M, Chen W, Ponce-Toledo RI, Savvides SN, Wienkoop S, Hartl M, Schleper C. 2023. Unexpected complexity of the ammonia monooxygenase in archaea. ISME J 17:588–599. doi: 10.1038/s41396-023-01367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wright CL, Schatteman A, Crombie AT, Murrell JC, Lehtovirta-Morley LE. 2020. Inhibition of ammonia monooxygenase from ammonia-oxidizing archaea by linear and aromatic alkynes. Appl Environ Microbiol 86:e02388-19. doi: 10.1128/AEM.02388-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schouten S, Hopmans EC, Sinninghe Damsté JS. 2013. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: a review. Organic Geochemistry 54:19–61. doi: 10.1016/j.orggeochem.2012.09.006 [DOI] [Google Scholar]
- 78. Elling FJ, Könneke M, Nicol GW, Stieglmeier M, Bayer B, Spieck E, de la Torre JR, Becker KW, Thomm M, Prosser JI, Herndl GJ, Schleper C, Hinrichs K-U. 2017. Chemotaxonomic Characterisation of the Thaumarchaeal lipidome. Environ Microbiol 19:2681–2700. doi: 10.1111/1462-2920.13759 [DOI] [PubMed] [Google Scholar]
- 79. Lehtovirta-Morley LE, Sayavedra-Soto LA, Gallois N, Schouten S, Stein LY, Prosser JI, Nicol GW. 2016. Identifying potential mechanisms enabling acidophily in the ammonia-oxidizing archaeon “Candidatus Nitrosotalea devanaterra". Appl Environ Microbiol 82:2608–2619. doi: 10.1128/AEM.04031-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gao B, Zhu X, Xu C, Yue Q, Li W, Wei J. 2008. Influence of extracellular polymeric substances on microbial activity and cell hydrophobicity in biofilms. J Chem Technol Biotechnol 83:227–232. doi: 10.1002/jctb.1792 [DOI] [Google Scholar]
- 81. Jung M-Y, Kim J-G, Sinninghe Damsté JS, Rijpstra WIC, Madsen EL, Kim S-J, Hong H, Si O-J, Kerou M, Schleper C, Rhee S-K. 2016. A hydrophobic ammonia-oxidizing archaeon of the Nitrosocosmicus clade isolated from coal tar-contaminated sediment.. Environ Microbiol Rep 8:983–992. doi: 10.1111/1758-2229.12477 [DOI] [PubMed] [Google Scholar]
- 82. Lehtovirta-Morley LE, Ross J, Hink L, Weber EB, Gubry-Rangin C, Thion C, Prosser JI, Nicol GW. 2016. “Isolation of “Candidatus Nitrosocosmicus franklandus,” a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration”. FEMS Microbiol Ecol 92:fiw057. doi: 10.1093/femsec/fiw057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sauder LA, Albertsen M, Engel K, Schwarz J, Nielsen PH, Wagner M, Neufeld JD. 2017. Cultivation and characterization of Candidatus Nitrosocosmicus exaquare, an ammonia-oxidizing archaeon from a municipal wastewater treatment system. ISME J 11:1142–1157. doi: 10.1038/ismej.2016.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alves RJE, Kerou M, Zappe A, Bittner R, Abby SS, Schmidt HA, Pfeifer K, Schleper C. 2019. Ammonia oxidation by the arctic terrestrial Thaumarchaeote Candidatus Nitrosocosmicus arcticus is stimulated by increasing temperatures.. Front Microbiol 10:1571. doi: 10.3389/fmicb.2019.01571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu L, Liu M, Jiang Y, Lin W, Luo J. 2019. Physiological and Genomic analysis of “ Candidatus Nitrosocosmicus agrestis”, an ammonia tolerant ammonia-oxidizing archaeon from vegetable soil. Microbiology. doi: 10.1101/2019.12.11.872556 [DOI]
- 86. Powell SJ, Prosser JI. 1991. Protection of Nitrosomonas europaea colonizing clay minerals from inhibition by nitrapyrin.. J Gen Microbiol 137:1923–1929. doi: 10.1099/00221287-137-8-1923 [DOI] [PubMed] [Google Scholar]
- 87. Zorz JK, Kozlowski JA, Stein LY, Strous M, Kleiner M. 2018. Comparative proteomics of three species of ammonia-oxidizing bacteria. Front Microbiol 9:938. doi: 10.3389/fmicb.2018.00938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ecevit K, Barros AA, Silva JM, Reis RL. 2022. Preventing microbial infections with natural phenolic compounds. Fut Pharmacology 2:460–498. doi: 10.3390/futurepharmacol2040030 [DOI] [Google Scholar]
- 89. Kozlowski JA, Kits KD, Stein LY. 2016. Comparison of nitrogen oxide metabolism among diverse ammonia-oxidizing bacteria. Front Microbiol 7:1090. doi: 10.3389/fmicb.2016.01090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Daims H, Lücker S, Wagner M. 2016. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol 24:699–712. doi: 10.1016/j.tim.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Raun WR, Johnson GV. 1999. Improving nitrogen use efficiency for cereal production. Agronomy J 91:357–363. doi: 10.2134/agronj1999.00021962009100030001x [DOI] [Google Scholar]
- 92. Iizumi T, Mizumoto M, Nakamura K. 1998. A bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. Appl Environ Microbiol 64:3656–3662. doi: 10.1128/AEM.64.10.3656-3662.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hyman MR, Murton IB, Arp DJ. 1988. Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes . Appl Environ Microbiol 54:3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rasche ME, Hyman MR, Arp DJ. 1991. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea: cometabolic inactivation of ammonia monooxygenase and substrate specificity . Appl Environ Microbiol 57:2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Keener WK, Arp DJ. 1994. Transformations of aromatic compounds by Nitrosomonas europaea. Appl Environ Microbiol 60:1914–1920. doi: 10.1128/aem.60.6.1914-1920.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chang SW, Hyman MR, Williamson KJ. 2003. Cooxidation of naphthalene and other polycyclic aromatic hydrocarbons by the nitrifying bacterium, Nitrosomonas europaea. Biodegradation 13:373–381. https://link.springer.com/article/10.1023/A:1022811430030. [DOI] [PubMed] [Google Scholar]
- 97. Zhou LJ, Han P, Yu Y, Wang B, Men Y, Wagner M, Wu QL. 2019. Cometabolic biotransformation and microbial-mediated abiotic transformation of sulfonamides by three ammonia oxidizers. Water Res 159:444–453. doi: 10.1016/j.watres.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 98. Li S, Peng L, Yang C, Song S, Xu Y. 2022. Cometabolic biodegradation of antibiotics by ammonia oxidizing microorganisms during wastewater treatment processes. J Environ Manage 305:114336. doi: 10.1016/j.jenvman.2021.114336 [DOI] [PubMed] [Google Scholar]
- 99. Lehtovirta-Morley LE, Ge C, Ross J, Yao H, Nicol GW, Prosser JI. 2014. Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds. FEMS Microbiol Ecol 89:542–552. doi: 10.1111/1574-6941.12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. De Boer W, Gunnewiek PJ, Veenhuis M, Bock E, Laanbroek HJ. 1991. Nitrification at low pΗ by aggregated chemolithotrophic bacteria. Appl Environ Microbiol 57:3600–3604. doi: 10.1128/aem.57.12.3600-3604.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Skinner FA, Walker N. 1961. Growth of Nitrosomonas europaea in batch and continuous culture. Archiv. Mikrobiol 38:339–349. doi: 10.1007/BF00408008 [DOI] [PubMed] [Google Scholar]
- 102. Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. 2011. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci U S A 108:15892–15897. doi: 10.1073/pnas.1107196108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shinn MB. 1941. Colorimetric method for determination of nitrite. Ind Eng Chem Anal Ed 13:33–35. doi: 10.1021/i560089a010 [DOI] [Google Scholar]
- 104. Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water column sand sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688. doi: 10.1073/pnas.0506625102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rousidou C, Papadopoulou ES, Kortsinidou M, Giannakou IO, Singh BK, Menkissoglu-Spiroudi U, Karpouzas DG. 2013. Bio-pesticides: harmful or harmless to ammonia oxidizing microorganisms? The case of a paecilomyces lilacinus based nematicide. Soil Biol Biochem 67:98–105. doi: 10.1016/j.soilbio.2013.08.014 [DOI] [Google Scholar]
- 106. Vanparys B, Spieck E, Heylen K, Wittebolle L, Geets J, Boon N, De Vos P. 2007. The Phylogeny of the genus Nitrobacter based on comparative Rep-PCR, 16S rRNA and nitrite oxidoreductase gene sequence analysis. Syst Appl Microbiol 30:297–308. doi: 10.1016/j.syapm.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 107. Ritz C, Streibig JC.. 2005. Bioassay analysis using R. J Stat Softw 12:32875. 10.18637/jss.v012.i05. [DOI] [Google Scholar]
- 108. Kolovou M, Panagiotou D, Süße L, Loiseleur O, Williams S, Karpouzas DG, Papadopoulou ES. 2023. Assessing the activity of different plant-derived molecules and potential biological nitrification inhibitors on a range of soil ammonia- and nitrite- oxidizing strains. Microbiology. doi: 10.1101/2023.07.12.548655 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Results and Material and Methods.
Data Availability Statement
The data that support the findings of this study are available in the supplemental material.







