Abstract
The worldwide rise in the rates of antibiotic resistance of bacteria underlines the need for alternative antibacterial agents. A promising approach to the killing of gram-positive antibiotic-resistant bacteria of the skin uses light in combination with a photosensitizer to induce a phototoxic reaction. Different concentrations (0 to 100 μM) of porphyrin-based photosensitizers (CTP1, XF70, and XF73) and different incubation times (5 min, 1 h, and 4 h) were used to determine phototoxicity against two methicillin-resistant Staphylococcus aureus strains, one methicillin-sensitive S. aureus strain, one methicillin-resistant Staphylococcus epidermidis strain, one Escherichia coli strain, and human keratinocytes and fibroblasts. Incubation with 0.005 μM XF70 or XF73, followed by illumination, yielded a 3-log10 (≥99.9%) decrease in the viable cell numbers of all staphylococcal strains, indicating that the XF drugs have high degrees of potency against gram-positive bacteria and also that the activities of these novel drugs are independent of the antibiotic resistance pattern of the staphylococci examined. CTP1 was less potent against the staphylococci under the same conditions. At 0.005 μM, XF70 and XF73 demonstrated no toxicity toward fibroblasts or keratinocytes. No inactivation of E. coli was detected at this concentration. XF73 was confirmed to act via a reactive oxygen species from the results of studies with sodium azide (a quencher of singlet oxygen), which reduced the killing of both eukaryotic and prokaryotic cells. When a quencher of superoxide anion and the hydroxyl radical was used, cell killing was not inhibited. These results demonstrate that the porphyrin-based photosensitizers had concentration-dependent differences in their efficacies of killing of methicillin-resistant staphylococcal strains via reactive oxygen species without harming eukaryotic cells at the same concentrations.
Oscar Raab published the first paper (52) on the photodynamic effects on the activities of chemical compounds against microorganisms more than 100 years ago. He observed that the toxicity of acridine hydrochloride against Paramecium caudatum was dependent on the amount of light incident on the experimental mixture. H. von Tappeiner later reported (65) that these toxic effects in the presence of light were not due to heat and, after further experiments in 1904 to exclude the direct influence of light, coined the term “photodynamic reaction” (67). Additional experiments demonstrated the contribution of oxygen in the killing of the bacteria, as in the absence of oxygen, the fluorescent dyes did not exert any antibacterial activity against the facultative anaerobic species Proteus vulgaris (65, 66).
Today, the worldwide rise in the rates of antibiotic resistance forces researchers to develop new antibacterial strategies. In 1996, the first clinical isolate of a methicillin-resistant Staphylococcus aureus (MRSA) with reduced susceptibility to vancomycin (MIC = 8 μg/ml; vancomycin-intermediate resistance type) was reported from Japan (29). Clinical infections caused by vancomycin-intermediate S. aureus isolates were also confirmed in the United States and France (26, 50, 62). The first documented case of an infection caused by vancomycin-resistant S. aureus (MIC ≥ 32 μg/ml) was reported in July 2002 (61). Recent reports have shown that the annual rate of resistance to methicillin increased from 13% in 1986 to 28% in 2000 (P < 0.001) and is still increasing (58).
Infections with MRSA can be difficult to treat, and infected patients may be colonized for many months and can require long hospital stays (15). Accordingly, the treatments range from local disinfectants to systemic antibiotics (45, 60, 64). In addition, the emergence of mupirocin (Bactroban) resistance in MRSA emphasizes the importance and urgency of developing new topical treatment alternatives to the standard antibiotic treatments for skin infections (18).
A photodynamic approach to the killing of bacteria on the skin uses light in combination with a photosensitizer (in our case, XF compounds) to induce a phototoxic reaction, identical to the use of photodynamic therapy (PDT) for skin cancer (24, 30, 63). The initial step is the absorption of light by a photosensitizer (5). In the presence of oxygen, the triplet state of the excited photosensitizer acts as the reactive intermediate and can follow two competitive mechanisms, either a type I or a type II reaction (25). In a typical type I reaction, electron transfer directly from the excited photosensitizer to a substrate occurs by the generation of different kinds of radical species; the latter are then intercepted by oxygen-yielding oxidized products, such as hydrogen peroxide, superoxide radical anion, or hydroxyl radical. In a type II reaction the excited photosensitizer reacts directly with molecular oxygen. Therefore, the excited photosensitizer may then react with normal triplet oxygen to produce singlet oxygen (1O2) when the photosensitizer returns to its ground state, the singlet state (31, 33). This highly reactive singlet oxygen initiates further oxidative reactions in a closed environment, like the bacterial cell wall, lipid membranes, enzymes, or nucleic acids (6, 28).
Different classes of chemical compounds with photoactive properties have been tested, with various results, against gram-positive and gram-negative bacteria (37, 68, 70). Nitzan and colleagues (47) have demonstrated that uncharged porphyrins and light illumination yield photodynamic activity against S. aureus and Escherichia coli in the presence of the membrane-disorganizing peptide polymyxin B. Anionic porphyrins showed no activity against either gram-positive or gram-negative bacteria, whereas positively charged photosensitizers, including phenothiazines (methylene blue and toluidine blue), phthalocyanines, and porphyrins, have so far been successfully tested as photoinactivating agents with activities against gram-positive and gram-negative bacteria (40-42). Photoinactivation of gram-positive and gram-negative bacteria is based on the concept that certain photosensitizers can accumulate in significant amounts in or at the cytoplasmic membrane, the critical target for the induction of irreversible damage in bacteria after illumination (40). Recently, Reddi et al. (54), in a study of meso-substituted cationic porphyrins, demonstrated that phototoxic activity was mainly mediated by the impairment of the enzymatic and transport functions of both the outer and the cytoplasmic membranes of gram-negative bacteria.
Therapy of skin wounds and infections with antibiotics has become unpopular because of the development of resistance (32). Colsky and colleagues (16, 17) compared the antibiotic resistance profiles of isolates from patients with skin wounds and revealed a marked increase in the rate of oxacillin resistance among S. aureus isolates.
Therefore, the challenge in the photodynamic inactivation of bacteria is to define the appropriate photosensitizers that have activities against bacteria but that do not harm the surrounding tissue in vivo. The aim of this study was to find concentration-dependent differences in the killing efficacies of new porphyrin-based photosensitizers in vitro so that primary human keratinocytes and human dermal fibroblasts are not harmed but multiresistant staphylococcal strains are efficiently killed.
MATERIALS AND METHODS
Bacterial strains.
Biochemical analysis and resistance testing of each bacterial strain were done with a VITEK 2 system (bioMérieux, Nürtingen, Germany), according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS, Wayne, Pa.). S. aureus and Staphylococcus epidermidis, prevalent members of the skin flora of serious concern in hospital-acquired infections, were the principal bacteria included in this study (69). The bacterial strains used in the study were methicillin-sensitive S. aureus (MSSA; ATCC 25923), MRSA (ATCC BAA-44 and ATCC 43300), methicillin-resistant S. epidermidis (MRSE; ATCC 700565), and E. coli (ATCC 25922), which were grown aerobically at 37°C in brain heart infusion broth (Gibco Life Technologies GmbH, Eggenstein, Germany). A 500-μl portion of an overnight cell culture (3 ml) was transferred to 50 ml of fresh brain heart infusion medium and grown at 37°C on an orbital shaker. When the cultures reached the stationary phase of growth, the cells were harvested by centrifugation (200 × g, 15 min), washed with 10 mM phosphate-buffered saline (PBS; Biochrom, Berlin, Germany) at pH 7.4 containing 2.7 mM KCl and 0.14 M NaCl, and suspended in PBS at an optical density of 0.7 at 650 nm, which corresponded to 108 to 109 cells/ml, for use in the phototoxicity experiments.
Cell cultures.
Primary normal human dermal keratinocytes (NHEKs) and primary normal human dermal fibroblasts (NHDFs) were purchased from CellSystems Biotechnologie (St. Katharinen, Germany). NHEKs and NHDFs were propagated in a KGM Bullet kit and a FGM-2 Bullet kit (Clonetics BioWhittaker, Verviers, Belgium), respectively. The media were supplemented with 10% fetal calf serum (Sigma Chemie, Deisenhofen, Germany), 1% l-glutamine, and 1% penicillin-streptomycin (Gibco, Eggenstein, Germany) in a humidified atmosphere containing 5% carbon dioxide at 37°C. The cells were washed with PBS and harvested by using treatment with 0.05% tryphotosensitizer and 0.02% EDTA (Gibco) in PBS for 10 min. The cells were reseeded at 105 cells/ml in 75-cm3 tissue culture flasks and were used between passages 2 and 10.
Photosensitizer and light source.
Three porphyrin-based photosensitizers were synthesized by Xiangdong Feng (Solvias Company, Basel, Switzerland) and were kindly provided by Destiny Pharma Ltd. (Brighton, United Kingdom). Two of the porphyrin-based photosensitizers were novel cationic diporphyrin-based compounds (compounds XF70 and XF73), while the third compound (compound CTP1) was a cationic triporphyrin compound. The chemical structures of the three porphyrin-based photosensitizers used in the present investigation are shown in Fig. 1. All three photosensitizers were readily soluble in PBS at pH 7.4. In terms of hydrophilic and lipophilic characteristics, the porphyrin-based photosensitizers followed an order of increasing hydrophilicity of XF73 < XF70 < CTP1. XF70 and XF73 differ only in the meta or para position of the aminoalkyl chain. Samples were dissolved in bidistilled water at a concentration of 2 mM, passed through a 0.22-μm-pore-size filter, and stored at 4°C until use. The absorption spectrum of each probe was recorded at room temperature with a DU640 spectrophotometer (Beckman Instruments GmbH, Munich, Germany). Fluorescence emission spectra were measured at room temperature with a Bio-Tek Kontron Instruments (Zurich, Switzerland) spectrophotometer. The cells were illuminated with an incoherent light source (UV236; emission λ, 380 to 480 nm) provided by Waldmann Medizintechnik (Villingen-Schwenningen, Germany). The maximal fluence rate at the level of the illuminated samples was 15.2 mW/cm2. The samples were illuminated for 15 min (13.7 J/cm2).
FIG. 1.
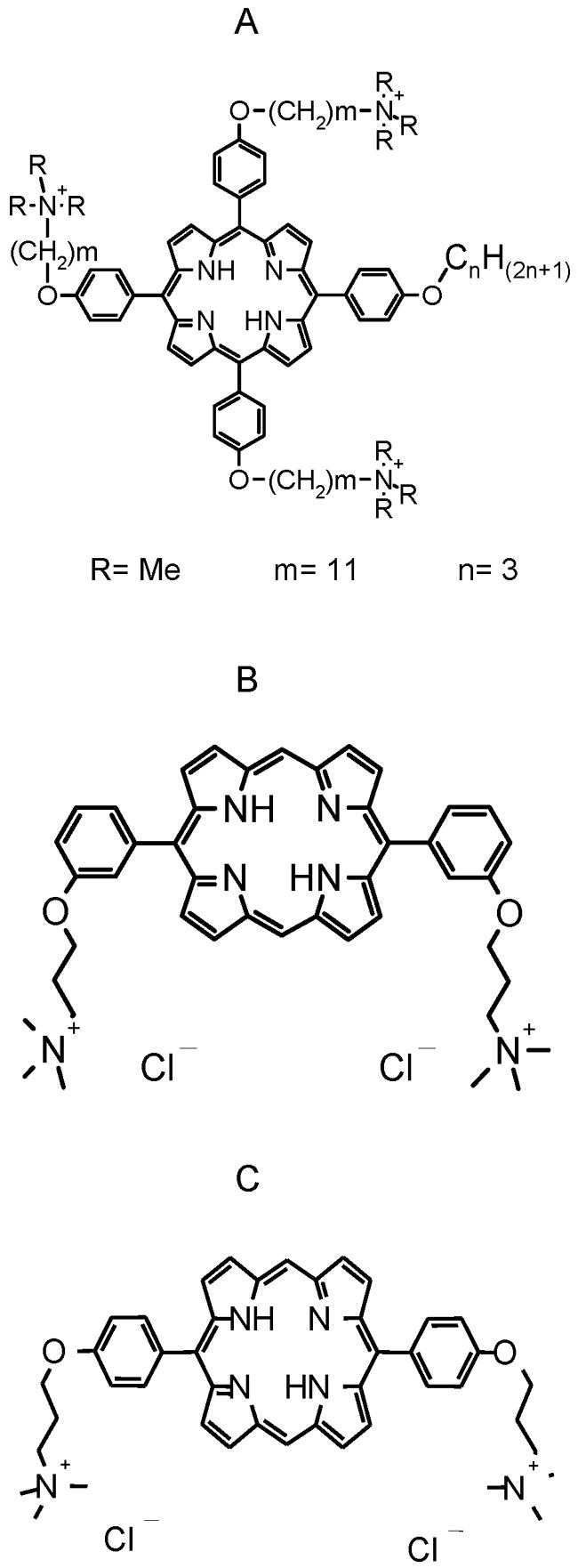
Chemical structures of CPT1 (A; molecular weight, 1,134.62), XF70 (B; molecular weight, 694.93), and XF73 (C; molecular weight, 694.93). Me, methyl.
Phototoxicity assay of the bacteria.
A total of 108 to 109 bacterial cells ml−1 were incubated with different concentrations of a porphyrin-based photosensitizer (0 to 10 μM) for 5 min in the dark. At the end of the incubation period, the cells were washed three times with PBS, suspended in PBS, transferred into a 96-well microtiter plate (200 μl/well), and illuminated for 15 min (15.2 mW/cm2; 13.7 J/cm2). The cells were illuminated from the bottom of the plate, which was lying on the light source. Control wells were neither sensitized with a photosensitizer nor exposed to the light source or were incubated with a photosensitizer only. After illumination the survival of the bacteria was determined by counting the numbers of CFU. Serially diluted aliquots of treated and untreated (no photosensitizer, no light) cells were plated on brain heart infusion agar, and the number of CFU milliliter−1 was counted after 18 to 24 h of incubation at 37°C.
Phototoxicity assay with eukaryotic cells.
For each experiment, NHEKs and NHDFs were grown to at least 70% confluence, harvested, and suspended in medium to a concentration of 104 cells/well (96-well microtiter plate). Three different incubation times (5 min, 1 h, and 4 h) and concentrations up to 100 μM were used to determine the phototoxicities of the compounds. After illumination, cell survival was determined 24 h later by a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (44). MTT is used as an indicator of metabolically active cells, in which a color reaction dependent on enzyme activity takes place in mitochondria, and this activity can be measured with an enzyme-linked immunosorbent assay reader (540 nm). The cell viability was normalized to 1; that is, the optical densities of the cells after illumination but without incubation with the photosensitizer were equal to 1. Every experiment was performed in triplicate.
Mechanism of cell killing by photooxidation.
Sodium azide and mannitol, which are effective physical quenchers of reactive oxygen species (ROS), were added to the NHDF culture 60 min prior to illumination (3, 43, 73). NHDFs were incubated with different concentrations (0, 0.001, 0.01, 0.1, and 1.0 μM) of XF73 (5 min) and illuminated as described above, but in the presence of sodium azide or mannitol (Merck, Darmstadt, Germany) at a concentration of 50 mM in PBS. Photooxidative effects are enhanced by using D2O in vitro (34). In a separate experiment, D2O was added to the cultures 60 min prior to illumination. NHDFs were incubated with XF73 as described above in the presence of D2O. Cell viability was assessed by the MTT assay, as described above. MRSA (ATCC BAA-44) was incubated with different concentrations of XF73 (0, 0.001, 0.005, and 0.1 μM) for 5 min. After three washing steps with PBS, bacterial cells incubated with XF73 were illuminated in the presence of sodium azide at a concentration of 5 mM. The numbers of CFU were determined as described above.
Subcellular localization.
NHDFs (106) were grown on microscope slides (Menzel-Gläser, Braunschweig, Germany) overnight. Next, the cells were incubated with 1 μM CTP1, XF70, or XF73 for 5 min, 1 h, or 4 h alone or incubated cells were costained with organelle-specific dyes. For labeling of the lysosomes and the mitochondria, 8 μM LysoTrackerGreen DND-26 (2 h; Molecular Probes, Eugene, Oreg.) and 50 ng of rhodamine 6G (5 min; Sigma-Aldrich, Taufkirchen, Germany) per ml, respectively, were used (14, 21, 49). Nuclear staining was performed with 10 μM Hoechst 33342 (Molecular Probes) for 10 min (4). Following incubation with the organelle-specific dye Hoechst 33342 and CTP1, XF70, or XF73, the slides were washed twice with medium without phenol red. Subcellular localization was examined by fluorescence microscopy (Zeiss Vario-AxioTech, Goettingen, Germany) with an appropriate dual-band filter set for excitation and emission (Omega Optical, Brattleboro, Vt.).
Data analysis and statistics.
Each experiment was performed at least in triplicate. All primary data are presented as means with standard deviations of the mean. Differences were tested for statistical significance by Student's t test. Probability values less than 5% were considered significant.
RESULTS
Phototoxicity against methicillin-sensitive staphylococci.
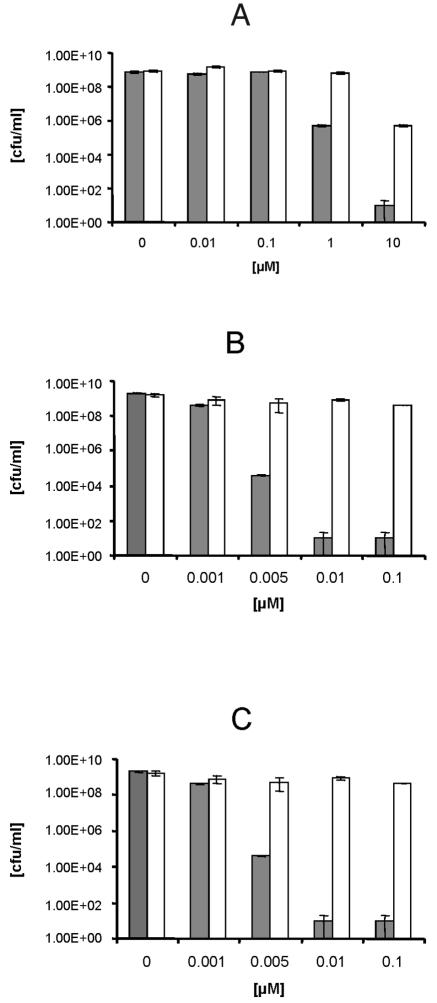
Three different S. aureus strains, one MRSE strain, and one E. coli strain were used to determine the antibacterial toxicities of CTP1, XF70, and XF73. Illumination of MSSA following incubation with different concentrations (0 to 0.1 μM) of CTP1, XF70, and XF73 caused a significant decrease (P < 0.005) in viability, as determined by the numbers of CFU per milliliter (Fig. 2). CTP1 at a concentration of 1.0 μM exhibited significant antibacterial activity after illumination for 5 min (killing efficacy, 99.93%), and the antibacterial activity was further increased with increasing concentrations (Fig. 2A). However, a concentration of 10 μM caused a marked decrease in the numbers of CFU per milliliter without illumination (Fig. 2A). In contrast, XF70 or XF73 at concentrations of only 0.005 μM killed 99.9% of the MSSA isolates when the compounds were illuminated (Fig. 2B and C). At concentrations of 0.001 μM, the photosensitizers did not yield an effect upon illumination. Incubation with higher concentrations (up to 0.1 μM) of XF70 and XF73 showed a decrease in cell survival of 5 log10 after illumination. Similar to CTP1, higher concentrations (5 to 10 μM) of XF70 and XF73 led to a reduction of >6 log10 CFU/ml, even without light exposure (data not shown). All bacterial samples that were incubated without photosensitizers exhibited normal growth with or without illumination, demonstrating that the maximal fluence rate (15.2 mW/cm2) at the level of the illuminated samples alone had no antibacterial effects. Longer times of incubation (up to 4 h) with CTP1, XF70, or XF73 did not show a significant increase in the rate of killing of these bacterial cells (data not shown).
FIG. 2.
Efficacies of killing of MSSA (ATCC 25923) by CPT1 (A), XF70 (B), and XF73 (C) after 5 min of incubation with (gray bars) and without (white bars) illumination (15.2 mW/cm2; 13.7 J/cm2). Survival of MSSA was determined by the CFU assay. Each bar is the mean ± standard deviation of three experiments (P < 0.005).
Phototoxicity against methicillin-resistant staphylococci.
In order to investigate whether the observed growth reduction of MSSA was independent of the antibiotic resistance pattern, two MRSA strains and one MRSE strain were photosensitized under conditions identical to those used for the MSSA strain. In repeated experiments, both MRSA strains showed similar decreases in the numbers of CFU per milliliter as the MSSA cells after incubation with CTP1, XF70, or XF73 and illumination (Table 1). CTP1 at a concentration of 0.005 μM did not show a significant killing effect against either MRSA strain (Table 1). Illumination of the two MRSA strains and the MRSE strain in the presence of 0.005 μM XF70 or XF73 resulted in a substantial decrease in the numbers of CFU per milliliter in all cases (Table 1). The mean reductions in the numbers of CFU per milliliter were 3 log10 with XF70 and 3.2 log10 with XF73 upon illumination of MRSA. When MRSE was treated with 0.005 μM CTP1, XF70, or XF73 and illuminated, CTP1 showed no killing efficacy (<0.8 log10 CFU/ml), whereas at the same concentration XF70 achieved a 3.0-log10 reduction upon illumination and XF73 achieved a 3.1-log10 reduction upon illumination (Table 1). As shown in Table 1, higher concentrations of all the photodynamic compounds (up to 10 μM) caused a reduction in the growth of the bacteria compared to the growth of the controls without illumination.
TABLE 1.
Killing efficacies of CPT1, XF70, and XF73 for different methicillin-resistant bacteria after illumination
| Organism | Log10 reduction ofa:
|
||||||
|---|---|---|---|---|---|---|---|
| Untreated controlb | 0.005 μM
|
10 μM
|
|||||
| CPT1 | XF70 | XF73 | CPT1 | XF70 | XF73 | ||
| MSSA (ATCC 25923) | <0.5 | <0.3 | 3.1 | 3.2 | 4.1 | >5.6 | >5.8 |
| MRSA (ATCC BAA44) | <0.5 | <0.3 | 3.1 | 3.3 | 3.9 | >5.7 | >5.5 |
| MRSA (ATCC 43300) | <0.5 | <0.25 | 2.9 | 3.1 | 4.0 | >5.55 | >5.4 |
| MRSE (ATCC 700565) | <0.5 | <0.8 | 3.0 | 3.1 | 4.1 | >5.2 | >5.25 |
| E. coli (ATCC 25922) | <0.3 | <1 | <1 | <1 | 3.5 | 4.5 | 4.5 |
A total of 108 bacteria were incubated with the photosensitizer and illuminated. The log10 reductions of the viable bacterial cell numbers were determined by the CFU assay. Control experiments without illumination have shown that these concentrations have no antibacterial activity under the same conditions (data not shown). The results are the mean values of at least three independent experiments (P < 0.005).
Incubation without photosensiters but illuminated.
Phototoxicity against E. coli.
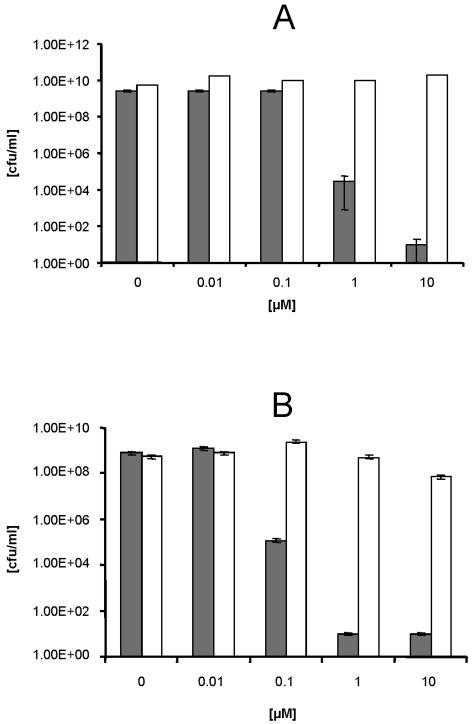
Illumination of the gram-negative bacterium E. coli after incubation with photosensitizers at concentrations identical to those in the experiments mentioned above revealed a smaller decrease in the log10 numbers of CFU per milliliter compared with the decreases in the numbers of staphylococcal strains. Upon illumination, incubation of E. coli with CTP1 at a concentration up to 10 μM showed no significant decrease in the numbers of CFU per milliliter (data not shown). Illumination of E. coli in the presence of higher concentrations of XF70 (1.0 to 10 μM) or XF73 (0.1 μM) resulted in decreases in the numbers of CFU per milliliter of 5.6 and 4.2 log10, respectively (Fig. 3A and B). In summary, XF70 and XF73 killed gram-positive bacterial strains (MRSA, MRSE, and MSSA) and the gram-negative bacterium E. coli more efficiently than CTP1 did.
FIG. 3.
Effect of XF70 or XF73 after illumination on the survival of E. coli. The antibacterial efficacies of XF70 (A) and XF73 (B) against E. coli after 5 min of incubation and illumination are shown. Gray bars, E. coli cells after 5 min of incubation with different concentrations of XF70 or XF73; open bars, E. coli cells after 5 min of incubation with different concentrations of XF70 or XF73 without illumination (15.2 mW/cm2; 13.7 J/cm2). The survival of the bacterial cells was determined by the CFU assay. Each bar is the mean ± standard deviation of three experiments.
Phototoxicity for eukaryotic cells.
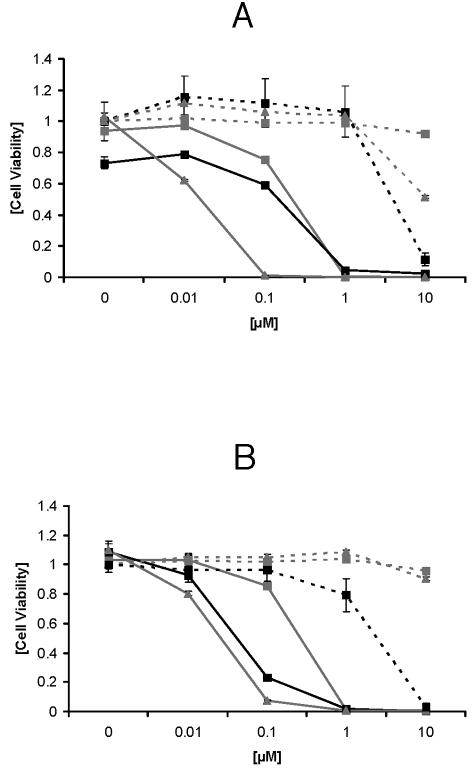
The toxicities of different concentrations of CTP1, XF70, or XF73 for human skin cells were tested by using NHEKs and NHDFs after illumination. As shown in Fig. 4A, incubation of NHEKs with CTP1, XF70, or XF73 yielded reduced cell viability upon illumination. The corresponding 50% effective concentrations (EC50s) after 5 min of incubation and illumination were 0.25 μM for CTP1, 0.4 μM for XF70, and 0.028 μM for XF73. Incubation of NHEKs with CTP1, XF70 or XF73 (at concentrations up to at least 1 μM) without illumination did not influence cell viability (Fig. 4A). However, NHEKs incubated with 10 μM CTP1, XF70, or XF73 without illumination did show decreases in viability. Figure 4B shows the viabilities of NHDFs following incubation with CTP1, XF70, or XF73 and illumination. Concentrations up to 1 μM resulted in a significant decrease in cell viability upon illumination (Fig. 4B). The EC50s were 0.065 μM for CTP1, 0.47 μM for XF70, and 0.047 μM for XF73. Again, NHDFs incubated with different concentrations of CTP1, XF70, or XF73 at concentrations up to 1 μM without illumination did not show reduced viability (Fig. 4B).
FIG. 4.
Toxicities of CPT1, XF70, and XF73 for NHEKs and NHDFs after 5 min of incubation. NHEKs (A) and NHDFs (B) were incubated with different concentrations (0, 0.01, 0.1, 1.0, or 10 μM) of CPT1, XF70, and XF73 for 5 min. After illumination (15.2 mW/cm2; 13.7 J/cm2), the cells were incubated for 24 h in the dark. Phototoxicity was tested by the standard MTT assay. Cell viability was normalized to 1; that is, the values for control cells without illumination were 1. Black line, CPT1 with illumination; gray line and ▪, XF70 with illumination; gray line and ▴, XF73 with illumination; black dotted line, CPT1 without illumination; gray dotted line and ▪, XF70 without illumination; gray dotted line and ▴, XF73 without illumination. Values are the means and standard deviations of three experiments (P < 0.001).
Importantly, the concentrations of XF70 and XF73 used in the bacterial and cell toxicity experiments clearly showed concentration-dependent differences upon illumination when the two MRSA strains as well as the MRSE strain were efficiently killed (Table 1 and Fig. 4A and B). XF70 or XF73A at a concentration of 0.005 μM reduced the numbers of CFU per milliliter after illumination, whereas at this concentration NHDFs and NHEKs were still viable with and without illumination (Fig. 4A and B). In contrast, concentrations of XF73 more than 20 times higher (0.005 and 0.1 μM, respectively) were necessary to kill 99.9% of the E. coli cells (Fig. 3B and Table 1). At this concentration the viabilities of both cell types were reduced. Following longer periods of incubation (1 or 4 h) of NHEKs and NHDFs with CTP1, XF70, or XF73, cell viability was markedly decreased compared to that after 5 min of incubation (Table 2).
TABLE 2.
Toxicities of CPT1, XF70, and XF73 against fibroblasts and keratinocytes
| Compound | EC50 (μM) at the following incubation timea:
|
|||
|---|---|---|---|---|
| 1 h
|
4 h
|
|||
| Fibroblasts | Keratinocytes | Fibroblasts | Keratinocytes | |
| CPT1 | 0.1 ± 0.009 | 0.1 ± 0.002 | 0.01 ± 0.03 | 0.09 ± 0.03 |
| XF70 | 0.01 ± 0.008 | 0.09 ± 0.03 | 0.08 ± 0.02 | 0.017 ± 0.02 |
| XF73 | 0.009 ± 0.04 | 0.009 ± 0.01 | 0.009 ± 0.01 | 0.009 ± 0.009 |
Fibroblasts and keratinocytes were incubated with different concentrations (0 to 10 μM) of each photosensitizer and incubated for 1 or 4 h prior to illumination. The values are the averages of three independent experiments ± standard deviations.
Mechanism of action.
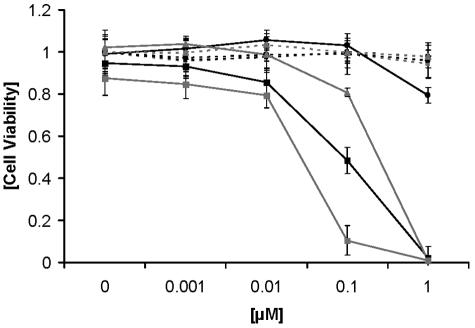
Mannitol, a quencher of superoxide anion and hydroxyl radical (type I reaction), or sodium azide, a quencher of singlet oxygen (type II reaction), were used to investigate if type I or type II photooxidation is the predominant mechanism of action. NHDFs were incubated with different concentrations of XF73 in the presence of 50 mM mannitol. After illumination, cell viability increased slightly from 0.48 ± 0.063 to 0.8 ± 0.041 (Fig. 5). Incubation of NHDFs with XF73 and 50 mM sodium azide increased the cell viability from 0.48 ± 0.063 to 1.0 ± 0.058. Incubation of NHDF with XF73 in the presence of sodium azide or mannitol without illumination did not show any effect on cell viability. The photooxidative effects of the type II reaction are enhanced in the presence of D2O. The addition of D2O yielded a dramatic decrease in cell viability to 0.1 ± 0.03 with XF73 at a concentration of 0.1 μM (Fig. 5).
FIG. 5.
Effects of mannitol (50 mM), sodium azide (50 mM), and D2O on NHDFs incubated with XF73 with and without illumination (15.2 mW/cm2; 13.7 J/cm2). Black squares, XF73 alone with illumination; black square and dotted line, XF73 alone without illumination; gray square, XF73 in combination with D2O and illumination; gray square and dotted line, XF73 in combination with D2O without illumination; gray triangle, XF73 in combination with mannitol; gray triangle and dotted line, XF73 in combination with mannitol without illumination; black circle, XF73 in combination with sodium azide and illumination; black circle and dotted line, XF73 in combination with sodium azide without illumination. Each point is the mean of three determinations ± standard deviation.
When MRSA (ATCC BAA-44) was incubated with 0.005 μM XF73 alone or in the presence of 5 mM sodium azide, the survival of MRSA cells increased from 0.015% ± 0.01% to 8.6% ± 0.2% compared to that with incubation with XF73 alone after illumination (Table 3). By adding D2O to the bacteria incubated with XF73, the survival of the MRSA strain decreased from 0.00001% ± 0.000024% to 0.000001% ± 0.00001%. In contrast, the addition of 5 mM sodium azide to bacterial cells incubated with XF73 without light illumination did not significantly change the rate of survival, 95.3% ± 0.3% to 98.7% ± 0.28% (Table 3), thus demonstrating that the cytotoxic effects against NHDFs or MRSA following incubation with XF73 after illumination are due to the generation of ROS, in particular, singlet oxygen of the type II reaction.
TABLE 3.
Survival of XF73-incubated MRSA cells after 5 min of incubation with 5 mM sodium azide with and without illuminationa
| Condition | % Survival of MRSA cells with XF73 atb:
|
|||
|---|---|---|---|---|
| 0 μM | 0.001 μM | 0.005 μM | 0.1 μM | |
| XF73 alone + light | 100 ± 0.15 | 99.9 ± 0.24 | 0.015 ± 0.01 | 0.00001 ± 0.000024 |
| XF73 + sodium azide + light | 100 ± 0.15 | 99.98 ± 0.18 | 8.6 ± 0.2 | 0.0001 ± 0.00015 |
| XF73 + sodium azide without light | 100 ± 0.21 | 99.98 ± 0.21 | 95.3 ± 0.3c | 78 ± 0.5 |
| XF73 alone without light | 100 ± 0.13 | 99.98 ± 0.20 | 98.7 ± 0.28c | 48 ± 0.9 |
Illumination was at 15.2 mW/cm2 for 15 min.
The results are the means of at least three independent experiments ± standard deviations.
P < 0.1.
Subcellular localization.
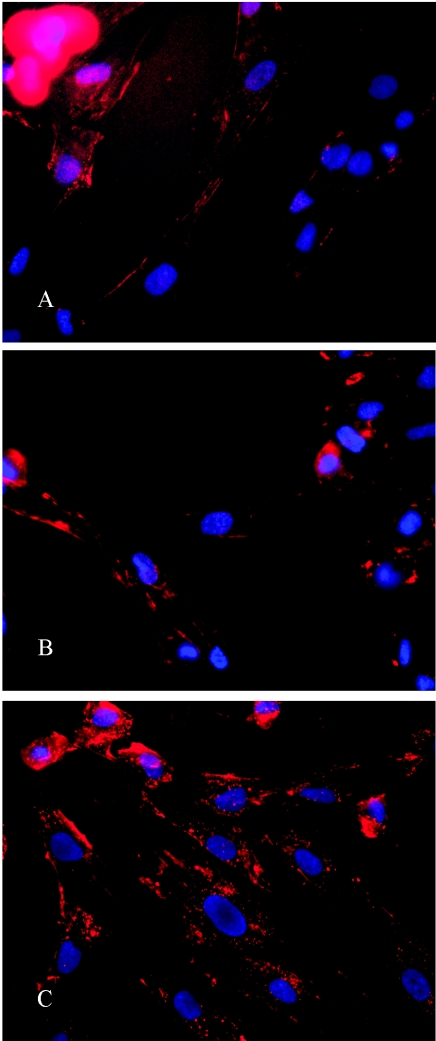
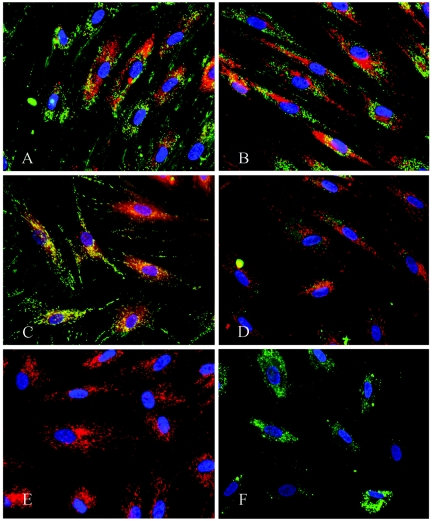
The subcellular localization of CTP1, XF70, and XF73 was examined by fluorescence microscopy. Figure 6 shows NHDFs incubated with XF73 at different time points. Control images showed no autofluorescence in these cells (data not shown). After 5 min of incubation with XF73, fluorescence was detected only around the cytoplasmic membrane (Fig. 6A). Longer incubation times led to further intracellular accumulation of XF73 (Fig. 6B and C). Similar images were recorded at these times when the cells were incubated with CTP1 and XF70 (data not shown). Next, colocalization of CTP1 was examined after 4 h of incubation of NHDFs (Fig. 7 A). Spotty perinuclear fluorescence signals could be detected throughout the cytoplasm. Costaining with a lysosome-specific dye revealed a colocalization (yellow) of CTP1 fluorescence and lysosomal fluorescence (Fig. 7A). No costaining with a mitochondrion-specific dye could be detected (Fig. 7B). Figure 7C shows the subcellular distribution of XF73 in NHDFs after 4 h of incubation with 1 μM XF73. The XF73 fluorescence (red) is localized outside the nucleus (blue), and there is a colocalization (yellow) with the mitochondrion-specific dye rhodamine 6G (green) (Fig. 7C). Colocalization with the lysosome-specific dye was not detected (Fig. 7D). No difference in the subcellular localization of XF70 compared to that of XF73 was detected (data not shown). Control staining of mitochondria and lysosomes without costaining is shown in Fig. 7E and F, respectively. Spotty fluorescence signals in the stained mitochondria were evenly distributed throughout the cytoplasm (Fig. 7E), whereas staining of lysosomes showed a different pattern (Fig. 7F).
FIG. 6.
Localization of XF73 at different time points. NHDFs were incubated with 1 μM XF73 (red), and nuclei were stained with 10 μM Hoechst 33342 dye (blue). (A) Five minutes of incubation with XF73; (B) 1 h of incubation with XF73; (C) 4 h of incubation with XF73.
FIG. 7.
Subcellular localization of CPT1 and XF73. NHDFs were incubated with 1 μM CPT1 or XF73 for 4 h. (A) CPT1 (red) with costaining of lysosomes (green); (B) CPT1 (green) with costaining of mitochondria (red); (C) XF73 (red) with costaining of mitochondria (green); (D) XF73 (red) with costaining of lysosomes (green); (E) staining of mitochondria alone using rhodamine 6G (red); (F) staining of lysosomes alone using LysoTrackerGreen (green). Nuclei are stained with Hoechst 33342 dye (blue).
DISCUSSION
The challenge in antimicrobial PDT is to find a therapeutic window in which bacteria are killed without harming the surrounding tissue. Porphyrin-based photosensitizers exhibit significant photosensitizing activities against both eukaryotic and prokaryotic cells only in the presence of light and oxygen (36, 56). The results of the present study show that the photosensitization with three porphyrin-based photosensitizers, namely, CTP1, XF70, and XF73, was concentration dependent after illumination. An extremely low concentration, 0.005 μM, of XF70 and XF73 was effective in killing a range of gram-positive staphylococcal strains, two MRSA strains (ATCC BAA-44 and ATCC 43300), one MRSE strain (ATCC 700565), and one MSSA strain (ATCC 25923), against which it achieved 3-log10 reductions. CTP1 was less efficient in this respect. At the same concentration (0.005 μM) and with the same incubation time, after illumination XF70 and XF73 had little effect on skin cells, with ≥90% of NHEKs and NHDFs still viable in vitro. In contrast, after illumination a concentration of XF73 more than 20 times higher was necessary to efficiently kill the gram-negative bacterium E. coli by the same amount. Therefore, potent antibacterial properties have been demonstrated with nanomolar concentrations of the photosensitizers upon illumination without any toxicity against eukaryotic cells.
All three porphyrin-based photosensitizers killed MRSA and MRSE to the same extent as MSSA after the photosensitizers were illuminated, indicating that the mechanisms of methicillin resistance did not affect photosensitizer uptake or efficacy. This is important, because gram-positive bacteria may become resistant to antibiotics or biocides due to limited penetration, as shown for vancomycin-intermediate-resistant S. aureus strains, which produce a markedly thicker peptidoglycan layer (12, 39). Thus, cell wall changes, e.g., an increased cell wall thickness or different patterns of cross-linking of the peptidoglycan layer, did not lead to a reduced efficacy of the photosensitizer used. However, whether bacteria could develop resistance to ROS, e.g., singlet oxygen, once the photosensitizer is in the cell is questionable. Until now, no report has shown a potential specific mechanism of resistance to ROS. Recent studies with the XF porphyrin derivatives have shown that MRSA developed no resistance to these dyes after PDT treatment, even after 10 exposures to XF treatment (E. Reddi et al., unpublished data). This finding is important, because in the clinic the treatment of chronic and/or recurrent infections by repeated antimicrobial PDT is very likely. Additionally, clinical results with topical PDT (up to 30 mW/cm2) have shown improvements of inflammatory dermatoses without adverse effects, even after multiple treatments (1). In principle, the entire body can be treated with an appropriate incoherent light source (white light), like the UV light cabinet used for clinical dermatological use.
In this investigation, we demonstrated clear concentration-dependent differences in vitro, as 0.005 μM XF70 or XF73 efficiently killed all MRSA and MRSE strains used without causing significant damage to NHDFs and NHEKs at that concentration. Embleton et al. (23) also reported a lethal photosensitization of methicillin-resistant S. aureus using an immunoglobulin G-tin(IV)chlorin e6 conjugate as a photosensitizer. Protein A, an S. aureus cell wall protein which is expressed and localized on the cell surface by different MRSA strains, binds to many isotypes of immunoglobulin G through the Fc region of the immunoglobulin (55). Therefore, the efficacy of killing was dependent in part on the MRSA strain used due to differences in the composition of the cell wall, because the amount of protein A can vary among different MRSA strains (55). In our experiments, unconjugated XF70 and XF73 showed toxicities upon illumination for two MRSA strains as well as one MRSE strain. Therefore, the phototoxicities of these dyes are independent of the composition of the cell wall, whether methicillin-resistant or methicillin-sensitive staphylococcal strains are used.
An important goal in the investigation of photosensitization processes in antimicrobial PDT is elucidation of the mechanism of action of a selected photosensitizer to determine whether a specific reactions proceeds via a type I or a type II pathway. In our study, killing was mediated predominantly by singlet oxygen, the type II pathway. Phototoxicity efficacy was not affected when mannitol was used as a type I scavenger. This observation is in contrast to that from an earlier study that used 1,4-diazabicyclo-(2,2,2)octane (DABCO) and sodium azide as type II scavengers (27). In that study killing was mediated by singlet oxygen and/or free radicals, but the type I mechanism was not analyzed. Sodium azide is known to be a specific scavenger of singlet oxygen (7). An explanation for this could be that not enough of the type II scavenger (DABCO) could reach the localization site of the dye. It is not known if sodium azide or DABCO is a more effective quenching singlet oxygen. However, combinations of type I and type II photosensitization processes with different photosensitizers may enhance the efficacy.
To date, a broad spectrum of chemically distinct photosensitizers have been studied, including phenothiazines, phthalocyanines, and porphyrins, all of which have been shown to have activities against gram-positive as well as gram-negative bacteria upon illumination (41, 42). The efficacies of killing of all of these photosensitizers have been confirmed to be significantly different between gram-positive and gram-negative bacteria, with greater efficacies against gram-positive bacteria (38, 40, 46, 48, 51). The results of our experiments are thus in accordance with the published data. However, where the results for the XF porphyrin derivatives differ from the published results is in their potencies against gram-positive bacteria, in which a 3-log10 reduction in bacterial cell number is achieved at nanomolar concentrations of XF70 and XF73. The difference in the efficacies of the XF derivatives against gram-negative bacteria may be explained by the difference in their outer membrane structures compared with those of gram-positive bacteria. It is generally accepted that the peptidoglycan layer of staphylococcal strains has a much higher permeability, e.g., for antibiotics, compared with that of the outer membranes of gram-negative bacteria (35). As a consequence, significant concentrations of photosensitizer reach the cytoplasmic membrane of gram-positive bacteria, a critical target, and induce irreversible damage via singlet oxygen after illumination. Thus, it is not surprising that in the present study 20- to 100-fold higher concentrations of the XF derivatives were necessary to achieve the same log10 decrease in the numbers of CFU per milliliter for gram-negative bacteria as that for gram-positive strains. The main physiological difference between gram-positive and gram-negative bacteria is that the cell wall of gram-negative bacteria has an outer membrane (lipid bilayer) outside of the peptidoglycan layer (38). The photosensitizer CTP1 used in our study has a molecular mass of ∼1,100 Da. Porphyrins with molecular masses of 1 kDa or greater cannot diffuse through the narrow porin channels, which selectively allow the influx of low-molecular-mass nutrients (57). As a consequence, the generation of the ROS induced after photosensitization, in particular, singlet oxygen, takes place only at the outer cell membrane, where the cell killing effect is marginal. This finding is supported by the short diffusion length of 1O2, because it has been shown that the 1O2 generated outside the bacterial cell wall of Salmonella enterica serovar Typhimurium does not react significantly with the bacterial chromosome, leading to DNA damage (19, 20, 22). In addition, Dahl and colleagues (19) showed that a deep rough strain of S. enterica serovar Typhimurium, which lacks nearly all of the cell wall lipopolysaccharide coat, responded to singlet oxygen with a faster inactivation than did the S. enterica serovar Typhimurium wild-type strain, indicating that the outer membrane barrier of gram-negative bacteria plays an important role in susceptibility to singlet oxygen. In contrast to CTP1, the photosensitizers XF70 and XF73 have molecular masses of only ∼700 Da and thus may diffuse through the outer membrane of gram-negative bacteria. It is therefore not surprising that XF70 and XF73 were more effective in killing E. coli. In our opinion, the molecular mass that allows transport through the outer cell membrane is more important for the antibacterial activity than the substitution with three positive charges of CTP1. Why this structural feature was effective in meso-substituted cationic porphyrins is not clear (40). Also, additional experiments must be performed to determine whether the chemical structure or the molecular mass of CTP1, XF70, or XF73 is the relevant factor for transport into E. coli, since the para-substituted derivative XF73 was also more efficient than the meta-substituted derivative XF70 at killing gram-positive bacteria. Previous observations (41) have shown that meso-substituted cationic porphyries can efficiently inactivate bacteria independently of the number of positive charges.
Nevertheless, the symmetric orientations of both XF70 and XF73, which do not contain a hydrocarbon chain, could promote a more efficient photoinactivation than that of the meso-substituted cationic derivative CPT1, which contains a long hydrocarbon chain, which normally enhances its affinity for bacterial cells, as shown by previous investigators (54). In our case, the presence of a low molecular mass and a symmetric orientation of positive charges could bring about an additional effect, namely, that XF70 or XF73 could interfere with the morphology of the bacterial cells to a greater extent, resulting in a more efficient photoinactivation compared to that of CPT1.
Nevertheless, it is important to underline the fact that upon illumination XF73 and XF70 were shown to have activities against gram-negative bacteria, like E. coli.
Compared to standard topical antibiotic treatment, which can require application for several weeks to kill bacteria, only a few minutes of XF incubation and illumination is sufficient to kill bacteria. This advantage is clearly shown in the present study, which yielded a significant reduction of pathogens (3 to 5.8 log10 CFU/ml) after only 5 min of incubation with a photosensitizer. Typically, the reductions of test bacteria from preparations used for hand hygiene range from 3 to 5 log10 CFU/ml (11). Longer incubation times (1 or 4 h) did not increase the phototoxicities of the XF derivatives for the different staphylococcal strains or E. coli. This result is in accordance with those of previous studies (40), which demonstrated an increase in phototoxicity against bacteria only when higher fluence rates were used at the level of the illuminated samples. In contrast to the findings obtained with bacteria, the uptake of XF73 by the eukaryotic cells used in this study, keratinocytes and fibroblasts, was time dependent. A longer incubation with significantly lower photosensitizer concentrations yielded equal phototoxicities. Active uptake by mammalian cells via endocytosis may explain this observation (13, 59).
Fluorescence microscopy revealed different localization patterns for CTP1 compared with those for XF70 and XF73. Fibroblasts showed granular, spotty fluorescence signals of CTP1 within the cells after 4 h of incubation. The colocalization of CTP1 with the LysotrackerGreen staining pattern of fibroblasts revealed that CTP1 is localized more or less in lysosomes. It is likely that hydrophilicity or lipophilicity, electric charge, molecular size, and nonspecific protein binding influence the uptake and distribution of the dye used for PDT. Hydrophilic dyes such as sulfonated tetraphenyl porphines are photosensitizers that localize mainly in lysosomes (8). At the moment it is unclear why the majority of the spotty intracellular distribution of CTP1 was not localized with lysosome-specific or mitochondrion-specific dyes. A possible explanation may be that following transport through the cytoplasmic membrane, CTP1 binds to cytoplasmic proteins. This assumption is supported by the fact that indocyanine green, another dye known to be a photosensitizer, is bound to gluthathione S-transferase, as shown in hepatocytes, and the fluorescence signals of indocyanine green were also found in the cytosols of keratinocytes and were not colocalized in lysosomes or mitochondria (2, 10, 53). In contrast to these findings for CTP1, the fluorescence signals of XF70 and XF73 were colocalized with the mitochondria. This observation is very likely caused by the different degrees of hydrophilicity of CTP1, XF70, and XF73; solubility in water decreases in the order CTP1 > XF70 > XF73. Thus, the more lipophilic dyes, like XF70 and XF73, may associate with the mitochondrial membrane, which has been shown for lipophilic porphyrin-based photosensitizers with cationic side chains (9, 71, 72).
The results obtained in this study show clear concentration-dependent differences in vitro between the photodynamic inactivation of eukaryotic cells and both antibiotic-sensitive and -resistant bacteria strains by the novel XF porphyrin derivatives.
In our opinion, this is a prerequisite for antibacterial PDT as an alternative to standard topical antibiotic treatment. Despite these promising experimental results, only a controlled, randomized clinical trial can prove the efficacy of PDT for the inactivation of bacteria in vivo. Indications for these new porphyrin-based photosensitizers would be for the control of bacterial colonization in patients and staff in hospitals, wound sterilization, and disinfection of skin and skin lesions, if the enhanced killing efficacy against bacteria compared to that against eukaryotic cells in vitro can also be demonstrated in vivo. Ongoing experiments with an ex vivo skin model and the same gram-positive multiresistant bacterial strains have confirmed the in vitro results (unpublished data). Moreover, the restriction of the phototoxic activity to the site of illumination would not disrupt the microflora at other sites.
In summary, the XF porphyrin series represent the first photosensitizers with selectivity for gram-positive bacteria which are active in vitro at nanomolar concentrations.
Acknowledgments
This work was funded by European Union grant Dynamicro-CRAF-1999-70306.
We thank X. Feng (Solvias Company) for synthesizing the photosensitizers.
REFERENCES
- 1.Abels, C., G. Ackermann, C. Alge, B. Aveline, W. Baumler, K. Berg, R. Bissonnette, W. H. Boehncke, P. Calzavara-Pinton, P. Curry, G. De Panfilis, C. C. Dierickx, J. Draeger, R. Fink-Puches, M. R. Hamblin, D. Hunt, S. Karrer, M. Landthaler, O. Larko, H. Lui, A. Moor, C. Morton, J. North, B. Ortel, Q. Peng, A. Sidoroff, C. R. Taylor, M. Trehan, A. Wennberg, P. Wolf, and C. Zane. 2001. Photodynamic therapy and fluorescence diagnosis in dermatology, vol. 2. Elsevier, London, United Kingdom.
- 2.Abels, C., S. Fickweiler, P. Weiderer, W. Baumler, F. Hofstadter, M. Landthaler, and R. M. Szeimies. 2000. Indocyanine green (ICG) and laser irradiation induce photooxidation. Arch. Dermatol. Res. 292:404-411. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal, R., M. Athar, S. A. Urban, D. R. Bickers, and H. Mukhtar. 1991. Involvement of singlet oxygen in chloroaluminum phthalocyanine tetrasulfonate-mediated photoenhancement of lipid peroxidation in rat epidermal microsomes. Cancer Lett. 56:125-129. [DOI] [PubMed] [Google Scholar]
- 4.Arndt-Jovin, D. J., and T. M. Jovin. 1989. Fluorescence labeling and microscopy of DNA. Methods Cell Biol. 30:417-448. [DOI] [PubMed] [Google Scholar]
- 5.Aveline, B. 2001. Primary processes in photosensitization mechanisms. Comprehensive Ser. Photosci. 2:17-34. [Google Scholar]
- 6.Baumler, W., C. Abels, S. Karrer, T. Weiss, H. Messmann, M. Landthaler, and R. M. Szeimies. 1999. Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br. J. Cancer 80:360-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellus, D. 1978. Quenchers of singlet oxygen—a critical review, p. 61. In R. Ranby (ed.), Singlet oxygen, reactions with organic compounds and polymers. John Wiley & Sons, Chichester, United Kingdom.
- 8.Berg, K., K. Prydz, and J. Moan. 1993. Photochemical treatment with the lysosomally localized dye tetra(4-sulfonatophenyl)porphine results in lysosomal release of the dye but not of beta-N-acetyl-d-glucosaminidase activity. Biochim. Biophys. Acta 1158:300-306. [DOI] [PubMed] [Google Scholar]
- 9.Berns, M. W., A. Dahlman, F. M. Johnson, R. Burns, D. Sperling, M. Guiltinan, A. Siemens, R. Walter, W. Wright, M. Hammer-Wilson, and A. Wile. 1982. In vitro cellular effects of hematoporphyrin derivative. Cancer Res. 42:2325-2329. [PubMed] [Google Scholar]
- 10.Blacker, K. L., E. Olson, D. A. Vessey, and T. D. Boyer. 1991. Characterization of glutathione S-transferase in cultured human keratinocytes. J. Investig. Dermatol. 97:442-446. [DOI] [PubMed] [Google Scholar]
- 11.Boyce, J. M., and D. Pittet. 2002. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am. J. Infect. Control 30:S1-S46. [DOI] [PubMed] [Google Scholar]
- 12.Boyle-Vavra, S., H. Labischinski, C. C. Ebert, K. Ehlert, and R. S. Daum. 2001. A spectrum of changes occurs in peptidoglycan composition of glycopeptide-intermediate clinical Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 45:280-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown, V. I., and M. I. Greene. 1991. Molecular and cellular mechanisms of receptor-mediated endocytosis. DNA Cell Biol. 10:399-409. [DOI] [PubMed] [Google Scholar]
- 14.Bunting, J. R., T. V. Phan, E. Kamali, and R. M. Dowben. 1989. Fluorescent cationic probes of mitochondria. Metrics and mechanism of interaction. Biophys. J. 56:979-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbon, C. 1999. Costs of treating infections caused by methicillin-resistant staphylococci and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 44(Suppl. A):31-36. [DOI] [PubMed] [Google Scholar]
- 16.Colsky, A. S., R. S. Kirsner, and F. A. Kerdel. 1998. Analysis of antibiotic susceptibilities of skin wound flora in hospitalized dermatology patients. The crisis of antibiotic resistance has come to the surface. Arch. Dermatol. 134:1006-1009. [DOI] [PubMed] [Google Scholar]
- 17.Colsky, A. S., R. S. Kirsner, and F. A. Kerdel. 1998. Microbiologic evaluation of cutaneous wounds in hospitalized dermatology patients. Ostomy Wound Manage. 44:40-42, 46. [PubMed] [Google Scholar]
- 18.Cookson, B. D. 1998. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J. Antimicrob. Chemother. 41:11-18. [DOI] [PubMed] [Google Scholar]
- 19.Dahl, T. A., W. R. Midden, and P. E. Hartman. 1989. Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J. Bacteriol. 171:2188-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahl, T. A., W. R. Midden, and P. E. Hartman. 1988. Pure exogenous singlet oxygen: nonmutagenicity in bacteria. Mutat. Res. 201:127-136. [DOI] [PubMed] [Google Scholar]
- 21.Dietzmann, K., G. Letko, and A. Sokolowski. 1987. Mitochondrial membrane potential in living cells: evidence from studies with rhodamine 6 G as fluorescent probe. Exp. Pathol. 31:47-51. [DOI] [PubMed] [Google Scholar]
- 22.Ehrenberg, B., J. L. Anderson, and C. S. Foote. 1998. Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media. Photochem. Photobiol. 68:135-140. [PubMed] [Google Scholar]
- 23.Embleton, M. L., S. P. Nair, B. D. Cookson, and M. Wilson. 2002. Selective lethal photosensitization of methicillin-resistant Staphylococcus aureus using an IgG-tin (IV) chlorin e6 conjugate. J. Antimicrob. Chemother. 50:857-864. [DOI] [PubMed] [Google Scholar]
- 24.Fink-Puches, R., A. Hofer, J. Smolle, H. Kerl, and P. Wolf. 1997. Primary clinical response and long-term follow-up of solar keratoses treated with topically applied 5-aminolevulinic acid and irradiation by different wave bands of light. J. Photochem. Photobiol. B 41:145-151. [DOI] [PubMed] [Google Scholar]
- 25.Foote, C. S. 1991. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 54:659. [DOI] [PubMed] [Google Scholar]
- 26.Fridkin, S. K. 2001. Vancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin. Infect. Dis. 32:108-115. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths, M. A., B. W. Wren, and M. Wilson. 1997. Killing of methicillin-resistant Staphylococcus aureus in vitro using aluminium disulphonated phthalocyanine, a light-activated antimicrobial agent. J. Antimicrob. Chemother. 40:873-876. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell, B., and J. M. Gutteridge. 1984. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet i:1396-1397. [DOI] [PubMed] [Google Scholar]
- 29.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 30.Jeffes, E. W., J. L. McCullough, G. D. Weinstein, P. E. Fergin, J. S. Nelson, T. F. Shull, K. R. Simpson, L. M. Bukaty, W. L. Hoffman, and N. L. Fong. 1997. Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid. A pilot dose-ranging study. Arch. Dermatol. 133:727-732. [PubMed] [Google Scholar]
- 31.Jones, L. R., and L. I. Grossweiner. 1994. Singlet oxygen generation by Photofrin in homogeneous and light-scattering media. J. Photochem. Photobiol. B 26:249-256. [DOI] [PubMed] [Google Scholar]
- 32.Kaye, E. T. 2000. Topical antibacterial agents: role in prophylaxis and treatment of bacterial infections. Curr. Clin. Top. Infect. Dis. 20:43-62. [PubMed] [Google Scholar]
- 33.Kilger, R., M. Maier, R. M. Szeimies, and W. Bäumler. 2001. Bidirectional energy transfer between the triplet T1 state of photofrin and singlet oxygen in deuterium oxide. Chem. Phys. Lett. 343:543-548. [Google Scholar]
- 34.Lin, C. W., J. R. Shulok, Y. K. Wong, C. F. Schanbacher, L. Cincotta, and J. W. Foley. 1991. Photosensitization, uptake, and retention of phenoxazine Nile blue derivatives in human bladder carcinoma cells. Cancer Res. 51:1109-1116. [PubMed] [Google Scholar]
- 35.Livermore, D. M. 1990. Antibiotic uptake and transport by bacteria. Scand. J. Infect. Dis. Suppl. 74:15-22. [PubMed] [Google Scholar]
- 36.Ma, L., J. Moan, and K. Berg. 1994. Evaluation of a new photosensitizer, meso-tetra-hydroxyphenyl-chlorin, for use in photodynamic therapy: a comparison of its photobiological properties with those of two other photosensitizers. Int. J. Cancer 57:883-888. [DOI] [PubMed] [Google Scholar]
- 37.Malik, Z., J. Hanania, and Y. Nitzan. 1990. Bactericidal effects of photoactivated porphyrins—an alternative approach to antimicrobial drugs. J. Photochem. Photobiol. B 5:281-293. [DOI] [PubMed] [Google Scholar]
- 38.Malik, Z., H. Ladan, and Y. Nitzan. 1992. Photodynamic inactivation of gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14:262-266. [DOI] [PubMed] [Google Scholar]
- 39.Menezes, S., M. A. Capella, and L. R. Caldas. 1990. Photodynamic action of methylene blue: repair and mutation in Escherichia coli. J. Photochem. Photobiol. B 5:505-517. [DOI] [PubMed] [Google Scholar]
- 40.Merchat, M., G. Bertolini, P. Giacomini, A. Villanueva, and G. Jori. 1996. meso-Substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 32:153-157. [DOI] [PubMed] [Google Scholar]
- 41.Merchat, M., J. D. Spikes, G. Bertoloni, and G. Jori. 1996. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J. Photochem. Photobiol. B 35:149-157. [DOI] [PubMed] [Google Scholar]
- 42.Minnock, A., D. I. Vernon, J. Schofield, J. Griffiths, J. H. Parish, and S. T. Brown. 1996. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J. Photochem. Photobiol. B 32:159-164. [DOI] [PubMed] [Google Scholar]
- 43.Moan, J., E. O. Pettersen, and T. Christensen. 1979. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br. J. Cancer 39:398-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mossman, B. T. 1983. In vitro approaches for determining mechanisms of toxicity and carcinogenicity by asbestos in the gastrointestinal and respiratory tracts. Environ. Health Perspect. 53:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichols, R. L. 1999. Optimal treatment of complicated skin and skin structure infections. J Antimicrob. Chemother. 44(Suppl. A):19-23. [DOI] [PubMed] [Google Scholar]
- 46.Nitzan, Y., and H. Ashkenazi. 2001. Photoinactivation of Acinetobacter baumannii and Escherichia coli B by a cationic hydrophilic porphyrin at various light wavelengths. Curr. Microbiol. 42:408-414. [DOI] [PubMed] [Google Scholar]
- 47.Nitzan, Y., R. Dror, H. Ladan, Z. Malik, S. Kimel, and V. Gottfried. 1995. Structure-activity relationship of porphines for photoinactivation of bacteria. Photochem. Photobiol. 62:342-347. [DOI] [PubMed] [Google Scholar]
- 48.Nitzan, Y., M. Gutterman, Z. Malik, and B. Ehrenberg. 1992. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 55:89-96. [DOI] [PubMed] [Google Scholar]
- 49.Palmiter, R. D., T. B. Cole, and S. D. Findley. 1996. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 15:1784-1791. [PMC free article] [PubMed] [Google Scholar]
- 50.Ploy, M. C., C. Grelaud, C. Martin, L. de Lumley, and F. Denis. 1998. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet 351:1212. [DOI] [PubMed] [Google Scholar]
- 51.Polo, L., A. Segalla, G. Bertoloni, G. Jori, K. Schaffner, and E. Reddi. 2000. Polylysine-porphycene conjugates as efficient photosensitizers for the inactivation of microbial pathogens. J. Photochem. Photobiol. B 59:152-158. [DOI] [PubMed] [Google Scholar]
- 52.Raab, O. 1900. Ueber die Wirkung fluorizierender Stoffe auf Infusorien. Z. Biol. 39:524-546. [Google Scholar]
- 53.Raza, H., Y. C. Awasthi, M. T. Zaim, R. L. Eckert, and H. Mukhtar. 1991. Glutathione S-transferases in human and rodent skin: multiple forms and species-specific expression. J. Investig. Dermatol. 96:463-467. [DOI] [PubMed] [Google Scholar]
- 54.Reddi, E., M. Ceccon, G. Valduga, G. Jori, J. C. Bommer, F. Elisei, L. Latterini, and U. Mazzucato. 2002. Photophysical properties and antibacterial activity of meso-substituted cationic porphyrins. Photochem. Photobiol. 75:462-470. [DOI] [PubMed] [Google Scholar]
- 55.Roberts, J. I., and M. A. Gaston. 1987. Protein A and coagulase expression in epidemic and non-epidemic Staphylococcus aureus. J. Clin. Pathol. 40:837-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rovaldi, C. R., A. Pievsky, N. A. Sole, P. M. Friden, D. M. Rothstein, and P. Spacciapoli. 2000. Photoactive porphyrin derivative with broad-spectrum activity against oral pathogens in vitro. Antimicrob. Agents Chemother. 44:3364-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanderson, K. E., T. MacAlister, J. W. Costerton, and K. J. Cheng. 1974. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can. J. Microbiol. 20:1135-1145. [DOI] [PubMed] [Google Scholar]
- 58.Seal, J. B., B. Moreira, C. D. Bethel, and R. S. Daum. 2003. Antimicrobial resistance in Staphylococcus aureus at the University of Chicago Hospitals: a 15-year longitudinal assessment in a large university-based hospital. Infect. Control Hosp. Epidemiol. 24:403-408. [DOI] [PubMed] [Google Scholar]
- 59.Selbo, P. K., A. Hogset, L. Prasmickaite, and K. Berg. 2002. Photochemical internalisation: a novel drug delivery system. Tumour Biol. 23:103-112. [DOI] [PubMed] [Google Scholar]
- 60.Sharma, S., and K. K. Verma. 2001. Skin and soft tissue infection. Indian J. Pediatr. 68(Suppl. 3):S46-S50. [PubMed] [Google Scholar]
- 61.Sievert, D. 2002. Staphylococcus aureus resistant to vancomycin. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 62.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, W. R. Jarvis, et al. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 63.Szeimies, R. M., S. Karrer, A. Sauerwald, and M. Landthaler. 1996. Photodynamic therapy with topical application of 5-aminolevulinic acid in the treatment of actinic keratoses: an initial clinical study. Dermatology 192:246-251. [DOI] [PubMed] [Google Scholar]
- 64.Veien, N. K. 1998. The clinician's choice of antibiotics in the treatment of bacterial skin infection. Br. J. Dermatol. 139(Suppl. 53):30-36. [DOI] [PubMed] [Google Scholar]
- 65.von Tappeiner, H. 1904. Zur Kenntnis der lichtwirkenden (fluoreszierenden) Stoffe. Dtsch. Med. Wochenschau 1:579-580. [Google Scholar]
- 66.von Tappeiner, H., and A. Jesionek. 1903. Therapeutische Versuche mit fluoreszierenden Stoffen. Münch. Med. Wochenschau 50:2042-2044. [Google Scholar]
- 67.von Tappeiner, H., and A. Jesionek. 1904. Über die Wirkung der photodynamischen (fluoreszierenden) Stoffe auf Infusorien. Dtsch. Arch. Klin. Med. 80:427-487. [Google Scholar]
- 68.Wainwright, M. 1998. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 69.Wenzel, R. P. 1986. Methicillin-resistant S. aureus and S. epidermidis strains: modern hospital pathogens. Infect. Control 7:118-119. [DOI] [PubMed] [Google Scholar]
- 70.Wilson, M. 1993. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease. J. Appl. Bacteriol. 75:299-306. [DOI] [PubMed] [Google Scholar]
- 71.Woodburn, K. W., S. Stylli, J. S. Hill, A. H. Kaye, J. A. Reiss, and D. R. Phillips. 1992. Evaluation of tumour and tissue distribution of porphyrins for use in photodynamic therapy. Br. J. Cancer 65:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woodburn, K. W., N. J. Vardaxis, J. S. Hill, A. H. Kaye, J. A. Reiss, and D. R. Phillips. 1992. Evaluation of porphyrin characteristics required for photodynamic therapy. Photochem. Photobiol. 55:697-704. [DOI] [PubMed] [Google Scholar]
- 73.Zaidi, S. I., R. Agarwal, G. Eichler, B. D. Rihter, M. E. Kenney, and H. Mukhtar. 1993. Photodynamic effects of new silicon phthalocyanines: in vitro studies utilizing rat hepatic microsomes and human erythrocyte ghosts as model membrane sources. Photochem. Photobiol. 58:204-210. [DOI] [PubMed] [Google Scholar]