ABSTRACT
The maintenance of cellular calcium homeostasis in response to calcium supply is required for a wide range of physiological processes in fungi, including growth, development, and virulence. In Aspergillus, the transcription factor CrzA is involved in efficient regulation of calcium homeostasis, especially under calcium-repleted conditions. However, the transcriptional regulatory mechanisms under calcium-limited conditions remain unclear. Here, we found that a highly conserved transcription factor, SltA, known for its roles in salt tolerance and azole resistance, can confer adaptation to calcium-limited conditions in Aspergillus fumigatus. Loss of sltA caused severe growth defects with abnormal expressions of calcium metabolism-related genes, which resulted in a decreased cytosolic calcium transient under calcium-limited conditions, while the addition of calcium rescued all defective phenotypes in the ΔsltA mutant. Moreover, A. fumigatus SltA undergoes a proteolysis modification that depends on SltB, a chymotrypsin-like serine protease. Strikingly, A. fumigatus SltA plays a reverse role to CrzA in the regulation of expressions of calcium metabolism-related genes. Deletion of crzA significantly suppressed the growth defect of the ΔsltA mutant. Deletion of sltA led to highly expressed CrzA with a constant nuclear location under calcium-limited conditions, demonstrating that there exists a mutually restricted network regulated by SltA and CrzA to adapt to calcium-limited conditions. Electrophoretic mobility shift assays and chromatin immunoprecipitation sequencing revealed that SltA directly binds to a subset of genes involved in calcium metabolism via a conserved motif. These findings expand knowledge on the regulatory circuits of the Ca2+ signaling pathway.
IMPORTANCE
Calcium ions are ubiquitous intracellular signaling molecules for many signaling pathways regulating the fungal response to stress and antifungal drugs. The concentration of intracellular calcium is tightly regulated in its storage, release, and distribution. CrzA is the best-studied transcription factor that regulates this process under sufficient calcium or other external signals. However, CrzA was excluded from nuclei and then lost transcriptional activation under calcium-limited conditions. The regulators in the Ca2+ signaling pathway under calcium-limited conditions remain unclear. Here, we identified SltA as a key regulator in the Ca2+ signaling pathway under calcium-limited conditions, and the underlying mechanisms were further explored in Aspergillus fumigatus. These findings reveal a transcriptional control pathway that precisely regulates calcium homeostasis under calcium-limited conditions.
KEYWORDS: Aspergillus fumigatus, calcium signaling pathway, CrzA, SltA, calcium-limited conditions
INTRODUCTION
Aspergillus fumigatus is a filamentous fungus that can cause disease in humans (1 – 3). The Ca2+ signaling pathway plays a fundamental role in A. fumigatus development, drug resistance, and virulence (4, 5). Distinct from other secondary messengers, calcium (Ca2+) is not dependent on biosynthesis but rather on effective regulation of calcium concentration (6 – 8). The free cytosolic calcium concentrations are maintained at a resting level between 50 and 200 nM, but their concentrations in the intracellular storage organelles such as the endoplasmic reticulum, vacuoles, and mitochondria are much higher (9). Calcium homeostasis is precisely controlled by various channels, transporters, and pumps located in the plasma membrane and intracellular storage organelles. The best-studied Ca2+-responsive signaling pathway in A. fumigatus and other fungi involves the calcium sensor protein calmodulin, phosphatase calcineurin, and zinc finger transcription factor CrzA (10 – 12). In response to calcium-repleted conditions and other external signals, the increased cytosolic Ca2+ binds to calmodulin and subsequently activates the phosphatase calcineurin, which then dephosphorylates the transcription factor CrzA, leading to its nuclear translocation and subsequent transcriptional activation (13 – 15). Nuclear-localized CrzA could activate the expression of pmcA, pmcB, and pmcC (encoding vacuolar Ca2+ ATPases) as well as vcxA (encoding a vacuolar Ca2+/H+ exchanger) to promote vacuolar calcium sequestration (13, 16, 17). In addition to calcium metabolism, the direct targets of CrzA are involved in asexual development, cell wall biosynthesis, and stress tolerance (18, 19). Consistent with its multiple regulatory roles, CrzA deficiency causes defects in conidial production, calcium homeostasis regulation, cell wall biosynthesis, and virulence (13, 20). However, CrzA was apparently excluded from nuclei under calcium-limited conditions, and the transcriptional regulatory mechanisms that maintain calcium homoeostasis under calcium-limited conditions remain unclear (21).
SltA (involved in the salt tolerance pathway) was initially identified as a C2H2 zinc finger transcription factor necessary for tolerance to high levels of Na+ and K+ in Aspergillus nidulans (22 – 24). Further studies revealed that loss of sltA causes sensitivity to high concentrations of the monovalent cations lithium and rubidium in addition to sodium and potassium and to the divalent cations manganese and magnesium but not to calcium and was recently reported to affect the trivalent metal boron in A. nidulans (23, 25 – 27). In addition, SltA plays an important role in DNA repair, arginase activity, and sterigmatocystin biosynthesis in A. nidulans (28). Different from CrzA, which can be found in most ascomycetes, SltA is exclusive to the subphylum Pezizomycotina, and no identifiable SltA ortholog was detected in Saccharomyces cerevisiae (28, 29). In the pathogenic filamentous fungus Colletotrichum gloeosporioides, loss of the sltA ortholog inhibited appressorium formation and reduced pathogenicity (30). SltA exhibits substantial similarity to the transcriptional repressor Ace1 from Trichoderma reesei, which regulates the expression of genes encoding cellulase and xylanase by binding to the consensus 5′-AGGCA-3′ in the promotor region (31). Interestingly, this consensus 5′-AGGCA-3′ motif appeared on the promoters of SltA targets in A. nidulans, including sltB (encoding a chymotrypsin-like serine protease) (22), vcxA (32), and enaA (encoding a sodium pump ATPase) (24). Recently, SltA was reported to function in the regulation of the production of secondary metabolites and mycotoxins in A. fumigatus (33). SltA was also shown to be involved in azole resistance by coregulating the expression of genes involved in ergosterol biosynthesis (erg11A, erg13A, and erg24A) and drug pumps (mdr1, mfsC, and abcE) in A. fumigatus by directly binding to the conserved 5′-AGGCA-3′ motif in electrophoretic mobility shift assays (EMSAs) (34). However, the direct targets and binding sites of SltA in vivo have not been systematically identified in fungi.
The work in A. nidulans and other fungi suggested that SltA plays a role in calcium metabolism. First, a combination of null mutations of sltA and halA, encoding a protein kinase, resulted in a growth defect under calcium-limited conditions in A. nidulans (32, 35). Second, deletion of sltA caused an increase in pmcA and pmcB transcript levels and resulted in hypertrophy of the vacuolar system under calcium-limited conditions. Furthermore, the addition of calcium rescued the growth defects and toxin production of the ΔsltA mutant in A. nidulans (28, 36). In addition, calcium supplementation rescued the appressorium formation defect of the ΔsltA mutant in Colletotrichum gloeosporioides (30). However, the regulatory mechanisms of SltA on calcium homeostasis in fungi remain unclear.
In this study, by screening a transcription factor mutant library (37), we identified SltA and its conserved Cys2His2 DNA-binding domain as playing an important role in adaptation to calcium-limited conditions in A. fumigatus. Moreover, SltA reversely regulates calcium metabolism with the well-known zinc finger transcription factor CrzA. Findings in this work shed light on a regulatory circuit of the Ca2+ signaling pathway under calcium-limited conditions.
RESULTS
The transcription factor SltA is required for the adaptation of A. fumigatus to calcium-limited conditions
To globally assess transcription factors involved in the adaptation of A. fumigatus to calcium-limited conditions, we screened a library of transcription factor null mutants (37). The mutants of transcription factor PacC, which mediates the ambient pH regulatory pathway (38, 39), and the cation homeostasis responsive factor SltA (32) exhibited increased sensitivity to ethylene glycol tetra acetic acid (EGTA, calcium chelator) compared to the wild-type (WT) strain (Fig. S1A through C). Since the ΔsltA mutant displayed a more severe growth defect than the ΔpacC mutant, we then only selected SltA for the following studies.
To further validate the phenotypes of the ΔsltA mutant in the transcription factor mutant library, the asexual development and colony growth of the reconstructed sltA deletion and its complemented strains were assayed. The number of conidia produced per unit area (cm2) by the ΔsltA mutant on minimal medium (MM, referred to as a calcium-limited condition) was lower than that of the WT and reconstituted strains (Fig. 1A and B). The addition of 1 mM EGTA to MM (a harsh calcium-limited condition) strongly inhibited the colony growth of the ΔsltA mutant. However, the addition of 5 mM calcium to MM (calcium-repleted condition) completely restored the colony growth of the ΔsltA mutant to the WT level (Fig. 1A and B). This result indicates that SltA is required for colony growth and conidiation under calcium-limited conditions.
FIG 1.

The transcription factor SltA is required for the adaptation of A. fumigatus to calcium-limited conditions. (A) Growth phenotypes of the indicated strains on MM or MM with 5 mM Ca2+ or 1 mM calcium chelator EGTA at 37°C for 5 days. (B) Bar graphs show the quantitative analysis of colony diameter and spore production per unit area of the indicated strains. Data are shown as the means ± SD from three independent experiments. A schematic representation of the domains of the SltA protein (the C2H2 DNA-binding domain is shown in red) with the introduced site-directed mutations. (C) Western blot analysis of SltA-FLAG protein levels using FLAG antibody. All strains were cultured on liquid MM for 24 h at 37°C. Actin was used as a loading control. (D) Quantification of the signal intensity ratio of SltA-FLAG protein to actin. The expression levels of full-length SltA-FLAG (105 kDa) were quantified in the SltA-FLAGΔsltB strain, and the expression levels of truncated SltA-FLAG (70 kDa) were quantified in the SltA-FLAG strain. Data are shown as the means ± SD from three independent experiments. Statistical analysis was performed using one-tailed, unpaired t-tests. ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Three classic Cys2His2 zinc fingers mediate the binding of DNA to SltA (34). To assess whether the conserved cysteine and histidine within the DNA-binding domain of SltA are necessary for the adaptation of A. fumigatus to calcium-limited conditions, we assessed the colony growth and conidia production of the site-directed mutants sltAC502S and sltAH518A (the Cys residue at position 502 was mutated to Ser, and the His residue at position 518 was mutated to Ala). The sltAC502S and sltAH518A strains displayed colony growth and conidiation defects similar to those of the full-length ΔsltA deletion strain (Fig. 1A and B), suggesting that the conserved cysteine and histidine within the Cys2His2 zinc-finger DNA-binding domain are indispensable for SltA-mediated adaptation to calcium-limited conditions.
As SltA confers adaptation to calcium-limited conditions, we next questioned whether the expression of sltA could be induced under these conditions. Unexpectedly, RT-qPCR (reverse transcription quantitative PCR) data showed that there was no difference in sltA mRNA expression between samples collected from MM and MM plus 5 mM calcium (Fig. S2A). To further test the expression of SltA at the protein level, we generated a C-terminal FLAG-tagged SltA strain. The SltA-FLAG strain had no apparent growth defects under calcium-limited conditions, indicating that introduction of the FLAG tag did not affect SltA function (Fig. S2B and C). Using an antibody against FLAG, we found that the amount of SltA was much higher when cultured on MM than when cultured on MM plus 5 mM calcium, indicating that the concentrations of calcium ions may affect the stability of the SltA protein (Fig. 1C and D; Fig. S2D). It is important to note that the detected molecular weight of the SltA-FLAG (approximately 70 kDa) was less than that expected from the calculated 105 kDa mass of the SltA-FLAG fusion protein (99 kDa of SltA + 6 kDa of FLAG).
Previous studies have shown that the SltA ortholog in A. nidulans undergoes a proteolysis modification and this procedure depends on SltB, a chymotrypsin-like serine protease (36). To further verify whether SltA in A. fumigatus undergoes a proteolysis modification, and whether the SltA-FLAG band at 70 kDa in A. fumigatus found in this study is equivalent to the SltA 32 kDa in A. nidulans in the previous report, additional experiments were performed. We then constructed the sltB-deleted mutant strain in the SltA-FLAG background strain. This sltB null mutant displayed a similar extent of sensitive phenotype under calcium-limited conditions to the sltA null mutant (Fig. S2E and F). The western blotting result showed that the band corresponding to the truncated SltA-FLAG (70 kDa) disappeared in the sltB null mutant. Instead, the low intensity of bands corresponding to the full-length SltA-FLAG (about 105 kDa) were detected in the sltB null mutant (Fig. 1C and D). Those results suggest that SltB is required for the expression and proteolysis modification of SltA in A. fumigatus. Collectively, those results suggest that SltA orthologs in both A. nidulans and A. fumigatus could undergo proteolysis post-translational modification, and this procedure depends on SltB. However, in previous published information for A. nidulans, two bands corresponding to the truncated SltA-FLAG and the full-length SltA-FLAG can be detected simultaneously (36), which is different from that in A. fumigatus found in this study. Those results indicated that in the presence of SltB in A. fumigatus, SltA could be completely proteolysis modified, resulting in all truncated SltA with no detectable full-length SltA. This finding might suggest that there are some conserved and varied functions for both SltA and SltB in A. fumigatus and in A. nidulans.
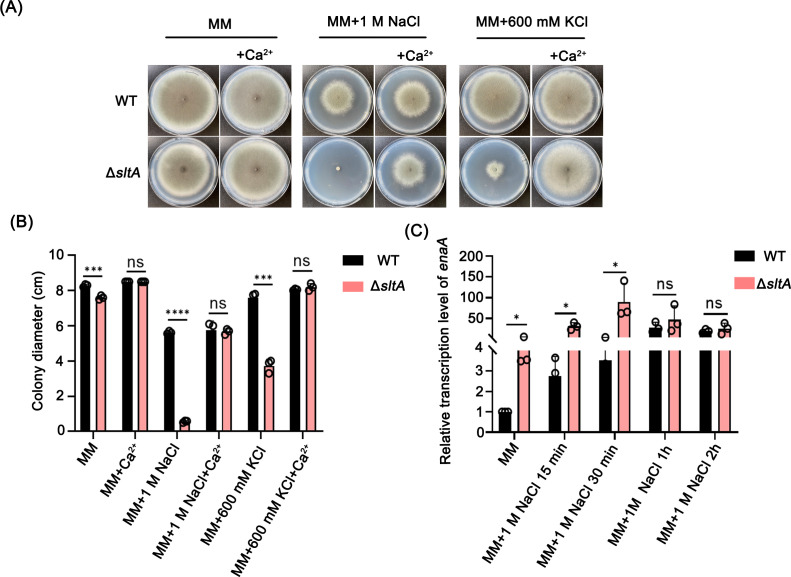
Sensitivity to salt stress in the ΔsltA mutant can be restored by extracellular calcium
SltA has been previously identified as an alkali metal ion-responsive factor in A. nidulans (22, 27, 36). The loss of sltA confers A. nidulans sensitivity to high concentrations of alkali metal ions (24, 29). Consistent with the report in A. nidulans, the loss of sltA in A. fumigatus caused a dramatic colony growth defect when cultured on MM supplemented with NaCl and KCl (Fig. 2A and B). Modulation of cytoplasmic cation homeostasis is mainly achieved by active transporters located in the plasma membrane and intracellular compartment (40, 41). RT-qPCR assays showed that the expression of enaA, a gene encoding a sodium pump ATPase (24, 29), was markedly increased but with no significant decreased expression in the ΔsltA mutant compared to that in WT under treatment with 1 M NaCl (Fig. 2C), suggesting that the expression of enaA is not dependent on SltA. Furthermore, the expression of additional genes encoding ion transporters was assayed by RT-qPCR. Those genes include trkA, which encodes a high-affinity sodium and potassium uptake transporter (42); mdm38, an essential K+/H+ exchanger that localizes in the inner mitochondrial membrane (43); vnxA, an antiporter for vacuolar alkali metal cations (44, 45); and nhaA, an electrogenic proton antiporter localized in the plasma membrane (46). The RT-qPCR results showed that the expression of all four selected genes encoding ion transporters increased to varying degrees when cultured on MM as well as under treatment with 1 M NaCl for 15 and 30 min. We deduce that these increased expressions of these genes may play a compensatory role for the deletion of sltA under the treatment of 1 M NaCl. However, the increased expression levels almost returned to the WT level when treated for 2 h, indicating that induced mRNA-enhanced expressions were transient (Fig. S3). These data also suggest that the expressions of tested ion transporters (enaA, trkA, mdm38, etc.) are not dependent on SltA. However, surprisingly, the salt stress-sensitive phenotype of the ΔsltA strain could be rescued after adding 5 mM calcium ions (Fig. 2A and B). These results suggest that SltA-mediated regulation of alkali metal ion homeostasis is related to calcium absorption, so adding extracellular calcium is able to rescue all defective phenotypes induced by salt stress in the ΔsltA mutant, while the underlying mechanisms of SltA-mediated salt stress adaptation need to be further explored in A. fumigatus.
FIG 2.
Sensitivity of the ΔsltA mutant to alkali metal ions can be restored by extracellular calcium. (A) Growing phenotypes of WT and ΔsltA on solid MM with different metal ions. WT and ΔsltA conidia were grown in MM and MM with 1 M NaCl or 0.6 M KCl in the presence or absence of 5 mM calcium at 37°C for 5 days. (B) Quantification of the colony diameter of the indicated strains with three independent biological repeats. (C) Relative transcript levels of enaA in WT and ΔsltA strains grown in liquid MM at 37°C for 20 h, and 1 M NaCl was added to further culture for 15 min, 30 min, 1 h, and 2 h. The mRNA levels were normalized to the reference gene tubA. The data are shown as the means ± SD of three independent experiments. Statistical analysis was performed using one-tailed, unpaired t-tests. ns, not significant (P > 0.05); ***, P < 0.001; ****, P < 0.0001.
Loss of sltA causes decreased cytosolic Ca2+ transients, probably resulting from overexpression of vacuolar Ca2+ transporter-encoding genes
To further explore the role of SltA in regulating cytosolic calcium homeostasis, we monitored the transient cytosolic Ca2+ levels in response to extracellular calcium by expressing codon optimized aequorin in living cells of A. fumigatus (47, 48). After an extracellular calcium stimulus, the cytosolic Ca2+ ([Ca2+]c) amplitude in the ΔsltA AEQ mutant showed reduced cytosolic Ca2+ transients with approximately 40 ± 4% of the WTAEQ peak value under the same detection conditions (Fig. 3A). However, the basal resting cytosolic calcium concentration (prior to extracellular calcium stimulus) was not significantly different between the ΔsltA AEQ and WTAEQ strains. Those results imply that the resting Ca2+ concentration in the cytosol was complicated and not dependent on SltA only, while the cytosolic Ca2+ transient in response to extracellular stimulus was affected by SltA.
FIG 3.
Loss of sltA results in reduced transient cytosolic Ca2+ levels and abnormal expression of calcium transport genes. (A) [Ca2+]c responses in the WTAEQ and ΔsltA AEQ strains following a stimulus of 0.1 M CaCl2. The bar graph shows the peak values of the [Ca2+]c amplitude. Data are shown as the means ± SD from three independent experiments. (B) The relative transcript levels of the indicated genes in WT and ΔsltA strains grown in liquid MM with/without 5 mM CaCl2 for 24 h. The mRNA levels were normalized to the reference gene tubA. Data are shown as the means ± SD from three independent experiments. Statistical analysis was performed using one-tailed, unpaired t-tests. ns, not significant (P > 0.05); **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Previous studies in A. nidulans revealed that SltA negatively regulates the expression of the vacuolar Ca2+ transporter-encoding genes vcxA, pmcA, and pmcB (32, 35). We next wondered whether the loss of sltA in A. fumigatus changes the expression of vacuolar Ca2+ transporter-encoding genes, which may contribute to the decreased cytosolic Ca2+ transients. As shown in Fig. 3B, overexpression of vcxA, pmcA, pmcB, and pmcC occurred in the ΔsltA mutant when cultured on MM. Strikingly, the addition of calcium (5 mM) to MM greatly restored the expression of vacuolar Ca2+ transporter-encoding genes pmcA and pmcB but not pmcC in the ΔsltA mutant close to the level of the WT. The results indicated that SltA regulates the expression of calcium-related genes specifically under calcium-limited conditions. The overexpression of genes encoding vacuolar Ca2+ transporters may lead to excessive vacuolar storage of calcium and cytosolic deficits accompanied by decreased cytosolic Ca2+ transients, which may be the cause of the sensitive phenotypes of the ΔsltA mutant under calcium-limited conditions. However, double knockout of pmcA and pmcB [pmcC is an essential gene (16)] in the ΔsltA mutant background could not rescue the defective phenotypes in the ΔsltA mutant (Fig. S4A through C), suggesting that the overexpression of the vacuolar Ca2+ transporters encoded by the genes pmcA and pmcB may not be the only reason for the phenotype of ΔsltA.
RNA sequencing analysis identifies a core set of SltA target genes involved in calcium metabolism
To gain insight into the genome-wide targets of SltA, we performed RNA sequencing (RNA-seq) on the WT and ΔsltA mutant under both MM (calcium-limited condition) and MM plus 5 mM calcium (calcium-repleted condition). A total of 2,073 genes (976 up- and 1,097 downregulated) were differentially expressed (fold change >2, P < 0.05) in the ΔsltA mutant compared to the WT when cultured on MM medium (Table S2). In comparison, only 781 genes (596 up- and 185 downregulated) were differentially expressed (fold change >2, P < 0.05) when cultured on MM plus 5 mM calcium (Fig. 4A). The results indicate that SltA regulates more targets under calcium-limited conditions than calcium-repleted conditions.
FIG 4.
RNA-seq analysis identifies a core set of SltA target genes involved in calcium metabolism. (A) Volcano plot showing statistical significance (−log10 P value) versus fold change (log2 fold change) of RNA-seq data from WT and ΔsltA mutants cultured on MM and MM plus 5 mM calcium at 37°C for 24 h. (B-C) KEGG enrichment tables of upregulated and downregulated genes in the ΔsltA versus WT cultured in liquid MM (B) or MM plus 5 mM calcium (C). P values and gene numbers are represented using a gradient of color and bubble size, respectively. (D) Heatmap depicting the relative expression levels of the selected genes putatively involved in calcium metabolism from the RNA-seq results of the ΔsltA mutant and WT strain cultured in liquid MM with and without 5 mM calcium at 37°C for 24 h.
To identify the potential roles of SltA-dependent genes in specific fungal processes, we subjected these differentially expressed genes to pathway analysis using Kyoto Encyclopedia of Genes and Genomes (KEGG) (Table S3). The results showed that differentially expressed genes under the MM condition were mainly enriched in different amino acid metabolic pathways, ribosome biogenesis, and lipid metabolism (Fig. 4B). In comparison, when cultured on MM plus 5 mM calcium, differentially expressed genes were enriched in amino sugar and nucleotide sugar metabolism, pyruvate metabolism, and starch and sucrose metabolism (Fig. 4C). Consistent with its role in calcium metabolism, the differentially expressed genes of the ΔsltA mutant were highly enriched in functions related to calcium metabolism, including calcium transporters, channels, protein kinases, and transcription factors. In particular, the expression of vacuolar Ca2+ transporters encoding gene pmcB was significantly increased 8.9-fold in the ΔsltA mutant compared to that in the WT under calcium-limited conditions, which may result in excessive vacuolar storage of calcium. In addition, the normal expression of mcuA (AFUB_067410) and pmrA (AFUB_072670) depended on SltA. McuA is a mitochondrial Ca2+ uniporter that responds to Ca2+ uptake into the mitochondria in A. fumigatus (49). The lack of sltA leads to 2.4-fold increased transcription of mcuA, specifically under calcium-limited conditions. PmrA, a P-type ATPase, localizes to the Golgi apparatus, where it mediates Ca2+ transport from the cytoplasm into the Golgi (50). The ΔsltA mutant exhibited 2.2-fold decreased expression of pmrA compared to that of the WT (Fig. 4D). These results suggest that SltA regulates calcium homeostasis by influencing the expression of a subset of genes involved in calcium storage, release, and distribution.
SltA and CrzA reversely regulate the expression of genes encoding vacuolar Ca2+ transporters
Previous studies have reported that the transcription factor CrzA is involved in calcium homeostasis by positively regulating the expression of pmcA-C and vcxA (13, 16, 51). The aforementioned data suggest that SltA is a negative regulator for the expression of pmcA-C and vcxA. Thus, this result indicated that SltA and CrzA have reverse regulatory effects on some overlapping targets. To further analyze the regulatory relationship between SltA and CrzA to those downstream targets involved in calcium metabolism, we constructed a ΔsltAΔcrzA double knockout mutant, and its transcriptomic profile was determined by RNA-seq. A comparison between differentially expressed genes revealed that approximately 35% (n = 342 out of 976) of the upregulated genes in the ΔsltA mutant were suppressed in the ΔsltAΔcrzA mutant, indicating that the overexpression of those genes in the ΔsltA mutant depends on crzA (Fig. 5A). Notably, these putative CrzA-dependent subsets of genes include many calcium metabolism-related genes, such as the vacuolar calcium transporter genes pmcB and pmcC, the calcium response transcription factor zfpA (AFUB_082490), and the calcineurin-binding protein encoding gene rcnA (AFUB_028690). In addition, the expression of the vacuolar calcium transporter gene pmcA and the vacuolar Ca2+/H+ exchanger gene vcxA showed moderately increased expression in the ΔsltA mutant compared to the WT, as determined by RT-qPCR but not by RNA-seq analysis (Fig. 5A and B). The loss of crzA restored the overexpressed pmcA and vcxA in the ΔsltA mutant to the WT level, which indicates that their overexpression in the ΔsltA mutant depends on crzA. However, we also observed that the expression of some differentially expressed genes in the ΔsltA mutant was not significantly affected by the loss of crzA. These genes included the mitochondrial calcium transporter mcuA and the Golgi calcium transporter gene pmrA (Fig. 5A and B).
FIG 5.
The aberrant gene expression of the ΔsltA mutant under calcium-limited conditions partly depends on CrzA. (A) Venn diagram showing the distribution of differentially expressed genes (fold change >2; P < 0.05) in the ΔsltA and ΔsltAΔcrzA mutants relative to the WT strain in liquid MM at 37°C for 24 h. The number 342 represents the intersection of upregulated genes in the ΔsltA mutant (n = 976) and nonupregulated (downregulated and no significant difference, n = 8,647) genes in the ΔsltAΔcrzA mutant, and the number 297 represents the intersection of the ΔsltA mutant downregulated gene (n = 1,097) and nondownregulated (upregulated and no significant difference, n = 8,596) genes in the ΔsltAΔcrzA mutant. (B) RT-qPCR analysis of the relative transcript levels of the selected genes involved in calcium metabolism in the indicated strains grown in liquid MM for 24 h. The mRNA levels were normalized to the reference gene tubA. (C) Growth phenotypes of the indicated strains on MM and MM with 1 mM calcium chelator EGTA at 37°C for 5 days. The bar graph shows quantitative results of colony diameter and conidia production per unit area (cm2). Statistical analysis was performed using one-tailed, unpaired t-tests. ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Considering that the loss of crzA could partly restore the aberrant expression of genes related to calcium metabolism, we hypothesized that knockout of crzA in the ΔsltA mutant background might alleviate the growth defect of the ΔsltA mutant under calcium-limited conditions. As expected, the ΔsltAΔcrzA mutant exhibited increased conidiation and hyphal growth compared with the ΔsltA mutant when cultured on MM plus 1 mM EGTA (Fig. 5C). However, the hyphal growth and conidiation of the ΔsltAΔcrzA mutant were still weaker than those of the WT under the same conditions. Taken together, the above data indicate that the aberrant gene expression of the ΔsltA mutant under calcium-limited conditions is partly dependent on CrzA, and that the deletion of crzA could partially restore the sensitivity phenotypes of the ΔsltA mutant.
The loss of sltA leads to high expression of CrzA as well as abnormal nuclear localization under calcium-limited culture conditions
The location and activity of CrzA protein are regulated by Ca2+, and CrzA protein is apparently excluded from nuclei under calcium-limited conditions so that it cannot activate downstream targets (13, 16, 52). Given that the aberrant expression of a subset of genes in the ΔsltA mutant depends on CrzA under calcium-limited conditions, we speculated that SltA may have an effect on CrzA translocation. To test this hypothesis, we generated C-terminally GFP-labeled CrzA (CrzA-GFP) strains in both WT strain and ΔsltA mutant backgrounds. As expected, when the WTCrzA-GFP strain was cultured on MM, CrzA-GFP was mostly excluded from the nuclei. When 200 mM CaCl2 was added to MM, almost all of the CrzA-GFP was translocated into the nuclei (Fig. 6A). In comparison, CrzA-GFP showed persistent nuclear localization when cultured on MM in the ΔsltA CrzA-GFP strain, suggesting that loss of sltA caused CrzA constitutive nuclear localization under calcium-limited conditions. Moreover, the deletion of sltA resulted in the overexpression of crzA/CrzA under MM (Fig. 6B and C). Overall, SltA has an effect on the localization and expression of crzA/CrzA under calcium-limited conditions in A. fumigatus.
FIG 6.
The loss of sltA leads to CrzA nuclear localization and overexpression under low-calcium conditions. (A) Fluorescence-microscopic images demonstrating CrzA-GFP distributions in WT and ΔsltA on MM and MM treated with 200 mM calcium for 15 min as a stimulus. Then, 1 × 105 spores of WTCrzA-GFP and ΔsltA CrzA-GFP on coverslips with 1 mL liquid MM at 37°C for 15 h. Hoechst dye was used as a nuclear localization signal to visualize the nuclear localization. The merged images of GFP and Hoechst staining showed the nuclear localization of CrzA-GFP. Images are representative of three replicate cultures; at least 200 hyphae were observed in each replicate. Bar, 10 µm. (B) The relative mRNA level of crzA in WT and ΔsltA strains cultured in liquid MM at 37°C for 24 h. Gene expression was normalized to the reference gene tubA, and all results represent data from three independent biological experiments. (C) Western blotting analysis of CrzA-GFP expression levels in WT and ΔsltA strains cultured in liquid MM at 37°C for 24 h. Actin was used as a loading control. The bar graph shows quantification of the signal intensity ratio for CrzA protein to Actin. Data are shown as the means ± SD from three independent experiments. Statistical analysis was performed using one-tailed, unpaired t-tests. *, P < 0.05; ****, P < 0.0001.
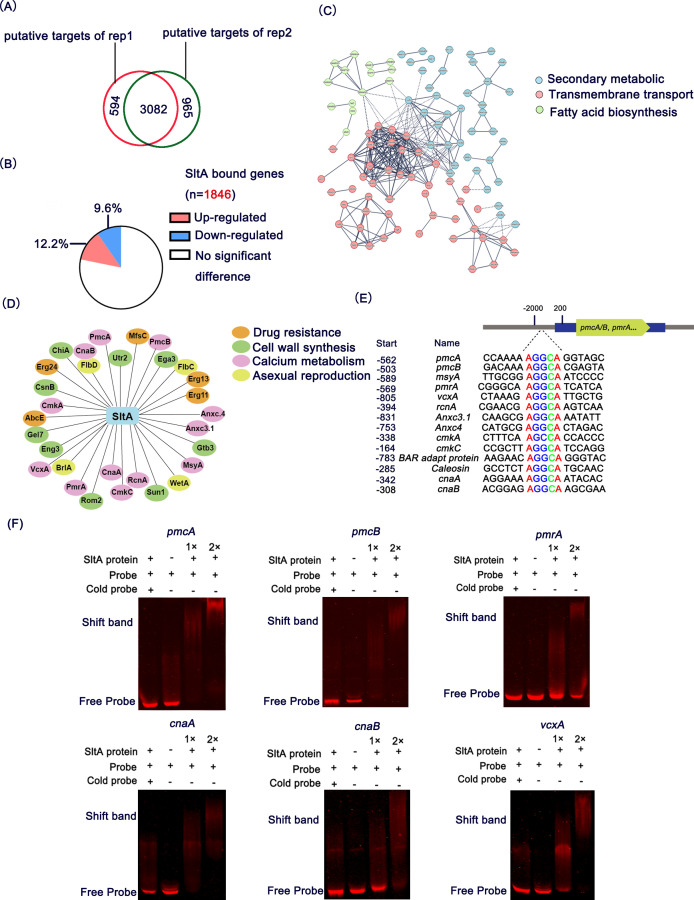
SltA directly binds to genes involved in calcium metabolism
To gain further insight into the genes directly regulated by SltA in A. fumigatus when cultured on MM, we identified SltA-binding sites by chromatin immunoprecipitation coupled to DNA sequencing (ChIP-seq) of a SltA-FLAG-tagged strain. The two biological repeats were performed under MM conditions, and we found 3,676 and 4,047 potential target genes, with 3,082 common targets (q < 0.001, P < 0.001) (Fig. 7A). We further investigated the correlation between SltA occupancy and mRNA levels by comparing the ChIP-seq and RNA-seq data sets. Of the 2,073 differentially expressed genes, 405 were identified as SltA occupancies (Fig. 7B). These results suggest that many of these SltA-dependent genes are likely indirectly regulated by SltA. Next, we combined the target genes of SltA with the A. fumigatus PPI (STRING) database to further understand the overall regulatory function of SltA. Using the highest confidence (0.900) and k-means clustering, we identified 725 nodes and 278 edges. SltA downstream target genes were mainly divided into three clusters: secondary metabolism, transmembrane transport, and fatty acid biosynthesis (Fig. 7C).
FIG 7.
SltA directly binds to genes involved in calcium metabolism. (A) Number of putative SltA target genes identified by two biological replicates of ChIP-seq analyses (q < 0.001, P < 0.001). (B) The pie chart shows the ratio of genes differentially expressed in RNA-seq and genes identified as directly bound by SltA in ChIP-seq analyses. (C) STRING software generated genome-wide regulatory networks of SltA (highest confidence and k-means clustering). (D) Genes with SltA-binding regions in ChIP-seq analyses. Genes involved in drug resistance are shown in orange, genes involved in cell wall synthesis are shown in green, genes involved in calcium metabolism are shown in pink, and genes involved in asexual reproduction are shown in yellow. (E) A conserved “AGGCA” motif is present in the promoters of the indicated genes. The distance between the binding site and the ATG is shown in the start column. (F) EMSA showed that SltA is able to bind to the promoter region of calcium-related genes in vitro.
As expected, some calcium-related genes, such as pmcA, pmcB, vcxA, pmrA, cnaA (AFUB_056900, catalytic A subunit of calcineurin), and cnaB (AFUB_093760, regulatory B subunit of calcineurin) (53), were enriched in the ChIP-seq results, indicating the direct regulation of these genes by SltA (Fig. 7D). In addition, ChIP-seq analyses showed that SltA can directly bind to genes involved in drug resistance, such as erg11 (AFUB_063960), erg24 (AFUB_003560), erg13 (AFUB_080560), abcE (AFUB_087060), and mfsC (AFUB_003610), which is consistent with the previous EMSA results (34). In agreement with the conidiation defect and susceptibilities to multiple cell wall stressors of the ΔsltA mutant (28, 54), ChIP-seq analysis also indicated that several genes involved in asexual development, such as brlA (AFUB_015960), wetA (AFUB_070140), flbC (AFUB_029400), and flbD (AFUB_003630), and cell wall biosynthesis genes, such as rom2 (AFUB_056090), gel7 (AFUB_078410), csnB (AFUB_101780), and sun1 (AFUB_091030), are direct targets of SltA (Fig. 7D).
Previous studies have shown that SltA regulates the expression of its downstream targets, such as SltB (chymotrypsin-like serine protease), Mdr1 (ABC multidrug transporter), and Erg11A (ergosterol biosynthesis), by directly binding to the “AGGCA” motif in the promoter region in A. fumigatus and A. nidulans (22, 34). Most interestingly, many SltA-binding calcium-related genes shared the consensus“AGGCA” sites in their promoters, as shown by MEME software analysis (Fig. 7E). To further investigate whether SltA can directly bind to the predicted “AGGCA” motif, we expressed and purified the DNA-binding domain of SltA for EMSAs. EMSA results confirmed that SltA binds to the promoter regions of pmcA, pmcB, pmrA, vcxA, cnaA, and cnaB through the “AGGCA” motif (Fig. 7F). Excess unlabeled DNA fragments (cold probe) blocked the interaction of SltA with the promoter region, underscoring the specificity of the protein/DNA interaction. Collectively, these results indicated that SltA mediates calcium metabolism via a conserved “AGGCA” motif, and that SltA may also be a direct regulator of the above calcium transporter genes, thereby regulating intracellular calcium homeostasis.
DISCUSSION
In all eukaryotic cells, the cytosolic free calcium ([Ca2+]c) concentration is strictly and precisely controlled in its storage, release, and distribution (55 – 57). The transcription factor CrzA has been extensively studied as a key regulator that mainly functions under calcium-repleted conditions (7). However, the transcription factor responses for calcium-limited conditions remain unclear. In this study, we demonstrated that the transcription factor SltA contributes to A. fumigatus adaptation to calcium-limited conditions.
Previous studies in A. nidulans revealed that transcription factors PacC and SltA are necessary for the growth of A. nidulans at alkaline pH (29). In addition, it has been reported that the transcription factor CrzA, which mainly functions under conditions of sufficient calcium, is involved in the growth of A. nidulans under alkaline pH conditions (29, 58). Those findings suggest a possible link between the pH regulatory pathway and the calcium signaling pathway. In addition, it has been reported that in A. nidulans, the overexpression of vacuolar Ca2+ transporter-encoding genes vcxA, pmcA, and pmcB could be inhibited by SltA, which may avoid excessive vacuolar storage of calcium under calcium-limited conditions (32, 35). However, in this study, double knockout of pmcA and pmcB in the ΔsltA mutant could not rescue the defective phenotypes of the ΔsltA mutant in A. fumigatus. Those results indicate that SltA in A. fumigatus mediates cytosolic calcium homeostasis by regulating multiple targets in addition to vacuolar transporters. As expected, RNA-seq combined with ChIP-seq revealed that SltA regulates the expression of some calcium-related genes, such as pmrA, mucA, cnaA, and cnaB, in addition to pmcA, pmcB, and vcxA, in a direct or indirect manner. Thus, SltA has multiple targeted genes that play roles in regulating the calcium homeostasis of multiple organelles, including the vacuolar, Golgi, and mitochondria. Multiple lines of evidence suggest that SltA acts as a master regulator in the Ca2+ signaling pathway, specifically under calcium-limited conditions. (i) The expression of SltA could be induced under calcium-limited conditions. (ii) Loss of sltA caused a severe growth defect under low-calcium conditions, and the addition of calcium could restore all the defects of the mutant. (iii) Loss of sltA decreased transient cytosolic Ca2+ levels in response to extracellular calcium. (iv) RNA-seq combined with ChIP-seq revealed that SltA governed the expression of a subset of genes involved in calcium metabolism in a direct and indirect manner.
It has previously been reported that the loss of some genes causes persistent nuclear localization of CrzA even under low calcium conditions. For example, the loss of cox10 causes persistent nuclear localization of CrzA, which contributes to drug resistance (59). In addition, the CBC complex HapB could directly bind to the “CCAAT” motif upstream of the crzA promoter, and deletion of hapB increased the expression of crzA as well as CrzA-GFP with constant nuclear localization (60). Loss of both cox10 and hapB led to an increased transient cytoplasmic Ca2+ level, which subsequently activated and translocated CrzA to the nucleus. The loss of sltA also caused CrzA nuclear localization under calcium-limited conditions. However, this effect is not caused by the increased transient cytoplasmic Ca2+ level in the ΔsltA mutant. In fact, loss of sltA decreased transient cytosolic Ca2+ levels in response to extracellular calcium. The loss of sltA caused increased expression of crzA/CrzA under low-calcium conditions in A. fumigatus, but the crzA was not enriched in the SltA-FLAG ChIP-seq analyses. Instead, the genes cnaA and cnaB encoding the crzA upstream phosphatase calcineurin are both direct targets of SltA. In addition, the loss of sltA led to a moderate but significant overexpression of cnaA (1.6-fold, P < 0.01). Given that the translocation of CrzA is dependent on calcineurin, SltA may influence the localization and expression of CrzA mainly through its direct regulatory role on calcineurin. Further studies should confirm the effect of SltA on the activity of calcineurin and the phosphorylation level of CrzA. A possible working model of sltA-mediated calcium homeostasis under calcium-limited conditions in A. fumigatus is presented in Fig. 8.
FIG 8.
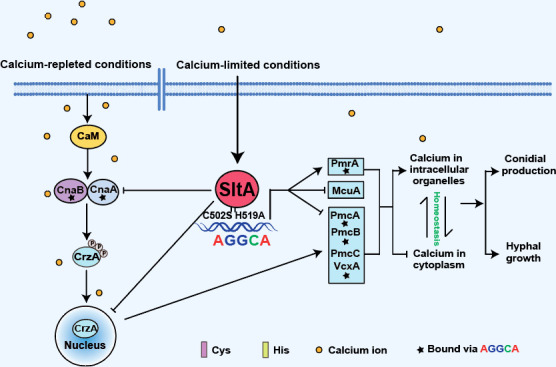
Working model showing how SltA regulates calcium homeostasis under calcium-limited conditions. The transcription factor SltA plays a dual role in maintaining calcium homeostasis under calcium-limited conditions. First, SltA represses the expression and nuclear localization of CrzA, probably by affecting the activity of calcineurin, which turns off the expression of the vacuolar calcium transporter-encoding gene pmcA-C and the vacuolar Ca2+/H+ exchanger-encoding gene vcxA to prohibit the excessive vacuolar storage of calcium. Second, SltA regulates the expression of pmcA, pmcB, vcxA, and the Golgi calcium transporter-encoding gene pmrA by directy binding to the conserved AGGCA motif. The cysteine and histidine within the C2H2 DNA-binding domain of SltA are required for its function. In addition, SltA can regulate the expression of pmcC and the mitochondrial calcium transporter-encoding gene mcuA in an indirect manner. Collectively, SltA enhances hyphal growth and conidial formation of A. fumigatus under calcium-limited conditions by inhibiting the excessive storage of calcium ions in intracellular organelles and maintaining calcium homeostasis in the cytoplasm.
Consistent with the report in A. nidulans, the loss of sltA in A. fumigatus caused a dramatic growth defect when cultured on MM plus 1 M NaCl or 0.6 M KCl. However, the sick colony phenotype in the sltA deletion mutant induced by 1 M NaCl was not a result of decreased expression of enaA in A. fumigatus. Strikingly, findings in this study demonstrated that extracellular calcium is able to rescue all defective phenotypes of the sltA null mutant induced by salt stress, indicating that calcium absorption is tightly related to SltA-mediated regulation of alkali metal ion homeostasis. However, the underlying mechanisms of SltA-mediated salt stress adaptation need to be further explored in A. fumigatus. Moreover, this result suggests a relationship between the calcium signaling pathway and alkali metal cation stress adaptation. This hypothesis was further supported by evidence in A. nidulans, in which the deletion of nhaA (encoding a plasma membrane Na+/H+ antiporter for Na+ and K+ efflux) or trkB (encoding a plasma membrane high affinity K+ transporter) suppressed the calcium auxotrophy of the ΔhalAΔsltA mutant (35). It has been previously reported that the calcium-mediated signaling pathway plays a role in salt tolerance. In yeast, NaCl can induce [Ca2+]cyt transients (61). Yvc1p, a vacuolar membrane protein with homology to transient receptor potential channels, mediates hyperosmolarity-induced calcium ion release from vacuoles. After that, vacuolar calcium ions are replenished through the activity of Vcx1p, a Ca2+/H+ exchanger (62). Collectively, these results indicated that SltA-mediated calcium homeostasis plays important roles in A. fumigatus adaptation to salt stress.
MATERIALS AND METHODS
Strains and media
The strains used in this study are listed in Table 1. In general, A. fumigatus strains were cultured on MM (a calcium-limited condition) (63, 64). For the harsh calcium-limited condition, 1 mM calcium-specific chelator EGTA was added to MM. For calcium repletion, 5 mM calcium ions were added to MM.
TABLE 1.
The list of A. fumigatus strains used in this study
| Strain | Genotype and source | Reference |
|---|---|---|
| A1160 | Δku80, pyrG1 | FGSC a |
| WT (A1161) | A1160, pyr4 | (65) |
| ΔsltA | Δku80, pyrG1, ΔsltA::pyr4 | (34) |
| sltAC502S | Δku80, pyrG1, ΔsltA::pyr4, sltA (P)::sltAC502S::hph | (34) |
| sltAH518A | Δku80, pyrG1, ΔsltA::pyr4, sltA (P)::sltAH518A::hph | (34) |
| sltAC | Δku80, pyrG1, ΔsltA::pyr4, sltA::hph | (34) |
| SltA-FLAG | Δku80, pyrG1, sltA::5 × FLAG::pyr4 | (34) |
| WTAEQ | Δku80, pyrG1, AMA1::PgpdA::Aeq::pyr4 | (66) |
| ΔsltA AEQ | Δku80, pyrG1, ΔsltA::hph, AMA1::PgpdA::Aeq::pyr4 | (34) |
| WTCrzA-GFP | Δku80, pyrG1, crzA::GFP::ptrA | (59) |
| ΔsltAΔcrzA | Δku80, pyrG1, ΔsltA::hph, ΔcrzA::pyr4 | This study |
| ΔcrzA | Δku80, pyrG1, ΔcrzA::pyr4 | This study |
| ΔsltAΔpmcAΔpmcB | Δku80, pyrG1, ΔsltA::pyr4, ΔpmcA::hph, ΔpmcB::ptrA | This study |
| ΔsltA CrzA-GFP | Δku80, pyrG1, crzA::GFP::ptrA, ΔsltA::hph | This study |
| ΔsltB | Δku80, pyrG1, ΔsltB::hph | This study |
| ΔsltB SltA-FLAG | Δku80, pyrG1, SltA::FLAG::pyr4, ΔsltB::hph | This study |
FGSC, Fungal Genetics Stock Center.
Construction of genetic mutant strains
Homologous recombination was used to knock out genes, and pyr4 was used as a marker for the ΔcrzA mutant. First, an approximately 1.0 kb upstream and downstream flanking sequence of crzA was amplified from A1160 genomic DNA using the primers p1/p3 and p2/p6. The pyr4 gene was amplified from plasmid pAL5 using the primers pyr4-F and pyr4-R. Next, the three PCR products were combined to generate the gene knockout cassette with primers p2/p5. Finally, the transformation of A. fumigatus A1160 was performed as described previously (65). The ΔsltB, ΔsltB SltA-FLAG, ΔsltAΔcrzA, and ΔsltAΔpmcAΔpmcB mutants were constructed using the same strategy with pyr4, hph, and ptrA as screening markers, respectively.
For the generation of the ΔsltA CrzA-GFP strain, WTCrzA-GFP was used as a parent strain constructed by the CRISPR system (59), and the GFP-ptrA fusion product with CrzA-GFP-F and CrzA-ptrA-R primers and the synthesized sgRNA were cotransformed into A. fumigatus A1160 with Cas9 expression. Finally, sltA was deleted by the same method as described above, with hph as the screening gene.
For the generation of the ΔsltA AEQ strain, pAMA1-PgpdA-Aeq was transformed into A. fumigatus A1160 with pyr4 as a marker, and then hph was used as a marker to knock out sltA. All of the primers used in this study are listed in Table S1.
Plate assays
Two microliters of conidial suspensions (1 × 107 conidia/mL) of the indicated strains was spotted on the indicated medium plates, grown at 37°C for 5 days, and then observed and imaged. The colony growth diameter and spore production per unit area (The total number of spores produced by the colony divided by the colony area) of the indicated strains were determined in three independent biological experiments.
For the evaluation of the effect of extracellular calcium on the growth phenotype, 5 mM calcium ions and 1 mM EGTA were added to the prepared MM solid medium. The ionic stress condition was established by the addition of 600 mM KCl, 600 mM KCl plus 5 mM Ca2+, 1 M NaCl, and 1 M NaCl plus 5 mM Ca2+ to the minimal medium. The growth conditions were similar to those described above.
Cytoplasmic Ca2+ measurement
The strains expressing aequorin were cultured at 37°C for 2 days, fresh spores were harvested, and 1 × 106 spores were added to 100 µL of MM medium and incubated at 37°C for 24 h. The cytoplasmic Ca2+ concentrations were determined by a previously described method (48, 66). Eight parallel wells were used for each treatment. Each experiment was repeated three times independently.
RNA isolation, RT-qPCR, and RNA-seq
Conidia (1 × 107 spores) from WT, ΔsltA, and ΔsltAΔcrzA strains were cultured in liquid MM or MM plus 5 mM calcium ions at 200 rpm and 37°C for 24 h, and the mycelia were harvested and frozen in liquid nitrogen for RNA extraction. Next, total RNA was extracted and purified as described in the UNlQ-10 column TRIzol total RNA isolation kit (Sangon Biotech; B511361) (60). For RT-qPCR, cDNA was synthesized according to the instructions of HiScript II Q Select RT SuperMix (Vazyme, R233-01). The primers used in this procedure are listed in Table S1. The transcription levels were calculated by the 2−ΔΔCT method and normalized to the mRNA level of the reference gene tubA. For RNA-seq, after RNA extraction, purification, and library construction, the samples were sequenced with next-generation sequencing technology based on the Illumina sequencing platform (Nanjing Personal Gene Technology Co., Ltd.). For each treatment, three independent biological repeats were conducted. Fold change >2 and P < 0.05 were set as the threshold values to select differentially expressed genes.
Fluorescence microscopy analyses
To investigate the localization of CrzA-GFP, we cultured conidia (1 × 105 spores) of WTCrzA-GFP and ΔsltA CrzA-GFP on coverslips with 1 mL of liquid MM at 37°C for 15 h. The hyphae were then treated with 200 mM calcium for 15 min as an extracellular calcium stimulus to induce the CrzA-GFP nuclear location (67). Cultivated cells were then fixed with 4% paraformaldehyde for 30 min at room temperature and washed three times with phosphate-buffered saline (PBS). After that, the nuclear dye Hoechst dissolved in PBS was used at a final concentration of 1 µg/mL and incubated for 30 min at 37°C. The Hoechst dye was removed, and the coverslips were washed three times with 1× PBS buffer. Images were captured using a Zeiss Axio imager A1 microscope (Zeiss, Jena, Germany) (68).
Protein extraction and western blotting
A. fumigatus conidia (1 × 107 spores) were cultured in MM and MM plus 5 mM calcium ions at 37°C and 200 rpm for 24 h. Mycelia were then harvested in liquid nitrogen and ground with lysis buffer (0.2 M NaOH and 0.2% β-mercaptoethanol). Then, 75 µL of 100% (wt/vol) trichloroacetic acid was added to precipitate the protein. After centrifugation at 4°C for 15 min, the supernatant was discarded, and the precipitate was vortexed in 100 µL of 1 M Tris and 100 µL of 2× sodium dodecyl sulfate protein sample buffer to complete dissolution and heated to 95°C (69, 70). The blots were probed with the appropriate primary antibodies: antimouse FLAG (1:5,000, Sigma-Aldrich Co.), antirabbit actin (1:50,000, ABclonal Technology Co.), and antimouse GFP (1:5,000, Sigma-Aldrich Co.) (45). The secondary antibodies were peroxidase-labeled goat antimouse (1:5,000, ABclonal Technology Co.) and goat antirabbit (1:5,000, ABclonal Technology Co.). Finally, images were captured using a Tanon 4200 chemiluminescence imaging system.
ChIP-seq
The strain harboring FLAG-tagged SltA was grown in MM conditions for 24 h and crosslinked by the addition of 1% formaldehyde for 20 min at room temperature with slow shaking. The addition of 2.5 M glycine terminated the experiment after crosslinking, and the supernatant was removed. The mycelia were collected as described above, 50 mL of PBS was added, and the samples were mixed several times. DNA sonication, chromatin immunoprecipitation, DNA purification, and ChIP-seq were performed by Biotech & Consult (Shanghai) Co., Ltd. Peaks were called using Model-based Analysis for ChIP-Seq (MACS2, version 2.2.7.1). A cutoff q-value of 0.001 and a P value of 0.001 were applied to the output data.
Recombinant SltA protein purification and EMSAs
The protein purification steps were performed as described previously (34, 60). EMSAs were performed as previously described with minor modifications (71, 72). Fragments containing the AGGCA sequence in target gene promoter regions were amplified by the primers listed in Table S1. Next, the Cy5 sequence was synthesized with a fluorescently labeled Cy5 probe for the second round of amplification. The basic reaction mixtures consisted of 6 µL of 5× EMSA buffer, 1.5 µL of 1 mg/mL salmon sperm DNA, 50 ng Cy5-labeled probe (double-stranded DNA), and 0.5 µg SltA DNA-binding domain expression protein. The reaction mixtures were incubated at 37°C for 30 min and then separated by a 5% polyacrylamide gel in 1× tris-borate-EDTA buffer. Finally, an Odyssey machine was used to detect the fluorescence signal and generate pictures.
ACKNOWLEDGMENTS
We thank Michael J. Bromley (Faculty of Biology, Medicine and Health, University of Manchester, UK) for kindly providing the A. fumigatus transcription factor deletion library.
This work was financially supported by the National Natural Science Foundation of China (NSFC) (grants 82172292 and 31861133014 to L.L. and 32170040 to S.Z.) and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
M.D., S.Z., and L.L. designed the experiments. M.D. and W.D. performed the experiments. M.D., S.Z., and L.L. analyzed the data and wrote the manuscript.
Contributor Information
Ling Lu, Email: linglu@njnu.edu.cn.
Shizhu Zhang, Email: szzhang@njnu.edu.cn.
Haruyuki Atomi, Kyoto University, Kyoto, Japan .
DATA AVAILABILITY
RNA-seq and ChIP-seq data are deposited in the NCBI Sequence Read Archive under accession numbers PRJNA948373 and PRJNA948579, respectively. This article and its supplementary material provide other relevant information to support the findings of this study.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.01170-23.
The ΔsltA mutant is the most sensitive strain in the A. fumigatus transcription factor deletion library under calcium-limited conditions.
The expression levels of sltA/SltA and the growth phenotypes of the indicated strains.
Relative transcription levels of alkali metal ion transporter genes in WT and ΔsltA mutants under NaCl stress conditions.
Double deletion of pmcA and pmcB in the ΔsltA mutant fails to restore its growth defects under calcium-limited conditions.
Primers used in this study.
RNA-seq analysis of differentially expressed genes.
KEGG enrichment of RNA-seq results.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Abad A, Fernández-Molina JV, Bikandi J, Ramírez A, Margareto J, Sendino J, Hernando FL, Pontón J, Garaizar J, Rementeria A. 2010. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27:155–182. doi: 10.1016/j.riam.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 2. Latgé JP. 1999. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12:310–350. doi: 10.1128/CMR.12.2.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormick A, Loeffler J, Ebel F. 2010. Aspergillus fumigatus: contours of an opportunistic human pathogen. Cell Microbiol 12:1535–1543. doi: 10.1111/j.1462-5822.2010.01517.x [DOI] [PubMed] [Google Scholar]
- 4. Liu Y, Gianinazzi-Pearson V, Arnould C, Wipf D, Zhao B, van Tuinen D. 2013. Fungal genes related to calcium homeostasis and signalling are upregulated in symbiotic arbuscular mycorrhiza interactions. Fungal Biol 117:22–31. doi: 10.1016/j.funbio.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 5. Zheng J, Zeng X, Wang S. 2015. Calcium ion as cellular messenger. Sci China Life Sci 58:1–5. doi: 10.1007/s11427-014-4795-y [DOI] [PubMed] [Google Scholar]
- 6. Bencina M, Bagar T, Lah L, Krasevec N. 2009. A comparative genomic analysis of calcium and proton signaling/homeostasis in Aspergillus species. Fungal Genet Biol 46 Suppl 1:S93–S104. doi: 10.1016/j.fgb.2008.07.019 [DOI] [PubMed] [Google Scholar]
- 7. Espeso EA. 2016. The CRaZy calcium cycle. Adv Exp Med Biol 892:169–186. doi: 10.1007/978-3-319-25304-6_7 [DOI] [PubMed] [Google Scholar]
- 8. Jackson SL, Heath IB. 1993. Roles of calcium ions in hyphal tip growth. Microbiol Rev 57:367–382. doi: 10.1128/mr.57.2.367-382.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Zhang Y, Lu L. 2019. Calcium signaling pathway is involved in non-CYP51 azole resistance in Aspergillus fumigatus. Med Mycol Open Access 57:S233–S238. doi: 10.1093/mmy/myy075 [DOI] [PubMed] [Google Scholar]
- 10. Thewes S. 2014. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot Cell 13:694–705. doi: 10.1128/EC.00038-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cyert MS. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun 311:1143–1150. doi: 10.1016/s0006-291x(03)01552-3 [DOI] [PubMed] [Google Scholar]
- 12. Hernández-Ortiz P, Espeso EA. 2013. Phospho-regulation and nucleocytoplasmic trafficking of CrzA in response to calcium and alkaline-pH stress in Aspergillus nidulans. Mol Microbiol 89:532–551. doi: 10.1111/mmi.12294 [DOI] [PubMed] [Google Scholar]
- 13. Soriani FM, Malavazi I, da Silva Ferreira ME, Savoldi M, Von Zeska Kress MR, de Souza Goldman MH, Loss O, Bignell E, Goldman GH. 2008. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol 67:1274–1291. doi: 10.1111/j.1365-2958.2008.06122.x [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Xie P, Li Y, Bi Y, Prusky DB. 2022. Updating insights into the regulatory mechanisms of calcineurin-activated transcription factor Crz1 in pathogenic fungi. JoF 8:1082. doi: 10.3390/jof8101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta S, Kumar A, Tamuli R. 2022. Crz1 transcription factor is involved in cell survival, stress tolerance, and virulence in fungi. J Biosci 47:66. doi: 10.1007/s12038-022-00294-3 [DOI] [PubMed] [Google Scholar]
- 16. Dinamarco TM, Freitas FZ, Almeida RS, Brown NA, dos Reis TF, Ramalho LNZ, Savoldi M, Goldman MHS, Bertolini MC, Goldman GH. 2012. Functional characterization of an Aspergillus fumigatus calcium transporter (PmcA) that is essential for fungal infection. PLoS One 7:e37591. doi: 10.1371/journal.pone.0037591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soriani FM, Malavazi I, Savoldi M, Espeso E, Dinamarco TM, Bernardes LAS, Ferreira MES, Goldman MHS, Goldman GH. 2010. Identification of possible targets of the Aspergillus fumigatus CRZ1 homologue, CrzA. BMC Microbiol 10:12. doi: 10.1186/1471-2180-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colabardini AC, Wang F, Dong Z, Pardeshi L, Rocha MC, Costa JH, Dos Reis TF, Brown A, Jaber QZ, Fridman M, Fill T, Rokas A, Malavazi I, Wong KH, Goldman GH. 2022. Heterogeneity in the transcriptional response of the human pathogen Aspergillus fumigatus to the antifungal agent caspofungin. Genetics 220:iyab183. doi: 10.1093/genetics/iyab183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Castro PA, Chen C, de Almeida RSC, Freitas FZ, Bertolini MC, Morais ER, Brown NA, Ramalho LNZ, Hagiwara D, Mitchell TK, Goldman GH. 2014. ChIP-seq reveals a role for CrzA in the Aspergillus fumigatus high-osmolarity glycerol response (HOG) signalling pathway. Mol Microbiol 94:655–674. doi: 10.1111/mmi.12785 [DOI] [PubMed] [Google Scholar]
- 20. Shwab EK, Juvvadi PR, Waitt G, Soderblom EJ, Barrington BC, Asfaw YG, Moseley MA, Steinbach WJ. 2019. Calcineurin-dependent dephosphorylation of the transcription factor CrzA at specific sites controls conidiation, stress tolerance, and virulence of Aspergillus fumigatus. Mol Microbiol 112:62–80. doi: 10.1111/mmi.14254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernández-Ortiz P, Espeso EA. 2017. Spatiotemporal dynamics of the calcineurin target CrzA. Cell Signal 29:168–180. doi: 10.1016/j.cellsig.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 22. Mellado L, Calcagno-Pizarelli AM, Lockington RA, Cortese MS, Kelly JM, Arst HN, Espeso EA. 2015. A second component of the SltA-dependent cation tolerance pathway in Aspergillus nidulans. Fungal Genet Biol 82:116–128. doi: 10.1016/j.fgb.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clement DJ, Attwell NA, Stanley MS, Clipson NJ, Hooley P, Fincham DA. 1995. Salt sensitivity and arginine toxicity in Aspergillus nidulans. Biochem Soc Trans 23:24S. doi: 10.1042/bst023024s [DOI] [PubMed] [Google Scholar]
- 24. Markina-Iñarrairaegui A, Spielvogel A, Etxebeste O, Ugalde U, Espeso EA. 2020. Tolerance to alkaline ambient pH in Aspergillus nidulans depends on the activity of ENA proteins. Sci Rep 10:14325. doi: 10.1038/s41598-020-71297-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Villarino M, Etxebeste O, Mendizabal G, Garzia A, Ugalde U, Espeso EA. 2017. Boron tolerance in Aspergillus nidulans is sustained by the SltA pathway through the SLC-family transporters SbtA and SbtB. Genes (Basel) 8:188. doi: 10.3390/genes8070188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calcagno-Pizarelli AM, Hervás-Aguilar A, Galindo A, Abenza JF, Peñalva MA, Arst HN Jr. 2011. Rescue of Aspergillus nidulans severely debilitating null mutations in ESCRT-0, I, II and III genes by inactivation of a salt-tolerance pathway allows examination of ESCRT gene roles in pH signalling. J Cell Sci 124:4064–4076. doi: 10.1242/jcs.088344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Neil JD, Bugno M, Stanley MS, Barham-Morris JB, Woodcock NA, Clement DJ, Clipson NJW, Whitehead MP, Fincham DA, Hooley P. 2002. Cloning of a novel gene encoding a C2H2 zinc finger protein that alleviates sensitivity to abiotic stresses in Aspergillus nidulans. Mycol Res 106:491–498. doi: 10.1017/S0953756202005701 [DOI] [Google Scholar]
- 28. Shantappa S, Dhingra S, Hernández-Ortiz P, Espeso EA, Calvo AM. 2013. Role of the zinc finger transcription factor SltA in morphogenesis and sterigmatocystin biosynthesis in the fungus Aspergillus nidulans. PLoS One 8:e68492. doi: 10.1371/journal.pone.0068492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Picazo I, Etxebeste O, Requena E, Garzia A, Espeso EA. 2020. Defining the transcriptional responses of Aspergillus nidulans to cation/alkaline pH stress and the role of the transcription factor SltA. Microb Genom 6:mgen000415. doi: 10.1099/mgen.0.000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dubey AK, Barad S, Luria N, Kumar D, Espeso EA, Prusky DB. 2016. Cation-stress-responsive transcription factors SltA and CrzA regulate morphogenetic processes and pathogenicity of Colletotrichum gloeosporioides. PLoS One 11:e0168561. doi: 10.1371/journal.pone.0168561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saloheimo A, Aro N, Ilmén M, Penttilä M. 2000. Isolation of the ace1 gene encoding a Cys2-His2 transcription factor involved in regulation of activity of the cellulase promoter cbh1 of Trichoderma reesei. J Biol Chem 275:5817–5825. doi: 10.1074/jbc.275.8.5817 [DOI] [PubMed] [Google Scholar]
- 32. Spielvogel A, Findon H, Arst HN, Araújo-Bazán L, Hernández-Ortíz P, Stahl U, Meyer V, Espeso EA. 2008. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochem J 414:419–429. doi: 10.1042/BJ20080344 [DOI] [PubMed] [Google Scholar]
- 33. Liu H, Xu W, Bruno VM, Phan QT, Solis NV, Woolford CA, Ehrlich RL, Shetty AC, McCraken C, Lin J, Bromley MJ, Mitchell AP, Filler SG, Cramer RA. 2021. Determining Aspergillus fumigatus transcription factor expression and function during invasion of the mammalian lung. PLoS Pathog 17:e1009235. doi: 10.1371/journal.ppat.1009235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du W, Zhai P, Wang T, Bromley MJ, Zhang Y, Lu L. 2021. The C2H2 transcription factor SltA contributes to azole resistance by coregulating the expression of the drug target Erg11A and the drug efflux pump Mdr1 in Aspergillus fumigatus. Antimicrob Agents Chemother 65:e01839-20. doi: 10.1128/AAC.01839-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Findon H, Calcagno-Pizarelli A-M, Martínez JL, Spielvogel A, Markina-Iñarrairaegui A, Indrakumar T, Ramos J, Peñalva MA, Espeso EA, Arst HN. 2010. Analysis of a novel calcium auxotrophy in Aspergillus nidulans. Fungal Genet Biol 47:647–655. doi: 10.1016/j.fgb.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mellado L, Arst HN, Espeso EA. 2016. Proteolytic activation of both components of the cation stress-responsive Slt pathway in Aspergillus nidulans. Mol Biol Cell 27:2598–2612. doi: 10.1091/mbc.E16-01-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furukawa T, van Rhijn N, Fraczek M, Gsaller F, Davies E, Carr P, Gago S, Fortune-Grant R, Rahman S, Gilsenan JM, Houlder E, Kowalski CH, Raj S, Paul S, Cook P, Parker JE, Kelly S, Cramer RA, Latgé J-P, Moye-Rowley S, Bignell E, Bowyer P, Bromley MJ. 2020. The negative cofactor 2 complex is a key regulator of drug resistance in Aspergillus fumigatus. Nat Commun 11:427. doi: 10.1038/s41467-019-14191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caracuel Z, Casanova C, Roncero MIG, Di Pietro A, Ramos J. 2003. pH response transcription factor PacC controls salt stress tolerance and expression of the P-Type Na+ -ATPase Ena1 in Fusarium oxysporum. Eukaryot Cell 2:1246–1252. doi: 10.1128/EC.2.6.1246-1252.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. da Silva LG, Martins MP, Sanches PR, Peres N de A, Martinez-Rossi NM, Rossi A. 2020. Saline stress affects the pH-dependent regulation of the transcription factor PacC in the dermatophyte Trichophyton interdigitale. Braz J Microbiol 51:1585–1591. doi: 10.1007/s42770-020-00313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller AJ, Vogg G, Sanders D. 1990. Cytosolic calcium homeostasis in fungi: roles of plasma membrane transport and intracellular sequestration of calcium. Proc Natl Acad Sci U S A 87:9348–9352. doi: 10.1073/pnas.87.23.9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cyert MS, Philpott CC. 2013. Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193:677–713. doi: 10.1534/genetics.112.147207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaber RF, Styles CA, Fink GR. 1988. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol 8:2848–2859. doi: 10.1128/mcb.8.7.2848-2859.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ariño J, Ramos J, Sychrová H. 2010. Alkali metal cation transport and homeostasis in yeasts. Microbiol Mol Biol Rev 74:95–120. doi: 10.1128/MMBR.00042-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cagnac O, Aranda-Sicilia MN, Leterrier M, Rodriguez-Rosales MP, Venema K. 2010. Vacuolar cation/H+ antiporters of Saccharomyces cerevisiae. J Biol Chem 285:33914–33922. doi: 10.1074/jbc.M110.116590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cunningham KW, Fink GR. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16:2226–2237. doi: 10.1128/MCB.16.5.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prior C, Potier S, Souciet JL, Sychrova H. 1996. Characterization of the NHA1 gene encoding a Na+/H+-antiporter of the yeast Saccharomyces cerevisiae. FEBS Lett 387:89–93. doi: 10.1016/0014-5793(96)00470-x [DOI] [PubMed] [Google Scholar]
- 47. Greene V, Cao H, Schanne FAX, Bartelt DC. 2002. Oxidative stress-induced calcium signalling in Aspergillus nidulans. Cell Signal 14:437–443. doi: 10.1016/s0898-6568(01)00266-2 [DOI] [PubMed] [Google Scholar]
- 48. Nelson G, Kozlova-Zwinderman O, Collis AJ, Knight MR, Fincham JRS, Stanger CP, Renwick A, Hessing JGM, Punt PJ, van den Hondel CAMJJ, Read ND. 2004. Calcium measurement in living filamentous fungi expressing codon-optimized aequorin. Mol Microbiol 52:1437–1450. doi: 10.1111/j.1365-2958.2004.04066.x [DOI] [PubMed] [Google Scholar]
- 49. Song J, Liu X, Zhai P, Huang J, Lu L. 2016. A putative mitochondrial calcium uniporter in A. fumigatus contributes to mitochondrial Ca2+ homeostasis and stress responses. Fungal Genet Biol 94:15–22. doi: 10.1016/j.fgb.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 50. Pinchai N, Juvvadi PR, Fortwendel JR, Perfect BZ, Rogg LE, Asfaw YG, Steinbach WJ. 2010. The Aspergillus fumigatus P-type Golgi apparatus Ca2+/Mn2+ ATPase PmrA is involved in cation homeostasis and cell wall integrity but is not essential for pathogenesis. Eukaryot Cell 9:472–476. doi: 10.1128/EC.00378-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Castro PA, Colabardini AC, Manfiolli AO, Chiaratto J, Silva LP, Mattos EC, Palmisano G, Almeida F, Persinoti GF, Ries LNA, Mellado L, Rocha MC, Bromley M, Silva RN, de Souza GS, Loures FV, Malavazi I, Brown NA, Goldman GH. 2019. Aspergillus fumigatus calcium-responsive transcription factors regulate cell wall architecture promoting stress tolerance, virulence and caspofungin resistance. PLoS Genet 15:e1008551. doi: 10.1371/journal.pgen.1008551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ries LNA, Rocha MC, de Castro PA, Silva-Rocha R, Silva RN, Freitas FZ, de Assis LJ, Bertolini MC, Malavazi I, Goldman GH, Alspaugh JA. 2017. The Aspergillus fumigatus CrzA transcription factor activates chitin synthase gene expression during the caspofungin paradoxical effect. mBio 8:e00705-17. doi: 10.1128/mBio.00705-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fox DS, Heitman J. 2002. Good fungi gone bad: the corruption of calcineurin. Bioessays 24:894–903. doi: 10.1002/bies.10157 [DOI] [PubMed] [Google Scholar]
- 54. Liu Z, Raj S, van Rhijn N, Fraczek M, Michel J-P, Sismeiro O, Legendre R, Varet H, Fontaine T, Bromley M, Latgé J-P, Alspaugh JA. 2021. Functional genomic and biochemical analysis reveals pleiotropic effect of congo red on Aspergillus fumigatus. mBio 12:e00863-21. doi: 10.1128/mBio.00863-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fischer M, Schnell N, Chattaway J, Davies P, Dixon G, Sanders D. 1997. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett 419:259–262. doi: 10.1016/s0014-5793(97)01466-x [DOI] [PubMed] [Google Scholar]
- 56. Pitt D, Barnes JC. 1993. Calcium homeostasis, signalling and protein phosphorylation during calcium-induced conidiation in Penicillium notatum. J Gen Microbiol 139:3053–3063. doi: 10.1099/00221287-139-12-3053 [DOI] [PubMed] [Google Scholar]
- 57. Cui J, Kaandorp JA, Sloot PMA, Lloyd CM, Filatov MV. 2009. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res 9:1137–1147. doi: 10.1111/j.1567-1364.2009.00552.x [DOI] [PubMed] [Google Scholar]
- 58. Loss O, Bertuzzi M, Yan Y, Fedorova N, McCann BL, Armstrong-James D, Espeso EA, Read ND, Nierman WC, Bignell EM. 2017. Mutual independence of alkaline- and calcium-mediated signalling in Aspergillus fumigatus refutes the existence of a conserved druggable signalling nexus. Mol Microbiol 106:861–875. doi: 10.1111/mmi.13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li Y, Zhang Y, Zhang C, Wang H, Wei X, Chen P, Lu L. 2020. Mitochondrial dysfunctions trigger the calcium signaling-dependent fungal multidrug resistance. Proc Natl Acad Sci U S A 117:1711–1721. doi: 10.1073/pnas.1911560116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ren Y, Zhang C, Chen Z, Lu L, Fischer R. 2021. The heterotrimeric transcription factor CCAAT-binding complex and Ca2+-CrzA signaling reversely regulate the transition between fungal hyphal growth and asexual reproduction. mBio 12:e0300721. doi: 10.1128/mBio.03007-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Serrano R, Rodriguez-Navarro A. 2001. Ion homeostasis during salt stress in plants. Curr Opin Cell Biol 13:399–404. doi: 10.1016/s0955-0674(00)00227-1 [DOI] [PubMed] [Google Scholar]
- 62. Denis V, Cyert MS. 2002. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol 156:29–34. doi: 10.1083/jcb.200111004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AWJ. 1953. The genetics of Aspergillus nidulans. Adv Genet 5:141–238. doi: 10.1016/s0065-2660(08)60408-3 [DOI] [PubMed] [Google Scholar]
- 64. Zhang C, Lu L. 2017. Precise and efficient in-frame integration of an exogenous GFP tag in Aspergillus fumigatus by a CRISPR system, p 249–258. In Vaccines for invasive fungal infections. doi: 10.1007/978-1-4939-7104-6 [DOI] [PubMed] [Google Scholar]
- 65. Jiang H, Shen Y, Liu W, Lu L. 2014. Deletion of the putative stretch-activated ion channel Mid1 is hypervirulent in Aspergillus fumigatus. Fungal Genet Biol 62:62–70. doi: 10.1016/j.fgb.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 66. Zhang C, Ren Y, Gu H, Gao L, Zhang Y, Lu L. 2021. Calcineurin-mediated intracellular organelle calcium homeostasis is required for the survival of fungal pathogens upon extracellular calcium stimuli. Virulence 12:1091–1110. doi: 10.1080/21505594.2021.1909954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhai P, Ma Y, Du W, Lu L. 2022. The metal chaperone protein MtmA plays important roles in antifungal drug susceptibility in Aspergillus fumigatus. Front Microbiol 13:1062282. doi: 10.3389/fmicb.2022.1062282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou X, Ye J, Zheng L, Jiang P, Lu L. 2019. A new identified suppressor of Cdc7p/SepH kinase, PomA, regulates fungal asexual reproduction via affecting phosphorylation of MAPK-HogA. PLoS Genet 15:e1008206. doi: 10.1371/journal.pgen.1008206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Wang Y, Fan J, Zhu G, Lu L. 2022. Aspergillus fumigatus elongator complex subunit 3 affects hyphal growth, adhesion and virulence through wobble uridine tRNA modification. PLoS Pathog 18:e1010976. doi: 10.1371/journal.ppat.1010976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang Y, Fan J, Ye J, Lu L. 2021. The fungal-specific histone acetyltransferase Rtt109 regulates development, DNA damage response, and virulence in Aspergillus fumigatus. Mol Microbiol 115:1191–1206. doi: 10.1111/mmi.14665 [DOI] [PubMed] [Google Scholar]
- 71. Long N, Orasch T, Zhang S, Gao L, Xu X, Hortschansky P, Ye J, Zhang F, Xu K, Gsaller F, Straßburger M, Binder U, Heinekamp T, Brakhage AA, Haas H, Lu L, Freitag M. 2018. The Zn2Cys6-type transcription factor LeuB cross-links regulation of leucine biosynthesis and iron acquisition in Aspergillus fumigatus. PLoS Genet 14:e1007762. doi: 10.1371/journal.pgen.1007762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang W, Hong S, Tang G, Lu Y, Wang C. 2019. Unveiling the function and regulation control of the DUF3129 family proteins in fungal infection of hosts. Philos Trans R Soc Lond B Biol Sci 374:20180321. doi: 10.1098/rstb.2018.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The ΔsltA mutant is the most sensitive strain in the A. fumigatus transcription factor deletion library under calcium-limited conditions.
The expression levels of sltA/SltA and the growth phenotypes of the indicated strains.
Relative transcription levels of alkali metal ion transporter genes in WT and ΔsltA mutants under NaCl stress conditions.
Double deletion of pmcA and pmcB in the ΔsltA mutant fails to restore its growth defects under calcium-limited conditions.
Primers used in this study.
RNA-seq analysis of differentially expressed genes.
KEGG enrichment of RNA-seq results.
Data Availability Statement
RNA-seq and ChIP-seq data are deposited in the NCBI Sequence Read Archive under accession numbers PRJNA948373 and PRJNA948579, respectively. This article and its supplementary material provide other relevant information to support the findings of this study.