Abstract
The pharmacokinetics of tigecycline, when given as a 100-mg loading dose followed by 50 mg every 12 h, were determined in serum and blister fluid. The peak tigecycline concentration and half-life in serum were greater than those in blister fluid. Tigecycline penetrates into blister fluid well, with a mean penetration rate of 74%.
Tigecycline is the first of the glycylcyclines, a novel class of antimicrobials structurally related to the tetracyclines, to undergo clinical development (19). Tigecycline is an expanded broad-spectrum antibiotic with activity against gram-negative, gram-positive, anaerobic, and atypical pathogens. It shows in vitro activity against tetracycline-resistant organisms containing genes responsible for efflux or ribosomal protection resistance mechanisms, as well as other resistant pathogens, such as methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci (1, 2, 5, 6, 12).
Limited pharmacokinetic data are available for tigecycline, and no studies evaluating tigecycline's ability to penetrate the skin have been published. In phase 2 studies, tigecycline has demonstrated good clinical efficacy in the treatment of skin and skin structure infections (17).
In the context of skin and soft tissue infections, the evaluation of drug concentrations by using the cantharidin-induced skin blister model mimics situations within an infected tissue (13). Previous studies using this model have been successfully performed at the Center for Anti-Infective Research and Development, Hartford Hospital (14). The purpose of this study was to determine the steady-state pharmacokinetic profile of tigecycline in serum and blister fluid when tigecycline is administered intravenously over 30 min as a 100-mg loading dose followed by 50 mg every 12 h for a total of seven doses.
This study was approved by Hartford Hospital's Institutional Review Board. All subjects were given a detailed description of the study, and all provided written informed consent. Ten healthy subjects were enrolled in this single-center, multiple-dose, open-label study. The subjects were 20 to 37 years of age (mean age, 26.7 years), 172 to 185 cm in height (mean height, 177.3 cm), and 69.5 to 89.1 kg in weight (mean weight, 80.1 kg). Participation included a screening evaluation within 3 weeks of tigecycline administration on day 1 and a 6-day (5-night) inpatient period. Subjects were enrolled after the screening evaluation, and laboratory evaluations (including hematologies, blood chemistries, and urinalyses) and electrocardiograms revealed no clinically significant abnormalities.
Each subject received a loading dose of 100 mg of tigecycline (Wyeth Pharmaceuticals, Collegeville, Pa.) infused over 30 min on study day 1, followed by 50 mg infused over 30 min every 12 h, for a total of seven doses. Subjects were served medium-fat meals approximately 30 min before tigecycline administration. Fluids were allowed ad libitum. Consumption of any caffeine-containing products, grapefruit, grapefruit-containing products, or alcoholic beverages was not permitted.
Approximately 14 h before the start of the final dose of tigecycline, 5 drops (0.2 ml each) of 0.25% cantharidin ointment made from cantharidin powder (Sigma-Aldrich Laboratories, St. Louis, Mo.) and standard ointment base were applied to the anterior forearms of the subjects to produce a total of five blisters per subject. The blisters were sprayed with a fast-drying plastic dressing (New-Skin liquid bandage spray; Medtech Laboratories, Inc., Jackson, Wyo.) in order to maintain their integrity. The sixth and final blister was induced by using the method described above approximately 14 h before the hour 24 sample collection time point.
Blood samples were collected from an indwelling catheter in blood collection tubes containing no anticoagulant. Blood sampling was performed just before tigecycline administration (time zero) and at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after the start of the final infusion. Blood samples were immediately placed on ice until they clotted and were then centrifuged at 4°C at 1,000 × g for 10 min. Serum samples were then collected and stored at −80°C until they were shipped for analysis. Blister fluid samples (100 to 200 μl) were collected by using a 28-gauge needle at simultaneous time points, with the exception of hour 0, and were stored at −80°C until they were shipped for analysis.
Concentrations of tigecycline in serum and skin blister fluid were determined by validated liquid chromatography methods with tandem mass spectrometer (LC-MS-MS) detection. Analyses were performed by BioAssay Laboratory, Inc. (Houston, Tex.).
Briefly, a 0.2-ml aliquot of serum sample was mixed with 0.60 ml of internal standard working solution (150 ng of [tert-butyl-d9] tigecycline/ml). After vortexing and centrifugation, the supernatant was transferred to a set of clean culture tubes and evaporated to dryness under a gentle stream of air. The residue was reconstituted in 200 μl of mobile phase, and a 10-μl aliquot was injected onto the API 3000 LC-MS-MS system. The calibration curves ranged from 10 to 2,000 ng/ml, and each assay run contained independent quality control (QC) samples at 25, 200, and 1,500 ng/ml. During the assays of the serum samples from this study, the mean accuracies (percent bias) and precisions (coefficients of variation) of the QC samples were +8.52% and 5.38% for the 25-ng/ml QC samples, +5.50% and 2.18% for the 200-ng/ml QC samples, and +4.29% and 3.97% for the 1,500-ng/ml QC samples. Similarly, a 40-μl aliquot of blister fluid sample was mixed with 0.30 ml of internal standard working solution (150 ng/ml of [tert-butyl-d9] tigecycline). After vortexing and centrifugation, the supernatant was transferred to a set of clean culture tubes and evaporated to dryness under a gentle stream of air. The residue was reconstituted in 100 μl of mobile phase, and a 7-μl aliquot was injected onto the API 3000 LC-MS-MS system. The calibration curves ranged from 10 to 2,000 ng/ml, and each assay run contained independent QC samples at 25, 200, and 1,500 ng/ml. During the assays of the blister fluid samples, the mean accuracies and precisions were −12.60% and 1.44% for the 25-ng/ml QC samples, −7.02% and 4.73% for the 200-ng/ml QC samples, and +5.36% and 4.14% for the 1,500-ng/ml QC samples.
Noncompartmental pharmacokinetic methods were used to analyze the concentrations of tigecycline in serum and blister fluid for each subject (11). The following pharmacokinetic parameters were determined by using a validated SAS macro: the maximum concentration of tigecycline in serum or blister fluid (Cmax), the time at which the maximum concentration was reached (Tmax), the area under the tigecycline serum or blister fluid concentration-time curve from 0 to 12 h (AUC0-12), and the elimination half-life (t1/2). The penetration of tigecycline into blister fluid was determined by comparing the AUC0-12 in blister fluid with that in serum.
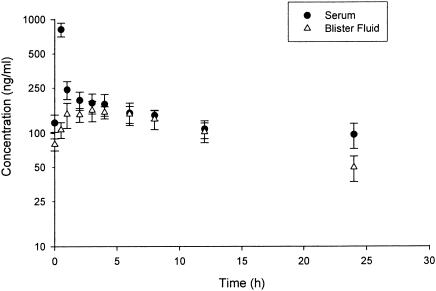
The mean concentration-time profile of serum and blister fluid for the 10 subjects is illustrated in Fig. 1. The pharmacokinetic parameters for tigecycline in serum and blister fluid for each individual subject are displayed in Table 1. All parameters described below are reported as means ± standard deviations.
FIG. 1.
Mean (± standard deviation [error bars]) steady-state tigecycline concentrations in serum and blister fluid, versus time, after administration of a 100-mg loading dose followed by 50 mg infused over 30 min every 12 h.
TABLE 1.
Pharmacokinetic parameters of tigecycline in serum and blister fluid
| Subject no. | Value for tigecycline in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Serum
|
Blister fluid
|
|||||||
| Tmax (h) | Cmax (ng/ml) | AUC0-12 (ng · h/ml) | t1/2 (h)a | Tmax (h) | Cmax (ng/ml) | AUC0-12 (ng · h/ml) | t1/2 (h) | |
| 1 | 0.5 | 804 | 2,408 | ND | 6.0 | 1,157 | 1,649 | 9.5 |
| 2 | 0.5 | 853 | 2,552 | 47.3 | 3.0 | 194 | 1,932 | 12.6 |
| 3 | 0.5 | 864 | 2,356 | 60.5 | 6.0 | 183 | 1,766 | 13.6 |
| 4 | 0.5 | 928 | 2,243 | 45.4 | 1.0 | 223 | 1,731 | 9.7 |
| 5 | 0.5 | 967 | 2,073 | 56.6 | 4.0 | 146 | 1,396 | 9.9 |
| 6 | 0.5 | 771 | 2,617 | ND | 3.0 | 201 | 1,823 | 11.7 |
| 7 | 0.5 | 716 | 1,786 | 26.2 | 0.5 | 129 | 1,296 | 16.8 |
| 8 | 0.5 | 674 | 1,819 | 27.2 | 2.0 | 162 | 1,330 | 9.0 |
| 9 | 0.5 | 954 | 2,268 | ND | 1.0 | 177 | 1,583 | 14.2 |
| 10 | 0.5 | 662 | 1,730 | 51.0 | 1.0 | 160 | 1,584 | 10.7 |
| Mean | 0.5 | 819 | 2,185 | 44.9 | 2.8 | 273 | 1,609 | 11.8 |
| SD | 0.0 | 113 | 320 | 13.5 | 2.0 | 312 | 214 | 2.5 |
ND, not determined in individual subject because of insufficient data points.
The tigecycline Cmax in serum was 819.4 ± 112.6 ng/ml, the mean tigecycline t1/2 in serum was 44.9 ± 13.5 h, and the mean tigecycline AUC0-12 in serum was 2,185 ± 320 ng · h/ml. The tigecycline Cmax and Tmax in blister fluid were 273 ± 312 ng/ml and 2.8 ± 2.0 h, respectively. The tigecycline t1/2 in blister fluid was 11.8 ± 2.5 h, and the tigecycline AUC0-12 in blister fluid was 1,609 ± 214 ng · h/ml. By 4 h after dose administration, the tigecycline concentrations in serum and blister fluid were equivalent and remained so until hour 12. The ratio of the AUC0-12 for tigecycline in blister to that in serum was individually calculated for each subject. The mean percent penetration of tigecycline into blister fluid was 74% (±7%).
No clinically important changes in laboratory test results, vital signs, electrocardiograms, or physical examinations were noted during the study. There were no deaths or serious adverse events. The most frequently reported adverse events were nausea (nine subjects), vomiting (four subjects), and dyspepsia (three subjects). All resolved and all were mild, with the exception of nausea of moderate severity experienced by two subjects. No patient withdrew from this study due to adverse events (all 10 patients completed the study).
Three subjects experienced transient elevations in alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase levels. These elevations all returned to normal levels within the study period.
Antimicrobial resistance is spreading globally at an alarming rate (8, 18). Therefore, the continued development of new antimicrobial agents with the ability to overcome current resistance mechanisms is essential. Tigecycline is the first member of the glycylcycline class to be developed for clinical use. It displays potent in vitro activity against gram-positive and certain gram-negative pathogens, with MICs at which 90% of strains are inhibited (MIC90s) being ≤0.5 μg/ml for S. aureus (including methicillin-resistant S. aureus) and S. pneumoniae (including penicillin-resistant S. pneumoniae) (7, 9, 15, 16). Furthermore, it displays activity against those pathogens resistant to tetracyclines (7, 19).
Skin and soft tissue infections are often primarily caused by gram-positive pathogens, such as S. aureus, Streptococcus pyogenes, and Streptococcus agalactiae (3, 4, 10). Tigecycline exhibits excellent in vitro activity against these pathogens. MIC90s for S. pyogenes and S. agalactiae are 0.6 μg/ml and 0.06 μg/ml, respectively (15). According to the SENTRY Antimicrobial Surveillance Program, S. aureus is the most prevalent pathogen causing skin and soft tissue infections in hospitalized patients (18), followed by Pseudomonas aeruginosa and Enterococcus spp. Although tigecycline displays less activity against P. aeruginosa, it possesses potent activity against Enterococcus spp., with MIC90s for Enterococcus faecalis and Enterococcus faecium of 0.5 μg/ml and 0.25 μg/ml, respectively (18).
These pharmacokinetic data indicate a considerable difference in half-life between tigecycline in serum and that in blister fluid. In this study, each subject received six blisters, each of which was sampled no more than two times. For the 24-h sample, the blister for each subject was punctured only once. This scheme was used in an attempt to minimize the influence of sampling on the subsequent refilling and/or accumulation within the blister. While a clear explanation for this observed difference is not presently available, the apparently faster elimination of tigecycline from the blister may represent a more rapid redistribution from this peripheral compartment.
Protein binding of tigecycline, as measured in blood, is approximately 68% in humans (19). Protein binding in blister fluid was not measured in this study; thus, the influence of protein binding on the disposition of tigecycline in this fluid is currently unknown. Theoretically, if only free drug can penetrate across the skin blister interface, then the concentrations in the blister fluid may more appropriately represent the bioactive free-drug concentration-time profile of this agent.
The elimination half-lives in sera from three subjects were not calculated because of insufficient data in the terminal elimination phase of the pharmacokinetic profile. A limitation in the calculation of the half-life itself must also be considered. Because the observation time in this study was relatively short compared with the long half-life, our estimate may not be truly accurate. However, the mean estimated half-life (44.5 h) does approximate that which has been reported in another human pharmacokinetic study (G. Muralidharan, J. Getsy, P. Mayer, I. Paty, M. Micalizzi, J. Speth, B. Wester, and P. Mojaverian, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 416, 1999). The short observation time also brings to question whether the subjects were truly at steady state when pharmacokinetic sampling occurred. Based on the serum half-lives found in this study, it may take approximately 5 days to reach steady state without the utilization of a bolus dose. In the present study, a bolus dose was incorporated into the dosing scheme, and when the mean concentration values among the 10 subjects at 0 and 12 h were compared, no significant difference (P = 0.123) was observed, thus confirming that steady state was achieved prior to the blood and blister fluid samplings.
This study was the first to investigate tigecycline's pharmacokinetics and penetration into skin blister fluid. Treatment for skin and soft tissue infections can be problematic if the antibiotic does not achieve adequate concentrations at the site of infection. Our study showed that tigecycline penetrates into blister fluid well (74% penetration) when given as a 100-mg loading dose followed by 50 mg administered every 12 h.
Although phase 2 clinical studies evaluating tigecycline's efficacy and safety in the treatment of skin and skin structure infections and intra-abdominal infection have been published (17; J. Murray, S. Wilson, S. Klein, A. Yellin, and E. Loh, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., L-739, 2003), further phase 3 clinical studies must be performed to further establish tigecycline's role in the treatment of skin and soft tissue infections.
REFERENCES
- 1.Betriu, C., I. Rodriguez-Avial, B. A. Sanchez, M. Gomez, J. Alvarez, J. J. Picazo, and Spanish Group of Tigecycline. 2002. In vitro activities of tigecycline (GAR-936) against recently isolated clinical bacteria in Spain. Antimicrob. Agents Chemother. 46:892-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biedenbach, D. J., M. L. Beach, and R. N. Jones. 2001. In vitro antimicrobial activity of GAR-936 tested against antibiotic-resistant gram-positive blood stream infection isolates and strains producing extended-spectrum beta-lactamases. Diagn. Microbiol. Infect. Dis. 40:173-177. [DOI] [PubMed] [Google Scholar]
- 3.Eron, L. J., B. A. Lipsky, D. E. Low, D. Nathwani, A. D. Tice, and G. A. Volturo. 2003. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J. Antimicrob. Chemother. 52(Suppl. 1):3-17. [DOI] [PubMed] [Google Scholar]
- 4.File, T. M., Jr., and J. S. Tan. 1995. Treatment of skin and soft-tissue infections. Am. J. Surg. 169:27S-33S. [PubMed] [Google Scholar]
- 5.Gales, A. C., and R. N. Jones. 2000. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn. Microbiol. Infect. Dis. 36:19-36. [DOI] [PubMed] [Google Scholar]
- 6.Gales, A. C., J. B. Silva, S. S. Andrade, H. S. Sader, and R. N. Jones. 2003. In vitro activity of the tigecycline, a new glycylcycline, tested against 1,326 clinical bacterial strains isolated from the Latin American region. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1537, p. 207.
- 7.Guay, D. R. P. 2004. Oritavancin and tigecycline: investigational antimicrobials for multidrug-resistant bacteria. Pharmacotherapy 24:58-68. [DOI] [PubMed] [Google Scholar]
- 8.Hoban, D., K. Waites, and D. Felmingham. 2003. Antimicrobial susceptibility of community-acquired respiratory tract pathogens in North America in 1999-2000: findings of the PROTEKT surveillance study. Diagn. Microbiol. Infect. Dis. 45:251-259. [DOI] [PubMed] [Google Scholar]
- 9.Hoellman, D. B., G. A. Pankuch, M. R. Jacobs, and P. C. Appelbaum. 2000. Antipneumococcal activities of GAR-936 (a new glycylcycline) compared to those of nine other agents against penicillin-susceptible and -resistant pneumococci. Antimicrob. Agents Chemother. 44:1085-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, M. E., J. A. Karlowsky, D. C. Draghi, C. Thornsberry, D. F. Sahm, and D. Nathwani. 2003. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int. J. Antimicrob. Agents 22:406-419. [DOI] [PubMed] [Google Scholar]
- 11.Jusko, W. J. 1992. Guidelines for collection and analysis of pharmacokinetic data, p. 21-43. In W. E. Evans, J. J. Schentag, and W. J. Jusko (ed.), Applied pharmacokinetics: principles of therapeutic drug monitoring, 3rd ed. Applied Therapeutics, Vancouver, Wash.
- 12.Labthavikul, P., P. J. Petersen, and P. A. Bradford. 2003. In vitro activity of tigecycline against Staphylococcus epidermidis growing in an adherent-cell biofilm model. Antimicrob. Agents Chemother. 47:3967-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maglio, D., C. H. Nightingale, and D. P. Nicolau. 2003. Production and resolution of cantharidin-induced inflammatory blisters. Int. J. Antimicrob. Agents 22:77-80. [DOI] [PubMed] [Google Scholar]
- 14.Maglio, D., R. Teng, P. T. Thyrum, C. H. Nightingale, and D. P. Nicolau. 2003. Pharmacokinetic profile of meropenem, administered at 500 milligrams every 8 hours, in plasma and cantharidin-induced skin blister fluid. Antimicrob. Agents Chemother. 47:1771-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milatovic, D., F.-J. Schmitz, J. Verhoef, and A. C. Fluit. 2003. Activities of the glycylcycline tigecycline (GAR-936) against 1,924 recent European clinical bacterial isolates. Antimicrob. Agents Chemother. 47:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen, P. J., P. A. Bradford, W. J. Weiss, T. M. Murphy, P. E. Sum, and S. J. Projan. 2002. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob. Agents Chemother. 46:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postier, R. G., S. L. Green, S. R. Klein, E. J. Ellis-Grosse, E. Loh, and the Tigecycline 200 Study Group. 2004. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin. Ther. 26:704-714. [DOI] [PubMed] [Google Scholar]
- 18.Rennie, R. P., R. N. Jones, and A. H. Mutnick. 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn. Microb. Infect. Dis. 45:287-293. [DOI] [PubMed] [Google Scholar]
- 19.Zhanel, G. G., K. Homenuik, K. Nichol, A. Noreddin, L. Vercaigne, J. Embil, A. Gin, J. A. Karlowsky, and D. J. Hoban. 2004. The glycylcyclines: a comparative review with the tetracyclines. Drugs 64:63-88. [DOI] [PubMed] [Google Scholar]