Abstract
Clinical treatment failures have been reported to occur in early Lyme borreliosis (LB) for many suitable antimicrobial agents. Investigations of possible resistance mechanisms of the Borrelia burgdorferi complex must analyze clinical isolates obtained from LB patients, despite their receiving antibiotic treatment. Here, borrelial isolates obtained from five patients with erythema migrans (EM) before the start of antibiotic therapy and again after the conclusion of treatment were investigated. The 10 isolates were characterized by restriction fragment length polymorphism analysis and plasmid profile analysis and subjected to susceptibility testing against a variety of antimicrobial agents including those used for initial chemotherapy. Four out of five patients were infected by the same genospecies (Borrelia afzelii, n = 3; Borrelia garinii, n = 1) at the site of the EM lesion before and after antimicrobial therapy. In one patient the genospecies of the initial isolate (B. afzelii) differed from that of the follow-up isolate (B. garinii). No significant changes in the in vitro susceptibilities became obvious for corresponding clinical isolates before the start and after the conclusion of antimicrobial therapy. This holds true for the antimicrobial agents used for specific chemotherapy of the patients, as well as for any of the additional agents tested in vitro. Our study substantiates borrelial persistence in some EM patients at the site of the infectious lesion despite antibiotic treatment over a reasonable time period. Borrelial persistence, however, was not caused by increasing MICs or minimal borreliacidal concentrations in these isolates. Therefore, resistance mechanisms other than acquired resistance to antimicrobial agents should be considered in patients with LB resistant to treatment.
Human Lyme borreliosis (LB) represents a multisystem disorder caused by the Borrelia burgdorferi complex (36). In much of Europe, LB does not constitute a notifiable disease, but incidence estimations range between 3.9 and 137/100,000 inhabitants/year (23, 35). In Slovenia, the incidence was 168/100,000 in 2002 (National Notifiable Communicable Diseases Surveillance System, Slovenia, unpublished data). Erythema migrans (EM), which develops at the site of the tick bite, occurs in 77 to 90% of LB patients (4, 8). Antimicrobial treatment of early LB manifestations such as EM is commonly successful in >90% of cases (8, 34). However, similar to failures of chemotherapy for Treponema pallidum in syphilis (24), clinical treatment failures have been reported to occur in early LB cases for almost every suitable antimicrobial agent (10, 12, 28, 38, 42). Furthermore, the currently available diagnostic techniques do not reliably discriminate among possible reinfection, true endogenous relapse, and coinfection with other tick-borne pathogens (12). These drawbacks together with the phenomenon of resistance to therapy in individual patients undoubtedly contribute to the inconsistencies surrounding the optimal treatment regimens for LB and are often misinterpreted and misused to support prolonged antibiotic treatment regimens. However, relatively few cases of culture-proven treatment failure have been published (19, 22, 28, 29, 37, 38, 39), and the underlying mechanisms of antimicrobial resistance in B. burgdorferi sensu lato remain unresolved. The overall culture detection rate of the pathogen in clinical specimens obtained from cutaneous lesions does not usually exceed 40 to 70% of cases under routine laboratory conditions (1, 19, 32, 38, 44). The culture-positive rate falls to <1 and 20% in cases with Lyme arthritis and neuroborreliosis, respectively. Unfortunately, culture is rarely successful after antimicrobial therapy is initiated (18, 21). Despite this challenge, investigations that explore possible resistance mechanisms in B. burgdorferi sensu lato must focus on isolates obtained from patients receiving antibiotic therapy. Here, we examined the in vitro susceptibility and molecular biology of B. burgdorferi sensu lato isolates cultured from skin biopsy samples of EM patients before and after antimicrobial chemotherapy to explore whether the persistence of the LB spirochete may be caused by increasing acquired antibiotic resistance.
(This study was part of the CAPSTONE project of K.-P.H. performed in partial fulfillment of the requirements for his Master of Public Health degree.)
MATERIALS AND METHODS
Clinical information and primary culture of clinical isolates.
Between 1995 and 2000 a total of 3,421 patients >18 years of age were diagnosed clinically with typical EM by experienced physicians at the LB Outpatients' Clinic, Department of Infectious Diseases, University Medical Centre, Ljubljana, Slovenia, according to slightly modified Centers for Disease Control and Prevention criteria as outlined by Arnez et al. (2). Most of these individuals were enrolled in prospective studies on the assessment of clinical and microbiological efficacy of treatment with different antimicrobial agents. The study protocols included initial biopsy of EM at first visit before the institution of antibiotic therapy and a second biopsy at the same anatomic site approximately 2 months (range, 1.3 to 3 months) later as reported elsewhere (39, 40). Biopsy samples were taken under sterile conditions and immediately cultured in modified Barbour-Stoenner-Kelly (BSK) medium at 33°C for 9 weeks as described previously (27). Weekly subcultures were inoculated into fresh modified BSK medium and were examined by dark-field microscopy. The overall recovery rate for B. burgdorferi sensu lato in the initial skin biopsy samples from these patients was 50%. In 19 out of 1,148 (1.7%) skin-culture-positive EM patients diagnosed during the 6-year-period spirochetes could be cultured not only from the site of EM before the start of empirical antibiotic therapy but again after the conclusion of treatment from the original site of the lesion. Stock cultures of 10 clinical isolates obtained from five immunocompetent patients out of these 19 individuals were still available for analysis in this in vitro study. None of the patients reported a second tick bite, and all declared having taken their medication as prescribed at the first visit. No clinical signs of treatment resistance were obvious in these patients at the time of second biopsy. The 10 isolates were then subjected to further molecular typing and to detailed susceptibility testing. Information on available clinical and laboratory data for the patients is summarized in Table 1.
TABLE 1.
Clinical information and laboratory data for five patients with EM and culture-confirmed persistent B. burgdorferi sensu lato infection after conclusion of antimicrobial chemotherapya
| Characteristic | Clinical data for EM patient:
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Clinical information | |||||
| Sex | F | F | F | F | M |
| Age (yrs) | 43 | 38 | 53 | 68 | 36 |
| Symptom(s) at first visit | MEM | EM | EM | EM, BL, PFP | EM, BL |
| Tick bite remembered | Yes | Yes | No | No | Yes |
| Time (days) between tick bite and onset of EM | 10 | 26 | NK | NK | 19 |
| No. of EM lesions | 2 | 1 | 1 | 1 | 1 |
| Size of EM (cm) | 7 by 12; 6 by 8 | 10 by 16 | 7 by 11 | 9 by 12 | 7 by 12 |
| Systemic complaint(s)b | No | No | Yes | No | No |
| Time (days) between onset of symptoms and treatment | 1 | 11 | 5 | 7 | 4 |
| Initial treatment | Ceftriaxone, 1 dose, 2 g, i.v., 14 days | Amoxicillin, 3 doses, 500 mg, p.o., 14 days | Cefuroxime, 2 doses, 500 mg, p.o., 14 days | Cefuroxime, 2 doses, 500 mg, p.o., 14 days | Azithromycin, 2 doses, 500 mg, p.o., 1 day; 1 dose, 500 mg, p.o., 4 days |
| Duration of EM (days) after initiation of treatment | 4 | 12 | 14 | 10 | 3 |
| Laboratory data | |||||
| IgM-IFT (titerc) | 256 | 128 | Neg. | Neg. | Neg. |
| IgG-IFT (titerc) | 128 | 128 | Neg. | Neg. | Neg. |
| Time (wks) between first biopsy and positive culture | 4 | 4 | 4 | 4 | 2 |
| Genospecies cultured after first biopsy | B. afzelii | B. garinii | B. afzelii | B. afzelii | B. afzelii |
| Time (days) between start of treatment and second biopsy | 40 | 39 | 55 | 56 | 69 |
| Time (wks) between second biopsy and positive culture | 4 | 5 | 2.5 | 4 | 6 |
| Genospecies cultured after second biopsy | B. afzelii | B. garinii | B. afzelii | B. afzelii | B. garinii |
Abbreviations: F, female; M, male; MEM, multiple EM; BL, borrelial lymphocytoma; PFP, peripheral facial palsy; NK, not known; i.v., intravenously; p.o., orally; Neg., negative; IFT, indirect fluorescent antibody test; IgM, immunoglobulin M; IgG, immunoglobulin G.
Fever, headache, and/or myalgia.
No significant titer change was noted at the second biopsy.
Subculture procedures.
Stock cultures of the B. burgdorferi sensu lato isolates analyzed in the present in vitro study previously had been stored at −80°C. Cultures of these isolates then were further propagated in modified BSK medium as follows. Aliquots of 1.5 ml of the stock were resuspended in modified BSK medium, and subcultures were repeatedly incubated at 33°C for 5 days until conventional cell counts indicated an increase of live borreliae up to 108/ml. Further propagation of subcultures was performed by inoculation of 0.5 to 1 ml of a late-log-phase culture into 10 ml of fresh BSK medium. All molecular typing experiments and in vitro susceptibility tests utilized isolates that had not been processed for more than 10 passages from the stock. All subcultures were monitored for vitality of spirochetes and possible contamination by conventional dark-field microscopy.
Genotyping of borrelial isolates.
The clinical isolates were genotyped by two independent methods. First, pulsed-field gel electrophoresis (PFGE) was applied as the “gold standard” in combination with genomic DNA restriction fragment length polymorphism (RFLP) analysis as previously described (26) with some modification. Briefly, DNA of the borrelial isolates cast in agarose plugs was digested overnight at 37°C by incubation in restriction buffer containing 40 U of MluI. The DNA fragments were then separated in a 1% agarose gel by applying a voltage of 6 V/cm at a pulse time ramped from 1 to 10 s for 22 h with a contour-clamped homogeneous electric field model CHEF-DRII apparatus (Bio-Rad Laboratories). Bands were visualized by use of a UV light gel imaging system (Cybertech). Lambda concatemers (New England Biolabs) with a monomer size of 48.5 kb were used as a standard for exact size determination. Genospecies designation of the isolates was carried out after analysis of species-specific RFLP patterns according to the work of Belfaiza et al. (3).
Second, restriction pattern analysis of the rrfA-rrlB spacer region of the clinical isolates was performed after PCR amplification as described by Postic et al. (26). The rrfA-rrlB spacer region was amplified using primers BB5S (5′-CTGCGAGTTCGCGGGAGA-3′) and BB23S (5′-TCCTAGGCATTCACCATA-3′). Each PCR mixture (100 μl) contained 1 μl of sample DNA, 20 mM Tris-HCl (pH 8.4), 1.5 mM MgCl2, 50 mM KCl, each of four deoxynucleoside triphosphates at a concentration of 100 μM, 2.5 U of Taq DNA polymerase (Invitrogen), and 100 pmol of primers BB5S and BB23S. Amplification reactions were carried out for 30 cycles with an amplification profile of denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 2 min, followed by a final extension step at 72°C for 7 min on a GeneAmp PCR System 2400 (Perkin-Elmer). Negative controls (water blanks) were also included in the experiment. PCR products (10 μl) were digested with 5 U of MseI (New England Biolabs) in a total volume of 20 μl. The resulting species-specific PCR-RFLP patterns were analyzed by electrophoresis on a 12% polyacrylamide gel stained with ethidium bromide, and the fragment size was determined by comparison to DNA fragments of a pBR322 molecular weight standard digested with MspI (New England Biolabs) and a 123-bp marker (Gibco-BRL Life Technologies). Genospecies identification of the isolates was performed according to the work of Postic et al. (26). Reference strains B31 (B. burgdorferi sensu stricto ATCC 35210), PBi (Borrelia garinii), and VS461 (Borrelia afzelii) were included for quality control purposes.
Plasmid profile analysis.
Plasmid profile analysis was performed on passage 4 of all isolates as described by Xu and Johnson (43). Purified plasmid DNA was loaded on a 1% agarose gel and run by applying a voltage of 6 V/cm and a pulse time ramped from 0.9 to 2.5 s for 26 h with a CHEF-DRII apparatus (Bio-Rad Laboratories). To determine the plasmid sizes, low-range and MidRange I PFG markers (Biolabs) were used as molecular weight standards. The gels were stained as outlined above and photographed, and the relative sizes of plasmid bands were calculated by Wincam gel styler software version 1.0 (Cybertech, Berlin, Germany).
Broth microdilution susceptibility testing.
Borrelia stock cultures were cultured in modified BSK medium at 33°C until log phase of growth and adjusted to 2.5 × 107 borreliae/ml as determined by enumeration with a Kova counting chamber (Hycor, Garden Grove, Calif.) in combination with dark-field microscopy. Final concentrations of the lyophilized antibiotics were reconstituted by adding 200 μl of the final inoculum suspension (5 × 106 cells/well) in BSK medium containing phenol red (25 μg/ml) as a growth indicator. Cells were cultured at 33°C in 5% CO2 (17). The antimicrobial substances and test ranges appear in Table 2. Ceftriaxone and doxycycline served as control substances, and strain B31 (ATCC 35210) served as control organism in order that our data could be related to our recent publications on the in vitro susceptibility of borreliae (11-14, 17).
TABLE 2.
Antibiotic susceptibilities (MICs and MBCs in micrograms per milliliter) of clinical B. burgdorferi sensu lato isolates obtained before and after antimicrobial chemotherapy and reference strain B31 to seven antimicrobial agents as determined in BSK mediuma
| Patient and isolateb | Drug (test range [μg/ml])
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythromycin (0.0002-0.5)
|
Azithromycin (0.0002-0.5)
|
Telithromycin (0.0002-0.5)
|
Amoxicillin (0.0078-16)
|
Cefuroxime (0.0156-16)
|
Ceftriaxone (0.0078-16)
|
Doxycycline (0.125-8)
|
||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Patient 1 | ||||||||||||||
| 1A | 0.0156 | >0.5 | 0.0019 | 0.125 | ≤0.0002 | 0.0312 | 0.25 | 4 | 0.0625 | 16 | 0.0156 | 0.25 | ≤0.125 | 1 |
| 1B | 0.0078 | >0.5 | 0.0009 | 0.0625 | ≤0.0002 | 0.0312 | 0.25 | 8 | 0.0625 | 16 | 0.0312 | 0.5 | ≤0.125 | 0.5 |
| Patient 2 | ||||||||||||||
| 2A | 0.0039 | 0.5 | 0.0004 | 0.0312 | ≤0.0002 | 0.0039 | 0.0312 | 0.5 | 0.0625 | 8 | 0.0156 | 0.5 | ≤0.125 | 0.25 |
| 2B | 0.0019 | 0.5 | 0.0004 | 0.0156 | ≤0.0002 | 0.0019 | 0.0625 | 1 | 0.0312 | 8 | 0.0156 | 0.5 | 0.25 | 0.25 |
| Patient 3 | ||||||||||||||
| 3A | 0.0078 | 0.5 | 0.0009 | 0.0156 | ≤0.0002 | 0.0312 | 1 | 8 | 0.0625 | 8 | 0.0156 | 1 | 0.25 | 0.5 |
| 3B | 0.0078 | 0.5 | 0.0019 | 0.0156 | ≤0.0002 | 0.0625 | 0.5 | 4 | 0.125 | 16 | 0.0312 | 1 | ≤0.125 | 0.25 |
| Patient 4 | ||||||||||||||
| 4A | 0.0019 | 0.125 | 0.0004 | 0.0312 | ≤0.0002 | 0.0312 | 0.5 | 4 | 0.0625 | 8 | 0.0156 | 0.25 | ≤0.125 | 0.5 |
| 4B | 0.0019 | 0.062 | 0.0004 | 0.0312 | ≤0.0002 | 0.0156 | 0.5 | 8 | 0.0625 | 8 | 0.0156 | 0.25 | ≤0.125 | 0.5 |
| Patient 5 | ||||||||||||||
| 5A | 0.0156 | >0.5 | 0.0039 | 0.125 | 0.0004 | 0.0312 | 0.0312 | 2 | 0.0312 | 8 | 0.0625 | 0.5 | ≤0.125 | 0.25 |
| 5B | 0.0078 | >0.5 | 0.0019 | 0.125 | 0.0009 | 0.0312 | 0.0625 | 4 | 0.0625 | 16 | 0.0312 | 0.25 | ≤0.125 | 0.5 |
| Range | 0.0019-0.0156 | 0.062->0.5 | ≤0.0002-0.0039 | 0.0156-0.125 | ≤0.0002-0.0009 | 0.0019-0.0625 | 0.0156-2 | 0.25-16 | 0.0312-0.125 | 8->16 | 0.0078-0.0625 | 0.125-2 | ≤0.125-0.25 | 0.125-1 |
| Reference strain B31 (ATCC 35210)c | 0.0156 | >0.5 | 0.0019 | 0.125 | ≤0.0002 | 0.06 | 0.5 | 8 | 0.125 | 16 | 0.0625 | 1 | 0.25 | 0.5 |
Antimicrobial susceptibility was determined on three different days, and the MIC and MBC for each isolate are reported as the median of three experiments. Boldface indicates the initial antibiotic treatment regimen.
In isolate designations, A refers to the initial borrelial isolate and B refers to the borrelial isolate obtained after the conclusion of therapy.
Determination of MICs.
For quantification of bacterial growth we applied kinetic measurement of indicator color shift at 562 and 630 nm by use of a commercially available enzyme-linked immunosorbent assay reader (PowerWave 200; Bio-Tec Instruments) in combination with a calculation program (Microwin 3.0; Microtek) at 0, 24, 48, and 72 h of incubation. Growth of samples and controls finally was determined by decrease of absorbance after 72 h (Et72) in comparison to the initial values (Et0). The well was reported negative for growth if Et72 > (Et0 − 10%) (17). Colorimetric MICs were reported as the medians of three experiments performed on different days.
Determination of MBCs.
Following 72 h of incubation with the antibiotic, aliquots (20 μl) were taken from all vials without growth and were diluted 1:1,000-fold with BSK medium below the MIC. Subcultures were incubated at 33°C in 5% CO2 for an additional 3 weeks (14). After gentle agitation, 5 to 10 high-power fields were then examined by dark-field microscopy for the presence or absence of spirochetes. The minimal borreliacidal concentration (MBC) was defined as the lowest concentration of the antimicrobial where no spirochetes could be detected (100% killing) after 3 weeks of subculture (14, 17). MBCs were reported as the medians of three experiments performed on different days (Table 1).
Statistical analysis.
To detect possible differences in MIC and MBC data of the different genospecies, the Kruskal-Wallis test was applied using Primer of Biostatistics software, version 5.0 (The McGraw-Hill Companies), for statistical calculation.
RESULTS
Identification of genospecies.
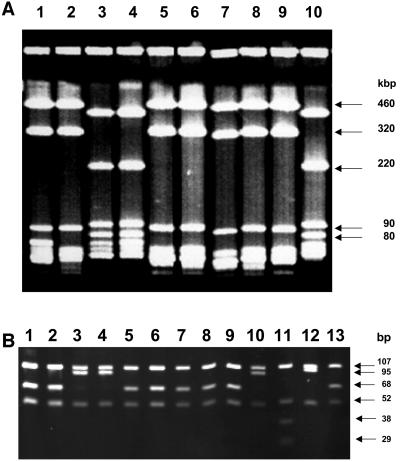
The results of genomic DNA RFLP analysis and the PCR-RFLP analysis of the rrfA-rrlB spacer region of the clinical isolates are depicted in Fig. 1. Three out of five patients (Table 1, patients 1, 3, and 4) were infected by B. afzelii at the site of the EM lesion before and after antimicrobial therapy as shown by the presence of species-specific 460-, 320-, and 90-kb bands in PFGE-RFLP analysis (Fig. 1, top). In patient 2 B. garinii was cultured from the first skin biopsy sample and from the follow-up specimen as demonstrated by the characteristic 220- and 80-kb bands. Interestingly, B. afzelii was cultured from the primary skin biopsy sample from patient 5 followed by growth of B. garinii from the follow-up specimen sampled from the initial site of the EM lesion after conclusion of antimicrobial chemotherapy (Fig. 1, top). These results were confirmed by the genospecies determination of the clinical isolates with the use of highly specific PCR-RFLP analysis of the rrfA-rrlB spacer region after digestion with MseI (Fig. 1, bottom). Isolates of patients 1, 3, and 4 and the initial isolate of patient 5 were identified as B. afzelii by generation of fragment sizes of 107, 68, and 50 bp. B. garinii-specific fragment sizes of 107, 95, and 50 bp were recovered from the first and second isolates of patient 2 and the second isolate of patient 5.
FIG. 1.
(Top) RFLP of genomic DNA of clinical B. burgdorferi sensu lato isolates from Slovenia after MluI digestion and separation by PFGE. DNA was electrophoresed on a 1% acrylamide gel, stained with ethidium bromide, and UV illuminated. Restriction patterns of lanes 1, 2, and 5 to 9 show an RFLP pattern characteristic for B. afzelii. The restriction patterns of lanes 3, 4, and 10 are typical for B. garinii. The molecular sizes of the characteristic DNA fragments are depicted to the right. Lanes 1 and 2, 3 and 4, 5 and 6, 7 and 8, and 9 and 10 show pairs of isolates obtained from patients 1 to 5, respectively. Isolates within each pair are designated A (before treatment) and B (after treatment) as depicted in Table 2. Patient designations correspond to clinical information given in Table 1. (Bottom) MseI restriction polymorphism of the amplified rrf-rrl spacer region of the same clinical isolates. DNA was electrophoresed on a 12% acrylamide gel. Restriction patterns of lanes 1, 2, 5 to 9, and 13 show fragments of 107, 68, and 50 bp characteristic for B. afzelii. Restriction patterns of lanes 3, 4, 10, and 12 show fragments of 107, 95, and 50 bp typical for B. garinii. Lane 11 shows fragments of 107, 52, 38, and 29 bp characteristic for B. burgdorferi sensu stricto. The molecular sizes of DNA fragments are depicted to the right. Lanes 1 and 2, 3 and 4, 5 and 6, 7 and 8, and 9 and 10 show pairs of isolates obtained from patients 1 to 5, respectively. Within each pair, isolates are designated A (before treatment) and B (after treatment) as depicted in Table 2. Lanes 11 to 13 show B. burgdorferi sensu stricto strain B31, B. garinii strain PBi, and B. afzelii strain VS461, respectively. Patient designations correspond to clinical information given in Table 1.
Plasmid profile analysis.
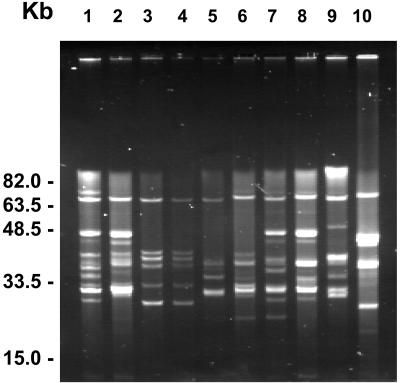
The plasmid profiles of the 10 clinical isolates as determined by PFGE are depicted in Fig. 2. The number of plasmids present in each isolate varied from 6 to 11, and the plasmid size ranged from approximately 5 to 62 kb. The majority of plasmids were in the 30- to 39-kb size range (n = 26), followed by the 20- to 29-kb range (n = 20), and only one plasmid was in the 50- to 59-kb range. The average number of plasmids per strain was higher for the B. afzelii isolates (7.7; range, 6 to 11) than for the B. garinii isolates, all of which contained six plasmids. The plasmid pattern of the B. afzelii and B. garinii isolates obtained before and after treatment from patient 5 revealed major differences (Fig. 2, lanes 9 and 10) and therefore paralleled the results of the DNA RFLP analysis and PCR-RFLP of the rrfA-rrlB spacer region in these isolates (see above). The B. garinii isolates obtained from patient 2 before and after therapy showed exactly the same plasmid pattern (Fig. 2, lanes 3 and 4). In the remaining patients (1, 3, and 5) the plasmid pattern appeared closely related for each pair of strains. However, the number and size of plasmids varied in the isolates obtained before and after antimicrobial chemotherapy (Fig. 2, lanes 1 and 2, 5 and 6, and 7 and 8).
FIG. 2.
Plasmid profiles of B. burgdorferi sensu lato isolates as determined by PFGE. Plasmid profiles are of genospecies B. garinii (lanes 1, 2, and 5 to 9) and B. afzelii (lanes 3, 4, and 10). Plasmids of 32 and 21 kb occur with high frequency in B. garinii; plasmids of 36, 27, 26, 25, and 24 kb occur with high frequency in B. afzelii. A scale of molecular DNA sizes as determined by use of low-range and MidRange I PFG markers (New England Biolabs) is depicted to the left. Lanes 1 and 2, 3 and 4, 5 and 6, 7 and 8, and 9 and 10 show pairs of isolates from patients 1 to 5, respectively. Within each pair, isolates are designated A (before treatment) and B (after treatment) as depicted in Table 2. Patient designations correspond to clinical information as given in Table 1.
MIC and MBC determination.
The individual in vitro susceptibilities of the 10 clinical isolates to seven commonly used antimicrobial agents including the drugs used for initial treatment of the patients with EM are summarized in Table 2. Clearly, there was some variability in the individual MICs and MBCs of the various antimicrobial agents belonging to the classes macrolides, β-lactams, and tetracyclines for the different pairs of isolates. Erythromycin and amoxicillin revealed the largest amount of interstrain variability, with MICs and MBCs varying over an 8- to 100-fold range for the different isolates, respectively. Overall, B. garinii isolates tended to be more susceptible than B. afzelii isolates, for which in part β-lactam agents showed higher MICs and MBCs. For amoxicillin, MICs and MBCs were significantly higher for the B. afzelii isolates than for the B. garinii isolates (P < 0.05). After parallel examinations on three different days, however, no significant differences, i.e., no increase or decrease for ≥2 log2 unit dilutions in the median MICs and MBCs, became obvious for corresponding clinical isolates obtained from the same patient before the start and after the conclusion of antimicrobial therapy for EM. This holds true for the antimicrobial agents that have been used in vivo for specific chemotherapy in the patients with EM, as well as for any of the additional substances that were tested in vitro throughout our study. For all antimicrobial agents except amoxicillin and doxycycline the MIC at which 90% of the isolates tested were inhibited was found to be ≤0.0625 μg/ml even though the MBCs as determined under very restrictive conditions (100% killing) were higher (Table 2).When determined on three different days, the MICs and MBCs of the antibiotics tested for the same isolate spanned a maximum range of only 1 log2-unit dilution around the median, thereby indicating high reproducibility. For the reference strain B31 ATCC 35210, the MIC and MBC ranges of the drugs tested (Table 2) were within the ranges specified for these antimicrobial agents in our recent publications by use of our assay described for in vitro susceptibility testing of B. burgdorferi sensu lato under controlled test conditions (11, 13, 14, 17).
DISCUSSION
Despite the presence of well-conducted and compelling trials (2, 10, 16, 25, 34, 39, 42), considerable controversy remains on optimal chemotherapy of patients with LB (12). Clinically, treatment failures occur in 5 to 10% of EM patients (12, 34). Macrolides fail more often than β-lactam agents and tetracyclines do (9). Here, the existence of persistent borrelial infection is further substantiated in seropositive and seronegative EM patients (Table 1) despite highly active antimicrobial chemotherapy. As the number of individuals with persistent infection after treatment accounts for only 1.7% of culture-positive EM patients, we think that bacterial persistence is a rare phenomenon that is probably the exception rather than the rule. However, the rate of persistent infections might be underestimated due to the limits of culture sensitivity in these patients. In the context of the ongoing discussion on the possible misuse of antibiotics in so-called post-LB syndrome (16), LB patients suffering from protracted nonspecific complaints clearly should be distinguished from cases with culture-confirmed relapse or persistent borrelia infection, which, although infrequent, can occur and may warrant further treatment (19, 22, 28, 29, 38, 39). The clinical data presented here and the results of our molecular typing clearly substantiate that all but one patient with a positive follow-up culture remained persistently infected with the same borrelial genospecies at the same peripheral location for several weeks despite antibiotic treatment. Our observations corroborate with the findings of Bockenstedt et al., which could demonstrate borrelial persistence in the mouse model by use of xenodiagnosis in 4 out of 10 animals up to 3 months after prolonged therapy with doxycycline and ceftriaxone (5). In our study, the plasmid pattern also differed in three out of four pairs of isolates belonging to the same genospecies cultured from the corresponding patient's EM site before and after chemotherapy. This observation requires further investigation but is more likely to result from adaptation of borrelial clones during persistent infection rather than from reinfection of the same body site due to a second and unobserved tick bite within 5 to 10 weeks. Vector-borne pathogens have evolved to adapt and persist in their various hosts (5). Similarly, such adaptation during antibiotic chemotherapy may rapidly result in selection of clonal subtypes of the same borrelial genospecies. Our findings in antibiotically treated EM patients indeed suggest that the population of spirochetes detected after chemotherapy may genetically differ from the initial bacterial population initiating the infection. This observation is in accordance with recent findings that survival of infectious borrelial isolates in antibiotically treated mice is correlated with genetic recombination and diminished levels or complete loss of lp25 and lp28-1, plasmids that are known to carry genes which are important for the infectiousness of borreliae (5). Such attenuated residual borreliae were no longer infectious when transmitted to new mammalian hosts (5). In our strains, we did not test for a potential loss of infectiousness, but our patients did not present with clinical signs of treatment failure or relapse. Similarly, the persistence of group A streptococci and Chlamydia spp. after chemotherapy of infection is not necessarily equivalent to clinical treatment failure (15, 41). In patient 2, however, the plasmid pattern of the two subsequent B. garinii isolates did not change at all despite antibiotic treatment (Fig. 2). Therefore, survival of small numbers of bacteria may result in persisting clinical complaints in some patients. Further investigations are clearly warranted to elucidate effects of potential changes in the infectiousness of persisting borreliae on the clinical course of human LB treated with antimicrobial agents.
In Europe, coinfections with more than one borrelial genospecies have been well documented in molecular epidemiological studies of patients with EM (30, 31). In one of our EM patients (patient 5, Table 1) B. afzelii was cultured from the primary skin biopsy sample, followed by growth of B. garinii from the follow-up specimen after conclusion of antimicrobial chemotherapy (Fig. 1, top). Although these findings could result from a possible double or concomitant infection with B. garinii and B. afzelii following the initial infectious tick bite, a second tick bite that went unnoted cannot be excluded based on our clinical and molecular biological findings. In Europe, human coinfections may be more frequent than previously believed, as 45% of Ixodes ricinus ticks have been shown to be infected with more than one borrelial genospecies (20).
To date, neither MIC definitions, test conditions, nor the inocula for the in vitro susceptibility testing of B. burgdorferi sensu lato are standardized (7, 12). Previous studies (6, 7) and our own experience (11-14, 17), however, clearly indicate that a microdilution method with BSK medium and incubation for 72 h holds promise for standardization of antimicrobial susceptibility testing of borreliae. To relate our study data to our recent publications on the in vitro susceptibility of borreliae (11-14, 17), we used our approach for the susceptibility testing of newly cultured clinical isolates from patients with EM. There is speculation whether the number of passages may influence the susceptibility of isolates tested in vitro (7). A careful analysis carried out by Dever et al., however, did not reveal significant differences in the in vitro susceptibilities of low- and high-passage-number B31 and HB19 strains for penicillin and ceftriaxone after 9 years of continuous passage (7). Instead, the MICs were within 3 log2 unit dilutions, i.e., within the anticipated precision of an assay that uses serial log2 dilutions (7, 17). Although low-passage-number strains may grow more slowly than high-passage-number strains (7), a maximum of 10 passages in vitro is unlikely to have significantly influenced the test results obtained for the isolates of this study. B. burgdorferi sensu lato isolates may vary in respect to their in vitro susceptibilities to some antimicrobial agents on the genospecies level (11, 17, 29, 33). Correspondingly, there was a trend towards lower MICs and MBCs of several antimicrobial agents for the B. garinii isolates compared to the B. afzelii isolates tested in our study (Table 2). As shown in this study and demonstrated earlier by Preac-Mursic et al. (29), isolates can also differ in their individual susceptibilities to various antimicrobial agents. These minor differences, however, are of no clinical relevance, as they commonly do not exceed the critical concentrations for these substances to become ineffective and therefore cannot explain survival of spirochetes during prolonged effective antibiotic therapy (33). In summary, our study provides compelling evidence that, although rare, survival of B. burgdorferi sensu lato can occur in antibiotically treated individuals with EM after antimicrobial chemotherapy. Spirochete persistence in these patients was not caused by increasing MICs or MBCs for B. burgdorferi sensu lato. Instead, our findings corroborate those of Hansen et al. (9) and Pfister et al. (25) in relapsed patients with early LB, demonstrating that isolates cultured after the conclusion of roxithromycin and ceftriaxone therapy remain fully susceptible to these agents in vitro. These findings, however, do not rule out phenotypic resistance mechanisms similar to those assumed to cause relapse in syphilis and leptospirosis (24, 37).
REFERENCES
- 1.Aberer, E., A. Kersten, H. Klade, C. Poitschek, and W. Jurecka. 1996. Heterogeneity of Borrelia burgdorferi in the skin. Am. J. Dermatopathol. 18:571-579. [DOI] [PubMed] [Google Scholar]
- 2.Arnez, M., A. Radsel-Medvescek, D. Pleterski-Rigler, E. Ruzic-Sabljic, and F. Strle. 1999. Comparison of cefuroxime axetil and phenoxymethyl penicillin for the treatment of children with solitary erythema migrans. Wien. Klin. Wochenschr. 111:916-922. [PubMed] [Google Scholar]
- 3.Belfaiza, J., D. Postic, E. Bellenger, G. Baranton, and I. Saint-Girons. 1993. Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed-field gel electrophoresis. J. Clin. Microbiol. 31:2873-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglund, J., R. Eitrem, K. Ornstein, A. Lindberg, Å. Rignér, H. Elmrud, M. Carlsson, A. Runehagen, K. Svanborg, and R. Norrby. 1995. An epidemiological study of Lyme diseases in southern Sweden. N. Engl. J. Med. 333:1319-1324. [DOI] [PubMed] [Google Scholar]
- 5.Bockenstedt, L. K., J. Mao, E. Hodzic, S. W. Barthold, and D. Fish. 2002. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J. Infect. Dis. 186:1430-1437. [DOI] [PubMed] [Google Scholar]
- 6.Boerner, J., K. Failing, and M. M. Wittenbrink. 1995. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: influence of test conditions on minimal inhibitory concentration (MIC) values. Zentbl. Bakteriol. 283:49-60. [DOI] [PubMed] [Google Scholar]
- 7.Dever, L. L., J. H. Jorgensen, and A. G. Barbour. 1992. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J. Clin. Microbiol. 30:2692-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber, M. A., E. D. Shapiro, G. S. Burke, V. J. Parcells, G. L. Bell, et al. 1996. Lyme disease in children in southeastern Connecticut. N. Engl. J. Med. 335:1270-1274. [DOI] [PubMed] [Google Scholar]
- 9.Hansen, K., A. Hovmark, A. M. Lebech, K. Lebech, I. Olsson, L. Halkier-Sorensen, L., E. Olsson, and E. Asbrink. 1992. Roxithromycin in Lyme borreliosis: discrepant results of an in vitro and in vivo animal susceptibility study and a clinical trial in patients with erythema migrans. Acta Dermato-Venereol. 72:297-300. [PubMed] [Google Scholar]
- 10.Hassler, D., L. Zöller, M. Haude, H. D. Hufnagel, F. Heinrich, and H. G. Sonntag. 1990. Cefotaxime versus penicillin in the late stage of Lyme disease—a prospective, randomized therapeutic study. Infection 18:16-20. [DOI] [PubMed] [Google Scholar]
- 11.Hunfeld, K.-P., P. Kraiczy, T. A. Wichelhaus, V. Schäfer, and V. Brade. 2000. New colorimetric microdilution method for in vitro susceptibility testing of Borrelia burgdorferi against antimicrobial substances. Eur. J. Clin. Microbiol. Infect. Dis. 19:27-32. [DOI] [PubMed] [Google Scholar]
- 12.Hunfeld, K.-P., P. Kraiczy, E. Kekoukh, V. Schäfer, and V. Brade. 2002. Standardised in vitro susceptibility testing of Borrelia burgdorferi against well-known and newly developed antimicrobial agents—possible implications for new therapeutic approaches to Lyme disease. Int. J. Med. Microbiol. 291(Suppl. 33):125-137. [DOI] [PubMed] [Google Scholar]
- 13.Hunfeld, K.-P., R. Rodel, and T. A. Wichelhaus. 2003. In vitro activity of eight oral cephalosporins against Borrelia burgdorferi. Int. J. Antimicrob. Agents 21:313-318. [DOI] [PubMed] [Google Scholar]
- 14.Hunfeld, K.-P., T. A. Wichelhaus, R. Rodel, G. Acker, V. Brade, and P. Kraiczy. 2004. Comparison of in vitro activities of ketolides, macrolides, and an azalide against the spirochete Borrelia burgdorferi. Antimicrob. Agents Chemother. 48:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan, E. L., A. S. Gastanaduy, and B. B. Huwe. 1981. The role of the carrier in treatment failures after antibiotic for group A streptococci in the upper respiratory tract. J. Lab. Clin. Med. 98:326-335. [PubMed] [Google Scholar]
- 16.Klempner, M. S., L. T. Hu, J. Evans, C. H. Schmid, G. M. Johnson, R. P. Trevino, D. Norton, L. Levy, D. Wall, J. McCall, M. Kosinski, and A. Weinstein. 2001. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 345:85-92. [DOI] [PubMed] [Google Scholar]
- 17.Kraiczy, P., J. Weigand, T. A. Wichelhaus, P. Heisig, H. Backes, V. Schäfer, G. Acker, V. Brade, and K.-P. Hunfeld. 2001. In vitro activities of fluoroquinolones against the spirochete Borrelia burgdorferi. Antimicrob. Agents Chemother. 45:2486-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logar, M., S. Lotric-Furlan, V. Maraspin, J. Cimperman, T. Jurca, E. Ruzic-Sabljic, and F. Strle. 1999. Has the presence or absence of Borrelia burgdorferi sensu lato as detected by skin culture any influence on the course of erythema migrans? Wien. Klin. Wochenschr. 111:945-950. [PubMed] [Google Scholar]
- 19.Lomholt, H., A. M. Lebech, K. Hansen, F. Brandrup, and L. Halkier-Sorensen. 2000. Long-term serological follow-up of patients treated for chronic cutaneous borreliosis or culture-positive erythema migrans. Acta Dermato-Venereol. 80:362-366. [DOI] [PubMed] [Google Scholar]
- 20.Misonne, M.-C., G. van Impe, and P. P. Hoet. 1998. Genetic heterogeneity of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in Belgium. J. Clin. Microbiol. 36:3352-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadelman, R. B., J. Nowakowski, G. Forseter, S. Bittker, D. Cooper, N. Goldberg, D. McKenna, and G. P. Wormser. 1993. Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: a report of a prospective study. Am. J. Med. 94:583-588. [DOI] [PubMed] [Google Scholar]
- 22.Nowakowski, J., D. McKenna, R. B. Nadelman, D. Cooper, S. Bittker, D. Holmgren, C. Pavia, R. C. Johnson, and G. P. Wormser. 2000. Failure of treatment with cephalexin for Lyme disease. Arch. Fam. Med. 9:563-567. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell, S., M. Granström, J. S. Gray, and G. Stanek. 1998. Epidemiology of European Lyme borreliosis. Zentbl. Bakteriol. 287:229-240. [DOI] [PubMed] [Google Scholar]
- 24.Panconesi, E., G. Zuccati, and A. Cantini. 1981. Treatment of syphilis: a short critical review. Sex. Transm. Dis. 8:321-325. [PubMed] [Google Scholar]
- 25.Pfister, H. W., V. Preac-Mursic, B. Wilske, E. Schielke, F. Sorgel, and K. M. Einhaupl. 1991. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J. Infect. Dis. 163:311-318. [DOI] [PubMed] [Google Scholar]
- 26.Postic, D., M. V. Assous, P. A. D. Grimont, and G. Baranton. 1994. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf(5S)-rrl(23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 44:743-752. [DOI] [PubMed] [Google Scholar]
- 27.Preac-Mursic, V., B. Wilske, and G. Schierz. 1986. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentbl. Bakteriol. Mikrobiol. Hyg. 1 Abt. Orig. A 263:112-118. [DOI] [PubMed] [Google Scholar]
- 28.Preac-Mursic, V., K. Weber, H. W. Pfister, B. Wilske, B. Gross, A. Baumann, and J. Prokop. 1989. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection 17:355-359. [DOI] [PubMed] [Google Scholar]
- 29.Preac-Mursic, V., W. Marget, U. Busch, D. Pleterski-Riegler, and S. Hagel. 1996. Kill kinetics of Borrelia burgdorferi and bacterial findings in relation to the treatment of Lyme borreliosis. Infection 24:9-16. [DOI] [PubMed] [Google Scholar]
- 30.Rijpkema, S. G., D. J. Tazelaar, M. J. Molkenboer, G. T. Noordhoek, G. Plantinga, L. M. Schouls, and J. F. Schellekens. 1997. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin. Microbiol. Infect. 3:109-116. [DOI] [PubMed] [Google Scholar]
- 31.Ruzic-Sabljic, E., M. Arnez, S. Lotric-Furlan, V. Maraspin, J. Cimperman, and F. Strle. 2001. Genotypic and phenotypic characterisation of Borrelia burgdorferi sensu lato strains isolated from human blood. J. Med. Microbiol. 50:896-901. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz, I., G. P. Wormser, J. J. Schwartz, D. Cooper, P. Weissensee, A. Gazumyan, E. Zimmermann, N. S. Goldberg, S. Bittker, G. L. Campbell, and C. S. Pavia. 1992. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J. Clin. Microbiol. 30:3082-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sicklinger, M., R. Wienecke, and U. Neubert. 2003. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J. Clin. Microbiol. 41:1791-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, R. P., R. T. Schoen, D. W. Rahn, V. K. Sikand, J. Nowakowski, D. L. Parenti, M. S. Holman, D. H. Persing, and A. C. Steere. 2002. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann. Intern. Med. 136:421-428. [DOI] [PubMed] [Google Scholar]
- 35.Stanek, G., S. O'Connell, M. Cimmino, E. Aberer, W. Kristoweritsch, M. Granström, E. Guy, and J. Gray. 1996. European Union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien. Klin. Wochenschr. 108:741-747. [PubMed] [Google Scholar]
- 36.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 37.Straubinger, R. K., B. A. Summers, Y. F. Chang, and M. J. G. Appel. 1997. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 35:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strle, F., V. Preac-Mursic, J. Cimperman, E. Ruzic, V. Maraspin, and M. Jereb. 1993. Azithromycin versus doxycycline for treatment of erythema migrans: clinical and microbiological findings. Infection 21:83-88. [DOI] [PubMed] [Google Scholar]
- 39.Strle, F., V. Maraspin, S. Lotric-Furlan, E. Ruzic-Sabljic, and J. Cimperman. 1996. Azithromycin and doxycycline for treatment of Borrelia culture-positive erythema migrans. Infection 24:64-68. [DOI] [PubMed] [Google Scholar]
- 40.Strle, F., J. A. Nelson, E. Ruzic-Sabljic, J. Cimperman, V. Maraspin, S. Lotric-Furlan, Y. Cheng, M. M. Picken, G. M. Trenholme, and R. N. Picken. 1996. European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin. Infect. Dis. 23:61-65. [DOI] [PubMed] [Google Scholar]
- 41.Van den Brule, A. J., C. Munk, J. F. Winther, S. K. Kjaer, H. O. Jorgensen, C. J. Meijer, and S. A. Morre. 2002. Prevalence and persistence of asymptomatic Chlamydia trachomatis infections in urine specimens from Danish male military recruits. Int. J. STD AIDS 13(Suppl. 2):19-22. [DOI] [PubMed] [Google Scholar]
- 42.Wormser, G. P., R. Ramanathan, J. Nowakowski, D. McKenna, D. Holmgren, P. Visintainer, R. Dornbush, B. Singh, and R. B. Nadelman. 2003. Duration of antibiotic therapy for early Lyme disease. Ann. Intern. Med. 138:697-704. [DOI] [PubMed] [Google Scholar]
- 43.Xu, Y., and R. C. Johnson. 1995. Analysis and comparison of plasmid profiles of Borrelia burgdorferi sensu lato strains. J. Clin. Microbiol. 33:2679-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zore, A., E. Ruzic-Sabljic, V. Maraspin, J. Cimperman, S. Lotric-Furlan, A. Pikelj, T. Jurca, M. Logar, and F. Strle. 2002. Sensitivity of culture and polymerase chain reaction for the etiologic diagnosis of erythema migrans. Wien. Klin. Wochenschr. 114:606-609. [PubMed] [Google Scholar]