Abstract
Background
It has been observed that cancer and venous thromboembolism (VTE) are associated, but anticancer therapy may violate the causality. Therefore, this study aimed to elucidate the causal relationship of various cancers to VTE using Mendelian randomization (MR).
Methods
Three MR methods were used to estimate causal effects: Inverse variance weighted (IVW), MR‐Egger and weighted median. Sensitivity analyses included Cochran's Q‐test, MR‐Egger intercept test and MR‐PRESSO. Gene ontology enrichment analysis was performed to elucidate the underlying mechanisms of VTE development in cancer patients.
Results
The primary IVW approach showed that non‐Hodgkin's lymphoma (NHL) might increase the risk of VTE (odds ratio [OR]: 1.20, 95% confidence interval [95% CI]: 1.00–1.44, p = 0.045), while melanoma possibly reduced the risk of VTE (OR: 0.89, 95% CI: 0.82–0.97, p = 0.006), although there was no significance after adjustment for multiple testing. No association was observed between VTE risk and other site‐specific cancers. Gene ontology enrichment analysis revealed that vitamin D played an important role in the development of VTE in cancer patients.
Conclusions
Our findings suggested that genetically predicted NHL was associated with higher VTE risk, whereas melanoma had lower VTE risk compared with other site‐specific cancers. Moreover, this study suggested that anticancer therapy and increased extensive examination might play a more important role in VTE development than the nature of cancer.
Keywords: cancer, venous thromboembolism, Mendelian randomization, causal effect
Using Mendelian randomization study, we elucidated the causal effect between 14 site‐specific cancers and venous thromboembolism. Non‐Hodgkin's lymphoma increases risk of venous thromboembolism while melanoma decreases its risk. footnotes: VTE: venous thromboembolism; some icons were referenced from the Biorender (https://biorender.com/).
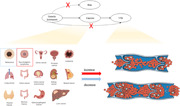
Abbreviations
- BP
biological processes
- CAT
cancer‐associated thrombosis
- DVT
deep vein thrombosis
- GER
the Kaiser permanente genetic epidemiology research on adult health and aging
- GO
gene ontology
- GWAS
genome‐wide association study
- IVW
Inverse Variance Weighted
- LD
linkage disequilibrium
- MR
Mendelian randomization
- NHL
non‐Hodgkin's lymphoma
- PE
pulmonary embolism
- RCTs
randomized controlled trials
- SNP
single nucleotide polymorphisms
- VTE
venous thromboembolism
1. INTRODUCTION
According to the World Health Organization's 2020 estimates, cancer incidence and mortality are rapidly increasing and represent a major obstacle to increasing life expectancy globally [1]. Venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is the second leading cause of disability and death in cancer patients after disease progression [2]. In fact, cancer patients have a four‐ to seven‐fold increased risk of VTE compared with patients without cancer [3, 4]. Cancer‐associated thrombosis (CAT) can lead to bleeding, hospitalization, postthrombotic syndrome, morbidity, delays in cancer treatment and death [5]. Therefore, strategies such as early diagnosis and optimal prevention management of VTE are of crucial for cancer patients.
Cancers are known to be at particularly high risk for VTE, but reported risk events varied widely by cancer type, stage of malignancy and antineoplastic drug treatment [6, 7]. Although previous observational studies report an increased incidence of VTE in cancer patients, the causal relationship between various cancers and VTE remains uncertain. Cross‐sectional studies fail to differentiate between precancerous CAT and VTE cases [8]. In addition, confirmed cases in patients with unprovoked VTE and no underlying or directly predisposing risk factors were more likely to have underlying cancer [9, 10]. Moreover, new cancer therapies (e.g., antiangiogenic agents, multitargeted tyrosine kinase inhibitors, immunomodulatory drug combinations and immunotherapy regimens) and the accompanying extensive examinations (e.g., high‐resolution imaging and central venous catheters) might increase the risk of VTE in cancer patients over the past decade [11, 12, 13]. Furthermore, close clinical monitoring of cancer patients might lead to relatively earlier detection and subsequent higher incidence of VTE. Although pharmacologic thromboprophylaxis (low‐molecular‐weight heparin and oral anticoagulants) has been repeatedly shown to reduce the risk of VTE, long‐term anticoagulation therapy also increases the risk of bleeding and the financial burden on patients [5, 7]. It remains to be determined whether cancer contributes to an increased risk of VTE compared to subsequent testing and treatment [11].
Mendelian randomization (MR) is an epidemiologic method in which genetic variation (as a surrogate for modifiable risk factors) is used to make causal inferences about a disease of interest. Because the study design parallels the random assignment of treatments in randomized controlled trials (RCTs), it is less susceptible to the typical biases encountered in observational studies, such as confounding and reverse causality [14, 15]. More importantly, RCTs that exclude interference from other treatment regimens are not ethically feasible in cancer patients; therefore, MR is necessary. However, no previous study applied MR to address this issue.
Therefore, this study aimed to investigate the impact of various cancers on VTE using publicly available genome‐wide association study (GWAS) data with MR.
2. MATERIALS AND METHODS
2.1. Data sources
The exposed GWAS aggregated data included 14 cancer types (bladder, breast, cervical, colon, gastricesophageal, kidney, leukemia, lung, melanoma, non‐Hodgkin's lymphoma [NHL], pancreatic, prostate, rectal, and thyroid cancers) and included 408,786 people of European ancestry from two large, independent and modern cohorts that weren't phenotypic screened—UK Biobank and Kaiser Permanente Genetic Epidemiology Research on Adult Health and Aging (GERA) [16]. Cancer cases in the UK Biobank were identified through linked to various national cancer registries. GERA cancer cases were identified using the Kaiser Permanente Northern California Cancer Registry. Due to the low number of cases in GERA, testicular cancer data were only obtained from UK Biobank. Controls were limited to individuals without any cancer records in the relevant registry. After a separate GWAS for each cohort, the association results for 7,846,216 single nucleotide polymorphisms (SNPs) in the two cohorts were combined by meta‐analysis. For variants examined in only one cohort (22% of the total SNPs), raw summary statistics were combined with the meta‐analyzed SNPs to create a set of SNP statistics. Detailed methods can be found in a meta‐analysis by Rashkin et al. [16].
GWAS summary statistics on VTE were collected from the FinnGen consortium, which included 130,235 control cases and 5403 patients with the disease, all European ancestry (these data are available at https://finngen.gitbook.io/documentation/). Detailed methods such as data collection, participation in cohorts, genotyping and data analysis are described on FinnGen's webpage. For sex‐specific cancers (prostate, cervical and breast cancers), we used additional sex‐specific VTE summary GWAS data downloaded from UK Biobank to avoid sex as a confounding factor.
2.2. Instrumental variable selection
The complete research framework was shown in Figure 1. As shown in our previous study, the selection of instrumental variables was based on (i) a GWAS‐correlation p < 5 × 10−8, and (ii) a linkage disequilibrium [LD] r 2 < 0.001, clump_kb = 10,000 [17]. The corresponding SNPs in the VTE were retrieved. Proximal SNPs with r 2 > 0.8 were selected if corresponding SNPs were absent in the VTE [15]. Therefore, 2–45 SNPs were selected as instrumental variables with F statistics between 15 and 45, indicating that the instrumental variables used in our study were strong (Table 1) [18]. Detailed SNP information was listed in Supporting Information: Table S1.
Figure 1.
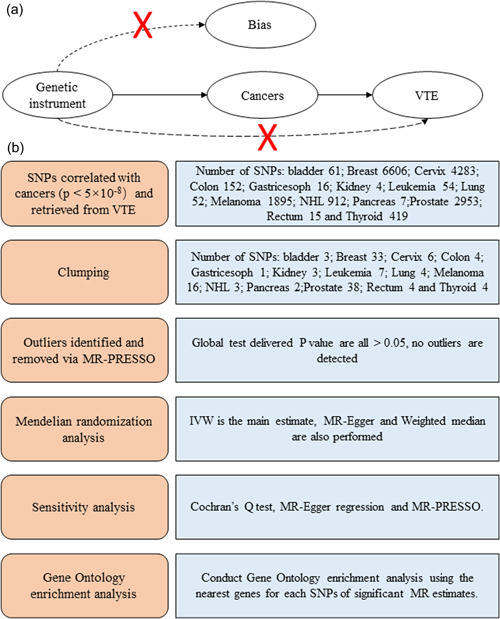
Schematic overview of the Mendelian randomization (MR) framework. (a) Conceptual framework for the MR analysis of cancers and risk of venous thromboembolism (VTE) and (b) flowchart and main methods of this MR study. IVW, inverse‐variance weighted; NHL, non‐Hodgkin's lymphoma; SNP, single‐nucleotide polymorphism.
Table 1.
Description of instrumental variable for various cancers
| Exposures | No. of SNPs for the IV | Variance explained by the IV (R 2) | Sample size of exposure GWAS | Number of cases | Consortium | Adjustment | Population | F‐statistics |
|---|---|---|---|---|---|---|---|---|
| Bladder | 3 | 2.99 × 10−04 | 412,592 | 2242 | All were from meta‐analysis of UK Biobank and GERA | Age at specimen collection, sex (nonsex‐specific cancers only), first 10 ancestry PCs, genotyping array (UKB only), and reagent kit used for genotyping (Axiom v1 or v2; GERA only) | European | 41 |
| Breast | 44 | 6.33 × 10−03 | 237,537 | 17,881 | European | 34 | ||
| Cervix | 6 | 1.07 × 10−03 | 226,219 | 6563 | European | 40 | ||
| Colon | 5 | 3.60 × 10−04 | 414,143 | 3793 | European | 29 | ||
| Gastricesophageal | 2 | 8.34 × 10−05 | 411,441 | 1091 | European | 17 | ||
| Kidney | 3 | 2.90 × 10−04 | 411,688 | 1338 | European | 39 | ||
| Leukemia | 7 | 6.95 × 10−04 | 411,202 | 852 | European | 41 | ||
| Lung | 4 | 3.39 × 10−04 | 412,835 | 2485 | European | 35 | ||
| Melanoma | 17 | 1.85 × 10−03 | 417,127 | 6777 | European | 45 | ||
| NHL | 3 | 2.54 × 10−04 | 412,750 | 2400 | European | 35 | ||
| Pancreas | 5 | 2.01 × 10−02 | 360,296 | 663 | European | 15 | ||
| Prostate | 45 | 1.01 × 10−02 | 201,486 | 10,792 | European | 45 | ||
| Rectum | 4 | 3.48 × 10−04 | 412,441 | 2091 | European | 36 | ||
| Thyroid | 4 | 4.42 × 10−04 | 411,112 | 762 | European | 45 |
Abbreviations: GWAS, genome‐wide association studies; IV, instrumental variable; NHL, non‐Hodgkin's lymphoma; SNPs, single nucleotide polymorphisms.
2.3. MR analysis
Three MR methods were used to provide robust MR estimates: Inverse‐Variance Weighted (IVW), MR‐Egger, and Weighted Median. Since IVW has been recommended compared to the other two methods, it was selected as the primary method, while the others were used to obtain more robust estimates. In IVW, the MR estimate was represented by the weighted regression slope of the effect of SNP‐VTE on the SNP‐cancer effect, and the intercept was fixed at zero. Weighted Median provided a consistent estimate of causality, even when 50% of the instrumental variables are invalid. MR‐Egger assumed that SNP‐exposure effects did not involve directed pleiotropic effects, whereas MR‐PRESSO identified potential horizontal pleiotropic effects of SNPs to detect and correct for possible outliers; both were used for the sensitivity analyses [19]. All results were presented as odds ratio (OR) and 95% confidence interval (95% CI), representing the risk of VTE in cancer patients compared to non‐cancer cases. Since our data included 14 cancer types, we used Bonferroni correction to adjust the significance threshold and set it to 0.05/14 for multiple analyses.
2.4. Sensitivity analysis
In an MR study, three main assumptions must be met to generate robust causal estimates: (1) the selected genetic variant is strongly associated with exposure, (2) the genetic variant is not associated with any confounding factors for exposure and outcome, and (3) the genetic variant should independently affect outcome only through exposure and not through any other causal pathway (Figure 1). The first assumption was easily satisfied by using strong instrumental variables, while the second assumption was satisfied for SNPs randomly assigned during pregnancy and independent of the environment. To determine whether the third assumption was satisfied and to assess the possible effect of horizontal pleiotropy, we performed a sensitivity analysis using MR‐Steiger. In addition, the MR‐Egger regression intercept test, MR‐PRESSO, leave‐one‐out ,and funnel plots were performed to detect outliers, and Cochran's Q test was performed to detect heterogeneity. In addition, PhenoScanner (www.phenoscanner.medschl.cam.ac.uk) is a platform for comprehensive genotype and phenotype association information to determine if our SNPs are associated with any potential confounders, such as obesity, BMI and other cardiovascular diseases [20]. The MR analyses were subsequently reperformed to ensure the robustness of causal effects after the removal of SNPs associated with potential confounders of genome‐wide significance.
2.5. Gene ontology (GO) enrichment analysis
To better understand the mechanism of CAT development, we combined all significant estimated SNPs and performed GO enrichment analysis using the nearest genes for each SNP. GO enrichment analysis is widely used for gene annotation concerning biological processes (BP), cellular components, and molecular functions. Here, we focused on BP. Metascape (http://metascape.org/gp/index.html) is a customer‐friendly web‐based portal designed to provide a comprehensive resource for gene list annotation and analysis, which was used in this study for GO enrichment analysis [21].
2.6. Statistics analysis
All analyses were conducted using the TwoSampleMR (version 0.4.25) and MR‐PRESSO (version 1.0) packages in R (version 3.6.1). Two‐sided p < 0.05 was considered significant. In this report, a specific p‐value threshold (e.g., 0.05) was not a goal, rather we were interested in finding causal relationship between different cancers and VTE.
3. RESULTS
3.1. Causal effect from site‐specific cancers to VTE
Our MR study showed that genetically predisposed melanoma may be associated with an 11% lower risk of VTE compared with non‐cancer cases (OR: 0.89, 95% CI: 0.82–0.97; p = 0.006), while genetically predisposed NHL may increase risk of VTE with 20% (OR: 1.20, 95% CI: 1.00–1.44; p = 0.045). However, neither reached a significant level after correction by multiple testing. In addition, genetically predicted breast cancer may slightly increase the risk of VTE by 9% (OR: 1.09, 95% CI: 1.00–1.20; p = 0.054; Table 2). The estimates from MR‐Egger, Weighted Median, and MR‐PRESSO analyses were consistent with the direction and magnitude of the IVW estimates (Figure 2). The scatter plots of melanoma and NHL were shown in Supporting Information: Figure S1a,b.
Table 2.
MR estimates of IVW, MR‐Egger, and weighted median for various cancers on VTE
| Exposure | IVW | MR‐egger | Weighted median | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | SE | p | OR | SE | p | OR | SE | p | |
| Bladder | 0.980 | 0.069 | 0.769 | 0.956 | 0.200 | 0.860 | 0.980 | 0.075 | 0.787 |
| Breast | 1.095 | 0.047 | 0.054 | 1.351 | 0.171 | 0.088 | 1.089 | 0.065 | 0.190 |
| Cervix | 0.964 | 0.082 | 0.653 | 0.779 | 0.238 | 0.352 | 0.914 | 0.088 | 0.309 |
| Colon | 0.940 | 0.064 | 0.329 | 0.801 | 0.507 | 0.704 | 0.980 | 0.078 | 0.791 |
| Gastricesophageal | 0.759 | 0.230 | 0.229 | / | / | / | / | / | / |
| Kidney | 0.985 | 0.029 | 0.604 | 0.967 | 0.079 | 0.748 | 0.986 | 0.031 | 0.648 |
| Leukemia | 0.987 | 0.028 | 0.639 | 0.969 | 0.091 | 0.748 | 0.981 | 0.034 | 0.567 |
| Lung | 1.073 | 0.055 | 0.198 | 1.212 | 0.180 | 0.396 | 1.066 | 0.065 | 0.326 |
| Melanoma | 0.888 | 0.043 | 0.006 | 0.935 | 0.100 | 0.509 | 0.881 | 0.057 | 0.026 |
| NHL | 1.201 | 0.091 | 0.045 | 3.054 | 0.646 | 0.334 | 1.134 | 0.103 | 0.224 |
| Pancreas | 0.983 | 0.048 | 0.726 | / | / | / | / | / | / |
| Prostate | 0.994 | 0.029 | 0.830 | 0.961 | 0.094 | 0.677 | 0.980 | 0.045 | 0.663 |
| Rectum | 1.004 | 0.051 | 0.941 | 0.677 | 0.659 | 0.614 | 1.006 | 0.059 | 0.913 |
| Thyroid | 1.009 | 0.034 | 0.789 | 1.443 | 0.265 | 0.301 | 0.987 | 0.035 | 0.697 |
Abbreviations: IVW, inverse‐variance weighted; MR, Mendelian randomization; NHL, non‐Hodgkin's lymphoma; OR, odds ratio; SE, standard error; VTE, venous thromboembolism.
Figure 2.
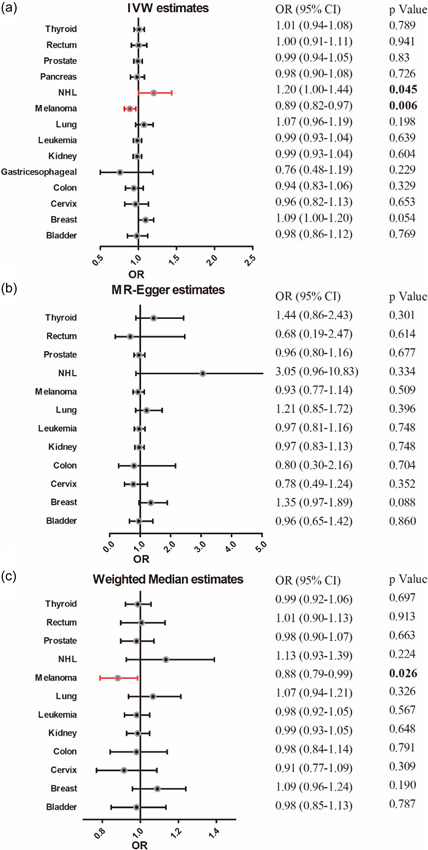
Forest plot of the Mendelian randomization (MR) analysis with odds ratio (OR) and 95% confidence intervals (95% CIs) of the causal effect of site‐specific cancers on venous thromboembolism in regression analyses, including (a) inverse‐variance weighted (IVW), (b) MR‐Egger, and (c) weighted median. NHL, non‐Hodgkin's lymphoma.
For sex‐specific cancers (prostate, cervix, and breast), we also performed MR estimates using sex‐specific aggregated data downloaded from UK Biobank. IVW estimates for these cancers remained insignificant (prostate cancer, p = 0.632; cervical cancer, p = 0.282 and breast cancer, p = 0.744), which were consistent with estimates from FinnGen consortium data.
3.2. Sensitivity analysis
To make our estimates robust, we performed a series of sensitivity analyses. The Cochran's Q test indicated no heterogeneity (p > 0.05). Likewise, the intercepts of the MR‐Egger regression indicated no directional pleiotropy (p > 0.05), and no outlier or horizontal pleiotropic effects of cancer on VTE risk were detected in the MR‐PRESSO global test (Table 3). MR‐Steiger was applied and no SNP bias orientation was found. In the leave‐one‐out analysis, the estimated risk of VTE due to melanoma remained negative and significant, while the estimated risk of NHL remained positive but no longer significant (Supporting Information: Figure S1c,d). The funnel plots were shown in Supporting Information: Figure 1e,f. No SNP was associated with any known risk factors for VTE, suggesting that our hypothesis was not violated.
Table 3.
Sensitivity analysis for the MR analyses concerning causal effect from various cancers on VTE
| Cochran Q test derived p‐value (IVW) | MR‐egger | MR‐PRESSO | |||
|---|---|---|---|---|---|
| Exposure | Q | p‐Value | Intercept | p‐Value | p‐Value |
| Bladder | 0.223 | 0.894 | −0.005 | 0.918 | / |
| Breast | 24.219 | 0.836 | 0.019 | 0.204 | 0.859 |
| Cervix | 7.743 | 0.171 | −0.029 | 0.393 | 0.192 |
| Colon | 1.595 | 0.660 | −0.026 | 0.781 | 0.515 |
| Gastricesophageal | / | / | / | / | / |
| Kidney | 0.087 | 0.957 | −0.021 | 0.845 | / |
| Leukemia | 6.966 | 0.324 | −0.007 | 0.844 | 0.344 |
| Lung | 2.726 | 0.436 | 0.027 | 0.547 | 0.448 |
| Melanoma | 8.286 | 0.912 | 0.008 | 0.578 | 0.909 |
| NHL | 2.635 | 0.268 | 0.193 | 0.383 | / |
| Pancreas | 0.002 | 0.965 | / | / | / |
| Prostate | 34.895 | 0.568 | −0.004 | 0.711 | 0.635 |
| Rectum | 0.533 | 0.912 | −0.074 | 0.610 | 0.923 |
| Thyroid | 4.376 | 0.224 | 0.122 | 0.307 | 0.213 |
Abbreviations: IVW, inverse‐variance weighted; MR, Mendelian randomization; NHL, non‐Hodgkin's lymphoma; OR, odds ratio; SE, standard error; VTE, venous thromboembolism.
3.3. Gene ontology enrichment
Sixteen SNPs from melanoma and three SNPs from NHL were combined and their nearest genes were determined. These 19 genes were used for GO enrichment analysis in Metascape. Figure 3 showed the top four GO‐BP enriched terms, including miRNA regulation of gene silencing, vitamin D receptor pathway, regulation of cellular amide metabolic processes and negative regulation of phosphate metabolic processes.
Figure 3.
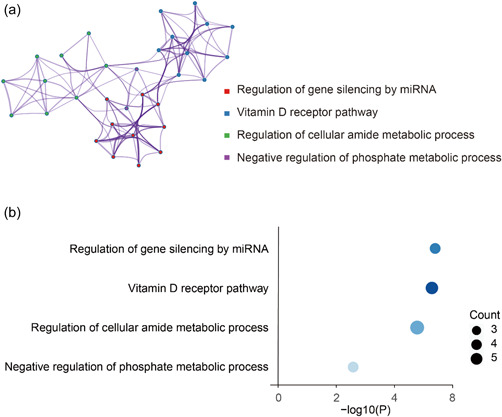
Gene ontology enrichment analysis of nearest genes for single‐nucleotide polymorphisms used on significant Mendelian randomization (MR) analysis results. (a) enriched ontology clusters, where every cluster is represented in a single color and shown as a circle; (b) enrichment heatmap where count means enriched number of genes.
4. DISCUSSION
In this study, we performed MR using a population of European ancestry to comprehensively reveal the causal relationship between various cancers and VTE. Our findings suggested that genetically predisposed melanoma may reduce VTE risk, while genetically predisposed NHL and breast cancer may increase VTE risk, supporting previous observational studies. Conversely, no causal relationship was found between VTE and most types of cancer, such as bladder, cervical, colon, gastricesophageal, kidney, leukemia, lung, pancreatic, prostate, rectal and thyroid cancers. Furthermore, our study suggested that vitamin D may play an important role in CAT development and thus may be a potential therapeutic target for its management.
Cancer has been reported to increase the risk of VTE, an important cause of death in cancer patients, by seven times [22]. However, whether the association between cancer and VTE is primarily causal or confounded by common risk factors remains incompletely understood. Importantly, it has been observed that VTE risk varies widely between different types of cancer subgroups. Moreover, increases in CAT may be due in part to interindividual differences (e.g., advanced age, smoking, obesity and activity), tumor‐related factors (e.g., cancer type and stage), testing methods (e.g., computerized tomography or magnetic resonance) and treatments (e.g., chemotherapy, anticoagulant and hormonal therapy, immunotherapy, surgery, or central venous catheter use, of which VTE is a common complication) [11, 13, 23, 24]. Since optimal thrombosis prevention strategies target cancer patients at high risk for VTE while avoiding low‐risk patients, well‐designed methods for predicting CAT risk are critical. However, to date, few studies have been able to determine whether VTE is associated with individual differences in cancer type or patients. To our knowledge, this is the first study to use MR to investigate the causal relationship between site‐specific cancer and VTE.
The reported incidence of VTE from multiple cross‐sectional and cohort studies varied by cancer type [11, 25, 26, 27]. Yohei et al. broadly divided cancer into three groups based on the risk of VTE: High risk (pancreatic, ovarian, brain, stomach, gynecologic and hematologic cancers), intermediate risk (colon and lung cancers) and low risk (breast and prostate cancer) [28]. Here, the causal relationship between most types of cancer and VTE risk was not statistically significant; this is in contrast to previous studies reporting cancer to be associated with a 4‐ to 7‐fold higher risk of VTE [3, 29]. In this study, only genetically predisposed melanoma significantly reduced VTE risk, while genetically predisposed NHL cancer increased VTE risk. This difference may be due in part to differences in methodologies, sample populations, and methods of detecting and reporting VTE [7]. It has been reported that the biological aggressiveness of the tumor as reflected by the high tumor grade may lead to a hypercoagulable state in cancer patients. A meta‐analysis of 29 independent cohorts find that the OR for thrombosis increased proportionally to the disease stage [30]. Here, the VTE risk was estimated using genetics rather than tumor grade, which may explain some of the differences from previous observational studies [31]. Another explanation could be an association between cancer types and specific VTE pathways. Levels of leukocytes, platelets, heparinase and tissue factor‐positive microvesicles were all potential factors (alone or in combination) to increase CAT, explaining some of the differences in CAT risk [28].
The relationship between melanoma and VTE has been under the spotlight for many years. In a large Danish population cohort study of 499,092 newly diagnosed cancer patients and 1,497,276 cancer‐free controls, pancreatic cancer (4.4%; 95% CI, 4.1%–4.8%) was associated with the highest 6‐month cumulative incidence of VTE, while melanoma (0.36%; 95% CI, 0.30%–0.43%) was associated with the lowest risk [27]. Similarly, a hospital‐based study of 3,558,660 cancer patients in England reported the lowest risk for melanoma (0.37%) compared with the highest risk for pancreatic cancer (4.89%) [11]. In contrast, we showed a causal relationship between melanoma and a lower risk of VTE at the genetic level, with an 11% lower risk of VTE in IVW and MR‐PRESSO. A plausible explanation for this difference might be that most melanomas are clinically benign. Therefore, a lower incidence of malignant melanoma might be associated with a lower risk of VTE [32]. Interestingly, Sparsa et al. found a high prevalence of VTE in stage IV melanomas (e.g., lung and gastrointestinal cancers), suggesting that comprehensive VTE testing and aggressive anticoagulation in patients with stage IV melanoma may be relevant [32]. In a meta‐analysis including 18,018 lymphoma patients from 29 cohorts, the incidence of VTE was found to be 5.3%, of which 95% occurred during treatment; 3.8% were present at disease diagnosis before initiation of chemotherapy. Only 1.2% appeared after treatment was completed [30].
Furthermore, in a cohort study using the UK healthcare databases, Walker et al. reported that the risk of VTE to be increased by more than 10 times during chemotherapy, and women receiving tamoxifen were five times higher than those before starting treatment [33]. Meanwhile, a 10‐year study observed a doubling of the incidence of VTE and breast cancer, likely a result of more aggressive cancer screening or treatment [33]. This finding was consistent with our findings, which suggested that while some cancers, such as breast cancer, may not increase the incidence of VTE, associated treatments do. In a retrospective cohort study matching cancer patients receiving therapeutic anticoagulation with those not receiving therapeutic anticoagulation, Wood and colleagues reported that melanoma had a higher risk of intracerebral hemorrhage than other primary malignancies (HR 6.46 vs. 1.36, p = 0.02) [34]. This finding supported our MR estimates. Summarizing our findings and those in the existing literature, cancer nature (except NHL) was not a significant risk factor for the development of VTE, but anticancer therapy was. Therefore, promotion of empirical anticoagulation for most cancer patients may lead to bleeding risk rather than VTE avoidance. Moreover, further studies should be conducted to clarify the role of different anticancer treatment regimens in the development of VTE.
VTE and cancer are interconnected through multiple pathophysiological mechanisms. About 150 years ago, Trousseau first identified a particular condition of blood that predisposes it to spontaneously clot in the cachexia [35]. The idea that cancer cells can activate the coagulation cascade is gaining acceptance. This activation may occur directly, by increasing the expression of tissue factor and plasminogen activator inhibitor‐1, or indirectly, facilitated by secretion of inflammatory cytokines such as tumor necrosis factor‐α and interleukin‐1β, promoting procoagulant activity, inhibits the antithrombotic response of endothelial cells [36, 37]. However, the exact mechanism of VTE is not entirely consistent across different cancer types. For instance, malignant melanoma is one of the most aggressive tumors. Highly aggressive melanoma cells can activate the vascular endothelium and promote the release of coagulation and angiogenesis‐related factors (von Willebrand factor, P‐selectin, angiopoietin‐2), thereby promoting cancer progression [38, 39]. In this study, we performed GO enrichment analysis of melanoma and NHL‐related genes, and the results indicated that vitamin D may be involved in the development of coronary artery disease. There was growing evidence that vitamin D is important in reducing the risk of cardiovascular diseases and VTE [40, 41, 42, 43]. Blondon et al. reported associations between serum 25‐hydroxyvitamin D, plasminogen activator inhibitor‐1 concentrations and tissue factor pathway inhibitors. In addition, the same group found that vitamin d‐deficient participants had a propensity for hypofibrinolysis, suggesting a higher risk of VTE [44]. However, Hejazi and colleagues reported in their pilot randomized clinical trial that there was no significant reduction in C‐reactive protein or P‐selectin after vitamin D supplementation. Interestingly, vitamin D supplementation did allow the use of lower doses of warfarin to control the international normalized ratio (INR) [45]. These results taken together suggested that the role of vitamin D in CAT development should be further investigated, especially in patients with melanoma and NHL.
Given that this study showed no causal relationship between most types of cancer and VTE risk, careful consideration should be given to patients who would and would not benefit from screening and prophylaxis after cancer diagnosis. Moreover, our results supported the notion that certain iatrogenic factors increased the risk of VTE, such as screening imaging techniques, indwelling central venous catheters, history of thrombosis and surgeries, chemotherapy and hormone therapy [11, 13, 46]. Although thromboprophylaxis is an effective means of VTE prevention and treatment, it also increases the risk of bleeding, morbidity and mortality [47]. In fact, according to the American Society of Clinical Oncology guidelines, clinicians should not provide routine anticoagulant therapy to all cancer patients, but should only provide routine anticoagulant therapy to patients at high risk for VTE [47]. Being able to accurately quantify the risk of VTE in patients with different types of cancer will allow for optimal thromboprophylaxis strategies, allowing treatment to be targeted to high‐risk patients while avoiding low‐risk patients.
This study found a causal relationship between NHL and VTE risk. Therefore, patients with NHL require more stringent VTE screening. However, this may not be clinically feasible; instead, from a public health and clinical viewpoint, more attention should be paid to prophylactic thromboembolic interventions and more rigorous testing for thrombosis in NHL patients [48]. Unprovoked VTE events in the absence of major thrombotic risk factors may be the earliest signs of cancer [10]. If risk factors associated with occult cancer can be distinguished, patients can be stratified by different cancer risks. Detection of unprovoked VTE can serve as the basis for effective screening and prevention strategies. In addition, patients with occult malignant melanoma may benefit from early VTE testing and intervention when unprovoked VTE occurs.
Our work had several advantages. First, MR provided an opportunity to efficiently and reliably investigate the potential causal relationship between cancer types and VTE, regardless of reverse causality and confounding factors inherent in observational studies. This methodological difference may also explain why we found results that were inconsistent with those of previous studies. Second, this study included a heterogeneous population of VTE patients, and we also obtained and pooled data from the UK Biobank and GERA cohorts, particularly for the investigation of VTE in sex‐specific cancers (e.g., prostate, cervical and breast cancers). To our knowledge, this is the first comprehensive MR analysis to assess the causal relationship between various cancers and VTE in a population of approximately 370,000 individuals of European ancestry. Such a large cohort may be particularly important for stratifying cancer patient groups. Our results can guide clinicians on what aspects of tumor screening should be performed in patients with unprovoked VTE. What more, policymakers could use the study's findings to inform testing guidelines based on cancer types, such as more stringent testing for thrombosis and even preventive anticoagulation therapy.
However, this study also has several limitations. First, because this study was conducted only in a population of single European ancestry, the results may not apply to other ethnic populations. Future research should focus on including more diverse cohorts. Second, some misclassification may have been introduced, since exposed parts were defined by validated self‐reports. Furthermore, endpoints were obtained from a computerized registry and were not personally verified. Although we have shown that different genes predict cancers with different VTE risk, we were unable to explore the precise physiological mechanisms that might mediate the relationship between these cancers and VTE risk.
5. CONCLUSION
In conclusion, our estimates suggested that genetically predicted melanoma was associated with a lower risk of VTE, whereas NHL increases this risk. In contrast, cancers of multiple sites, including bladder, cervix, colon, stomach and esophagus, kidney, leukemia, lung, pancreas, prostate, rectum, thyroid and breast cancers, were not associated with VTE. Understanding the causal relationship between various cancers and VTE can lay the foundation for targeted therapy and prevention guidelines for CAT in selected high‐risk patient populations.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: Xiong Chen, Xiaosi Hong, Kai Huang, Guochang Liu, and Qinchang Chen. Performed and analyzed the experiments: Xiong Chen, Xiaosi Hong, Jiahao Cai, and Guiwu Huang. Wrote the manuscript: Xiong Chen and Xiaosi Hong. Read and approved the final manuscript: Jiahao Cai, Guiwu Huang, Gaochen Bai, Wen Fu, Kai Huang, Guochang Liu, and Qinchang Chen.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was conducted with publicly available data and was previously approved by corresponding consortiums.
INFORMED CONSENT
Not applicable.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
We thank the participants of UK Biobank, FinnGen and GERA consortium.
Chen X, Hong X, Luo S, Cai J, Huang G, Shen R, et al. Causal relationship between 14 site‐specific cancers and venous thromboembolism. Cancer Innovation. 2022;1:316–327. 10.1002/cai2.36
Xiong Chen and Xiaosi Hong contributed equally to this study.
Contributor Information
Guochang Liu, Email: starbless2003@126.com.
Kai Huang, Email: huangk37@mail.sysu.edu.cn.
Qinchang Chen, Email: chenqinchang@gdph.org.cn.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are publicly available and accessible. Data derived from public domain resources.
REFERENCES
- 1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–30. 10.1002/cncr.33587 [DOI] [PubMed] [Google Scholar]
- 2. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122(10):1712–23. 10.1182/blood-2013-04-460121 [DOI] [PubMed] [Google Scholar]
- 3. Fuentes HE, Tafur AJ, Caprini JA. Cancer‐associated thrombosis. Dis Mon. 2016;62(5):121–58. 10.1016/j.disamonth.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 4. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):3–14. 10.1007/s11239-015-1311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang TF, Billett HH, Connors JM, Soff GA. Approach to cancer‐associated thrombosis: challenging situations and knowledge gaps. Oncologist. 2021;26(1):e17–23. 10.1002/onco.13570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. The Lancet. 2014;383(9921):955–62. 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 7. Chen DY, Tseng CN, Hsieh MJ, Lan WC, Chuang CK, Pang ST, et al. Comparison between non‐vitamin k antagonist oral anticoagulants and low‐molecular‐weight heparin in Asian individuals with cancer‐associated venous thromboembolism. JAMA Netw Open. 2021;4(2):e2036304. 10.1001/jamanetworkopen.2020.36304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robertson L, Yeoh SE, Stansby G, Agarwal R. Effect of testing for cancer on cancer‐ and venous thromboembolism (VTE)‐related mortality and morbidity in people with unprovoked VTE. Cochrane Database Syst Rev. 2017;8(8):Cd010837. 10.1002/14651858.CD010837.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Es N, Le Gal G, Otten HM, Robin P, Piccioli A, Lecumberri R, et al. Screening for occult cancer in patients with unprovoked venous thromboembolism: a systematic review and meta‐analysis of individual patient data. Ann Intern Med. 2017;167(6):410–7. 10.7326/M17-0868 [DOI] [PubMed] [Google Scholar]
- 10. Ihaddadene R, Corsi DJ, Lazo‐Langner A, Shivakumar S, Zarychanski R, Tagalakis V, et al. Risk factors predictive of occult cancer detection in patients with unprovoked venous thromboembolism. Blood. 2016;127(16):2035–7. 10.1182/blood-2015-11-682963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mulder FI, Horváth‐Puhó E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137(14):1959–69. 10.1182/blood.2020007338 [DOI] [PubMed] [Google Scholar]
- 12. Bar J, Markel G, Gottfried T, Percik R, Leibowitz‐Amit R, Berger R, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single‐institute retrospective study. Eur J Cancer. 2019;120:122–31. 10.1016/j.ejca.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 13. Swan D, Rocci A, Bradbury C, Thachil J. Venous thromboembolism in multiple myeloma ‐ choice of prophylaxis, role of direct oral anticoagulants and special considerations. Br J Haematol. 2018;183(4):538–56. 10.1111/bjh.15684 [DOI] [PubMed] [Google Scholar]
- 14. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Gen. 2014;23(R1):R89–98. 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X, Kong J, Diao X, Cai J, Zheng J, Xie W, et al. Depression and prostate cancer risk: a Mendelian randomization study. Cancer Med. 2020;9(23):9160–7. 10.1002/cam4.3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rashkin SR, Graff RE, Kachuri L, Thai KK, Alexeeff SE, Blatchins MA, et al. Pan‐cancer study detects genetic risk variants and shared genetic basis in two large cohorts. Nat Commun. 2020;11(1):4423. 10.1038/s41467-020-18246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X, Kong J, Pan J, Huang K, Zhou W, Diao X, et al. Kidney damage causally affects the brain cortical structure: a Mendelian randomization study. EBioMedicine. 2021;72:103592. 10.1016/j.ebiom.2021.103592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–52. 10.1093/ije/dyq151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype‐phenotype associations. Bioinformatics. 2016;32(20):3207–9. 10.1093/bioinformatics/btw373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist‐oriented resource for the analysis of systems‐level datasets. Nat Commun. 2019;10(1):1523. 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buka RJ, Chandra D, Sutton DJ. Cancer is not a single disease: is it safe to extrapolate evidence from trials of direct oral anticoagulants in cancer‐associated venous thromboembolism to patients with haematological malignancies? Br J Haematol. 2021;193(1):194–7. 10.1111/bjh.17123 [DOI] [PubMed] [Google Scholar]
- 23. van Dam LF, Dronkers CEA, Gautam G, Eckerbom Å, Ghanima W, Gleditsch J, et al. Magnetic resonance imaging for diagnosis of recurrent ipsilateral deep vein thrombosis. Blood. 2020;135(16):1377–85. 10.1182/blood.2019004114 [DOI] [PubMed] [Google Scholar]
- 24. Roopkumar J, Swaidani S, Kim AS, Thapa B, Gervaso L, Hobbs BP, et al. Increased incidence of venous thromboembolism with cancer immunotherapy. Med. 2021;2(4):423–434.e3. 10.1016/j.medj.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falanga A, Marchetti M, Russo L. Venous thromboembolism in the hematologic malignancies. Curr Opin Oncol. 2012;24(6):702–10. 10.1097/CCO.0b013e3283592331 [DOI] [PubMed] [Google Scholar]
- 26. Paneesha S, McManus A, Arya R, Scriven N, Farren T, Nokes T, et al. Frequency, demographics and risk (according to tumour type or site) of cancer‐associated thrombosis among patients seen at outpatient DVT clinics. Thromb Haemost. 2010;103(2):338–43. 10.1160/TH09-06-0397 [DOI] [PubMed] [Google Scholar]
- 27. Ratib S, Walker AJ, Card TR, Grainge MJ. Risk of venous thromboembolism in hospitalised cancer patients in England‐a cohort study. J Hematol Oncol. 2016;9(1):60. 10.1186/s13045-016-0291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hisada Y, Mackman N. Cancer‐associated pathways and biomarkers of venous thrombosis. Blood. 2017;130(13):1499–506. 10.1182/blood-2017-03-743211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahajan A, Brunson A, White R, Wun T. The epidemiology of cancer‐associated venous thromboembolism: an update. Semin Thromb Hemost. 2019;45(4):321–5. 10.1055/s-0039-1688494 [DOI] [PubMed] [Google Scholar]
- 30. Caruso V, Di Castelnuovo A, Meschengieser S, Lazzari MA, de Gaetano G, Storti S, et al. Thrombotic complications in adult patients with lymphoma: a meta‐analysis of 29 independent cohorts including 18 018 patients and 1149 events. Blood. 2010;115(26):5322–8. 10.1182/blood-2010-01-258624 [DOI] [PubMed] [Google Scholar]
- 31. Ahlbrecht J, Dickmann B, Ay C, Dunkler D, Thaler J, Schmidinger M, et al. Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2012;30(31):3870–5. 10.1200/JCO.2011.40.1810 [DOI] [PubMed] [Google Scholar]
- 32. Sparsa A, Durox H, Doffoel‐Hantz V, Munyangango EM, Bédane C, Cendras J, et al. High prevalence and risk factors of thromboembolism in stage IV melanoma. J Eur Acad Dermatol Venereol. 2011;25(3):340–4. 10.1111/j.1468-3083.2010.03795.x [DOI] [PubMed] [Google Scholar]
- 33. Walker AJ, West J, Card TR, Crooks C, Kirwan CC, Grainge MJ. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood. 2016;127(7):849–57. 10.1182/blood-2015-01-625582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wood P, Boyer G, Mehanna E, Cagney D, Lamba N, Catalano P, et al. Intracerebral haemorrhage in patients with brain metastases receiving therapeutic anticoagulation. J Neurol Neurosurg Psychiatry. 2021;92:655–61. 10.1136/jnnp-2020-324488 [DOI] [PubMed] [Google Scholar]
- 35. Trousseau A. Clinique médicale de l'Hôtel‐Dieu de Paris. Trousseau: Tome 2/par A; 1865. [Google Scholar]
- 36. Sussman TA, Li H, Hobbs B, Funchain P, McCrae KR, Khorana AA. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother Cancer. 2021;9(1):e001719. 10.1136/jitc-2020-001719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muhsin‐Sharafaldine MR, Saunderson SC, Dunn AC, Faed JM, Kleffmann T, McLellan AD. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget. 2016;7(35):56279–94. 10.18632/oncotarget.10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desch A, Gebhardt C, Utikal J, Schneider SW. D‐dimers in malignant melanoma: association with prognosis and dynamic variation in disease progress. Int J Cancer. 2017;140(4):914–21. 10.1002/ijc.30498 [DOI] [PubMed] [Google Scholar]
- 39. Cañadas I, Taus Á, Villanueva X, Arpí O, Pijuan L, Rodríguez Y, et al. Angiopoietin‐2 is a negative prognostic marker in small cell lung cancer. Lung Cancer. 2015;90(2):302–6. 10.1016/j.lungcan.2015.09.023 [DOI] [PubMed] [Google Scholar]
- 40. Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–41. 10.1016/j.jacc.2011.03.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brøndum‐Jacobsen P, Benn M, Tybjaerg‐Hansen A, Nordestgaard BG. 25‐Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18,791 participants. J Thromb Haemostasis. 2013;11(3):423–31. 10.1111/jth.12118 [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, et al. Circulating 25‐hydroxy‐vitamin D and risk of cardiovascular disease: a meta‐analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–29. 10.1161/CIRCOUTCOMES.112.967604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berwick M, Armstrong BK, Ben‐Porat L, Fine J, Kricker A, Eberle C, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97(3):195–9. 10.1093/jnci/dji019 [DOI] [PubMed] [Google Scholar]
- 44. Blondon M, Cushman M, Jenny N, Michos ED, Smith NL, Kestenbaum B, et al. Associations of serum 25‐hydroxyvitamin D with hemostatic and inflammatory biomarkers in the multi‐ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2016;101(6):2348–57. 10.1210/jc.2016-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hejazi ME, Modarresi‐Ghazani F, Hamishehkar H, Mesgari‐Abbasi M, Dousti S, Entezari‐Maleki T. The effect of treatment of vitamin D deficiency on the level of P‐selectin and hs‐CRP in patients with thromboembolism: a pilot randomized clinical trial. J Clin Pharmacol. 2017;57(1):40–7. 10.1002/jcph.774 [DOI] [PubMed] [Google Scholar]
- 46. Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566–81. 10.1016/S1470-2045(19)30336-5 [DOI] [PubMed] [Google Scholar]
- 47. Stockler MR. ASCO updated recommendations for preventing and treating VTE in adults with cancer. Ann Intern Med. 2020;172(2):JC2. 10.7326/ACPJ202001210-002 [DOI] [PubMed] [Google Scholar]
- 48. Luo S, Au Yeung SL, Zuber V, Burgess S, Schooling CM. Impact of genetically predicted red blood cell traits on venous thromboembolism: multivariable mendelian randomization study using UK biobank. J Am Heart Assoc. 2020;9(14):e016771. 10.1161/JAHA.120.016771 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The datasets used and/or analyzed during the current study are publicly available and accessible. Data derived from public domain resources.


