Abstract
Recent studies have shown that individual amino acid exchanges within elongation factor G (EF-G) cause fusidic acid resistance in Staphylococcus aureus. The data from the present study illustrate that the fusidic acid resistance-mediating amino acid substitutions P406L and H457Y are associated with a marked impairment of the biological fitness of S. aureus. In particular, strains producing EF-G derivatives with these mutations showed reduced growth, decreased plasma coagulase activity, and an impaired capability to compete with the isogenic wild-type strain. Second-site mutations within EF-G, such as A67T and S416F, that have been encountered in clinical fusidic acid-resistant isolates containing the amino acid exchanges P406L and H457Y, respectively, were shown not to contribute to resistance. Furthermore, the substitution A67T had no impact on the biological fitness in vitro. The exchange S416F, however, was found to function as a fitness-compensating mutation in S. aureus carrying the substitution H457Y in EF-G. In conclusion, the data presented in this report provide evidence at the molecular level that the deleterious effects of fusidic acid resistance-mediating exchanges within EF-G of S. aureus can be reduced considerably by specific compensating mutations in this target protein. This compensatory adaptation most likely plays a significant role in the stabilization of resistant bacteria within a given population.
Fusidic acid is an effective antibiotic for the treatment of severe Staphylococcus aureus infections. It inhibits bacterial protein synthesis by interacting with a complex of elongation factor G (EF-G) and the ribosome. In the presence of fusidic acid, the release of EF-G-GDP from the ribosome is prevented, and consequently, the next stage in translation is sterically blocked (6, 16). Alteration of the target protein EF-G by specific individual amino acid substitutions confers resistance to fusidic acid and is the major mechanism of resistance, in addition to plasmid-mediated resistance, which is believed to be due to the decreased permeability of the bacterial envelope (3, 8, 22, 27).
In vitro, fusidic acid-resistant S. aureus strains can readily be selected by exposure to this antibiotic (24). In the clinical setting, however, resistance to fusidic acid is uncommon, despite its clinical use for the treatment of staphylococcal infections for more than 35 years (10, 23). Recently, however, several publications have described a striking increase in the incidence of resistance to fusidic acid among clinical isolates of methicillin-susceptible S. aureus (7). Furthermore, the emergence of community-acquired methicillin-resistant S. aureus strains that show fusidic acid resistance and that produce the virulence factor Panton-Valentine leukocidin has been observed (9, 29).
This spread of fusidic acid-resistant S. aureus strains is remarkable, as fusidic acid resistance was speculated to be associated with a biological fitness cost for the resistant bacteria (1). Indeed, chromosomal mutations that cause alteration of essential proteins often have deleterious effects on bacterial fitness, resulting in reduced growth, impaired virulence, and poor survival (26, 28). Some bacterial species, however, can compensate for these fitness costs during growth in vitro as well as in vivo, usually without a loss of resistance (2). In Salmonella enterica serovar Typhimurium, for example, this compensatory adaptation was suggested to be associated with second-site mutations within the target protein (20). Likewise, in S. aureus individual amino acid substitutions in EF-G have been supposed to compensate for the fitness costs of fusidic acid resistance (21), but the causal relationship between such mutations and fitness compensation has not been demonstrated so far at the molecular level.
Recently, we demonstrated that the amino acid exchanges P406L, H457Y, and L461K within EF-G lead to different levels of fusidic acid resistance in S. aureus (3). Furthermore, we observed that clinical S. aureus isolates harboring these resistance-mediating mutations frequently carry second-site mutations within EF-G (e.g., A67T and P406L, or S416F and H457Y). Hence, this study was aimed at (i) analyzing the biological costs of the fusidic acid resistance-mediating exchanges P406L and H457Y and (ii) investigating whether the additional substitutions A67T and S416F in EF-G function as fitness-compensating mutations.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
E. coli strain DH5α [F′ φ80 dlacZΔM15 Δ(lacZYA argF)U169 deoR recA1 endA1 phoA hsdR17 (rK− mK+) supE44 λ− thi-1 gyrA96 relA1] was used as the host strain for the construction and propagation of plasmids as well as for site-directed mutagenesis. Fusidic acid-susceptible S. aureus strain RN4220 (14) was used to determine the effects of amino acid substitutions in EF-G on the resistance and fitness of S. aureus. All plasmids used in this study are listed in Table 1. The bacteria were grown aerobically at 37°C on yeast extract-tryptone (YT) medium (yeast extract, 5 g/liter; tryptone, 8 g/liter; NaCl, 5 g/liter) solidified with 1.5% (wt/vol) agar or in 2× YT liquid medium. Antibiotics were used at the following final concentrations: ampicillin, 100 μg/ml; chloramphenicol, 10 μg/ml; erythromycin, 10 μg/ml.
TABLE 1.
Plasmids used in this work
| Plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| pUC19 | Cloning vector; Ampr | New England Biolabs |
| pSB1 | pUC19 carrying fusA of S. aureus RN4220 | 3 |
| pHPS9 | E. coli-S. aureus shuttle and expression vector; Eryr Cmr | 11 |
| pSB2 | pHPS9 carrying fusA of S. aureus RN4220 | 3 |
| pSB2-P406L | pSB2 with mutant fusA (P406L) | 3 |
| pSB2-H457Y | pSB2 with mutant fusA (H457Y) | 3 |
| pSB2-A67T | pSB2 with mutant fusA (A67T) | This work |
| pSB2-A67T/P406L | pSB2 with mutant fusA (A67T, P406L) | This work |
| pSB2-S416F | pSB2 with mutant fusA (S416F) | This work |
| pSB2-S416F/H457Y | pSB2 with mutant fusA (S416F, H457Y) | This work |
The mutant fusA alleles on the pSB2 derivatives encode EF-G with the amino acid exchanges given in parentheses.
Standard DNA techniques.
DNA manipulations and cloning procedures were carried out by standard protocols (25). Site-directed mutagenesis was performed with the QuikChange site-directed mutagenesis kit (Stratagene). The nucleotide sequences of DNA fragments were determined by cycle sequencing with an ABI PRISM DNA sequencer (Applied Biosystems).
Construction of plasmids.
Plasmid pSB1, which carries the wild-type fusA gene of fusidic acid-susceptible S. aureus strain RN4220 (3), was used for construction of mutant fusA alleles encoding EF-G derivatives with defined amino acid substitutions. Site-directed mutagenesis was used to construct fusA alleles encoding EF-G with the amino acid exchanges A67T, A67T and P406L, S416F, and S416F and H457Y. The presence of all mutations generated was confirmed by DNA sequence analysis. Subsequently, mutant fusA alleles were excised from the corresponding pSB1 derivatives by cleavage with SphI and BamHI and cloned into the E. coli-S. aureus shuttle expression vector pHPS9. In the resulting plasmids, pSB2-A67T, pSB2-A67T/P406L, pSB2-S416F, and pSB2-S416F/H457Y, fusA is under the control of the lactococcal promoter 59 that is provided by the vector (11).
Isolation of RNA and RT-PCR.
To isolate the total RNA of S. aureus RN4220 carrying pSB2 or pSB2 derivatives, the strains were grown in 2× YT medium supplemented with erythromycin and chloramphenicol. The bacteria were harvested in the mid-logarithmic growth phase (optical density at 600 nm [OD600] = 0.7 to 0.9). Before cell lysis, the bacteria were treated with RNAprotect Bacteria Reagent (Qiagen), according to the instructions of the manufacturer. This procedure provides immediate stabilization of the RNA. Lysis of the cells was achieved by suspending the bacteria in TE buffer (10 mM Tris-HCl, 1 mM sodium EDTA [pH 8.0]) containing lysostaphin at a concentration of 1 mg/ml, followed by incubation for 30 min at 37°C. Subsequently, the total RNA of the bacteria was isolated with the RNeasy Mini kit (Qiagen), according to the protocol provided by the manufacturer. Residual amounts of DNA were removed during RNA isolation by on-column DNase I digestion with the RNase-free DNase set from Qiagen.
Reverse transcription (RT)-PCR analysis of the expression of plasmid-encoded wild-type and mutant fusA was performed with the Qiagen OneStep RT-PCR kit, according to the recommendations of the manufacturer. The fusA-specific oligonucleotide P10 (5′-GAATTCAATGTGAACATCACCG-3′) was used as the primer for RT of the fusA mRNA; PCR amplification of the generated cDNA was conducted with the fusA-specific forward primer P9 (5′-GCGGTAGGTCTTAAAGATACAGG-3′) and the reverse primer P10 to yield a PCR product of 410 bp. The best results were obtained when approximately 100 pg of template RNA was used for the RT-PCR.
Testing of susceptibility to fusidic acid.
The susceptibilities of the S. aureus strains to fusidic acid were analyzed by determining the MIC of this antibiotic. MICs were determined with Etest strips (AB Biodisk, Solna, Sweden), according to the recommendations of the manufacturer.
Determination of biological fitness.
The influences of amino acid exchanges within EF-G on the biological fitness of S. aureus RN4220 were analyzed by measuring growth kinetics as well as by means of plasma coagulase activity assays and pairwise competition assays.
To analyze growth kinetics, the recombinant S. aureus strains were grown to the logarithmic growth phase (OD600 = 1). A total of 400 μl of each culture with an OD600 of 1 was inoculated into 10 ml of fresh medium and incubated with shaking (200 rpm) at 37°C. Bacterial growth was recorded for a total period of 10 h by measuring the OD600 of the cultures at intervals of 1 h.
The plasma coagulase activities of the recombinant S. aureus strains were measured with BBL Coagulase Plasma (Becton Dickinson), according to the recommendations of the manufacturer. For each assay, 500 μl of coagulase plasma was mixed with 50 μl of an overnight culture of the corresponding S. aureus strain and the mixture was incubated at 37°C. The plasma coagulase activity was determined every half hour by recording the formation of fibrin clots. Evaluation was as follows: 0, no evidence of fibrin formation; 1+, small unorganized clots; 2+, small organized clots; 3+, large organized clots; 4+, entire coagulation of the content of the test tube without dislocation after inversion.
For competition assays, 10 ml of 2× YT medium was inoculated with equal amounts (200 μl of a culture grown to an OD600 of 1) of a fusidic acid-resistant strain (strain X) harboring a mutant fusA allele on its multicopy plasmid and an isogenic fusidic acid-susceptible strain (strain Y) carrying the wild-type fusA gene. Subsequently, the mixture was incubated with shaking at 37°C for 16 h. The numbers of CFU were determined at the beginning and at the end of the incubation period by plating serial dilutions of the culture on YT agar plates containing either no fusidic acid or 1 μg of fusidic acid per ml. The number of generations (G) of the competing strains was calculated by the formula (log B − log A)/log2, with A and B being the numbers of CFU per milliliter at the beginning and at the end of the culture period, respectively (4). Finally, the relative fitness of the fusidic acid-resistant strain was determined by the formula Gstrain X/Gstrain Y. The experiments were performed in triplicate.
RESULTS
To study the influence of amino acid substitutions in EF-G on the fusidic acid susceptibility and fitness of S. aureus, plasmid pSB2 harboring the wild-type fusA gene and pSB2 derivatives carrying distinct mutant fusA alleles were introduced into fusidic acid-susceptible S. aureus strain RN4220. The different RN4220 clones were subsequently analyzed with regard to growth kinetics, plasma coagulase activity, and competition competence, as shown in greater detail below. The pSB2 derivatives pSB2-P406L and pSB2-H457Y, which contained site-directed mutations in fusA that cause in EF-G the amino acid substitutions P406L and H457Y, respectively, were available from a previous study (3). Other pSB2 derivatives encoding mutant EF-G with the amino acid substitutions A67T (pSB2-A67T), A67T and P406L (pSB2-A67T/P406L), S416F (pSB2-S416F), and S416F and H457Y (pSB2-S416F/H457Y) were constructed by site-directed mutagenesis, as described in Materials and Methods.
Fusidic acid susceptibilities of recombinant S. aureus strains.
As shown in Table 2, recombinant S. aureus RN4220 clones expressing EF-G derivatives with the single amino acid exchanges P406L (EF-GP406L) and H457Y (EF-GH457Y) exhibited fusidic acid resistance, insofar as the MICs of 8 and 64 μg/ml, respectively, were far above the MIC determined for the isogenic strain expressing wild-type EF-G (0.032 μg/ml). In contrast, the individual amino acid exchanges A67T and S416F in EF-G were demonstrated not to contribute to fusidic acid resistance in S. aureus, as deduced from the following findings. First, the fusidic acid MICs for the recombinant RN4220 clones expressing EF-G derivatives with the single amino acid exchanges A67T (EF-GA67T) and S416F (EF-GS416F) were identical to the MIC measured for the isogenic fusidic acid-susceptible strain producing wild-type EF-G. Second, recombinant RN4220 clones expressing EF-G with the double amino acid exchanges A67T and P406L (EF-GA67T/P406L) and S416F and H457Y (EF-GS416F/H457Y) exhibited the same levels of fusidic acid resistance as those clones containing only the single substitutions P406L and H457Y, respectively.
TABLE 2.
Fusidic acid susceptibilities of S. aureus strains
| S. aureus strain | Substitution(s) in EF-Ga | Fusidic acid MIC (μg/ml)b |
|---|---|---|
| T52 (clinical isolate) | A67T, P406L | 8 |
| T121 (clinical isolate) | S416F, H457Y | 64 |
| RN4220 | 0.032 | |
| RN4220/pSB2 | 0.032 | |
| RN4220/pSB2-A67T | A67T | 0.032 |
| RN4220/pSB2-P406L | P406L | 8 |
| RN4220/pSB2-A67T/P406L | A67T, P406L | 8 |
| RN4220/pSB2-S416F | S416F | 0.032 |
| RN4220/pSB2-H457Y | H457Y | 64 |
| RN4220/pSB2-S416F/H457Y | S416F, H457Y | 64 |
The amino acid exchanges given for the recombinant RN4220 derivatives refer to those in plasmid-encoded EF-G.
MICs were measured with Etest strips.
Analysis of fusA expression by RT-PCR.
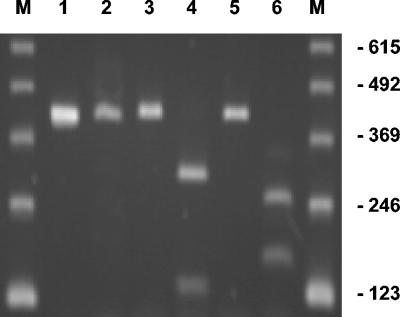
To confirm that the wild-type and mutant fusA alleles present in pSB2 and the pSB2 derivatives are indeed expressed in the recombinant S. aureus RN4220 strains harboring these plasmids, the fusA-specific mRNA levels in log-phase cultures of three of these strains, i.e., S. aureus RN4220/pSB2, S. aureus RN4220/pSB2-S416F, and S. aureus RN4220/pSB2-H457Y, were analyzed by RT-PCR. When oligonucleotides P9 and P10 were used as RT-PCR primers, the expected 410-bp fragment of fusA was amplified from all three strains (Fig. 1). A unique DraI restriction site in fusA-S416F and a unique EcoRV site in fusA-H457Y were used to discriminate between RT-PCR products that were amplified from transcripts of the chromosomal wild-type fusA gene of S. aureus RN4220 and those that were amplified from transcripts of the plasmid-encoded fusA gene. The DraI site, which is located 120 bp downstream from the 5′ end of the 410-bp RT-PCR product, and the EcoRV site, which is located 248 bp downstream from the 5′ end of the 410-bp RT-PCR product, were introduced together with the mutations causing the amino acid substitutions S416F and H457Y, respectively, to permit identification of mutant clones by restriction analysis. Indeed, most of the 410-bp fragments amplified from total RNA of S. aureus RN4220/pSB2-S416F and S. aureus RN4220/pSB2-H457Y were cleaved into two fragments by DraI and EcoRV, respectively. In both cases, the sizes of these fragments corresponded to those of the anticipated cleavage products (fusA-S416F, 120- and 290-bp fragments; fusA-H457Y, 248- and 162-bp fragments), showing that the plasmid-encoded mutant fusA genes were efficiently transcribed (Fig. 1). In contrast, the 410-bp RT-PCR product amplified from the RNA of S. aureus RN4220/pSB2 was not cleaved by either DraI or EcoRV, which is consistent with the fact that pSB2 contains the wild-type fusA gene.
FIG. 1.
Analysis of the expression of the plasmid-encoded fusA alleles in S. aureus RN4220/pSB2, S. aureus RN4220/pSB2-S416F, and RN4220/pSB2-H457Y by RT-PCR. RT-PCR was performed with the fusA-specific forward primer P9 and the reverse primer P10 with 100 pg of total RNA as the template. The PCR products obtained from S. aureus RN4220/pSB2 (lanes 1 and 2), S. aureus RN4220/pSB2-S416F (lanes 3 and 4), and S. aureus RN4220/pSB2-H457Y (lanes 5 and 6) were either directly separated by agarose gel electrophoresis (lanes 1, 3, and 5) or incubated with DraI (lanes 2 and 4) or EcoRV (lanes 2 and 6) before separation on the agarose gel. Lanes M, 123-bp DNA ladder (Invitrogen); the sizes of the marker fragments are given in base pairs.
Growth kinetics of recombinant S. aureus strains.
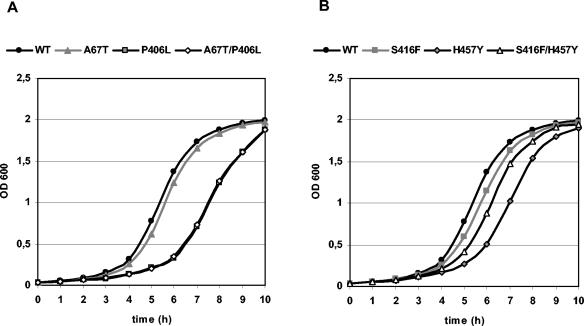
Evaluation of the growth kinetics of the recombinant S. aureus RN4220 derivatives harboring wild-type or mutant fusA revealed that the fusidic acid resistance-conferring single amino acid exchanges P406L and H457Y cause marked decreases in the growth rates (Fig. 2; Table 3). In contrast, the fusidic acid-susceptible clones expressing EF-GA67T and EF-GS416F, respectively, grew nearly as fast as the strain producing wild-type EF-G. Strikingly, however, a significant increase in the growth rate was observed for the fusidic acid-resistant clone producing the EF-G derivative with the double exchange S416F and H457Y compared with that of the strain containing solely the resistance-mediating substitution H457Y in EF-G. Hence, it can be concluded that the additional substitution S416F can compensate for the biological costs associated with the fusidic acid resistance-mediating amino acid exchange H457Y. The substitution A67T, however, was not able to compensate for the fitness loss caused by the resistance-mediating amino acid exchange P406L since the clone expressing EF-GA67T/P406L and the strain expressing EF-GP406L showed similar growth kinetics.
FIG. 2.
Growth kinetics of recombinant S. aureus strains. (A) RN4220/pSB2 (wild type [WT]), RN4220/pSB2-A67T (A67T), RN4220/pSB2-P406L (P406L), RN4220/pSB2-A67T/P406L (A67T/P406L); (B) RN4220/pSB2 (WT), RN4220/pSB2-S416F (S416F), RN4220/pSB2-H457Y (H457Y), and RN4220/pSB2-S416F/H457Y (S416F/H457Y). Experiments were performed in triplicate. The results of one experiment are shown as an example.
TABLE 3.
Comparison of growth of recombinant S. aureus strains after 6 h
| S. aureus strain | Ratio of OD600 for EF-G mutant vs OD600 for RN4220/pSB2a |
|---|---|
| RN4220/pSB2-A67T | 0.9 ± 0.04 |
| RN4220/pSB2-P406L | 0.24 ± 0.01 |
| RN4220/pSB2-A67T/P406L | 0.27 ± 0.03 |
| RN4220/pSB2-S416F | 0.86 ± 0.06 |
| RN4220/pSB2-H457Y | 0.37 ± 0.06 |
| RN4220/pSB2-S416F/H457Y | 0.64 ± 0.09b |
Results are expressed as the means ± SEMs of three experiments.
P < 0.05 compared with the results for RN4220/pSB2-H457Y.
Plasma coagulase activities of recombinant S. aureus strains.
The recombinant S. aureus strain harboring the wild-type fusA gene and the fusidic acid-susceptible strains expressing EF-GA67T and EF-GS416F, respectively, exhibited the highest activities of plasma coagulase, which is known to be one of the most important virulence factors of S. aureus (Table 4). In contrast, clones containing only the resistance-mediating substitutions P406L and H457Y, respectively, as well as the strain harboring the double exchange A67T and P406L in EF-G showed the lowest plasma coagulase activities. The fusidic acid-resistant strain expressing EF-GS416F/H457Y, however, displayed stronger plasma coagulase activity than the strain containing only the resistance-mediating amino acid substitution H457Y, although its activity did not reach that of the strain expressing wild-type EF-G. In line with the results of the growth kinetics studies, these data further confirm that the amino acid substitution S416F in EF-G can compensate for the biological costs associated with the resistance-mediating exchange H457Y, whereas the substitution A67T cannot reduce the deleterious effects caused by the resistance-conferring exchange P406L.
TABLE 4.
Plasma coagulase activities of recombinant S. aureus strainsa
| S. aureus strain | Plasma coagulase activity after incubation for:
|
|||||
|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 1.5 h | 2 h | 2.5 h | 3 h | |
| RN4220/pSB2 | 0 | 0 | 3+ | 4+ | 4+ | 4+ |
| RN4220/pSB2-A67T | 0 | 0 | 2+ | 4+ | 4+ | 4+ |
| RN4220/pSB2-P406L | 0 | 0 | 0 | 2+ | 3+ | 4+ |
| RN4220/pSB2-A67T/P406L | 0 | 0 | 0 | 2+ | 3+ | 4+ |
| RN4220/pSB2-S416F | 0 | 0 | 2+ | 4+ | 4+ | 4+ |
| RN4220/pSB2-H457Y | 0 | 0 | 0 | 2+ | 3+ | 4+ |
| RN4220/pSB2-S416F/H457Y | 0 | 0 | 0 | 3+ | 4+ | 4+ |
Experiments were performed in triplicate. The results of one experiment are shown as a representative example. The different levels of plasma coagulase activity are defined in Materials and Methods.
Competition assays.
Competition assays were performed to further demonstrate that the amino acid substitution S416F in EF-G compensates for the fitness loss associated with the fusidic acid resistance-conferring amino acid substitution H457Y. In particular, the recombinant S. aureus strain expressing wild-type EF-G (RN4220/pSB2) was cocultivated either with the isogenic clone RN4220/pSB2-H457Y producing EF-GH457Y or with the clone RN4220/pSB2-S416F/H457Y expressing EF-GS416F/H457Y. After incubation for 18 h the abilities of the clones producing mutant EF-G derivatives to compete with the wild-type strain were analyzed by determining the numbers of generations for each strain and calculating their relative fitness, as described in Materials and Methods. The relative fitness of the fusidic acid-resistant clone expressing EF-GH457Y was determined to be −0.75 (standard error of the mean [SEM], ±0.17). This negative fitness value indicates that the cell number of the fusidic acid-resistant clone declined during the incubation period, whereas the cell number of the strain expressing wild-type EF-G increased substantially. In contrast, the relative fitness of the fusidic acid-resistant clone expressing EF-GS416F/H457Y was determined to be -0.19 (SEM, ±0.09), which indicates a significant increase in fitness compared to that of the strain producing EF-GH457Y (P < 0.05).
DISCUSSION
The data presented in this study clearly demonstrate that distinct fusidic acid resistance-mediating mutations within EF-G cause a marked impairment of the biological fitness in S. aureus and that second-site mutations in EF-G can compensate for these resistance costs.
In particular, the resistance-conferring amino acid substitutions P406L and H457Y were shown to perturb the functionality of EF-G, with detrimental effects on biological fitness, as documented by reduced growth, decreased plasma coagulase activity, and an impaired capability to compete with the isogenic strain expressing wild-type EF-G. Interestingly, the level of fusidic acid resistance appears to have no influence on the degree of the fitness burden since the low-level resistance-conferring mutation P406L and the intermediate-level resistance-mediating mutation H457Y similarly affected the biological fitness.
It has been convincingly argued that bacteria which are resistant to antibiotics due to chromosomal mutations may acquire certain second-site mutations that ameliorate these resistance-associated deleterious effects, usually without a loss of resistance (5, 17). Hence, we investigated the function of the additional EF-G mutations A67T and S416F, which were found in clinical S. aureus isolates in combination with the fusidic acid resistance-mediating mutations P406L and H457Y, respectively, and which demonstrably had no influence on fusidic acid susceptibility on their own. The substitution S416F indeed turned out to function as a fitness-compensating mutation in an S. aureus strain carrying the fusidic acid resistance-mediating exchange H457Y. It is likely that the exchange of the hydrophilic amino acid serine by the rather hydrophobic amino acid phenylalanine leads to conformational changes which substantially restore the functionality of EF-G. The exchange A67T, however, had no impact on biological fitness, at least in vitro.
Our findings are in line with those of previous studies concerning the influence of fusidic acid resistance-conferring mutations in fusA on the fitness of S. enterica serovar Typhimurium. Those reports indicated that distinct EF-G mutations are associated with a fitness burden for the resistant bacteria, as reflected by reduced translation rates, perturbed levels of the transcriptional signal molecule ppGpp, and reduced levels of RpoS (18, 19). Most fusidic acid resistance-mediating mutations found in EF-G of S. enterica serovar Typhimurium and S. aureus (including the exchanges P406L and H457Y described in this work) are located in domain III of this protein. This central domain is believed to be of crucial importance for conformational changes and possibly functions as the fusidic acid binding site (16). Hence, it is convincing that mutational changes within this sensitive region can result not only in fusidic acid resistance but also in a loss of biological fitness. Nagaev et al. (21) have also suggested that mutational changes within EF-G of S. aureus are associated with fusidic acid resistance and impaired fitness, but the causal relationship between the resistance-mediating mutations and reduced fitness has not been proven unequivocally, since second-site mutations outside EF-G could not be ruled out.
In S. enterica serovar Typhimurium second-site mutations compensating for the costs of fusidic acid resistance have been suggested to be situated in domains I and II of EF-G (12, 13). Moreover, in S. aureus an amino acid substitution in domain I, namely, A67V, has been supposed to ameliorate the fitness costs of fusidic acid resistance (21). The exchange A67T investigated in this work is also located in domain I but does not improve the fitness of S. aureus carrying the fusidic acid resistance-conferring mutation P406L in EF-G. This apparent inconsistency may, however, be explained as follows. First, different amino acid substitutions at the same position may have different effects on protein function. Second, the mutation A67V has been selected only in combination with another resistance-mediating exchange, namely, F88L (21). Third, the second-site mutation A67V in EF-G of S. aureus can be regarded only as putatively fitness compensating, since mutations outside the fusA gene could not be excluded.
In contrast to the amino acid substitution A67T, the second-site mutation S416F demonstrably functions as a fitness-compensating mutation in fusidic acid-resistant S. aureus isolates harboring the EF-G substitution H457Y. Interestingly, this exchange, which has not been described before in the context of fitness compensation, is located in the central domain III of EF-G, close to the resistance-conferring mutation H457Y. It is remarkable that the restoration of fitness via the exchange S416F is not complete. Although the S. aureus derivative expressing EF-G with the S416F and H457Y double amino acid substitution showed significantly enhanced fitness in comparison to that of the clone producing EF-GH457Y, it did not reach the fitness of the strain expressing wild-type EF-G. Nevertheless, it is probably not necessary for the resistant bacteria to restore the fitness completely, because the selection pressure in vivo within the clinical setting calls for both fusidic acid resistance and EF-G functionality. Consequently, in specific cases the fitness-compensated fusidic acid-resistant strain is probably able to outcompete the fit but fusidic acid-susceptible wild-type strain. In this context, it is further remarkable that compensation of resistance costs by second-site mutations is more likely than reversion to the fit wild type, as shown by Björkman et al. (5) and Levin et al. (17). As a result of such compensatory evolution, the resistant bacteria in a given population may be stabilized, as indicated by the slightly increasing incidence of clinical fusidic acid-resistant S. aureus strains. Accordingly, strategies to reduce resistance by decreased use of antibiotics might not be as successful as originally anticipated (15).
In conclusion, this is the first study to our knowledge that provides direct evidence at the molecular level that individual fusidic acid resistance-mediating EF-G mutations are associated with fitness costs in S. aureus and that compensatory EF-G mutations are able to ameliorate these detrimental effects. Since the resistance-mediating and fitness-compensating mutations investigated were found in clinical S. aureus isolates, it is very likely that the observed mechanisms are indeed of crucial importance for the evolution of antibiotic resistance in S. aureus under in vivo conditions.
Acknowledgments
We thank Denia Frank for excellent technical assistance.
This work was supported by a grant (grant 7345005) from the Klinikum der J. W. Goethe-Universität, Frankfurt am Main.
REFERENCES
- 1.Andersson, D. I., and B. R. Levin. 1999. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, D. I. 2003. Persistence of antibiotic resistant bacteria. Curr. Opin. Microbiol. 6:452-456. [DOI] [PubMed] [Google Scholar]
- 3.Besier, S., A. Ludwig, V. Brade, and T. A. Wichelhaus. 2003. Molecular analysis of fusidic acid resistance in Staphylococcus aureus. Mol. Microbiol. 47:463-469. [DOI] [PubMed] [Google Scholar]
- 4.Billington, O., T. D. McHugh, and S. H. Gillespie. 1999. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 43:1866-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björkman, J., I. Nagaev, O. G. Berg, D. Hughes, and D. I. Andersson. 2000. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287:1479-1482. [DOI] [PubMed] [Google Scholar]
- 6.Bodley, J. W., F. J. Zieve, L. Lin, and S. T. Zieve. 1969. Formation of the ribosome-G factor-GDP complex in the presence of fusidic acid. Biochem. Biophys. Res. Commun. 37:437-443. [DOI] [PubMed] [Google Scholar]
- 7.Brown, E. M., and P. Thomas. 2002. Fusidic acid resistance in Staphylococcus aureus isolates. Lancet 359:803. [DOI] [PubMed] [Google Scholar]
- 8.Chopra, I. 1976. Mechanisms of resistance to fusidic acid in Staphylococcus aureus. J. Gen. Microbiol. 96:229-238. [DOI] [PubMed] [Google Scholar]
- 9.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 10.Godtfredsen, W., K. Roholt, and L. Tybring. 1962. Fusidin. A new orally active antibiotic. Lancet i:928-931. [DOI] [PubMed] [Google Scholar]
- 11.Haima, P., D. van Sinderen, H. Schotting, S. Bron, and G. Venema. 1990. Development of a beta-galactosidase alpha-complementation system for molecular cloning in Bacillus subtilis. Gene 86:63-69. [DOI] [PubMed] [Google Scholar]
- 12.Johanson, U., and D. Hughes. 1994. Fusidic acid-resistant mutants define three regions in elongation factor G in Salmonella typhimurium. Gene 143:55-59. [DOI] [PubMed] [Google Scholar]
- 13.Johanson, U., A. Ævarson, A. Liljas, and D. Hughes. 1996. The dynamic structure of EF-G studied by fusidic acid resistance and internal revertants. J. Mol. Biol. 258:420-432. [DOI] [PubMed] [Google Scholar]
- 14.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 15.Kristinsson, K. G. 1997. Effect of antimicrobial use and other risk factors on antimicrobial resistance in pneumococci. Microb. Drug Resist. 3:117-123. [DOI] [PubMed] [Google Scholar]
- 16.Laurberg, M., O. Kristensen, K. Martemyanov, A. T. Gudkov, I. Nagaev, D. Hughes, and A. Liljas. 2000. Structure of a mutant EF-G reveals domain III and possibly the fusidic acid binding site. J. Mol. Biol. 303:593-603. [DOI] [PubMed] [Google Scholar]
- 17.Levin, B. R., V. Perrot, and N. Walker. 2000. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics 154:985-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macvanin, M., U. Johanson, M. Ehrenberg, and D. Hughes. 2000. Fusidic acid-resistant EF-G perturbs the accumulation of ppGpp. Mol. Microbiol. 37:98-107. [DOI] [PubMed] [Google Scholar]
- 19.Macvanin, M., J. Bjorkman, S. Eriksson, M. Rhen, D. I. Andersson, and D. Hughes. 2003. Fusidic acid-resistant mutants of Salmonella enterica serovar Typhimurium with low fitness in vivo are defective in RpoS induction. Antimicrob. Agents Chemother. 47:3743-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maisnier-Patin, S., O. G. Berg, L. Liljas, and D. I. Andersson. 2002. Compensatory adaptation to the deleterious effect of antibiotic resistance in Salmonella typhimurium. Mol. Microbiol. 2:355-366. [DOI] [PubMed] [Google Scholar]
- 21.Nagaev, I., J. Björkman, D. I. Andersson, and D. Hughes. 2001. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol. Microbiol. 40:433-439. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien, F. G., C. Price, W. B. Grubb, and J. E. Gustafson. 2002. Genetic characterization of the fusidic acid and cadmium resistance determinants of Staphylococcus aureus plasmid pUB101. J. Antimicrob. Chemother. 50:313-321. [DOI] [PubMed] [Google Scholar]
- 23.Philips, I., A. King, W. R. Gransden, and S. J. Eykyn. 1990. The antibiotic sensitivity of bacteria isolated from the blood of patients in St Thomas' Hospital, 1969-1988. J. Antimicrob. Chemother. 25:59-80. [DOI] [PubMed] [Google Scholar]
- 24.Price, C. T., and J. E. Gustafson. 2001. Increase in the mutation frequency at which fusidic acid-resistant Staphylococcus aureus arise with salicylate. J. Med. Microbiol. 50:104-106. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sander, P., B. Springer, T. Prammananan, A. Sturmfels, M. Kappler, M. Pletschette, and E. C. Böttger. 2002. Fitness cost of chromosomal drug resistance-conferring mutations. Antimicrob. Agents Chemother. 46:1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnidge, J., and P. Collignon. 1999. Resistance to fusidic acid. Int. J. Antmicrob. Agents 12:35-44. [DOI] [PubMed] [Google Scholar]
- 28.Wichelhaus, T. A., B. Böddinghaus, S. Besier, V. Schäfer, V. Brade, and A. Ludwig. 2002. Biological cost of rifampicin resistance from the perspective of Staphylococcus aureus. Antimicrob. Agents Chemother. 46:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witte, W., C. Cuny, B. Strommenger, C. Braulke, and D. Heuck. 2004. Emergence of a new community acquired MRSA strain in Germany. Euro. Surveill. 9:1-2. [DOI] [PubMed] [Google Scholar]