Abstract
The immunomodulatory effects of liposomal amphotericin B (LAMB), amphotericin B lipid complex (ABLC), and amphotericin B colloidal dispersion (ABCD) on mRNA and protein profiles of five cytokines and chemokines expressed by human monocyte-enriched mononuclear leukocytes (MNCs) were comprehensively evaluated by semiquantitative reverse transcription-PCR and enzyme-linked immunosorbent assays; they were compared to those of deoxycholate amphotericin B (DAMB). mRNAs of interleukin-1β (IL-1β), IL-1 receptor antagonist (IL-1ra), tumor necrosis factor alpha (TNF-α), monocyte chemotactic protein 1 (MCP-1), and macrophage inflammatory protein 1β (MIP-1β) were assessed after treatment of MNCs with each drug for 0.5, 2, 6, and 22 h. The cytokine protein profiles were obtained after incubation of MNCs with the drugs for 2 h (TNF-α) or 6 h (all the others). In the mRNA studies, DAMB resulted in an early increase of inflammatory cytokines or chemokines IL-1β, TNF-α, MCP-1, and MIP-1β (2 to 6 h) and in a late increase of anti-inflammatory IL-1ra (22 h). ABCD showed a general similar trend of inflammatory gene up-regulation. LAMB and ABLC decreased or did not affect IL-1β and TNF-α, whereas ABLC additionally decreased MIP-1β. In protein measurement studies, DAMB and ABCD up-regulated production of IL-1β (P < 0.05), decreased the IL-1ra/IL-1β ratio, and up-regulated the production of MCP-1 and MIP-1β. In comparison, LAMB and ABLC down-regulated or did not affect the production of these cytokines/chemokines compared to untreated MNCs; furthermore, ABLC tended to increase the IL-1ra/IL-1β ratio. These studies demonstrate that amphotericin B formulations differentially affect gene expression and release of an array of proinflammatory and anti-inflammatory cytokines that potentially may explain the differences in infusion-related reactions and dose-dependent nephrotoxicity as well as modulation of the host immune response to invasive fungal infections.
Historically, deoxycholate amphotericin B (DAMB) has been considered the “gold standard” of antifungal therapy, and it remains the drug with the broadest antifungal spectrum (21, 25). However, DAMB causes adverse infusion-related reactions and dose-dependent nephrotoxicity, which are clearly associated with increased morbidity in immunocompromised patients (1, 13, 19). The lipid-based amphotericin B formulations liposomal amphotericin B (LAMB), amphotericin B lipid complex (ABLC), and amphotericin B colloidal dispersion (ABCD) have been developed with the goal to decrease toxicity and improve drug tolerance and thus efficacy (17, 38). Patients with neutropenia and invasive fungal infections developed infusion-related adverse reactions less frequently with LAMB than with ABLC and ABCD, whereas nephrotoxic tolerability was improved with all three lipid formulations compared to with DAMB (8, 17, 22, 41).
In vivo, amphotericin B-related toxicity has been previously correlated with increased levels in plasma of interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-1 receptor antagonist (IL-1ra) (4, 36). In vitro, amphotericin B-responsive cytokines and chemokines have been identified to be expressed either in THP-1 cells, a leukemic monocytic cell line (26-29, 39), or in human peripheral blood mononuclear cells (PBMCs) (40). Little is known, however, about the cytokine gene expression in primary human monocytes in response to lipid formulations of amphotericin B. We therefore investigated the immunomodulatory effects of DAMB, LAMB, ABLC, and ABCD on gene expression of the cytokines IL-1β, IL-1ra, and TNF-α as well as of chemokines monocyte chemotactic protein 1 (MCP-1) and macrophage inflammatory protein 1β (MIP-1β), which affect either positively or negatively the acute and chronic inflammatory processes (24, 31).
MATERIALS AND METHODS
Reagents.
DAMB was purchased from Bristol Myers Squibb (La Grande Nord, Paris, France), LAMB was obtained from Gilead Sciences (San Dimas, Calif.), ABLC was obtained from Enzon Pharmaceuticals (Piscataway, N.J.), and ABCD was obtained from Sequus Pharmaceuticals (Menlo Park, Calif.). RPMI 1640 medium, fetal calf serum, penicillin, streptomycin, Hanks' balanced solution without Ca2+ and Mg2+ (HBSS−), and Ficoll (Lymphocyte Separation Medium) were obtained from Gibco BRL, Life Technologies, Ltd. (Paisley, Scotland). Trizol reagent, Superscript one-step RT-PCR system, agarose gel, 10× Tris-borate-EDTA (TBE) gel electrophoresis buffer, ethidium bromide, a 100-bp DNA ladder, loading buffer, RNase-away, and DNase I were supplied by Gibco BRL. Triton X-100, HEPES, EDTA, MgCl2, isopropanol, isoamyl alcohol, NaN2, phenylmethylsulfonyl fluoride (PMSF), and aprotinin were purchased from Sigma Chemical (St. Louis, Mo.). The six sets of primers used for the reverse transcription-PCRs (RT-PCRs) were obtained from TIB MOLBIOL (Dahlem, Germany). The enzyme-linked immunosorbent assay (ELISA) kits for cytokine measurements were purchased from R and D Systems (Minneapolis, Minn.).
Preparation of human monocyte-enriched mononuclear leukocytes.
Human mononuclear cells were obtained from blood of healthy adult volunteers and separated by centrifugation over Ficoll, as previously described in detail (30). Briefly, the cells were washed and resuspended in HBSS−. They were counted on a hemocytometer by trypan blue staining, and the percentage of monocytes over the total number of PBMCs was calculated after staining with May-Grunwald-Giemsa. Monocytes were adjusted to 106 cells/ml in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (FCM). Monocyte-enriched cell populations (MNCs) were obtained from mononuclear leukocytes by adherence on plastic surfaces in 12-well plates during incubation in a humidified CO2 incubator at 37°C for 2 h as described previously (30), yielding >85% monocytes among the cell populations. The enrichment procedure was necessary, since the main objective of this study was to investigate production of cytokines and chemokines by primary human monocytes. Following adherence, the cells were washed with warm HBSS− and incubated in fresh FCM at 37°C for 24 h prior to incubation with the drugs in order to avoid cell activation and enhanced cytokine expression due to handling. Cell viability of untreated and amphotericin B-treated MNCs after the long incubation period (22 h) was checked by including additional control wells containing either untreated or treated cells and counting the percentage of viable cells by trypan blue staining. The viability of the untreated cells and cells that had been treated with the concentrations of the drugs stated below was >95% at the end of each experiment.
Amphotericin B formulations and cell culture treatment.
Lipid formulations were stored at 4°C. DAMB dissolved in sterile water at a concentration of 1 mg/ml was stored at −20°C for a month. The drug concentrations used in the experiments were selected to be within a range of therapeutically achievable concentrations in plasma and tissue, as well as by preliminary ELISA experiments with different drug concentrations. After the 24-h preparatory incubation, FCM was removed from each well and was replaced with 1 ml of fresh FCM containing DAMB, LAMB, ABLC, or ABCD at the appropriate concentrations. To determine the mRNA levels of the cytokines under study, MNCs were incubated at 37°C with FCM containing 5 μg of DAMB per ml or 25 μg of LAMB, ABLC, or ABCD per ml for 0.5, 2, 6, and 22 h. Low concentrations of amphotericin B formulations were not used for mRNA assessment. To measure TNF-α release, MNCs were incubated with 1 or 5 μg of DAMB per ml and 5 or 25 μg of LAMB, ABLC, or ABCD per ml, respectively, at 37°C for 2 h; whereas, to measure production of IL-1β, IL-1ra, MCP-1, and MIP-1β, the incubation time with the same concentrations of drugs was 6 h. The incubation periods used to measure production of TNF-α as well as the rest of cytokines were based on preliminary time response experiments.
RNA isolation.
After each incubation period, the supernatants were replaced with 1 ml of Trizol reagent. The cells were scraped, and total RNA was extracted by the Chomczynski and Sacchi method with slight modifications (10). Briefly, 0.2 ml of chloroform was added to 1 ml of cell lysates, and each sample was shaken vigorously by hand and incubated at room temperature for 2 to 5 min. The aqueous phase was separated after centrifugation at 4°C and 12,000 × g for 15 min. Total RNA was precipitated with 0.5 ml of isopropyl alcohol and washed once with 80% ethanol, and the RNA pellet was dissolved in diethylpyrocarbonate-treated sterile water and quantified by spectrophotometry (UV101, Pocklington, United Kingdom).
RT-PCR analysis of cytokine mRNAs.
Three independent experiments were performed. One donor was used for each experiment. For each sample, 0.5 μg of total RNA was reverse transcribed in a final reaction volume of 50 μl containing 800 μM each deoxynucleoside triphosphate (dNTP), 2.2 mM MgSO4, 0.2 μM each sense and antisense primer, and 1 U of Superscript II RT/Platinum-Taq polymerase mix. Reverse transcription was performed at 48°C for 30 min, followed by a 94°C denaturation step for 2 min and 29 PCR cycles, with each cycle consisting of 94°C for 15 s, 56.8°C for 30 s (for TNF-α amplification), or 53°C for 30 s (for IL-1β, IL-1ra, MCP-1, and MIP-1β amplification) and 68°C for 1 min. Each PCR was completed with 1 cycle at 68°C for 7 min. Sequences of the primer sets used for the five human cytokines and the housekeeping gene aldolase A as well as the sizes of expected PCR products are listed in Table 1. Aldolase A was selected as an internal RNA control, used to normalize variations in sample concentration. A negative control was also included to rule out genomic contamination. All PCR products were separated on 1.5% agarose gels by electrophoresis and visualized on a UV transilluminator after ethidium bromide staining. The DNA amounts on agarose gels were quantified with the UviDoc software program (DOC-008.TFT; UVItec, Cambridge, United Kingdom). Band intensities were expressed in relative absorbance units and converted to nanograms per milliliter. The ratios between the sample cytokine mRNA's and aldolase A were calculated to correct for fluctuation among the samples.
TABLE 1.
Forward and reverse sets of primers used for mRNA amplification of the cytokines or chemokinesa
| Primer | Nucleotide sequence | Size (bp) | Tm (°C) | No. of cycles |
|---|---|---|---|---|
| Aldolase Ab | ||||
| Forward 1 | 5′-GTG CTG GCT GCT GTC TAC-3′ | 184 | 53 | 29 |
| Reverse 1 | 5′-AGG TGA TCC CAG TGA CAG-3′ | |||
| IL-1β | ||||
| Forward | 5′-GTG GCA ATG AGG ATG ACT T-3′ | 534 | 53 | 29 |
| Reverse | 5′-TGG GCT TAT CAT CTT TCA A-3′ | |||
| IL-1ra | ||||
| Forward | 5′-TCC GCA GTC ACC TAA TCA-3′ | 370 | 53 | 29 |
| Reverse | 5′-CTG TCT GAG CGG ATG AAG-3′ | |||
| MCP-1 | ||||
| Forward | 5′-CAA ACT GAA GCT CGC ACT-3′ | 317 | 53 | 29 |
| Reverse | 5′-GTT TGG GTT TGC TTG TCC-3′ | |||
| MIP-1β | ||||
| Forward | 5′-GAA GCT CTG CGT GAC TGT-3′ | 246 | 53 | 29 |
| Reverse | 5′-TGG ACC CAG GAT TCA CTG-3′ | |||
| Aldolase Ac | ||||
| Forward 2 | 5′-GTG CTG GCT GCT GTC TAC AA-3′ | 218 | 56.8 | 29 |
| Reverse 2 | 5′-GAC GCC TCC TCC TCA CTC TG-3′ | |||
| TNF-α | ||||
| Forward | 5′-CTT GTT CCT CAG CCT CTT CT-3′ | 578 | 56.8 | 29 |
| Reverse | 5′-ACT CGG CAA AGT CGA GAT AG-3′ |
The size of the expected PCR products, the annealing temperature (melting temperature [Tm]) for each set of primers, and the number of PCR cycles used are indicated.
A pair of aldolase A (internal control) primers was used for the coamplification of aldolase A, IL-1β, IL-1ra, MCP-1, and MIP-1β.
A different pair of aldolase A primers was designed for the coamplification of aldolase A and TNF-α due to GC-rich sequence of TNF-α and Tm incompatibility with the rest of the cytokine gene sequences.
Measurement of cytokine protein production.
After incubation of MNCs with the drugs, supernatants were collected and intracellular extracts of attached MNCs were recovered by adding 1 ml of cold lysis buffer containing 1% Triton X-100, 50 mM HEPES (pH 7.4), 10 mM EDTA, 2 mM MgCl2, 0.02% NaN2, 10 μg of aprotinin per ml, and 1 mM PMSF. The plates were then incubated on ice for 10 min, scraped, and the contents were collected. Culture supernatants and cell extracts were combined and centrifuged at 12,000 × g for 10 min at 4°C to remove cell debris. Aliquots were stored at −20°C until assayed. The protein concentration of the five cytokines was determined in duplicate by the quantitative sandwich ELISA method. Absorbance was read on a microplate reader set at 450 nm with a reference wavelength of 540 nm. A total of eight donors were used for cytokine measurements. Specifically, seven donors were used for IL-1β and IL-1ra measurements, eight donors for MCP-1, six donors for MIP-1β, and five donors for TNF-α. Each donor was used for a single experiment.
Statistical analysis.
The averages of the duplicate wells from each ELISA experiment were used in the data analysis to calculate the mean ± standard error of the mean (SE) of all the experiments for each particular cytokine or donor. The statistics program Instat (GraphPad, Inc., San Diego, Calif.) was used. Parametric analysis of variance (ANOVA) with Dunn's multiple comparisons test was used for statistical comparisons of cytokine concentrations between drug-treated and untreated cells. A P value of < 0.05 was considered statistically significant.
RESULTS
Effects of amphotericin B formulations on cytokine and chemokine mRNA levels.
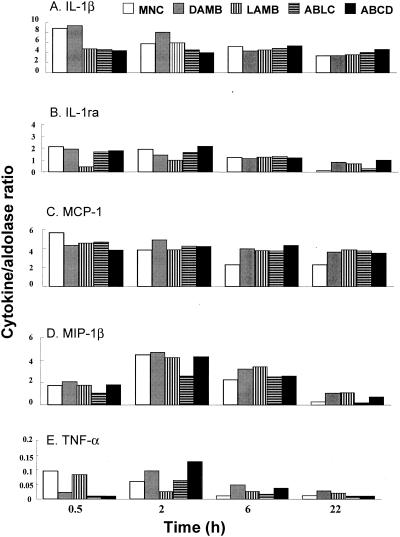
The mRNA profiles of the genes of the five cytokines/chemokines were generated after incubating MNCs with 5 μg of DAMB per ml or 25 μg of LAMB, ABLC, and ABCD per ml for 0.5, 2, 6, and 22 h by semiquantitative RT-PCR. There was a progressive decrease of mRNAs of all five cytokines or chemokines expressed by untreated MNCs between 0.5 and 22 h. DAMB induced a 39% increase of IL-1β mRNA after incubation for 2 h, but this difference was eliminated by 6 h. In contrast, IL-1ra mRNA was approximately equal to that of MNCs alone, but it increased by 640% compared to the level in untreated MNCs after 22 h simultaneously with the return of IL-1β mRNA levels to values equal to those of untreated MNCs (Fig. 1A and B). The same drug induced maximal increases of MCP-1, MIP-1β, and TNF-α at 6 h (Fig. 1C to E).
FIG. 1.
Profiles of IL-1β (A), IL-1ra (B), MCP-1 (C), MIP-1β (D), and TNF-α (E) mRNA expression as assessed by semiquantitative RT-PCR after exposure of MNCs to no drugs (open bars), DAMB (5 μg/ml; gray bars), or LAMB (vertical striped bars), ABLC (horizontal striped bars) and ABCD (25 μg/ml; black bars) for 0.5, 2, 6 and 22 h (time course). Sample concentrations were normalized to aldolase A to eliminate mRNA concentration variations among samples. Each value is presented as a ratio of a cytokine per aldolase A (internal control) and is derived from three independent donors or experiments.
LAMB, ABLC, and ABCD induced decreases in the IL-1β mRNAs at 0.5 h by approximately 48% compared to untreated MNCs. Thereafter, the levels of IL-1β mRNAs returned to values similar to those of untreated cells and remained as such until 22 h (Fig. 1A).
Amphotericin B formulations did not have an enhancing effect on the expression of IL-1ra within 0.5 to 6 h, as the mRNA levels did not exceed the corresponding mRNA levels in the untreated cells. IL-1ra was much more stable in DAMB-, LAMB-, and ABCD-treated cells at 22 h than in untreated cells where the mRNA was reduced to undetectable levels by 22 h. Although IL-1ra seemed to be stable at the time interval from 0.5 to 6 h in ABLC-treated MNCs, transcript levels fell below detectable limits at 22 h (Fig. 1B).
While MCP-1 mRNA decreased progressively in untreated cells, reaching a value at 22 h half of that measured at 0.5 h (Fig. 1C), all amphotericin B formulations resulted in a maintenance of MCP-1 mRNA production equal to that measured in untreated cells at 0.5 h. Peak levels of MIP-1β mRNA in unstimulated MNCs were reached at 2 h but decreased to barely detectable values at 22 h (Fig. 1D). Although DAMB, LAMB, and ABCD induced increased production of MIP-1β mRNA at 6 to 22 h compared to the values obtained in untreated cells, ABLC did not cause this increase and induced a rapid decrease of MIP-1β to undetectable levels at 22 h (Fig. 1D).
TNF-α mRNA in both untreated and treated MNCs was expressed at very small amounts compared to the other cytokines. TNF-α mRNA levels declined progressively to undetectable levels by 22 h. DAMB and ABCD induced peak levels of TNF-α mRNA at 2 and 6 h. LAMB and ABLC did not affect TNF-α mRNA expression, since comparable expression profiles were observed in treated and untreated cells (Fig. 1E).
Effects of amphotericin B formulations on cytokine and chemokine protein concentrations.
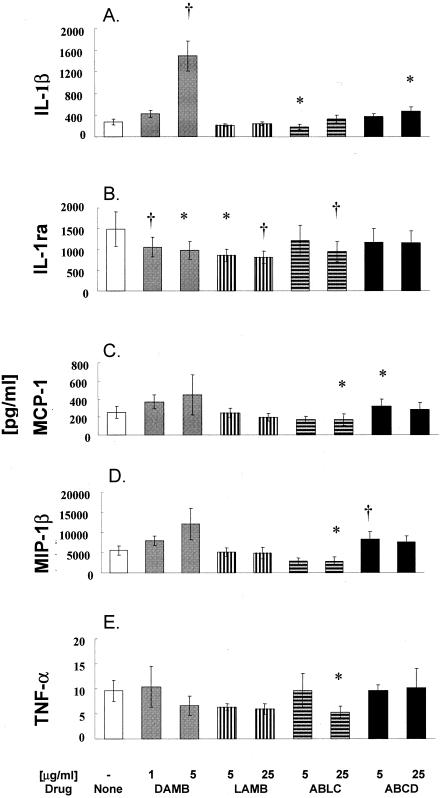
After incubation of MNCs with 1 and 5 μg of DAMB per ml, or 5 and 25 μg of LAMB, ABLC, and ABCD per ml for 2 h (TNF-α) or 6 h (all the other cytokines or chemokines), supernatants and cell extracts were combined for total protein measurement (Fig. 2). The incubation times used were selected from a preliminary time course experiment performed at 2, 6, or 22 h on selected cytokines and drug concentrations (data not shown). Relatively high concentrations of DAMB and ABCD caused significant increases in the levels of IL-1β (n = 7, P < 0.01 and P < 0.05, respectively; Fig. 2A). In comparison, neither LAMB nor ABLC induced an increase of IL-1β concentration. Indeed, 5 μg of ABLC per ml reduced IL-1β by approximately 42% compared to that in untreated MNCs (n = 7, P < 0.05). There also was a tendency for significant decrease with 5 μg of LAMB per ml.
FIG. 2.
Profiles of IL-1β (A), IL-1ra (B), MCP-1 (C), MIP-1β (D), and TNF-α (E) levels after treatment of human MNCs with the antifungal drugs DAMB (1 and 5 μg/ml) or LAMB, ABLC, and ABCD (5 and 25 μg/ml) for 2 h (TNF-α) or 6 h (all of the other cytokines/chemokines). Data are presented as means ± SE derived from 5 to 8 donors or experiments. Comparisons between drug-treated and control cells were performed by ANOVA with Dunn's test for multiple comparisons. Statistically significant differences from untreated MNCs (controls) with P < 0.05 are indicated by asterisks, and those with P < 0.01 are indicated by a dagger (†).
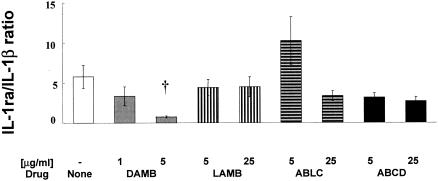
In contrast, IL-1ra was significantly decreased by DAMB and LAMB at both concentrations and by ABLC at 25 μg/ml (n = 7, P < 0.05; Fig. 2B). To determine whether there is an inverse relationship between IL-1ra and IL-1β indicating an imbalance between the production of anti-inflammatory IL-1ra and proinflammatory IL-1β, we expressed these two cytokines as a ratio. DAMB at 5 μg/ml significantly decreased the IL-1ra/IL-1β ratio (P < 0.01), indicating an increased inflammatory response (Fig. 3). In comparison, LAMB did not affect this ratio and ABLC at 5 μg/ml increased the IL-1ra/IL-1β ratio. This increase, however, did not reach statistical significance.
FIG. 3.
IL-1ra/IL-1β ratio of total cytokines produced by human MNCs incubated with no drug (MNC) or with amphotericin B formulations DAMB (1 and 5 μg/ml) or LAMB, ABLC, and ABCD (5 and 25 μg/ml) for 6 h. The values are means ± SE of values derived from five experiments. Comparisons between drug-treated and untreated control cells (MNCs) were performed by ANOVA with Dunn's test for multiple comparisons. Statistically significant difference from untreated MNCs (controls) with P < 0.01 is indicated by a dagger (†).
Chemokines MCP-1 and MIP-1β were not affected by LAMB or were decreased by 25 μg of ABLC per ml (n = 8 and 6, respectively, P < 0.05). In comparison, increased production of these chemokines was induced by ABCD at 5 μg/ml (P < 0.05; Fig. 1C and D). DAMB showed a tendency to increase both chemokines.
Overall, release of TNF-α was similar or somewhat decreased in the presence of amphotericin B and lipid formulations. TNF-α release was significantly decreased by 25 μg of ABLC per ml (n = 5, P < 0.05; Fig. 2D).
DISCUSSION
Amphotericin B is a microbial product that is recognized by Toll-like receptor 2 and CD14 on the surface of MNCs, and through MyD88 and NF-κB it induces the expression of cytokine genes (33). In this study, we compared the immunomodulating activities of amphotericin B formulations on gene expression of IL-1β, IL-1ra, MCP-1, MIP-1β, and TNF-α in MNCs. The mRNA studies showed that there is a progressive decline of most of these molecules in untreated MNCs, reaching very low concentrations at 22 h. In general, DAMB resulted in an early increase of IL-1β, TNF-α, MCP-1, and MIP-1β and a late increase of IL-1ra. ABCD exhibited a general similar trend of inflammatory gene up-regulation. LAMB and ABLC decreased or did not affect IL-1β and TNF-α, whereas ABLC additionally decreased MIP-1β, a potent macrophage inflammatory chemokine. The protein measurement studies showed that DAMB and ABCD up-regulate production of IL-1β and decrease the ratio of IL-1ra to IL-1β. In addition, these two drugs up-regulate the production of the chemokines MCP-1 and MIP-1β. By comparison, LAMB and ABLC down-regulate or do not affect the production of these cytokines or chemokines as compared to untreated MNCs. Moreover, ABLC tends to increase the IL-1ra/IL-1β ratio.
The concentrations of the amphotericin B formulations evaluated in this study were those that are, in general, therapeutically achievable in plasma and tissue. Higher concentrations of 25 μg/ml were used to investigate the effects of amphotericin B lipid formulations on cytokine mRNAs in MNCs, as these formulations are administered in approximately fivefold-higher dosages (e.g., 5 mg/kg/day) than that of DAMB (e.g., 1 mg/kg/day). Indeed, increased doses and treatment duration are part of the therapeutic regimens in neutropenic and immunocompromised patients (38, 41). In this study, we used primary human MNCs instead of cell lines, as the former cells have not been altered genetically and therefore responses to amphotericin B formulations accurately represent clinical conditions. Our experimental strategy also investigated both mRNA expression and protein synthesis. One should note, however, that mRNA and protein levels of each cytokine in treated cells may not necessarily correlate, as dissociation between transcription and translation has been documented for at least IL-1β and TNF-α (34).
Patients receiving DAMB show infusion-related toxic adverse effects, such as fever, chills, and hypotension, symptoms that are mediated by proinflammatory cytokines, such as IL-1β (2, 9, 37). In this study, DAMB at 5 μg/ml and ABCD at 25 μg/ml up-regulated IL-1β synthesis in MNCs while decreasing IL-1ra levels. In addition, IL-1ra mRNA increased in DAMB-treated cells only after 22 h, with a corresponding decrease in IL-1β mRNA relative to that in untreated cells. Taking into consideration the adverse infusion-related reactions following DAMB administration, an imbalance in the protein synthesis of IL-1β and IL-1ra could indeed be responsible for the observed toxic adverse effects. DAMB-mediated up-regulation of IL-1β protein synthesis has previously been reported for THP-1 cells after incubation of DAMB at 5 μg/ml for 2 and 6 h, respectively (26, 39). However, stimulation of MNCs with 1 μg of DAMB per ml for 24 h did not result in IL-1β synthesis (40) suggesting an optimum time window for IL-1β expression between 0.5 and 6 h. Keeping in mind the different conditions used, results from the present study and the previously mentioned reports are essentially in agreement with clinical data, where DAMB-induced IL-1β synthesis occurs between 2 and 6 h, which coincides with the time interval that adverse reactions are observed in humans (11).
We also focused our studies on the potential immunomodulatory properties of these formulations that may provide insight into their mechanisms of antifungal activity. A long duration of antifungal therapy is usually needed for the management of invasive fungal infections in immunocompetent or immunocompromised patients. IL-1β is a proinflammatory protein, which stimulates synthesis of itself, TNF-α, and several other proteins associated with inflammation and autoimmune diseases (12, 14, 16). Although IL-1β up-regulates host defense mechanisms, continued gene expression has unacceptable toxic effects in humans and leads to debilitation of normal host functions (15). The potent biologic activities of IL-1β are inhibited by IL-1ra, which blocks further IL-1β synthesis and inhibits gene expression of other inflammatory cytokines by competitively binding to the IL-1 receptors on the surface of monocytes (20). Maintenance of a balance between IL-1β and IL-1ra is essential in reducing the severity of inflammation and autoimmune processes (3). Extensive clinical and experimental studies suggest that an imbalance between IL-1ra and IL-1β production may be a crucial factor predisposing to initiation or perpetuation of chronic inflammatory diseases (5, 19, 40).
TNF-α has a central role in infection and inflammation, but deregulation of TNF-α expression may contribute to disease processes (35). It is involved in the pathogenesis of early arthritis, as well as autoimmune and infectious diseases, and cancer (18, 44). TNF-α production and release from effector cells is related to adverse drug reactions of DAMB (4). Although we measured TNF-α after 2 h of exposure of MNCs to amphotericin B formulations, comparable TNF-α protein profiles have been reported after exposure of THP-1 cultures to amphotericin B formulations of 2 to 5 μg/ml for 6 h (39). In contrast, we showed increased TNF-α mRNA in MNCs treated with 5 μg of DAMB per ml from 2 top 6 h. Similarly, Rogers et al. (26) showed TNF-α mRNA expression in the THP-1 cell line exposed to 5 μg of DAMB per ml within 2 to4 h, decreasing to undetectable levels after 6 h.
MCP-1 and MIP-1β are members of the CC subfamily of chemokines and are involved in acute and chronic inflammatory processes by attracting leukocytes to the site of inflammation or activating cell immune responses (24, 32). Production of MCP-1 and MIP-1β were increased by DAMB and ABCD and decreased by LAMB and ABLC. To the best of our knowledge, this is the first report of the effects of amphotericin B lipid formulations on gene expression of MIP-1β. Previous observations on gene expression of MCP-1 in DAMB-treated MNCs or THP-1 cell lines (29) and on MIP-1β expression after THP-1 exposure to the four amphotericin B formulations (39) are consistent with our studies. Collectively these data suggest that DAMB and ABCD formulations may augment host response through MIP-1β release. At the same time, however, MIP-1β may also contribute to the acute infusion-related reactions observed with these amphotericin B formulations. Studies evaluating the efficacy of DAMB and ABCD in persistent neutropenia and invasive aspergillosis have shown similar infusion-related toxicity (7, 38, 43), while LAMB and ABLC have shown reduced adverse effects in comparison to those of DAMB (6, 23, 42).
The time course of observation of protein concentrations was based on preliminary time response experiments, at which protein levels were accurately detected within the concentration range provided by the manufacturer of the protein assay kits. The time course of observation for mRNA was based on previously published studies (26, 29). Except for IL-1β and MCP-1, the mRNA studies showed that there is a progressive decline of gene expression over 22 h, leading to the assumption that gene expression is either an early event, with mRNA levels gradually declining over 22 h, or housekeeping genes, like IL-1β and MCP-1, are constitutively expressed over the 22-h time interval.
Conventional amphotericin B binds on Toll-like receptor 2 of human mononuclear cells and induces the production of cytokines/chemokines by the cell. It is unclear whether the differential effect of lipid formulations of AMB is due to differential exposure of AMB to the surface receptors or the lipids (deoxycholate/sodium cholesteryl sulfate) of the different formulations modulate the effect.
In this study, we have demonstrated that DAMB and ABCD up-regulate the gene expression and production of proinflammatory cytokines/chemokines. In comparison, LAMB and ABLC generally down-regulate or do not affect gene expression of proinflammatory cytokines/chemokines, which may explain the relatively lower frequency of adverse infusion-related reactions of these agents reported in clinical trials. Moreover, these studies demonstrate that DAMB and its lipid formulations affect gene expression and cytokine release of IL-1β, IL-1ra, MCP-1, MIP-1β, and TNF-α differently, which may further modulate host response to invasive fungal infections.
Acknowledgments
This study was supported in part by research grants from Gilead Sciences, San Dimas, Calif., to the Aristotle University of Thessaloniki and from the Technological Educational Institute, Thessaloniki, Greece.
REFERENCES
- 1.Abu-Salah, K. M. 1996. Amphotericin B: an update. Br. J. Biomed. Sci. 53:122-133. [PubMed] [Google Scholar]
- 2.Arend, W. P. 2001. Cytokine imbalance in the pathogenesis of rheumatoid arthritis: the role of interleukin-1 receptor antagonist. Semin. Arthritis Rheum. 30:1-6. [DOI] [PubMed] [Google Scholar]
- 3.Arend, W. P., and C. J. Guthridge. 2000. Biological role of interleukin 1 receptor antagonist isoforms. Annu. Rheum. Dis. 59(Suppl. 1):i60-i64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arning, M., K. O. Kliche, A. H. Heer-Sonderhoff, and A. Wehmeier. 1995. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses 38:459-465. [DOI] [PubMed] [Google Scholar]
- 5.Arondel, J., M. Singer, A. Matsukawa, A. Zychlinsky, and P. J. Sansonetti. 1999. Increased interleukin-1 (IL-1) and imbalance between IL-1 and IL-1 receptor antagonist during acute inflammation in experimental shigellosis. Infect. Immun. 67:6056-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blau, I. W., and A. A. Fauser. 2000. Review of comparative studies between conventional and liposomal amphotericin B (Ambisome) in neutropenic patients with fever of unknown origin and patients with systemic mycosis. Mycoses 43:325-332. [DOI] [PubMed] [Google Scholar]
- 7.Bowden, R., P. Chandrasekar, M. H. White, X. Li, L. Pietrelli, M. Gurwith, J. A. van Burik, M. Laverdiere, S. Safrin, and J. R. Wingard. 2002. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 35:359-366. [DOI] [PubMed] [Google Scholar]
- 8.Cagnoni, P. J. 2002. Liposomal amphotericin B versus conventional amphotericin B in the empirical treatment of persistently febrile neutropenic patients. J. Antimicrob. Chemother. 49(Suppl. 1):81-86. [DOI] [PubMed] [Google Scholar]
- 9.Casini-Raggi, V., L. Kam, Y. J. Chong, C. Fiocchi, T. T. Pizarro, and F. Cominelli. 1995. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J. Immunol. 154:2434-2440. [PubMed] [Google Scholar]
- 10.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 11.Cleary, J. D., D. Weisdorf, and C. V. Fletcher. 1988. Effect of infusion rate on amphotericin B-associated febrile reactions. Drug Intell. Clin. Pharm. 22:769-772. [DOI] [PubMed] [Google Scholar]
- 12.Dayer, J. M. 2002. Evidence for the biological modulation of IL-1 activity: the role of IL-1Ra. Clin. Exp. Rheumatol. 20:S14-S20. [PubMed] [Google Scholar]
- 13.Deray, G. 2002. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 49(Suppl. 1):37-41. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello, C. A. 2002. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 20:S1-S13. [PubMed] [Google Scholar]
- 15.Dinarello, C. A.1997. Interleukin-1. Cytokine Growth Factor Rev. 8:253-265. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello, C. A. 1992. Interleukin-1 and tumor necrosis factor: effector cytokines in autoimmune diseases. Semin. Immunol. 4:133-145. [PubMed] [Google Scholar]
- 17.Dupont, B. 2002. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemother. 49(Suppl. 1):31-36. [DOI] [PubMed] [Google Scholar]
- 18.Emery, P., and Y. Seto. 2003. Role of biologics in early arthritis. Clin. Exp. Rheumatol. 21:S191-S194. [PubMed] [Google Scholar]
- 19.Goodwin, S. D., J. D. Cleary, C. A. Walawander, J. W. Taylor, and T. H. Grasela, Jr. 1995. Pretreatment regimens for adverse events related to infusion of amphotericin B. Clin. Infect. Dis. 20:755-761. [DOI] [PubMed] [Google Scholar]
- 20.Granowitz, E. V., B. D. Clark, E. Vannier, M. V. Callahan, and C. A. Dinarello. 1992. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis. I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood 79:2356-2363. [PubMed] [Google Scholar]
- 21.Groll, A. H., J. C. Gea-Banacloche, A. Glasmacher, G. Just-Nuebling, G. Maschmeyer, and T. J. Walsh. 2003. Clinical pharmacology of antifungal compounds. Infect. Dis. Clin. N. Am. 17:159-191, ix. [DOI] [PubMed] [Google Scholar]
- 22.Hann, I. M., and H. G. Prentice. 2001. Lipid-based amphotericin B: a review of the last 10 years of use. Int. J. Antimicrob. Agents 17:161-169. [DOI] [PubMed] [Google Scholar]
- 23.Martino, R. 2004. Efficacy, safety and cost-effectiveness of Amphotericin B Lipid Complex (ABLC): a review of the literature. Curr. Med. Res. Opin. 20:485-504. [DOI] [PubMed] [Google Scholar]
- 24.Moser, B., M. Wolf, A. Walz, and P. Loetscher. 2004. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 25:75-84. [DOI] [PubMed] [Google Scholar]
- 25.Ostrosky-Zeichner, L., K. A. Marr, J. H. Rex, and S. H. Cohen. 2003. Amphotericin B: time for a new “gold standard.” Clin. Infect. Dis. 37:415-425. [DOI] [PubMed] [Google Scholar]
- 26.Rogers, P. D., J. K. Jenkins, S. W. Chapman, K. Ndebele, B. A. Chapman, and J. D. Cleary. 1998. Amphotericin B activation of human genes encoding for cytokines. J. Infect. Dis. 178:1726-1733. [DOI] [PubMed] [Google Scholar]
- 27.Rogers, P. D., R. E. Kramer, S. W. Chapman, and J. D. Cleary. 1999. Amphotericin B-induced interleukin-1beta expression in human monocytic cells is calcium and calmodulin dependent. J. Infect. Dis. 180:1259-1266. [DOI] [PubMed] [Google Scholar]
- 28.Rogers, P. D., M. M. Pearson, J. D. Cleary, D. C. Sullivan, and S. W. Chapman. 2002. Differential expression of genes encoding immunomodulatory proteins in response to amphotericin B in human mononuclear cells identified by cDNA microarray analysis. J. Antimicrob. Chemother. 50:811-817. [DOI] [PubMed] [Google Scholar]
- 29.Rogers, P. D., J. K. Stiles, S. W. Chapman, and J. D. Cleary. 2000. Amphotericin B induces expression of genes encoding chemokines and cell adhesion molecules in the human monocytic cell line THP-1. J. Infect. Dis. 182:1280-1283. [DOI] [PubMed] [Google Scholar]
- 30.Roilides, E., I. Kadiltsoglou, A. Dimitriadou, M. Hatzistilianou, A. Manitsa, J. Karpouzas, P. A. Pizzo, and T. J. Walsh. 1997. Interleukin-4 suppresses antifungal activity of human mononuclear phagocytes against Candida albicans in association with decreased uptake of blastoconidia. FEMS Immunol. Med. Microbiol. 19:169-180. [DOI] [PubMed] [Google Scholar]
- 31.Roilides, E., C. G. Lamaignere, and E. Farmaki. 2002. Cytokines in immunodeficient patients with invasive fungal infections: an emerging therapy. Int. J. Infect. Dis. 6:154-163. [DOI] [PubMed] [Google Scholar]
- 32.Rollins, B. J. 1996. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol. Med. Today 2:198-204. [DOI] [PubMed] [Google Scholar]
- 33.Sau, K., S. S. Mambula, E. Latz, P. Henneke, D. T. Golenbock, and S. M. Levitz. 2003. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J. Biol. Chem. 278:37561-37568. [DOI] [PubMed] [Google Scholar]
- 34.Schindler, R., B. D. Clark, and C. A. Dinarello. 1990. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J. Biol. Chem. 265:10232-10237. [PubMed] [Google Scholar]
- 35.Schottelius, A. J., L. L. Moldawer, C. A. Dinarello, K. Asadullah, W. Sterry, and C. K. Edwards III. 2004. Biology of tumor necrosis factor-alpha-implications for psoriasis. Exp. Dermatol. 13:193-222. [DOI] [PubMed] [Google Scholar]
- 36.Shadkchan, Y., Y. Keisari, and E. Segal. 2004. Cytokines in mice treated with amphotericin B-intralipid. Med. Mycol. 42:123-128. [DOI] [PubMed] [Google Scholar]
- 37.Shimauchi, H., S. Takayama, T. Imai-Tanaka, and H. Okada. 1998. Balance of interleukin-1 beta and interleukin-1 receptor antagonist in human periapical lesions. J. Endod. 24:116-119. [DOI] [PubMed] [Google Scholar]
- 38.Tiphine, M., V. Letscher-Bru, and R. Herbrecht. 1999. Amphotericin B and its new formulations: pharmacologic characteristics, clinical efficacy, and tolerability. Transplant. Infect. Dis. 1:273-283. [DOI] [PubMed] [Google Scholar]
- 39.Turtinen, L. W., D. N. Prall, L. A. Bremer, R. E. Nauss, and S. C. Hartsel. 2004. Antibody array-generated profiles of cytokine release from THP-1 leukemic monocytes exposed to different amphotericin B formulations. Antimicrob. Agents Chemother. 48:396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vonk, A. G., M. G. Netea, N. E. Denecker, I. C. Verschueren, J. W. van der Meer, and B. J. Kullberg. 1998. Modulation of the pro- and anti-inflammatory cytokine balance by amphotericin B. J. Antimicrob. Chemother. 42:469-474. [DOI] [PubMed] [Google Scholar]
- 41.Walsh, T. J., R. W. Finberg, C. Arndt, J. Hiemenz, C. Schwartz, D. Bodensteiner, P. Pappas, N. Seibel, R. N. Greenberg, S. Dummer, M. Schuster, J. S. Holcenberg et al. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 340:764-771. [DOI] [PubMed] [Google Scholar]
- 42.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, M. H., R. A. Bowden, E. S. Sandler, M. L. Graham, G. A. Noskin, J. R. Wingard, M. Goldman, J. A. van Burik, A. McCabe, J. S. Lin, M. Gurwith, and C. B. Miller. 1998. Randomized, double-blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia. Clin. Infect. Dis. 27:296-302. [DOI] [PubMed] [Google Scholar]
- 44.Younes, A., and M. E. Kadin. 2003. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J. Clin. Oncol. 21:3526-3534. [DOI] [PubMed] [Google Scholar]