Abstract
This article summarizes the drug therapy progress of advanced hepatocellular carcinoma, biliary tract cancer, and pancreatic cancer in 2022, including chemotherapy, molecular targeted therapy, and immunotherapy, to provide reference information for current clinical treatment and future clinical research, and to better improve prognosis and quality of life in patients with hepatobiliary and pancreatic cancer.
Keywords: hepatocellular carcinoma, biliary tract cancer, cholangiocarcinoma, pancreatic cancer, chemotherapy, targeted therapy, immunotherapy
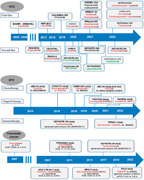
Key studies in the development of systemic therapy for advanced hepatocellular carcinoma (HCC), biliary tract cancer (BTC), and pancreatic cancer. The development of immunotherapy and targeted therapy has raised the survival rate of advanced HCC to a new high. Research on targeted therapy for specific gene mutations has made great progress in precise therapy of BTC, and immunotherapy has also achieved positive results, adding new treatment options. Chemotherapy is still the main treatment for pancreatic cancer, while targeted therapy and immunotherapy have also seen some dawn in exploratory research.
Abbreviations
- AEs
adverse events
- AFP
alpha‐fetoprotein
- ASCO
American Society of Clinical Oncology
- BTC
biliary tract cancer
- CTLA‐4
cytotoxic T‐lymphocyte antigen 4
- DCR
disease control rate
- dMMR
deficient of mismatch repair
- DOR
duration of response
- ESMO
European Society for Medical Oncology
- FDA
Food and Drug Administration
- FGFR
fibroblast growth factor receptor
- GI
gastrointestinal
- HCC
hepatocellular carcinoma
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- IHC
immunohistochemistry
- ISH
in situ hybridization
- MSI‐H
microsatellite instability‐high
- NMPA
National Medical Products Administration
- ORR
objective response rate
- OS
overall survival
- PD‐1
programmed death protein‐1
- PD‐L1
programmed death ligand‐1
- PFS
progression‐free survival
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial‐derived growth factor
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is a tumor with high incidence, especially in China, while biliary tract cancer (BTC) and pancreatic cancer are tumors with relatively low incidence, while HCC, BTC, and pancreatic cancer are tumors with high mortality [1, 2]. In the past, the treatment drugs were very limited, resulting in a great bottleneck in the treatment of advanced HCC. In recent years, the development of immunotherapy and targeted therapy has brought more treatment options for HCC, raising the survival rate of advanced HCC to a new high [3]. BTC is clinically and genetically heterogeneous. Genomic and molecular profiling analysis reveals potential targetable molecular aberrations. Research on targeted therapy for specific gene mutations (e.g., isocitrate dehydrogenase 1, human epidermal growth factor receptor 2 [HER2], fibroblast growth factor receptor [FGFR], and other mutated molecules) has made great progress in the field of biliary tract malignancies [4]. At present, precisely targeted therapy guided by different driver genes has become an important strategy for the clinical treatment of BTC, enriching the treatment options for biliary tract tumors. Immunotherapy has also achieved positive results in BTC, adding new treatment options [5]. However, chemotherapy is still the main treatment for pancreatic cancer, and optimization of the chemotherapy regimens is still one of the exploration directions for pancreatic cancer, while targeted therapy and immunotherapy have also seen some dawn in exploratory research [6]. This article reviews and summarizes the main progress of advanced hepatobiliary and pancreatic tumors in 2022, hoping to provide references for current clinical treatment and future clinical research.
2. HCC
2.1. Targeted therapy
Both the SHARP study in 2008 and the ORIENTAL study in 2009 confirmed that compared with placebo, first‐line sorafenib improved the survival of patients with advanced HCC, thus establishing sorafenib as the first‐line standard treatment for inoperable HCC [7, 8]. Until 2018, no other drug could replace sorafenib. The REFLECT study confirmed that first‐line lenvatinib was not inferior to sorafenib in overall survival (OS), and is superior to sorafenib in progression‐free survival (PFS) and objective response rate (ORR) [9]. In 2020, the results of the ZGDH3 study confirmed that donafenib was superior to sorafenib in the first‐line treatment of advanced HCC in OS, but only achieved noninferiority in ORR and PFS [10]. However, in many clinical studies, sorafenib is still the standard control for the first‐line treatment of HCC. There was no standard second‐line treatment for HCC until the RESORCE results of regorafenib in 2017 [11], and the CELESTIAL results of cabozantinib in 2018 [12]. The REACH study was negative, but a subgroup analysis found a benefit for ramucirumab in those with serum alpha‐fetoprotein (AFP) concentrations of 400 ng/mL or higher, and the subsequent REACH‐2 studies were conducted in those with AFP levels greater than 400 and achieved positive results for ramucirumab [13]. In addition, apatinib, a novel oral tyrosine kinase inhibitor (TKI) targeting vascular endothelial‐derived growth factor (VEGF)‐2, also achieved a significant improvement in OS compared with placebo in the second‐line treatment of HCC patients in the Chinese population [14]. However, there is still no progress in targeted therapy for HCC in 2022 (Tables 1, 2).
Table 1.
Results of phase III clinical trials of first‐line treatment for advanced HCC.
| Trial | Treatment | Control | n | ORR (%) | Median PFS/TTP (mo), HR (95% CI) | Median OS (mo), HR (95% CI) | Result |
|---|---|---|---|---|---|---|---|
| Chemotherapy | |||||||
| EACH | FOLFOX | Doxorubicin | 371 | 8.15 vs. 2.67 | 2.93 vs. 1.77 | 6.4 vs 4.97 | Negative |
| 0.62 (0.49–0.79) | 0.80 (0.63–1.02) | ||||||
| Targeted therapy | |||||||
| SHARP | Sorafenib | Placebo | 602 | 2.0 vs. 1.0 | 5.5 vs. 2.8 | 10.7 vs. 7.9 | Positive |
| 0.58 (0.45–0.74) | 0.69 (0.55–0.87) | ||||||
| ORIENTAL | Sorafenib | Placebo | 226 | 3.3 vs. 1.3 | 2.8 vs. 1.4 | 6.5 vs. 4.2 | Positive |
| 0.57 (0.42–0.79) | 0.68 (0.50–0.93) | ||||||
| REFLECT | Lenvatinib | Sorafenib | 954 | 24.1 vs. 9.2 | 7.4 vs. 3.7 | 13.6 vs. 12.3 | Positive |
| 0.66 (0.57–0.77) | 0.92 (0.79–1.06) | ||||||
| ZGDH3 | Donafenib | Sorafenib | 668 | 4.6 vs. 2.7 | 3.7 vs. 3.6 | 12.1 vs. 10.3 | Positive |
| 0.91 (0.76–1.08) | 0.83 (0.699–0.988) | ||||||
| Immunotherapy | |||||||
| CheckMate459 | Nivolumab | Sorafenib | 743 | 15.0 vs. 7.0 | 3.7 vs. 3.8 | 16.4 vs. 14.7 | Negative |
| 0.93 (0.79–1.10) | 0·85 (0.72–1.02) | ||||||
| RATIONALE‐301 | Tislelizumab | Sorafenib | 674 | 14.3 vs. 5.4 | 2.1 vs. 3.4 | 15.9 vs. 14.1 | Positive |
| 1.11 (0.92–1.33) | 0.85 (0.71–1.02) | ||||||
| HIMALAYA | Durvalumab | Sorafenib | 778 | 17.0 vs. 5.1 | 3.65 vs. 4.07 | 16.6 vs. 13.8 | Positive |
| 1.02 (0.88–1.19) | 0.86 (0.73–1.03) | ||||||
| IMbrave150 | Atezolizumab + bevacizumab | Sorafenib | 501 | 30.0 vs. 11.0 | 6.9 vs. 4.3 | 19.2 vs. 13.4 | Positive |
| 0.65 (0.53–0.81) | 0.66 (0.52–0.85) | ||||||
| ORIENT‐32 | Sintilimab + bevacizumab | Sorafenib | 571 | 21.0 vs. 4.0 | 4.6 vs. 2.8 | NR vs. 10.4 | Positive |
| 0.56 (0.46–0.70) | 0.57 (0.43–0.75) | ||||||
| HIMALAYA | Trimetrelizumab + durvalumab | Sorafenib | 782 | 20.1 vs. 5.1 | 3.78 vs. 4.07 | 16.4 vs. 13.8 | Positive |
| 0.90 (0.77–1.05) | 0.78 (0.65–0.92) | ||||||
| NCT03764293 | Camrelizumab + apatinib | Sorafenib | 543 | 25.4 vs. 5.9 | 5.6 vs. 3.7 | 22.1 vs. 15.2 | Positive |
| 0.52 (0.41–0.65) | 0.62 (0.49–0.80) | ||||||
| COSMIC‐312 | Atezolizumab + cabozantinib | Sorafenib | 649 | 11.0 vs. 4.0 | 6.8 vs. 4.2 | 15.4 vs. 15.5 | Negative |
| 0.63 (0.44–0.91) | 0.90 (0.69–1.18) | ||||||
| LEAP‐002 | Pembrolizumab + lenvatinib | Lenvatinib | 794 | 26.1 vs. 17.5 | 8.2 vs. 8.0 | 21.1 vs. 19.0 | Negative |
| 0.867 (0.734–1.024) | 0.84 (0.708–0.997) | ||||||
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; NR, not reached; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TTP, time to progression.
Table 2.
Results of clinical trials of second‐line treatment for advanced HCC.
| Trial | Treatment | Control | n | ORR (%) | Median PFS/TTP (mo), HR (95% CI) | Median OS (mo), HR (95% CI) | Result |
|---|---|---|---|---|---|---|---|
| Targeted therapy | |||||||
| RESORCE (III) | Regorafenib | Placebo | 573 | 7.0 vs. 3.0 | 3.1 vs. 1.5 | 10.6 vs. 7.8 | Positive |
| 0.46 (0.37–0.56) | 0.63 (0.50–0.79) | ||||||
| REACH‐2 (III) | Ramucirumab | Placebo | 292 | 4.6 vs. 1.1 | 2.8 vs. 1.6 | 8.5 vs. 7.3 | Positive |
| 0.45 (0.34–0.60) | 0.71 (0.53–0.95) | ||||||
| CELESTIAL (III) | Cabozantinib | Placebo | 760 | 4.0 vs. <1.0 | 5.2 vs. 1.9 | 10.2 vs. 8.0 | Positive |
| 0.44 (0.36–0.52) | 0.76 (0.63–0.92) | ||||||
| AHELP (III) | Apatinib | Placebo | 393 | 10.7 vs. 1.5 | 4.5 vs. 1.9 | 8.7 vs. 6.8 | Positive |
| 0.47 (0.37–0.60) | 0.79 (0.617–0.998) | ||||||
| Immunotherapy | |||||||
| CheckMate040 (I/II) | Nivolumab | – | 145 | 14.0 | 4.0 | 15.6 | Approved by FDA |
| KeyNote224 (II) | Pembrolizumab | – | 104 | 17.0 | 4.9 | 12.9 | Approved by FDA |
| NCT02989922 (II) | Camrelizumab | Placebo | 217 | 14.7 | 2.1 | 13.8 | Approved by NMPA |
| RATIONALE‐208 (II) | Tislelizumab | Placebo | 249 | 12.4 | 2.7 | 12.4 | Approved by NMPA |
| KeyNote240 (III) | Pembrolizumab | Placebo | 413 | 18.3 vs. 4.4 | 3.0 vs. 2.8 | 13.8 vs. 10.6 | Negative |
| 0.72 (0.57–0.90) | 0.78 (0.611–0.998) | ||||||
| KeyNote 394 (III) | Pembrolizumab | Placebo | 453 | 12.7 vs. 1.3 | 2.6 vs. 2.3 | 14.6 vs. 13.0 | Positive |
| 0.74 (0.60–0.92) | 0.79 (0.63–0.99) | ||||||
Note: –, not available or applicable.
Abbreviations: CI, confidence interval; FDA, Food and Drug Administration; HCC, hepatocellular carcinoma; HR, hazard ratio; NMPA, National Medical Products Administration; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TTP, time to progression.
2.2. Immunotherapy
2.2.1. Single‐drug immunotherapy
2.2.1.1. First‐line treatment
Single‐drug immunotherapy has been explored in many phase III studies in HCC (Table 1). CheckMate459 study head‐to‐head comparing nivolumab and sorafenib as first‐line therapy failed to demonstrate superiority for nivolumab over sorafenib in terms of OS, which median OS (mOS) was 16.4 months (95% [confidence interval] CI: 13.9–18.4) in the nivolumab group and 14.7 months (95% CI: 11.9–17.2) in the sorafenib group, with a hazard ratio of 0.85 (95% CI: 0.72–1.02, p = 0.075), but a favorable safety profile was observed in the nivolumab arm [15]. However, the indication for nivolumab in HCC was withdrawn due to negative results from CheckMate459. Tislelizumab is a monoclonal antibody with a high binding affinity to programmed death protein‐1 (PD‐1). RATIONALE‐301 is a global multicenter phase III study. The final analysis was published at the European Society for Medical Oncology (ESMO) 2022. Tislelizumab met the primary endpoint of OS in a noninferiority efficacy test compared to sorafenib as a first‐line treatment for unresectable HCC (15.9 vs. 14.1 months, hazard ratio [HR] = 0.85, p = 0.040). However, the ORR in the tislelizumab group was significantly better than that in the sorafenib group (14.3% vs. 5.4%), especially in the median duration of response (DOR) (36.1 vs. 11.0 months). There were also fewer treatment‐related adverse events (AEs) and grade 3 or higher treatment‐related AEs with tislelizumab [16]. Additionally, durvalumab is a programmed death ligand‐1 (PD‐L1) monoclonal antibody. In the HIMALAYA study, durvalumab monotherapy was compared with sorafenib, and achieved OS with noninferiority and non‐superiority (16.56 vs. 13.77 months, HR = 0.86, p = 0.0398) [17]. The results of the above three studies are almost consistent: first‐line single‐drug immunotherapy is noninferior to but not superior to sorafenib, but the ORR and tolerability are better than sorafenib. Therefore, the above three drugs can be used as treatment options for patients who are contraindicated or at greater risk of TKIs and antiangiogenic drugs.
2.2.1.2. Second‐line treatment
CheckMate040 (phase I/II) (ORR: 14%, median PFS [mPFS]: 4.0 months, mOS: 15.6 months) and KeyNote224 (phase II) (ORR: 17%, mPFS: 4.9 months, mOS: 12.9 months) have initiated HCC immunotherapy [18, 19] (Table 2). Based on these two studies, nivolumab and pembrolizumab received Food and Drug Administration (FDA) approval in July 2017 and November 2018, respectively, for the treatment of HCC patients who had failed sorafenib. The National Medical Products Administration (NMPA) of China has also approved two PD‐1 antibodies for the second‐line treatment of HCC based on the results of two phase II studies. In a study (NCT02989922) published in 2018, the ORR of camrelizumab in the second‐line treatment of HCC was 14.7%, the mPFS was 2.1 months, and the mOS was 13.8 months [20]. Another study of RATINALE‐208 is an open‐label, global multicenter, phase II clinical study (NCT03419897), which was presented at the American Society of Clinical Oncology gastrointestinal (ASCO‐GI) in 2022. The results showed that tislelizumab monotherapy showed favorable clinical activity and was well tolerated in previously treated patients with advanced HCC, with an ORR of 13.3% (95% CI: 9.3–18.1), the mPFS was 2.7 months (95% CI: 1.4–2.8), and the mOS was 13.2 months (95% CI: 10.8–15.0) [21]. KeyNote‐240 is a phase III, randomized controlled, global multicenter study based on KeyNote224, designed to evaluate the efficacy and safety of pembrolizumab versus placebo in patients with advanced HCC treated with sorafenib. However, the results published by ASCO in 2019 did not meet the preset common endpoints of OS and PFS. At the final analysis, the mOS was 13.9 and 10.6 months, mPFS was 3.0 and 2.8 months, and ORR was 18.3% and 4.4% in the pembrolizumab group and placebo group, respectively [22]. A longer follow‐up in 2021 also did not reach the statistical endpoint [23]. However, a similar study in the Asian population, the KeyNote394 study presented at the ASCO‐GI meeting in 2022, achieved positive results on the expected endpoint compared with placebo plus best supportive care. The mOS was 14.6 months (95% CI: 12.6–18.0), and there was a 21% reduction in the risk of death (HR = 0.79, 95% CI: 0.63–0.99, p = 0.018) in the pembrolizumab group of previously treated patients with advanced HCC. Long‐term survival was also significantly improved in the pembrolizumab group compared with the placebo group, with 2‐year survival rates of 34.3% and 24.9%, respectively [24].
2.2.2. Combined immunotherapy
Combined immunotherapy has become the first‐line standard treatment for HCC. The IMbrave150 study confirmed that atezolizumab (PD‐L1 antibody) combined with bevacizumab was significantly superior to sorafenib in terms of OS, PFS, and ORR in the first‐line treatment of advanced HCC [25, 26]. Similarly, the ORIENT‐32 study demonstrated that first‐line sintilimab (PD‐1 antibody) plus bevacizumab was superior to sorafenib [27]. And these two regimens have been approved by the NMPA of China for the first‐line treatment of advanced HCC. Several other immunotherapy studies have also been successful (Table 1). The HIMALAYA study is a multicohort phase III study exploring the first‐line efficacy of the combined immunotherapy (STRIDE protocol): durvalumab (PD‐L1 antibody) plus trimetrelizumab (cytotoxic T‐lymphocyte antigen 4 [CTLA‐4] antibody) in advanced HCC. The final results reported at the ASCO‐GI meeting in 2022 showed that the mOS of the STRIDE regimen was 16.4 months, while the mOS of sorafenib was 13.8 months (HR = 0.78, p = 0.004), reaching the primary endpoint of the superior efficacy in terms of OS. The ORR of the STRIDE regimen was higher (20.1% vs. 5.1%), but the mPFS was not superior to that of sorafenib (3.78 vs. 4.07, HR = 0.90, 95% CI: 0.77–1.05), and the safety of single starting dose of tremelimumab plus regularly spaced durvalumab was manageable, resulting in a lower incidence of treatment‐related adverse events than sorafenib [17]. The final results of a phase III study (NCT03764239) reported at ESMO in 2022 showed that camrelizumab (anti‐PD‐1 IgG4 antibody) plus apatinib (small‐molecule TKI targeting VEGF receptor type 2) was superior to sorafenib: OS (22.1 vs. 15.2 months, HR = 0.62, 95% CI: 0.49–0.80, p < 0.0001), PFS (5.6 vs. 3.7 months, HR = 0.52, 95% CI: 0.41–0.65, p < 0.0001), and ORR (25.4% vs. 5.9%, p < 0.0001) were significantly improved, and the combination of camrelizumab and apatinib was also well tolerated [16]. However, in the COSMIC‐312 study published in 2021, atezolizumab (anti‐PD‐L1 antibody) plus cabozantinib (a multitargeted small‐molecule TKI) versus sorafenib in the first‐line treatment of advanced HCC showed improved mPFS (6.8 vs. 4.2 months, HR = 0.63, 95% CI: 0.44–0.91, p = 0.001) in the combination group, but mOS (15.4 vs. 15.5, HR = 0.90, 95% CI: 0.69–1.18, p = 0.440) and ORR (11% vs. 4%) did not improve significantly [28]. A phase III study of LEAP‐002 was widely anticipated given the excellent ORR and PFS results of lenvatinib plus pembrolizumab in a phase Ib study (NCT03006926) [29]. Regrettably, the primary results of the LEAP‐002 study presented at the ESMO meeting in 2022 showed that the combination regimen first‐line treatment did not significantly improve OS (21.1 months vs. 19.0 months, HR = 0.84, p = 0.023) and PFS (8.2 months vs. 8.0 months, HR = 0.87, p = 0.047) compared to lenvatinib alone (failed to reach prespecified statistical difference), and only improvements were observed in ORR (26.1% vs. 17.5%) and DOR (11.2 vs. 8.5 months) [30]. The three similar studies above yielded different results, adding to the complexity of the HCC immunotherapy puzzle. Whether the results are different due to different populations, different immunotherapy drugs, different TKI drugs, or different control groups is still unclear and needs to be further explored.
3. BTC
3.1. Targeted therapy
3.1.1. HER2 targeted therapy
HER2 mutations, including amplification, overexpression, or both, were observed in approximately 19% of gallbladder tumors, 17% of extrahepatic cholangiocarcinomas, 13% of ampullary carcinomas, and 5% of intrahepatic cholangiocarcinomas [4, 31]. In the earlier MyPathway study, trastuzumab plus pertuzumab had an ORR of 23% in HER2‐mutated advanced BTC, with mPFS and OS of 4.0 and 10.9 months, respectively [32] (Table 3). In a phase I study of zanidatamab (ZW25), a HER2 bispecific antibody, was used in 21 patients with HER2‐mutated advanced BTC, and the ORR was 38% [33, 34]. Neratinib is an irreversible pan‐HER TKI. In the SUMMIT study, 25 patients with HER2‐mutated advanced biliary tumors treated with neratinib had an ORR of 16%, a mPFS of 2.8 months, and a mOS of 5.4 months [35]. The 2022 ASCO meeting reported trastuzumab deruxtecan (DS‐8201) in the treatment of patients with HER2‐expressing unresectable or recurrent BTC. The investigator‐initiated multicenter phase II study (HERB trial) in a total of 22 HER2‐positive patients had an ORR of 36.4%, a mPFS of 4.4 months, and a mOS of 7.1 months. For the eight patients with low HER2 expression (immunohistochemistry [IHC]/in situ hybridization status 0/+, 1+/−, 1+/+, 2+/−), the ORR was 12.5%, and the mPFS and OS were 4.2 and 8.9 months, respectively. However, the incidence of grade 3/4 AEs in this study was as high as 81.3%, and eight patients complicated with interstitial lung disease or pneumonia, suggesting that special attention should be paid to the adverse drug reactions of DS‐8201 [36]. In addition, a multicenter phase II trial (KCSG‐HB19‐14) conducted by the Korea Cancer Research Group reported at ASCO 2022 that the ORR of trastuzumab plus FOLFOX in gemcitabine/cisplatin refractory HER2‐positive BTC reached 29.4% of 34 patients. The mPFS and OS were 5.1 and 10.7 months, respectively, with HER2 expressing IHC3+ (n = 23, 67.6%) showing a trend toward better PFS (5.5 vs. 4.9 months, HR = 0.52, 95% CI: 0.23–1.16) [37].
Table 3.
Results of clinical trials of treatment for advanced BTC.
| Trial | Treatment | Phase | Line | n | ORR (%) | Median PFS (mo), HR (p‐value) | Median OS (mo), HR (p‐value) | Result |
|---|---|---|---|---|---|---|---|---|
| Chemotherapy | ||||||||
| ABC‐02 | GemCis vs. Gemcitabine | III | 1st | 410 | 26.1 vs. 15.5 | 8.0 vs. 5.0 (p < 0.001) | 11.7 vs. 8.1 (p < 0.001) | Positive |
| JCOG1113 | Gemcitabine + S‐1 vs. GemCis | III | 1st | 354 | 29.8 vs. 32.4 | 6.8 vs. 5.8 | 15.1 vs. 13·4 (p = 0.046) | Noninferiority |
| KHBO1401 | GemCis + S‐1 vs. GemCis | III | 1st | 246 | 41.5 vs. 15.0 | 7.4 vs. 5.5 (p = 0.0015) | 13.5 vs. 12.6 (p = 0.046) | Positive |
| ABC‐06 | FOLFOX + ASC vs. ASC | III | 2nd | 292 | 5.0 | 4.0 | 6.2 vs. 5.3 | Positive |
| HER2 targeted | ||||||||
| MyPathway | Trastuzumab + pertuzumab | II | 2nd | 39 | 23.0 | 4.0 | 10.9 | – |
| NCT02892123 | Zanidatamab | I | 2nd | 21 | 38.0 | 3.5 | – | – |
| SUMMIT (II) | Neratinib | II | 2nd | 25 | 16.0 | 2.8 | 5.4 | – |
| KCSG‐HB19‐14 | Trustuzumab + FOLFOX | II | 2nd | 34 | 29.4 | 5.1 | 10.7 | – |
| HERB | T‐DXd (DS‐8201) | II | 2nd | 30 | 36.4 | 5.1 | 7.1 | – |
| IDH1 targeted | ||||||||
| ClarlDHy | Ivosidenib vs. placebo | III | 2nd | 187 | 2.4 | 2.7 vs. 1.4 (p < 0.001) | 10.3 vs. 7.5 (p = 0.093) | Positive |
| FGFR trageted | ||||||||
| FIGHT202 | Pemigatinib | II | 2nd | 107 | 37.0 | 7.0 | 17.5 | Approved by FDA |
| CIBI375A201 | Pemigatinib | II | 2nd | 30 | 60.0 | 9.1 | – | Approved by NMPA |
| NCT02150967 | Infigratinib | II | 2nd | 108 | 23.1 | 7.3 | 12.2 | – |
| NCT02699606 | Erdafitinib | II | 2nd | 22 | 40.9 | 5.6 | 40.2 | – |
| FIDES‐01 | Derazantinib | II | 2nd | 103 | 21.4 | 8.0 | 17.2 | – |
| FOENIX‐CCA2 | Futibatinib | II | 2nd | 28 | 41.7 | 8.9 | 20.0 | – |
| ReFocus | RLY‐4008 | II | 2nd | 38 | 63.2 | – | – | – |
| BRAF‐V600E targeted | ||||||||
| NCT02034110 | Dabrafenib + trametinib | II | 2nd | 43 | 47.0 | 9.0 | 14.0 | – |
| Immunotherapy (MSI‐H/dMMR) | ||||||||
| KEYNOTE‐016 | Pembrolizumab | II | 2nd | 4 | 53.0 | – | – | – |
| KEYNOTE‐158 | Pembrolizumab | II | 2nd | 9 | 37.0 | – | – | – |
| Immunotherapy (MSS/pMMR) | ||||||||
| Kim et al. | Nivolumab | II | 2nd | 54 | 22.0 | 3.68 | 14.24 | – |
| Ueno et al. | Nivolumab | I | 1st | 30 | 3.3 | 1.4 | 5.2 | – |
| KEYNOTE‐158 | Pembrolizumab | II | 2nd | 104 | 5.8 | 2.0 | 7.4 | – |
| Doki et al. | Durvalumab | II | 2nd | 42 | 4.8 | 1.5 | 8.1 | – |
| NCT03092895 | Camrelizumab + Gemox | II | 1st | 63 | 19.0 | 3.8 | 13.6 | – |
| Camrelizumab + FOLFOX | II | 1st | 29 | 10.3 | 5.5 | 12.0 | – | |
| NCT03486678 | Camrelizumab + Gemox | II | 1st | 38 | 54.0 | 6.1 | 11.8 | – |
| JapicCTI‐153098 | Nivolumab + GemCis vs. nivolumab | I | 1st | 60 | 37.0 vs. 3.0 | 4.2 vs. 1.4 | 15.4 vs. 5.2 | – |
| NCT03796429 | Toripalimab + Gemcitabine + S‐1 | II | 1st | 50 | 30.6 | 7.0 | 15.0 | – |
| TCOG T1219 | Nivolumab + Gemcitabine + S‐1 | II | 1st | 48 | 43.8 | 9.1 | – | – |
| TOPAZ‐1 | Durvalumab + GemCis vs. GemCis | III | 1st | 685 | 26.7 vs. 18.7 | 7.2 vs. 5.7 (p = 0.001) | 12.8 vs. 11.5 (p = 0.021) | Positive |
Note: –, not available or applicable.
Abbreviations: ASC, active symptom control; BTC, biliary tract cancer; CI, confidence interval; dMMR, deficient of mismatch repair; FDA, Food and Drug Administration; FGFR, fibroblast growth factor receptor; GemCis, gemcitabine plus cisplatin; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; IDH1, isocitrate dehydrogenase 1; MSI‐H, microsatellite instability‐high; MSS, microsatellite stable; NMPA, National Medical Products Administration; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; pMMR, proficient mismatch repair; T‐DXd, trastuzumab deruxtecan.
3.1.2. FGFR targeted therapy
FGFR 1–4 gene alterations are one of the common oncogenic drivers of BTC, especially intrahepatic cholangiocarcinomas, where FGFR2 mutations are detectable in ∼14% of patients, the vast majority of which are fusion mutations [5, 38]. Pemigatinib, a pan‐FGFR (FGFR 1–3) inhibitor, was approved by the FDA on April 17, 2020, for the treatment of adult patients with FGFR2 fusion cholangiocarcinoma based on the results of the FIGHT‐202 study [39] (Table 3). The results of the FIGHT‐202 study were updated at ESMO 2022. In 107 patients with FGFR2 fusion/rearrangement mutations, ORR was 37%, disease control rate (DCR) was 82%, and mPFS and mOS were 7.0 and 17.5 months [16]. Based on the results of the Phase II CIBI375A201 bridging trial of pemigatinib in China, pemigatinib was officially approved by the NMPA of China in April 2022 for the treatment of adults with advanced, metastatic or inoperable cholangiocarcinoma, who have received at least one prior systemic therapy and have detected FGFR2 fusion or rearrangement. In this study, a total of 30 patients with advanced cholangiocarcinoma and FGFR2 fusion/rearrangement mutations who failed standard therapy received pemigatinib, resulting in an ORR of 60%, DCR of 100%, and mPFS of 9.1 months, as updated at ASCO 2022 [40]. In addition, multiple pan‐FGFR inhibitors including infigratinib, erdafitinib, derazantinib, and futibatinib were tested in phase II studies in advanced BTC patients with FGFR2 fusion/rearrangement mutations, resulting in ORRs of 21.4%–41.7%, DCR of 75.7%–84.3%, mPFS of 5.6–8.9 months and mOS of 12.2–40.2 months [41, 42, 43, 44]. Whereas, RLY‐4008, a highly selective FGFR2 inhibitor, is a potent and selective FGFR2 inhibitor compared with pan‐FGFR inhibitors, which exhibited strong activity in FGFRi‐sensitive or drug‐resistant exosomal model of cholangiocarcinoma [45]. Preliminary efficacy data from the ReFocus trial of RLY‐4008, which were reported at the 2022 ESMO congress, in patients with FGFR2 fusion/rearranged BTC not previously treated with FGFR inhibitors showed an ORR of 63.2% and a DCR of 94.7% in a total of 38 patients across all dose groups [45]. The 70 mg dose group was the recommended dose in the phase II study, in which the 17 patients who received the 70 mg dose had an ORR of 88.2% and a DCR of 100%, supporting further expansion of the study.
3.2. Immunotherapy
For patients of cholangiocarcinoma with microsatellite instability‐high (MSI‐H) or deficient mismatch repair (dMMR) mutations, pembrolizumab alone achieved ORR of 53% and 37% in KEYNOTE‐016 [46] and KEYNOTE‐158 study [47], but the proportion of MSI‐H/dMMR in cholangiocarcinoma was very low [48]. However, for patients of cholangiocarcinoma with non‐MSI‐H/dMMR, the efficacy of single‐agent immunotherapy is still unclear, and only small sample studies have been reported (Table 3). Kim et al. reported that the ORR of nivolumab in the second‐line or beyond the treatment of advanced cholangiocarcinoma was 22%, and mPFS and mOS were 3.68 and 14.24 months, respectively [49]. In contrast, Ueno et al. reported an ORR of 3.3% with first‐line nivolumab, and mPFS and mOS were 1.4 and 5.2 months, respectively [50]. In the KEYNOTE‐158 study, 104 patients with advanced cholangiocarcinoma who received single‐agent pembrolizumab had an ORR of 5.8%, mPFS and mOS of 2.0 and 7.4 months, respectively [51]. Doki et al. reported an ORR of 4.8%, mPFS, and mOS of 1.5 and 8.1 months, respectively, in second‐line or beyond durvalumab therapy in 42 patients with advanced cholangiocarcinoma [52].
However, improved ORR has been observed in many phase II studies of immunotherapy combined with chemotherapy. In two phase II studies (NCT03092895 and NCT03486678) [53, 54], camrelizumab in combination with GEMOX or FOLFOX had an ORR of 10.3%–54%, while in the JapicCTI‐153098 study, the ORR of gemcitabine plus cisplatin (GemCis) plus nivolumab was 37%, which was significantly improved compared with nivolumab monotherapy (ORR was 3%) [50]. In two other phase II studies (NCT03796429 and TCOG T1219), the ORR of toripalimab or nivolumab combined with gemcitabine and TS‐1 (tegafur, gimeracil, and oteracil potassium capsules) were 30.6% and 43.8%, respectively, and mPFS were 7.0 and 9.1 months, respectively [55, 56]. Nevertheless, TOPAZ‐1 is the only phase III randomized controlled study with definitively positive results demonstrating a significant survival benefit of durvalumab plus chemotherapy compared with standard chemotherapy. The results published by ASCO‐GI in 2022 showed that compared with GemCis, durvalumab plus GemCis significantly improved ORR (26.7% vs. 18.7%), PFS (7.2 vs. 5.7 months, HR = 0.75, 95% CI: 0.64–0.89, p = 0.001), and OS (12.8 vs. 11.5 months, HR = 0.80, 95% CI: 0.66–0.97, p = 0.021). The safety of combination therapy is controllable, and durvalumab combined with GemCis provides a new option for the first‐line treatment of advanced BTC [57]. In addition to the above‐mentioned schemes, there are many other immunocombined treatment schemes for advanced BTC, such as PD‐1 antibody or PD‐L1 antibody combined with antiangiogenic TKI drugs or CTLA4 antibody, or on the basis of the above combination chemotherapy. However, most of them are small‐sample studies, and there is no definite conclusion yet.
4. PANCREATIC CANCER
4.1. Chemotherapy
At the 2021 ESMO meeting, Taieb et al. reported a European real‐world study on the effect of treatment sequence on prognosis in metastatic pancreatic adenocarcinoma, finding that the longest OS line was found in the sequential treatment‐naive FOLFIRINOX and gemcitabine‐based second‐line combination, with mOS reaching 20.0 months [58]. In 2022, multiple studies on optimizing chemotherapy regimens for pancreatic cancer have been reported (Table 4). The SEQUENCE phase III study reported by Carrato et al. at ASCO 2022 showed that the first‐line gemcitabine combined with nab‐paclitaxel (AG) regimen sequentially modified FOLFOX significantly improved ORR (39.7% vs. 20.3%, p = 0.009), PFS (7.9 vs. 5.2 months, p < 0.001), and OS (13.2 vs. 9.7 months, p = 0.023) [59]. The PRODIGE 65‐UCGI 36‐GEMPAX UNICANCER study, reported by de la Fouchardiere et al. at the 2022 ESMO conference, was a phase III randomized trial comparing gemcitabine plus paclitaxel versus gemcitabine in patients with metastatic pancreatic cancer who failed or intolerant to FOLFIRINOX, and the results showed improvements in ORR (19.2% vs. 4.8%), PFS (3.1 vs. 2.0 months, HR = 0.64, 95% CI: 0.47–0.89) compared with gemcitabine alone, but failed to improve OS (6.4 vs. 5.9 months, HR = 0.87, 95% CI: 0.63–1.20, p = 0.410) [60]. However, gemcitabine plus paclitaxel is not a common clinical combination. The HR‐IRI‐APC study reported by Wang et al. is a multicenter, randomized, double‐blind, parallel‐controlled phase III trial. The results showed that HR070803 (liposome irinotecan) combined with 5‐fluorouracil/leucovorin (FU/LV) compared with placebo combined with 5‐FU/LV in the second‐line treatment of gemcitabine refractory advanced pancreatic cancer, the combination significantly prolonged the mPFS (4.21 vs. 1.48 months, HR = 0.36, 95% CI: 0.27–0.48, p < 0.0001) and OS (7.39 vs. 4.99 months, HR = 0.63, 95% CI: 0.48–0.84, p = 0.002), and safety was manageable [61].
Table 4.
Results of clinical trials of treatment for advanced pancreatic cancer.
| Trial | Treatment | Control | Phase | Line | n | ORR (%) | Median PFS/TTP (mo), HR (95% CI) | Median OS (mo), HR (95% CI) | Result |
|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | |||||||||
| Burris HA et al. | Gemcitabine | 5‐FU | II | 1st | 124 | 5.4 vs. 0 | – | 5.65 vs. 4.41 | Positive |
| NCT00112658 | FOLFIRINOX | Gemcitabine | III | 1st | 342 | 31.6 vs. 9.4 | 6.4 vs. 3.3 | 11.1 vs. 6.8 | Positive |
| 0.47 (0.37–0.59) | 0.57 (0.45–0.73) | ||||||||
| MPACT | AG | Gemcitabine | III | 1st | 861 | 23.0 vs. 7.0 | 5.5 vs. 3.7 | 8.5 vs. 6.7 | Positive |
| 0.69 (0.581–0.821) | 0.72 (0.617–0.835) | ||||||||
| SEQUENCE | AG‐mFOLFOX | AG | III | 1st | 157 | 39.7 vs. 20.3 | 7.9 vs. 5.2 | 13.2 vs. 9.7 | Positive |
| 0.51 (0.36–0.73) | 0.68 (0.48–0.95) | ||||||||
| NCT03943667 | Gemcitabine + palitaxol | Gemcitabine | III | 2nd | 210 | 19.2 vs. 4.8 | 3.1 vs. 2.0 | 6.4 vs. 5.9 | Negative |
| 0.64 (0.47, 0.89) | 0.87 (90.63–1.20) | ||||||||
| HR‐IRI‐APC | HR070803 + 5‐FU/LV | Placebo + 5‐FU/LV | III | 2nd | 298 | – | 4.21 vs. 1.48 | 7.39 vs. 4.99 | Positive |
| 0.36 (0.27, 0.48) | 0.63 (0.48–0.84) | ||||||||
| Targeted therapy | |||||||||
| POLO | Olaparib | Placebo | III | 1st | 154 | – | 7.4 vs. 3.8 | 19.0 vs. 19.2 | Positive |
| 0.53 (0.35–0.82) | 0.79 (0.55–1.15) | ||||||||
| KRYSTAL‐1 | Adagrasib | – | I | ≧2nd | 12 | 50.0 | 6.6 | ||
| NOTABLE | Nimotuzumab + gemcitabine | Gemcitabine | III | 1st | 90 | 7.3 vs. 9.8 | 4.2 vs. 3.6 | 10.9 vs. 8.5 | Positive |
| 0.56 (0.12–0.99) | 0.50 (0.06–0.94) | ||||||||
| Immunotherapy | |||||||||
| Henriksen A et al. | Ipilimumab | – | II | ≧2nd | 27 | 0 | – | 4.5 | – |
| NCT02054806 | Pembrolizumab | – | Ib | ≧2nd | 24 | 0 | 1.7 | 3.9 | – |
| NCT02558894 | Durvalumab | – | II | ≧2nd | 32 | 0 | – | 3.6 | – |
| NCT00556023 | Tremelimumab + gemcitabine | – | I | 1st | 34 | 5.8 | – | 7.4 | – |
| NCT01473940 | Ipilimumab + gemcitabine | – | Ib | 1st | 21 | 14.0 | 2.78 | 6.90 | – |
| NCT02331251 | Pembrolizumab + AG | – | II | 1st | 17 | 17.7 | 9.1 | 15.0 | – |
| JapicCTI‐184230 | Nivolumab + mFOLFIRINOX | – | II | 1st | 31 | 32.3 | >7 | >13 | – |
| NCT04324307 | KN046 + AG | – | II | 1st | 31 | 45.2 | – | – | – |
| NCT03404960 | Niraparib + ipilimumab | Niraparib + nivolumab | Ib/II | 1st | 84 | 15.4 vs. 7.1 | 8.1 vs. 1.9 | 17.3 vs. 14.0 | – |
| CISPD3 | Sintilimab + mFOLFIRINOX | mFOLFIRINOX | III | 1st | 110 | 50.0 vs. 23.9 | 5.9 vs. 5.73 | 10.9 vs. 10.8 | Negative |
| 0.93 (0.62–1.41) | 1.083 (0.68–1.69) | ||||||||
Note: –, not available or applicable.
Abbreviations: 5‐FU, 5‐fluorouracil; AG, Nab‐paclitaxel/gemcitabine; CI, confidence interval; HR, hazard ratio; LV, leucovorin; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TTP, time to progression.
4.2. Targeted therapy
Pancreatic cancer is developing slowly toward precision therapy. It was once believed that targeted drugs could not be used, but in recent years, with the advancement of pharmaceutical methods and targeted therapy, breakthroughs have been made (Table 4). The study targeting the KRAS G12C mutation was reported at the 2022 ASCO GI meeting. The KRYSTAL‐1 (NCT03785249) study is a multicohort phase I/II study evaluating the efficacy of adgrasib monotherapy or in combination in patients with advanced solid tumors with KRAS G12C mutations. Adgrasib is a highly selective KRAS G12C inhibitor that selectively binds to and inactivates KRAS G12C irreversibly. In this study, 12 pancreatic cancer patients were enrolled after the failure of multiple lines of therapy. Clinical activity was assessed in 10 patients, of whom 5 achieved a partial response and 5 were stable, with a DCR of 100% and an mPFS of 6.6 months (95% CI: 1.0–9.7).
Across the entire cohort, the most common AEs were nausea (48%), diarrhea (43%), vomiting (43%), and fatigue (29%). Grade 3/4 AEs occurred in 21% of patients, and none grade 5 AEs, indicating an overall good safety profile [62]. KRAS G12C will be the most promising direction in the history of targeted therapy for pancreatic cancer, but unfortunately, this mutation only accounts for about 2% of KRAS. For other mutations of KRAS, drugs are expected to be developed soon. On the other hand, the NOTABLE study for KRAS wild‐type pancreatic cancer was reported at the 2022 ASCO meeting. The efficacy of nimotuzumab (EGFR monoclonal antibody) combined with gemcitabine versus gemcitabine in the treatment of KRAS wild‐type locally advanced or metastatic pancreatic cancer was evaluated. In this prospective, randomized, double‐blind, multicenter, phase III study, a positive result was reached. In the full analysis set (FAS) and the protocol analysis set (PPS), the mOS of the experimental group was significantly longer than that of the control group (FAS group: 10.9 vs. 8.5 months, HR = 0.50, p = 0.024; PPS population: 11.5 vs. 8.5 months, HR = 0.60, p = 0.039). In the FAS population, the mPFS was significantly longer in the experimental arm (4.2 months vs. 3.6 months, HR = 0.56, p = 0.013) [63]. Therefore, the combination of nimotuzumab and gemcitabine may be an option for patients with KRAS wild‐type pancreatic cancer who are not candidates for other combination therapy.
4.3. Immunotherapy
Pancreatic cancer itself has an immune escape, and at the same time, immunosuppressive factors such as CD47 and VEGF are often highly expressed [64]. Pancreatic cancer patients are generally in a highly immunosuppressive state, and various immunotherapy methods such as immune checkpoint inhibitors, chimeric antigen receptor T, and tumor vaccines have been tried, but no positive results have been found [65, 66, 67] (Table 4). KN046 is a PD‐L1/CTLA‐4 bispecific antibody. The results of the phase II study (NCT04324307) are encouraging. The ORR of 31 evaluable patients was 45.2%, and the DCR was 93.5%. The phase III study of KN046 combined with standard chemotherapy has been conducted in first‐line patients with advanced pancreatic cancer, which is worth looking forward to [68]. The CISPD3 study is a single‐center, randomized, open‐label phase III trial comparing the efficacy and safety of sintilimab combined with a modified FOLFRINOX regimen versus FOLFIRINOX alone as first‐line or second‐line treatment for patients with metastatic or recurrent pancreatic cancer. A total of 110 patients were enrolled, and sintillimab combined with chemotherapy failed to prove superior to chemotherapy alone, and the mOS and PFS were similar in the combination group and chemotherapy alone group (10.9 vs. 10.8 months, 5.9 vs. 5.73 months). However, the ORR of sintilimab combined with chemotherapy was significantly higher (50% vs. 23.9%) [69]. The addition of immune drugs improved ORR, but this did not translate into a survival benefit, although there was no significant increase in adverse reactions in the combination group. However, a randomized phase Ib/II study of niraparib plus nivolumab or ipilimumab in patients with advanced platinum‐sensitive pancreatic cancer reported at ASCO 2022 showed that maintenance therapy with niraparib plus ipilimumab was effective in patients with platinum‐sensitive advanced pancreatic cancer. In the patients, the mPFS was 8.1 months, the 6‐month PFS rate was 59.6%, and continued to be effective in patients without any known DNA damage repair subtype while niraparib combined with nivolumab was ineffective under the same conditions [70], suggesting that the combined use of niraparib and ipilimumab is worth further exploration. The failure of immunotherapy to achieve breakthroughs may be related to the inherent immunosuppressive properties of pancreatic cancer and the complexity of the tumor microenvironment. It is necessary to further study the immune microenvironment of pancreatic cancer and screen the population sensitive to immunotherapy to improve the poor prognosis of pancreatic cancer.
5. CONCLUSIONS AND FUTURE OUTLOOK
Hepatobiliary and pancreatic tumors are tumors with poor prognoses. However, for advanced diseases, systematic therapy can clearly bring survival benefits to patients. Looking back to 2022, these achievements in the field of medical treatment of hepatobiliary and pancreatic tumors will have an important impact on future clinical practice and guide future clinical research.
Patients with HCC are often accompanied by underlying liver disease, resulting in poor tolerance to chemotherapy, poor efficacy of chemotherapy, and lack of standardization of chemotherapy drugs. Due to the lack of standard chemotherapy drugs, the first‐line and second‐line treatment of HCC is mainly targeted drug therapy. At present, first‐line single‐drug immunotherapy has not been proven to be superior to sorafenib, but not inferior to sorafenib. However, studies have shown higher ORR and better immunotherapy tolerance than sorafenib. Therefore, for the first‐line treatment of HCC, immunotherapy can be considered as an alternative treatment option for patients in whom TKIs and antiangiogenic drugs are contraindicated or at significant risk. Atezolizumab or sintilimab plus bevacizumab have both been shown to be superior to sorafenib alone and have become the first‐line standard of care. In 2022, the regimens of tremelimumab plus durvalumab or camrelizumab plus apatinib are significantly better than sorafenib alone, which will add more options for the first‐line treatment of HCC. However, LEAP‐002 study failed. In terms of the PD‐1 antibody combined with TKI drugs, camrelizumab plus apatinib and pembrolizumab plus lenvatinib have achieved different test results. The lenvatinib monotherapy OS in the LEAP‐002 study control group exceeded expectations, and the different populations of the two studies may be an important factor leading to the different results. The biggest difference between KEYNOTE‐240 and KEYNOTE‐394 is the population. In KEYNOTE‐394, where the population was predominantly Asian, the proportions of hepatitis B virus (HBV)‐associated HCC in the experimental and placebo groups were 78.7% and 81%, respectively [24], compared with 25.9% and 21.5% in the KEYNOTE‐240 group [22]. The results of the subgroup analysis showed that HBV‐associated HCC was more likely to benefit from immunotherapy for survival. Similar subgroup analysis results were seen in the CheckMate459, COSMIC‐312, and IMbrave150 studies. In a phase III study of camrelizumab combined with rivoceranib (apatinib), the percentages of HBV‐associated HCC were 76.5% and 72.7% in the experimental group and sorafenib group, respectively [16], but only 48.6% and 48.4% in the LEAP‐002 study [30]. Pfister et al. found that nonviral HCC, especially NASH‐HCC, may respond poorly to immunotherapy, which may be due to tissue damage caused by abnormal T cell activation associated with NASH, resulting in impaired immune surveillance [71]. A first‐line retrospective study found that in the general population of nonviral HCC, especially in the NASH/NAFLD population, the OS time of atezolizumab combined with bevacizumab was significantly worse than lenvatinib, but not sorafenib [72]. Such conclusions still need to be further verified through prospective studies, but future research on HCC may need to consider the impact of different etiologies on treatment outcomes. Based on the current research progress of HCC, more exploratory studies on combination therapy models, the rationality of the treatment sequence, and population screening for benefit should be expected.
For systemic treatment of BTC, GemCis has been established as the first‐line standard since the ABC‐02 study, and FOLFOX has been established as the second‐line treatment since the ABC‐06 study, which can improve the survival of patients with advanced BTC. However, the efficacy of chemotherapy alone for advanced BTC is very limited. Single‐agent immunotherapy also has very limited efficacy in non‐MSI‐H/dMMR biliary tract tumors. However, the TOPAZ‐1 study in 2022 confirmed that durvalumab combined with chemotherapy is significantly better than chemotherapy alone. Durvalumab plus GemCis will become a new option for first‐line treatment of advanced BTC. But, the addition of immunotherapy in the TOPAZ‐1 study was unsatisfactory in terms of survival improvement. Further exploration is needed to screen out the population that really benefits from immunotherapy, or to explore other combination schemes to find more effective treatment options. On the other hand, precisely targeted therapy guided by genetic characteristics is an important treatment strategy for advanced BTC. Phase II studies of trastuzumab combined with FOLFOX or DS‐8201 in 2022 all suggest that anti‐HER2 therapy is effective for advanced biliary tract tumors, and further studies are needed to clarify its efficacy and provide evidence to support its clinical application. In addition, as a highly selective FGFR2 inhibitor, LGY‐4008 showed a high ORR of up to 88.2% at the recommended dose against FGFR2 fusion/rearranged cholangiocarcinoma patients not receiving FGFR inhibitor therapy, which may provide a more effective response in patients with FGFR2 fusion/rearrangement mutations and further studies should be highly anticipated. In addition, two Phase III studies (NCT03656536, NCT03773302) evaluating FGFR inhibitors for first‐line treatment of advanced biliary tract tumors with FGFR2 gene fusion/rearrangement are noteworthy.
However, chemotherapy is still the main systemic treatment for pancreatic cancer, with FOLFIRINOX, gemcitabine combined with nab‐paclitaxel, gemcitabine, or FU monotherapy being the most commonly used regimens. In 2022, the SEQUENCE study showed that first‐line gemcitabine plus nab‐paclitaxel (AG) sequentially modified FOLFOX was significantly better than AG alone, suggesting that this sequential regimen can be used as a first‐line treatment option, but whether it is better than FOLFIRINOX remains to be explored. In terms of second‐line treatment, liposome irinovican (HR070803) combined with 5‐FU/LV is superior to 5‐FU/LV, and can be used as a new second‐line option to further enrich the evidence‐based data of chemotherapy for pancreatic cancer. As for targeted therapy, the results of the KRYSTAL‐1 study in 2022 are encouraging, and the follow‐up extended research is worth looking forward to. However, while KRAS mutations occur more frequently in pancreatic cancer, KRAS G12C mutation is very rare, and it is unclear whether the other mutations in KRAS can achieve the same result. For KRAS wild‐type pancreatic cancer, the results of the NOTABLE study support nimotuzumab plus gemcitabine as a first‐line option. For immunotherapy, there is currently no evidence to support its use in non‐MSI‐H/dMMR pancreatic cancer, but a randomized phase Ib/II study of niraparib combined with ipilimumab is considered a potential breakthrough. Further research results should be expected. Furthermore, given the excellent performance of the Phase II study, we are looking forward to the results of the phase III study of KN046 in combination with chemotherapy (NCT05149326).
To sum up, in the field of hepatobiliary and pancreatic tumors, more and more clinical studies have formed more and more evidence‐based medical evidence in the continuous exploration of new drugs and new treatment schemes, which will change clinical practice, promote the progress of clinical treatment and ultimately benefit patients.
AUTHOR CONTRIBUTIONS
Le Zhang: Conceptualization (lead); data curation (lead); investigation (lead); writing—original draft (lead); writing—review and editing (lead). Rui Liu: Data curation (supporting); investigation (supporting); writing—original draft (supporting); writing—review and editing (supporting). Ting Deng: Conceptualization (supporting); supervision (supporting); validation (supporting). Yi Ba: Conceptualization (lead); funding acquisition (lead); supervision (lead); validation (lead).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
ACKNOWLEDGMENTS
None.
Zhang L, Liu R, Deng T, Ba Y. Advances in medical treatment of advanced hepatobiliary and pancreatic cancer in 2022. Cancer Innovation. 2023;2:36–51. 10.1002/cai2.60
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time‐trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021;41(10):1037–48. 10.1002/cac2.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62. 10.1016/S0140-6736(22)01200-4 [DOI] [PubMed] [Google Scholar]
- 4. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943–62. 10.1158/2159-8290.CD-17-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol. 2021;6(11):956–69. 10.1016/S2468-1253(21)00171-0 [DOI] [PubMed] [Google Scholar]
- 6. Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic cancer: pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022;163(2):386–402. 10.1053/j.gastro.2022.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 8. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2009;10(1):25–34. 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 9. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018;391(10126):1163–73. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 10. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first‐line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open‐label, parallel‐controlled phase II‐III trial. J Clin Oncol. 2021;39(27):3002–11. 10.1200/JCO.21.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 12. Abou‐Alfa GK, Meyer T, Cheng AL, El‐Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. 10.1056/NEJMoa1717002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α‐fetoprotein concentrations (REACH‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96. 10.1016/S1470-2045(18)30937-9 [DOI] [PubMed] [Google Scholar]
- 14. Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second‐line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double‐blind, randomised, placebo‐controlled, phase 3 trial. The Lancet Gastroenterol Hepatol. 2021;6(7):559–68. 10.1016/S2468-1253(21)00109-6 [DOI] [PubMed] [Google Scholar]
- 15. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open‐label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. 10.1016/S1470-2045(21)00604-5 [DOI] [PubMed] [Google Scholar]
- 16. Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. LBA35 camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first‐line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. 2022;33:S1401–2. 10.1016/j.annonc.2022.08.032 [DOI] [Google Scholar]
- 17. Abou‐Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open‐label, multicenter study of tremelimumab (T) and durvalumab (D) as first‐line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40(4_suppl):379. 10.1200/JCO.2022.40.4_suppl.379 [DOI] [Google Scholar]
- 18. Yau T, Kang YK, Kim TY, El‐Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. 10.1001/jamaoncol.2020.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE‐224): a non‐randomised, open‐label phase 2 trial. Lancet Oncol. 2018;19(7):940–52. 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 20. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open‐label, parallel‐group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–80. 10.1016/S1470-2045(20)30011-5 [DOI] [PubMed] [Google Scholar]
- 21. Edeline J, Merle P, Fang W, Assenat E, Pan H, Rimassa L, et al. Clinical outcomes associated with tislelizumab in patients (pts) with advanced hepatocellular carcinoma (HCC) who have been previously treated with sorafenib (SOR) or lenvatinib (LEN) in RATIONALE‐208. J Clin Oncol. 2022;40(16_suppl):4072. 10.1200/JCO.2022.40.16_suppl.4072 [DOI] [Google Scholar]
- 22. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second‐line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE‐240: a randomized, double‐blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 23. Finn RS, Edeline J, Bouattour M, Cheng AL, Chan SL, Yau T, et al. Pembrolizumab (pembro) versus placebo (pbo) in patients (pts) with advanced hepatocellular carcinoma (aHCC) previously treated with sorafenib: updated data from the randomized, phase 3 KEYNOTE‐240 study. J Clin Oncol. 2021;39(15_suppl):4072. 10.1200/JCO.2021.39.15_suppl.4072 [DOI] [Google Scholar]
- 24. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second‐line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE‐394 study. J Clin Oncol. 2022;40(4_suppl):383. 10.1200/JCO.2022.40.4_suppl.383 [DOI] [Google Scholar]
- 25. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 26. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. 10.1016/j.jhep.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 27. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT‐32): a randomised, open‐label, phase 2–3 study. Lancet Oncol. 2021;22(7):977–90. 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 28. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC‐312): a multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. 10.1016/S1470-2045(22)00326-6 [DOI] [PubMed] [Google Scholar]
- 29. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–70. 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. LBA34 primary results from the phase III LEAP‐002 study: lenvatinib plus pembrolizumab versus lenvatinib as first‐line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401. 10.1016/j.annonc.2022.08.031 [DOI] [Google Scholar]
- 31. Hechtman JF, Liu W, Sadowska J, Zhen L, Borsu L, Arcila ME, et al. Sequencing of 279 cancer genes in ampullary carcinoma reveals trends relating to histologic subtypes and frequent amplification and overexpression of ERBB2 (HER2). Mod Pathol. 2015;28(8):1123–9. 10.1038/modpathol.2015.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou‐Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2‐positive, metastatic biliary tract cancer (MyPathway): a multicentre, open‐label, phase 2a, multiple basket study. Lancet Oncol. 2021;22(9):1290–300. 10.1016/S1470-2045(21)00336-3 [DOI] [PubMed] [Google Scholar]
- 33. Meric‐Bernstam F, Hanna DL, El‐Khoueiry AB, Kang YK, Oh DY, Chaves JM, et al. Zanidatamab (ZW25) in HER2‐positive biliary tract cancers (BTCs): results from a phase I study. J Clin Oncol. 2021;39(3_suppl):299. 10.1200/JCO.2021.39.3_suppl.299 [DOI] [Google Scholar]
- 34. Meric‐Bernstam F, Beeram M, Hamilton E, Oh DY, Hanna DL, Kang YK, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2‐expressing or HER2‐amplified cancers: a phase 1, dose‐escalation and expansion study. Lancet Oncol. 2022;23(12):1558–70. 10.1016/S1470-2045(22)00621-0 [DOI] [PubMed] [Google Scholar]
- 35. Harding JJ, Cleary JM, Quinn DI, Braña I, Moreno V, Borad MJ, et al. Targeting HER2 (ERBB2) mutation‐positive advanced biliary tract cancers with neratinib: results from the phase II SUMMIT ‘basket’ trial. J Clin Oncol. 2021;39(3_suppl):320. 10.1200/JCO.2021.39.3_suppl.320 [DOI] [Google Scholar]
- 36. Ohba A, Morizane C, Kawamoto Y, Komatsu Y, Ueno M, Kobayashi S, et al. Trastuzumab deruxtecan (T‐DXd; DS‐8201) in patients (pts) with HER2‐expressing unresectable or recurrent biliary tract cancer (BTC): an investigator‐initiated multicenter phase 2 study (HERB trial). J Clin Oncol. 2022;40(16_suppl):4006. 10.1200/JCO.2022.40.16_suppl.4006 [DOI] [Google Scholar]
- 37. Lee C, Chon HJ, Cheon J, Lee MA, Im HS, Jang JS, et al. Trastuzumab plus FOLFOX for HER2‐positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi‐institutional phase 2 trial of the Korean Cancer Study Group (KCSG‐HB19–14). Lancet Gastroenterol Hepatol. 2023;8(1):56–65. 10.1016/S2468-1253(22)00335-1. [DOI] [PubMed] [Google Scholar]
- 38. Ross JS, Wang K, Gay L, Al‐Rohil R, Rand JV, Jones DM, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by Next‐Generation sequencing. Oncologist. 2014;19(3):235–42. 10.1634/theoncologist.2013-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abou‐Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al‐Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open‐label, phase 2 study. Lancet Oncol. 2020;21(5):671–84. 10.1016/S1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi GM, Huang XY, Wen TF, Song TQ, Kuang M, Mou HB, et al. Pemigatinib in Chinese patients with advanced/metastatic or surgically unresectable cholangiocarcinoma including FGFR2 fusion or rearrangement: updated data from an open‐label, single‐arm, multicenter phase II study (CIBI375A201 study). Cancer Med. 2022;40:e16183. 10.1200/JCO.2022.40.16_suppl.e16183 [DOI] [Google Scholar]
- 41. Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase II study of BGJ398 in patients with FGFR‐altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276–82. 10.1200/JCO.2017.75.5009(2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meric‐Bernstam F, Bahleda R, Hierro C, Sanson M, Bridgewater J, Arkenau HT, et al. Futibatinib, an irreversible FGFR1–4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose‐expansion study. Cancer Discov. 2022;12(2):402–15. 10.1158/2159-8290.CD-21-0697(2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, et al. Multicenter phase I study of erdafitinib (JNJ‐42756493), oral pan‐fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res. 2019;25(16):4888–97. 10.1158/1078-0432.CCR-18-3334 [DOI] [PubMed] [Google Scholar]
- 44. Mazzaferro V, El‐Rayes BF, Droz Dit Busset M, Cotsoglou C, Harris WP, Damjanov N, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion‐positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120(2):165–71. 10.1038/s41416-018-0334-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hollebecque A, Borad M, Goyal L, Schram A, Park JO, Cassier PA, et al. LBA12 efficacy of RLY‐4008, a highly selective FGFR2 inhibitor in patients (pts) with an FGFR2‐fusion or rearrangement (f/r), FGFR inhibitor (FGFRi)‐naïve cholangiocarcinoma (CCA): ReFocus trial. Ann Oncol. 2022;33:S1381. 10.1016/j.annonc.2022.08.006 [DOI] [Google Scholar]
- 46. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science. 2017;357(6349):409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus‐Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair‐deficient cancer: results from the phase II KEYNOTE‐158 study. J Clin Oncol. 2020;38(1):1–10. 10.1200/JCO.19.02105(2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Javle MM, Murugesan K, Shroff RT, Borad MJ, Abdel‐Wahab R, Schrock AB, et al. Profiling of 3,634 cholangiocarcinomas (CCA) to identify genomic alterations (GA), tumor mutational burden (TMB), and genomic loss of heterozygosity (gLOH). J Clin Oncol. 2019;37(15_suppl):4087. 10.1200/JCO.2019.37.15_suppl.4087 [DOI] [Google Scholar]
- 49. Kim RD, Chung V, Alese OB, El‐Rayes BF, Li D, Al‐Toubah TE, et al. A phase 2 multi‐institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6(6):888–94. 10.1001/jamaoncol.2020.0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non‐randomised, multicentre, open‐label, phase 1 study. Lancet Gastroenterol Hepatol. 2019;4(8):611–21. 10.1016/S2468-1253(19)30086-X [DOI] [PubMed] [Google Scholar]
- 51. Piha‐Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE‐158 and KEYNOTE‐028 studies. Int J Cancer. 2020;147(8):2190–98. 10.1002/ijc.33013 [DOI] [PubMed] [Google Scholar]
- 52. Doki Y, Ueno M, Hsu CH, Oh DY, Park K, Yamamoto N, et al. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head‐and‐neck cancer. Cancer Med. 2022;11(13):2550–60. 10.1002/cam4.4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen X, Qin S, Gu S, Ren Z, Chen Z, Xiong J, et al. Camrelizumab plus oxaliplatin‐based chemotherapy as first‐line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer. 2021;149(11):1944–54. 10.1002/ijc.33751 [DOI] [PubMed] [Google Scholar]
- 54. Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single‐arm, open‐label, phase II trial. J Immunother Cancer. 2020;8(2):e001240. 10.1136/jitc-2020-001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li W, Wang Y, Yu Y, Li Q, Wang Y, Zhang C, et al. Toripalimab in advanced biliary tract cancer. Innovation. 2022;3(4):100255. 10.1016/j.xinn.2022.100255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chiang NJ, Tan KT, Bai LY, Hsiao CF, Huang CY, Hung YP, et al. Impaired chromatin remodeling predicts better survival to modified gemcitabine and S‐1 plus nivolumab in advanced biliary tract cancer: A phase II T1219 study. Clin Cancer Res. 2022;28(19):4248–57. 10.1158/1078-0432.CCR-22-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oh D‐Y, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. A phase 3 randomized, double‐blind, placebo‐controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ‐1. J Clin Oncol. 2022;40(4_suppl):378. 10.1200/JCO.2022.40.4_suppl.378 [DOI] [Google Scholar]
- 58. Taieb J, Carrato B, Westphalen D, Melisi G, Prager T, Macarulla Mercade ABC, et al. First‐line (1L) full dose (f) and modified (m) FOLFIRINOX and gemcitabine+nab‐paclitaxel (GN) treatment (tx) for metastatic pancreatic adenocarcinoma (mPAC) patients (pts) in routine clinical practice across Europe. Ann Oncol. 2018;29:viii244–5. 10.1093/annonc/mdy282.106 [DOI] [Google Scholar]
- 59. Carrato A, Pazo‐Cid R, Macarulla T, Gallego J, Jiménez‐Fonseca P, Rivera F, et al. Sequential nab‐paclitaxel/gemcitabine followed by modified FOLFOX for first‐line metastatic pancreatic cancer: the SEQUENCE trial. J Clin Oncol. 2022;40:4022. 10.1200/JCO.2022.40.16_suppl.4022 [DOI] [Google Scholar]
- 60. de la Fouchardiere C, Hammel P, Launay S, Vienot A, Raimbourg J, Perrier H, et al. 1566TiP PRODIGE 65—UCGI 36—GEMPAX: a unicancer phase III randomized study evaluating gemcitabine and paclitaxel versus gemcitabine alone after FOLFIRINOX failure or intolerance in metastatic pancreatic ductal adenocarcinoma. Ann Oncol. 2020;31:S954–55. 10.1016/j.annonc.2020.08.2049 [DOI] [Google Scholar]
- 61. Wang L, Qin S, Zhou Y, Zhang S, Sun X, Chen Z, et al. LBA61 HR070803 plus 5‐FU/LV versus placebo plus 5‐FU/LV in second‐line therapy for gemcitabine‐refractory locally advanced or metastatic pancreatic cancer: A multicentered, randomized, double‐blind, parallel‐controlled phase III trial (HR‐IRI‐APC). Ann Oncol. 2022;33:S1426. 10.1016/j.annonc.2022.08.063 [DOI] [Google Scholar]
- 62. Bekaii‐Saab TS, Spira AI, Yaeger R, Buchschacher GL, McRee AJ, Sabari JK, et al. KRYSTAL‐1: updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation. J Clin Oncol. 2022;40:519. 10.1200/JCO.2022.40.4_suppl.519 34878805 [DOI] [Google Scholar]
- 63. Qin S, Bai Y, Wang Z, Chen Z, Xu R, Xu J, et al. Nimotuzumab combined with gemcitabine versus gemcitabine in K‐RAS wild‐type locally advanced or metastatic pancreatic cancer: a prospective, randomized‐controlled, double‐blinded, multicenter, and phase III clinical trial. J Clin Oncol. 2022;40:LBA4011. 10.1200/JCO.2022.40.17_suppl.LBA4011 [DOI] [Google Scholar]
- 64. Bear AS, Vonderheide RH, O'Hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020;38(6):788–802. 10.1016/j.ccell.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gössling GCL, Zhen DB, Pillarisetty VG, Chiorean EG. Combination immunotherapy for pancreatic cancer: challenges and future considerations. Expert Rev Clin Immunol. 2022;18(11):1173–86. 10.1080/1744666X.2022.2120471 [DOI] [PubMed] [Google Scholar]
- 66. Bockorny B, Grossman JE, Hidalgo M. Facts and hopes in immunotherapy of pancreatic cancer. Clin Cancer Res. 2022;28(21):4606–17. 10.1158/1078-0432.CCR-21-3452 [DOI] [PubMed] [Google Scholar]
- 67. Zhu YH, Zheng JH, Jia QY, Duan ZH, Yao HF, Yang J, et al. Immunosuppression, immune escape, and immunotherapy in pancreatic cancer: focused on the tumor microenvironment. Cell Oncol. 2023;46:17– 48. 10.1007/s13402-022-00741-1 [DOI] [PubMed] [Google Scholar]
- 68. Jin G, Guo S, Xu J, Liu R, Liang Q, Yang Y, et al. A multicenter, randomized, double‐blind phase III clinical study to evaluate the efficacy and safety of KN046 combined with nab‐paclitaxel and gemcitabine versus placebo combined with nab‐paclitaxel and gemcitabine in patients with advanced pancreatic cancer (ENREACH‐PDAC‐01). J Clin Oncol. 2022;40:TPS4189. 10.1200/JCO.2022.40.16_suppl.TPS4189 [DOI] [Google Scholar]
- 69. Fu Q, Chen Y, Huang D, Guo C, Zhang Q, Li X, et al. Randomized phase III study of sintilimab in combination with modified folfrinox versus folfrinox alone in patients with metastatic and recurrent pancreatic cancer in China: the CISPD3 trial. J Clin Oncol. 2022;40:560. 10.1200/JCO.2022.40.4_suppl.560 [DOI] [Google Scholar]
- 70. Reiss KA, Mick R, Teitelbaum UR, O'Hara MH, Schneider CJ, Massa RC, et al. A randomized phase Ib/II study of niraparib (nira) plus nivolumab (nivo) or ipilimumab (ipi) in patients (pts) with platinum‐sensitive advanced pancreatic cancer (aPDAC). J Clin Oncol. 2022;40:4021. 10.1200/JCO.2022.40.16_suppl.4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti‐tumour surveillance in immunotherapy‐treated HCC. Nature. 2021;592(7854):450–6. 10.1038/s41586-021-03362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Casadei‐Gardini A, Rimini M, Rimassa L, Burgio V, Kudo M, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non‐viral unresectable hepatocellular carcinoma: an international study. J Clin Oncol. 2022;40:4069. 10.1200/JCO.2022.40.16_suppl.4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.


