Abstract
Background
No well‐performing nomogram has been developed specifically to predict individual‐patient cancer‐specific survival (CSS) and overall survival (OS) among patients with resectable colorectal liver metastasis (CRLM) who undergo simultaneous resection of primary and hepatic lesions without neoadjuvant chemotherapy (NAC). We aim to investigate the prognosis of patients with resectable CRLM undergoing simultaneous resection of primary and hepatic lesions without NAC.
Methods
Data of patients with CRLM in the Surveillance, Epidemiology and End Results Program (cohort, n = 225) were collected as the training set, and data of patients with CRLM treated at the National Cancer Center (cohort, n = 180) were collected as the validation set. The prognostic value of the clinicopathological parameters in the training cohort was assessed using Kaplan‒Meier curves and univariate and multivariate Cox proportional hazards models, and OS and CSS nomograms integrated with the prognostic variables were constructed. Calibration analyses, receiver operating characteristic (ROC) curves, and decision curve analyses (DCAs) were then performed to evaluate the performance of the nomograms.
Results
There was no collinearity among the collected variables. Three factors were associated with OS and CSS: the pretreatment carcinoembryonic antigen (CEA) concentration, pathologic N (pN) stage, and adjuvant chemotherapy (each p < 0.05). OS and CSS nomograms were constructed using these three parameters. The calibration plots revealed favorable agreement between the predicted and observed outcomes. The areas under the ROC curves were approximately 0.7. The DCA plots revealed that both nomograms had satisfactory clinical benefits. The ROC curves and DCAs also confirmed that the nomogram surpassed the tumor, node, and metastasis staging system.
Conclusion
The herein‐described nomograms containing the pretreatment CEA concentration, pN stage, and adjuvant chemotherapy may be effective models for predicting postoperative survival in patients with CRLM.
Keywords: nomogram, SEER, colorectal liver metastasis, cancer‐specific survival, overall survival
There is no consensus on the prognosis for patients with resectable colorectal liver metastasis (CRLM) who undergo simultaneous resection of primary and hepatic lesions without neoadjuvant chemotherapy (NAC). We aim to construct the nomogram models to investigate the prognosis of patients with resectable CRLM undergoing simultaneous resection of primary and hepatic lesions without NAC.
Abbreviations
- AUC
area under the ROC curve
- CEA
carcinoembryonic antigen
- CRC
colorectal cancer
- CRLM
colorectal liver metastasis
- CSS
cancer‐specific survival
- DCA
decision curve analyses
- NAC
neoadjuvant chemotherapy
- NCC
National Cancer Center
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- ROC
receiver operating characteristic
- SEER
Surveillance, Epidemiology and End Results
1. INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of morbidity and the second leading cause of death among patients with malignancies worldwide [1]. The liver is the most common site of metastatic disease in patients with CRC. When CRC is first diagnosed, 20%–25% of patients have concurrent liver metastasis [2]. Patients with untreated colorectal liver metastasis (CRLM) have a median survival duration of 4.5 months [3]. During the past few decades, simultaneous resection of primary and hepatic lesions in patients with CRLM has been regarded as the standard cure, and the reported 5‐year postoperative survival rate is 50% [4, 5]. Unfortunately, even when curative resection is performed, more than 70% of patients with CRLM develop recurrence within 5 years of the original resection, and the 5‐year survival rate is poor [6]. In recent years, neoadjuvant chemotherapy (NAC) has been used to improve the survival rates of patients with resectable metastases and to accomplish resectability in patients with nonresectable lesions [5]. Nonetheless, the distribution of NAC remains controversial because of the lack of reliable evidence proving a survival benefit and the danger of chemotherapy‐associated hepatotoxicity. For patients with obviously resectable CRLM, the revised European Society for Medical Oncology guideline recommends the immediate performance of hepatectomy [7].
As a result, the effectiveness of upfront simultaneous resection of primary and hepatic lesions without NAC remains unknown. Accurate prediction of the patient's prognosis is critical when considering perioperative chemotherapy strategies. A variety of prognostic models have been proposed to predict the postoperative outcomes of patients with CRLM. However, no well‐performing nomogram has been developed specifically for patients with CRLM who undergo simultaneous resection of primary and hepatic lesions without NAC. Furthermore, the majority of these studies lacked external independent validation, and the model assessment methods were inadequate.
A nomogram is a simple scoring tool that allows both doctors and patients to obtain personalized predictions in clinical practice. The current study was performed to develop simple and accurate nomograms for the prediction of overall survival (OS) and cancer‐specific survival (CSS) in patients with resectable CRLM undergoing simultaneous surgical intervention of both primary and metastatic lesions without NAC. First, we first established nomograms for survival prediction in 225 patients with CRLM who underwent concurrent resection of primary and hepatic lesions without NAC. Next, we independently tested this predictive model on 180 patients with CRLM.
2. METHODS
2.1. Study population
Data of patients with resectable CRLM who underwent simultaneous resection of primary and hepatic lesions from 2015 to 2019 in the Surveillance, Epidemiology and End Results Program (SEER) database (SEER Research Plus, 17 Registries, November 2021 Sub [2000–2019]) were included as the training set (n = 225). SEER Stat 8.3.6 was applied for SEER database patient screening as follows. (1) “Site recode ICD‐O‐3/WHO 2008” was collected to record information regarding the tumor location (ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, or rectum). (2) According to “Histologic Type ICD‐O‐3,” the following pathological types were included in this study: Adenocarcinoma (8010, 8020–8022, 8140–8141, 8144–8145, 8210–8211, 8220–8221, 8230–8231, and 8260–8263), mucinous adenocarcinoma (8472, 8473, 8480, and 8481), and signet ring cell carcinoma (8490). (3) The patients were aged 20–80 years. (4) The patients had been pathologically diagnosed with CRLM from 2015 to 2019. (5) The patients underwent synchronous resection of primary and hepatic lesions. (6) NAC was not used. The exclusion criteria were as follows: (1) incomplete clinical and pathological information (pathologic T [pT] stage, pathologic N [pN] stage, histology, chemotherapy record, survival month, and final cause of death), (2) incomplete surgical resection of the primary and hepatic lesions (R2 resection), and (3) extrahepatic metastasis.
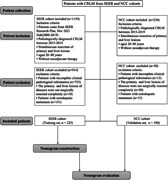
We enrolled patients with CRLM treated at the National Cancer Center (NCC cohort, n = 180) from 2015 to 2019 as an independent external validation set using the same exclusion and inclusion criteria applied to the SEER database. The current study was conducted in accordance with the Helsinki Declaration. The requirement for informed consent was waived. The NCC's Institute Research Medical Ethics Committee approved this study (NCC2021C‐125). The screening process is shown in Figure 1.
Figure 1.
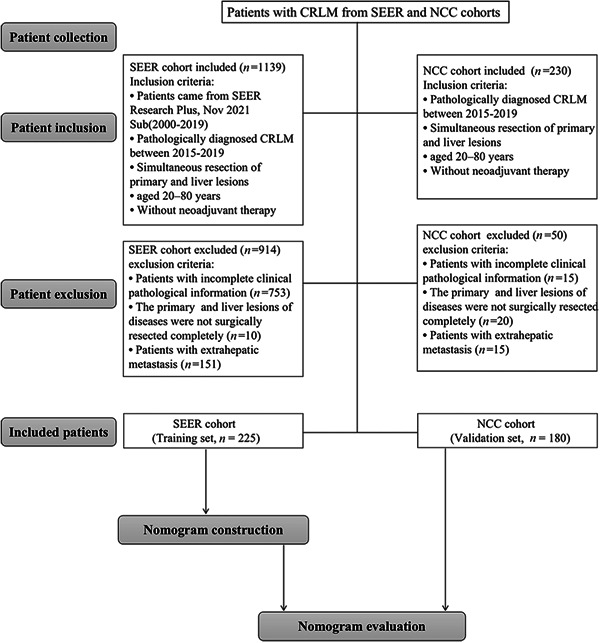
Flowchart of nomogram construction. CRLM, colorectal liver metastasis; NCC, National Cancer Center; SERR, Surveillance, Epidemiology and End Results Program.
When metastases were found during pretherapeutic staging or primary tumor surgery, CRLM was deemed synchronous. Factor H1 (i.e., one to four metastatic lesions with a maximum diameter of ≤5 cm), according to the Japanese Classification of Colorectal Carcinoma from the Japanese Society for Cancer of the Colon and Rectum was used to define resectable CRLM in the NCC cohort [8]. The following options were employed for CRLM that were resectable according to the SEER database: RXSumm‐SurgPrim, RXSumm—Surg Oth Reg/Dis, and SEER Combined Mets at DX‐liver [9].
Data on sex, age, primary site, tumor size, pretreatment carcinoembryonic antigen (CEA) concentration, pT stage, pN stage, differentiation grade, histology, adjuvant chemotherapy, and perineural invasion were retrieved. The seventh edition of the American Joint Committee on Cancer staging system was used to classify all clinicopathological factors. OS was calculated from the time of diagnosis to the time of death of any cause, and CSS was calculated from the time of diagnosis to the time of cancer‐specific death. The cutoff value of CEA was 5 ng/ml.
2.2. Treatment strategies and follow‐up in the NCC cohort
In our institute, patients were treated and monitored in accordance with protocols based on the National Comprehensive Cancer Network (NCCN) principles. Medical and surgical oncologists worked in multidisciplinary teams to develop a treatment plan for each patient in accordance with their clinical condition. Patients were informed that the efficacy of NAC for CRLM was under question, and they then had the option of whether to receive NAC. Before surgery, all patients had been histologically confirmed to have CRLM based on a colonoscopic specimen. The primary tumor and metastatic lesions were evaluated using dynamic magnetic resonance imaging and computed tomography. All procedures were performed under general anesthesia by an experienced group of surgeons from the hepatobiliary and colorectal departments. Adjuvant chemotherapy following hospital discharge was frequently suggested for patients with CRLM in our center based on the NCCN guidelines. Some patients did not receive adjuvant chemotherapy because of their economic status and intolerance to adjuvant chemotherapy. The following adjuvant chemotherapy regimens were available: FOLFOX, FOLFIRI, capecitabine, or XELOX.
In the first to second years, the patients in the NCC cohort received outpatient follow‐ups at least every 3 months. From the second to fifth years, the patients received follow‐ups every 6 months. At each follow‐up appointment, tumor markers and computed tomography images of the abdomen, pelvis, and thorax were examined.
2.3. Statistical analysis
Categorical variables are presented as numbers and percentages. Pearson's correlation analysis was used to determine the correlations between the variables. The Kaplan‒Meier method was used to calculate the cumulative rate of OS and CSS. A Cox proportional hazards model was used for both univariate and multivariate analyses, and statistically significant risk factors (p < 0.1) from the univariate Cox regression were included in the multivariate Cox proportional hazard models. All statistical analyses were performed using SPSS 26.0 (IBM Corp.). A p value of <0.05 was considered statistically significant.
Using R 4.2.0 (“rms” package, http://www.r-project.org), independent predictors in the multivariate Cox regression were kept to establish a nomogram. Receiver operating characteristic (ROC) curves, calibration curves, and decision curve analyses (DCAs) were used to evaluate the nomogram. The area under the ROC curve (AUC) efficiently summarizes the entire diagnostic accuracy, and the ROC curve displays the sensitivity versus 1—specificity of a diagnostic model [10]. The capacity to calibrate was assessed using calibration plots. The model forecasts survival probability more accurately when the predictive survival line is closer to the actual survival [11]. As two extreme scenarios, the curves for “ALL” (all patients will develop recurrence) and “NONE” (no patients will develop recurrence), which denote the highest clinical expenses and the absence of any treatment benefit, respectively, were displayed. Only if a model offers greater net benefits than “ALL” and “NONE” at a given threshold probability will it be clinically useful [12].
3. RESULTS
3.1. Basic patient characteristics
In this study, 405 patients with CRLM underwent simultaneous surgical treatment of primary and hepatic lesions, including 225 patients in the training set and 180 patients in the validation set. In the NCC cohort, 53 (29.4%) patients underwent liver surgery first, and 127 (70.6%) patients underwent colorectal surgery first. Of all patients, 58.0% were male, and 75.1% were older than 50 years. Of the primary tumor sites, a larger proportion involved colon cancer (n = 283, 69.9%), followed by rectal cancer (n = 122, 30.1%). When first diagnosed, the majority of patients with CRLM had stage N2 (42.2%) and T3 (57.5%) cancer. Postoperative adjuvant chemotherapy was performed in 340 (84.0%) patients. A total of 383 (94.6%) patients were diagnosed with adenocarcinoma. Perineural invasion was found in 181 (44.7%) patients. Pretreatment CEA‐positive patients accounted for 75.8%. The patients' baseline characteristics are presented in Table 1. Data from the cumulative incidence function subgroup assessment revealed that a higher cumulative incidence of CSS and OS occurred primarily in patients with CRLM aged >50 years with colon cancer or mucosal adenocarcinoma; with Grade IV, pN2, or CEA‐positive lesions; and with perineural invasion as well as in patients who did not receive chemotherapy (Supporting Information: Figures 1 and 2).
Table 1.
Patient demographics and pathological characteristics
| Variables | Total patients | Training set | Validation set |
|---|---|---|---|
| (N = 405) | (N = 225) | (N = 180) | |
| Age, n (%) | |||
| ≤50 | 101 (24.9) | 56 (24.9) | 45 (25.0) |
| >50 | 304 (75.1) | 169 (75.1) | 135 (75.0) |
| Sex, n (%) | |||
| Male | 235 (58.0) | 128 (56.9) | 107 (59.4) |
| Female | 170 (42.0) | 97 (43.1) | 73 (40.6) |
| Tumor size, n (%) | |||
| ≤5 cm | 182 (44.9) | 108 (48.0) | 74 (41.1) |
| >5 cm | 223 (55.1) | 117 (52.0) | 106 (58.9) |
| Primary Site, n (%) | |||
| Colon | 283 (69.9) | 159 (70.7) | 124 (68.9) |
| Rectum | 122 (30.1) | 66 (29.3) | 56 (31.1) |
| CEA, n (%) | |||
| Negative | 98 (24.2) | 29 (12.9) | 69 (38.3) |
| Positive | 307 (75.8) | 196 (87.1) | 111 (61.7) |
| Grade, n (%) | |||
| I | 175 (43.2) | 165 (73.3) | 10 (5.6) |
| II | 152 (37.5) | 11 (4.9) | 141 (78.3) |
| III | 68 (16.8) | 39 (17.3) | 29 (16.1) |
| IV | 10 (2.5) | 10 (4.4) | 0 (0) |
| pT, n (%) | |||
| T1 | 2 (0.5) | 1 (0.4) | 1 (0.6) |
| T2 | 9 (2.2) | 6 (2.7) | 3 (1.7) |
| T3 | 233 (57.5) | 130 (57.8) | 103 (57.2) |
| T4 | 158 (39.0) | 86 (38.2) | 72 (40.0) |
| Tx | 3 (0.7) | 2 (0.9) | 1 (0.6) |
| pN, n (%) | |||
| N0 | 87 (21.5) | 41 (18.2) | 46 (25.6) |
| N1 | 147 (36.3) | 87 (38.7) | 60 (33.3) |
| N2 | 171 (42.2) | 97 (43.1) | 74 (41.1) |
| Histology, n (%) | |||
| Mucosal adenocarcinoma | 22 (5.4) | 16 (7.1) | 6 (3.3) |
| adenocarcinoma | 383 (94.6) | 209 (92.9) | 174 (96.7) |
| Perineural invasion, n (%) | |||
| No | 224 (55.3) | 127 (56.4) | 97 (53.9) |
| Yes | 181 (44.7) | 98 (43.6) | 83 (46.1) |
| Adjuvant chemotherapy, n (%) | |||
| No | 65 (16.0) | 39 (17.3) | 26 (14.4) |
| Yes | 340 (84.0) | 186 (82.7) | 154 (85.6) |
Abbreviations: CEA, carcinoembryonic antigen; pN, pathologic N stage; pT, pathologic T stage.
3.2. Nomogram variable screening
Spearman's correlation analysis was used to ensure that there was no collinearity between the screened variables (Supporting Information: Figure 3). Univariate and multivariate Cox regression analyses were used to identify the potential prognostic factors for OS and CSS. The CEA concentration, histology, pN, and adjuvant chemotherapy all displayed highly significant differences in the univariate analysis of CSS and OS (each p < 0.1). These prognostic factors were then carefully assessed in further multivariate analysis. The multivariate Cox regression analysis showed that CEA (positive, hazard ratio [HR] = 2.03), pN (N1, HR = 1.10; N2, HR = 1.98), and adjuvant chemotherapy (yes, HR = 0.25) were independent predictors for OS. Similarly, CEA (positive, HR = 2.13), pN (N1, HR = 1.06; N2, HR = 2.00), and adjuvant chemotherapy (yes, HR = 0.26) were independently associated with CSS. Further details are presented in Tables 2 and 3.
Table 2.
Univariate and multivariate analyses of overall survival in the training set
| Factor | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| p Value | HR (95% CI) | p Value | HR (95% CI) | |
| Age | 0.333 | |||
| ≤50 | Reference | |||
| >50 | 1.21 (0.82–1.80) | |||
| Sex | 0.942 | |||
| Female | Reference | |||
| Male | 0.99 (0.71–1.37) | |||
| Tumor size | 0.828 | |||
| ≤5 cm | Reference | |||
| >5 cm | 0.96 (0.70–1.34) | |||
| Preimary site | 0.125 | |||
| Colon | Reference | |||
| Rectum | 0.71 (0.46–1.10) | |||
| CEA | ||||
| Negative | Reference | Reference | ||
| Positive | 0.015 | 2.11 (1.15–3.87) | 0.022 | 2.03 (1.11–3.71) |
| pT stage | ||||
| T1 | Reference | |||
| T2 | 0.877 | 6.45 (2.39–11.29) | ||
| T3 | 0.901 | 3.69 (3.01–9.08) | ||
| T4 | 0.911 | 9.12 (6.24–15.79) | ||
| pN stage | ||||
| N0 | Reference | Reference | ||
| N1 | 0.742 | 1.09 (0.66–1.79) | 0.698 | 1.10 (0.67–1.83) |
| N2 | 0.019 | 1.78 (1.10–2.87) | 0.006 | 1.98 (1.22–3.20) |
| Histology | 0.093 | 0.344 | 0.75 (0.41–1.36) | |
| Adenocarcinoma | Reference | |||
| Mucosal adenocarcinoma | 0.60 (0.33–1.09) | |||
| Perineural invasion | 0.270 | |||
| No | Reference | |||
| Yes | 1.20 (0.87–1.67) | |||
| Adjuvant chemotherapy | <0.001 | <0.001 | ||
| No | Reference | Reference | ||
| Yes | 0.28 (0.19–0.41) | 0.25 (0.17–0.38) | ||
Note: Statistically significant variables (p < 0.05) are highlighted in bold.
Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; pN, pathologic N stage; pT, pathologic T stage.
Table 3.
Univariate and multivariate analyses of cancer‐specific survival in the training set
| Factor | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| p Value | HR (95% CI) | p Value | HR (95% CI) | |
| Age | 0.397 | |||
| ≤50 | Reference | |||
| >50 | 1.19 (0.80–1.77) | |||
| Sex | 0.922 | |||
| Female | Reference | |||
| Male | 1.02 (0.73–1.43) | |||
| Tumor size | 0.524 | |||
| ≤5 cm | Reference | |||
| >5 cm | 0.90 (0.64–1.25) | |||
| Preimary site | 0.148 | |||
| Colon | Reference | |||
| Rectum | 0.72 (0.46–1.12) | |||
| CEA | ||||
| Negative | Reference | Reference | ||
| Positive | 0.013 | 2.22 (1.19–4.17) | 0.019 | 2.13 (1.13–4.00) |
| pT stage | ||||
| T1 | Reference | |||
| T2 | 0.781 | 7.01 (6.18–12.23) | ||
| T3 | 0.631 | 12.11 (6.10–25.36) | ||
| T4 | 0.990 | 15.35 (9.69–20.78) | ||
| pN stage | ||||
| N0 | Reference | Reference | ||
| N1 | 0.854 | 1.05 (1.63–1.76) | 0.815 | 1.06 (0.63–1.79) |
| N2 | 0.018 | 1.80 (1.11–2.94) | 0.006 | 2.00 (1.22–3.28) |
| Histology | 0.063 | 0.274 | ||
| Adenocarcinoma | Reference | Reference | ||
| Mucosal adenocarcinoma | 0.27 (1.31–1.03) | 0.72 (1.39–1.30) | ||
| Perineural Invasion | 0.267 | |||
| No | Reference | |||
| Yes | 1.21 (0.86–1.69) | |||
| Adjuvant chemotherapy | <0.001 | <0.001 | ||
| No | Reference | Reference | ||
| Yes | 0.28 (0.19–0.42) | 0.26 (0.17–0.38) | ||
Note: Statistically significant variables (p < 0.05) are highlighted in bold.
Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; pN, pathologic N stage; pT, pathologic T stage.
3.3. Nomogram construction
We used the three predictors mentioned above to construct two nomograms for patients with CRLM. Figure 2a depicts a model with which to predict the CSS probability of a given patient. Figure 2b assesses the probable 1‐ and 3‐year OS by incorporating the independent prognostic variables. The length of the line corresponding to each variable in these nomograms was used to measure how much a predictor contributed to survival. The nomograms illustrated that adjuvant chemotherapy contributed most significantly to the prognosis, followed by pN expression. Each variable had a score on the score scale. The total point estimates based on the sum of the abovementioned variables corresponded to the OS and CSS probabilities for patients at different time points after curative surgical resection of CRC. A lower score was associated with a worse outcome. For example, a patient with pN1 who was CEA‐positive and underwent adjuvant chemotherapy would have a total of 125 points based on the CSS nomogram (100 points for adjuvant chemotherapy, 25 points for pN1, and 0 points for being CEA‐positive). For this patient, the predicted 1‐ and 3‐year CSS rates were approximately 86.8% and 51.1%, respectively. Three identical criteria were included in both the OS and CSS nomograms for this investigation, but the risk scores for these categories were different (Supporting Information: Table 1).
Figure 2.
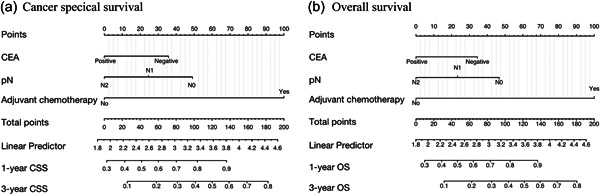
Nomograms for prognostic prediction of CSS and OS. CEA, carcinoembryonic antigen; CSS, cancer‐specific survival; pN, pathologic N stage; OS, overall survival.
3.4. Nomogram validation
The discriminative abilities, calibrating abilities, and clinical benefits were assessed internally and externally. ROC curves were produced to evaluate the accuracy of the prediction model. In both the training and validation cohorts, the AUC for the prediction of OS and CSS was >0.7 in most cases (Figure 3), demonstrating the ability of the nomograms to discriminate well. ROC analyses were also performed to further compare the nomograms with the tumor, node, and metastasis (TNM) stage. The AUC of our model was 0.722 and 0.717 for 1‐ and 3‐year CSS (TNM stage: 1‐year, 0.684; 3‐year, 0.694), respectively, and 0.728 and 0.711 for 1‐ and 3‐year OS (TNM stage: 1‐year, 0.684; 3‐year, 0.688), respectively, demonstrating that the nomogram was more accurate at predicting the prognosis than TNM‐based tumor staging (Figure 3). In both the training and validation sets, the calibration curves of these nomograms showed a good match between the actual survival rate and the nomogram‐predicted survival rate (Figure 4). The DCA plots for OS and CSS are presented in Figure 5. Additionally, among the majority of the threshold probabilities at various time intervals in the training set, DCA showed positive clinical benefits in the prediction model. Compared with the TNM staging system, the 1‐ and 3‐year DCA curves of the nomogram showed a more extensive net benefit within the risk of death in both cohorts. Taken together, our prediction models had satisfactory discriminative abilities, calibrating abilities, and clinical value in predicting OS and CSS at 1 and 3 years.
Figure 3.
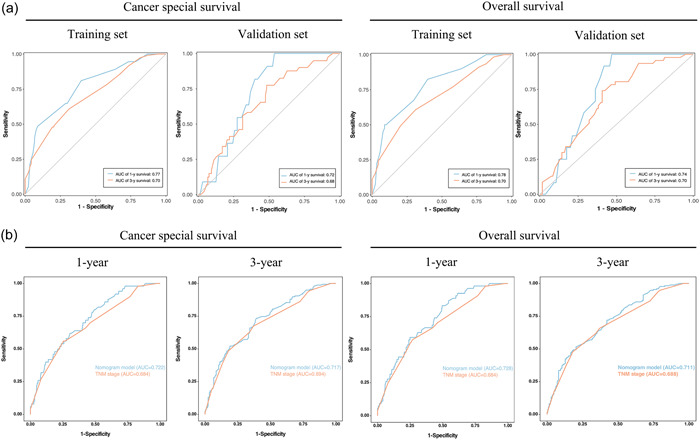
ROC curves of OS and CSS. (a) ROC curves corresponding to 1‐ and 3‐year CSS and OS in the training and validation cohorts. (b) ROC curves comparing the new nomograms with the TNM models. CSS, cancer‐specific survival; OS, overall survival; ROC, receiver operating characteristic; TNM, tumor, node, and metastasis.
Figure 4.

Calibration plots of the nomogram for 1‐ and 3‐year CSS and OS prediction in the training set and validation set. CSS, cancer‐specific survival; OS, overall survival.
Figure 5.
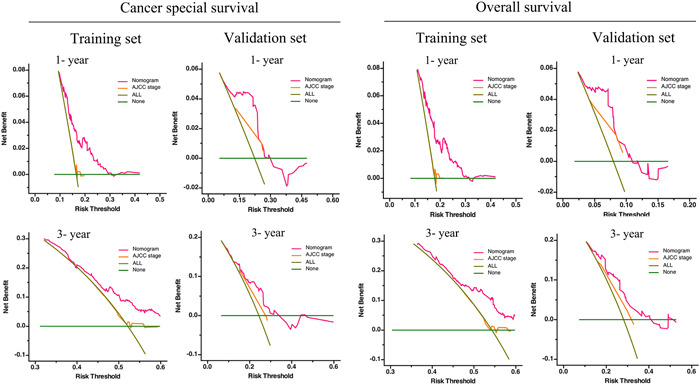
Validation of the prognostic nomograms using DCA curves. ALL, all patients died or relapsed; none, no patients died or relapsed. DCA, decision curve analyses.
4. DISCUSSION
The number of treatment choices for individuals with metastatic CRC has significantly increased during the last 2–3 decades. This has been facilitated by advancements in medical treatments and surgical procedures based on an increasingly customized approach to oncological care. However, the only option for treating CRLM remains surgical resection. Given the paucity of evidence demonstrating clear benefits in patients with resectable CRLM, the use of NAC for these patients is still debatable. Hepatotoxicity is a known adverse effect of the current generation of conventional chemotherapy drugs [4]. Upfront hepatectomy is a successful therapeutic approach. The prognosis of patients with CRLM who undergo simultaneous resection of primary and hepatic lesions without NAC has not received much attention in previous research, and the prognostic factors are still unclear. Therefore, we identified risk factors for these patients and constructed two models for the prediction of survival based on specific clinicopathological factors. For the first time, nomograms were built and validated to predict the OS and CSS of patients with resectable CRLM undergoing simultaneous resection of primary and liver metastatic lesions without NAC in the present study. The SEER database was applied to develop new prediction models and validate them internally as a training cohort, while a Chinese cohort was screened as an external validation set. Our findings suggest that the novel prognostic models have significant clinical utility, high specificity and sensitivity, and good prediction accuracy. The nomogram fared better than TNM staging with respect to predictive accuracy and prognostic clinical benefits in the ROC analysis and DCA.
The survival of individuals with CRLM may be impacted by numerous variables, including differentiation, clinical phenotype, and adjuvant therapies [13, 14]. Therefore, we tried to incorporate as many of these variables into the Cox regression analyses as possible. The OS and CSS nomograms consisted of three identical factors (the pretreatment CEA concentration, pN stage, and adjuvant chemotherapy), but these factors yielded different risk scores (Supporting Information: Table 1). From the perspective of tumor characteristics, independent prognostic factors determined the N stage and CEA concentration. CEA is a glycoprotein belonging to the immunoglobulin superfamily. Previous studies have shown that the serum CEA concentration has a solid predictive role in CRC [15, 16]. The addition of the preoperative serum CEA concentration as a predictive tool in CRC has been endorsed by both the European Group on Tumor Markers and the American Society of Clinical Oncology [17, 18]. Lymph node metastasis frequently occurs in patients with CRC, and a high incidence of lymph node metastases is also linked to a greater chance of having numerous metastatic sites [19]. The N stage was validated as a high‐risk factor for CRLM by Liu et al. [13]. Similar findings were found in our investigation, with the highest scores assigned to patients with advanced N stage (N+).
Previous research has shown that postoperative adjuvant therapy is associated with OS and disease‐free survival in patients who have CRC with liver metastases [20]. Postoperative chemotherapy was a high‐ranking protective measure in our OS and CSS nomograms, suggesting that it enhances the outcome of patients with CRLM; this is consistent with earlier reports [13]. The NCCN Colorectal Cancer Guidelines recommend providing chemotherapy to patients with CRLM [21].
Histologic type is an independent prognostic factor and is not included in the TNM stage [13]. Our findings demonstrated that there was, in fact, a negative correlation between mucosal adenocarcinoma and OS in the univariate regression analysis. However, the histologic type was not statistically significant for OS when the multivariate Cox regression was performed. Therefore, we did not include this indicator in the OS nomogram. The result was the same for the CSS nomogram. However, several researchers found that mucosal adenocarcinoma was associated with a poor prognosis in patients with CRC [9, 14]. This deviation in the current study may have been due to the small number of patients with mucinous adenocarcinoma.
Although several prediction models have already been built in earlier research, we believe that our study advances that work. Previous nomogram studies focusing on CRLM survival did not use external set validation when validating the model [14, 22]. We enrolled an independent validation set from China to prevent the overfitting of our nomograms. The nomograms were carefully examined for their accuracy and clinical value using a statistical analysis involving ROC analysis and DCA. These methods had not been applied in analogous investigations in the past [13, 23]. Although Guo et al. [9] conducted external validation, ROC analysis, and DCA, they did not include details on NAC. The present study is the first to establish survival nomograms for simultaneous resection of primary and liver lesions without NAC in patients with resectable CRLM.
Although the nomograms performed well, the present study had two main limitations. First, this was a retrospective study, and there was inevitable selection bias in the cohort. Second, because relevant data cannot be assessed from the SEER data, variables such as the number and size of liver metastases, the specific form and duration of chemotherapy, and gene mutations were not included in this study. As a result, a more thorough and comprehensive multicenter study with a larger sample size should be conducted to validate our findings.
5. CONCLUSION
We created two prognostic nomograms using the SEER database and a Chinese cohort. Our nomograms for survival prediction may aid in identifying patients with CRLM who will benefit from simultaneous resection of primary and hepatic lesions without NAC.
AUTHOR CONTRIBUTIONS
Yu‐Juan Jiang: Conceptualization (lead); formal analysis (lead); and writing – original draft (lead). Si‐Cheng Zhou: Conceptualization (supporting) and data curation (lead). Zi‐Xing Zhu: methodology (equal) and software (equal). Jing‐Hua Chen: Data curation (equal); formal analysis (equal); and software (lead). Jian‐Wei Liang: Funding acquisition (lead); supervision (lead); and writing – review and editing (lead).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of the Institutional Review Board of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College (NCC2021C‐125).
INFORMED CONSENT
Informed consent was not required because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
None.
Jiang Y‐J, Zhou S‐C, Zhu Z‐X, Chen J‐H, Liang J‐W. Survival nomograms for simultaneous resection of primary and hepatic lesions without neoadjuvant chemotherapy in patients with resectable colorectal liver metastasis. Cancer Innovation. 2023;2:240–252. 10.1002/cai2.45
This study has been reported in accordance with the guidelines of the STROBE Statement.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Mantke R, Schmidt U, Wolff S, Kube R, Lippert H. Incidence of synchronous liver metastases in patients with colorectal cancer in relationship to clinico‐pathologic characteristics. Results of a German prospective multicentre observational study. Eur J Surg Oncol. 2012;38(3):259–65. 10.1016/j.ejso.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 3. Kim YI, Park IJ, Kim JE, Kim SY, Park J‐H, Lee JH, et al. Hepatic resection after neoadjuvant chemotherapy for patients with liver metastases from colorectal cancer: need for cautious planning. Ann Surg Treat Res. 2019;97(5):245–53. 10.4174/astr.2019.97.5.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo M, Jin N, Pawlik T, Cloyd JM. Neoadjuvant chemotherapy for colorectal liver metastases: a contemporary review of the literature. World J Gastrointest Oncol. 2021;13(9):1043–61. 10.4251/wjgo.v13.i9.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirokawa F, Ueno M, Nakai T, Kaibori M, Nomi T, Iida H, et al. Neoadjuvant chemotherapy versus upfront surgery for resectable liver metastases from colorectal cancer: a multicenter, propensity score‐matched cohort study. J Gastrointest Surg. 2022;26(4):772–81. 10.1007/s11605-021-05175-y [DOI] [PubMed] [Google Scholar]
- 6. Ono K, Abe T, Oshita A, Sumi Y, Yano T, Okuda H, et al. Efficacy of upfront hepatectomy without neoadjuvant chemotherapy for resectable colorectal liver metastasis. World J Surg Oncol. 2021;19(1):97. 10.1186/s12957-021-02210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu R‐H, et al. Pan‐Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO‐ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70. 10.1093/annonc/mdx738 [DOI] [PubMed] [Google Scholar]
- 8. Dennosuke J. General rules for clinical and pathological studies on cancer of the colon, rectum and anus: part. I. Clinical classification. Jpn J Surg. 1983;13:557–73. 10.1007/BF02469505 [DOI] [PubMed] [Google Scholar]
- 9. Guo X, Liu Y, Liu L‐J, Li J, Zhao L, Jin X‐R, et al. Development and validation of survival nomograms in colorectal cancer patients with synchronous liver metastases underwent simultaneous surgical treatment of primary and metastatic lesions. Am J Cancer Res. 2021;11(6):2654–69. https://europepmc.org/article/PMC/8263634 [PMC free article] [PubMed] [Google Scholar]
- 10. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6. 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- 11. Deng GC, Lv Y, Yan H, Sun DC, Qu TT, Pan YT, et al. Nomogram to predict survival of patients with advanced and metastatic pancreatic cancer. BMC Cancer. 2021;21(1):1227. 10.1186/s12885-021-08943-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–74. 10.1177/0272989X06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu C, Hu C, Huang J, Xiang K, Li Z, Qu J, et al. A prognostic nomogram of colon cancer with liver metastasis: a study of the US SEER database and a Chinese cohort. Front Oncol. 2021;11:591009. 10.3389/fonc.2021.591009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng X, Li Y, Chen D, Xu X, Liu F, Zhao F. Nomogram predicting the survival of young‐onset patients with colorectal cancer liver metastases. Diagnostics. 2022;12(6):1395. 10.3390/diagnostics12061395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becerra AZ, Probst CP, Tejani MA, Aquina CT, González MG, Hensley BJ, et al. Evaluating the prognostic role of elevated preoperative carcinoembryonic antigen levels in colon cancer patients: results from the National cancer database. Ann Surg Oncol. 2016;23(5):1554–61. 10.1245/s10434-015-5014-1 [DOI] [PubMed] [Google Scholar]
- 16. Spindler BA, Bergquist JR, Thiels CA, Habermann EB, Kelley SR, Larson DW, et al. Incorporation of CEA improves risk stratification in stage II colon cancer. J Gastrointest Surg. 2017;21(5):770–7. 10.1007/s11605-017-3391-4 [DOI] [PubMed] [Google Scholar]
- 17. Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski‐Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43(9):1348–60. 10.1016/j.ejca.2007.03.021 [DOI] [PubMed] [Google Scholar]
- 18. Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313–27. 10.1200/JCO.2006.08.2644 [DOI] [PubMed] [Google Scholar]
- 19. Derwinger K, Gustavsson B. A study of lymph node ratio in stage IV colorectal cancer. World J Surg Oncol. 2008;6:127. 10.1186/1477-7819-6-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Portier G, Elias D, Bouche O, Rougier P, Bosset J‐F, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24(31):4976–82. 10.1200/JCO.2006.06.8353 [DOI] [PubMed] [Google Scholar]
- 21. Benson AB, Venook AP, Al‐Hawary MM, Arain MA, Chen Y‐J, Ciombor KK, et al. NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Compr Cancer Netw. 2020;18(7):806–15. 10.6004/jnccn.2020.0032 [DOI] [PubMed] [Google Scholar]
- 22. Wu Q, Wang W, Huang Y, Fang S, Guan Y. Nomograms for estimating survival in patients with liver‐only colorectal metastases: a retrospective study. Int J Surg. 2018;60:1–8. 10.1016/j.ijsu.2018.10.032 [DOI] [PubMed] [Google Scholar]
- 23. Liang J, Lin H, Liu J, Wang D, Yuan Y, Li B, et al. A novel prognostic nomogram for colorectal cancer liver metastasis patients with recurrence after hepatectomy. Cancer Med. 2021;10(5):1535–44. 10.1002/cam4.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


