Abstract
Effective treatment of cancer requires understanding the nature of the disease and accurately addressing the main root causes. General risk factors for cancer include poor nutrition, an acidogenic diet, an unhealthy lifestyle, and exposure to carcinogens such as toxins, chemicals, and radiation. The risk of developing cancers may be reduced by sufficient oxygenation and maintaining optimal alkalinity and nutritional balance at the cell level. The review paper summarizes some diet and lifestyle modifications that may potentially be considered for preventing and controlling some cancers. Moreover, worldwide statistical data for cancer incidence rates published by International Agency for Research on Cancer are analyzed for certain cancers regionally, concerning the effect of dietary habits and environmental factors that meaningfully correlate with the global trends of cancer. The study of cancer root causes integrated with analyzing the statistics related to cancer incidence rates suggests that the risk of developing cancer may be reduced by modifying dietary habits and lifestyle factors, as well as reducing exposure to carcinogens. Those with healthy balanced dietary habits may have a lower cancer risk than those who frequently have unhealthy diets; hence, considering a balanced natural diet and healthy lifestyle may be suggested as a complementary or alternative solution in cancer treatments.
Keywords: cancer, cancer prevention, cancer statistics, environmental factors, healthy diet, lifestyle
The age‐standardized cancer incidence rate data for the selected countries indicate a great areal variation and different global trends for each type of cancer. Analysis of the worldwide statistical data regionally for cancer incidence rates for certain cancers meaningfully correlate with diet, lifestyle, and environmental factors.
Abbreviation
- ASR
age‐standardized rate (for cancer incidence)
1. INTRODUCTION
Cancer prevention and cure based on natural solutions have poorly been researched due to the heavy focus of cancer studies on detection and medical treatments, causing most cancer prevention studies to remain inconclusive. As a result, epidemiological studies predict an increase in cancer incidence in the future [1, 2, 3].
Controlling cancer effectively requires understanding the nature of the disease and knowing the major root causes [1]. Investigations have shown that most people diagnosed with cancer do not have a family history of the disease, and only about 5%–10% of all cases of cancer are inherited via abnormal genes, meaning that most cancers may be preventable. In most cases, people in the same family get cancer because they share behaviors, lifestyles, or living conditions that raise their risk, not necessarily because they share genes. Cancer may also be caused by an abnormal gene that is passed down, which in this case, what is inherited is not cancer itself, but the abnormal gene that may or may not lead to cancer [1, 4, 5].
A fundamental cause of cancer is significant damage to cellular respiration, which can contribute to the development of tumors [2]. In an oxygen‐rich cellular environment with optimal alkaline pH, aerobic cellular respiration normally takes place, which is healthier and is associated with a lower risk of developing cancers [2, 6]. But in case of oxygen deficiency and the resulting acidosis at the cellular level in hypoxic regions of the body, anaerobic cellular respiration may take place where buffering mechanisms in the body fail to optimally maintain the acid‐base balance around the cells, which according to the Warburg effect, may lead to increased risk of developing cancers [2, 7].
When cellular respiration is damaged, some cells die, which is normally not a major problem, if not too many of them die, because they can be replaced with new cells. But some damaged cells may manage to resist cell death and survive by repairing themselves, leading to some targeted cell mutations building up in the cells. These cell mutations are not caused by a defect, but a result of activation of the survival mechanisms that help the cells gain resistance to survive under various conditions. However, some cells with too many mutations may stop working normally, grow out of control gradually, and eventually become cancerous [8, 9].
A considerable number of cells in our body may carry mutations due to occasional exposures to various carcinogens, which do not necessarily cause cancer in every case, all the time. Short temporary exposure to cancer‐causing substances may just result in some limited mutations that are not enough to cause cancer. In some cases, the cell mutations may just lead to the formation of noncancerous benign tumors that do not spread to other parts of the body, nonproblematic even for several years. But depending on the severity and accumulation rate of DNA mutations over time, some long‐term (chronic) exposure to certain human carcinogens may form aggressive malignant tumors, which can be life‐threatening [10, 11].
Some major risk factors of cancer may include unhealthy dietary habits, long‐lasting acidosis, smoking, consumption of alcohol and addictive drugs, sleep disorders and chronic insomnia, use of chemical cosmetics, infections, stress, physical inactivity, and environmental factors such as exposure to toxins and radiation [1, 2]. In addition, the consumption of supplements, that is, artificial sources of vitamins/minerals at high doses, may be linked to various cancers [2]. The other cause of cancer may be viruses, such as cervical cancer caused by certain types of human papillomavirus, and Kaposi's sarcoma cancer which often occurs in people with human immunodeficiency virus [12, 13]. Some vaccines also may increase the risk of developing certain cancers [14].
Dietary factors, particularly diet‐dependent acid load, may significantly influence the risk of developing cancers [2, 3, 15]. According to the food charts related to the dietary acid load [2], some foods form alkaline metabolic waste and have a negative dietary acid load (alkalizing effect), such as raw almonds, on‐tree sun‐ripened oranges, lemons, date fruits, figs, apples, mangoes, green beans, green peas, lettuce, red onion, garlic, tomato, carrot, cucumber, and olive oil. In contrast, some foods form acidic metabolic waste and have a positive dietary acid load (acidogenic), including peanuts, red meats such as pork and beef, commercial milk and dairy products, chocolates, coffee, table sugar, canola oil, fried potatoes, white flour, and sour plums. A high intake of acidogenic foods is associated with cancer [15], but following a balanced alkalizing diet that includes sufficient intake of alkalizing foods as a dietary habit may reduce dietary acid load, and lower the cancer risk [2]. It is noted that consuming alkaline substances such as alkaline water or baking soda does not have a proven health benefit in terms of cancer prevention or treatment, and frequent consumption may even have some major adverse effects [2].
It is believed that the Western modern dietary patterns may increase the risk of developing cancers as they are generally acidogenic and have negative health outcomes, due to being high in unhealthy animal proteins, trans or saturated fats, high synthetic sugars, refined carbs, processed cereals, and caffeinated sugary drinks. But traditional natural diets that have evidence‐based historical roots, are usually high in sun‐ripened fruits, fresh raw vegetables, legumes, whole grains, unprocessed nuts, and healthy fats, which can reduce the risk of cancer by providing a wide range of vitamins, minerals, enzymes, coenzymes, good sugars, antioxidants, alkalizing compounds, and various anticancer substances [2, 16].
This paper presents an integrated review of major cancer risk factors, focusing on the role of diet, lifestyle, and environmental factors. Some potential dietary solutions and lifestyle modifications to control cancer based on the root causes are also proposed. Furthermore, regional analysis of the International Agency for Research on Cancer (IARC) worldwide statistical data is performed for some cancers concerning the effect of dietary habits and environmental factors, to address the cancer root causes and solutions accordingly.
2. CANCER ROOT CAUSES AND SOLUTIONS
Some of the most common cancers worldwide include prostate cancer in men, breast cancer in women, colorectal cancer, lung cancer, stomach cancer, and skin cancer, which account for nearly half of all new cancer diagnoses [17]. The root causes and solutions for these common cancers are as follows.
2.1. Breast cancer
Breast cancer is the most common cancer in women and the second leading cause of their death due to cancer [17]. Breast cancer may be due to the presence of some hypoxic regions in the breast tissue that are poorly oxygenated [18], except during the breastfeeding period when the blood supply is richer [2, 19]. In general, in women who have never breastfed a baby or breast‐feed for short periods of a few months, their risk of breast cancer may be higher compared to the women who breast‐feed their newborns for 24 months and their breast tissues are repeatedly oxygenated for a sufficiently long period [19, 20]. Also, during pregnancy, the protecting effect of the fetal cells of the baby that migrate into the mother's bloodstream (fetal‐maternal microchimerism) helps repair the mother, while the mother builds the baby, which the protecting cells may remain active in the mother even for several years after the delivery, reducing the risk of cancer [21, 22].
Dietary risk factors for breast cancer in adults may include a high intake of commercial milk and dairy products, excessive consumption of red meats such as pork and beef, and eating processed fast foods frequently [2, 23]. The risk of developing cancer in the breast may also be increased by other factors, such as wearing a tight bra for many hours a day, consumption of contraceptives over the long term, frequent mammography screening (compression) [24, 25, 26], and/or regularly drinking water from plastic bottles that contain bisphenol A [2]. It is noted that a core needle biopsy may increase the risk of metastasis of breast cancer and spread it to other parts of the body [27].
2.2. Stomach cancer
A major root cause of stomach cancer is unhealthy dietary habits, particularly frequent consumption of pickled vegetables preserved in salt and vinegar (like pickled cucumber), which can gradually damage the stomach lining over the long term, making it vulnerable to bacteria such as Helicobacter pylori [28]. Most people may asymptomatically have H. pylori in their stomach, which is normally harmless or cause only minimal damage. But in case of a damaged stomach lining, the bacteria may develop severe gastric diseases and stomach cancer. Hence, H. pylori may greatly increase the risk of cancer when the stomach lining is damaged, which in this case, consuming salted preserved foods is a great risk factor [29, 30]. People who often have their plate of food with pickled vegetables as a side dish may have a higher risk of developing stomach cancer [28].
2.3. Colorectal cancer
Colorectal cancer is one of the major causes of morbidity and mortality throughout the world [17]. Most colorectal cancer cases occur in persons without a family history, indicating that inherited genetic risk is not a key factor [31]. Colorectal cancer is a disease strongly influenced by dietary habits, such as high intake of dairy products, red and processed meat, and alcohol [2, 32]. In addition, excessively consuming lacto‐fermented foods with too high probiotic content, or, taking too many probiotics and probiotic overdose, may contribute to digestive issues [33], and increase the risk of colorectal cancer. In contrast, eating a balanced diet with sufficient intake of raw fresh vegetables, sun‐ripened fruits, and nuts such as raw almonds, can significantly reduce the risk of colorectal cancer [2]. The dietary modifications can also help maintain a healthy gut microbiome and create significant amounts of butyric acid that reduce the viability of colorectal cancer cells [2], as well as produce some essential nutrients including vitamin B12 in the intestine [64].
As another risk factor, sitting on the toilet while pooping may increase the risk of colorectal cancer [34], a more common cancer among Western people [17]. But in people who usually squat in the toilet, such as Western Asians, East and South Asians, emptying their bowels is more complete and their risk of developing colorectal cancer is lower, compared to those who sit on the toilet [34].
2.4. Prostate cancer
Prostate cancer is one of the most frequently diagnosed cancers in men worldwide [17]. An unhealthy diet, smoking, alcohol abuse, and exposure to environmental contaminants may be some of the main root causes of prostate cancer [2, 35]. Another contributing factor to prostate cancer may be that the prostate gland can become overused by too frequent production of fluid for the ejaculate, which may result in a significant increase in the risk of contracting this type of cancer. It is hypothesized that this overuse can occur in men who engage in significant amounts of sex, more than that which would be considered healthy for a male, or frequent masturbation due to contributors such as porn addiction. Furthermore, if young boys are sexually overstimulated too early and frequently engage in sex or masturbation at an age before their bodies are sufficiently developed, this may then also be a contributing factor to prostate cancer later in life [36, 37, 38]. As opposed to that, lack of enough sexual activity in adults also may increase the risk of prostate cancer, as it results in persistent poor blood flow toward the prostate gland tissues, reduced oxygenation, and increased hypoxia risk [2, 39].
During ejaculation, the muscles around the urethra constrict to push the semen out through the penis and deep enough into the vagina [40]. This action involves some energy‐expensive processes that release large amounts of carbon dioxide and acidic metabolic wastes around the muscles near the prostate gland, leading to the acidosis that increases the risk of the development of prostate cancer. Some investigations also have shown that inhibition of external tumor acidity (enhanced buffering capacity by improved alkalinity) may reduce the risk of prostate cancer [41, 42].
Prostate cancer progression may also be affected by diet type and dietary habits. One contributing factor may be excessive consumption of acidifying foods, such as in a “Westernized” diet, including frequent high intake of processed meats, saturated animal fat, dairy products, chocolates, and alcoholic beverages (all form acidic metabolic wastes), which may make postejaculation acidosis around the prostate gland worse, and therefore, increase risk of developing prostate cancer [42]. In contrast, balanced dietary habits and sufficient intake of natural alkalizing foods such as date fruits, raw almonds, and mangoes, may help prevent prostate cancer by reducing acidosis [2]. Based on statistical data, prostate cancer incidence rates are significantly lower in Western Asian men who regularly consume date fruits, or, South Asian men who often eat sun‐ripened mangoes, compared to the men in Western countries whose acidogenic dietary habits and lifestyle may induce a shift towards increased prostate cancer risk [2, 17].
2.5. Lung cancer
Lung cancer is initiated by abnormal growth of cells in the lung, a disease that negatively affects the respiratory system. Lung cancer may be linked to airborne carcinogens, such as those in tobacco smoke, and emissions from motor vehicles, factories, and extraction mines [43, 44]. Each puff of each cigarette contains a mixture of thousands of compounds, including some well‐established carcinogens. According to considerable shreds of evidence, tobacco use is the leading cause of lung cancer, and most lung cancer deaths in women and men are due to smoking [45, 46]. In large cities, exposure to air pollutant emissions from vehicles and residential energy usage can significantly increase the risk of lung cancer [47, 48].
The risk of lung cancer may be significantly higher among miners, as well as in people living close to the extraction mines in case of chronically inhaling fine particles of dust or vapor containing heavy toxic metals such as uranium, copper, nickel, and lead [8, 49, 50]. An example is the island of New Caledonia, one of the world's largest producers of nickel [51], where age‐standardized rates (ASR) for lung cancer incidence also is one of the highest in the world [17]. The IARC has classified nickel compounds as carcinogenic to humans [50].
Another great risk factor for lung cancer is exposure to radon gas for a long period, which can induce lung cancer, even in nonsmokers [52]. Radon is a radioactive gas, a product in the uranium decay chain, which is found naturally everywhere on land with varying concentrations, as well as in ocean water [12, 53]. Human exposure to radium and radon is increased near the uranium extraction mines, or around oil/gas wells, where during oil and gas production, significant amounts of formation water with relatively high radium content also come from underground to the surface with the hydrocarbons. Radon can escape into the air from the soil particles that contain radium, and may also be released from the oceans, and reach the coastal areas with sea breeze depending on the wind direction and speed [12, 54].
Today, radon in buildings is an important indoor air pollutant. Inhaling radon and its highly radioactive daughters (including lead210 and lead214) can damage the cells in the lungs and develop lung cancer. Where soils have greater radium content (such as around uranium deposits), the houses in the area may have higher levels of radon in the indoor air. If doors and windows are kept closed for a long time, then high levels of radon are accumulated in the air inside the building, which increases the risk of radon‐caused lung cancer. In contrast, if windows are often kept open to allow the continuous flow of fresh air into the house, or, the effective ventilation system is utilized in the buildings to push the indoor air outside and replace it with fresh air (noting that radon is heavier than air and sits closer to the floor), then radon concentration in indoor air is lowered, reducing the risk of developing lung cancer. In the case of smoking and exposure to high radon levels in the building, the risk of developing lung cancer is significantly higher [12, 55, 56].
2.6. Skin cancer
Skin cancer, the abnormal growth of skin cells, may mainly be due to considerable exposure to cosmic rays and/or ultraviolet (UV) lights, as well as the development of acidic hypoxic regions in the skin. Human skin is slightly acidic to act as a barrier to bacteria and other potential contaminants, and also, slightly hypoxic and prone to oxygen tension. Another cause of oxygen deficiency in the skin cells may be a reduced blood flow to the skin in response to cold temperatures, or direct exposure of the skin to cold winds [57, 58, 59].
Some skin cancers are associated with cosmic radiation that continuously rains down on Earth. Cosmic rays may cause severe damage to the cells and result in gene mutations or cancer. Exposure to cosmic rays is lower around the equator, as well as in locations that have a latitude less than 37° north of the Earth's equatorial plane, because of the Earth's magnetic field. But at higher altitudes, as well as higher latitudes, particularly where severe ozone depletion has taken place, exposure to the damaging cosmic rays is more significant, regardless of the time of day or night. For this reason, airline pilots, cabin crew, and regular air travelers at aviation altitudes are more prone to cosmic radiation interaction, especially those who frequently fly over the North Pole, and are at a higher risk of developing skin cancers [60, 61].
Skin cancers can also be caused by UV rays that damage DNA and lead to genetic mutations [62]. The UV radiation in sunlight is primarily composed of UVA and UVB. Longer wavelength UVA penetrates deeply into the skin and damages the DNA in skin cells directly, but UVB penetrates and damages the outermost layers of the skin, causing sunburns. UVB also produces vitamin D3 in the skin, which protects against UV‐induced skin damage. Hence, while too much exposure to UV rays can damage the skin cells, sufficient exposure to direct midday sunlight on clear days, on average for 10–20 min each day, is a natural way to get enough vitamin D from the sun's UVB rays, and maintain healthy skin [63].
The National Academy of Medicine considers serum 25(OH)D level in the range of 20–50 ng/mL as sufficient (ideal), 12–20 ng/mL as moderate level (insufficient), and less than 12 ng/mL as deficient [64]. Normally, most people should be able to make all the vitamin D they need from sunlight during spring/summer time. Particularly, those living closer to the equator (such as in the Western Asia, Central America, North Africa, and South Asia) may be able to meet their requirements through direct sun exposure on clear sunny days of the year, as well as from their diet including products from animals that roam outside in the sunlight. But at high latitudes (such as in Scandinavian countries and Canada), very little vitamin D can be produced by the skin most of the year, and people living in these locations, as well as their farm animals, may be at a higher risk of vitamin D deficiency during fall/winter time. Vitamin D production from sunlight is reduced in case of darker skin or always wearing sun creams; however, no significant differences by age or sex have been found in vitamin D deficiencies [64, 65, 66, 67, 68].
Vitamin D deficiency (less than 12 ng/mL) has been linked to some major metabolic diseases, and we need adequate levels of vitamin D, provided from good sources, to maintain health. Dietary sources of vitamin D include D3 in animal products and D2 in mushrooms. The naturally occurring vitamin D from D3 is found in seafood, and farm products from animals that have sufficiently roamed outside in the sunlight, such as meat from farm lambs and eggs from farm chickens [64]. Animal products should be consumed in a balanced meal, including sufficient amounts of vegetables in the diet, to maintain a healthy nutritional balance [2]. Mushrooms also can contain vitamin D, but only after at least 1–2 h of direct exposure to UV sunlight on a clear day. It is noted that the daily value for vitamin D may be 200–800 IU/day, and taking large doses of vitamin D from supplements may have significant adverse effects such as vitamin D toxicity and kidney damage [2, 64].
As the use of sunscreens increased during the past decades, observations have indicated that skin cancer incidence rates showed a rising trend. That is probably because those wearing sun creams spent more time in the sun per day, while sun creams only prevent sunburns by blocking UV rays, but cannot block cosmic rays that cause gene mutations [63, 69, 70]. Sun creams also block most of the UVB rays that produce protective vitamin D3 in the skin. Moreover, sun creams contain nano‐sized particles of titanium dioxide, which the IARC has designated as a carcinogen for human cells [71]. Titanium dioxide nanoparticles may be absorbed and slightly penetrate the skin, which due to the carcinogenic effect, the penetrated titanium dioxide may exacerbate the cell damage, increasing the risk of cancer in the skin cells [71, 72]. Hence, regular daily use of sunscreens is not recommended, except in specific conditions of intense sun exposure. A better approach to care against skin cancer is to use hats, shades, and long sleeves that are more effective than sunscreen [73]. To provide extra protection, applying pure coconut oil, sesame oil, or extra virgin olive oil on the skin may be considered, which also have nourishing and hydrating effects, in addition to their natural SPF protection that blocks around 60%–80% of UVB rays [74, 75].
Skin cancer incidence is significantly lower in Middle‐Eastern countries where people generally use protective clothing in their lifestyle, than in people living the Western lifestyle such as in the United States and Australia, who often rely mainly on sun‐creams and often do not cover their bodies with long sleeves and hats [17].
3. WORLD CANCER INCIDENCE DATA ANALYSIS
In this study, cancer statistics are analyzed using the data published by the IARC in the year 2020, which have resulted from more than a decade of comprehensive research and advanced data analysis by some of the world's top scientists in the research center [17, 76, 77]. For analyzing the cancer data, looking at crude rates of cancer incidence may be misleading, as cancer incidence rates are higher in older people than in younger age groups. Hence, cancer statistics need to be analyzed using ASRs, which in this case, the data are corrected for the effect of age, and can meaningfully be analyzed independent of the median age or life expectancy values related to each country.
The statistical data related to an ASR per 100 000 (100 K) for cancer incidence in some countries are summarized in Table 1. The table presents a review of ASRs of cancer incidence, as well as the availability of population‐based cancer registry, for all cancers, stomach cancer, lung cancer, colorectal cancer, prostate cancer, breast cancer, Kaposi sarcoma, and skin cancer (melanoma and nonmelanoma), which indicate great variations and significant differences regionally for each type of cancer [17, 76, 77].
Table 1.
| Region | Country | ASR of cancer incidence per 100 000 (100K) | Availability of Population‐Based Cancer Registry, 2019 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All cancers | Stomach cancer | Lung cancer | Colorectal cancer | Prostate cancer | Breast cancer | Skin cancer | Kaposi sarcoma | |||
| Europe, North America, and Australia | Australia | 452 | 4.5 | 25.3 | 33.1 | 72 | 96 | 142 | 0.11 | High quality PBCR |
| Canada | 348 | 4.4 | 28.9 | 31.2 | 80 | 82 | 60 | 0.08 | High quality PBCR | |
| United States | 362 | 4.2 | 33.1 | 25.6 | 72 | 90 | 58 | 0.25 | High quality PBCR | |
| Norway | 328 | 4 | 27.2 | 41.9 | 96 | 83 | 42 | 0.05 | Registration activity | |
| Sweden | 289 | 3.3 | 17.7 | 27.8 | 100 | 84 | 40 | 0.11 | High quality PBCR | |
| Denmark | 351 | 4.4 | 36.8 | 40.9 | 76 | 98 | 47 | 0.03 | High quality PBCR | |
| France | 342 | 4.7 | 34.9 | 30.1 | 99 | 99 | 30 | 0.23 | High quality PBCR | |
| Belgium | 349 | 5.8 | 38.3 | 35.3 | 68 | 113 | 45 | 0.28 | High quality PBCR | |
| Germany | 313 | 7.0 | 31.9 | 25.8 | 67 | 82 | 44 | 0.11 | High quality PBCR | |
| Hungary | 338 | 7.8 | 50.1 | 45.3 | 66 | 77 | 20 | 0.04 | Registration activity | |
| Belarus | 253 | 15.4 | 24.3 | 30.2 | 58 | 52 | 12 | 0.11 | High quality PBCR | |
| Russia | 234 | 13.5 | 24 | 27.8 | 44 | 55 | 10 | 0.10 | High quality PBCR | |
| Western Asia | Iran | 153 | 17.5 | 12.6 | 13.9 | 21 | 36 | 2.6 | 0.17 | High quality PBCR |
| Saudi Arabia | 96 | 2.7 | 5.1 | 13.9 | 7 | 29 | 2.7 | 0.26 | High quality PBCR | |
| Qatar | 107 | 5.2 | 9.2 | 15.7 | 21 | 43 | 1.0 | 0.14 | High quality PBCR | |
| Oman | 104 | 8.0 | 5.4 | 9.9 | 14 | 38 | 1.8 | 0.34 | PBCR | |
| Eastern and Southern Asia | Japan | 285 | 31.6 | 32.1 | 38.5 | 52 | 76 | 2.1 | 0.03 | High quality PBCR |
| China | 205 | 20.6 | 34.8 | 23.9 | 10.2 | 39.1 | 1.2 | 0.01 | High quality PBCR | |
| Korea | 243 | 27.9 | 25.5 | 27.2 | 27 | 64 | 3.5 | 0.06 | High quality PBCR | |
| Malaysia | 144 | 4.3 | 15.4 | 19.6 | 13 | 49 | 1.5 | 0.07 | High quality PBCR | |
| Singapore | 233 | 7.2 | 26.5 | 33.0 | 34 | 78 | 3.2 | 0.04 | High quality PBCR | |
| India | 97 | 4.5 | 5.4 | 4.8 | 5 | 26 | 0.9 | 0.00 | High quality PBCR | |
| Bhutan | 82 | 17.7 | 7.5 | 3.8 | 1 | 5 | 0.4 | 0.26 | PBCR | |
| Africa | Nigeria | 110 | 2.8 | 1.9 | 7.3 | 35 | 49 | 2.6 | 0.71 | PBCR |
| Zimbabwe | 200 | 9.4 | 5.3 | 8.9 | 71 | 39 | 9.0 | 8.30 | High quality PBCR | |
| Botswana | 110 | 1.8 | 3.5 | 4.5 | 30 | 20 | 6.1 | 8.40 | PBCR | |
| Egypt | 159 | 4.1 | 8.0 | 6.1 | 14 | 49 | 2.1 | 0.03 | PBCR | |
Note: “Bold” numbers indicate countries with the highest incidence rate of specific cancer. Incidence rates for breast cancer are related to women only, prostate cancer to men, and other cancers to the average for both men and women. Skin cancer incidence rates are summation for both melanoma and nonmelanoma cases.
Abbreviations: ASR, age‐standardized rate; IARC, International Agency for Research on Cancer. PBCR, Population‐Based Cancer Registry.
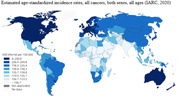
Furthermore, ASRs for all cancers for the selected countries from different regions are shown in Figure 1, and cancer incidence rates for different age groups are presented in Figure 2. As can be seen, the ASR of incidence for all cancers is three to four times higher in Western countries such as Australia and Denmark, than in countries in Arabia such as Oman and Qatar [17], not just in older people (to be due to only life expectancy), but in all age groups including younger people, meaning that the cancer incidence rates are unrelated to the life expectancy at birth reported for each country [78]. For instance, the average life expectancy is around 79.0 years in Qatar and 81.5 years in Denmark, but the total ASR for cancer incidence in Qatar is 107 per 100 K, but in Denmark, it is 351 per 100 K [17, 78], which the nearly three times higher ASR of cancer incidence in Denmark than in Qatar cannot be justified by just 1.5 years difference in their average life expectancy at birth.
Figure 1.
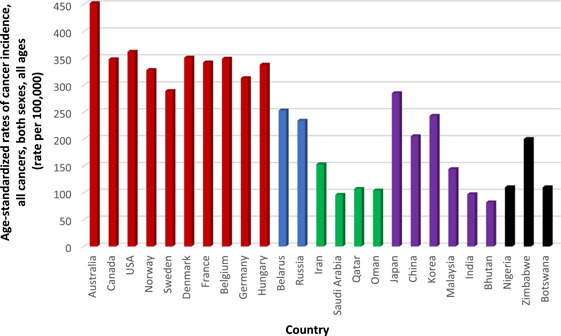
Age‐standardized cancer incidence rates for selected countries, all cancers, both sexes, all ages [17].
Figure 2.
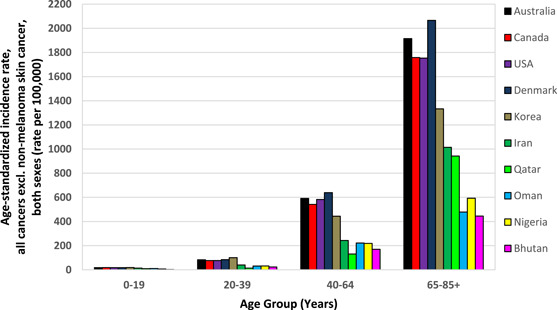
Age‐standardized rate of cancer incidence for different age groups, both sexes, all cancers excl. nonmelanoma skin cancer [17].
As additional pieces of evidence, ASRs of incidence for breast cancer, prostate cancer, and colorectal cancer (as reported in Table 1) are significantly higher in the United States, Australia, Western and Northern Europe, and Canada, where people often follow the Western diet including high intakes of processed meats, commercial dairy products, fast foods, and caffeinated sugar‐loaded drinks, as well as are industrialized. But in South Asia, North Africa, and Arabia, where people often consider balanced meals that also include foods primarily from plants such as vegetables, legumes, nuts, herbs, and spices, as well as have a sufficient intake of sun‐ripened fruits, the average rates of incidence for these cancer types are significantly lower [2, 17].
In countries like Japan, Korea, Bhutan, and Iran, the incidence rate of stomach cancer is considerably higher (as reported in Table 1), where salted preserved foods or pickled vegetables in vinegar and salt are often consumed as a side dish. But in countries like the United States, Canada, and Australia, stomach cancer incidence rate is significantly lower where salt preservation is not very common anymore and it has been mostly replaced by refrigeration [17, 79, 80, 81]. Even Saudi Arabia and Nigeria, despite their very high prevalence of H. pylori, have one of the lowest incidences of stomach cancer in the world [17, 82].
In Norway, the incidence rate of lung cancer is nearly 50% higher than in Sweden [17] (as reported in Table 1), while in the neighboring countries separated by Scandinavian mountains, average life expectancy is almost similar [78], which in this case, life expectancy does not correlate with the great differences in the lung cancer incidence rates. Likewise, stomach cancer incidence rates are eight times lower in Sweden than in the Korea, and there are almost similar incidence rates of colorectum cancer in Sweden and the Korea (as reported in Table 1), which these cannot directly or inversely be correlated with the small differences they have in life expectancy levels [17, 78].
In African countries where a significantly higher number of people have acquired immunodeficiency syndrome, the incidence rate of Kaposi sarcoma is significantly higher [17], in this case, 50–100 times higher incidence rate of Kaposi sarcoma cancer in Zimbabwe and Botswana, than in Canada and Australia (as reported in Table 1), is not because these African countries have more accurate detection of cancer cases to have higher incidence reports recorded, but simply because of significantly higher prevalence of infections related to HIV among them [83, 84]. The same interpretation applies to the incidence rate of brain cancer (central nervous system) in Iran, which is significantly higher than in Australia, not because of a more accurate detection system to be assumed in Iran, but because significantly more people are diagnosed with this type of cancer in Iran, than in Australia, unrelated to their life expectancy differences [17].
The global trends of age‐standardized cancer incidence rates are in agreement with the main root causes of cancers as discussed in the earlier sections of the paper. Although cancer risk under similar conditions is generally higher in older people, the variations in global trends of age‐standardized cancer incidence rate data for each cancer type reported by IARC are not correlated with life expectancy levels when people from different countries are compared. Likewise, although advanced medical facilities and modern screening systems, offered to the public, help detect cancers earlier, and therefore, reduce cancer death rates as a result of a higher percentage of cancer cases detected in early stages than in late stages [85], this factor is not a determinant that controls global trends for overall incidence rates. Indeed, the results of IARC advanced analysis on statistical cancer data have logical trends, and although some of the data may not be very accurate, they are qualitatively correct and in meaningful agreement with the root causes as per the scientific literature.
Studies on cancer incidence data for immigrants also have confirmed that the role of environmental factors and lifestyle may be more significant than the role of genes or life expectancy. For instance, some reports have shown that the incidence rate of breast cancer in Iranian immigrants to Canada increased fourfold and colorectal cancer by twofold, while a dramatic decrease was found in the incidence rate of stomach cancer [86]. The IARC cancer statistical data also showed that the ASR of breast cancer and colorectal cancer are two times higher in Canada, but stomach cancer is four times higher in Iran [17]. The data interestingly highlight that the incidence of cancer in Iranians who immigrated to Canada has more similarities to the local Canadians living in Canada, than the Iranians living in Iran, confirming the significant effect of changes in lifestyle and environmental factors. The studies regarding cancer incidence in Japanese immigrants to the United States had similar conclusions, as they observed a reduced stomach cancer rate and increased breast cancer rate in Japanese women who immigrated to the United States compared to Japanese women in Japan [87].
In summary, the key controlling factors of cancer incidence, in general, are unhealthy diet and lifestyle, as well as environmental factors and exposure to carcinogens. Hence, most cancers may be preventable by modifying dietary and lifestyle factors and reducing exposure to environmental carcinogens. Furthermore, a healthy balanced diet with alkalizing effect may be beneficiary for cancer patients as they often experience a significant metabolic acidosis as per the Warburg effect [1, 2, 3, 15, 88].
4. DISCUSSIONS
This study tried to find the hypothesized relationship between lifestyle and environmental factors that are associated with the risk of cancer. More detailed multidisciplinary integrated scientific research studies are recommended to be performed in the future to accurately address the cancer root causes, as well as propose effective dietary recommendations for cancer patients as a complementary or alternative solution.
5. CONCLUSIONS
The main highlights of the study are as follows:
-
(a)
Diet type is a key risk factor for cancer; hence, balanced healthy diets rich in suitable plant‐based foods may help improve health and reduce the risk of developing cancers.
-
(b)
The global trends of IARC statistical cancer incidence data meaningfully correlate with diet, lifestyle, and environmental factors. Therefore, considering a healthy diet and lifestyle, as well as reducing exposure to carcinogens, are suggested as complementary solutions in cancer treatments.
-
(c)
Controlling cancer effectively requires addressing the major root causes. For each cancer type, risk factors and root causes are specific and different, which taking them into account in treatments may be beneficiary for cancer patients in terms of better recovery from side effects of medical treatments, improving survival rates, and prevention of the second cancers.
-
(d)
Cancer patients may often experience metabolic acidosis as per the Warburg effect. Hence, maintaining an acid–base balance is important in cancer management. A sufficient intake of alkalizing (but not alkaline) foods and drinks, such as sun‐ripened fruits and fresh natural vegetables, may reduce dietary acid load and reduce the risk of cancer.
AUTHOR CONTRIBUTIONS
Hassan Bahrami: Conceptualization (equal); formal analysis (lead); investigation (lead); methodology (lead); validation (equal); visualization (equal); writing—original draft (lead); writing—review and editing (equal). Majid Tafrihi: Conceptualization (equal); investigation (supporting); methodology (supporting); validation (equal); writing—original draft (supporting); writing—review and editing (supporting).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was performed independently, using the publicly available data on the IARC website that everyone has free access to the database (available at: https://gco.iarc.fr/today/online-analysis), and not using any data from any specific human participants or patient material. Hence, ethical approval is not applicable to this study.
INFORMED CONSENT
Not applicable.
ACKNOWLEDGMENTS
The authors would like to thank and show gratitude to the International Agency for Research on Cancer (IARC), Cancer Research UK, Ted Greiner (World Nutrition), Thomas Corriher (Health‐Wyze USA), Dr. Ali Karami (National Foundation of Healthy Lifestyle), Dr. Roghayeh Javanmard, Hanieh Habibfar, Kian Bahrami, Nikan Bahrami, Gemma Byfield, Soraya Mohamadzadeh, Mazdafar Momeni, Sahar Mosaffa, and Lucas Waters for their help and/or sharing valuable information that was used in the study. This research was performed independently and did not receive any grant from funding agencies, commercial, or not‐for‐profit sectors.
Bahrami H, Tafrihi M. Global trends of cancer: the role of diet, lifestyle, and environmental factors. Cancer Innovation. 2023;2:290–301. 10.1002/cai2.76
DATA AVAILABILITY STATEMENT
The analyzed data that support the findings of this study are publicly available on the IARC website. The tabulated data presented in this study can be provided by the corresponding author upon reasonable request.
REFERENCES
- 1. Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–116. 10.1007/s11095-008-9661-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahrami H, Tafrihi M, Mohamadzadeh S. Reversing the Warburg effect to control cancer: a review of diet‐based solutions. J Curr Oncol Med Sci. 2022;2(3):234–48. [Google Scholar]
- 3. Key TJ, Bradbury KE, Perez‐Cornago A, Sinha R, Tsilidis KK, Tsugane S. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ. 2020;2020(368):m511. 10.1136/bmj.m511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Cancer Society . Explore your family cancer history. https://www.cancer.org/latest-news/explore-your-family-cancer-history.html. (20019). Accessed 13 June 2019.
- 5. National Cancer Institute . The genetics of cancer. https://www.cancer.gov/about-cancer/causes-prevention/genetics. (2022). Accessed 17 Aug 2022.
- 6. Ohishi T, Nukuzuma C, Seki A, Watanabe M, Tomiyama‐Miyaji C, Kainuma E, et al. Alkalization of blood pH is responsible for survival of cancer patients by mild hyperthermia. Biomed Res. 2009;30(2):95–100. 10.2220/biomedres.30.95 [DOI] [PubMed] [Google Scholar]
- 7. Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82:103–26. 10.1146/annurev-physiol-021119-034627 [DOI] [PubMed] [Google Scholar]
- 8. Edwards G. Uranium: known facts and hidden dangers. Salzburg, Austria: The World Uranium Hearing Conference; 1992. [Google Scholar]
- 9. Moritz A. Cancer is not a disease—it's a survival mechanism, USA: Ener‐chi.com; 2009. [Google Scholar]
- 10. Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell. 4th ed. New York: Garland Science; 2002. https://www.ncbi.nlm.nih.gov/books/NBK26891 [Google Scholar]
- 11. Ledford H. The human body is a mosaic of different genomes. https://www.nature.com/articles/d41586-019-01780-9. (2019). Accessed 6 June 2019.
- 12. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. 10.1128/CMR.16.1.1-17.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonçalves PH, Uldrick TS, Yarchoan R. HIV‐associated Kaposi sarcoma and related diseases. AIDS. 2017;31(14):1903–16. 10.1097/QAD.0000000000001567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomey R. Children develop leukemia after getting injected with COVID shots. Available from: https://www.naturalnews.com/2022-03-15-chinese-children-develop-leukemia-after-covid-vaccination.html. (2022). Accessed 15 Mar 2022.
- 15. Park YMM, Steck SE, Fung TT, Merchant AT, Elizabeth Hodgson M, Keller JA, et al. Higher diet‐dependent acid load is associated with risk of breast cancer: findings from the sister study. Int J Cancer. 2019;144(8):1834–43. 10.1002/ijc.31889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooper MG, Housman RE. The cell: a molecular approach. 5th ed. USA: Sinauer Associates Inc.; 2009. [Google Scholar]
- 17. International Agency for Research on Cancer (IARC) . Cancer incidence rate data tables and maps for each country. Available from: https://gco.iarc.fr/today/. (2020).
- 18. Gilkes DM, Semenza GL. Role of hypoxia‐inducible factors in breast cancer metastasis. Future Oncol. 2013;9(11):1623–36. 10.2217/fon.13.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woodman I. Breast feeding reduces risk of breast cancer, says study. BMJ. 2002;325(7357):184. 10.1136/bmj.325.7357.184/c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akbari ME, Sayad S, Sayad S, Khayamzadeh M, Shojaee L, Shormeji Z, et al. Breast cancer status in Iran: statistical analysis of 3010 cases between 1998 and 2014. Int J Breast Cancer. 2017;2017:2481021. 10.1155/2017/2481021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dawe GS, Tan XW, Xiao ZC. Cell migration from baby to mother. Cell Adh Migr. 2007;1(1):19–27. 10.4161/cam.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ASU News . Fetal cells influence mom's health during pregnancy and long after. Available from: https://news.asu.edu/content/fetal-cells-influence-moms-health-during-pregnancy-%E2%80%94-and-long-after. (2005). Accessed 28 Aug 2015.
- 23. Lo JJ, Park YMM, Sinha R, Sandler DP. Association between meat consumption and risk of breast cancer: findings from the sister study. Int J Cancer. 2020;146(8):2156–65. 10.1002/ijc.32547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Busch DR, Choe R, Durduran T, Friedman DH, Baker WB, Maidment AD, et al. Blood flow reduction in breast tissue due to mammographic compression. Academic Radiol. 2014;21(2):151–61. 10.1016/j.acra.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soroush A, Farshchian N, Komasi S, Izadi N, Amirifard N, Shahmohammadi A. The role of oral contraceptive pills on increased risk of breast cancer in Iranian populations: a meta‐analysis. J Cancer Prev. 2016;21(4):294–301. 10.15430/JCP.2016.21.4.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rios S, Chen AC. Wearing a tight bra for many hours a day is associated with increased risk of breast cancer. J Oncol Res Treat. 2016;1:1–5. 10.13140/RG.2.2.10742.19525 [DOI] [Google Scholar]
- 27. Fu Y, Guo F, Chen H, Lin Y, Fu X, Zhang H, et al. Core needle biopsy promotes lung metastasis of breast cancer: an experimental study. Mol Clin Oncol. 2018;10(2):253–60. 10.3892/mco.2018.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brunicardi FC, Andersen DK. Schwartz principles of surgery. 10th ed. USA: McGraw Hill Professional; 2014. [Google Scholar]
- 29. Abadi AT, Ierardi E, Lee YY. Why do we still have Helicobacter pylori in our stomachs. Malays J Med Sci. 2015;22(5):70–5. [PMC free article] [PubMed] [Google Scholar]
- 30. Alexander SM, Retnakumar RJ, Chouhan D, Devi TNB, Dharmaseelan S, Devadas K, et al. Helicobacter pylori in human stomach: the inconsistencies in clinical outcomes and the probable causes. Front Microbiol. 2021;12:713955. 10.3389/fmicb.2021.713955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haggar F, Boushey R. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–7. 10.1055/s-0029-1242458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vieira AR, Abar L, Chan DSM, Vingeliene S, Polemiti E, Stevens C, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta‐analysis of cohort studies, an update of the evidence of the WCRF‐AICR continuous update project. Ann Oncol. 2017;28(8):1788–802. 10.1093/annonc/mdx171 [DOI] [PubMed] [Google Scholar]
- 33. Coyle D. What is fermentation? The lowdown on fermented foods. Available from: https://www.healthline.com/nutrition/fermentation (2020). Accessed 20 Aug 2020.
- 34. ETIMES . Bowel cancer: sitting the wrong way while pooping could put you at risk. Available from: https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/bowel-cancer-sitting-the-wrong-way-while-pooping-could-put-you-at-risk/photostory/93114475.cms (2022). Accessed 31 Jul 2022.
- 35. Pacheco S, Pacheco F, Zapata G, Garcia J, Previale C, Cura H, et al. Food habits, lifestyle factors, and risk of prostate cancer in central Argentina: a case control study involving self‐motivated health behavior modifications after diagnosis. Nutrients. 2016;8(7):419. 10.3390/nu8070419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hyun JS. Prostate cancer and sexual function. World J Mens Health. 2012;30(2):99–107. 10.5534/wjmh.2012.30.2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jian Z, Ye D, Chen Y, Li H, Wang K. Sexual activity and risk of prostate cancer: a dose‐response meta‐analysis. J Sex Med. 2018;15(9):1300–9. 10.1016/j.jsxm.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 38. Rapaport L. Does sex protect men against prostate cancer? Reuters Health News. Available from: https://www.reuters.com/article/us-health-ejaculation-prostate-cancer-idUSKCN0XJ1YC (2016). Accessed 22 April 2016.
- 39. Marignol L, Coffey M, Lawler M, Hollywood D. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat Rev. 2008;34(4):313–27. 10.1016/j.ctrv.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 40. Singh O, Bolla SR. Anatomy abdomen and pelvis prostate [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [updated 2021 Jul 26; cited 2021]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540987/. Accessed 26 Jul 2021. [Google Scholar]
- 41. Ahmad F, Cherukuri MK, Choyke PL. Metabolic reprogramming in prostate cancer. Br J Cancer. 2021;125(9):1185–96. 10.1038/s41416-021-01435-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El‐Kenawi A, Gatenbee C, Robertson‐Tessi M, Bravo R, Dhillon J, Balagurunathan Y, et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br J Cancer. 2019;121(7):556–66. 10.1038/s41416-019-0542-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson EG. Lung cancer. University of Michigan Health, Michigan Medicine. Available from: https://www.uofmhealth.org/health-library/hw183816 (2020). Accessed 4 May 2022.
- 44. Lewtas J. Airborne carcinogens. Pharmacol Toxicol. 1993;72(Suppl 1):55–63. 10.1111/j.1600-0773.1993.tb01670.x [DOI] [PubMed] [Google Scholar]
- 45. O'Keeffe LM, Taylor G, Huxley RR, Mitchell P, Woodward M, Peters SAE. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta‐analysis. BMJ Open. 2018;8(10):e021611. 10.1136/bmjopen-2018-021611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cancer Research UK . https://www.cancerresearchuk.org/about-cancer/causes-of-cancer/smoking-and-cancer/whats-in-a-cigarette-0 (25 Mar 2021).
- 47. Chen G, Wan X, Yang G, Zou X. Traffic‐related air pollution and lung cancer: a meta‐analysis. Thorac Cancer. 2015;6(3):307–18. 10.1111/1759-7714.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yun X, Shen G, Shen H, Meng W, Chen Y, Xu H, et al. Residential solid fuel emissions contribute significantly to air pollution and associated health impacts in China. Sci Adv. 2020;6(44):eaba7621. 10.1126/sciadv.aba7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen R, Wei L, Huang H. Mortality from lung cancer among copper miners. Occup Environ Med. 1993;50(6):505–9. 10.1136/oem.50.6.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grimsrud TK. Exposure to different forms of nickel and risk of lung cancer. Am J Epidemiol. 2002;156(12):1123–32. 10.1093/aje/kwf165 [DOI] [PubMed] [Google Scholar]
- 51.New Caledonia agrees to sale of controversial nickel mine, with Tesla as partner. Available from: https://www.rfi.fr/en/france/20210306-new-caledonia-agrees-to-sale-of-controversial-nickel-mine-with-tesla-as-partner (2020). Accessed 6 Mar 2021.
- 52. Ou JY, Fowler B, Ding Q, Kirchhoff AC, Pappas L, Boucher K, et al. A statewide investigation of geographic lung cancer incidence patterns and radon exposure in a low‐smoking population. BMC Cancer. 2018;18(1):115. 10.1186/s12885-018-4002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kang JK, Seo S, Jin YW. Health effects of radon exposure. Yonsei Med J. 2019;60(7):597–603. 10.3349/ymj.2019.60.7.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Snavely ES. Radionuclides in produced water; vol. 4504. USA: American Petroleum Institute; 1989. [Google Scholar]
- 55. Kreuzer M, McLaughlin J. WHO guidelines for indoor air quality: selected pollutants. Geneva: World Health Organization; 2010. p. 7. (Chapter 7: Radon). Available from: https://www.ncbi.nlm.nih.gov/books/NBK138712/ [PubMed] [Google Scholar]
- 56. EPA . Consumer's guide to radon reduction: how to fix your home, USA: Environmental Protection Agency; 2010. [Google Scholar]
- 57. Bedogni B, Powell MB. Hypoxia, melanocytes and melanoma—survival and tumor development in the permissive microenvironment of the skin. Pigm Cell Melanoma Res. 2009;22(2):166–74. 10.1111/j.1755-148X.2009.00553.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagpal BM, Sharma R. Cold injuries: the chill within. Med J. Armed Forces India. 2004;60(2):165–71. 10.1016/S0377-1237(04)80111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149. 10.1155/2015/893149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Di Trolio R, Di Lorenzo G, Fumo B, Ascierto PA. Cosmic radiation and cancer: is there a link? Future Oncol. 2015;11(7):1123–35. 10.2217/fon.15.29 [DOI] [PubMed] [Google Scholar]
- 61. Enyinna P. Radiological risk assessment of cosmic radiation at aviation altitudes (a trip from Houston Intercontinental Airport to Lagos International Airport). J Med Phys. 2016;41(3):205–209. 10.4103/0971-6203.189491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Narayanan DL, Saladi RN, Fox JL. Review: ultraviolet radiation and skin cancer: UVR and skin cancer. Int J Dermatol. 2010;49(9):978–86. 10.1111/j.1365-4632.2010.04474.x [DOI] [PubMed] [Google Scholar]
- 63. Chauhan K, Shahrokhi M, Huecker MR. Vitamin D [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [updated 2021 Aug 26; cited 2022]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441912/. Accessed 26 Aug 2021. [Google Scholar]
- 64. Ahmadnia H, Bahrami H, Mohamadzadeh S. Effect of diet on depression: a review of nutritional solutions. World Nutrition. 2023;14(1):28–57. 10.26596/wn.202314128-57 [DOI] [Google Scholar]
- 65. Leary PF, Zamfirova I, Au J, McCracken WH. Effect of latitude on vitamin D levels. J Osteopath Med. 2017;117(7):433–9. 10.7556/jaoa.2017.089 [DOI] [PubMed] [Google Scholar]
- 66. Wallis GC. Vitamin D deficiency in dairy cows. Bulletins Paper 372. Available from: http://openprairie.sdstate.edu/agexperimentsta_bulletins/372 (1946). Accessed 6 Jan 2016.
- 67. NIH . Vitamin D: fact sheet for health professionals. Available from: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional (2022).
- 68. Daphale DA, Daphale DA, Daphale DS, Thakare DV. Comparative analysis of vitamin D levels in younger and older populations in rural vs. urban areas of Amravati district: an epidemiological survey. World Nutrition. 2022;13(3):31–9. 10.26596/wn.202213331-39 [DOI] [Google Scholar]
- 69. Gallagher RP. Sunscreens in melanoma and skin cancer prevention. Can Med Assoc J. 2005;173(3):244–5. 10.1503/cmaj.050762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loscalzo J, Fauci A, Hauser S, Longo D, Kasper D, Jameson JL. Harrison's principles of internal medicine. 19th ed. USA: McGraw Hill Professional; 2015. [Google Scholar]
- 71. Newman MD, Stotland M, Ellis JI. The safety of nano‐sized particles in titanium dioxide– and zinc oxide–based sunscreens. J Am Acad Dermatol. 2009;61(4):685–92. 10.1016/j.jaad.2009.02.051 [DOI] [PubMed] [Google Scholar]
- 72.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC monographs on the evaluation of carcinogenic risks to humans; vol. 93. World Health Organization, International Agency for Research on Cancer. [PMC free article] [PubMed]
- 73. Linos E, Keiser E, Fu T, Colditz G, Chen S, Tang JY. Hat, shade, long sleeves, or sunscreen? Rethinking US sun protection messages based on their relative effectiveness. Cancer Causes Control. 2011;22(7):1067–71. 10.1007/s10552-011-9780-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Saraf S, Kaur C. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacognosy Res. 2010;2(1):22–5. 10.4103/0974-8490.60586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lin TK, Zhong L, Santiago J. Anti‐inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19(1):70. 10.3390/ijms19010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. World Health Organization (2020) Cancer country profiles. https://www.who.int/teams/noncommunicable-diseases/surveillance/data/cancer-profiles
- 77. Bray F, Znaor A, Cueva P, Korir A, Swaminathan R, Ullrich A, Wang SA, et al. Planning and developing population‐based cancer registration in low‐ or middle‐income settings. Chapter 2: the role and status of population‐based cancer registration, International Agency for Research on Cancer, Technical Report No. 43; 2014. [PubMed] [Google Scholar]
- 78. World Data . Life expectancy for men and women. Available from: https://www.worlddata.info/life-expectancy.php (2019).
- 79. Singh A, Singh RK, Sureja AK. Cultural significance and diversities of ethnic foods of Northeast India. Indian J Tradit Knowl. 2007;6(1):79–94. [Google Scholar]
- 80. Uechi K, Asakura K, Masayasu S, Sasaki S. Within‐country variation of salt intake assessed via urinary excretion in Japan: a multilevel analysis in all 47 prefectures. Hypertension Res. 2017;40(6):598–605. 10.1038/hr.2016.185 [DOI] [PubMed] [Google Scholar]
- 81. Yan S, Gan Y, Song X, Chen Y, Liao N, Chen S, et al. Association between refrigerator use and the risk of gastric cancer: a systematic review and meta‐analysis of observational studies. PLoS One. 2018;13(8):e0203120. 10.1371/journal.pone.0203120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Burkitt MD, Duckworth CA, Williams JM, Pritchard DM. Helicobacter pylori‐induced gastric pathology: insights from in vivo and ex vivo models. Dis Model Mech. 2017;10(2):89–104. 10.1242/dmm.027649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kandala NB, Campbell EK, Rakgoasi SD, Madi BC. The geography of HIV/AIDS prevalence rates in Botswana. HIV AIDS (Auckl). 2012;4:95–102. 10.2147/HIV.S30537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Campbell TB, Borok M, White IE, Gudza I, Ndemera B, Taziwa A, et al. Relationship of Kaposi sarcoma (KS)‐associated herpesvirus viremia and KS disease in Zimbabwe. Clin Infect Dis. 2003;36(9):1144–51. 10.1086/374599 [DOI] [PubMed] [Google Scholar]
- 85. Gu X, Zheng R, Xia C, Zeng H, Zhang S, Zou X, et al. Interactions between life expectancy and the incidence and mortality rates of cancer in China: a population‐based cluster analysis. Cancer Commun. 2018;38(1):44. 10.1186/s40880-018-0308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yavari P, Hislop TG, Bajdik C, Sadjadi A, Nouraie M, Babai M, et al. Comparison of cancer incidence in Iran and Iranian immigrants to British Columbia, Canada. Asian Pac J Cancer Prev. 2006;7(1):86–90. [PubMed] [Google Scholar]
- 87. Dunn JE Jr. Breast cancer among American Japanese in the San Francisco Bay area. Natl Cancer Inst Monogr. 1977;47:157–60. [PubMed] [Google Scholar]
- 88. Bahrami H, Greiner T. The alkaline diet and the Warburg effect. World Nutrition. 2021;12(1):20–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data that support the findings of this study are publicly available on the IARC website. The tabulated data presented in this study can be provided by the corresponding author upon reasonable request.


