Abstract
Species of the genus Bacillus have been widely used for the biocontrol of plant diseases in the demand for sustainable agricultural development. New mechanisms underlying Bacillus biocontrol activity have been revealed with the development of microbiome and microbe‐plant interaction research. In this review, we first briefly introduce the typical Bacillus biocontrol mechanisms, such as the production of antimicrobial compounds, competition for niches/nutrients, and induction of systemic resistance. Then, we discussed in detail the new mechanisms of pathogen quorum sensing interference and reshaping of the soil microbiota. The “cry for help” mechanism was also introduced, in which plants can release specific signals under pathogen attack to recruit biocontrol Bacillus for root colonization against invasion. Finally, two emerging strategies for enhancing the biocontrol efficacy of Bacillus agents, including the construction of synthetic microbial consortia and the application of rhizosphere‐derived prebiotics, were proposed.
In this review, we first briefly introduced the typical Bacillus biocontrol mechanisms. Then, we discussed in detail the new mechanisms of pathogen QS interference and reshaping of the soil microbiota, and the “cry for help” mechanism was also introduced. Finally, two emerging strategies for enhancing the biocontrol efficacy of Bacillus agents, including the construction of synthetic microbial consortia and the application of rhizosphere‐derived prebiotics, were proposed.
INTRODUCTION
The rapid increase in the world population has led to an intense requirement for both the quality and quantity of agricultural products. Correspondingly, the modern intensified agriculture accompanied with a large input of chemical fertilizers usually suffers high incidence of plant diseases that cause considerable economic losses (Morales‐Cedeno et al., 2021; Raza et al., 2017). Traditional crop protection largely depends on the use of chemical pesticides, which are usually challenged by drug tolerance and environmental pollution (Raza et al., 2017). The application of biocontrol agents such as antagonistic bacteria and fungi, based on their environment friendly properties and high efficacy, is becoming an alternative strategy for the management of plant diseases and is receiving increasing attention (Lahlali et al., 2022).
Currently, the typical and widely used biocontrol agents mainly include bacterial Bacillus spp., Pseudomonas spp., Streptomyces spp., and Burkholderia spp., and fungal Trichoderma spp. and Penicillium spp. (Lahlali et al., 2022). In particular, the genus Bacillus represents a large group of rod‐shaped, endospore‐forming, and catalase‐positive bacteria that can be isolated from a large variety of ecological niches, such as soil, rhizosphere, compost, fermented food, and clinical specimens (Fira et al., 2018). With the advantages of secreting diverse secondary metabolites, excellent root colonization, and sporulation ability, Bacillus spp. and their derivative products have been widely used for the biocontrol of plant pathogens (Soni & Keharia, 2021). Accompanied by the successful application of Bacillus spp. in suppressing soilborne and foliar diseases, the underlying biocontrol mechanisms have been intensively studied (Blake et al., 2021; Fira et al., 2018). Firstly, the biocontrol mechanisms of plant pathogens by Bacillus agents, including both direct and indirect manners, are addressed in this review. Plant hosts are known to actively recruit or stimulate biocontrol agents to resist pathogen invasion, and this “cry for help” scenario is emphatically introduced (Griffiths & Ton, 2019). Finally, several biotechnology strategies for promoting the biocontrol efficacy of Bacillus agents were also proposed.
BIOCONTROL MECHANISMS OF PLANT PATHOGENS BY BACILLUS SPP.
As commonly used biocontrol agents, Bacillus spp. engages a number of patterns to protect plants from various pathogens (Blake et al., 2021; Chowdhury et al., 2015; Erlacher et al., 2014; Kloepper et al., 2004; Tao et al., 2020; Wang et al., 2015; Figure 1). Since the traditional biocontrol mechanisms by Bacillus spp., including antibiosis, competition, and induced systemic resistance (ISR), have been comprehensively summarized in several recent reviews (Adeniji et al., 2019; Blake et al., 2021; Hou & Kolodkin‐Gal, 2020; Khan et al., 2022; Kulkova et al., 2023; Salazar et al., 2023), these sections are briefly addressed in this review. Comparatively, two novel mechanisms as interfering with pathogen quorum sensing (QS) and reshaping of the soil microbiome are emphatically discussed in detail.
FIGURE 1.
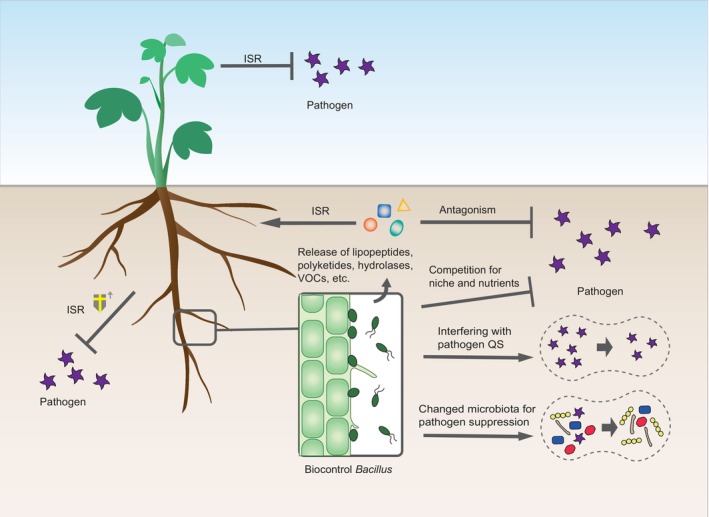
Schematic representation of the multiple biological protective mechanisms of Bacillus spp. Biocontrol Bacillus spp. suppresses plant pathogens directly by releasing antagonistic metabolites including antimicrobial peptides (e.g., bacteriocins and lipopeptides), polyketides, lytic enzymes, and volatile organic compounds (VOCs), or indirectly by niche/nutrients competition and induced systemic resistance (ISR). Importantly, species of Bacillus can secrete specific compounds or enzymes (e.g., AHL‐lactonase and AHL‐oxidoreductases) to interfere with quorum sensing (QS) signals produced by pathogens, thereby impacting their aggregation and pathogenicity. Finally, exogenous Bacillus strains are able to enrich specific indigenous soil microbes with direct biocontrol activity or cooperative performance with other beneficial agents, to enhance plant disease suppression.
Antagonism, competition, and ISR
Bacillus spp. can synthesize a large variety of secondary metabolites for direct inhibition of plant pathogens, including antimicrobial peptides (AMPs), polyketides, lytic enzymes, and volatiles, where the related functional genes can account for approximately 5%–10% of the whole genome (Chowdhury et al., 2015). AMPs synthesized through ribosomes are usually called bacteriocins with strong antagonistic effects against a variety of Gram‐positive bacteria (Wu, Huang, et al., 2018; Wu, Liu, et al., 2018), such as subtilisin, amylocyclicin, and subtilin produced by B. subtilis, B. velezensis, and B. cereus strains (Abriouel et al., 2011; Parisot et al., 2008; Scholz et al., 2014), respectively. Comparatively, lipopeptide is an amphiphilic molecule composed of a short hydrophilic peptide chain and a hydrophobic fatty acid chain that is synthesized by nonribosomal peptide synthetase (Dimkic et al., 2022). Bacillus‐produced lipopeptides mainly include: (1) surfactin family with surfactant property, which can be isolated from B. subtilis, B. velezensis, B. amyloliquefaciens, B. pumilus, and B. licheniformis strains (Li et al., 2014; Salazar et al., 2023); (2) iturin family with outstanding antifungal activity (e.g., Fusarium oxysporum and F. graminearum), including iturin A/D/E, bacillomycin D/F/L, mycosubtilin, and mojavensin, which were usually produced by B. subtilis, B. velezensis, and B. amyloliquefaciens (Salazar et al., 2023; Xu et al., 2013); (3) fengycin family (synonymous to plipastatin) also with antifungal activity (e.g., Verticillium dahliae kleb, F. oxysporum, and Phytophthora parasitica; Li et al., 2014; Salazar et al., 2023). The general antimicrobial mechanism of lipopeptides is penetrating the cell membrane and forming aggregates with phospholipids, thereby changing the permeability of the cell membrane of target pathogens (Dimkic et al., 2022; Sevugapperumal et al., 2019). Polyketides are a large class of natural products produced by continuous condensation reactions of lower carboxylic acids under the biocatalysis of polyketide synthases (Chen et al., 2006). Difficidin produced by B. velezensis FZB42 has excellent biocontrol effects on rice diseases caused by Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola, which significantly downregulated the expression of genes related to pathogen virulence, cell division, and cell wall/protein synthesis (Wu et al., 2015). Macrolactin can suppress multiple stages of cell division and interfere with the process of cell wall synthesis in gram‐positive bacterial pathogens (Yuan et al., 2012). Additionally, Bacillus spp. also produces hybrid polyketide‐nonribosomal peptide metabolites with antimicrobial activity, such as bacillaene produced by B. subtilis that inhibits a broad spectrum of bacteria (Butcher et al., 2007; Patel et al., 1995). Bacillus strains can engage hydrolases, such as chitinase, cellulase, xylanase, glucanase, and protease, to attack the glycosidic bonds of the fungal pathogen's main cell wall components (e.g., chitin, glucan, and protein) to inhibit their growth (Bardin et al., 2015; Jamali et al., 2020). Interestingly, in addition to contact‐dependent antimicrobial metabolites, Bacillus spp. also produces volatile organic compounds (VOCs) that can distantly inhibit target pathogens, including 2‐nonanone, 2‐decanone, 2‐propanone, benzaldehyde, 1,2‐benzothiazole‐3(2H)‐one, and 1,3‐butadiene (Asari et al., 2016; Garrido et al., 2020; Ryu et al., 2004; Saxena et al., 2020; Tahir et al., 2017; Wu, Huang, et al., 2018; Wu, Liu, et al., 2018; Yuan et al., 2012; Zhang et al., 2021; Zhang, Wei, et al., 2020; Zhang, Yu, et al., 2020).
Plant rhizosphere accommodates a large density of diverse microorganisms, where competition with pathogens for niches and nutrients is recognized as an important mechanism involved in biocontrol (Lugtenberg & Kamilova, 2009). The successful colonization of biocontrol agents on the root surface is a crucial step in repelling soil‐borne pathogens from occupying ecological spaces and invasion sites (Soni & Keharia, 2021). Specifically, Bacillus spp. usually colonize plant roots as cell aggregates embedded in a self‐produced extracellular matrix, which is called a biofilm (Arnaouteli et al., 2021). Combining fluorescence reporter and microscopy observations, a series of studies have highlighted that biofilm formation on roots by Bacillus agents contributes to spatial competition with plant pathogens and disease suppression (Berlanga‐Clavero et al., 2022; Chen et al., 2013; Molina‐Santiago et al., 2019; Pandin et al., 2017; Xu et al., 2019). Exploration of rhizosphere nutrients is another type of competition that contribute to pathogen suppression (Xia et al., 2022). Specifically, iron is an essential micronutrient that is present in a high percentage of soils; however, it has low solubility in soil with a pH >6 and is not suitable for uptake by microorganisms (Lugtenberg & Kamilova, 2009). Therefore, iron bioavailability usually becomes a limiting factor that causes nutrient competition among living microbes; under this circumstance, Bacillus agents that synthesize iron‐chelating compounds siderophores (e.g., bacillibactin) can effectively constrain the available iron to pathogens and inhibit their growth (Dimopoulou et al., 2021; Ghazy & El‐Nahrawy, 2021; Lahlali et al., 2022; Yu et al., 2011).
The interaction of some Bacillus strains with plant roots can trigger host defences by eliciting ISR, in which inoculation with beneficial bacteria enhances the resistance of the entire plant against pathogenic bacteria, fungi, and viruses (Kloepper et al., 2004). Lipopeptides, polyketides, specific proteins/peptides, exopolysaccharides (EPS), and VOCs from the genus Bacillus can all act as “elicitors” to activate salicylic acid (SA), jasmonic acid (JA), or ethylene (ET) signalling pathways, thereby priming JA/ET‐dependent defence genes, callose deposition, and stomatal closure, to resist pathogen infection and reduce disease degree (Gowtham et al., 2018; Niu et al., 2012; Vanthana et al., 2019). For example, pure surfactin and fengycin play a significant ISR‐mediated protective role against Botrytis cinerea infection on bean leaves, where the activation of lipoxygenase appeared to be the most important pathway involved in pathogen resistance (Ongena et al., 2007). Root inoculation of B. velezensis SQR9 induced Arabidopsis systemic resistance and significantly reduced the disease incidence of the foliar pathogens P. syringae DC3000 and B. cinerea. In detail, strain SQR9‐produced lipopeptides, polyketide, VOCs, and EPS act synergistically to activate plant ISR through different signalling pathways (Wu, Huang, et al., 2018; Wu, Liu, et al., 2018).
Interference of pathogen QS
The induction of virulence genes in many pathogenic bacteria relies on QS system that monitors the population density (Dong et al., 2000). Accordingly, several Bacillus agents are able to interfere with QS regulation to suppress pathogen invasion and reduce disease incidence, which is known as quorum quenching (QQ; Blake et al., 2021). Interruption of pathogen QS by Bacillus spp. generally includes impeding signals’ transduction and enzymatic hydrolyzing/inactivating the signals (Faure & Dessaux, 2007). With regard to the former step, Kachhadia et al. (2022) identified B. cereus RC1 as a potential QQ agent, based on its inhibition of violacein and pyocyanin production by QS biosensor strains. The ethyl acetate extracts of strain RC1 significantly inhibited soft rot caused by Lelliottia amnigena, as attenuating the macerated tissue in potato, carrot, and cucumber by 91.22%, 97.59%, and 88.78%, respectively. The following gas chromatography–mass spectrometry (GC–MS) analysis showed that diketopiperazines (DKPs), viz. cyclo (D‐phenylalanyl‐L‐prolyl), Cyclo Phe‐Val, Cyclo (Pro‐Ala), Cyclo (L‐prolyl‐L‐valine), Cyclo (Leu‐Leu), and Cyclo (−Leu‐Pro), are prominent metabolites that could modulate the pathogenicity in L. amnigena, which can act as mimicking molecules to compete with QS signals N‐acyl‐homoserine lactone (AHL) for the binding sites of LuxR receptors (Hanzelka & Greenberg, 1995; Kachhadia et al., 2022).
Enzymatic hydrolysis/inactivation of pathogen‐produced QS signals, such as AHLs, is the major mechanism involved in QQ exerted by Bacillus agents (Grandclement et al., 2016). To the best of our knowledge, most of the AHL‐degrading enzymes are lactonases (Leadbetter, 2001; Zhu et al., 2023). AiiA, the AHL‐lactonase that hydrolyzes AHL to inactivated signal N‐3‐oxohexanoyl‐L‐homoserine, was first identified from Bacillus sp. 240B1 and proved to attenuate E. carotovora pathogenicity in potato, eggplant, Chinese cabbage, carrot, celery, cauliflower, and tobacco (Dong et al., 2000); plants expressing the AHL‐lactonase from strain 240B1 successfully quenched pathogen QS signalling and showed enhanced resistance to E. carotovora infection (Dong et al., 2001). B. subtilis BS‐1, also carrying an aiiA gene, can suppress potato soft rot caused by E. carotovora, and the supernatant from Escherichia coli expressing the aiiA gene revealed similar biocontrol effects (Pan et al., 2008). Other bacterial isolates capable of enzymatic inactivation of AHLs were also identified, which belong to the species B. thuringiensis, B. cereus, B. mycoides, and so on (Dong et al., 2002; Huma et al., 2011). Recently, Garge and Nerurkar (2017) isolated 20 AHL‐degrading Bacillus spp. from soil and yielded three agents, As30, Gs42, and Gs52, which demonstrated appreciable attenuation of potato/carrot pathogenicity caused by Pectobacterium carotovorum subsp. carotovorum (Pcc), a pathogen dependent on AHL‐mediated QS for virulence. Similarly, using AHL compound N‐tetradecanoyl homoserine produced by pathogenic P. syringae pv. passiflorae as target substrate, Jose et al. (2019) screened 11 Bacillus strains with AHL degradation activity, and pronounced that these agents can both inhibit the virulence factors and disease induction of P. syringae pv. passiflorae. Interestingly, despite the well‐known disease suppression effect by AHL degradation Bacillus strains, they might also interfere with the QS signalling of other potential biocontrol agents (e.g., Pseudomonas spp.) and affect their antibiotic (2,4‐diacetylphloroglucinol and phenazine) production (Molina et al., 2003). In general, AHL degradation has been recognized as a novel biocontrol mechanism used by Bacillus strains, but the potential of non‐target interactions might also interfere with the efficacy of other biocontrol agents.
The AHL‐lactonase contains zinc‐binding motif that is conserved in several groups of metallo‐hydrolases, and was therefore proposed as a member of the metallo‐hydrolase superfamily (Dong et al., 2000). Site‐directed mutagenesis based on alignment of homology sequence indicated that the motif “His106‐X‐Asp108‐His109‐59X‐His169‐21X‐Asp191” of AiiA in Bacillus sp. 240B1 is essential for the hydrolase activity (Dong et al., 2000, 2002, 2007). The three‐dimensional structure analysis demonstrated the AHL‐lactonase from B. thuringiensis contain two zinc ions in their active sites, which are coordinated to a number of ligands, including a single oxygen of a bridging carboxylate and a bridging water/hydroxide ion (Kim et al., 2005; Liu et al., 2005). Specifically, AHL‐lactonase hydrolyses both short‐ and long‐chain AHL signals with similar efficiency, but has no or little residue activity to other chemicals, including non‐acyl lactones and aromatic carboxylic acid esters (Wang et al., 2004).
Another QQ enzyme produced by Bacillus is known as AHL‐oxidoreductases (Chen et al., 2013). The bacterial cytochrome P450 monooxygenase CYP102A1 from B. megaterium, a previously known enzyme with fatty acids as the substrate, was found to be capable of efficient oxidation of AHLs and their lactonloysis products acyl homoserines (Chowdhary et al., 2007). AHL oxidation primarily occurs at the ω‐1, ω‐2, and ω‐3 carbons of the acyl chain. In detail, the QS activity of the subterminally hydroxylated AHLs is 18‐fold less than the parent compound (Chowdhary et al., 2007). In summary, these studies reveal the role of QS‐quenching bacteria/enzymes in pathogen suppression and provide new insight for screening biocontrol agents or developing management strategies.
Reshaping of plant microbiome
The complex plant‐associated microbiota forms a holobiont with the host, which is referred to as the second genome of the plant and is crucial for plant health (Berendsen et al., 2012; Trivedi et al., 2021). Several recent studies have demonstrated that Bacillus agents can regulate the indigenous rhizosphere microbiome, such as stimulating specific taxa with direct biocontrol activity and cooperative performance with other beneficial microbes, to enhance plant disease suppression (Fu et al., 2017; Morales Moreira et al., 2023; Tao et al., 2020; Xiong et al., 2017). For instance, inoculation of B. amyloliquefaciens W19, an efficient antagonistic bacterium against banana wilt caused by Fusarium oxysporum f. sp. cubense (FOC), significantly impacted the resident soil microbial communities, especially enriched specific Pseudomonas spp. A higher percentage of Pseudomonas isolates with FOC inhibition ability was recovered from the W19‐inoculated soil as compared with non‐inoculated control, where a specific isolate, Pseudomonas sp. PSE78, positively interacted with W19 in biofilm formation and strongly antagonized against FOC. Interestingly, the cooperative biofilm formation between other beneficial Bacillus and Pseudomonas strains was attributed to the metabolic cross‐feeding, as Bacillus provides valeric acid and levulinic acid for Pseudomonas while Pseudomonas return branched‐chain amino acids (BCAAs) back to Bacillus (Sun et al., 2022). Importantly, pot experiments indicated that the positive interactions between B. amyloliquefaciens W19 and Pseudomonas sp. PSE78 synergistically inhibit FOC invasion and reduce disease incidence, highlighting the correlation between Bacillus inoculation and stimulation of indigenous Pseudomonas spp. with pathogen suppression potential (Tao et al., 2020). Similarly, Wang et al. (2023) selected B. velezensis BER1 and tomato bacterial wilt caused by Ralstonia solanacearum as models to investigate whether the biocontrol agent‐induced microbiome shift would contribute to disease suppression. Strain BER1 application significantly inhibited the rhizosphere colonization of R. solanacearum by 36.3% and effectively suppressed tomato wilt by over 49.0%. Interestingly, two specific Flavobacterium amplicon sequence variants (ASVs) were detected to be significantly enriched in response to B. velezensis BER1 inoculation. Using novel colony loop‐mediated isothermal amplification (LAMP) assay system, culturable Flavobacterium isolates were obtained from tomato rhizosphere. Among them, although Flavobacterium C45 did not inhibit R. solanacearum directly, it significantly increased the biofilm formation of strain BER1 under co‐culture condition. As compared with mono‐inoculation of B. velezensis BER1, co‐inoculation of BER1 and Flavobacterium C45 reduced the rhizosphere abundance of R. solanacearum in the rhizosphere by 43.1% and elevated the transcription level of plant defence gene PR1α by 45.4%, finally improved the biocontrol efficiency of bacterial wilt by 46.0% (Wang et al., 2023).
Several recent studies also demonstrated that the application of different Bacillus biocontrol agents can reshape the soil microbiome by stimulating a couple of microbial groups with antifungal activity, such as Streptomyces, Lysobacter, Burkolderia, Sphingobium, Dyadobacter, and Cryptococcus (Fu et al., 2016, 2017; Han et al., 2019; Xiong et al., 2017; Xu et al., 2020). Specifically, integrated Bacillus‐derived biofertilizer application after ammonia fumigation was highlighted to be an alternative strategy for reconstructing soil microbiome and suppressing banana Panama disease, which can further deplete pathogen abundance and reduce disease incidence (Shen et al., 2019); in detail, although the abundance of Bacillus (including the inoculated strain) did not significantly increase after biofertilization, putative beneficial taxa, such as Paenibacillus, Virgibacillus, Nitrosomonas, and Nitrobacter, were significantly enriched by fumigation and biofertilizer application and were significantly correlated with disease suppression or increased plant biomass (Shen et al., 2019). The involvement of mediating rhizosphere microbiome in disease suppression was also demonstrated after inoculating endophytic Bacillus strains (Cheng et al., 2020; Uwaremwe et al., 2022).
In addition to the mediation of rhizosphere/soil microbiome, biocontrol agents can also suppress foliage disease by reshaping phyllosphere microbiota (Liu et al., 2022). Biocontrol strain B. velezensis SYL‐3 significantly reduced the foliage disease index caused by Alternaria alternata and tobacco mosaic virus (TMV). Intriguingly, strain SYL‐3 treatment dramatically changed the phyllosphere microbial community of Nicotiana tabacum plants, where the abundance of beneficial taxa, including Pseudomonas, Sphingomonas, Massilia, and Cladosporium, increased by 19.0%, 9.5%, 3.3%, and 12.3%, respectively, while the abundance of Pantoea, Enterobacter, Sampaiozyma, and Rachicladosporium decreased. Furthermore, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) predication indicated that pathways such as gene replication and repair, energy metabolism, and biosynthesis of secondary metabolites were enriched in response to SYL‐3 application. Therefore, the stimulation of beneficial phyllosphere microbiota and their biocontrol function in response to strain SYL‐3 are involved in suppression of the foliage diseases, although the detailed contribution needs further confirmation (Liu et al., 2022). Taken together, these findings present new perspectives with regard to biocontrol mechanisms, and disease suppression can also be achieved by introducing keystone species to reshape rhizosphere microbiota structure and function.
CRY FOR HELP: PLANTS RECRUIT BIOCONTROL AGENTS UNDER PATHOGEN INVASION
Plant colonization and community assemblage by rhizosphere microbiome depend on several key steps such as uptake of plant metabolites, chemotaxis and cell motility, and biofilm production, which are manipulated by specific compounds in the released root exudates (e.g., carbohydrates and organic acids; Trivedi et al., 2021). Intriguingly, during the lengthy coevolutionary period between plant and microbiome, the host has adapted to biotic stress by an active assemblage of health‐promoting microbiota, which is an enhanced recruitment of beneficial microbes than ordinary state (Griffiths & Ton, 2019). This phenomenon is called the “cry for help” mechanism and provides a mechanistic explanation for plant feedback to pathogen invasion (Griffiths & Ton, 2019). Root exudates are acknowledged to be extremely crucial for recruiting biocontrol agents in response to plant diseases (Griffiths & Ton, 2019; Figure 2). A classic case is the malic acid‐mediated recruitment of beneficial soil bacteria by A. thaliana (Rudrappa et al., 2008). Compared with mock or nonpathogenic P. syringae treatments, leaf infection by the foliar pathogen P. syringae pv tomato (Pst DC3000) significantly stimulated the root colonization of beneficial rhizobacterium B. subtilis FB17. Pst DC3000 infection enhanced root secretion of the tricarboxylic acid cycle (TCA) intermediate L‐malic acid through intraplant long‐distance signalling, which induced chemotaxis and biofilm formation of B. subtilis FB17 in a dose‐dependent manner; L‐malic acid‐triggered biofilm formation by B. subtilis is dependent on the sensor histidine kinase KinD and particularly on an extracellular CACHE domain (Chen et al., 2012). As a result, the induced root binding of rhizobacterium B. subtilis FB17 can activate systemic resistance of A. thaliana plants for defence response and disease suppression (Rudrappa et al., 2008). Afterwards, the active recruitment of “enforcements” by plants under pathogen attack has been raised in different interaction scenes. For example, cucumber root invasion by F. oxysporum f. sp. cucumerinum J. H. Owen (FOCO) increased the concentrations of citric acid, fumaric acid, and tryptophan in root exudates (Liu et al., 2014, 2017). Citric acid acts as a chemoattractant, and fumaric acid serves as a biofilm stimulator for the biocontrol strain B. velezensis SQR9; being the precursor for indole 3‐acetic acid (IAA) synthesis, tryptophan can also contribute to enhanced rhizosphere colonization of the IAA‐producing strain SQR9 (Liu et al., 2014, 2017); additionally, the plant commensal type VII secretion system in strain SQR9 causes iron leakage from roots to promote its own colonization and subsequent plant beneficial functions (Liu, Shu, et al., 2023; Liu, Zhao, et al., 2023). Therefore, induced root secretion of different molecular signals can recruit the beneficial rhizobacterium SQR9 to resist FOCO infection (Liu et al., 2014).
FIGURE 2.
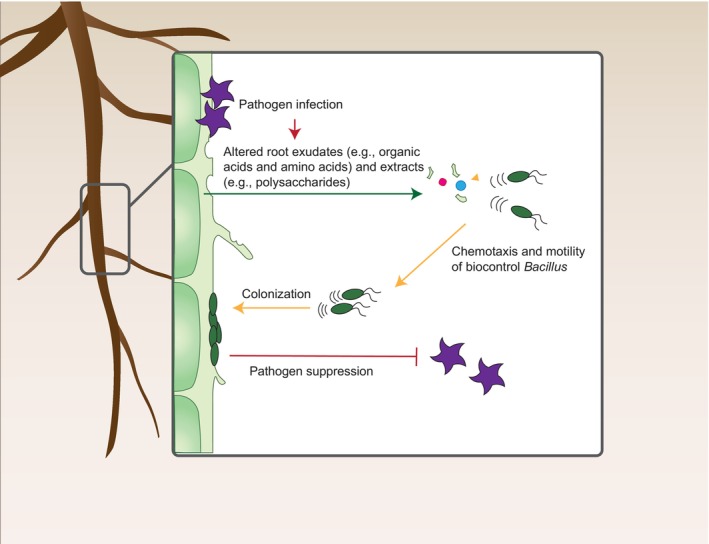
Schematic representation of plant recruitment of Bacillus biocontrol agents under pathogen invasion. In response to pathogen infection, plant roots can both actively secrete specific signals (e.g., malic acid and citric acid) or passively release polysaccharides (e.g., pectin and xylan) to recruit biocontrol Bacillus spp. by inducing their chemotaxis and biofilm formation. This plant feedback to pathogen invasion for enhancing resistance is called the “cry for help” mechanism, which is also used to explain the formation of pathogen‐induced disease‐suppressive soil.
Correspondingly, these “cry for help” examples have been used to explain pathogen‐induced disease‐suppressive soil (Griffiths & Ton, 2019). It is well known that successive plant monocultures can enrich relevant pathogens and lead to serious obstacles. However, continued cultivation in infested soil assembles health‐promoting microbiomes that can specifically inhibit the pathogen, therefore generating (specific) disease‐suppressive soil (Gao et al., 2021). The induction model summarized by Griffiths and Ton (2019) includes several stages: first, pathogen infection activates local and systemic signals that trigger root immunity; then, changes occur in root exudation profiles of primary or/and secondary metabolites; after that, the altered root‐secreted chemicals recruit antagonistic microbes and result in reshaped root microbiome functions and activities; finally, the enriched antagonists suppress the pathogens via mechanisms such as direct inhibition, niche/nutrient competition, and ISR. Recently, several studies have deeply explored the dynamic characteristics of soil microbial structure and function under pathogen challenge (Gao et al., 2021; Wen et al., 2023; Figure 2). During continuous growth of cucumber plants for eight generations inoculated with FOCO in a split‐root system, disease incidence gradually decreased upon pathogen infection and enrichment of the genus Bacillus and Sphingomonas. These key microbes can suppress pathogen infection by inducing reactive oxygen species’ (ROS; mainly OH.) accumulation in roots through activation of several biological pathways, including a two‐component system, a bacterial secretion system, and flagellar assembly. Importantly, threonic acid and lysine were determined to be the key metabolites for attracting beneficial Bacillus spp. and Sphingomonas spp. (Wen et al., 2023). Accompanied by Fusarium wilt disease on chilli pepper (Capsicum annuum L.), plant microbiome assembly and functions were significantly altered in the below‐ and above‐ground compartments (Gao et al., 2021). Several potential beneficial bacteria from genera including Bacillus were obviously enriched in the diseased plants for pathogen suppression, which was identified to be the core taxa in both healthy and diseased plants. The enrichment of Bacillus spp. also corresponded to the induction of functional genes involved in detoxification, biofilm formation, and chemotaxis (Gao et al., 2021). These “cry for help” cases also offer opportunities for screening biocontrol agents. Mülner et al. (2019) isolated Bacillus agents from the sclerotia of S. sclerotiorum and R. solani, which exhibited high antagonistic activity towards both pathogens by producing unidentified novel volatiles.
Despite the disease‐induced alternation of root exudation, the root extracts released upon pathogen invasion, such as polysaccharides, can also enhance rhizosphere colonization and antagonism exerted by Bacillus spp. (Bakker et al., 2018). B. subtilis biofilm formation can be triggered by certain plant polysaccharides, including pectin, xylan, and arabinogalactan (AG), via the kinase‐mediated phosphorylation transformation of the master regulator Spo0A; these polysaccharides also serve as a source of sugars for synthesizing the matrix exopolysaccharide of bacterial biofilms (Beauregard et al., 2013). Plant polysaccharides, especially AG and pectin, also enhance the synthesis of lipopeptide surfactin in B. subtilis and B. velezensis, which can simultaneously inhibit pathogens, promote root colonization by optimizing motility and biofilm formation, and induce host resistance (Debois et al., 2015; Hoff et al., 2021). In the specific nutritional context containing root exudates and polysaccharides, B. velezensis can even qualitatively modulate the pattern of surfactin homologues co‐produced in planta and forms mainly variants that are the most active at triggering plant immunity (Hoff et al., 2021). To summarize, both root exudate components (e.g., specific organic acids and amino acids) and polysaccharides (AG, xylan, and pectin) can be applied as elicitors for recruiting antagonistic Bacillus spp. and stimulating their biocontrol activities.
PRACTICAL AND EMERGING STRATEGIES FOR ENHANCING THE BIOCONTROL EFFICIENCY OF BACILLUS AGENTS
The use of Bacillus strains and their derived products for the biocontrol of plant disease has achieved certain benefits worldwide. However, practical utilization of these agents is usually confronted with unstable disease suppression efficacy under field conditions, which limits the application and extension of biocontrol products (Ling et al., 2010). Complicated and dynamic factors, such as soil characteristics, plant genotypes, and indigenous microbiota, can all influence the colonization and functional efficacy of inoculated Bacillus agents (Xiong et al., 2017). Accordingly, a few approaches have been attempted to enhance biocontrol efficiency, such as the combination of Bacillus strains with organic amendments to make bioorganic fertilizers (Cao et al., 2011; Chen et al., 2020; Ling et al., 2010; Qiu et al., 2012; Xiong et al., 2017; Zhang et al., 2011), the use of antagonistic metabolites instead of/mixed with biocontrol agents (Kang et al., 2021; Medeot et al., 2017; Mihalache et al., 2018; Ravi et al., 2021), and the combined application of bacterial isolates and chemical agents (e.g., fungicides; Ji et al., 2019; Liu, Shu, et al., 2023; Liu, Zhao, et al., 2023). These strategies have been summarized to improve the biocontrol efficacy of Bacillus agents on different levels, and two emerging strategies, including synthetic microbial consortia and rhizosphere‐derived prebiotics, are introduced here (Du et al., 2021; Figure 3).
FIGURE 3.
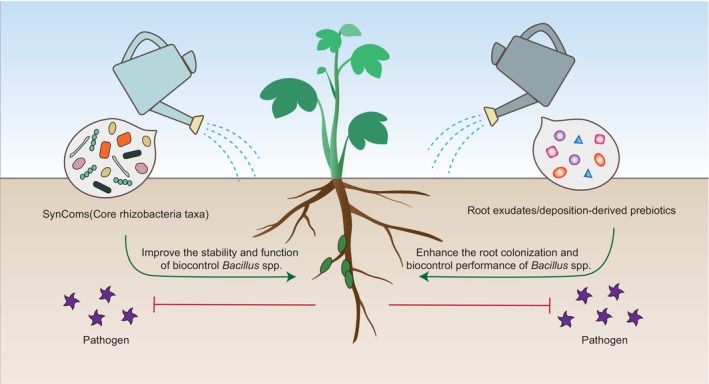
Emerging strategies for enhancing the biocontrol efficiency of Bacillus agents. Synthetic microbial consortium (SynCom) refers to a community that consists of a limited number of strains with multiple functions; compared with individual biocontrol agents, a rationally designed SynCom can reveal higher metabolic diversity and environmental adaptation ability, as well as wider/stronger functions. In another way, exogenous application of root exudates/deposition‐derived prebiotics (e.g., carbohydrates, L‐glutamic acid, and riboflavin) promote the growth and/or antimicrobial metabolites production by biocontrol Bacillus, thus enhancing their biocontrol efficacy against plant pathogens.
Construction of synthetic microbial consortia
Synthetic microbial consortium (SynCom) refers to a community that consists of a limited number of strains with multiple functions (Liu et al., 2018; Yang et al., 2023; Zhang, Wei, et al., 2020; Zhang, Yu, et al., 2020). Compared with individual biocontrol agents, a rationally designed SynCom can reveal higher metabolic diversity and environmental adaptation ability, as well as wider/stronger functions (Johns et al., 2016; Li et al., 2021; Niu et al., 2017; Orozco‐Mosqueda et al., 2018; Tsolakidou et al., 2019; Figure 3). As introduced above, co‐inoculation of biocontrol agents B. amyloliquefaciens W19 and Pseudomonas sp. PSE78 inhibited banana Fusarium wilt much more significantly than a single strain or other strain combination (Tao et al., 2020). Santhanam et al. (2015) constructed a bacterial consortium including the plant growth‐promoting rhizobacteria B. megaterium B55, B mojavensis K1, and three other strains, which effectively protect host plant N. attenuata from sudden wilt disease in a synergistic manner (Santhanam et al., 2015). Using microbiological culturomic approach, Zhou et al. (2022) screened 205 unique strains to design different SynComs and highlighted the cross‐kingdom SynComs, including both bacterial strains such as Bacillus spp. and fungal isolates, were more effective in inhibiting tomato Fusarium wilt than those of bacterial or fungal SynComs. This effect could be attributed to the positive interactions between bacterial and fungal communities, as well as the induction of plant immunity. Liu et al. (2018) assembled different mixtures using four biocontrol strains and found that the specific combination of B. velezensis AP197 and B. velezensis AP298 can enhance the biological control efficacy of multiple plant diseases compared with the individual strain or other mixtures.
Currently, rational construction of a SynCom usually follows the design‐build‐test‐learn (DBTL) cycle and can be achieved via a “top‐down” or “bottom‐up” approach (Lawson et al., 2019). The top‐down strategy refers to the gradual selection of key microbial agents from environmental samples based on physiological and functional screening (Lawson et al., 2019). Xun et al. (2023) used two strategies, progressive dilution and rhizodepositional attraction, to identify the core rhizobacteria taxa responsible for the suppression of maize seed‐borne Fusarium. Consequently, a bacterial consortium was constructed using these keystone strains and optimized according to superior community stability and disease suppression capability, resulting in a simplified SynCom composed of eight strains from the genus of Bacillus, Burkholderia, Enterobacter, Lysobacter, Stenotrophomonas, Pseudoxanthomonas, Pseudomonas, and Acinetobacter. SynCom can effectively inhibit seed‐borne Fusarium with superior efficacy compared to a single strain and randomly formed microbiota. Similarly, based on stepwise optimization based on internal interactions and biocontrol efficacy of banana Fusarium wilt, Prigigallo et al. (2022) gradually simplified a biocontrol SynCom 1.0 (44 isolates) to SynCom 1.1 (7 isolates), and finally to SynCom 1.2, including B. velezensis BN8.2, P. chlororaphis PS5, and Trichoderma virens T2C1.4. The bottom‐up strategy refers to picking potential strains and assembling a desired functional microbiome with known backgrounds based on their metabolic, functional, and interaction traits (Lawson et al., 2019). Palmieri et al. (2016) selected four strains of B. amyloliquefaciens, Serratia marcescens, P. fluorescens, and Rahnella aquatilis from 150 bacterial isolates to construct a SynCom based on their mutual compatibility when grown in a mixture and their antagonistic activity against two Fusarium pathogens. As a result, the microbial consortium significantly inhibited both Fusarium pathogens, with a consistently higher efficacy compared to individual isolates (Palmieri et al., 2016).
Interestingly, the synergetic mechanisms involved in the high‐efficacy biocontrol of SynCom have also been illustrated in several recent studies. A distinctive cooperative pattern was revealed in SynCom for suppressing maize seed‐borne pathogens; that is, the Bacillus strain serves as the key agent for direct Fusarium suppression by producing the lipopeptide fengycin, while other members intensify SynCom functional performance by promoting microbiota stability and Bacillus antagonistic/plant growth‐promoting function (Xun et al., 2023). Similarly, in a consortium consisting of five bacterial isolates for controlling fungal sudden wilt of N. attenuate, three members of P. azotoformans A70, P. frederiksbergensis A176, and Arthrobacter nitroguajacolicus E46 can form enhanced biofilms when growing together with B. megaterium B55 and B. mojavensis K1 in the community, where strain K1 produces the antifungal compound surfactin to inhibit fungal growth both in vitro and in vivo (Santhanam et al., 2019). These highlighted synergetic mechanisms of SynCoM provide theoretical foundations for designing synthetic microbiota with outstanding community stability and biocontrol performance.
Application of root exudates/deposition‐derived prebiotics to improve the biocontrol efficiency of Bacillus
Biocontrol agents, which can be recognized as a special invasive species when directly applied in field conditions, usually fail to adapt to the local soil matrix or compete with the native microbiome, leading to unsuccessful rhizosphere colonization and unsatisfactory biocontrol efficacy (Kim et al., 2021). As summarized above, specific signals released from root exudates or root deposition recruit Bacillus strains and induce their activities; therefore, relevant compounds can be developed as prebiotics for enhancing root colonization and biocontrol performance, similar to those widely applied for stimulating beneficial bacteria in the human gut (Du et al., 2021; Yang et al., 2023; Figure 3). For instance, exogenous addition of sucrose significantly promoted rhizosphere colonization by beneficial B. subtilis (Tian et al., 2021). In another practical example, microbiome collapse and grey mould disease occurrence along with the aging of strawberry plants were found to be closely associated with a decrease in the L‐glutamic acid concentration in root exudates (Kim et al., 2021). Correspondingly, exogenous addition of L‐glutamic acid reshaped the rhizosphere microbiota and enriched the core taxa in the strawberry anthosphere, especially the relative abundance of Bacillaceae, Streptomyces, and Burkholderiaceae in the tomato rhizosphere, contributing to a significant reduction in Botrytis and Fusarium‐caused diseases in both habitats (Kim et al., 2021). Similarly, riboflavin was identified to be a multifunctional prebiotic for enhancing the biocontrol activity of B. subtilis Tpb55 against tobacco black shank caused by P. nicotianae (Zhang et al., 2017). In detail, riboflavin not only stimulated the in vitro growth and rhizosphere colonization of B. subtilis Tpb55 but also induced the activity of catalase, peroxidase, superoxide dismutase, and β‐1,3‐glucanase in the roots of Tpb55‐inoculated tobacco seedlings. Based on the response surface quadratic model, the application of 2 × 108 CFU mL−1 Tpb55 combined with 0.2 mg mL−1 riboflavin showed the highest biocontrol efficacy of 70.1% (Zhang et al., 2017).
In addition to the use of individual compounds, the efficacy and mechanism of applying a mixture of prebiotics for disease control have received attention in recent years. Zhou et al. (2023) isolated eight residential bacterial strains from tomato‐planted soil, including B. velezensis H56 and P. stutzeri H3, and collected 36 carbon sources, including 12 carbohydrates, 12 organic acids, and 12 amino acids, from root exudates. Based on a microcosm study using different synthetic communities and prebiotic mixtures, carbon diversity was significantly positively correlated with the inhibition rate of the pathogen R. solanacearum. It was demonstrated that increasing carbon diversity mediated trophic network architecture, increased microbial evenness, and thus contributed to efficient pathogen repression. Furthermore, a pot experiment indicated that the application of available resources with high diversity also led to successful biocontrol by inducing the evenness and connection of dominant species and activating plant immune system regulation‐relevant microbial functions (Zhou et al., 2023).
CONCLUSION AND PERSPECTIVES
Currently, Bacillus spp. have played considerable roles in plant disease suppression, and the involved biological mechanisms have been intensively investigated. However, facing the rigorous challenge of biotic stresses under the global scenario of climate change, both theoretical and technological studies are still needed to deeply understand the biocontrol mechanisms and enhance the application efficacy in agricultural production (Fu et al., 2017). In particular, basic research covering SynCom assemblages and plant‐antagonist interactions is important to illustrate the biocontrol principle from a comprehensive perspective (Prigigallo et al., 2022). The former refers to how the beneficial members interact with each other to establish a stable community and how they cope with each other to conduct better biocontrol performance; the latter involves the mechanism of mediating plant local/systemic immunity for balancing Bacillus colonization and pathogen resistance (Minchev et al., 2021), as well as host genotype (e.g., resistant variety)‐mediated microbiota assemblage (Kwak et al., 2018). With regard to field applications, based on the novel scientific findings discussed above, designing practical SynCom and screening associated prebiotics, as well as their combination recognized as “synbiotic”, will become fundamental approaches for disease biocontrol in agricultural production (Santhanam et al., 2015). In summary, with advances in genomics, proteomics, metabolomics, phenomics, and bioinformatics, the underlying mechanisms and application potential of Bacillus agents will be adequately exploited for managing plant diseases in a green and environment friendly way.
AUTHOR CONTRIBUTIONS
Nan Zhang: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Zhengqi Wang: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Jiahui Shao: Data curation (supporting); formal analysis (equal); writing – original draft (supporting). Zhihui Xu: Data curation (supporting); formal analysis (equal); writing – original draft (supporting). Yunpeng Liu: Data curation (supporting); formal analysis (equal); writing – original draft (equal). Weibing Xun: Data curation (supporting); formal analysis (equal); writing – review and editing (equal). Youzhi Miao: Data curation (supporting); formal analysis (equal); writing – review and editing (supporting). Qirong Shen: Conceptualization (equal); funding acquisition (supporting); supervision (supporting); validation (equal); writing – review and editing (supporting). Ruifu Zhang: Conceptualization (equal); formal analysis (equal); funding acquisition (lead); investigation (equal); project administration (lead); resources (lead); supervision (lead); validation (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
ACKNOWLEDGEMENTS
This work was financially supported by the National Natural Science Foundation of China (32072665, 42090064, and 31972512).
Zhang, N. , Wang, Z. , Shao, J. , Xu, Z. , Liu, Y. , Xun, W. et al. (2023) Biocontrol mechanisms of Bacillus: Improving the efficiency of green agriculture. Microbial Biotechnology, 16, 2250–2263. Available from: 10.1111/1751-7915.14348
Nan Zhang and Zhengqi Wang contributed equally to this paper.
REFERENCES
- Abriouel, H. , Franz, C. , Ben Omar, N. & Galvez, A. (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiology Reviews, 35, 201–232. [DOI] [PubMed] [Google Scholar]
- Adeniji, A.A. , Loots, D.T. & Babalola, O.O. (2019) Bacillus velezensis: phylogeny, useful applications, and avenues for exploitation. Applied Microbiology and Biotechnology, 103, 3669–3682. [DOI] [PubMed] [Google Scholar]
- Arnaouteli, S. , Bamford, N.C. , Stanley‐Wall, N.R. & Kovacs, A.T. (2021) Bacillus subtilis biofilm formation and social interactions. Nature Reviews. Microbiology, 19, 600–614. [DOI] [PubMed] [Google Scholar]
- Asari, S. , Matzen, S. , Petersen, M.A. , Bejai, S. & Meijer, J. (2016) Multiple effects of Bacillus amyloliquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiology Ecology, 92, fiw070. [DOI] [PubMed] [Google Scholar]
- Bakker, P. , Pieterse, C.M.J. , de Jonge, R. & Berendsen, R.L. (2018) The soil‐borne legacy. Cell, 172, 1178–1180. [DOI] [PubMed] [Google Scholar]
- Bardin, M. , Ajouz, S. , Comby, M. , Lopez‐Ferber, M. , Graillot, B. , Siegwart, M. et al. (2015) Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Frontiers in Plant Science, 6, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard, P.B. , Chai, Y. , Vlamakis, H. , Losick, R. & Kolter, R. (2013) Bacillus subtilis biofilm induction by plant polysaccharides. Proceedings of the National Academy of Sciences of the United States of America, 110, E1621–E1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen, R.L. , Pieterse, C.M. & Bakker, P.A. (2012) The rhizosphere microbiome and plant health. Trends in Plant Science, 17, 478–486. [DOI] [PubMed] [Google Scholar]
- Berlanga‐Clavero, M.V. , Molina‐Santiago, C. , Caraballo‐Rodriguez, A.M. , Petras, D. , Diaz‐Martinez, L. , Perez‐Garcia, A. et al. (2022) Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti‐fungal resistance in melon. Nature Microbiology, 7, 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, C. , Christensen, M.N. & Kovacs, A.T. (2021) Molecular aspects of plant growth promotion and protection by Bacillus subtilis . Molecular Plant‐Microbe Interactions, 34, 15–25. [DOI] [PubMed] [Google Scholar]
- Butcher, R.A. , Schroeder, F.C. , Fischbach, M.A. , Straight, P.D. , Kolter, R. , Walsh, C.T. et al. (2007) The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis . Proceedings of the National Academy of Sciences of the United States of America, 104, 1506–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Zhang, Z. , Ling, N. , Yuan, Y. , Zheng, X. , Shen, B. et al. (2011) Bacillus subtilis SQR9 can control Fusarium wilt in cucumber by colonizing plant roots. Biology and Fertility of Soils, 47, 495–506. [Google Scholar]
- Chen, F. , Gao, Y. , Chen, X. , Yu, Z. & Li, X. (2013) Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing‐dependent infection. International Journal of Molecular Sciences, 14, 17477–17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Vater, J. , Piel, J. , Franke, P. , Scholz, R. , Schneider, K. et al. (2006) Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. Journal of Bacteriology, 188, 4024–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Cao, S. , Chai, Y. , Clardy, J. , Kolter, R. , Guo, J.H. et al. (2012) A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Molecular Microbiology, 85, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Xu, Y. , Zhou, T. , Akkaya, M.S. , Wang, L. , Li, S. et al. (2020) Biocontrol of Fusarium wilt disease in strawberries using bioorganic fertilizer fortified with Bacillus licheniformis X‐1 and Bacillus methylotrophicus Z‐1. 3 Biotech, 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, T. , Yao, X. , Wu, C.Y. , Zhang, W. , He, W. & Dai, C. (2020) Endophytic Bacillus megaterium triggers salicylic acid‐dependent resistance and improves the rhizosphere bacterial community to mitigate rice spikelet rot disease. Applied Soil Ecology, 156, 103710. [Google Scholar]
- Chowdhary, P.K. , Keshavan, N. , Nguyen, H.Q. , Peterson, J.A. , Gonzalez, J.E. & Haines, D.C. (2007) Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry, 46, 14429–14437. [DOI] [PubMed] [Google Scholar]
- Chowdhury, S.P. , Hartmann, A. , Gao, W. & Borriss, R. (2015) Biocontrol mechanism by root‐associated Bacillus amyloliquefaciens FZB42 – a review. Frontiers in Microbiology, 6, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debois, D. , Fernandez, O. , Franzil, L. , Jourdan, E. , de Brogniez, A. , Willems, L. et al. (2015) Plant polysaccharides initiate underground crosstalk with Bacillis by inducing synthesis of the immunogenic lipopeptide surfactin. Environmental Microbiology Reports, 7, 570–582. [DOI] [PubMed] [Google Scholar]
- Dimkic, I. , Janakiev, T. , Petrovic, M. , Degrassi, G. & Fira, D. (2022) Plant‐associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms – a review. Physiological and Molecular Plant Pathology, 117, 101754. [Google Scholar]
- Dimopoulou, A. , Theologidis, I. , Benaki, D. , Koukounia, M. , Zervakou, A. , Tzima, A. et al. (2021) Direct antibiotic activity of bacillibactin broadens the biocontrol range of Bacillus amyloliquefaciens MBI600. mSphere, 6, e0037621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Gusti, A. , Zhang, Q. , Xu, J. & Zhang, L. (2002) Identification of quorum‐quenching N‐acyl homoserine lactonases from Bacillus species. Applied and Environmental Microbiology, 68, 1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Wang, L. , Xu, J. , Zhang, H. , Zhang, X. & Zhang, L. (2001) Quenching quorum‐sensing‐dependent bacterial infection by an N‐acyl homoserine lactonase. Nature, 411, 813–817. [DOI] [PubMed] [Google Scholar]
- Dong, Y. , Wang, L. & Zhang, L. (2007) Quorum‐quenching microbial infections: mechanisms and implications. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 362, 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Xu, J. , Li, X. & Zhang, L. (2000) AiiA, an enzyme that inactivates the acylhomoserine lactone quorum‐sensing signal and attenuates the virulence of Erwinia carotovora . Proceedings of the National Academy of Sciences of the United States of America, 97, 3526–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, J. , Li, Y. , Ur‐Rehman, S. , Mukhtar, I. , Yin, Z. , Dong, H. et al. (2021) Synergistically promoting plant health by harnessing synthetic microbial communities and prebiotics. iScience, 24, 102918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher, A. , Cardinale, M. , Grosch, R. , Grube, M. & Berg, G. (2014) The impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Frontiers in Microbiology, 5, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure, D. & Dessaux, Y. (2007) Quorum sensing as a target for developing control strategies for the plant pathogen Pectobacterium . European Journal of Plant Pathology, 119, 353–365. [Google Scholar]
- Fira, D. , Dimkic, I. , Beric, T. , Lozo, J. & Stankovic, S. (2018) Biological control of plant pathogens by Bacillus species. Journal of Biotechnology, 285, 44–55. [DOI] [PubMed] [Google Scholar]
- Fu, L. , Penton, C.R. , Ruan, Y. , Shen, Z. , Xue, C. , Li, R. et al. (2017) Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biology and Biochemistry, 104, 39–48. [Google Scholar]
- Fu, L. , Ruan, Y. , Tao, C. , Li, R. & Shen, Q. (2016) Continous application of bioorganic fertilizer induced resilient culturable bacteria community associated with banana Fusarium wilt suppression. Scientific Reports, 6, 27731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, M. , Xiong, C. , Gao, C. , Tsui, C.K.M. , Wang, M. , Zhou, X. et al. (2021) Disease‐induced changes in plant microbiome assembly and functional adaptation. Microbiome, 9, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garge, S.S. & Nerurkar, A.S. (2017) Evaluation of quorum quenching Bacillus spp. for their biocontrol traits against Pectobacterium carotovorum subsp. carotovorum causing soft rot. Biocatalysis and Agricultural Biotechnology, 9, 48–57. [Google Scholar]
- Garrido, A. , Atencio, L.A. , Bethancourt, R. , Bethancourt, A. , Guzman, H. , Gutierrez, M. et al. (2020) Antibacterial activity of volatile organic compounds produced by the octocoral‐associated bacteria Bacillus sp. BO53 and Pseudoalteromonas sp. GA327. Antibiotics, 9, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazy, N. & El‐Nahrawy, S. (2021) Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Archives of Microbiology, 203, 1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowtham, H.G. , Murali, M. , Singh, S.B. , Lakshmeesha, T.R. , Narasimha Murthy, K. , Amruthesh, K.N. et al. (2018) Plant growth promoting rhizobacteria‐ Bacillus amyloliquefaciens improves plant growth and induces resistance in chilli against anthracnose disease. Biological Control, 126, 209–217. [Google Scholar]
- Grandclement, C. , Tannieres, M. , Morera, S. , Dessaux, Y. & Faure, D. (2016) Quorum quenching: role in nature and applied developments. FEMS Microbiology Reviews, 40, 86–116. [DOI] [PubMed] [Google Scholar]
- Han, L. , Wang, Z. , Li, N. , Wang, Y. , Feng, J. & Zhang, X. (2019) Bacillus amyloliquefaciens B1408 suppresses Fusarium wilt in cucumber by regulating the rhizosphere microbial community. Applied Soil Ecology, 136, 55–66. [Google Scholar]
- Hanzelka, B.L. & Greenberg, E.P. (1995) Evidence that the N‐terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer‐binding domain. Journal of Bacteriology, 177, 815–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff, G. , Arguelles Arias, A. , Boubsi, F. , Prsic, J. , Meyer, T. , Ibrahim, H.M.M. et al. (2021) Surfactin stimulated by pectin molecular patterns and root exudates acts as a key driver of the Bacillus‐plant mutualistic interaction. mBio, 12, e0177421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Q. & Kolodkin‐Gal, I. (2020) Harvesting the complex pathways of antibiotic production and resistance of soil Bacilli for optimizing plant microbiome. FEMS Microbiology Ecology, 96, fiaa142. [DOI] [PubMed] [Google Scholar]
- Huma, N. , Shankar, P. , Kushwah, J. , Bhushan, A. , Joshi, J. , Mukherjee, T. et al. (2011) Diversity and polymorphism in ahl‐lactonase gene (aiiA) of Bacillus . Journal of Microbiology and Biotechnology, 21, 1001–1011. [DOI] [PubMed] [Google Scholar]
- Jamali, H. , Sharma, A. , Roohi & Srivastava, A. K. (2020) Biocontrol potential of Bacillus subtilis RH5 against sheath blight of rice caused by Rhizoctonia solani . Journal of Basic Microbiology, 60, 268–280. [DOI] [PubMed] [Google Scholar]
- Ji, X. , Li, J. , Meng, Z. , Zhang, S. , Dong, B. & Qiao, K. (2019) Synergistic effect of combined application of a new fungicide fluopimomide with a biocontrol agent Bacillus methylotrophicus TA‐1 for management of gray mold in tomato. Plant Disease, 103, 1991–1997. [DOI] [PubMed] [Google Scholar]
- Johns, N.I. , Blazejewski, T. , Gomes, A.L. & Wang, H. (2016) Principles for designing synthetic microbial communities. Current Opinion in Microbiology, 31, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose, P.A. , Krishnamoorthy, R. , Kwon, S.W. , Janahiraman, V. , Senthilkumar, M. , Gopal, N.O. et al. (2019) Interference in quorum sensing and virulence of the phytopathogen Pseudomonas syringae pv. Passiflorae by Bacillus and Variovorax species. BioControl, 64, 423–433. [Google Scholar]
- Kachhadia, R. , Kapadia, C. , Singh, S. , Gandhi, K. , Jajda, H. , Alfarraj, S. et al. (2022) Quorum sensing inhibitory and quenching activity of Bacillus cereus RC1 extracts on soft rot‐causing bacteria Lelliottia amnigena . ACS Omega, 7, 25291–25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, B. , Song, Y. & Jung, W. (2021) Differential expression of bio‐active metabolites produced by chitosan polymers‐based Bacillus amyloliquefaciens fermentation. Carbohydrate Polymers, 260, 117799. [DOI] [PubMed] [Google Scholar]
- Khan, A.R. , Mustafa, A. , Hyder, S. , Valipour, M. , Rizvi, Z.F. , Gondal, A.S. et al. (2022) Bacillus spp. as bioagents: uses and application for sustainable agriculture. Biology, 11, 1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.R. , Jeon, C.W. , Cho, G. , Thomashow, L.S. , Weller, D.M. , Paik, M.J. et al. (2021) Glutamic acid reshapes the plant microbiota to protect plants against pathogens. Microbiome, 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.H. , Choi, W.C. , Kang, H.O. , Lee, J.S. , Kang, B.S. , Kim, K.J. et al. (2005) The molecular structure and catalytic mechanism of a quorum‐quenching N‐acyl‐L‐homoserine lactone hydrolase. Proceedings of the National Academy of Sciences of the United States of America, 102, 17606–17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepper, J. , Ryu, C.‐M. & Zhang, S. (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology, 94, 1259–1266. [DOI] [PubMed] [Google Scholar]
- Kulkova, I. , Dobrzynski, J. , Kowalczyk, P. , Belzecki, G. & Kramkowski, K. (2023) Plant growth promotion using Bacillus cereus . International Journal of Molecular Sciences, 24, 9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak, M.J. , Kong, H. , Choi, K. , Kwon, S.K. , Song, J. , Lee, J. et al. (2018) Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nature Biotechnology, 36, 1117. [DOI] [PubMed] [Google Scholar]
- Lahlali, R. , Ezrari, S. , Radouane, N. , Kenfaoui, J. , Esmaeel, Q. , El Hamss, H. et al. (2022) Biological control of plant pathogens: a global perspective. Microorganisms, 10, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, C.E. , Harcombe, W.R. , Hatzenpichler, R. , Lindemann, S.R. , Loffler, F.E. , O'Malley, M.A. et al. (2019) Common principles and best practices for engineering microbiomes. Nature Reviews. Microbiology, 17, 725–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter, J.R. (2001) Quieting the raucous crowd. Nature, 411, 748–749. [DOI] [PubMed] [Google Scholar]
- Li, B. , Li, Q. , Xu, Z. , Zhang, N. , Shen, Q. & Zhang, R. (2014) Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Frontiers in Microbiology, 5, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Bai, X. , Jiao, S. , Li, Y. , Li, P. , Yang, Y. et al. (2021) A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant‐induced systemic resistance. Microbiome, 9, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, N. , Xue, C. , Huang, Q. , Yang, X. , Xu, Y. & Shen, Q. (2010) Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt. BioControl, 55, 673–683. [Google Scholar]
- Liu, D. , Lepore, B.W. , Petsko, G.A. , Thomas, P.W. , Stone, E.M. , Fast, W. et al. (2005) Three‐dimensional structure of the quorum‐quenching N‐acyl homoserine lactone hydrolase from Bacillus thuringiensis . Proceedings of the National Academy of Sciences of the United States of America, 102, 11882–11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Jiang, J. , An, M. , Li, B. , Xie, Y. , Xu, C. et al. (2022) Bacillus velezensis SYL‐3 suppresses Alternaria alternata and tobacco mosaic virus infecting Nicotiana tabacum by regulating the phyllosphere microbial community. Frontiers in Microbiology, 13, 840318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , McInroy, J.A. , Hu, C. & Kloepper, J.W. (2018) Mixtures of plant‐growth‐promoting rhizobacteria enhance biological control of multiple plant diseases and plant‐growth promotion in the presence of pathogens. Plant Disease, 102, 67–72. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Zhao, K. , Cai, L. , Zhang, Y. , Fu, Q. & Huang, S. (2023) Combination effects of tebuconazole with Bacillus subtilis to control rice false smut and the related synergistic mechanism. Pest Management Science, 79, 234–243. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Chen, L. , Wu, G. , Feng, H. , Zhang, G. , Shen, Q. et al. (2017) Identification of root‐secreted compounds involved in the communication between cucumber, the beneficial Bacillus amyloliquefaciens, and the soil‐borne pathogen Fusarium oxysporum . Molecular Plant‐Microbe Interactions, 30, 53–62. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Shu, X. , Chen, L. , Zhang, H. , Feng, H. , Sun, X. et al. (2023) Plant commensal type VII secretion system causes iron leakage from roots to promote colonization. Nature Microbiology, 8, 1434–1449. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Zhang, N. , Qiu, M. , Feng, H. , Vivanco, J.M. , Shen, Q. et al. (2014) Enhanced rhizosphere colonization of beneficial Bacillus amyloliquefaciens SQR9 by pathogen infection. FEMS Microbiology Letters, 353, 49–56. [DOI] [PubMed] [Google Scholar]
- Lugtenberg, B. & Kamilova, F. (2009) Plant‐growth‐promoting rhizobacteria. Annual Review of Microbiology, 63, 541–556. [DOI] [PubMed] [Google Scholar]
- Medeot, D.B. , Bertorello‐Cuenca, M. , Liaudat, J.P. , Alvarez, F. , Flores‐Cáceres, M.L. & Jofré, E. (2017) Improvement of biomass and cyclic lipopeptides production in Bacillus amyloliquefaciens MEP218 by modifying carbon and nitrogen sources and ratios of the culture media. Biological Control, 115, 119–128. [Google Scholar]
- Mihalache, G. , Balaes, T. , Gostin, I. , Stefan, M. , Coutte, F. & Krier, F. (2018) Lipopeptides produced by Bacillus subtilis as new biocontrol products against Fusariosis in ornamental plants. Environmental Science and Pollution Research International, 25, 29784–29793. [DOI] [PubMed] [Google Scholar]
- Minchev, Z. , Kostenko, O. , Soler, R. & Pozo, M.J. (2021) Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Frontiers in Plant Science, 12, 756368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina, L. , Constantinescu, F. , Michel, L. , Reimmann, C. , Duffy, B. & Defago, G. (2003) Degradation of pathogen quorum‐sensing molecules by soil bacteria: a preventive and curative biological control mechanism. FEMS Microbiology Ecology, 45, 71–81. [DOI] [PubMed] [Google Scholar]
- Molina‐Santiago, C. , Pearson, J.R. , Navarro, Y. , Berlanga‐Clavero, M.V. , Caraballo‐Rodriguez, A.M. , Petras, D. et al. (2019) The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co‐colonization. Nature Communications, 10, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales Moreira, Z.P. , Chen, M. , Yanez Ortuno, D.L. & Haney, C.H. (2023) Engineering plant microbiomes by integrating eco‐evolutionary principles into current strategies. Current Opinion in Plant Biology, 71, 102316. [DOI] [PubMed] [Google Scholar]
- Morales‐Cedeno, L.R. , Orozco‐Mosqueda, M.D.C. , Loeza‐Lara, P.D. , Parra‐Cota, F.I. , de Los Santos‐Villalobos, S. & Santoyo, G. (2021) Plant growth‐promoting bacterial endophytes as biocontrol agents of pre‐ and post‐harvest diseases: fundamentals, methods of application and future perspectives. Microbiological Research, 242, 126612. [DOI] [PubMed] [Google Scholar]
- Mülner, P. , Bergna, A. , Wagner, P. , Sarajlić, D. , Gstöttenmayr, B. , Dietel, K. et al. (2019) Microbiota associated with sclerotia of soilborne fungal pathogens – a novel source of biocontrol agents producing bioactive volatiles. Phytobiomes Journal, 3, 125–136. [Google Scholar]
- Niu, B. , Paulson, J.N. , Zheng, X. & Kolter, R. (2017) Simplified and representative bacterial community of maize roots. Proceedings of the National Academy of Sciences of the United States of America, 114, E2450–E2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, D. , Wang, C. , Guo, Y. , Jiang, C. , Zhang, W. , Wang, Y. et al. (2012) The plant growth‐promoting rhizobacterium Bacillus cereus AR156 induces resistance in tomato with induction and priming of defence response. Biocontrol Science and Technology, 22, 991–1004. [Google Scholar]
- Ongena, M. , Jourdan, E. , Adam, A. , Paquot, M. , Brans, A. , Joris, B. et al. (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environmental Microbiology, 9, 1084–1090. [DOI] [PubMed] [Google Scholar]
- Orozco‐Mosqueda, M.D.C. , Rocha‐Granados, M.D.C. , Glick, B.R. & Santoyo, G. (2018) Microbiome engineering to improve biocontrol and plant growth‐promoting mechanisms. Microbiological Research, 208, 25–31. [DOI] [PubMed] [Google Scholar]
- Palmieri, D. , Vitullo, D. , De Curtis, F. & Lima, G. (2016) A microbial consortium in the rhizosphere as a new biocontrol approach against fusarium decline of chickpea. Plant and Soil, 412, 425–439. [Google Scholar]
- Pan, J. , Huang, T. , Yao, F. , Huang, Z. , Powell, C.A. , Qiu, S. et al. (2008) Expression and characterization of aiiA gene from Bacillus subtilis BS‐1. Microbiological Research, 163, 711–716. [DOI] [PubMed] [Google Scholar]
- Pandin, C. , Le Coq, D. , Canette, A. , Aymerich, S. & Briandet, R. (2017) Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microbial Biotechnology, 10, 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisot, J.L. , Carey, S. , Breukink, E. , Chan, W. , Narbad, A. & Bonev, B. (2008) Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic. Antimicrobial Agents and Chemotherapy, 52, 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, P.S. , Huang, S. , Fisher, S. , Pirnik, D. , Aklonis, C. , Dean, L. et al. (1995) Bacillaene, a novel inhibitor of prokaryotic protein‐synthesis produced by Bacillus‐subtilis‐production, taxonomy, isolation, physicochemical characterization and biological‐activity. The Journal of Antibiotics, 48, 997–1003. [DOI] [PubMed] [Google Scholar]
- Prigigallo, M.I. , Cabanas, C.G.L. , Mercado‐Blanco, J. & Bubici, G. (2022) Designing a synthetic microbial community devoted to biological control: the case study of Fusarium wilt of banana. Frontiers in Microbiology, 13, 967885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, M. , Zhang, R. , Xue, C. , Zhang, S. , Li, S. , Zhang, N. et al. (2012) Application of bio‐organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biology and Fertility of Soils, 48, 807–816. [Google Scholar]
- Ravi, A. , Nandayipurath, V.V.T. , Rajan, S. , Salim, S.A. , Khalid, N.K. , Aravindakumar, C.T. et al. (2021) Effect of zinc oxide nanoparticle supplementation on the enhanced production of surfactin and iturin lipopeptides of endophytic Bacillus sp. Fcl1 and its ameliorated antifungal activity. Pest Management Science, 77, 1035–1041. [DOI] [PubMed] [Google Scholar]
- Raza, W. , Ling, N. , Zhang, R. , Huang, Q. , Xu, Y. & Shen, Q. (2017) Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Critical Reviews in Biotechnology, 37, 202–212. [DOI] [PubMed] [Google Scholar]
- Rolfe, S.A. , Griffiths, J. & Ton, J. (2019) Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health‐promoting soil microbiomes. Current Opinion in Microbiology, 49, 73–82. [DOI] [PubMed] [Google Scholar]
- Rudrappa, T. , Czymmek, K.J. , Pare, P.W. & Bais, H.P. (2008) Root‐secreted malic acid recruits beneficial soil bacteria. Plant Physiology, 148, 1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, C.M. , Farag, M.A. , Hu, C. , Reddy, M.S. , Kloepper, J.W. & Pare, P.W. (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiology, 134, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar, B. , Ortiz, A. , Keswani, C. , Minkina, T. , Mandzhieva, S. , Singh, S.P. et al. (2023) Bacillus spp. as bio‐factories for antifungal secondary metabolites: innovation beyond whole organism formulations. Microbial Ecology, 86, 1–24. [DOI] [PubMed] [Google Scholar]
- Santhanam, R. , Luu, V.T. , Weinhold, A. , Goldberg, J. , Oh, Y. & Baldwin, I.T. (2015) Native root‐associated bacteria rescue a plant from a sudden‐wilt disease that emerged during continuous cropping. Proceedings of the National Academy of Sciences of the United States of America, 112, E5013–E5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam, R. , Menezes, R.C. , Grabe, V. , Li, D. , Baldwin, I.T. & Groten, K. (2019) A suite of complementary biocontrol traits allows a native consortium of root‐associated bacteria to protect their host plant from a fungal sudden‐wilt disease. Molecular Ecology, 28, 1154–1169. [DOI] [PubMed] [Google Scholar]
- Saxena, A.K. , Kumar, M. , Chakdar, H. , Anuroopa, N. & Bagyaraj, D.J. (2020) Bacillus species in soil as a natural resource for plant health and nutrition. Journal of Applied Microbiology, 128, 1583–1594. [DOI] [PubMed] [Google Scholar]
- Scholz, R. , Vater, J. , Budiharjo, A. , Wang, Z. , He, Y. , Dietel, K. et al. (2014) Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. Journal of Bacteriology, 196, 1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevugapperumal, N. , Senthilraja, S. & Renukadevi, C. (2019) Antimicrobial peptides of Bacillus species: biosynthesis, mode of action and their role in plant disease management. In: Pandey, R.N. , Chakraborty, B.N. , Singh, D. & Sharma, P. (Eds.) Microbial antagonists: their role in biological control of plant diseases. New Delhi: Today & Tomorrow's Printers and Publishers, pp. 487–514. [Google Scholar]
- Shen, Z. , Xue, C. , Penton, C.R. , Thomashow, L.S. , Zhang, N. , Wang, B. et al. (2019) Suppression of banana Panama disease induced by soil microbiome reconstruction through an integrated agricultural strategy. Soil Biology and Biochemistry, 128, 164–174. [Google Scholar]
- Soni, R. & Keharia, H. (2021) Phytostimulation and biocontrol potential of Gram‐positive endospore‐forming Bacilli . Planta, 254, 49. [DOI] [PubMed] [Google Scholar]
- Sun, X. , Xu, Z. , Xie, J. , Hesselberg‐Thomsen, V. , Tan, T. , Zheng, D. et al. (2022) Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. The ISME Journal, 16, 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir, H.A.S. , Gu, Q. , Wu, H. , Niu, Y. , Huo, R. & Gao, X. (2017) Bacillus volatiles adversely affect the physiology and ultra‐structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Scientific Reports, 7, 40481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, C. , Li, R. , Xiong, W. , Shen, Z. , Liu, S. , Wang, B. et al. (2020) Bio‐organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome, 8, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, T. , Sun, B. , Shi, H. , Gao, T. , He, Y. , Li, Y. et al. (2021) Sucrose triggers a novel signaling cascade promoting Bacillus subtilis rhizosphere colonization. The ISME Journal, 15, 2723–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi, P. , Leach, J.E. , Tringe, S.G. , Sa, T. & Singh, B.K. (2021) Plant‐microbiome interactions: from community assembly to plant health. Nature Reviews Microbiology, 18, 607–621. [DOI] [PubMed] [Google Scholar]
- Tsolakidou, M.D. , Stringlis, I.A. , Fanega‐Sleziak, N. , Papageorgiou, S. , Tsalakou, A. & Pantelides, I.S. (2019) Rhizosphere‐enriched microbes as a pool to design synthetic communities for reproducible beneficial outputs. FEMS Microbiology Ecology, 95, fiz138. [DOI] [PubMed] [Google Scholar]
- Uwaremwe, C. , Yue, L. , Wang, Y. , Tian, Y. , Zhao, X. , Liu, Y. et al. (2022) An endophytic strain of Bacillus amyloliquefaciens suppresses Fusarium oxysporum infection of chinese wolfberry by altering its rhizosphere bacterial community. Frontiers in Microbiology, 12, 782523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanthana, M. , Nakkeeran, S. , Malathi, V.G. , Renukadevi, P. & Vinodkumar, S. (2019) Induction of in planta resistance by flagellin (Flg) and elongation factor‐TU (EF‐TU) of Bacillus amyloliquefaciens VB7 against groundnut bud necrosis virus in tomato. Microbial Pathogenesis, 137, 103757. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Weng, L. , Dong, Y. & Zhang, L. (2004) Specificity and enzyme kinetics of the quorum‐quenching N‐acyl homoserine lactone lactonase (AHL‐lactonase). The Journal of Biological Chemistry, 279, 13645–13651. [DOI] [PubMed] [Google Scholar]
- Wang, N. , Ding, J. , Chen, Y. , Zhu, Y. , Zhang, L. , Wei, Y. et al. (2023) Bacillus velezensis BER1 enriched Flavobacterium daejeonense‐like bacterium in the rhizosphere of tomato against bacterial wilt. FEMS Microbiology Ecology, 99, fiad054. [DOI] [PubMed] [Google Scholar]
- Wang, T. , Liang, Y. , Wu, M. , Chen, Z. , Lin, J. & Yang, L. (2015) Natural products from Bacillus subtilis with antimicrobial properties. Chinese Journal of Chemical Engineering, 23, 744–754. [Google Scholar]
- Wen, T. , Ding, Z. , Thomashow, L.S. , Hale, L. , Yang, S. , Xie, P. et al. (2023) Deciphering the mechanism of fungal pathogen‐induced disease‐suppressive soil. The New Phytologist, 238, 2634–2650. [DOI] [PubMed] [Google Scholar]
- Wu, G. , Liu, Y. , Xu, Y. , Zhang, G. , Shen, Q. & Zhang, R. (2018) Exploring elicitors of the beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 to induce plant systemic resistance and their interactions with plant signaling pathways. Molecular Plant‐Microbe Interactions, 31, 560–567. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Huang, Z. , Li, X. , Ma, L. , Gu, Q. , Wu, H. et al. (2018) Stomatal closure and SA‐, JA/ET‐signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana . Frontiers in Microbiology, 9, 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Wu, H. , Chen, L. , Yu, X. , Borriss, R. & Gao, X. (2015) Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Scientific Reports, 5, 12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, L. , Miao, Y. , Cao, A. , Liu, Y. , Liu, Z. , Sun, X. et al. (2022) Biosynthetic gene cluster profiling predicts the positive association between antagonism and phylogeny in Bacillus . Nature Communications, 13, 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, W. , Guo, S. , Jousset, A. , Zhao, Q. , Wu, H. , Li, R. et al. (2017) Bio‐fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biology and Biochemistry, 114, 238–247. [Google Scholar]
- Xu, W. , Wang, K. , Wang, H. , Liu, Z. , Shi, Y. , Gao, Z. et al. (2020) Evaluation of the biocontrol potential of Bacillus sp. WB against Fusarium oxysporum f. sp. niveum. Biological Control, 147, 104288. [Google Scholar]
- Xu, Z. , Mandic‐Mulec, I. , Zhang, H. , Liu, Y. , Sun, X. , Feng, H. et al. (2019) Antibiotic bacillomycin D affects iron acquisition and biofilm formation in Bacillus velezensis through a Btr‐mediated feuABC‐dependent pathway. Cell Reports, 29, 1192–1202. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Shao, J. , Li, B. , Yan, X. , Shen, Q. & Zhang, R. (2013) Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Applied and Environmental Microbiology, 79, 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun, W. , Ren, Y. , Yan, H. , Ma, A. , Liu, Z. , Wang, L. et al. (2023) Sustained inhibition of maize seed‐borne fusarium using a Bacillus‐dominated rhizospheric stable core microbiota with unique cooperative patterns. Advanced Science, 10, e2205215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Liu, H. , Xie, P. , Wen, T. , Shen, Q. & Yuan, J. (2023) Emerging pathways for engineering the rhizosphere microbiome for optimal plant health. Journal of Agricultural and Food Chemistry, 71, 4441–4449. [DOI] [PubMed] [Google Scholar]
- Yu, X. , Ai, C. , Xin, L. & Zhou, G. (2011) The siderophore‐producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. European Journal of Soil Biology, 47, 138–145. [Google Scholar]
- Yuan, J. , Li, B. , Zhang, N. , Waseem, R. , Shen, Q. & Huang, Q. (2012) Production of bacillomycin‐ and macrolactin‐type antibiotics by Bacillus amyloliquefaciens NJN‐6 for suppressing soilborne plant pathogens. Journal of Agricultural and Food Chemistry, 60, 2976–2981. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Gao, J. , Han, T. , Tian, X. & Wang, F. (2017) Integrated control of tobacco black shank by combined use of riboflavin and Bacillus subtilis strain Tpb55. BioControl, 62, 835–845. [Google Scholar]
- Zhang, D. , Yu, S. , Yang, Y. , Zhang, J. , Zhao, D. , Pan, Y. et al. (2020) Antifungal effects of volatiles produced by Bacillus subtilis against Alternaria solaniin potato. Frontiers in Microbiology, 11, 1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Yu, S. , Zhao, D. , Zhang, J. , Pan, Y. , Yang, Y. et al. (2021) Inhibitory effects of non‐volatiles lipopeptides and volatiles ketones metabolites secreted by Bacillus velezensis C16 against Alternaria solani . Biological Control, 152, 104421. [Google Scholar]
- Zhang, J. , Wei, L. , Yang, J. , Ahmed, W. , Wang, Y. , Fu, L. et al. (2020) Probiotic consortia: reshaping the rhizospheric microbiome and its role in suppressing root‐rot disease of panax notoginseng. Frontiers in Microbiology, 11, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N. , Wu, K. , He, X. , Li, S. , Zhang, Z. , Shen, B. et al. (2011) A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant and Soil, 344, 87–97. [Google Scholar]
- Zhou, X. , Liu, L. , Zhao, J. , Zhang, J. , Cai, Z. & Huang, X. (2023) High carbon resource diversity enhances the certainty of successful plant pathogen and disease control. The New Phytologist, 237, 1333–1346. [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Wang, J. , Liu, F. , Liang, J. , Zhao, P. , Tsui, C.K.M. et al. (2022) Cross‐kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nature Communications, 13, 7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Chen, W. , Bhatt, K. , Zhou, Z. , Huang, Y. , Zhang, L. et al. (2023) Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review. Frontiers in Plant Science, 13, 1063393. [DOI] [PMC free article] [PubMed] [Google Scholar]


