Abstract
The aim of the present study was to elucidate the effect of the macrolide antibiotic azithromycin on Pseudomonas aeruginosa. We studied the susceptibility to azithromycin in P. aeruginosa PAO1 using a killing assay. PAO1 cells at the exponential growth phase were resistant to azithromycin. In contrast, PAO1 cells at the stationary growth phase were sensitive to azithromycin. The divalent cations Mg2+ and Ca2+ inhibited this activity, suggesting that the action of azithromycin is mediated by interaction with the outer membranes of the cells, since the divalent cations exist between adjacent lipopolysaccharides (LPSs) and stabilize the outer membrane. The divalent cation chelator EDTA behaved in a manner resembling that of azithromycin; EDTA killed more PAO1 in the stationary growth phase than in the exponential growth phase. A 1-N-phenylnaphthylamine assay showed that azithromycin interacted with the outer membrane of P. aeruginosa PAO1 and increased its permeability while Mg2+ and Ca2+ antagonized this action. Our results indicate that azithromycin directly interacts with the outer membrane of P. aeruginosa PAO1 by displacement of divalent cations from their binding sites on LPS. This action explains, at least in part, the effectiveness of sub-MICs of macrolide antibiotics in pseudomonal chronic airway infection.
Macrolide antibiotics have been used in the treatment of infections caused by clinically important gram-positive cocci, such as Streptococcus spp. and Staphylococcus spp., and atypical pathogens, such as Mycoplasma spp., Chlamydia spp., and Legionella spp. The mechanism of antimicrobial activity of macrolides is generally considered to be inhibition of microbial protein synthesis by acting on the 50S subunit of the 70S ribosome (17). In the 1990s, the “new” macrolides, clarithromycin and azithromycin, were released. The new macrolides have an expanded spectrum of activity, including fastidious gram-negative bacilli, such as Haemophilus influenzae and Neisseria spp. However, macrolides have poor activity against Pseudomonas aeruginosa from the aspect of MIC in vitro.
P. aeruginosa is one of the most important pathogens in patients with chronic airway infection, such as cystic fibrosis (CF) (12, 19) and diffuse panbronchiolitis (DPB) (6). When P. aeruginosa colonizes the airways of these patients, it is difficult to eradicate with antibiotics because of its ability to develop antibiotic resistance. In 1984, Kudoh et al. (8) reported the efficacy of macrolides in chronic airway infections, including DPB. Since then, most patients with DPB in Japan have been treated with macrolide antibiotics, and this therapy has been found to markedly improve the mortality of patients with DPB (7). In addition, recent studies reported that azithromycin also shows clinical efficacy in CF patients (14, 16).
Because the DPB patients are usually treated with sub-MIC concentrations of macrolide antibiotics, it has been considered that macrolides may affect the host defense mechanism and/or the expression of virulence of P. aeruginosa but that it has no bactericidal activity against P. aeruginosa.
In the present study, we show that azithromycin has a bactericidal effect against P. aeruginosa in certain bacterial growth phases. Our results also show that the direct effect of azithromycin on the outer membrane of P. aeruginosa might contribute to its bactericidal activity against this organism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. aeruginosa strain PAO1 was used in all experiments. It was grown in peptone trypticase soy broth. For the experiments described below, fresh medium was inoculated with an overnight culture to a final dilution of 1:30 and grown with vigorous shaking at 37°C.
Antibiotics and chemicals.
Azithromycin was provided by Pfizer Inc. (New York, N.Y.). Clarithromycin was provided by Abbott Japan Co. (Tokyo, Japan). Erythromycin was purchased from Sigma (St. Louis, Mo.). Gentamicin, EDTA, 1-N-phenylnaphthylamine (NPN), and KCN were purchased from Wako (Osaka, Japan).
Killing assay.
The bactericidal effects of compounds on P. aeruginosa were examined in two bacterial growth phases: the exponential phase and the stationary phase. After growth to an optical density at 600 nm of 0.5 (exponential phase) or 2.0 (stationary phase), azithromycin or EDTA was added to each phase of cells to the appropriate concentration with or without divalent cations. After coincubation for 8 h with vigorous shaking at 37°C, numbers of viable bacterial cells were determined by serial 10-fold dilution of samples plated on peptone trypticase soy agar (PTSA) plates and incubated at 37°C for 24 h. Experiments were performed in triplicate, and the results are expressed as means ± standard deviations.
Permeabilization of outer membrane to NPN.
The NPN assay was performed as described previously (5, 10). The cells were cultured to an optical density at 600 nm of 0.4 to 0.6. The cells were centrifuged at 3,000 × g for 10 min and resuspended in 5 mM HEPES buffer (pH 7.2) with 1 mM KCN at an optical density at 600 nm of 0.5. The cells were placed in a cuvette, and NPN was added to a final concentration of 10 μM. Compounds tested for the ability to permeabilize cells to NPN were added at the appropriate concentrations, and the increase in the NPN fluorescence intensity was monitored with a spectrofluorophotometer (RF-5300PC; Simazu Co., Tokyo, Japan). The excitation and emission wavelengths were set at 350 and 420 nm, with slit widths of 5 and 3 nm, respectively.
RESULTS
Effects of azithromycin on P. aeruginosa in different growth phases.
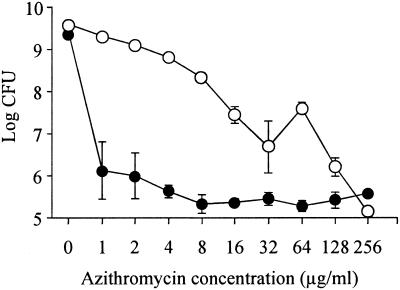
To determine the bactericidal activities of azithromycin against different growth phases of P. aeruginosa, we performed killing assays in the exponential phase and in stationary phase. The minimum bactericidal concentrations (MBCs, defined as the lowest concentration of antibiotics that caused >99.9% reduction of the bacterial count) of azithromycin against exponential- and stationary-phase PAO1 were 128 and 1 μg/ml, respectively (Fig. 1).
FIG. 1.
Numbers of viable P. aeruginosa cells cocultured with azithromycin. The cells were grown to exponential growth phase (open circles) or stationary growth phase (closed circles) and cocultured with 1 to 256 μg of azithromycin/ml. After coincubation for 8 h, viable-cell numbers were determined by serial 10-fold dilution of samples plated on PTSA plates and incubated at 37°C for 24 h. Experiments were performed in triplicate, and the results are expressed as mean ± standard deviation.
Effects of divalent cations on P. aeruginosa susceptibility to azithromycin.
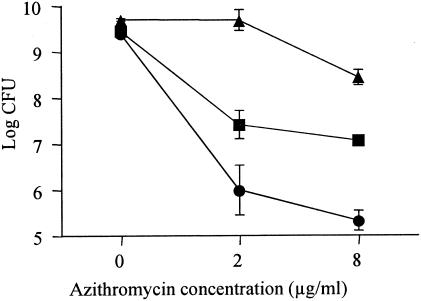
We evaluated the effects of magnesium (Mg2+) and calcium (Ca2+) ions on susceptibility to azithromycin in PAO1. Addition of 20 mg of Mg2+/liter or 20 mg of Ca2+/liter antagonized the bactericidal activity of azithromycin against PAO1 (Fig. 2).
FIG. 2.
Influence of divalent-cation addition on susceptibility of P. aeruginosa to azithromycin. Mg2+ (20 mg/liter) (squares), Ca2+ (20 mg/liter) (triangles), or no cation (circles) was added to stationary-phase PAO1 and coincubated with 2 or 8 μg of azithromycin/ml. After coincubation for 8 h, viable-cell numbers were determined by serial 10-fold dilution of samples plated on PTSA plates and incubated at 37°C for 24 h. Experiments were performed in triplicate, and the results are expressed as mean ± standard deviation.
Effect of EDTA on P. aeruginosa in stationary phase.
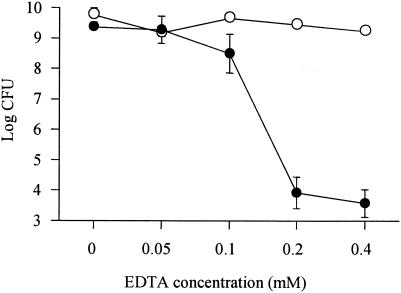
We examined the effect of EDTA on PAO1 in each growth phase. The MBCs of EDTA against exponential- and stationary-phase PAO1 were >4.0 and 0.2 mM, respectively (Fig. 3).
FIG. 3.
Numbers of viable P. aeruginosa cells cocultured with EDTA. The cells were grown to exponential growth phase (open circles) or stationary growth phase (closed circles) and cocultured with 0, 0.05, 0.1, 0.2, or 0.4 mM EDTA. After coincubation for 8 h, viable-cell numbers were determined by serial 10-fold dilution of samples plated on PTSA plates and incubated at 37°C for 24 h. Experiments were performed in triplicate, and the results are expressed as mean ± standard deviation.
Effect of azithromycin on permeability of P. aeruginosa outer membrane.
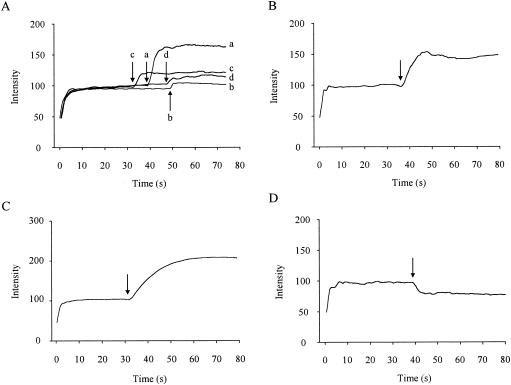
To determine whether azithromycin affected the outer membrane of P. aeruginosa, we performed the NPN assay. No fluorescence accumulation was evident when azithromycin was added to the buffer containing NPN without cells (data not shown). The addition of azithromycin to P. aeruginosa cells in the presence of NPN caused a time-dependent increase in fluorescence (Fig. 4A). The addition of 8 μg of azithromycin/ml to P. aeruginosa cells also caused an increase in fluorescence, but the activity was weaker than that of 128 μg of azithromycin/ml. This phenomenon was also observed when other macrolides, erythromycin and clarithromycin, were added separately. However, the activities of these agents were weaker than that of the same dose of azithromycin (Fig. 4A). EDTA (Fig. 4B) and gentamicin (Fig. 4C) also caused increases in fluorescence intensity. Ceftazidime, which showed bactericidal activity against exponential-phase PAO1 but not stationary-phase PAO1 in the killing assay (data not shown), had no effect on the fluorescence intensity (Fig. 4D).
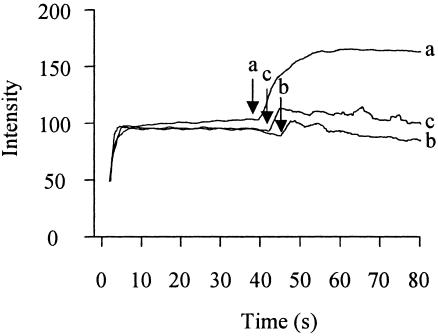
FIG. 4.
Time course of increase in NPN fluorescence intensity in P. aeruginosa cells treated with permeabilizers. At 0 s, 5 μM NPN was added to intact P. aeruginosa cells. (A) At the arrows, the following agents were added: a, azithromycin (128 μg/ml); b, azithromycin (8 μg/ml); c, clarithromycin (128 μg/ml); d, erythromycin (128 μg/ml). (B) At the arrow, 10 mM EDTA was added. (C) At the arrow, gentamicin (128 μg/ml) was added. (D) At the arrow, ceftazidime (128 μg/ml) was added. The data are representative of three separate experiments.
Inhibition of azithromycin enhancement of NPN fluorescence by divalent cations.
To confirm disrupted action on the outer membrane via replacement of divalent cations with azithromycin, we investigated whether the divalent cations inhibited the increase in NPN fluorescence. We found that addition of the divalent cations Mg2+ (1 mM) and Ca2+ (1 mM) inhibited the increase in NPN fluorescence induced by azithromycin.
DISCUSSION
P. aeruginosa is a major pathogen causing chronic airway infection, including DPB and CF. Because P. aeruginosa lives in microcolonies or biofilms in the airways of these patients (15), most of the organisms may be in stationary phase. The sensitivities of bacteria to antibiotics are usually tested by the MIC method, which is a measure of the drug concentration that inhibits bacterial growth during the exponential phase. As shown in Fig. 1, when P. aeruginosa PAO1 cells in the exponential phase were coincubated with azithromycin, the MBC of azithromycin against PAO1 was 128 μg/ml, indicating that azithromycin had no bactericidal activity against exponentially growing P. aeruginosa. However, when the bacteria had grown to stationary phase, the MBC decreased from 128 to 1 μg/ml, suggesting that P. aeruginosa became sensitive to azithromycin (Fig. 1). These data seem to provide the reason for the effectiveness of sub-MICs of macrolide antibiotics in DPB patients. The reason why P. aeruginosa in the stationary phase was more sensitive to azithromycin than in the exponential phase was not found in this study. Recently, it has been reported that there are many differences between the gene expression of P. aeruginosa in the stationary phase and that in the exponential phase (18). Further studies are needed to determine whether such differences in gene expression contribute to this phenomenon.
Polycationic antibiotics, such as aminoglycosides, are reported to disrupt the outer membrane of P. aeruginosa by acting to competitively displace divalent cations that cross-bridge adjacent lipopolysaccharide (LPS) molecules (3, 4). Antagonism of aminoglycosides by divalent cations is well documented for P. aeruginosa (1, 11, 20). Azithromycin is also a dicationic macrolide antibiotic. Farmer et al. (2) showed that azithromycin was capable of permeabilizing the outer membrane of Escherichia coli and that this action was antagonized by Mg2+. We also suspected that the bactericidal effect of azithromycin against P. aeruginosa was due to an aminoglycoside antibiotic-like action. Accordingly, we evaluated the effects of Mg2+ and Ca2+ on susceptibility to azithromycin in PAO1. We found that susceptibility to azithromycin in PAO1 was decreased by Mg2+ or Ca2+ (Fig. 2). These findings suggest that the mechanism of the bactericidal effect involves the displacement of divalent cations that cross-bridge adjacent LPS molecules. In addition, EDTA, which disrupts divalent-cation cross-bridges by chelation (4, 9), caused a similar bactericidal effect against P. aeruginosa (Fig. 3). These data supported the notion that displacement of divalent cations in the outer membrane caused bacterial cell death.
The outer monolayer of the outer membranes of gram-negative bacteria such as P. aeruginosa is constituted of LPS, and the outer membranes exclude hydrophobic compounds, including hydrophobic antibiotics (13) and the fluorescent probe NPN (10). Polycationic antibiotics can interact with the outer membrane at divalent-cation-binding sites on LPS, resulting in permeabilization of the outer membrane to hydrophobic compounds, such as NPN (5, 10). Thus, these probe molecules were utilized to observe the effect of azithromycin on outer membrane permeability. To determine whether azithromycin interacts with the outer membrane, we performed the NPN assay, which is commonly utilized to evaluate the interaction of compounds with the outer membrane. We demonstrated the enhancement of NPN uptake by azithromycin in intact bacterial cells, suggesting that azithromycin permeabilized the outer membrane of P. aeruginosa (Fig. 4A). Furthermore, Mg2+ or Ca2+ inhibited azithromycin-enhanced uptake of NPN (Fig. 5). This could be explained by competition between azithromycin and the divalent cations for a divalent-cation-binding site on the outer membrane.
FIG. 5.
Influence of divalent-cation addition on the azithromycin-promoted rise in NPN fluorescence intensity. At 0 s, 5 μM NPN was added to intact cells. At the arrows, the following agents were added: a, azithromycin (128 μg/ml); b, azithromycin (128 μg/ml) and 1 mM Ca2+; c, azithromycin (128 μg/ml) and 1 mM Mg2+. The data are representative of three separate experiments.
In conclusion, our data indicate that azithromycin has bactericidal activity against P. aeruginosa. Azithromycin directly disrupted the outer membrane of P. aeruginosa, probably by displacement of divalent cations from their binding sites on LPS on the outer membrane. This direct action of azithromycin may contribute to its bactericidal activity against P. aeruginosa.
Acknowledgments
We thank F. G. Issa (Word-Medex, Sydney, Australia) for his assistance with editing the manuscript.
This work was partially supported by a grant from CFF Therapeutics (IGLEWSOOGO).
REFERENCES
- 1.D'Amato, R., F. C. Thornsberry, C. N. Baker, and L. A. Kirven. 1975. Effect of calcium and magnesium ions on the susceptibility of Pseudomonas species to tetracycline, gentamicin, polymyxin B, and carbenicillin. Antimicrob. Agents Chemother. 7:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farmer, S., Z. S. Li, and R. E. Hancock. 1992. Influence of outer membrane mutations on susceptibility of Escherichia coli to the dibasic macrolide azithromycin. J. Antimicrob. Chemother. 29:27-33. [DOI] [PubMed] [Google Scholar]
- 3.Hancock, R. E. 1981. Aminoglycoside uptake and mode of action-with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J. Antimicrob. Chemother. 8:249-276. [DOI] [PubMed] [Google Scholar]
- 4.Hancock, R. E., V. J. Raffle, and T. I. Nicas. 1981. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 19:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancock, R. E., and P. G. Wong. 1984. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob. Agents Chemother. 26:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Homma, H., A. Yamanaka, S. Tanimoto, M. Tamura, Y. Chijimatsu, S. Kira, and T. Izumi. 1983. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest 83:63-69. [DOI] [PubMed] [Google Scholar]
- 7.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 8.Kudoh, S., T. Uetake, M. Hagiwara, L. H. Hus, H. Kimura, and Y. Sugiyama. 1987. Clinical effect of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn. J. Thorac. Dis. 25:632-642. (In Japanese with English abstract.) [PubMed] [Google Scholar]
- 9.Leive, L., V. K. Shovlin, and S. E. Mergenhagen. 1968. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J. Biol. Chem. 243:6384-6391. [PubMed] [Google Scholar]
- 10.Loh, B., C. Grant, and R. E. Hancock. 1984. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 26:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medeiros, A. A., T. F. O'Brien, W. E. Wacker, and N. F. Yulug. 1971. Effect of salt concentration on the apparent in-vitro susceptibility of Pseudomonas and other gram-negative bacilli to gentamicin. J. Infect. Dis. 124(Suppl.):59-64. [DOI] [PubMed] [Google Scholar]
- 12.Neu, H. C. 1983. The role of Pseudomonas aeruginosa in infections. J. Antimicrob. Chemother. 11(Suppl. B):1-13. [DOI] [PubMed] [Google Scholar]
- 13.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Campbell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 15.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 16.Southern, K. W., P. M. Barker, and A. Solis. 27May2003, posting date. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst. Rev.:CD002203. [Online.] [DOI] [PubMed]
- 17.Taubman, S. B., N. R. Jones, F. E. Young, and J. W. Corcoran. 1966. Sensitivity and resistance to erythromycin in Bacillus subtilis 168: the ribosomal binding of erythromycin and chloramphenicol. Biochim. Biophys. Acta 123:438-440. [DOI] [PubMed] [Google Scholar]
- 18.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood, R. E., T. F. Boat, and C. F. Doershuk. 1976. Cystic fibrosis. Am. Rev. Respir. Dis. 113:833-878. [DOI] [PubMed] [Google Scholar]
- 20.Zimelis, V. M., and G. G. Jackson. 1973. Activity of aminoglycoside antibiotics against Pseudomonas aeruginosa: specificity and site of calcium and magnesium antagonism. J. Infect. Dis. 127:663-669. [DOI] [PubMed] [Google Scholar]