Abstract
In this work, we report that gold nanorods coated with hydrophobically-modified mesoporous silica shells not only enhance photoacoustic (PA) signal over unmodified mesoporous silica coated gold nanorods, but that the relationship between PA amplitude and input laser fluence is strongly nonlinear. Mesoporous silica shells of ~14 nm thickness and with ~3 nm pores were grown on gold nanorods showing near infrared absorption. The silica was rendered hydrophobic with addition of dodecyltrichlorosilane, then re-suspended in aqueous media with a lipid monolayer. Analysis of the PA signal revealed not only an enhancement of PA signal compared to mesoporous silica coated gold nanorods at lower laser fluences, but also a nonlinear relationship between PA signal and laser fluence. We attribute each effect to the entrapment of solvent vapor in the mesopores: the vapor has both a larger expansion coefficient and thermal resistance than silica that enhances conversion to acoustic energy, and the hydrophobic porous surface is able to promote phase transition at the surface, leading to a nonlinear PA response even at fluences as low as 5 mJ cm−2. At 21 mJ cm−2, the highest laser fluence tested, the PA enhancement was >12-fold over mesoporous silica coated gold nanorods.
Keywords: Photoacoustic imaging, gold nanorod, mesoporous silica, cavitation, nonlinear optics
Photoacoustic (PA) imaging has garnered widespread interest in recent years due to its ability to image structural and functional properties of biological tissues and organs at depth inside of scattering media. Unlike purely optical techniques such as optical coherence tomography, PA imaging does not rely on ballistic light to create an image and rather exploits diffuse photons to excite acoustic waves that travel back to the specimen surface with minimal scattering.1–8 Advantages in imaging are derived from the ability of optical waves to capture wavelength-dependent optical information and transfer this information to a detector using acoustic waves. In general, a PA response occurs when a molecule or object absorbs directly incident light (typically of a pulsed laser with a few nanoseconds pulse width or modulated continuous wave laser) and converts this energy to acoustic waves through transient thermal expansion.9 In biomedical imaging applications, these acoustic waves are detected outside the body and processed to produce an image of the subsurface absorption profile, or used in sensing applications.1,2,5
While naturally-present biomolecules, like hemoglobin in blood, show endogenous PA contrast,1–3,5 there has been a significant amount of recent work on developing exogeneous contrast agents to increase imaging depth and sensitivity. Such agents can be engineered to enhance the PA response through improved photoacoustic conversion efficiency and increased absorption of excitation light at wavelengths with greater tissue penetration.10–16 Numerous types of exogenous contrast agents have been developed for PA imaging, including organic dyes, organic nanoparticles, graphene, carbon nanotubes, silver nanoparticles, gold nanoparticles and others.10,11,13,16–19 Of these, gold nanostructures are particularly popular because they are biocompatible, possess large absorption coefficients, and have plasmon absorption wavelengths that can be tuned into the near infrared based on the geometry of the nanostructure.10–16,20–22 Gold nanostructure contrast agents have shown great potential for biomedical imaging and have also been used as guide-star targets for optical imaging in scattering tissues through wavefront shaping.23–26
There have been a number of attempts to increase the PA response from gold nanostructures, including doping the shell with iodide and wrapping the gold with graphene oxide and porphyrins.17–19,27,28 Most relevant to this work, Emelianov and coworkers enhanced the PA response from gold nanorods (AuNRs) by adding a silica layer that both decreased thermal resistance at the particle surface and stabilized particle shape retention,22 with reports of three-fold PA enhancement as compared to uncoated AuNRs.12,29–31 The mechanism of acoustic emission was reported as being caused by the thermal expansion of the silica-coated nanorod and surrounding liquid, and the enhancement was associated with an increase in thermal conductance at the Au-silica and silica-water interfaces.
In addition to thermal expansion, cavitation, or the formation and collapse of bubbles on the nanostructure surface, can increase the PA signal significantly at elevated laser fluence and thereby impart a nonlinear characteristic to the signal.32 Cavitation has been demonstrated on AuNRs through the superheating of adjacent water but at laser fluences that are quite high with respect to the maximum permissible exposure for tissue.33–37 In order to reduce the cavitation threshold, Wilson et al. added AuNRs to a low boiling point perfluorocarbon droplet so that localized heating from the AuNRs would cause vaporization of the nanodroplet, leading to a much stronger photoacoustic response than AuNRs alone.38 In another example, Dixon et al. showed nonlinear PA enhancement from microbubbles coated with AuNRs through vaporization of the liquid around the AuNRs.39 Reports by O’Donnell and Pozzo also showed nonlinear enhancement by the combination of gold and microbubbles.40 However, obtaining similar modes of enhancement from sub-100 nm nanoparticles has been challenging.
In this paper, we report a unique contrast agent that is designed to induce cavitation on the surface at low laser fluence and thus impart a nonlinear PA response, while also maintaining a very strong photoacoustic response in the linear regime. Previously, we showed that hydrophobically modified mesoporous silica nanoparticles facilitate acoustic cavitation by stabilizing the formation of gas pockets on the surface.41–45 Here, gold nanorods with plasmon resonance in the NIR were coated first with silica, then covalently modified with hydrophobic alkyl chains. To impart dispersibility in aqueous solvents, the nanoparticles were resuspended with a monolayer of phospholipids (Figure 1). These hydrophobically-functionalized AuNRs showed greatly enhanced PA signal as compared to bare gold or gold coated with unfunctionalized silica, and the response transitioned from linear to non-linear at only 5 mJ cm−2. The presence of vapor bubbles trapped in the pores first increased the PA enhancement at laser fluences below the linear to nonlinear transition, then acted as surfaces to facilitate the liquid-vapor transition of surrounding water.45 The nonlinear PA response can be derived from vaporization and/or bubble oscillation.46,47 The particles showed good stability in biological media, and they exhibited a linear PA response with concentration. Ultimately, we envision the potential applications of these nanoparticles could include absolute measurements of nanoparticle concentration through illuminating the nanoparticles at different fluences in the nonlinear regime, and as guide-stars in photoacoustic guided wavefront shaping where the nonlinear response could lead to improvements in focusing through scattering media.
Figure 1.
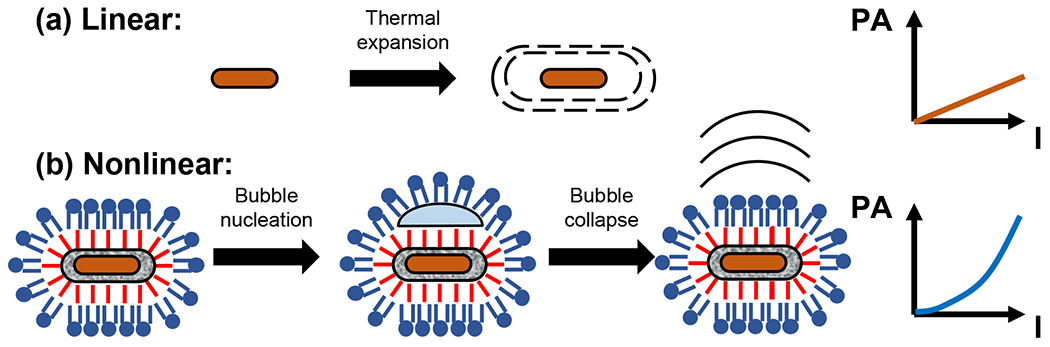
(a) The photoacoustic response of gold nanorods at low laser fluence is produced by the heating of the nanorods and surrounding fluid. If the physical properties of the particle and fluid are not temperature dependent, then a linear relationship between fluence and photoacoustic response is expected. (b) In surface modified silica coated nanorods, vaporization of the liquid surrounding the nanorods leads to bubble formation and a highly nonlinear photoacoustic response. Schematics are not drawn to scale.
Results and Discussion
Because previous research showed that rough, hydrophobic surfaces were optimal for stabilizing nascent bubbles, the synthesis of the nanostructures began by coating nanorods with mesoporous silica.12,22,29,30,45,48 First, bare gold AuNRs were synthesized from gold (III) trihydride, sodium oleate, and cetrimonium bromide (CTAB) by a seed-mediated process developed by Ye et al (Figure 2a).21 The aspect ratio of the rods was controlled using the pH of the growth solution. The concentration was measured by using the localized surface plasmonic resonance (LSPR) of ~765 nm and the extinction coefficient was determined from the peak wavelength (Figure 2d).49 Next, a modified Stöber method was used to coat the AuNRs with mesoporous silica to create MSiO2-AuNRs.48,50 After diluting the AuNRs to 1 nM, the colloidal solution was basified to pH~11 and tetraethylorthosilicate (TEOS) was added to produce a silica coating of ~14 nm (Figure 2b). The UV-Vis spectra (Figure 2d) showed that coating the AuNRs with mesoporous silica resulted in a red shift of ~15 nm. At the same time, dynamic light scattering (DLS) measurements showed an increase in particle diameter from 34±15 nm to 75±30 nm, though the AuNR value is likely underestimated due to the presence of excess CTAB micelles (Table S1).51,52 The zeta potential showed a large change from +24±1.7 mV to −41±1.6 mV, consistent with replacement of the CTAB stabilizing ligand with a silica shell.53
Figure 2.
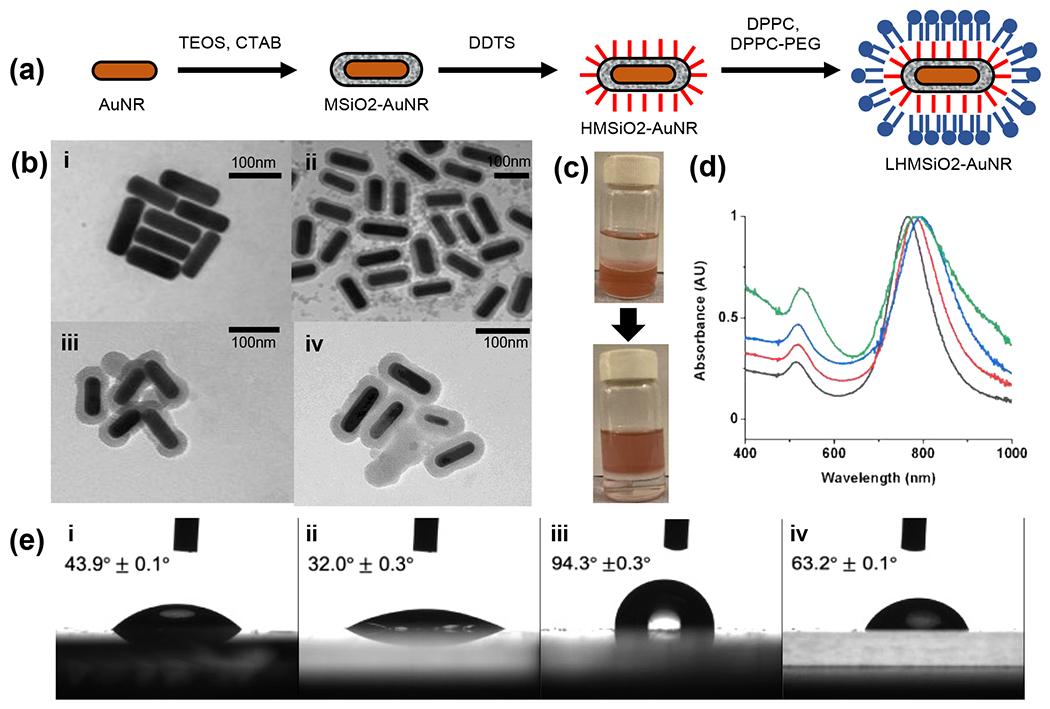
(a) Scheme for synthesis of lipid-coated, hydrophobically-modified mesoporous silica shells on gold nanorods (LHMSiO2-AuNR). (b) TEM images of (i) gold nanorods (AuNR), (ii) silica coated gold nanorods (MSiO2-AuNR), (iii) hydrophobically modified silica coated gold nanorods (HSiO2-AuNR), and (iv) lipid-coated hydrophobically-modified silica coated gold nanorods (LHMSiO2-AuNR). (c) Photographs of biphasic reaction transfer of MSiO2-AuNR from the acidic methanol layer into the hexane layer through DDTS functionalization to HMSiO2-AuNR. (d) Normalized UV-Vis spectra of AuNR (black curve), MSiO2-AuNR (red curve), HSiO2-AuNR (blue curve), and LHMSiO2-AuNR (green curve). (e) Contact angle measurements of (i) AuNR, (ii) MSiO2-AuNR, (iii) HSiO2-AuNR, and (iv) LHMSiO2-AuNR.
Next, a hydrophobic coating was deposited on the MSiO2-AuNRs to promote bubble stability during the cavitation process.45 The remaining bound CTAB was first removed with an acid wash, followed by resuspending the cleaned MSiO2-AuNR across a biphasic mixture of acidified methanol and hexane with dodecyltrichlorosilane (DDTS). Successful hydrophobic modification of the silica coating by DDTS was initially evidenced by a colorimetric phase transfer of the MSiO2-AuNRs from methanol to hexane (Figure 2c). UV-Vis analysis of the hydrophobically modified MSiO2-AuNRs (HMSiO2-AuNRs) showed a shift in the LSPR of about ~15 nm relative to the MSiO2-AuNR before surfactant extraction (Figure 2d), though this spectrum was obtained in chloroform due to insolubility in water. Beyond solubility profile, evidence for successful hydrophobic modification was found by depositing a film of the functionalized AuNRs on a glass surface and measuring the water contact angle (Figure 2e ii and iii). After addition of dodecyl groups to the nanoparticles, the static contact angle of the particles changed from 32.0° ± 0.3° for the MSiO2-AuNR to 94.3° ± 0.3° for the HMSiO2-AuNRs (HMSiO2-AuNR) (Figure 2). In addition, FTIR spectroscopy was performed to verify the hydrophobic modification. As shown in Figure S1, peaks were observed for C-H stretching at 2915 cm−1 and 2854 cm−1 and C-H bending at 1376 cm−1. Finally, an increase in average particle size was found by DLS measurements, which showed an increase from 75±30 nm to 137±61 nm, which indicates some possible agglomeration by the particles (Table S1). The zeta potential decreased slightly from −41±1.6 mV to −34±0.8 mV, which is consistent with changing a partially anionic silica layer to the net negatively charged DPPC/DPPC-PEG layer.
Finally, in order to disperse the HMSiO2-AuNR in aqueous media prior to photoacoustic analysis, the hydrophobic particles were coated in a lipid monolayer consisting of 1,2-dipalmitoylphosphatidylcholine (DPPC) and DPPC-polyethylene glycol (DPPC-PEG). Briefly, the HMSiO2-AuNRs were combined with DPPC and DPPC-PEG lipids in chloroform, dried, and rehydrated at 75°C in water. Addition of the lipid monolayer to the surface of the hydrophobically modified mesoporous silica coated gold nanorods (LHMSiO2-AuNR) significantly reduced the static contact angle from 94.3° ± 0.3° down to 63.2° ± 0.1° for the LHMSiO2-AuNR, which is consistent with the addition of an amphiphilic lipid to a hydrophobically modified surface. In addition, FTIR spectroscopy showed the presence of a lipid monolayer added to the surface of the HMSiO2-AuNR. As shown in Figure S1, FTIR peaks for DPPC and DPPC-PEG were observed at 1467 cm−1, 1737 cm−1, 2854 cm−1, and 2916 cm−1, corresponding to both C-H and C=O ester peaks.
The photoacoustic signals generated from irradiation of AuNRs, MSiO2-AuNRs, and LHMSiO2-AuNRs were next measured in a standard PA setup (Figure S2). Particles dispersed in water at a concentration of 0.45 nM were flowed at a rate of 80 μL min−1 through a polyethylene tube of 200 μm inner diameter and homogenously irradiated with a nanosecond pulsed laser beam of radius 1.6 mm. Single shots were recorded and averaged 500-1000 times to obtain mean PA signals; examples at 19 mJ cm−2 are shown in Figure S3. Mean peak-to-peak PA signals (Vpp) as a function of laser fluence were obtained for AuNR (green), MSiO2-AuNR (red), and LHMSiO2-AuNR (black) samples, as well as for the PE tube (magenta) filled with water solvent as a negative control (Figure 3). As expected, both AuNR and MSiO2-AuNR showed linear trends of PA amplitude vs. fluence up through 21 mJ cm−2, the largest fluence tested (Figure 3b). In contrast, the trend for LHMSiO2-AuNR particles was distinctly non-linear, fit by 3rd order polynomial (Figure 3b). Without any gold component, no PA response was observed over the tested laser fluence range (Figure S4).
Figure 3.
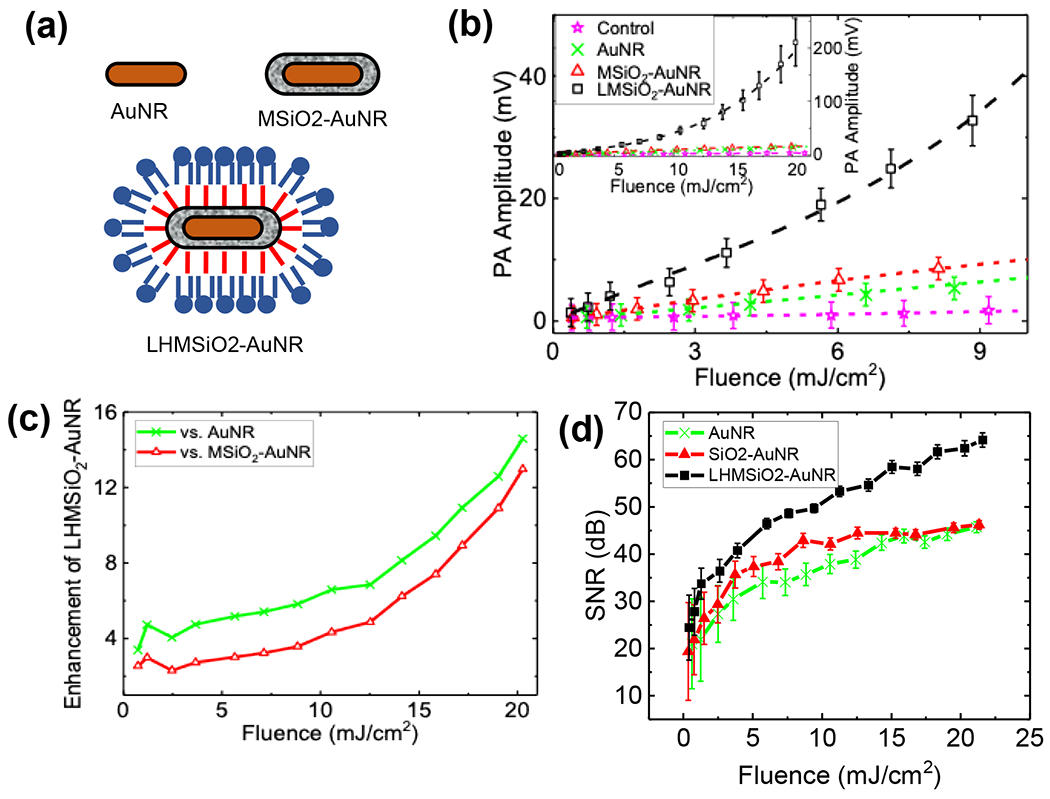
(a) Cartoon schematic of structures tested. (b) PA peak-to-peak amplitude (Vpp) as a function of laser fluence recorded for AuNR (green cross), MSiO2–AuNR (red triangles), and LHMSiO2–AuNR (black squares) particles in water. Signal from the control (PE tube filled with water) is displayed in magenta stars. All particle concentrations were 0.45 nM. Inset shows the results for a wider fluence range. Error bars show one standard deviation in both directions to the mean Vpp; all samples were run in triplicate. The connecting dashed lines show linear fits for AuNR in green, MSiO2–AuNR in red and for control in magenta. A third order polynomial fit for LHMSiO2–AuNR is displayed in black dashed line. Student’s t-test for significance showed p < 0.05 for LHMSiO2-AuNR vs. MSiO2-AuNR at all fluences tested. (c) Enhancement in PA response of LHMSiO2-AuNR vs AuNR (green cross) and MSiO2-AuNR (red triangles) as a function of laser fluence obtained by dividing corresponding amplitudes in Vpp. (d) Signal-to-noise ratios (SNRs) of each sample tested vs. laser fluence. The error bars represent the propagated standard deviations of the signal and noise.
Two trends were immediately apparent: the LHMSiO2-AuNR displayed linear behavior at about 5 mJ cm−2, and at higher laser fluences the signal increased in a nonlinear manner (Figure 3c). Within the linear portion, the LHMSiO2-AuNR possessed a stronger response to the laser fluence than either MSiO2-AuNR or AuNR, by 2-fold and 3-fold respectively, which was confirmed by comparing the slopes of the PA vs. fluence curve within the linear regime. We hypothesize that the enhancement at lower fluences is caused by the presence of solvent vapor contained within the hydrophobically-modified mesoporous silica pores. At room temperature, air has a thermal expansion coefficient β of ~3 x10−3 K−1, whereas silica’s would be closer to ~3 x10−5 K−1. Thus, increasing the nanorod temperature by laser induced heating may be expected to produce adjacent expansion of encapsulated water vapor that would push against the surrounding medium more strongly, leading to a larger acoustic response. Alternatively, water vapor has a lower thermal conductivity (~0.3 W m−1 K−1) than silica (1.3 W m−1 K−1), which could contribute to the increased PA response.
When fitted to the form of Ax + Bxd, where the Ax comprises the linear component and Bxd comprises the nonlinear component, the PA signal contained a power term, d = 3.17±0.16, with an adjusted R2 = 0.999 (Figure S5, Table S2). Additionally, a residual sum of the squares (RSS) analysis was performed to compare linear, quadratic, and cubic fits; in doing so, the RSS was reduced from 3571 to 172 to 21, respectively (Table S2). Thus, a cubic fit was most effective at accounting for the relationship between PA response and input laser fluence. The power term is also consistent with that found by Dixon et al,39 in which liquid vaporization was facilitated by the presence of microbubbles. The response of LHMSiO2-AuNRs in the nonlinear range resulted in a much stronger photoacoustic response when compared to bare or silica-coated nanorods, which both gave a linear PA response with laser fluence over the whole fluence range (Figure S6). The enhancement increased with laser fluence in the nonlinear regime to a final value of ~13-15 fold at 21 mJ cm−2. Similarly, the signal-to-noise ratio also increased significantly for LHMSiO2-AuNR, with a value of 64 dB at 21 mJ cm−2 as compared to 46 dB for both AuNR and MSiO2-AuNR (Figure 3d). In both the linear and nonlinear regimes, the signal enhancement is significant and would translate to improved imaging sensitivity and reduced measurement time.
As observed in the plots in Figure 3, the nonlinear transition in the PA response for the LHMSiO2-AuNR particles was estimated to occur at approximately 5 mJ cm−2, which is associated with the onset of a liquid-gas phase transformation in the liquid surrounding the nanoparticle. In these studies, AuNR and MSiO2-AuNR particles did not show evidence of cavitation through a non-linear PA response, indicating that the threshold for cavitation was higher than the fluence range considered in these studies. Both Dixon’s and our work observed a transition away from linearity at a similar fluence range, which they attributed to solvent vapor nanobubble formation.39 Here, the presence of rough, hydrophobic surfaces and the corresponding presence of pre-existing gas nuclei reduce the nucleation energy barrier and allow heterogeneous nucleation of solvent vapor. The phase transition of vapor, rather than expansion of air, was shown in a comparison study between LHMSiO2-AuNR in air-saturated and degassed water, which showed little difference in terms of PA response (Figure S7). Once phase transformation takes place, a gas bubble can grow rapidly (and potentially collapse) to produce a strong photoacoustic response. The nucleation and growth of a bubble is stochastic and its probability depends on the thermodynamic conditions of a system, such as temperature, gas concentration, and properties of the nucleating surface.54,55 Murray32 and others56,57 have shown that without an existing gas cavity, laser induced bubble formation begins when the superheated liquid around the nanoparticle approaches the spinodal temperature of water around 550 K. Our results indicate that the local nanoparticle surface conditions can promote heterogeneous nucleation at lower temperatures (decreased laser fluence), which have a dramatic effect on the PA response. When a single volume of LHMSiO2-AuNRs was irradiated as above and the PA response was monitored at the single shot level, there was an initial decrease in signal followed by a stochastic PA response to the laser (Figure S8). We interpret this result that the pre-existing gas bubbles are initially exhausted, but at later shots, vaporization and then nucleation of new bubbles can take place.
The concentration dependence of the photoacoustic response of the nanoparticles was also explored. Three concentrations of LHMSiO2-AuNRs were prepared: 0.11 nM (1X), 0.21 nM (2X), and 0.32 nM (3X). Not surprisingly, the PA response increased with nanostructure concentration, as more particles were irradiated in a given unit volume (Figure 4). At most laser fluences tested, the enhancement followed the ratio of particle concentrations closely; for example, 3X/2X produced an enhancement of ~1.5. Thus, dependence of PA signal on concentration was found to be roughly linear. This result provides evidence that the observed PA enhancement is likely not due to particle-particle interactions such as overlapping thermal fields or the presence of larger gas pockets stabilized by multiple nanoparticles, as we would expect both effects to be enhanced at higher concentrations. Because PA response appears linear in concentration but nonlinear in fluence, these nanoparticles may potentially be used to track absolute nanoparticle concentration within the body by, for example, measuring the PA response at two different fluences.58,59
Figure 4.
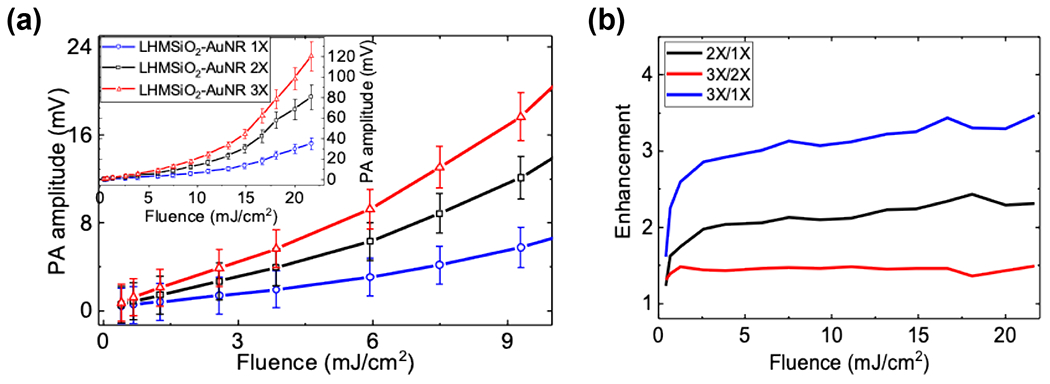
(a) PA response (Vpp) of LHMSiO2-AuNR at varying concentrations: 1 X (0.11nM, blue circles), 2X (0.21nM, black squares) and 3X (0.32nM, red triangles). Inset shows the same results at a broader fluence range. Error bars show 1 standard deviation in both directions to the mean Vpp. (b) Enhancement of PA signals for 2X/1X (black), 3X/2X (red) and 3X/1X(blue).
To see how the particles might respond in an environment more akin to the human body, the PA responses of LHMSiO2-AuNR were tested in phosphate buffer saline (PBS), human plasma (HPL), DI water, and bovine serum albumin (BSA) solution. The sample of LHMSiO2-AuNR dispersed in PBS had a PA response that was similar to that obtained in water (Figure 5). However, when LHMSiO2-AuNRs were dispersed in HPL or BSA solution, a PA response was recorded that showed greater nonlinearity, ultimately reaching a two-fold greater PA response than samples in water. This enhancement effect appears to be medium-dependent: AuNRs and MSiO2-AuNRs tested in HPL showed a similar two-fold enhancement in PA (Figure S9), which we hypothesize occurs because proteins adhering to the nanoparticle surface increase the thermal resistance in the nanorods. Enhancement against AuNR and MSiO2-AuNR was similar, ultimately reaching 17- and 11-fold enhancement respectively at 21 mJ cm−2. The LHMSiO2-AuNRs also possess low cytotoxicity: after incubation for 48 h and performing an MTT assay, MDA-MB-468 breast cancer cells showed 81±1.2%, 87.3±0.44%, and 94.2±0.5% cell viability at 0.45, 0.045, and 0.0045 nM LHMSiO2-AuNR, respectively (Figure S10).
Figure 5.
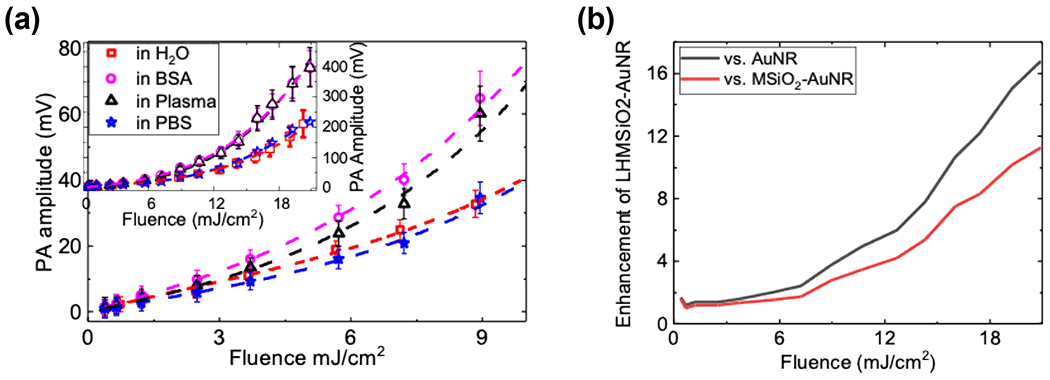
PA response (Vpp) plotted as a function of laser fluence for LHMSiO2-AuNR sample dispersed in water showed in red filled squares; in Bovine Serum Albumin (BSA) displayed in magenta circles; in human plasma (HPL) displayed in black triangle; and in phosphate buffer solution (PBS) displayed in blue star. Dotted lines are from a 3rd order polynomial fit. The inset shows the same results for an extended range of fluences. Error bars show one standard deviation above and below the mean value.
Based on these studies, the likeliest source of heterogeneous nucleation is the entrapment of vapor bubbles inside the hydrophobically-modified pores, which was demonstrated by Goodwin and co-workers for hydrophobically-modified mesoporous silica nanoparticles.26–30 If encapsulated vapor were primarily responsible for nonlinear enhancement, rather than the addition of surfactants capable of stabilizing nascent bubbles, then the addition of lipids without covalent hydrophobic particle modification would not impart significant nonlinearity to the particles. To test this theory, we compared the PA response of LHMSiO2-AuNR with that of MSiO2-AuNR stabilized with a lipid bilayer (LBMSiO2-AuNR), which is known to form around silica nanoparticles to improve their stability and biocompatibility.60 To determine if hydrophobic modification was necessary for non-linear PA response, a lipid bilayer was added to the MSiO2-AuNR in method similar to that reported in Liu, et al.60 Briefly, MSiO2-AuNRs were mixed with rehydrated liposomes of DPPC and DPPC-PEG in 0.5X PBS. Static contact angle measurements were performed to characterize the surface of lipid bilayer MSiO2-AuNR, which showed a decrease in contact angle from 23.0° ±1.0° to 17.1° ±0.2° for the surfactant extracted MSiO2-AuNR and LBMSiO2-AuNR, respectively. The decrease was likely caused by the zwitterionic phosphocholine producing a more hydrophilic surface. Additionally, the negative charge on nanorods became stronger, with zeta potential changing from −26.1 mV to −31.1 mV. Finally, FTIR spectroscopy showed peaks that closely matched with the peaks that were seen in the LHMSiO2-AuNR sample: a C=O stretch at 1738 cm−1, C-H bending at 1467 cm−1, and C-H stretches at 2854 cm−1 and 2915 cm−1 (Figure 6a). These results were distinguished from the LHMSiO2-AuNR by the lower intensity of the C-H stretches.
Figure 6.
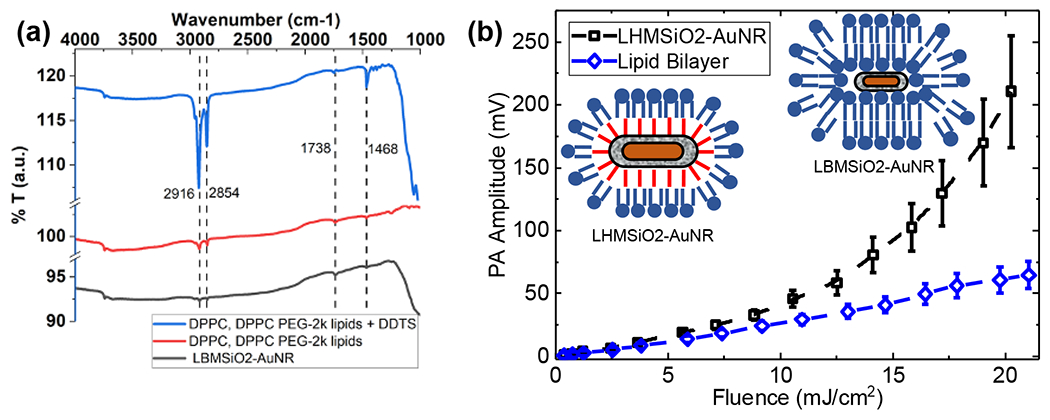
(a) FTIR spectra showing the presence of lipid bilayers on LBMSiO2-AuNR. (b) PA response as a function of fluence observed for LHMSiO2-AuNR particles coated with lipid bilayer (blue circles). PA response of LHMSiO2-AuNR (lipid monolayer) is also displayed for comparison. Water was used as the solvent in both cases. Error bars show one standard deviation above and below the mean value. Student’s t-test for significance showed p < 0.05 for LHMSiO2-AuNR vs. LBMSiO2-AuNR at all fluences tested.
Once the lipid bilayers were in place, the sample was subjected to the same PA response studies. As seen in Figure 6b, while LHMSiO2-AuNR achieves a nonlinear value, the LBMSiO2-AuNR sample is still linear. Thus, the hydrophobic modification plays a dominant role in the nonlinear relationship between PA response and laser fluence. The hydrophobic modification also improved the stability of the irradiated rods. As shown in Figure S11, TEM images of LHMSiO2-AuNRs recovered from water one month after irradiation showed little degradation of either the gold (from melting) or silica (from hydrolysis). In contrast, AuNRs showed significant deformation, as expected from melting by laser heating. Similarly, while silica coatings on MSiO2-AuNR were completely dissolved within 5 days, the LHMSiO2-AuNR showed no degradation within one week (Figure S12).
In conclusion, the lipid-coated, hydrophobically-modified gold nanorods (LHMSiO22-AuNR) presented here exhibit a nonlinear PA response with laser fluence at relatively low laser fluences. In functionalizing nanorods with hydrophobic, porous silica, vapor channels could be maintained in the pores that could facilitate the liquid-vapor transition of surrounding solvent and promote nonlinear enhancement. At low fluences, enhancement was observed due to either the increased thermal expansion coefficient or increased thermal resistance of the rods.29,61 At higher fluences, the data fit exponential curves that were consistent with data reported for microbubbles. The reported nanoparticles showed an approximately linear relationship between PA response and concentration, which allows the response to be distinguished in terms of either laser fluence or concentration. Finally, the LHMSiO2-AuNRs exhibited strong enhancement in human plasma, which shows promise for future in vivo imaging studies. Overall, this approach of hydrophobic functionalization of a mesoporous silica shell can be applied to many different types of nanostructures and represents a potential platform technology for imparting nonlinearity to other photoacoustic contrast agents at laser fluences compatible with in vivo imaging.
Methods
Synthesis of gold nanorods (AuNRs).
Gold nanorods were produced with a method that required the production of separate growth and seed solutions.17 The growth solution was was started by combining 0.7 g of cetrimmonium bromide (CTAB) and 25 mL of ultrapure water and dissolved by heating. Next, 0.1234 g of sodium oleate (NaOL) was added and dissolved by heating the solution. The solution was then cooled to 30°C for 15 min. 2.4 mL of 4 mM silver nitrate was added and left undisturbed for 15 min. Finally, 25 mL of 1 mM gold (III) trihydride HAuCl4 was added to the solution, which was mixed at 200 rpm for 90 min at 30°C.
While the growth solution was mixing, the seed solution was made by adding 1 mL of 0.5 mM HAuCl4 and 1 mL of 0.2 M CTAB to a 4 mL borosilicate vial. 200 μl of 0.0006 M NaBH4 in ice cold water is then added to the seed solution and stirred vigorously for 2 min. The seed solution was then aged for 30 min at RT. When the seed solution became clear, 175 μL of 37% hydrochloric acid (HCl) was added and the solution was mixed for 15 min. 125 μL of a 0.064 M ascorbic acid was added to the growth solution and the growth solution was stirred vigorously at room temperature for 30 s. Finally, 40 μL of the seed solution was added to the growth solution and left at 30°C for 12 h. After the reaction, the gold nanorods were centrifuge washed twice at 7500g for 20 min to remove excess CTAB.
Synthesis of mesoporous silica coated gold nanorods (MSiO2-AuNRs).
The AuNRs were diluted to 1 nM with water and 90 μl of 20 mM CTAB to create a 1 mL aqueous suspension of 1 nM AuNRs. The colloidal suspension was mixed for 12 h in a 4 mL borosilicate vial. 10 μl of 0.1 M sodium hydroxide (NaOH) was added, and the suspension was stirred vigorously for 30 min. 36 μl of a 5% volume solution of tetraethylorthosilicate (TEOS) dispersed in isopropoyl alocohol (IPA) was added to the suspension in four 9 μL doses over a 90 min period. The solution was then mixed overnight and centrifuged at 6708g for 15 min. To redisperse the nanoparticles, methanol was added, and the mixture was bath sonicated.
Extraction of CTAB from MSiO2-AuNRs.
80 μL of 37% HCl was added to 10 mL of MSiO2-AuNRs dispersed in methanol in a 20 mL borosilicate vial. The suspension was heated to and mixed at 65°C for 1 h. The sample was then centrifuged at 6708g for 15 min. The pellet was redispersed in methanol and the centrifuge washing was repeated twice to neutralize the sample for later storage.
Synthesis of hydrophobically modified mesoporous silica coated gold nanorods (HMSiO2-AuNRs).
80 μL of 37% HCl was added to a suspension of MSiO2-AuNRs dispersed in methanol. 8 mL of hexane was added to the solution. 500 μL dodecyltrichlorosilane was then added in five 100 μL portions to the mixture, which was capped and sealed with Parafilm and mixed vigorously for 5 h. The sample was then centrifuge washed twice with hexane and twice with chloroform. The sample was re-dispersed in 8 mL of chloroform and stored as a suspension.
Preparation of lipid-coated hydrophobically modified mesoporous silica coated gold nanorods (LHMSiO2-AuNRs).
300μL DPPC (4 mg mL−1) and 200 μL DPPC-PEG2000 (2 mg mL−1), each of which was dispersed in chloroform, then added to a suspension of HMSiO2-AuNRs in chloroform. The sample was evaporated at 65°C, re-dispersed in 8 mL of water, then mixed for 45 min at 65°C. The sample was collected as a pellet by centrifugation at 6700g and redispersed in water for PA measurement.
Preparation of lipid-bilayer mesoporous silica coated gold nanorods (LBMSiO2-AuNRs).
500 μL of 4 mg mL−1 DPPC in chloroform was added to 300 μL of 2 mg mL−1 DPPC and DPPC-PEG dispersed in chloroform. These samples were then dried under a stream of inert argon until the chloroform was evaporated. The liposomes were rehydrated in 1 mL 0.5X PBS at ~60°C for 1 h. The liposomes were sonicated with a probe sonicator for 1 min in a 10 second on and 10 second off cycle. 2 mL of CTAB-extracted MSiO2-AuNR suspension (see above) was centrifuged at 6708g for 15 min and redispersed in 0.5X PBS. The liposomes were then added to the CTAB-extracted MSiO2-AuNR and left at room temperature for 1 h. The lipid bilayer MSiO2-AuNR was then centrifuged at 6700g for 15 min and centrifuge washed an additional two times with water.
Characterization of nanorods by UV-Vis spectroscopy.
The AuNR concentration was first determined utilizing the maximum absorbance peak to determine the extinction coefficient from Orendorff et. al.49 The concentrations of other AuNR derivatives were then determined assuming AuNR as the only absorber and scatterer in the NIR.
FTIR measurements.
The FTIR measurements were performed using a Nicolet 6700 ATR. The colloidal solution was deposited onto a clean microscope glass slide, and then dried in an oven at 85 °C overnight. The sample was then placed on an ATR crystal and analyzed.
Contact angle measurements.
The contact angle measurements were performed utilizing a Rame-Hart 210-U1 Goniometer. To form the surfaces for measurement, the colloidal solutions were deposited onto a clean glass microscope slide, and then dried at 85 °C overnight to remove excess solvent.
Transmission electron microscopy (TEM) images.
TEM images were taken with a Technai T12 Spirit.
Measurements of dynamic light scattering (DLS) and zeta potential.
For DLS, 1 mL of AuNR, MSiO2-AuNR, or LHMSiO2-AuNR in ultrapure water was added to a disposable semi micro UV-cuvette. Using a Litesizer 500 with the particle size software, measurements were performed at 25°C. For zeta potential measurements, 300 μL suspension was added to an Omega cuvette Using a Litesizer 500 with the zeta potential analysis software, the zeta potential was measured at 25°C utilizing the Smoluchowski approximation..
Measurements of photoacoustic response.
Photoacoustic response of the synthesized particles was analyzed in a standard setup (Figure S2). An Optical Parametric Oscillator pumped by a Q-switched laser (model: SLI-20, Continuum Electro-Optics, Inc. 3150 Central Expressways, Santa Clara CA95051) with pulse repetition rate 20 Hz, tuned to operate at a chosen wavelength corresponding to the peak absorption of the sample (S) flowing through a transparent polyethylene tube (SCI Cat. # BB31695-PE/C 0.20mm x 0.36mm - inner x outer diameter) lie at the center of laser beam waist of radius 1.4 – 1.6 mm (at 1/e), at the focus of a lens (L3, focus = 25 cm). A mirror (M) was used to direct beam towards the sample and an engineered diffuser (D) was used to homogenize laser beam profile in order to avoid hotspots. Filter (F) blocks the fundamental (532 nm) wavelength from laser and allows to pass near infrared laser wavelengths. L1 and L2 (5 cm and 15 cm focal lengths, respectively) was used to expand the incoming beam to obtain a desired beam waist at the sample location. Wave plate (WP) along with the polarizing beam splitter cube (PBS) were used to control the laser energy hits in the sample. N is a neutral density filter for reducing laser power to an energy meter (EM, Thorlabs PM100D console and ES111C energy sensor).
A syringe pump (Harvard Apparatus, PhD Ultra) was in place to infuse particles in solution through the 200 μm inner diameter polyethylene tube (Cat.# BB31695-PE/C; Dimensions: I.D. x O.D.: 0.20 mm x 0.36 mm; Scientific Commodities, 2800 Sweetwater Ave., A105, Lake Havasu City, AZ 86406) in a controlled flow rate of 80 μL min−1, which was calculated to avoid repeated exposure of a flowing particles to subsequent laser pulses. For the single-shot fatigue experiments, no flow was applied in the tubing (see Figure S8). For long-term stability studies, S was collected post-irradiation for later analysis.
An ultrasound transducer (T), with focal length 19 mm and 10 MHz central frequency (Panametrics, V327) was used to capture the PA signal emitted by S. A water bath was used to facilitate acoustic coupling between S and T. Acoustic signals corresponding to each laser shots converted into electric pulses by T was pre-amplified (FEMTO, high speed GHz amplifier HSA-Y), and finally detected and digitized using an oscilloscope (Telydyne Lecroy, Model HDO4032) and stored into a computer. The EM was used to monitor pulse to pulse laser energy and a photodetector (PD) exposed to the beam reflected through a 2 mm thick microscope slide glass plate (G) was used to trigger the oscilloscope. A home-made data acquisition software using LabVIEW (NI, 11500 N Mopac Expwy, Austin, TX 78759-3504) was used to automize the experiments.
In order to ensure that the sample would behave as homogenous and randomly packed point absorbers, the particles were flowed through a transparent tube of inner diameter 200 μm and quasi-homogeneously irradiated by Gaussian profile laser pulses of 3 mm in diameter.62 In order to obtain PA signals as a function of fluence, laser pulse energy was adjusted using a waveplate combined with a polarizing beam splitter. For each sample, irradiation fluence was varied from 1 to 25 mJ cm−2 to match clinically relevant fluence levels. PA response for up to 1000 single shots of laser irradiation was captured at the lowest fluences to enhance the signal to noise ratio (SNR). The number of averages were decreased as the fluence level increased so that for the highest fluence only 300 single shot responses were captured. The tube wall absorption was negligible as evidenced by signals measured in water alone (Figure S3).
Degassed water experiments.
Water was degassed by heating water until it boiled and then letting it cool back to RT. The water was then sealed with Parafilm with minimal headspace. A suspension LHMSiO2-AuNR was then created as reported above, except the particles were washed 3x with degassed with water. The degassed water experiments were performed the same way as the main photoacoustic experiments.
Statistical analysis.
Utilizing Origin 2020b (OriginLab), a best fit was performed utilizing a nonlinear curve fit of Ax+Bx^d. In this statistical analysis, the goodness of fit was determined (R^2), as well as the residual sum of squares (RSS) for the nonlinear curve as well as to a linear, quadratic, and a cubic fit.
MDA-MB-468 Cell Viability Assay for LHMSiO2-AuNR Nanoparticles.
MDA-MB-468 breast carcinoma cells were procured from the American Type Culture Collection (ATCC) and grown to confluence in 75 cm2 culture flasks at 37°C under 5% CO2 in the presence of Dulbecco’s Modified Eagle Media (DMEM, with glucose and glutamine, phenol red but no sodium pyruvate, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin-streptomycin (Thermo Fisher). The cells were then trypsinized with 0.025% Trypsin-EDTA (Gibco), seeded in 12 wells of a 96 well cell culture plate with 10000 cells/well seeding density, and grown for 48 h. Next, cells were washed with 100 μL PBS twice, and then 100 μL of 0.45, 0.045, or 0.0045 nM of LHMSiO2-AuNR nanoparticle solution in DMEM media was added to the wells. Each nanoparticle concentration variant was studied in triplicate. As a control, fresh media was added instead of the nanorod suspension. After 48 h of incubation, cells were washed with 100 μL PBS thrice. 100 μL of 0.5 mg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was adding to the media in each of the wells. After incubating MTT solution to the cells for 4h, the formazan crystals were dissolved by adding isopropanol. Then UV absorbance of the wells was measured in a SynergyH1 microplate reader (BioTek, USA). Each of the absorbance values at 560 nm were background corrected by subtracting the reading at 650 nm from that of the 560 nm reading. Then, considering the UV absorbance of the control cells as 100% viable, the viability fraction of other variants was calculated.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge NIH R21EB027319 and NSF CBET 2025547 for support of this research. The authors thank Prof. Will Medlin for use of his FTIR spectrometer. Finally, the authors thank Fan Yang and Dr. Nicholas Blum for helpful discussions regarding silica-coated nanorod synthesis.
ABBREVIATIONS
- AuNR
gold nanorod
- BSA
bovine serum albumin
- CTAB
cetrimonium bromide
- DDTS
dodecyltrichlorosilane
- DI
deionized
- DPPC
1,2-dipalmitoylphosphatidylcholine
- FTIR
Fourier Transform Infrared
- HCl
hydrochloric acid
- HPL
human plasma
- HSiO2-AuNR
hydrophobically modified silica coated gold nanorods
- LBMSiO2-AuNR
lipid bilayer stabilized mesoporous silica coated gold nanorod
- LHMSiO2
lipid-coated, hydrophobically-modified mesoporous silica
- LSPR
localized surface plasmonic resonance
- MSiO2
mesoporous silica
- MSiO2-AuNR
mesoporous silica coated gold nanorods
- NaOL
sodium oleate
- PA
photoacoustic
- PBS
phosphate buffered saline
- PEG
polyethylene glycol
- TEM
transmission electron microscopy
- TEOS
tetraethylorthosilicate
- UV-Vis
UV and visible
- Vpp
peak-to-peak voltage
- WP
waveplate
Footnotes
Supporting Information. Additional FTIR spectra of nanorods, schematic of photoacoustic setup, typical pulse shapes, fits of PA vs. laser fluence data to models, PA response for control samples, PA response of sample without flow, TEM images of irradiated rods.
REFERENCES
- (1).Beard P Biomedical Photoacoustic Imaging. Interface Focus 2011, 1 (4), 602–631. 10.1098/rsfs.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Manohar S; Dantuma M Current and Future Trends in Photoacoustic Breast Imaging. Photoacoustics 2019, 16, 100134. 10.1016/j.pacs.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Emelianov SY; Li P-C; O’Donnell M Photoacoustics for Molecular Imaging and Therapy. Phys. Today 2009, 62 (8), 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Laufer JG; Zhang EZ; Treeby BE; Cox BT; Beard PC; Johnson P; Pedley B In Vivo Preclinical Photoacoustic Imaging of Tumor Vasculature Development and Therapy. J. Biomed. Opt 2012, 17 (5), 1. 10.1117/1.JBO.17.5.056016. [DOI] [PubMed] [Google Scholar]
- (5).Wang LV; Hu S Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335 (6075), 1458–1462. 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zhang HF; Maslov K; Stoica G; Wang LV Functional Photoacoustic Microscopy for High-Resolution and Noninvasive in Vivo Imaging. Nat. Biotechnol 2006, 24 (7), 848–851. 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- (7).Conkey DB; Caravaca-Aguirre AM; Dove JD; Ju H; Murray TW; Piestun R Super-Resolution Photoacoustic Imaging through a Scattering Wall. Nat. Commun 2015, 6 (1), 7902. 10.1038/ncomms8902. [DOI] [PubMed] [Google Scholar]
- (8).Xu M; Wang LV Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum 2006, 77 (4), 041101. 10.1063/1.2195024. [DOI] [Google Scholar]
- (9).Xia J; Yao J; Wang LV Photoacoustic Tomography: Principles and Advances. Electromagn. Waves Camb. Mass 2014, 147, 1–22. 10.2528/pier14032303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Weber J; Beard PC; Bohndiek SE Contrast Agents for Molecular Photoacoustic Imaging. Nat. Methods 2016, 13 (8), 639–650. 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- (11).Upputuri PK; Pramanik M Recent Advances in Photoacoustic Contrast Agents for in Vivo Imaging. WIREs Nanomedicine Nanobiotechnology 2020, 12 (4). 10.1002/wnan.1618. [DOI] [PubMed] [Google Scholar]
- (12).Kang J; Kim D; Wang J; Han Y; Zuidema JM; Hariri A; Park J-H; Jokerst JV; Sailor MJ Enhanced Performance of a Molecular Photoacoustic Imaging Agent by Encapsulation in Mesoporous Silicon Nanoparticles. Adv. Mater 2018, 30 (27), 1800512. 10.1002/adma.201800512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Luke GP; Yeager D; Emelianov SY Biomedical Applications of Photoacoustic Imaging with Exogenous Contrast Agents. Ann. Biomed. Eng 2012, 40 (2), 422–437. 10.1007/s10439-011-0449-4. [DOI] [PubMed] [Google Scholar]
- (14).Li W; Chen X Gold Nanoparticles for Photoacoustic Imaging. Nanomed. 2015, 10 (2), 299–320. 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Manohar S; Ungureanu C; Van Leeuwen TG Gold Nanorods as Molecular Contrast Agents in Photoacoustic Imaging: The Promises and the Caveats: GOLD NANORODS IN PHOTOACOUSTIC MOLECULAR IMAGING. Contrast Media Mol. Imaging 2011, 6 (5), 389–400. 10.1002/cmmi.454. [DOI] [PubMed] [Google Scholar]
- (16).Wu D; Huang L; Jiang M; Jiang H Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review. Int. J. Mol. Sci 2014, 15 (12), 23616–23639. 10.3390/ijms151223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Lyu Y; Fang Y; Miao Q; Zhen X; Ding D; Pu K Intraparticle Molecular Orbital Engineering of Semiconducting Polymer Nanoparticles as Amplified Theranostics for in Vivo Photoacoustic Imaging and Photothermal Therapy. ACS Nano 2016, 10 (4), 4472–4481. 10.1021/acsnano.6b00168. [DOI] [PubMed] [Google Scholar]
- (18).Zhen X; Feng X; Xie C; Zheng Y; Pu K Surface Engineering of Semiconducting Polymer Nanoparticles for Amplified Photoacoustic Imaging. Biomaterials 2017, 127, 97–106. 10.1016/j.biomaterials.2017.03.003. [DOI] [PubMed] [Google Scholar]
- (19).Huang J; Pu K Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed 2020, 59 (29), 11717–11731. 10.1002/anie.202001783. [DOI] [PubMed] [Google Scholar]
- (20).Amendola V; Pilot R; Frasconi M; Maragò OM; Iatì MA Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys. Condens. Matter 2017, 29 (20), 203002. 10.1088/1361-648X/aa60f3. [DOI] [PubMed] [Google Scholar]
- (21).Ye X; Zheng C; Chen J; Gao Y; Murray CB Using Binary Surfactant Mixtures To Simultaneously Improve the Dimensional Tunability and Monodispersity in the Seeded Growth of Gold Nanorods. Nano Lett. 2013, 13 (2), 765–771. 10.1021/nl304478h. [DOI] [PubMed] [Google Scholar]
- (22).Chen Y-S; Frey W; Kim S; Homan K; Kruizinga P; Sokolov K; Emelianov S Enhanced Thermal Stability of Silica-Coated Gold Nanorods for Photoacoustic Imaging and Image-Guided Therapy. Opt. Express 2010, 18 (9), 8867. 10.1364/OE.18.008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kong F; Silverman RH; Liu L; Chitnis PV; Lee KK; Chen YC Photoacoustic-Guided Convergence of Light through Optically Diffusive Media. Opt. Lett 2011, 36 (11), 2053. 10.1364/OL.36.002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lai P; Wang L; Tay JW; Wang LV Photoacoustically Guided Wavefront Shaping for Enhanced Optical Focusing in Scattering Media. Nat. Photonics 2015, 9 (2), 126–132. 10.1038/nphoton.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Chaigne T; Katz O; Boccara AC; Fink M; Bossy E; Gigan S Controlling Light in Scattering Media Non-Invasively Using the Photoacoustic Transmission Matrix. Nat. Photonics 2014, 8 (1), 58–64. 10.1038/nphoton.2013.307. [DOI] [Google Scholar]
- (26).Tzang O; Caravaca-Aguirre AM; Wagner K; Piestun R Adaptive Wavefront Shaping for Controlling Nonlinear Multimode Interactions in Optical Fibres. Nat. Photonics 2018, 12 (6), 368–374. 10.1038/s41566-018-0167-7. [DOI] [Google Scholar]
- (27).Mantri Y; Jokerst JV Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14 (8), 9408–9422. 10.1021/acsnano.0c05215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gao F; Bai L; Liu S; Zhang R; Zhang J; Feng X; Zheng Y; Zhao Y Rationally Encapsulated Gold Nanorods Improving Both Linear and Nonlinear Photoacoustic Imaging Contrast in Vivo. Nanoscale 2017, 9 (1), 79–86. 10.1039/C6NR07528B. [DOI] [PubMed] [Google Scholar]
- (29).Chen Y-S; Frey W; Kim S; Kruizinga P; Homan K; Emelianov S Silica-Coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Lett. 2011, 11 (2), 348–354. 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Inose T; Oikawa T; Shibuya K; Tokunaga M; Hatoyama K; Nakashima K; Kamei T; Gonda K; Kobayashi Y Fabrication of Silica-Coated Gold Nanorods and Investigation of Their Property of Photothermal Conversion. Biochem. Biophys. Res. Commun 2017, 484 (2), 318–322. 10.1016/j.bbrc.2017.01.112. [DOI] [PubMed] [Google Scholar]
- (31).Jokerst JV; Thangaraj M; Kempen PJ; Sinclair R; Gambhir SS Photoacoustic Imaging of Mesenchymal Stem Cells in Living Mice via Silica-Coated Gold Nanorods. ACS Nano 2012, 6 (7), 5920–5930. 10.1021/nn302042y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Farny CH; Wu T; Holt RG; Murray TW; Roy RA Nucleating Cavitation from Laser-Illuminated Nano-Particles. Acoust. Res. Lett. Online 2005, 6 (3), 138–143. 10.1121/1.1897823. [DOI] [Google Scholar]
- (33).Kotaidis V; Dahmen C; von Plessen G; Springer F; Plech A Excitation of Nanoscale Vapor Bubbles at the Surface of Gold Nanoparticles in Water. J. Chem. Phys 2006, 124 (18), 184702. 10.1063/1.2187476. [DOI] [PubMed] [Google Scholar]
- (34).Lukianova-Hleb EY; Santiago C; Wagner DS; Hafner JH; Lapotko DO Generation and Detection of Plasmonic Nanobubbles in Zebrafish. Nanotechnology 2010, 21 (22), 225102. 10.1088/0957-4484/21/22/225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lukianova-Hleb EY; Koneva II; Oginsky AO; La Francesca S; Lapotko DO Selective and Self-Guided Micro-Ablation of Tissue with Plasmonic Nanobubbles. J. Surg. Res 2011, 166 (1), e3–e13. 10.1016/j.jss.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Peeters S; Kitz M; Preisser S; Wetterwald A; Rothen-Rutishauser B; Thalmann GN; Brandenberger C; Bailey A; Frenz M Mechanisms of Nanoparticle-Mediated Photomechanical Cell Damage. Biomed. Opt. Express 2012, 3 (3), 435. 10.1364/BOE.3.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wei C; Xia J; Lombardo M; Perez C; Arnal B; Larson-Smith K; Pelivanov I; Matula T; Pozzo L; O’Donnell M Laser-Induced Cavitation in Nanoemulsion with Gold Nanospheres for Blood Clot Disruption: In Vitro Results. Opt. Lett 2014, 39 (9), 2599. 10.1364/OL.39.002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Wilson K; Homan K; Emelianov S Biomedical Photoacoustics beyond Thermal Expansion Using Triggered Nanodroplet Vaporization for Contrast-Enhanced Imaging. Nat. Commun 2012, 3 (1), 618. 10.1038/ncomms1627. [DOI] [PubMed] [Google Scholar]
- (39).Dixon AJ; Hu S; Klibanov AL; Hossack JA Oscillatory Dynamics and In Vivo Photoacoustic Imaging Performance of Plasmonic Nanoparticle-Coated Microbubbles. Small 2015, 11 (25), 3066–3077. 10.1002/smll.201403398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wei C; Lombardo M; Larson-Smith K; Pelivanov I; Perez C; Xia J; Matula T; Pozzo D; O’Donnell M Nonlinear Contrast Enhancement in Photoacoustic Molecular Imaging with Gold Nanosphere Encapsulated Nanoemulsions. Appl. Phys. Lett 2014, 104 (3), 033701. 10.1063/1.4862461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Blum NT; Yildirim A; Gyorkos C; Shi D; Cai A; Chattaraj R; Goodwin AP Temperature-Responsive Hydrophobic Silica Nanoparticle Ultrasound Contrast Agents Directed by Phospholipid Phase Behavior. ACS Appl. Mater. Interfaces 2019, 11 (17), 15233–15240. 10.1021/acsami.8b22659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yildirim A; Shi D; Roy S; Blum NT; Chattaraj R; Cha JN; Goodwin AP Nanoparticle-Mediated Acoustic Cavitation Enables High Intensity Focused Ultrasound Ablation Without Tissue Heating. ACS Appl. Mater. Interfaces 2018, 10 (43), 36786–36795. 10.1021/acsami.8b15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Yildirim A; Chattaraj R; Blum NT; Shi D; Kumar K; Goodwin AP Phospholipid Capped Mesoporous Nanoparticles for Targeted High Intensity Focused Ultrasound Ablation. Adv. Healthc. Mater 2017, 6 (18). 10.1002/adhm.201700514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Bull DS; Nelson N; Konetski D; Bowman CN; Schwartz DK; Goodwin AP Contact Line Pinning Is Not Required for Nanobubble Stability on Copolymer Brushes. J. Phys. Chem. Lett 2018, 9 (15), 4239–4244. 10.1021/acs.jpclett.8b01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yildirim A; Chattaraj R; Blum NT; Goodwin AP Understanding Acoustic Cavitation Initiation by Porous Nanoparticles: Toward Nanoscale Agents for Ultrasound Imaging and Therapy. Chem. Mater 2016, 28 (16), 5962–5972. 10.1021/acs.chemmater.6b02634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Kuriakose M; Borden MA Microbubbles and Nanodrops for Photoacoustic Tomography. Curr. Opin. Colloid Interface Sci 2021, 55, 101464. 10.1016/j.cocis.2021.101464. [DOI] [Google Scholar]
- (47).Gao R; Xu Z; Ren Y; Song L; Liu C Nonlinear Mechanisms in Photoacoustics—Powerful Tools in Photoacoustic Imaging. Photoacoustics 2021, 22, 100243. 10.1016/j.pacs.2021.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Zhang Z; Wang L; Wang J; Jiang X; Li X; Hu Z; Ji Y; Wu X; Chen C Mesoporous Silica-Coated Gold Nanorods as a Light-Mediated Multifunctional Theranostic Platform for Cancer Treatment. Adv. Mater 2012, 24 (11), 1418–1423. 10.1002/adma.201104714. [DOI] [PubMed] [Google Scholar]
- (49).Orendorff CJ; Murphy CJ Quantitation of Metal Content in the Silver-Assisted Growth of Gold Nanorods. J. Phys. Chem. B 2006, 110 (9), 3990–3994. 10.1021/jp0570972. [DOI] [PubMed] [Google Scholar]
- (50).Gorelikov I; Matsuura N Single-Step Coating of Mesoporous Silica on Cetyltrimethyl Ammonium Bromide-Capped Nanoparticles. Nano Lett 2008, 8 (1), 369–373. 10.1021/nl0727415. [DOI] [PubMed] [Google Scholar]
- (51).Khlebtsov BN; Khlebtsov NG On the Measurement of Gold Nanoparticle Sizes by the Dynamic Light Scattering Method. Colloid J. 2011, 73 (1), 118–127. 10.1134/S1061933X11010078. [DOI] [Google Scholar]
- (52).Rodríguez-Fernández J; Pérez–Juste J; Liz–Marzán LM; Lang PR Dynamic Light Scattering of Short Au Rods with Low Aspect Ratios. J. Phys. Chem. C 2007, 111 (13), 5020–5025. 10.1021/jp067049x. [DOI] [Google Scholar]
- (53).Monem AS; Elbialy N; Mohamed N Mesoporous Silica Coated Gold Nanorods Loaded Doxorubicin for Combined Chemo–Photothermal Therapy. Int. J. Pharm 2014, 470 (1–2), 1–7. 10.1016/j.ijpharm.2014.04.067. [DOI] [PubMed] [Google Scholar]
- (54).Carey VP Liquid-Vapor Phase-Change Phenomena; Taylor and Francis: Boca Raton, 2020. [Google Scholar]
- (55).Fisher JC The Fracture of Liquids. J. Appl. Phys 1948, 19 (11), 1062–1067. 10.1063/1.1698012. [DOI] [Google Scholar]
- (56).Visscher M; Lajoinie G; Blazejewski E; Veldhuis G; Versluis M Laser-Activated Microparticles for Multimodal Imaging: Ultrasound and Photoacoustics. Phys. Med. Biol 2019, 64 (3), 034001. 10.1088/1361-6560/aaf4a2. [DOI] [PubMed] [Google Scholar]
- (57).Arnal B; Perez C; Wei C-W; Xia J; Lombardo M; Pelivanov I; Matula TJ; Pozzo LD; O’Donnell M Sono-Photoacoustic Imaging of Gold Nanoemulsions: Part I. Exposure Thresholds. Photoacoustics 2015, 3 (1), 3–10. 10.1016/j.pacs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Schrof S; Pang G; Buchmann J; Laufer J Exploiting Nonlinear Photoacoustic Signal Generation in Gold Nanospheres for Selective Detection in Serial 3D PA Tomography. J. Imaging 2018, 4 (12), 146. 10.3390/jimaging4120146. [DOI] [Google Scholar]
- (59).Simandoux O; Prost A; Gateau J; Bossy E Influence of Nanoscale Temperature Rises on Photoacoustic Generation: Discrimination between Optical Absorbers Based on Thermal Nonlinearity at High Frequency. Photoacoustics 2015, 3 (1), 20–25. 10.1016/j.pacs.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Liu J; Stace-Naughton A; Jiang X; Brinker CJ Porous Nanoparticle Supported Lipid Bilayers (Protocells) as Delivery Vehicles. J. Am. Chem. Soc 2009, 131 (4), 1354–1355. 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Shahbazi K; Frey W; Chen Y-S; Aglyamov S; Emelianov S Photoacoustics of Core–Shell Nanospheres Using Comprehensive Modeling and Analytical Solution Approach. Commun. Phys 2019, 2 (1), 119. 10.1038/s42005-019-0216-7. [DOI] [Google Scholar]
- (62).Inzunza-Ibarra MA; Premillieu E; Grünsteidl C; Piestun R; Murray TW Sub-Acoustic Resolution Optical Focusing through Scattering Using Photoacoustic Fluctuation Guided Wavefront Shaping. Opt. Express 2020, 28 (7), 9823. 10.1364/OE.385320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


