Abstract
The antitumor compound camptothecin (CPT) is also recognized for its specific activity against Leishmania donovani topoisomerase I (Topo-I). In consequence, defining CPT resistance mechanisms represents an important strategic tool in the acquisition of a better understanding of its mode of action. In the present study, we selected a single highly resistant L. donovani strain termed LdRCPT.160 by stepwise exposure to CPT. Gene sequencing revealed two single nucleotide mutations in the LdRCPT.160 LdTOP1A gene, resulting in two amino acid substitutions (Gly185Arg and Asp325Glu) in the protein. Moreover, these two substitutions observed in the LdTOP1A protein were correlated with a decreased Topo-I DNA relaxation activity in these resistant parasites. Nevertheless, there was no change in the LdTOP1A gene expression level. Interestingly, transfection studies of the LdRCPT.160 LdTOP1A gene in its wild-type counterpart showed that it induced CPT resistance. Site-directed mutagenesis studies demonstrated that, despite a substantial level of resistance conferred by the Gly185Arg and Asp325Glu substitutions separately, both were essential to reach a high-resistance phenotype. Of interest, the amino acid substitutions observed in LdRCPT.160 LdTOP1A protein occurred near the amino acids previously predicted to interact with CPT, providing new insight into the mechanism of CPT molecular action.
Leishmaniasis, caused by the sand fly-transmitted intracellular protozoan parasite Leishmania, occurs worldwide. An estimated 12 million people are infected, with a yearly incidence of 1 to 1.5 million cases of cutaneous leishmaniasis and 500,000 cases of visceral leishmaniasis (12). If untreated, the visceral form, principally caused by Leishmania donovani, is usually fatal. In addition, even in treated patients the fatality rate may be as high as 30% because of late or missed diagnoses (11). Due to the lack of vaccines, difficulties in vector control, and the development of parasite resistance to several treatments, leishmaniasis represents a significant clinical and public health problem (16). Development of new antileishmania drug alternatives is required, but a better understanding of the resistance mechanisms is a priority.
Eukaryotic DNA topoisomerase I (Topo-I) is an essential enzyme that regulates the topological changes of DNA that accompany DNA replication, transcription, recombination, and chromosome segregation during mitosis (15, 39, 40). Topo-I introduces transient single-stranded DNA breaks in one of the phosphodiester backbones of the duplex DNA and results in a reversible Topo-I/DNA covalent complex (5-7). In 1999, we cloned and sequenced the first Topo-I gene from L. donovani (LdTOP1A) (4). More recently, a novel Topo-I gene has also been described for this parasite (LdTOP1B) (38). These latter observations suggested a dimeric Topo-I in Leishmania parasites, since the enzymatic activity was detected only when both genes (LdTOP1A and LdTOP1B) were coexpressed in a yeast expression system (38). Interestingly, a recent study demonstrated for the first time the in vitro reconstitution of the two recombinant proteins, LdTOP1L and LdTOP1S, corresponding to the large (LdTOP1A) and small (LdTOP1B) subunits, respectively (10). This study also revealed the location of the active enzyme (LdTOP1LS) in both the nucleus and the kinetoplast of the parasite (10).
Because Topo-I poisoning has been recognized as a promising pharmacological target for the development of therapeutic agents, a number of candidates have been identified. The potent antitumor compound camptothecin (CPT) represents the best-characterized Topo-I inhibitor. CPT has been shown to stabilize the Topo-I/DNA covalent complex in a cell-free simian virus 40 DNA replication system, which leads to DNA damage through collision with replication forks when replication occurs and subsequent cell death (17, 36). In addition to its antitumor effects, CPT has also been recognized to specifically target the L. donovani Topo-I (2). Moreover, we recently demonstrated the potential of a liposomal CPT formulation to inhibit the growth of L. donovani in vitro and to reduce the liver parasitic load of infected mice (29). Several CPT derivatives such as irinotecan and topotecan have recently been introduced for cancer therapy (37) and could also be of interest in leishmaniasis treatment. Therefore, defining the mechanisms of CPT resistance in Leishmania represents an important strategic tool in the acquisition of a better understanding of its mechanism of molecular action.
From previous studies performed on mammalian cells, the development of mechanisms of resistance to topoisomerase inhibitors appears to be a multifactorial event including drug transport, drug-target interaction, and drug detoxification. The resistance to Topo-I poisons, in contrast to Topo-II inhibitors, does not seem to involve the multiple drug resistance phenotype (28). For instance, as the number of DNA strand breaks induced by topoisomerase inhibitors is dependent on the amount of enzymes in the cells, one of the most conceivable resistance mechanisms to emerge is the decrease of topoisomerase content in the cells (25). Consequently, this mechanism leads to a reduced production of lethal enzyme-mediated DNA damage (14). The resistance to Topo-I inhibitors in cancer cells, particularly to CPT, is often associated with Topo-I gene mutations, which result in an altered protein leading to a decreased enzyme activity (26). Most of these mutations map to four conserved regions, including residues Gly717 to Asn722, which interact directly with the catalytic Tyr723 (30, 32). The reduction of Topo-I activity, without any gene mutation, is probably more frequent and representative of the clinical resistance observed. This phenomenon can be linked to rearrangement, deletion, or hypermethylation of one of the Topo-I alleles (19, 33, 34). Others have observed a modulation in Topo-I activity with no change in Topo-I expression, suggesting that the posttranscriptional events could have a significant impact on the Topo-I activity and on its susceptibility to inhibition (22).
Knowing that topoisomerases play a pivotal role in protozoan parasite replication (8) and that Topo-I is specifically targeted by CPT in L. donovani (2), we developed parasites resistant to this drug. We further attempted to determine the resistance mechanisms by which the parasites bypassed the cytotoxic effects of CPT. In the present study, we report the first observations on CPT resistance mechanisms occurring in protozoan parasites. Our findings may help to improve our knowledge of the mechanism of CPT molecular action on Leishmania Topo-I.
MATERIALS AND METHODS
Drug solutions.
CPT was purchased from Sigma, and stock solution (25 mM) in dimethyl sulfoxide (ICN Biomedicals, Inc.) was stored at −20°C.
Parasite cultures.
The parasites (L. donovani strain 1S2D [MHOM/SD/00/LS]) were grown at room temperature and transferred biweekly in SDM-79 culture medium (SDM) supplemented with 10% fetal bovine serum as previously described (23, 41). A highly resistant L. donovani strain called LdRCPT.160 was selected by stepwise exposure to CPT (5, 10, 20, 40, 80, and 160 μM) until reaching a resistance level of 32-fold over the wild-type strain. LdRCPT.160 was developed from a clone of L. donovani strain 1S2D (L. donovani C1).
Drug treatments.
To monitor the impact of CPT on Leishmania growth, parasites were transferred (2 × 106 log-phase promastigotes/ml) into 3 ml of SDM in the absence or presence of increasing concentrations (0 to 50 μM) of CPT. The growth was monitored over 6 days by measuring the absorbance at 600 nm with an automated microplate reader (Organon Teknika, Reader 510) (41).
Drug permeability.
Log-phase promastigote parasites (2 × 106 parasites/ml) treated with CPT (0 to 50 μM) were collected at different time points over 30 min. The fluorescent property of the CPT enabled the parasite fluorescence intensity to be directly evaluated by flow cytometry with an Epics Elite ESP flow cytometer (Coulter Electronics, Miami, Fla.).
LdTOP1A gene expression.
Total RNA from 5 × 108 log-phase promastigotes was extracted using Trizol reagent (Invitrogen). Briefly, RNA was resolved on a 1% agarose gel and transferred to a Nytran Plus nylon membrane. After material transfer, the membrane was UV exposed for 3 min on a transilluminator and prehybridized for 4 h at 42°C in a solution of 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 10× Denhardt's solution, 50 mM NaPO4, 0.2 mM dextran sulfate, 0.5% sodium dodecyl sulfate (SDS), 133 mM glycine, and 50% formamide with 150 μg of salmon sperm DNA/ml. The membrane hybridization was performed overnight at 42°C with a [α-32P]dCTP-labeled SacI DNA fragment from the L. donovani TOP1-like gene (LdTOP1A), as we previously described (4). After incubation, the membrane was washed three times with 2× SSC-0.1% SDS and three times with 0.1× SSC-0.1% SDS (15 min per wash, 42°C). Autoradiography was performed using X-ray film (Kodak).
Topo-I activity assays.
Parasite extracts were obtained from 5 × 108 log-phase promastigotes and subjected to the Topo-I activity assays (20, 35). Briefly, the parasites were resuspended in 100 to 200 μl of Topo-I assay buffer (10 mM Tris-HCl [pH 7.9], 1 mM EDTA, 150 mM NaCl, 0.1% bovine serum albumin, 0.1 mM spermidine, 5% glycerol) and lysed by repeated passage through a 25-gauge needle. The parasite extracts (2 μg of total proteins) were incubated for 1 h at 37°C in a 20-μl volume containing Topo-I assay buffer and 0.5 μg of supercoiled pBR322 (Roche). They were further incubated with 0.5 μg of proteinase K/μl-1% SDS-100 mM EDTA for 30 min at 50°C before the addition of 2.5 μl of stop mix (5% Sarkosyl, 0.0025% bromophenol blue, 25% glycerol). Supercoiled and relaxed forms of pBR322 were separated in a 1% agarose slab. After migration in 1× TBE buffer (89 mM Tris base, 89 mM boric acid, 2 mM EDTA, pH 8.0), the gel was soaked in ethidium bromide and UV illuminated to reveal the status of DNA coiling. To exclude the possibility of Topo-II activity, Mg2+ ions and ATP were omitted from the reaction mix, since both are recognized to be necessary for Topo-II activity (18, 31).
DNA constructs and gene transfections.
Parasite genomic DNA was extracted by the use of DNAzol reagent (Invitrogen) according to the manufacturer's protocol. The Topo-I gene (LdTOP1A) was amplified by PCR with the specific primers 5′-GCG AAG CTT ATG AAG GTG GAG AAT AGC-3′ and 5′-CGC TCT AGA CTA CAC GCT CAA AGC TGC-3′, which contained the HindIII and XbaI restriction sites, respectively (underlined). PCR amplification with Pwo DNA polymerase (Roche) included an initial denaturation step (2 min at 94°C) followed by 35 cycles of 30 s at 94°C, 30 s at 55°C, and 2 min at 72°C and a final elongation step (5 min at 72°C). Amplicons were visualized on a 1% agarose gel and purified with the QIAquick gel extraction kit (Qiagen). The gel-purified PCR fragment was digested with HindIII and XbaI and directionally cloned into pGEM7-αNEOα (24). This vector, kindly provided by Barbara Papadopoulou (Université Laval, Sainte-Foy, Québec, Canada), contains the neomycin phosphotransferase gene (NEO) flanked by two α-tubulin intergenic regions. The resulting plasmids were isolated using the HiSpeed Plasmid Midi kit (Qiagen) and independently introduced into L. donovani C1 promastigotes by electroporation according to standard procedures (24). Selections were done with 40 μg of Geneticin (Invitrogen)/ml.
Site-directed mutagenesis.
Mutagenesis was performed by using the QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. Plasmid pGEM7-αNEOα containing the mutated LdTOP1A gene from LdRCPT.160 was used as a template for all mutagenesis experiments. Each mutation was independently replaced by the wild-type nucleotide by using a specific pair of mutagenic primers. The correct nucleotide sequence from each mutated plasmid was verified by sequence analysis.
DNA sequencing.
Sequencing of the LdTOP1A gene was carried out with the same PCR primer described above, with a cycle sequencing kit (Big Dye Terminator Cycle Sequencing Ready Reaction; Perkin-Elmer) and an automated DNA sequencer (ABI Prism 377 DNA sequencer; Perkin-Elmer).
RESULTS
Sensitivity of wild-type and resistant parasites to CPT.
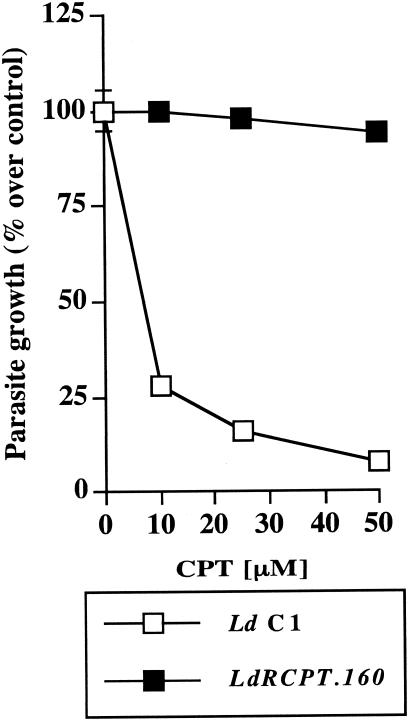
Drug resistance mechanisms in Leishmania parasites are usually investigated by analyzing mutants selected for resistance with increasing drug concentrations (3). In order to determine the mechanisms of CPT resistance, we selected parasites for resistance to this drug in a step-by-step manner until they reached a resistance level of 32-fold over their wild-type counterparts. More specifically, highly resistant L. donovani strain LdRCPT.160 was selected by stepwise exposure to increasing concentrations of CPT (5, 10, 20, 40, 80, and 160 μM). To compare their susceptibilities to CPT, we exposed freshly harvested parasites to increasing concentrations of the drug. Whereas LdRCPT.160 growth was hardly affected by CPT even at the highest concentration used, the growth of wild-type parasites was inhibited in a dose-dependent manner with a 50% inhibitory concentration of approximately 5 μM (Fig. 1). This result clearly established the development of resistance mechanisms in LdRCPT.160 to circumvent the cytotoxic effect of this compound. As the resistance to Topo-I inhibitors is usually multifactorial in mammalian cells, we further performed experiments at different cellular levels to establish the potential mechanism(s) of resistance to CPT in L. donovani.
FIG. 1.
Effect of CPT on L. donovani growth. L. donovani C1 and LdRCPT.160 parasites were grown in the presence of increasing concentrations (0, 10, 25, and 50 μM) of CPT. Optical density was monitored for 6 days. Proliferation of L. donovani C1 in the absence of any treatment was considered as a maximal growth control. The parasite growth is expressed as a percentage of the optical density compared with that for the control. Results (percent over control ± standard error) are representative of three experiments performed in triplicate.
Drug accumulation in LdRCPT.160.
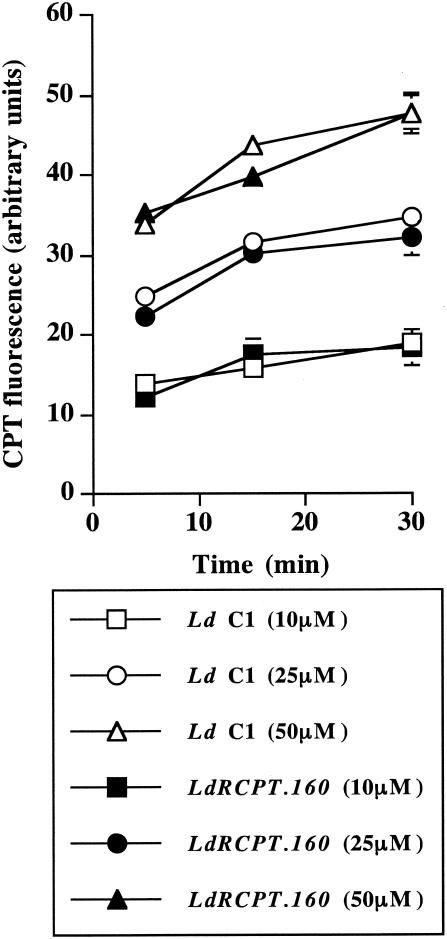
One possible way to increase the level of resistance could be by blocking the drug uptake or involving a drug efflux system. Since CPT has intrinsic fluorescence, we tested these possibilities by measuring its accumulation inside the parasites. Moreover, flow cytometry analysis of the drug kinetics was performed to determine whether the wild-type strain was more permeable to CPT than resistant parasites were. As reported in Fig. 2, no significant differences of permeability were detected between L. donovani C1 and LdRCPT.160, even at a higher CPT concentration (50 μM), suggesting no alteration in the drug accumulation for the resistant strain. As CPT entered both parasite strains similarly, we further investigated the molecular aspects of Topo-I and its interaction with the drug.
FIG. 2.
CPT fluorescence in LdRCPT.160. Parasites (L. donovani C1 and LdRCPT.160) treated with increasing concentrations (0, 10, 25, and 50 μM) of CPT were collected at different time points over 30 min and analyzed by flow cytometry. The fluorescence intensity results are expressed in arbitrary units (mean ± standard error). Results are representative of three experiments independently performed in triplicate.
Topo-I gene expression and enzymatic activity.
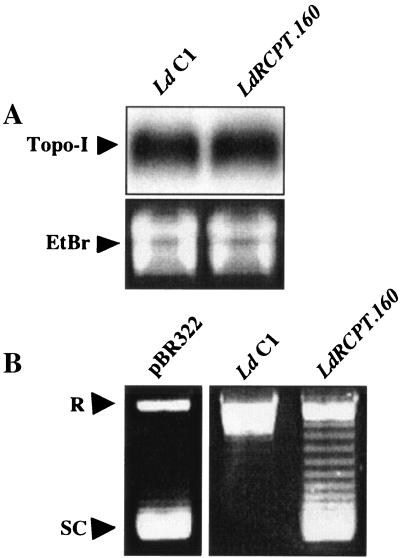
Since Topo-I is the specific target of CPT (2), the amount of LdTOP1A mRNA transcripts in the wild-type and resistant parasites was determined by Northern blot analysis. As reported in Fig. 3A, no difference in the LdTOP1A gene expression was observed between LdRCPT.160 and L. donovani C1.
FIG. 3.
(A) Topo-I gene expression in LdRCPT.160. (A) Northern blot of L. donovani C1 and LdRCPT.160. The probe used corresponds to the SacI DNA fragment (1,069 bp) from the TOP1-like gene (LdTOP1A) of L. donovani. The LdTOP1A mRNA transcript is 3.4 kb long according to molecular size markers. RNA integrity was verified by ethidium bromide (EtBr) staining. This result is representative of one out of three experiments independently performed. (B) Topo-I DNA relaxation activity of LdRCPT.160. Supercoiled pBR322 DNA alone is shown in lane 1. Supercoiled pBR322 DNA in the presence of parasite extracts is represented in lanes 2 and 3. R, relaxed DNA form; SC, supercoiled DNA form. Results are representative of three experiments independently performed.
However, as the number of DNA strand breaks induced by topoisomerase inhibitors is dependent on the amount of enzymes in the cells, one of the most conceivable mechanisms of resistance to emerge is the decrease of enzyme content in the cells (25), leading to a reduced production of lethal enzyme-mediated DNA damage (14). To test this hypothesis, we measured the Topo-I activity in the wild-type and resistant parasites by the ATP-independent relaxation of supercoiled pBR322 DNA. Interestingly, we observed a reduced Topo-I activity in LdRCPT.160 compared to that for L. donovani C1 (Fig. 3B), despite equivalent mRNA expression. Even though we could not totally exclude the possibility that the LdTOP1A enzyme level might be reduced in LdRCPT.160 cells, we therefore searched for mutations that could account for this reduced activity.
Topo-I gene sequencing.
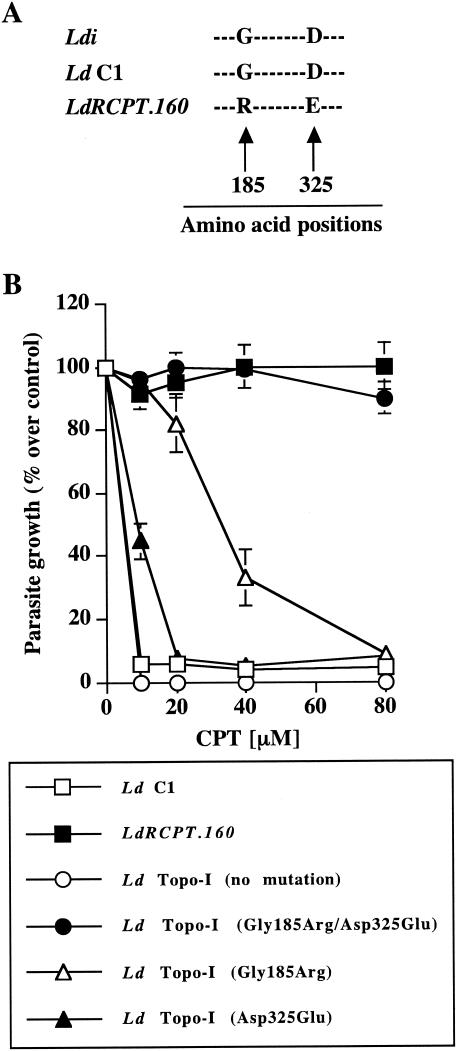
The resistance to Topo-I inhibitors in cancer cells, particularly to CPT, is commonly associated with Topo-I gene mutations (26). We reasoned that, during drug selection, we may have selected for point mutations within the Topo-I genes (LdTOP1A and LdTOP1B), which could be responsible for the observed resistance phenotype. To address this possibility, we sequenced the Topo-I genes (LdTOP1A and LdTOP1B) of the resistant parasites and compared these sequences to the sequences of the genes derived from the susceptible parasite strain. As reported in Fig. 4A, two amino acid substitutions resulting from two single nucleotide mutations were observed in the LdTOP1A protein sequence of LdRCPT.160. These two substitutions were detected in the highly conserved core domain of the LdTOP1A protein. More specifically, the glycine 185 (Gly185) and the aspartic acid 325 (Asp325) found in L. donovani C1 were mutated to arginine (Arg) and glutamic acid (Glu) in LdRCPT.160, respectively. Based on the three-dimensional model of the putative LdTOP1 protein (9), we determined that these mutations occurred near the amino acid residues (Arg190 and Asp353) predicted to interact with the essential lactone and 20(S)-hydroxyl moieties of CPT (9). This observation suggests that the amino acids Gly185 and Asp325 of the L. donovani LdTOP1A mediate the interaction between the drug and the enzyme. In addition, no mutations were observed for the LdTOP1B gene in the resistant parasites (LdRCPT.160) (data not shown).
FIG. 4.
(A) Sequence alignment of the Topo-I protein (LdTOP1A) at positions 185 and 325. Parasite DNA was extracted with DNAzol reagent, and the LdTOP1A gene (1,905 bp) was sequenced with specific primers as described in Materials and Methods. L. donovani C1 and LdRCPT.160 LdTOP1A sequences were compared with L. donovani infantum (Ldi) LdTOP1A sequence (GenBank accession number AF145121). G, glycine; D, aspartic acid; R, arginine; E, glutamic acid. (B) Effect of CPT on LdTOP1A mutant parasites. Parasites were grown in the presence of increasing concentrations of CPT (0 to 80 μM), and optical density was monitored for 6 days. L. donovani C1 was transfected with the mutated gene coding for the double-substituted LdTOP1A protein (L. donovani Topo-I Gly185Arg/Asp325Glu) or with the mutated genes coding for the single-substituted LdTOP1A proteins independently (L. donovani Topo-I Gly185Arg and L. donovani Topo-I Asp325Glu). L. donovani C1 overexpressing the wild-type LdTOP1A gene (no mutation) was used as a control. Proliferation of L. donovani C1 in the absence of any treatment was considered as a maximal growth control. The parasite growth is expressed as a percentage of the optical density compared with that for the control. Results (percent over control ± standard error) are representative of three experiments performed in triplicate.
Transfection of L. donovani C1 with the mutated LdRCPT.160 LdTOP1A gene.
To corroborate this potential resistance mechanism and to evaluate the contribution of the two mutations observed in CPT resistance, we cloned the mutated LdRCPT.160 LdTOP1A gene in a Leishmania expression vector. In parallel, we also performed site-directed mutagenesis on the LdRCPT.160 LdTOP1A gene, where each mutation, conferring an amino acid substitution in the resulting protein, was independently replaced by the wild-type nucleotide. As a control, we also used a construct without gene mutation to eliminate a possible effect of LdTOP1A overexpression in the resistance mechanisms. Indeed, upon transfection, no significant phenotype change was observed in L. donovani Topo-I parasites (no mutation) in response to CPT, whereas parasites containing both amino acid substitutions simultaneously (L. donovani Topo-I Gly185Arg/Asp325Glu) showed a high level of CPT resistance (Fig. 4B). Furthermore, we determined that the substitutions Gly185Arg (L. donovani Topo-I Gly185Arg) and Asp325Glu (L. donovani Topo-I Asp325Glu) independently conferred a substantial level of resistance to CPT with 50% inhibitory concentrations of approximately 35 and 10 μM, respectively (Fig. 4B). Nevertheless, even if the Gly185Arg substitution seems to confer more CPT resistance than the Asp325Glu substitution does in L. donovani, we demonstrated that both substitutions were essential to obtain a high-resistance phenotype.
DISCUSSION
In the present study, we characterized the resistance mechanisms developed by L. donovani against CPT. LdRCPT.160 parasites were around 32-fold more resistant to CPT than their wild-type strain was. As for other drugs, resistance to CPT may involve alterations in cellular drug accumulation, the drug target, or the response to the drug-target interaction. Firstly, as the Leishmania plasma membrane represents the first cellular barrier that CPT encounters before reaching its specific target, we determined the efficiency of the drug in accumulating inside the wild-type and resistant parasites, in order to verify differences in their CPT transport kinetics. Our results concerning cellular permeability established that CPT accumulated similarly within LdRCPT.160 and its wild-type parent, revealing no alteration in the drug accumulation for the resistant parasites.
To further study the mechanisms responsible for the development of resistance in LdRCPT.160, a series of quantitative and qualitative assays of Topo-I was performed. While Northern blot analysis showed no change in the LdTOP1A gene expression in LdRCPT.160 parasites in comparison with its wild-type counterpart, resistant parasites showed a reduced Topo-I DNA relaxation activity. Usually, CPT resistance mechanisms involving a decrease in Topo-I DNA relaxation activity are associated with Topo-I gene mutations (26). Moreover, this is consistent with genetic studies of CPT-resistant mammalian or yeast cells that identified point mutations in the Topo-I gene (1, 13, 21). To address this possibility, we sequenced the Topo-I genes (LdTOP1A and LdTOP1B) of the wild-type and resistant parasites. LdRCPT.160 revealed the presence of two amino acid substitutions (Gly185Arg and Asp325Glu) in the core domain of LdTOP1A protein, which is highly conserved among known members of the IB (eukaryotic) topoisomerase family (4). Based on recent evidence that the Leishmania Topo-I is an unusual bisubunit enzyme (LdTOP1L and LdTOP1S) (10) and that the mutations that we identified are localized to the gene coding for the long subunit, we hypothesized that this enzyme portion is crucial for the cytotoxic action of CPT in Leishmania. Furthermore, as we observed a reduced Topo-I DNA relaxation activity in the resistant parasites harboring the LdTOP1L (4) amino acid substitutions, we also speculated that this subunit could play an important role in the LdTOP1LS catalytic activity, even though the Topo-I active site (serine, lysine, X, X, tyrosine motif) is located in the LdTOP1S subunit (38). Nevertheless, both subunits are required to reconstitute an active dimeric Topo-I (LdTOP1LS) in L. donovani (10). Despite substantial resistance conferred by each amino acid substitution individually, site-directed mutagenesis studies indicated that both substitutions, performed simultaneously, were necessary to reach a high CPT resistance level. As a potential mechanism, we suggest that the transfected mutant LdTOP1A (LdTOP1L) competed with its wild-type enzyme for the association with the LdTOP1B (LdTOP1S) subunit, resulting in a limited number of CPT-stabilized Topo-I-DNA complexes and by consequence in decreased drug susceptibility. Due to the high level of homology between L. donovani and human Topo-I, a three-dimensional model of the putative LdTOP1 protein based on the crystal structure of human Topo-I was proposed (9). From this, the L. donovani Arg190 and Asp353, equivalent to Arg364 and Asp533 of human Topo-I, respectively, were predicted to interact with the essential lactone and 20(S)-hydroxyl moieties of CPT (9). Moreover, mutations involving amino acids 361 to 364 and 533 localized to two lip domains of the human enzyme, which bind DNA, are likely to undergo conformational changes during enzyme catalysis (27, 32). A model of the putative CPT-Topo-I ternary complex proposed specific interactions between amino acids in these two regions and the drug molecule, providing an explanation for the resistance conferred by mutations involving these residues (30). Interestingly, with respect to these models, we determined that the amino acid substitutions in the LdRCPT.160 LdTOP1A protein occurred near the amino acid residues recognized as being involved in human CPT resistance. Moreover, by comparing the Topo-I sequence alignment of L. donovani with the human enzyme (4), we observed that the Gly185 and Asp325 residues in L. donovani Topo-I are both conserved in humans (Table 1). Phe361, Arg364, Gly363, Asp533, and Gly503 are among the most important human Topo-I amino acids, where CPT-resistant mutations that have been observed are also conserved in the L. donovani enzyme (Table 1).
TABLE 1.
CPT resistance mutations in L. donovani and human Topo-I with their corresponding residues
| Residue type |
L. donovani mutation
|
Human mutation
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G185R | D325E | N722S | F361S | R364H | G363C | E418K | D533G | G503S | T729A | A653P | |
| Human | G359 | D500 | |||||||||
| L. donovani | A579 | F187 | R190a | G189 | D244 | D353a | G328 | A586 | Y474 | ||
Amino acid residues predicted to interact with CPT in L. donovani based on the human Topo-I crystal structure.
Taken together, our findings provide experimental evidence demonstrating that the CPT resistance in LdRCPT.160 is mediated by two amino acid substitutions in the large subunit (LdTOP1A) of the dimeric enzyme resulting from two single nucleotide mutations. In addition, we also observed a decrease in Topo-I DNA relaxation activity in LdRCPT.160 that is presumably due to the presence of these mutations. However, we cannot totally exclude the possibility that the LdTOP1A enzyme level might also be reduced in LdRCPT.160 cells, resulting in a decreased DNA relaxation activity. The two amino acid substitutions most likely prevented the formation of CPT-Topo-I-DNA complexes as usually observed in wild-type CPT-treated L. donovani, Trypanosoma brucei, and Trypanosoma cruzi (2). Interestingly, this is the first report concerning mechanisms of resistance to CPT in protozoan parasites. Moreover, based on previously reported studies, we identified two new Topo-I mutation sites conferring resistance to CPT. These amino acid substitutions together with previously defined Topo-I resistance mutation sites in other models provide new insight into CPT molecular interactions.
Acknowledgments
We thank Marc Ouellette (Centre de Recherche en Infectiologie du CHUQ, Université Laval, Québec, Canada) for participation in several research discussions and for useful comments on experimental approaches. We also thank David Gregory (McGill University, Montreal, Canada), who kindly reviewed the language used in the manuscript.
M.O. is supported by funds from the Canadian Institute of Health Research (CIHR) and is a member of a CIHR group in host-pathogen interactions. M.O. holds a CIHR investigator award and is a Burroughs Wellcome Fund awardee in molecular parasitology. J.-F.M. and I.H. are the recipients of CIHR doctoral studentships.
REFERENCES
- 1.Bjornsti, M. A., P. Benedetti, G. A. Viglianti, and J. C. Wang. 1989. Expression of human DNA topoisomerase I in yeast cells lacking yeast DNA topoisomerase I: restoration of sensitivity of the cells to the antitumor drug camptothecin. Cancer Res. 49:6318-6323. [PubMed] [Google Scholar]
- 2.Bodley, A. L., and T. A. Shapiro. 1995. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc. Natl. Acad. Sci. USA 92:3726-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borst, P., and M. Ouellette. 1995. New mechanisms of drug resistance in parasitic protozoa. Annu. Rev. Microbiol. 49:427-460. [DOI] [PubMed] [Google Scholar]
- 4.Broccoli, S., J. F. Marquis, B. Papadopoulou, M. Olivier, and M. Drolet. 1999. Characterization of a Leishmania donovani gene encoding a protein that closely resembles a type IB topoisomerase. Nucleic Acids Res. 27:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champoux, J. J. 1981. DNA is linked to the rat liver DNA nicking-closing enzyme by a phosphodiester bond to tyrosine. J. Biol. Chem. 256:4805-4809. [PubMed] [Google Scholar]
- 6.Champoux, J. J. 1976. Evidence for an intermediate with a single-strand break in the reaction catalyzed by the DNA untwisting enzyme. Proc. Natl. Acad. Sci. USA 73:3488-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champoux, J. J. 1978. Mechanism of the reaction catalyzed by the DNA untwisting enzyme: attachment of the enzyme to 3′-terminus of the nicked DNA. J. Mol. Biol. 118:441-446. [DOI] [PubMed] [Google Scholar]
- 8.Cheesman, S. 2000. The topoisomerases of protozoan parasites. Parasitol. Today 16:277-281. [DOI] [PubMed] [Google Scholar]
- 9.Das, A., C. Mandal, A. Dasgupta, T. Sengupta, and H. Majumder. 2002. An insight into the active site of a type I DNA topoisomerase from the kinetoplastid protozoan Leishmania donovani. Nucleic Acids Res. 30:794-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das, B. B., N. Sen, A. Ganguly, and H. K. Majumder. 2004. Reconstitution and functional characterization of the unusual bi-subunit type I DNA topoisomerase from Leishmania donovani. FEBS Lett. 565:81-88. [DOI] [PubMed] [Google Scholar]
- 11.De Beer, P., A. El Harith, M. Van Grootheest, and A. Winkler. 1990. Outbreak of kala-azar in the Sudan. Lancet 335:224. [DOI] [PubMed] [Google Scholar]
- 12.Desjeux, P. 1996. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 14:417-423. [DOI] [PubMed] [Google Scholar]
- 13.Eng, W. K., L. Faucette, R. K. Johnson, and R. Sternglanz. 1988. Evidence that DNA topoisomerase I is necessary for the cytotoxic effects of camptothecin. Mol. Pharmacol. 34:755-760. [PubMed] [Google Scholar]
- 14.Eng, W. K., F. L. McCabe, K. B. Tan, M. R. Mattern, G. A. Hofmann, R. D. Woessner, R. P. Hertzberg, and R. K. Johnson. 1990. Development of a stable camptothecin-resistant subline of P388 leukemia with reduced topoisomerase I content. Mol. Pharmacol. 38:471-480. [PubMed] [Google Scholar]
- 15.Gellert, M. 1981. DNA topoisomerases. Annu. Rev. Biochem. 50:879-910. [DOI] [PubMed] [Google Scholar]
- 16.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 17.Hsiang, Y. H., M. G. Lihou, and L. F. Liu. 1989. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 49:5077-5082. [PubMed] [Google Scholar]
- 18.Liu, L. F., C. C. Liu, and B. M. Alberts. 1979. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature 281:456-461. [DOI] [PubMed] [Google Scholar]
- 19.Madelaine, I., S. Prost, A. Naudin, G. Riou, F. Lavelle, and J. F. Riou. 1993. Sequential modifications of topoisomerase I activity in a camptothecin-resistant cell line established by progressive adaptation. Biochem. Pharmacol. 45:339-348. [DOI] [PubMed] [Google Scholar]
- 20.Marquis, J. F., M. Drolet, and M. Olivier. 2003. Consequence of Hoechst 33342-mediated Leishmania DNA topoisomerase-I inhibition on parasite replication. Parasitology 126:21-30. [DOI] [PubMed] [Google Scholar]
- 21.Nitiss, J., and J. C. Wang. 1988. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc. Natl. Acad. Sci. USA 85:7501-7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohira, T., K. Nishio, F. Kanzawa, T. Ishida, Y. Ohe, H. Arioka, Y. Funayama, H. Ogasawara, H. Kato, and N. Saijo. 1996. Hypersensitivity of NIH3T3 cells transformed by H-ras gene to DNA-topoisomerase-I inhibitors. Int. J. Cancer 67:702-708. [DOI] [PubMed] [Google Scholar]
- 23.Olivier, M., and C. E. Tanner. 1987. Susceptibilities of macrophage populations to infection in vitro by Leishmania donovani. Infect. Immun. 55:467-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadopoulou, B., G. Roy, and M. Ouellette. 1992. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J. 11:3601-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pessina, A. 1993. Topoisomerase I in multiple drug resistance. Cytotechnology 12:127-135. [DOI] [PubMed] [Google Scholar]
- 26.Pommier, Y., F. Leteurtre, M. R. Fesen, A. Fujimori, R. Bertrand, E. Solary, G. Kohlhagen, and K. W. Kohn. 1994. Cellular determinants of sensitivity and resistance to DNA topoisomerase inhibitors. Cancer Investig. 12:530-542. [DOI] [PubMed] [Google Scholar]
- 27.Pond, C. D., X. G. Li, E. H. Rubin, and L. R. Barrows. 1999. Effects of mutations in the F361 to R364 region of topoisomerase I (Topo I), in the presence and absence of 9-aminocamptothecin, on the Topo I-DNA interaction. Anti-Cancer Drugs 10:647-653. [DOI] [PubMed] [Google Scholar]
- 28.Prost, S. 1995. Mechanisms of resistance to topoisomerases poisons. Gen. Pharmacol. 26:1673-1684. [DOI] [PubMed] [Google Scholar]
- 29.Proulx, M. E., A. Désormeaux, J. F. Marquis, M. Olivier, and M. G. Bergeron. 2001. Treatment of visceral leishmaniasis with sterically stabilized liposomes containing camptothecin. Antimicrob. Agents Chemother. 45:2623-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redinbo, M. R., L. Stewart, P. Kuhn, J. J. Champoux, and W. G. Hol. 1998. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science 279:1504-1513. [DOI] [PubMed] [Google Scholar]
- 31.Stetler, G. L., G. J. King, and W. M. Huang. 1979. T4 DNA-delay proteins, required for specific DNA replication, form a complex that has ATP-dependent DNA topoisomerase activity. Proc. Natl. Acad. Sci. USA 76:3737-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart, L., M. R. Redinbo, X. Qiu, W. G. Hol, and J. J. Champoux. 1998. A model for the mechanism of human topoisomerase I. Science 279:1534-1541. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto, Y., S. Tsukahara, T. Oh-hara, L. F. Liu, and T. Tsuruo. 1990. Elevated expression of DNA topoisomerase II in camptothecin-resistant human tumor cell lines. Cancer Res. 50:7962-7965. [PubMed] [Google Scholar]
- 34.Tan, K. B., M. R. Mattern, W. K. Eng, F. L. McCabe, and R. K. Johnson. 1989. Nonproductive rearrangement of DNA topoisomerase I and II genes: correlation with resistance to topoisomerase inhibitors. J. Natl. Cancer Inst. 81:1732-1735. [DOI] [PubMed] [Google Scholar]
- 35.Tosh, K., S. Cheesman, P. Horrocks, and B. Kilbey. 1999. Plasmodium falciparum: stage-related expression of topoisomerase I. Exp. Parasitol. 91:126-132. [DOI] [PubMed] [Google Scholar]
- 36.Tsao, Y. P., A. Russo, G. Nyamuswa, R. Silber, and L. F. Liu. 1993. Interaction between replication forks and topoisomerase I-DNA cleavable complexes: studies in a cell-free SV40 DNA replication system. Cancer Res. 53:5908-5914. [PubMed] [Google Scholar]
- 37.Ulukan, H., and P. Swaan. 2002. Camptothecins: a review of their chemotherapeutic potential. Drugs 62:2039-2057. [DOI] [PubMed] [Google Scholar]
- 38.Villa, H., A. Marcos, R. Reguera, R. Balana-Fouce, C. Garcia-Estrada, Y. Perez-Pertejo, B. Tekwani, P. Myler, K. Stuart, M. Bjornsti, and D. Ordonez. 2003. A novel active DNA topoisomerase I in Leishmania donovani. J. Biol. Chem. 278:3521-3526. [DOI] [PubMed] [Google Scholar]
- 39.Wang, J. C. 1985. DNA topoisomerases. Annu. Rev. Biochem. 54:665-697. [DOI] [PubMed] [Google Scholar]
- 40.Wang, J. C. 1991. DNA topoisomerases: why so many? J. Biol. Chem. 266:6659-6662. [PubMed] [Google Scholar]
- 41.White, T. C., F. Fase-Fowler, H. van Luenen, J. Calafat, and P. Borst. 1988. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J. Biol. Chem. 263:16977-16983. [PubMed] [Google Scholar]