Supplemental Digital Content is Available in the Text.
Key Words: Hyper-CL, insulin eye drops, neurotrophic keratopathy, epithelial defect
Abstract
Purpose:
The aim of this study was to report the successful clinical outcome of recalcitrant neurotrophic keratopathy (NK) treated with insulin eye drops associated with therapeutic Hyper-CL soft contact lens (CL) (EyeYon Medical, Ness Ziona, Israel).
Methods:
This study is a case report.
Results:
A 40-year-old man was referred to our clinic for the management of severe recalcitrant NK developed after surgical and adjuvant radiotherapy treatment of adenoid cystic carcinoma of the nasal cavity with basicranial involvement. The patient presented with severe conjunctival hyperemia, a large (7 × 4 mm) central epithelial defect, corneal opacity and thinning, and deep corneal neovascularization. Unpreserved tear substitutes, vitamin A ointment, punctal plug, bandage CL, and autologous serum had been used for the previous 3 months without success. Patient was prescribed insulin eye drops (1 unit per mL), and therapeutic Hyper-CL soft CL was applied to increase the contact time between insulin eye drops and the corneal surface. Follow-up visits were performed at day 10 (T1) and day 20 (T2). A marked reduction in the epithelial defect size was noted at T1 and complete healing was reached at T2. Simultaneously, conjunctival hyperemia and corneal opacity markedly reduced over time with treatment.
Conclusions:
The combination of insulin eye drops and therapeutic Hyper-CL soft CL was effective in determining healing of recalcitrant NK not responsive to standard treatments and bandage CL. It is unclear whether the positive outcomes were determined by insulin eye drops, Hyper-CL, or the combination of both, and future randomized clinical trials are warranted to determine the contribution of each treatment.
Neurotrophic keratopathy (NK) is a degenerative corneal disease characterized by hypoesthesia/anesthesia and deficient corneal tropism, with consequent epithelial defect, ulcer formation, and possibly keratolysis and perforation. It is mostly caused by damage at the level of the V cranial nerve or its branches, herpetic infection, diabetic neuropathy, and corneal surgery.1 Traditionally, conservative therapeutic approaches for NK include withdrawal of therapies that are toxic for the corneal epithelium, frequent use of unpreserved lubricants, bandage contact lens (CL), blood-derived products, matrix metalloproteinase inhibitors in case of keratolysis, and, when available, recombinant human nerve growth factor; other reported successful approaches include the use of purified plasma fibronectin and a combination of substance-P-derived peptide with insulin-like growth factor-I (IGF-I).2–4
Recently, insulin eye drops used 4 times daily at the concentration of 1 unit per mL have been successfully implemented in the armamentarium of medical treatments used for recalcitrant cases of moderate to severe NK.5–7 Mechanisms of action are not clearly understood, but restoration of corneal nerves and/or improved epithelial cell migration are believed to be responsible for its biological effect.8,9 Therapeutic Hyper-CL (EyeYon Medical, Ness Ziona, Israel) is a novel soft CL designed to act as a drug depot to increase bioavailability of eye drops on the corneal surface. This lens has a peculiar shape characterized by a groove with fenestrations and a dual base curve that forms a drug reservoir while shielding the eye. Its use has been reported in combination with ophthalmic solutions (eg, antibiotics, corticosteroids, and autologous serum drops) for the treatment of bacterial keratitis, corneal edema, and severe Sjögren syndrome–associated dry eye disease.10–12 We hypothesize that applying therapeutic Hyper-CL in eyes receiving insulin eye drops could help to increase the chances and the speed of NK healing, and report herein the positive outcomes of the first case of recalcitrant stage III NK treated accordingly.
CASE REPORT
A 40-year-old man was referred to our clinic for the management of stage III NK in the right eye. Seven years before, he underwent endoscopic resection of adenoid cystic carcinoma of the nasal cavity with basicranial involvement. Residual malignant tissue was then successfully treated with carbon ion radiotherapy (total dose 68.8 Gray). The patient developed iatrogenic damage of the right V cranial nerve with the onset of symptoms of decreased vision and watery discharge. The diagnosis of NK was reached elsewhere and various conservative treatments were attempted with no success. In brief, in the first months, tear substitutes and vitamin A ointment were prescribed in association with punctal occlusion; then, owing to the worsening of the clinical image, autologous serum eye drops were administered and continued in combination with standard bandage CL. Short courses of topical corticosteroids (fluorometholone) were also used to control symptoms. At the time of presentation at our institution, Snellen best-corrected visual acuity was limited to 0.01, slitlamp examination revealed conjunctival hyperemia (Efron grade IV), a large epithelial defect (about 7 × 4 mm), diffuse opacity of the cornea with deep inferior neovascularization, and keratolysis in the 3-mm central area and thinning (about one third of the stroma). Blink was complete despite its rate being markedly reduced (6/min as average). Cochet–Bonnet esthesiometry revealed total anesthesia of the cornea in all quadrants with absence of corneal reflex. Contralateral (left) eye was normal with a preserved corneal sensitivity. Ongoing treatments were discontinued, and insulin eye drops (1 unit per mL) were prepared by a compounding pharmacy (Farmacia Europea, Catanzaro, Italy) in a grade D cleanroom and prescribed 4 times daily. Specifically, the formulation of insulin eye drops was obtained combining Humalogsc 5cart 3 mL 100 IU/mL (Eli Lilly, Indianapolis, IN) as source of insulin with artificial tears based on a hydroxypropyl guar that forms a galactomannan-borate polymer network in the presence of boric acid (borate source) in aqueous solution. Detailed description of the insulin eye drops formulation is reported in Table 1, and additional information on the preparation protocol is reported in Supplemental Digital Content 1 (http://links.lww.com/ICO/B575).
TABLE 1.
Detailed Description of Topical Insulin Eye Drops 1IU/mL Formulation
| Substance | Quantity | Description/Function | Producer |
| Humalog®sc 5cart 3 mL 100 IU/mL | 1 mL | Active substance | Eli Illy Italia S.p.A |
| Hydroxypropyl guar | 0.16% w/v | Galactomannan source | Acef Spa |
| Boric acid | 0.7% w/v | Borate source | Acef Spa |
| Sorbitol | 1.4% w/v | Cross-linking inhibitor | Acef Spa |
| Polyethylene glycol 400 | 0.4% w/v | Demulcent | Acef Spa |
| Propylene glycol | 0.3% w/v | Demulcent | Acef Spa |
| Potassium chloride | 0.12% w/v | Tonicity adjusting agent | Acef Spa |
| Sodium chloride | 0.1% w/v | Tonicity adjusting agent | Acef Spa |
| 2-Amino-2-methylpropanol (AMP) | 0.57% w/v | Buffering agent | Acef Spa |
| Sodium hydroxide/hydrochloric acid | q.s. pH 7.9 | pH modifier | Acef Spa |
| Purified water | q.s. 100 | To reach final volume | Acef Spa |
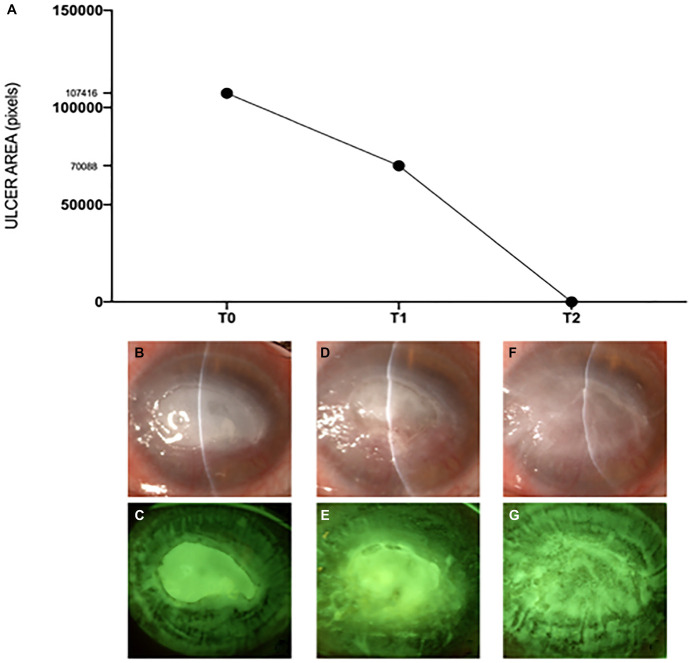
To try to improve the chance and the speed of NK healing while avoiding the progression to keratolysis and perforation, a therapeutic Hyper-CL was also applied (Fig. 1). Ten days later (T1), conjunctival hyperemia was reduced (Efron grade III), as well as the size of the epithelial defect (about 5 × 3 mm). Therapy was therefore continued unchanged until the subsequent follow-up that was scheduled 10 days later (T2). At T2, slitlamp examination showed a further reduction of conjunctival hyperemia (Efron grade II) and a complete healing of the epithelial defect of the cornea that appeared also clearer. Snellen best-corrected visual acuity improved to 0.05 at T2. Figure 2 shows a graph representing for each time point the changes in the area of the epithelial defect that was digitally analyzed using the public domain software ImageJ 1.53t (National Institutes of Health, Bethesda, MD) along with the corresponding slitlamp photographs with and without blue/yellow filters. After NK healing, insulin eye drops were continued and Hyper-CL was kept in place for further 10 days. Currently, 3 months after NK healing, patient is under unpreserved tear substitutes and corneal epithelium continues to be closed. No changes of corneal sensitivity were recorded during the entire course.
FIGURE 1.
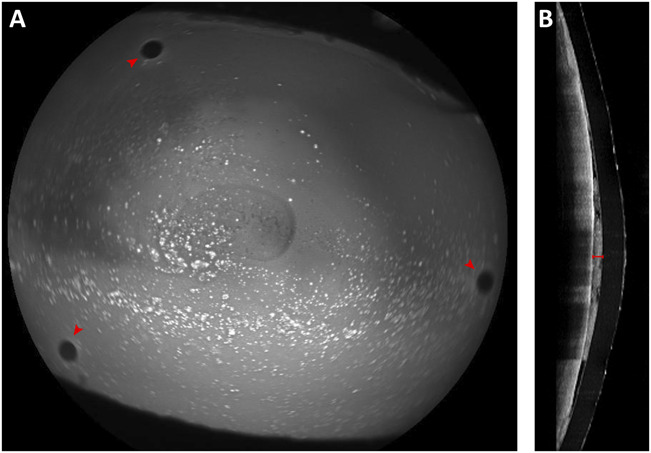
Therapeutic Hyper-CL lens applied on the affected eye evaluated by means of anterior segment optical coherence tomography (AS-OCT). A, Blue autofluorescence highlighting 3 fenestrations at the level of the groove (red arrowheads). B, AS-OCT scan showing the fluid reservoir between corneal surface and Hyper-CL (red caliper).
FIGURE 2.
Course over time of the corneal disease. A, Graph representing the changes of the area of the epithelial defect (expressed in pixels) for each time point (T0, T1, and T2) that was digitally analyzed using the public domain software ImageJ 1.53t (National Institutes of Health, Bethesda, MD). B and C, Slitlamp photographs of the cornea with and without blue/yellow filters at T0. D and E, Slitlamp photographs of the cornea with and without blue/yellow filters at T1. F and G, Slitlamp photographs of the cornea with and without blue/yellow filters at T2.
DISCUSSION
In the present case report, insulin eye drops used in combination with Hyper-CL allowed a fast healing of a recalcitrant case of severe NK characterized by completely abolished corneal sensitivity. Our findings, showing a progressive closure of the epithelial defect up to the complete closure after 20 days of therapy, confirm the interesting activity of insulin eye drops in the setting of complex corneal diseases and the utility of the application of the novel Hyper-CL to increase the residence time of the topical drug, thus providing a boost of the therapy.
The lack of established and readily available therapies makes the medical management of NK challenging in most cases, and surgical approaches may be required for recalcitrant or complicated cases.13 Recently, topical insulin therapy was shown to be effective for inducing corneal reepithelization in patients who were refractory to other therapies. This drug is easily retrievable and unexpensive while bearing an elevated safety profile.14 Because eye protection mechanisms such as blinking, basal and reflex tearing, and nasolacrimal draining are responsible for a reduced permanence of the drug on the ocular surface, improving contact time of insulin eye drops by application of therapeutic Hyper-CL may increase the chance and speed up the process of reepithelialization, especially in eyes at risk for keratolysis and corneal perforation. Because the central base curve of Hyper-CL is steeper than the peripheral curve, an elevated zone at the center creates a depot for ophthalmic solutions. Previous studies demonstrated that a drop applied on the surface of this lens is maintained at the epicorneal space up to 20 minutes.11 Moreover, oxygen supply is ensured by the holes present on the midperiphery of the cornea so the lens can be worn permanently for 7 days, after which it may be removed, cleaned, used for further 7 days, and then disposed. Previous studies evaluating the outcomes of insulin eye drops in the setting of persistent epithelial defect owing to different origins reported a success rate in the closure of the epithelium of 81% to 100% after a variable amount of time ranging from 3 to 124 days.5–7,14,15 Our hypothesis of the synergistic effect of the use of insulin eye drops combined with the wearing of therapeutic Hyper-CL seems to be reasonable considering the short time interval for the healing of NK despite the large area of the epithelial defect of the cornea and the refractory long-standing course of the disease.
Other treatments proved to be helpful in reepithelization of NK. Among these, autologous or allogeneic serum eye drops, fibronectin, a combination of substance-P-derived peptide with IGF-I, and recombinant human nerve growth factor all showed positive results.2–4,16–18 To note, purified plasma fibronectin was shown to be more effective than autologous serum eye drops, whereas a combination of substance-P-derived peptide with IGF-I (or the combination of the peptide derived from these 2 agents) showed, both in vitro and in vivo, a synergistic effect in facilitating corneal epithelial cell migration and corneal epithelial defect closure.2–4 It is therefore conceivable that the combination of the above-mentioned approaches with the therapeutic Hyper-CL might as well convey good clinical outcomes. However, the easy availability and the low cost of insulin eye drops play a major role in the current interest in this topical formulation. Other CL-based attempts have been conducted in the past to vault over the cornea protecting the ocular surface and providing a reservoir for hydration. Prosthetic replacement of the ocular surface ecosystem is a treatment that uses custom-designed and custom-fabricated prosthetic devices to replace or augment the impaired ocular surface functions in complex corneal diseases.19 Although a good efficacy has been reported thanks to high oxygen permeability, fluid-filled reservoir, lack of corneal contact, positional stability on the eye, and protection from lid-related shear forces, limitations to the wider adoption of prosthetic replacement of the ocular surface ecosystem treatment include cost and availability at a limited number of tertiary eye care centers.
In conclusion, insulin eye drops used in combination with Hyper-CL soft CL were safe and effective in inducing in a short time the healing of a case of NK resistant to various previous treatments, including autologous serum. This combined approach represents a promising strategy for the management of severe NK at high risk for keratolysis and corneal perforation. However, it is unclear whether the positive outcomes reported herein are due to the insulin eye drop, the use of Hyper-CL, or the combination of both, and future randomized clinical trials are warranted to determine the contribution of each treatment.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Rosanna Verdiglione, Federica Squillace, and Federico Gallicchio for their technical support in the insulin eye drops preparation.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
Contributor Information
Giuseppe Giannaccare, Email: giuseppe.giannaccare@gmail.com.
Costanza Rossi, Email: costanzarossi9@gmail.com.
Massimiliano Borselli, Email: mborselli93@gmail.com.
Andrea Lucisano, Email: andrealucisano@alice.it.
Sabrina Vaccaro, Email: sabrina.vaccaro1@studenti.unicz.it.
Mario Verdiglione, Email: laboratorio.farmaciaeuropea@gmail.com.
Vincenzo Scorcia, Email: vscorcia@unicz.it.
REFERENCES
- 1.Bonini S, Rama P, Olzi D, et al. Neurotrophic keratitis. Eye (Lond). 2003;17:989–995. [DOI] [PubMed] [Google Scholar]
- 2.Nishida T, Ohashi Y, Awata T, et al. Fibronectin. A new therapy for corneal trophic ulcer. Arch Ophthalmol. 1983;101:1046–1048. [DOI] [PubMed] [Google Scholar]
- 3.Chikama T, Fukuda K, Morishige N, et al. Treatment of neurotrophic keratopathy with substance-P-derived peptide (FGLM) and insulin-like growth factor I. Lancet. 1998;351:1783–1784. [DOI] [PubMed] [Google Scholar]
- 4.Nishida T. Neurotrophic mediators and corneal wound healing. Ocul Surf. 2005;3:194–202. [DOI] [PubMed] [Google Scholar]
- 5.Soares RJDSM, Arêde C, Sousa Neves F, et al. Topical insulin-utility and results in refractory neurotrophic keratopathy in stages 2 and 3. Cornea. 2022;41:990–994. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Valle D, Burgos-Blasco B, Rego-Lorca D, et al. Comparison of the efficacy of topical insulin with autologous serum eye drops in persistent epithelial defects of the cornea. Acta Ophthalmol. 2022;100:e912–e919. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Valle D, Burgos-Blasco B, Gegundez-Fernandez JA, et al. Topical insulin for refractory persistent corneal epithelial defects. Eur J Ophthalmol. 2021;31:2280–2286. [DOI] [PubMed] [Google Scholar]
- 8.Shanley LJ, McCaig CD, Forrester JV, et al. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyu J, Lee KS, Joo CK. Transactivation of EGFR mediates insulin-stimulated ERK1/2 activation and enhanced cell migration in human corneal epithelial cells. Mol Vis. 2006;12:1403–1410. [PubMed] [Google Scholar]
- 10.Daniel Raj Ponniah LR, Ranilakshmi V, Anandan H, et al. Novel drug-repository contact lens for prolonging the antimicrobial-cornea interaction for bacterial keratitis treatment: randomised controlled trial results. BMJ Open Ophthalmol. 2022;7:e001093. [Google Scholar]
- 11.Daphna O, Mimouni M, Keshet Y, et al. Therapeutic HL-contact lens versus standard bandage contact lens for corneal edema: a prospective, multicenter, randomized, crossover study. J Ophthalmol. 2020;2020:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romano V, Romano D, Semeraro P, et al. Therapeutic Hyper-CL soft contact lens in Sjögren's syndrome. Am J Ophthalmol Case Rep. 2022;28:101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Versura P, Giannaccare G, Pellegrini M, et al. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. 2018;10:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang AL, Weinlander E, Metcalf BM, et al. Use of topical insulin to treat refractory neurotrophic corneal ulcers. Cornea. 2017;36:1426–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balal S, Din N, Ashton C, et al. Healing of chemical injury-related persistent corneal epithelial defects with topical insulin. Cornea. 2022;42:1000–1004. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111:1115–1120. [DOI] [PubMed] [Google Scholar]
- 17.Versura P, Buzzi M, Giannaccare G, et al. Targeting growth factor supply in keratopathy treatment: comparison between maternal peripheral blood and cord blood as sources for the preparation of topical eye drops. Blood Transfus. 2016;14:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127:14–26. [DOI] [PubMed] [Google Scholar]
- 19.Lim P, Ridges R, Jacobs DS, et al. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol. 2013;156:1095–1101. [DOI] [PubMed] [Google Scholar]