Abstract
We have found that broadband light (380 to 520 nm) rapidly and selectively kills oral black-pigmented bacteria (BPB) in pure cultures and in dental plaque samples obtained from human subjects with chronic periodontitis. We hypothesize that this killing effect is a result of light excitation of their endogenous porphyrins. Cultures of Prevotella intermedia and P. nigrescens were killed by 4.2 J/cm2, whereas P. melaninogenica required 21 J/cm2. Exposure to light with a fluence of 42 J/cm2 produced 99% killing of P. gingivalis. High-performance liquid chromatography demonstrated the presence of various amounts of different porphyrin molecules in BPB. The amounts of endogenous porphyrin in BPB were 267 (P. intermedia), 47 (P. nigrescens), 41 (P. melaninogenica), and 2.2 (P. gingivalis) ng/mg. Analysis of bacteria in dental plaque samples by DNA-DNA hybridization for 40 taxa before and after phototherapy showed that the growth of the four BPB was decreased by 2 and 3 times after irradiation at energy fluences of 4.2 and 21 J/cm2, respectively, whereas the growth of the remaining 36 microorganisms was decreased by 1.5 times at both energy fluences. The present study suggests that intraoral light exposure may be used to control BPB growth and possibly benefit patients with periodontal disease.
Dental plaque is a biofilm that develops naturally on teeth. It consists of aggregates of 500 to 600 different bacterial taxa embedded in a matrix of polymers of bacterial and salivary origin (30). In healthy subjects, dental plaque remains stable for prolonged periods of time because of a dynamic balance among the resident members of its microbial community (12). Disease arises when the microbial homeostasis within the plaque breaks down because of disruption of the habitat's ecology (14). In periodontal disease, there is a shift in the composition of subgingival plaque's microflora that colonizes tooth surfaces and epithelial cells in the periodontal pocket to a more proteolytic gram-negative anaerobic community including the pigmented rods in the genera Porphyromonas and Prevotella (19, 29). Black-pigmented anaerobes such as Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens have been implicated as pathogens associated with the initiation and progression of periodontitis (3-5, 31, 32). These species depend largely on external heme as an iron source for their growth (20) and accumulate a cell surface black pigment that mainly consists of μ-oxobisheme of iron protoporphyrin IX (PpIX) in P. gingivalis (25) and monomeric iron PpIX (hematin) in P. intermedia and P. nigrescens (26). Although iron PpIX is the predominant pigment in black-pigmented bacteria (BPB), these species also accumulate various amounts of iron-free PpIX (23, 24), which is photosensitive (18). The UV-visible absorption spectrum of porphyrins exhibits an intense peak at around 405 nm, followed by several weaker peaks at 505, 540, 575, and 630 nm (27). As a result, excitation of PpIX by light causes energy transfer from the PpIX triplet state to molecular oxygen to produce the excited-state singlet oxygen (type II photoprocess), which can then oxidize and destroy various biological molecules such as lipids, proteins, and nucleic acids (22). Inactivation of oral BPB by visible light has been reported previously with an argon laser (6, 7) and a helium-neon laser (9) at high-energy fluences ranging of up to 360 J/cm2.
In the present study, we have investigated the effect of broadband light (380 to 520 nm) on BPB in pure cultures, as well as in dental plaque samples obtained from humans with chronic periodontitis. Our hypothesis was that blue light could achieve rapid and selective elimination of periodontopathogenic BPB by exciting their endogenous porphyrins.
MATERIALS AND METHODS
Microorganisms.
The pure bacterial strains used in this study were Porphyromonas gingivalis ATCC 33277, P. intermedia ATCC 25611, P. nigrescens ATCC 33563, Prevotella melaninogenica ATCC 25845, and Streptococcus constellatus ATCC 27823. Cultures were maintained by weekly subculture in Trypticase soy agar with 5 μg of hemin per ml, 0.3 μg of vitamin K per ml, and 5% sheep blood (manufactured plates from Northeast Labs, Waterville, Maine). Cultures were grown in the presence of 80% N2, 10% H2, and 10% CO2 at 35°C in an anaerobic chamber for 48 to 72 h. On the day of the experiment, the cells were harvested by centrifugation and resuspended in brain heart infusion broth (Becton Dickson & Company, Sparks, Md.). Cells were dispersed by sonication and repeated passage through Pasteur pipettes. For adjustment of inoculum density, cell numbers were estimated in a spectrophotometer (wavelength, 600 nm; 0.1 optical density [OD] unit equals approximately 108 cells/ml) in 1-ml cuvettes.
HPLC analysis.
For extraction of total porphyrins from P. gingivalis, P. intermedia, P. nigrescens, and P. melaninogenica, a two-phase method was used that included the use of acidified ethyl acetate (ethyl acetate-glacial acetic acid at 2:1), followed by 1 M HCl. Iron-containing porphyrins (heme) was extracted into the organic solvent but not extracted back into the acid phase. Thus, heme compounds were excluded. Porphyrins were quantified by scanning from 640 to 670 nm with an excitation wavelength of 400 nm with a Fluoromax-3 spectrofluorometer (Jobin Yvon, Edison, N.J.). The level of total porphyrins was calculated on the basis of a reference porphyrin mixture standard (see Fig. 3). Porphyrins were fractionated by a reversed-phase high-performance liquid chromatography (HPLC) method (11). The Waters HPLC system (Waters, Milford, Mass.) consisted of a 600E system controller, a 717 autosampler, a 470 fluorescence detector, and a 745B data module for peak integration. Separation of porphyrins was performed on a Phenomenex C18 Bondclone column (150 by 3.9 mm; Phenomenex, Torrance, Calif.).
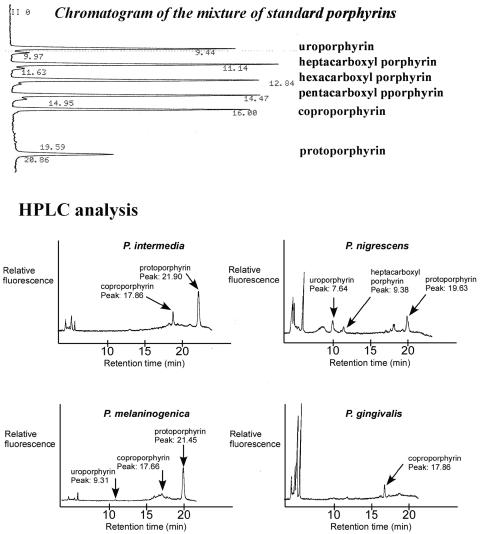
FIG. 3.
Chromatogram of a mixture of standard porphyrins in the order of decreasing retention time and HPLC analysis of the porphyrin content of BPB.
Subjects and plaque samples.
Samples of subgingival plaque were taken from 15 patients. Permission to collect dental plaque samples was authorized by Institutional Review Board-approved informant consent. All patients were diagnosed as having chronic periodontitis with pockets greater than 3 mm in depth. None of them used antibiotics or had undergone periodontal treatment during the 3 months prior to sampling. Dental plaque samples were taken from the supra- and subgingival mesiobuccal aspects of premolars or molars in each patient with individual sterile Gracey curettes. After their removal, the samples were placed immediately into an Eppendorf tube with 5 ml of prereduced anaerobically sterilized Ringer's solution. Cells were dispersed by sonication and repeated passage through Pasteur pipettes. Cell numbers were measured in a spectrophotometer with 1-ml tubes (1 OD unit equals approximately 109 cells/ml at 600 nm).
Light source.
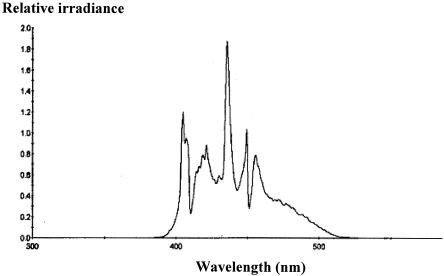
The irradiation source (BriteSmile model BS3000; BriteSmile, Inc., Walnut Creek, Calif.) consisted of two Mejiro metal halide gas plasma lamps with reflecting elements. The lamps are attached to two (one each) optical fiber bundles that lead to a “front end” that breaks each bundle up into three rectangular emitting output areas. The spectral range of the light source was 380 to 520 nm (Fig. 1). A strong peak occurred at 435 nm, and secondary peaks occurred at 405, 420, 450, and 455 nm (Fig. 1). The light source emitted 1.7% of the total energy in the UV A range of the spectrum (380 to 400 nm).
FIG. 1.
Emission spectrum of the light source provided by BriteSmile, Inc., Walnut Creek, Calif.
Phototherapy studies. (i) Bacterial cultures.
Suspensions of bacteria (108/ml) were placed in the wells of 24-well plates. All four BPB and S. constellatus (non-black-pigmented control species) were exposed to light from the halogen lamp at room temperature from above with fluences ranging from 0 to 42 J/cm2 at an irradiance of 70 mW/cm2. The measured temperature rise in the medium was less than 3°C during exposure to an irradiation fluence of 42 J/cm2. All plates were kept covered during illumination in order to maintain the sterility of the culture. After illumination of the appropriate wells, serial dilutions of the contents of each well were prepared in brain heart infusion broth, and 100-μl aliquots were spread over the surfaces of blood agar plates enriched with vitamin K, N-acetylmuramic acid, and hemin. The plates were incubated anaerobically at 35°C for 7 days. Survival fractions in each well were calculated by counting the CFU on the plates and dividing by the number of colonies from control plates that were not exposed to light and kept at room temperature for periods equal to the irradiation times.
(ii) Pooled dental plaque.
Dispersed dental plaque (108/ml) was placed in the wells of 24-well plates and exposed to light with fluences of 4.2 and 21 J/cm2 at an irradiance of 70 mW/cm2. After illumination, survival was estimated by two methods, i.e., by counting CFU as described above and then performing total DNA probe counts of 40 bacterial species (Table 1) by checkerboard DNA-DNA hybridization (28). For DNA probe analysis, Tris-EDTA buffer (1.5 ml) was added to the plates and the bacterial colonies were scraped off the surface with sterile L-shaped glass rods. The suspensions were placed into individual Eppendorf tubes and sonicated for 10 s to break up clumps. Each suspension was adjusted to a final OD of 1.0, which corresponded to approximately 109 cells. Ten microliters of the suspension (107 cells) was removed and placed in another Eppendorf tube with 140 μl of TE buffer and 150 μl of 0.5 M NaOH. The samples were lysed, and the DNA was placed in lanes on positively charged nylon membrane with a Minislot device (Immunetics, Cambridge, Mass.). After fixation of the DNA to the membrane, the membrane was placed in a Miniblotter 45 (Immunetics) with the lanes of DNA perpendicular to the lanes of the device. Digoxigenin-labeled whole genomic DNA probes for 40 bacterial taxa (Table 1) were hybridized in individual lanes of the Miniblotter. After hybridization, the membranes were washed at high stringency and the DNA probes were detected with antibody to digoxigenin conjugated with alkaline phosphatase for chemifluorescence detection. Signals were detected with AttoPhos substrate (Amersham Life Science, Arlington Heights, Ill.) and scanned with a Storm Fluorimager (Molecular Dynamics, Sunnyvale, Calif.). Computer-generated images were analyzed to determine the fluorescence intensity associated with each sample and probe. Two lanes in each membrane contained DNA standards with 1 ng (105 bacteria) and 10 ng (106 bacteria) of each species. The sensitivity of the assay was adjusted to permit detection of 104 cells of a given species by adjusting the concentration of each DNA probe. The measured fluorescence intensities were converted to absolute counts by comparison with the standards on the same membrane. Failure to detect a signal was recorded as zero. Inhibition of BPB growth was defined as the ratio of DNA probe counts before exposure to light to those after exposure to light. Differences between mean growth inhibition ratios or percentages were tested for statistical significance with Student's t test.
TABLE 1.
Strains used for the development of DNA probesa
| Bacterium | Strain(s) |
|---|---|
| Actinobacillus actinomycetemcomitans | 43718, 29523 |
| Actinomyces gerencseriae | 23860 |
| Actinomyces israelii | 12102 |
| Actinomyces naeslundii genospecies 1 | 12104 |
| Actinomyces naeslundii genospecies 2 | 43146 |
| Actinomyces odontolyticus | 17929 |
| Tannerella forsythensis | 43037 |
| Campylobacter gracilis | 33236 |
| Campylobacter rectus | 33238 |
| Campylobacter showae | 51146 |
| Capnocytophaga gingivalis | 33624 |
| Capnocytophaga ochracea | 33596 |
| Capnocytophaga sputigena | 33612 |
| Eikenella corrodens | 23834 |
| Eubacterium nodatum | 33099 |
| Eubacterium saburreum | 33271 |
| Fusobacterium nucleatum subsp. nucleatum | 25586 |
| Fusobacterium nucleatum subsp. polymorphum | 10953 |
| Fusobacterium nucleatum subsp. vincentii | 49256 |
| Fusobacterium periodonticum | 33693 |
| Gemella morbillorum | 27824 |
| Leptotrichia buccalis | 14201 |
| Neisseria mucosa | 19696 |
| Peptostreptococcus micros | 33270 |
| Porphyromonas gingivalis | 33277 |
| Prevotella intermedia | 25611 |
| Prevotella melaninogenica | 25845 |
| Prevotella nigrescens | 33563 |
| Propionibacterium acnes | 11827, 11828 |
| Selenomonas noxia | 43541 |
| Streptococcus anginosus | 33397 |
| Streptococcus constellatus | 27823 |
| Streptococcus gordonii | 10558 |
| Streptococcus intermedius | 27335 |
| Streptococcus mitis | 49456 |
| Streptococcus oratis | 35037 |
| Streptococcus sanguis | 10556 |
| Treponema denticola | BI |
| Treponema socranskii | SI |
| Veillonella parvula | 10790 |
All strains were obtained from the American Type Culture Collection except T. denticola B1 and T. socranskii S1, which were obtained from The Forsyth Institute.
RESULTS
Photodestruction of bacterial cultures.
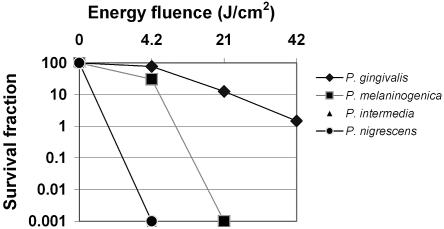
The effects of increasing light doses from the light source on cultures of BPB are shown in Fig. 2. P. intermedia and P. nigrescens were completely killed by exposure to light with a fluence of 4.2 J/cm2 (1 min of irradiation). P. melaninogenica was reduced by 70% by exposure to 4.2 J/cm2 (P < 0.008) and completely killed by exposure to 21 J/cm2 (5 min of irradiation). The P. gingivalis survival fractions were 77.25% (P < 0.001), 12.55% (P < 0.00002), and 1.48% (P < 0.000001) after exposure to light with fluences of 4.2, 21, and 42 J/cm2, respectively. S. constellatus, a nonpigmented species, was unaffected by irradiation (data not shown).
FIG. 2.
Survival fraction of oral species following irradiation of planktonic cell suspensions. A survival fraction of 100% represents 108 microorganisms. Each plotted point is the mean of triplicate determinations.
HPLC analysis.
HPLC revealed that BPB expressed different porphyrin patterns (Fig. 3). The percent porphyrin content in BPB is shown in Table 2. The amounts of porphyrin were 267, 47, 41, and 2.2 ng/mg of protein in P. intermedia, P. nigrescens, P. melaninogenica, and P. gingivalis, respectively. The large signals appearing at the solvent front in the chromatograms of P. nigrescens and P. gingivalis represent low-molecular-weight fluorescent compounds of bacterial origin (Fig. 3).
TABLE 2.
Porphyrin contents expressed in BPB
| Porphyrin | % of porphyrin content of:
|
|||
|---|---|---|---|---|
| P. intermedia | P. nigrescens | P. melaninogenica | P. gingivalis | |
| Uroporphyrin | 33 | 2 | ||
| Heptacarboxyl porphyrin | 16 | |||
| Hexacarboxyl porphyrin | ||||
| Pentacarboxyl porphyrin | ||||
| Isocoproporphyrin | ||||
| Coproporphyrin | 17 | 12 | 100 | |
| Protoporphyrin | 83 | 51 | 85 | |
Phototherapy of dental plaque microorganisms. (i) CFU.
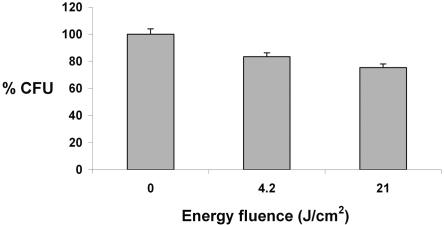
Figure 4 shows the reduction in the total number of CFU after exposure of dental plaque samples to light with energy fluences of 4.2 and 21 J/cm2. The survival fractions were reduced by 17% (P < 0.00002) and 25% (P < 0.0000007), respectively.
FIG. 4.
Reduction in total CFU counts after exposure of pooled dental plaque samples to visible light at 4.2 and 21 J/cm2. Each bar is the mean of values obtained from 15 patients (data from each patient were representative of three independent experiments). Error bars denote the standard error of the mean.
(ii) Checkerboard DNA-DNA hybridization.
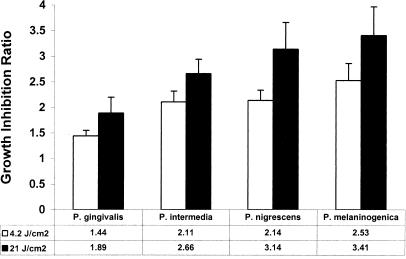
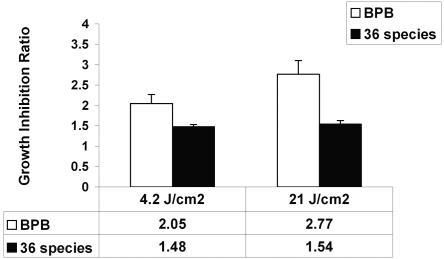
Figure 5 shows the growth inhibition ratios of BPB after exposure of dental plaque samples to light with energy fluences of 4.2 and 21 J/cm2. The order of growth inhibition was P. melaninogenica > P. nigrescens > P. intermedia > P. gingivalis for both energy fluencies. The growth inhibition ratios of all BPB were statistically significantly different from those of controls at both energy fluences (P < 0.05). On the other hand, the growth inhibition ratios of BPB at 21 J/cm2 were not statistically significantly different from those obtained at 4.2 J/cm2 (P > 0.05), with the exception of P. intermedia (P < 0.02). The growth of all four BPB was suppressed 2 and 2.8 times at energy fluences of 4.2 and 21 J/cm2, respectively (P < 0.05), whereas the remaining 36 microorganisms (Table 1) were inhibited 1.5 times at both energy fluences (Fig. 6).
FIG. 5.
Inhibition of BPB growth after exposure to light. The bars represent the ratio of DNA counts before exposure to light to those after exposure to light (mean ± standard error of the mean, n = 15 subjects).
FIG. 6.
Suppression of the BPB group (four species) and that of 36 other microorganisms after exposure of dental plaque to light. The bars represent the mean growth inhibition ratios (ratios of DNA counts before exposure to light to those after exposure to light) obtained from 15 patients (data from each patient were representative of three independent experiments), and the error bars represent the standard error of the mean.
DISCUSSION
In healthy subjects, dental plaque remains stable for prolonged periods of time because of a dynamic balance among the resident members of its microbial community (15). A major disturbance of the local habitat can cause a breakdown of this microbial homeostasis that may lead to enrichment of the microbial community by pathogens (13, 14). The primary goal of a strategy for disease prevention and control should be specific suppression of key pathogens, such as P. gingivalis, which may result in an increase in the microbial flora that is associated with health. The specific hypothesis of this study was that blue light could achieve a rapid and selective elimination of oral BPB by exciting their endogenous porphyrins.
Inactivation of oral BPB, such as P. intermedia and P. gingivalis, by visible light from an argon laser (wavelength range, 488 to 514 nm) (6, 7) and a helium-neon laser (wavelength, 633 nm) (9) at high-energy fluences ranging from 200 to 360 J/cm2 has been reported previously. The 380- to 520-nm spectral range of light used in our studies matches the strongest porphyrin photoexcitation band at 405 to 415 nm and a small band at 505 nm (27). The green and red lights used in the above-mentioned studies (6, 7, 9) targeted only two small absorption peaks of endogenous porphyrins, and thus high-energy fluences were required to achieve bacterial inactivation. Blue light has also been used for eradication of Propionibacterium acnes, the gram-positive species that causes acne (1, 21), which produces endogenous porphyrins (mainly coproporphyrin and PpIX) that absorb energy in the near-UV and blue parts of the light spectrum (8, 10, 16, 17). A significant improvement in inflammatory lesions of patients with acne vulgaris after exposure to blue light with peaks at 405 and 420 nm has been demonstrated (21). However, the cumulative energy fluences used (320 J/cm2) were much higher than those in our studies (21).
Our results showed the presence of different porphyrin patterns expressed in BPB (Table 2). The amount of endogenous porphyrin produced in P. intermedia was 120, 6.5, and 5.5 times higher than those in P. gingivalis, P. melaninogenica, and P. nigrescens, respectively. Although P. gingivalis and P. melaninogenica showed less susceptibility to blue light than P. intermedia did, as expected, both P. nigrescens and P. intermedia were completely killed after 1 min of irradiation. There are two ways to explain this discrepancy. It is possible that P. intermedia requires a lower-energy fluence for complete killing than P. nigrescens does and/or the porphyrin content of microorganisms may not be the sole determinant of photosensitivity.
The checkerboard DNA-DNA hybridization technique was used for identification and enumeration of bacterial species in dental plaque samples before and after exposure to light with whole genomic probes for 40 test taxa (28). This method offers advantages over the reverse-capture oligonucleotide method (2), which uses synthetic oligonucleotide probes. The latter, although highly specific, has proven difficult to use in applications requiring quantitative estimates of bacterial numbers because of difficulties in controlling the PCR step required for multiple samples and the lack of a suitable universal probe for oral bacteria. The whole-genomic method has permitted estimation of bacterial numbers by adjusting the average level of alkaline phosphatase labeling. With probit modeling, the response function of each of the probes was found to be linear in log10 N with a correlation coefficient of 0.97. The detection limit of this method was estimated by computing the number equivalent to 1.97 times the standard deviation of background samples, the lowest value that one can say is statistically significantly different from zero (P < 0.05).
When dental plaque samples from human subjects were irradiated, P. melaninogenica showed the highest susceptibility to light, followed by P. nigrescens, P. intermedia, and P. gingivalis (Fig. 5). All of the Prevotella species showed similar patterns of susceptibility to light, with growth inhibition ratios ranging between 2.1 (4.2 J/cm2) and 3.4 (21 J/cm2). The growth of P. gingivalis was inhibited 1.4 (4.2 J/cm2) to 1.9 (21 J/cm2) times. These data are in accordance with those obtained in a previous study in which exposure of human subgingival plaque samples to red light at 633 nm led to 60 and 40% elimination of Prevotella species and P. gingivalis, respectively (9). However, the energy fluence delivered to the species was 360 J/cm2 since the red light corresponded to the long-wavelength absorption maximum of porphyrins. The same study demonstrated a reduction in the number of CFU of other anaerobic and aerobic dental plaque microorganisms by 50% as a result of their exposure to red light (9). In our study, the reduction of CFU in dental plaque samples was 25% (Fig. 4) after exposure to light with an energy fluence of 21 J/cm2. The microbial analysis showed that the growth of the remaining 36 taxa was suppressed 1.5 times at both energy fluences whereas the growth of all four BPB was inhibited 2 to 2.8 times (Fig. 6). Some of these non-black-pigmented species may also contain porphyrins and/or other cell pigments, which can explain their susceptibility to light.
These data suggest that visible light could be used prophylactically to stabilize the normal microbial composition of plaque by suppressing potentially pathogenic BPB. Compared with other forms of periodontal therapy (scaling, mouthwashes, surgery), this form of treatment would offer many advantages; it is painless, rapid, and devoid of drug toxicity; has no effect on taste; and is selective in its effect.
Acknowledgments
We thank Phil Stewart, The Center for Biofilm Engineering, Montana State University—Bozeman, for critically reading the manuscript and Gordon Row, Healthcare and Bioscience Practice, IDEO, Lexington, Mass., and John Warner, BriteSmile, Inc., Walnut Creek, Calif., for useful advice and suggestions.
This work was supported in part by the National Institute of Dental and Craniofacial Research (DE-14360) and by BriteSmile, Inc.
REFERENCES
- 1.Ashkenazi, H., Z. Malik, Y. Harth, and Y. Nitzan. 2003. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 35:17-24. [DOI] [PubMed] [Google Scholar]
- 2.Becker, M. R., B. J. Paster, E. J. Leys, M. L. Moeschberger, S. G. Kenyon, J. L. Galvin, S. K. Boches, F. E. Dewhirst, and A. L. Griffen. 2002. Molecular analysis of bacterial species associated with childhood caries. J. Clin. Microbiol. 40:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlén, G. G. 1993. Black-pigmented gram-negative anaerobes in periodontitis. FEMS Immunol. Med. Microbiol. 6:181-192. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee, A. D., and S. S. Socransky. 1994. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 5:78-111. [DOI] [PubMed] [Google Scholar]
- 5.Haffajee, A. D., M. A. Cugini, A. Tanner, R. P. Pollack, C. Smith, R. L. Kent, Jr., and S. S. Socransky. 1998. Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. J. Clin. Periodontol. 25:346-353. [DOI] [PubMed] [Google Scholar]
- 6.Henry, C. A., M. Judy, B. Dyer, M. Wagner, and J. L. Matthews. 1995. Sensitivity of Porphyromonas and Prevotella species in liquid media to argon laser. Photochem. Photobiol. 61:410-413. [DOI] [PubMed] [Google Scholar]
- 7.Henry, C. A., B. Dyer, M. Wagner, M. Judy, and J. L. Matthews. 1996. Phototoxicity of argon laser irradiation on biofilms of Porphyromonas and Prevotella species. J. Photochem. Photobiol. B: Biol. 34:123-128. [DOI] [PubMed] [Google Scholar]
- 8.Kjeldstad, B., and A. Johnsson. 1986. An action spectrum for blue and near ultraviolet inactivation of Propionibacterium acnes: with emphasis on a possible porphyrin photosensitization. Photochem. Photobiol. 43:67-70. [DOI] [PubMed] [Google Scholar]
- 9.König, K., M. Teschke, B. Sigusch, E. Glockmann, S. Eick, and W. Pfister. 2000. Red light kills bacteria via photodynamic action. Cell. Mol. Biol. 46:1297-1303. [PubMed] [Google Scholar]
- 10.Lee, W. L. S., A. R. Shalita, and M. B. Poh-Fitzpatrick. 1978. Comparative studies of porphyrin production in Propionibacterium acnes and Propionibacterium granulosum. J. Bacteriol. 133:811-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim, C. K., and T. J. Peters. 1984. Urine and faecal porphyrins profiles by reversed-phase high-performance liquid chromatography in the porphyrias. Clin. Chim. Acta 139:55-63. [DOI] [PubMed] [Google Scholar]
- 12.Marsh, P. D. 1989. Host defenses and microbial homeostasis: role of microbial infections. J. Dent. Res. 68:1567-1575. [Google Scholar]
- 13.Marsh, P. D. 1994. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 8:263-271. [DOI] [PubMed] [Google Scholar]
- 14.Marsh, P. D., and D. J. Bradshaw. 1997. Physiological approaches to the control of oral biofilms. Adv. Dent. Res. 11:176-185. [DOI] [PubMed] [Google Scholar]
- 15.Marsh, P. D. 2003. Plaque as a biofilm: pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 9:16-22. [DOI] [PubMed] [Google Scholar]
- 16.McGinley, K. J., G. F. Webster, and J. J. Leyden. 1980. Facial follicular porphyrin fluorescence—correlation with age and density of Propionibacterium acnes. Br. J. Dermatol. 102:437-445. [DOI] [PubMed] [Google Scholar]
- 17.Meló, T. B., and M. Johnsson. 1982. In vivo porphyrin fluorescence from Propionibacterium acnes—a characterization of fluorescing pigments. Dermatologica 164:167-174. [PubMed] [Google Scholar]
- 18.Meló, T. B. 1987. Uptake of protoporphyrin and violet light photodestruction of Propionibacterium acnes. Z. Naturforsch. 42:123-128. [DOI] [PubMed] [Google Scholar]
- 19.Moore, W. E. C., and L. V. H. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000 5:66-77. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto, K., K. Nakayama, T. Kadowaki, N. Abe, D. B. Ratnayake, and K. Yamamoto. 1998. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J. Biol. Chem. 273:21225-21231. [DOI] [PubMed] [Google Scholar]
- 21.Papageorgiou, P., A. Katsambas, and A. Chu. 2000. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br. J. Dermatol. 142:973-978. [DOI] [PubMed] [Google Scholar]
- 22.Redmond, R. W., and J. N. Gamlin. 1999. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 70:391-475. [PubMed] [Google Scholar]
- 23.Shah, H. N., R. Bonnett, B. Mateen, and R. A. D. Williams. 1979. Porphyrin pigmentation of subspecies of Bacteroides melaninogenicus. Biochem. J. 180:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah, H. N., and S. E. Gharbia. 1993. Biochemical and chemical analyses of black-pigmented gram-negative anaerobes. FEMS Immunol. Med. Microbiol. 6:89-96. [DOI] [PubMed] [Google Scholar]
- 25.Smalley, J. W., J. Silver, P. J. Marsh, and A. J. Birss. 1998. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the μ-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem. J. 331:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smalley, J. W., J. Silver, A. J. Birss, R. Withnall, and P. J. Titler. 2003. The haem pigment of the oral anaerobes Prevotella nigrescens and Prevotella intermedia is composed of iron (III) protoporphyrin IX in the monomeric form. Microbiology 149:1711-1718. [DOI] [PubMed] [Google Scholar]
- 27.Smith, K. M. 1975. Porphyrins and metalloporphyrins. Elsevier, Amsterdam, The Netherlands.
- 28.Socransky, S. S., C. Smith, L. Martin, B. J. Paster, F. E. Dewhirst, and A. E. Levin. 1994. Checkerboard DNA-DNA hybridization. BioTechniques 17:788-792. [PubMed] [Google Scholar]
- 29.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 30.Socransky, S. S., and A. D. Haffajee. 2002. Dental biofilms: difficult therapeutic targets. Periodontol. 2000 28:12-55. [DOI] [PubMed] [Google Scholar]
- 31.Ximenez-Fyvie, L. A., A. D. Haffajee, S. Som, M. Thompson, G. Torresyap, and S. S. Socransky. 2000. The effect of repeated professional supragingival plaque removal on the composition of the supra- and subgingival microbiota. J. Clin. Periodontol. 27:637-647. [DOI] [PubMed] [Google Scholar]
- 32.Ximenez-Fyvie, L. A., A. D. Haffajee, and S. S. Socransky. 2000. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J. Clin. Periodontol. 27:722-732. [DOI] [PubMed] [Google Scholar]