Abstract
In Helicobacter pylori, the contribution of efflux proteins to antibiotic resistance is not well established. As translocases that act in parallel may have overlapping substrate specificities, the loss of function of one such translocase may be compensated for by that of another translocase with no effect on susceptibilities to antibiotics. The genome of H. pylori 26695 was assessed for the presence of putative translocases and outer membrane efflux or TolC-like proteins which could interact to form efflux systems involved in drug resistance. Twenty-seven translocases were identified, of which HP1184 was the sole representative of the multidrug and toxic compound extrusion family of translocases and which could thus have a unique substrate specificity. In addition, four TolC-like proteins (HP0605, HP0971, HP1327, and HP1489) were identified. Thus, it is feasible that inactivation of a TolC-like protein would affect the functions of multiple translocases. We aimed to determine whether efflux systems contribute to antimicrobial susceptibility by evaluation of the susceptibility profiles of an HP1184-knockout mutant, four mutants in which one of the four TolC homologs was inactivated, as well as a mutant in which both HP0605 and HP0971 were inactivated. The HP1184- and HP1489-knockout mutants both showed increased susceptibilities to ethidium bromide, while the HP0605-knockout mutant exhibited increased susceptibilities to novobiocin and sodium deoxycholate. The HP0605 and HP0971 double-knockout mutant was also more susceptible to metronidazole, in addition to being susceptible to novobiocin and sodium deoxycholate. Thus, active efflux is an eminent means of resistance to antimicrobials in H. pylori and resembles the situation in other bacteria.
Helicobacter pylori colonizes the gastric mucus layer of approximately half of the world's population (9, 10). Infection with this bacterium results in a superficial gastritis, which can progress into peptic ulceration. Moreover, H. pylori infection is strongly associated with the development of atrophic gastritis and gastric cancer (9).
Present treatments for H. pylori infection include a proton pump inhibitor in combination with amoxicillin or metronidazole (MTZ) and clarithromycin (14). Bacterial resistance to MTZ or clarithromycin hampers the treatment of H. pylori infections. Also, additional treatment with, for example, tetracycline (TET), ultimately results in increasing rates of resistance to the drug. Possible mechanisms of intrinsic drug resistance involve decreased drug uptake or increased drug efflux (22).
Five families of multidrug efflux transporters have been described: small multidrug resistance (SMR) proteins, multidrug and toxic compound extrusion (MATE) proteins, the major facilitator superfamily (MFS), the ATP-binding cassette (ABC) superfamilies, and the resistance-nodulation-cell division (RND) family (23). Generally, in gram-negative bacteria, the last three efflux pumps or translocases are located in the inner membrane and are therefore also called inner membrane efflux proteins (IEPs), which act with two other components, a periplasmic efflux protein (PEP), which facilitates the interaction with the other component, and an outer membrane efflux protein (OEP), which is TolC or a TolC homolog (13). Bacteria may have a number of different translocases and may act with only a limited number of OEPs. In Escherichia coli, four TolC-like proteins may act with an eightfold higher number of translocases (16). Poor sequence conservation of the OEP family in bacterial efflux systems in part hampers functional characterization, but the presence of structural domains within these proteins as well as genetic clustering with a translocase and an accessory component facilitate their identification (13).
Johnson and Church (13) identified two TolC homologs, HP0605 and HP1489, in H. pylori on the basis of their structural similarities with OEP domains. In addition, Bina and coworkers (6) identified three RND efflux systems, each of which consisted of a translocase, an accessory protein, and a TolC homolog (HP0605 to HP0607, HP0971 to HP0969, and HP1327 to HP1329). Nevertheless, they could not establish a role for these efflux systems in antibiotic resistance, since knockout mutants for each of the three translocases (HP0607, HP0969, and HP1329) displayed profiles of susceptibility to 19 different antibiotics identical to that of their wild-type strains (6). Still, since parallel translocases may have overlapping substrate specificities (16), the loss of function in a knockout mutant for one translocase may be compensated for by the activity of another translocase.
In silico, we identified 26 putative translocases belonging to the ABC, MFS, and RND families of translocases but only one putative translocase belonging to the MATE family of translocases. In addition to the four known OEPs or TolC homologs (HP0605, HP0971, HP1327, and HP1489), other OEPs were not identified. We aimed to determine whether efflux systems contribute to antibacterial compound susceptibility by evaluation of the susceptibility profiles after insertional inactivation of the MATE translocase and each of the four TolC homologs. Overlapping substrate specificity was further assessed by the construction of a double-knockout mutant by insertional inactivation of both HP0605 and HP0971.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
H. pylori strain 1061 (12) was cultured on Columbia agar medium plates containing 7% lysed horse blood (Rottier, Kloosterzande, The Netherlands). The bacteria were grown at 37°C under microaerobic conditions (5% O2, 10% CO2, 85% N2) by using CampyPAK pouches (Fisher Scientific, Pittsburgh, Pa.) for 48 h. Putative multidrug efflux-knockout mutants of H. pylori were grown on horse blood agar plates containing 50 μg of kanamycin (KAN) per ml. An HP0605 and HP0971 double-knockout mutant was grown on horse blood agar plates containing 50 μg of KAN per ml and 10 μg of chloramphenicol (CHL) per ml.
DNA techniques.
All standard DNA techniques, transformation of Escherichia coli, and DNA analysis procedures were performed as described by Ausubel (5). Plasmid DNA was isolated by use of a Wizard Plus SV miniprep kit (Promega, Mannheim, Germany). All restriction endonucleases were obtained from Roche (Palo Alto, Calif.). H. pylori DNA isolation and natural transformation of H. pylori 1061 were performed essentially as described previously (1, 31).
H. pylori 1061 knockout mutants for (putative) efflux proteins were constructed by insertion of the KAN resistance cassette from pJMK30 (30) into HP0605, HP0971, HP1184, HP1327, and HP1489. An HP0605 and HP0971 double-knockout mutant was made by natural transformation of the KAN-resistant HP0605-knockout strain with pBSII KS+ containing an internal HP0971 fragment interrupted with a cat cassette from pAV35 (kindly provided by A. van Vliet), with selection for both KAN- and CHL-resistant colonies. Insertion of the KAN and/or cat resistance cassette at the desired location(s) in the H. pylori putative efflux genes was confirmed by PCR (Table 1).
TABLE 1.
Primers used in this study
| Primer namea | Locus name (reference) | Sequence (5′-3′)b | Amplicon size (bp)c |
|---|---|---|---|
| HP0605f | hefA (6) | ACGCCTCGAGTAAAAGCGCAAGGGAATTTG | 410 |
| HP0605r | hefA | ACGCTCTAGATTCGCTAATTGGCCTAGCAT | |
| HP0971f | hefD (6) | ACGCCTCGAGAAACCAACGTGGAAACCAAA | 402 |
| HP0971r | hefD | ACGCTCTAGACGCTTTGCAAATCCAAGAAT | |
| HP1184f | ACGCTCTAGACTCAAAGCACGGCAGAATTT | 445 | |
| HP1184r | ACGCCTCGAGGAACTTTTGGTGCGTTGGAT | ||
| HP1327f | hefG (6) | ACGCCTCGAGCGCTATCTGCCAGCCATTAT | 327 |
| HP1327r | hefG | ACGCTCTAGATTTGCTCCACCAATTTAGCC | |
| HP1489f | ACGCCTCGAGCAAAAAGCCACCAACCAGAT | 462 | |
| HP1489r | ACGCTCTAGAATAAGCCACTTGAGCGCCTA |
The frxA (HP0642) and rdxA (HP0954) genes were amplified with primer pairs frx1-frx4 and rdx1-rdx4, respectively (17). The amplicons were sequenced by using a Thermo Sequenase II dye terminator sequencing premix kit (Amersham, Uppsala, Sweden), according to the instructions by the manufacturer, by using the same primers. Sequences were obtained with an ABI Prism 3100 automated sequencer (Perkin-Elmer, Norwalk, Conn.), and sequence analysis was performed with a CodonCode Corporation (Dedham, Mass.) aligner (version 1.2.4).
Assessment of susceptibilities to antimicrobials.
H. pylori cells grown for 48 h on horse blood agar plates were resuspended in phosphate-buffered saline (Life Technologies, Gaithersburg, Md.). Suspensions of H. pylori were adjusted to an optical density at 540 nm of 0.65. Of these suspensions, 100 μl, which contained approximately 107 CFU/ml, was spread on horse blood agar plates. The MICs of CHL, erythromycin, gentamicin, MTZ, TET, trimethoprim, and vancomycin were determined by Etest (AB Biodisk, Solna, Sweden). Susceptibilities to acriflavine (10 μg), ethidium bromide (100 μg), nalidixic acid (10 μg), nickel chloride (1.2 mg), polymyxin B sulfate (1 mg), sodium dodecyl sulfate (1 mg), and sodium deoxycholate (1 mg) were determined on 6-mm Whatman (Maidstone, United Kingdom) disks. Susceptibility to novobiocin was measured with a 5-μg novobiocin disk, and susceptibility to norfloxacin was measured with a 10-μg norfloxacin disk (Oxoid, Basingstoke, United Kingdom). The plates were incubated for 48 to 72 h under microaerobic conditions.
RT-PCR.
RNA from H. pylori 1061 and 11 clinical isolates recovered from biopsy specimens (Department of Gastroenterology and Hepatology, Academic Medical Center, Amsterdam, The Netherlands) was isolated with Trizol reagents (Promega) with an additional phenol-chloroform extraction. Prior to reverse transcriptase (RT) PCR, the RNA samples were treated with RQ1 RNase-free DNase (Promega), followed by another phenol-chloroform extraction. RT-PCR was performed according to the instructions for the Superscript First Strand Synthesis system (Life Technologies). The primers used in the RT-PCR are given in Table 1.
RESULTS
Putative multidrug efflux pumps in H. pylori.
In the H. pylori 26695 genome, 27 putative translocases were identified by assessment of the UniProt (Universal Protein Resource) database (3) with InterPro accession numbers IPR003439 (ABC family of transporters), IPR007114 (MFS translocases), IPR001936 (RND family of transporters), and IPR002584 (MATE family of transporters). While the families of ABC transporters, RND transporters, and MFS translocases are represented by more than one example in the H. pylori genome, only one representative of the MATE family of translocases, HP1184, was identified. This putative translocase displays a high degree of similarity (E value, 9e−51) to NorM, which was first identified in Vibrio parahaemolyticus (20). Since HP1184 is the sole representative of the MATE family of translocases in H. pylori, a unique function not paralleled by those of other translocases was inferred.
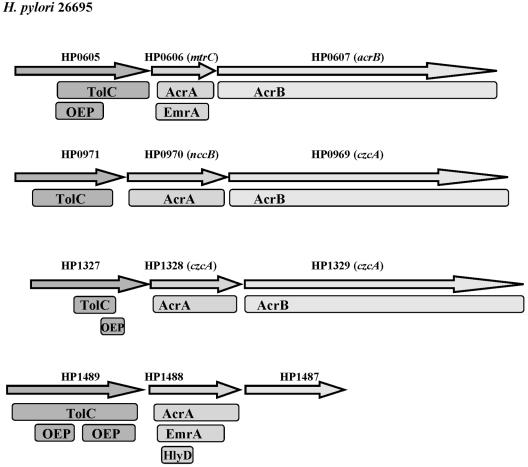
Only four putative H. pylori OEPs or TolC homologs were identified in the UniProt database with accession number IPR003423 (OEP family). These results are consistent with the notion that parallel translocases may function with only a limited number of OEPs (15). Genes encoding a TolC homolog are often found in one cluster with genes encoding an IEP and a PEP. Indeed, the four TolC homolog-encoding genes of H. pylori are located adjacent to genes encoding a putative PEP (AcrA, EmrA, HlyD), while three of them are located in a cluster with genes encoding a putative PEP and an IEP (AcrB) (Fig. 1), emphasizing their potential function as OEPs in efflux systems. Since each of the four TolC homologs may combine with many translocases, their inactivation was presumed to affect the functions of many efflux systems and, hence, resistance to antimicrobials.
FIG. 1.
Genetic organization of putative H. pylori drug efflux proteins. Similarities with OEPs (TolC), PEPs (AcrA, EmrA, HlyD), and IEPs (AcrB) are indicated for proteins with E values <10−5 in a search of the sequences in the National Center for Biotechnology Information Conserved Domain Database (18) with the RPS BLAST program.
Genes encoding the TolC and NorM homologs are transcribed in vitro.
Transcription of the genes encoding putative efflux proteins for which knockout mutants were constructed was assessed by RT-PCR with cDNA obtained from 12 different H. pylori strains. All 12 strains displayed transcripts for HP0605, HP0971, HP1184, HP1327, and HP1489, confirming the in vitro expression of these genes (data not shown).
Active efflux systems in H. pylori revealed by susceptibility profiles of the HP1184-, HP0605-, and HP1489-knockout mutants.
The susceptibilities of each of the five H. pylori mutants and their wild-type strain to CHL, erythromycin, gentamicin, TET, trimethoprim, and vancomycin were identical. However, an HP0605-knockout mutant of H. pylori was more susceptible to both novobiocin and deoxycholate than wild-type strain H. pylori 1061, while the HP1489-knockout and the HP1184-knockout mutants displayed increased susceptibilities to the dye ethidium bromide (Table 2). Independent transformation events resulted in identical susceptibility profiles for each of the knockout mutants.
TABLE 2.
Susceptibilities of H. pylori 1061 and knockout mutants for putative multidrug efflux proteins to toxic compounds
| Compound | Susceptibility of H. pylori (growth inhibition zone [mm])a
|
||||||
|---|---|---|---|---|---|---|---|
| Wild type | Wild type with the following inactivated gene:
|
||||||
| HP0605 | HP0971 | HP1184 | HP1327 | HP1489 | HP0605 and HP0971 | ||
| Acriflavine | 10 | 11 | 11.5 | 11.5 | 11 | 11 | 11.5 |
| Ethidium bromide | 9 | 9 | 8.5 | 18 | 8 | 18 | 9 |
| Nalidixic acid | 8.5 | 8.5 | 7 | 7 | 7 | 7 | 8.5 |
| Nickel chloride | 13 | 13 | 12 | 13 | 13 | 13 | 13 |
| Norfloxacin | 20 | 20 | 21 | 21 | 20 | 20 | 21 |
| Novobiocin | 0 | 10 | 0 | 0 | 0 | 0 | 10 |
| Polymyxin B sulfate | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Sodium dodecyl sulfate | 5.5 | 5.5 | 5.5 | 5 | 5 | 5 | 5.5 |
| Sodium deoxycholate | 4 | 11 | 4 | 4 | 4 | 4 | 11 |
HP numbers correspond to the H. pylori 26695 genome sequence (28) and indicate in which gene the KAN and/or cat resistance gene cassette(s) was inserted. Values in boldface are markedly different from the value for the H. pylori 1061 wild-type control strain.
Simultaneous inactivation of HP0605 and HP0971 confer MTZ resistance in H. pylori.
Similar to parallel acting translocases, some of the four OEPs could also form efflux systems with overlapping specificities. To examine this hypothesis, a double-knockout mutant was constructed in which both HP0605 and HP0971 were inactivated. This double-knockout mutant was, like the HP0605 single-knockout mutant, more susceptible to novobiocin and sodium deoxycholate (Table 2). In addition, the susceptibility to MTZ was increased. The MICs of MTZ for wild-type H. pylori strain 1061 and the five single-knockout mutants were >256 μg/ml, while that of the HP0605 and HP0971 double-knockout mutant was only 8 μg/ml. Independent transformation events, in which either the HP0605 or the HP0971 single-knockout mutant was used as the acceptor strain, resulted in HP0605 and HP0971 double-knockout mutants with identical susceptibility profiles, indicating that secondary mutations are not the cause of the loss of high-level MTZ resistance in the double-knockout mutant strain.
In H. pylori, mutations in the rdxA and frxA genes are implicated in MTZ resistance (17). Therefore, the rdxA and frxA sequences of three mutant strains (the HP0605-knockout, HP0971-knockout, and HP0605 and HP0971 double-knockout mutant strains) and their wild-type strain were compared. The sequences of each of the two genes were identical in all four strains, excluding the possibility that the increased susceptibility of the HP0605 and HP0971 double-knockout strain to MTZ was caused by mutations in rdxA or frxA.
DISCUSSION
Efflux systems have been identified in H. pylori, yet the possibility that these systems are implicated in multidrug resistance has not been established previously (6, 21). This study for the first time provides evidence that active efflux systems are involved in H. pylori susceptibility to antimicrobials. In silico, we identified HP1184 as the sole representative of the MATE family of translocases in the H. pylori 26695 genome, which might indicate that its activity is not covered by other translocases. The H. pylori HP1184-knockout strain was more susceptible to ethidium bromide, indicating that HP1184 is involved in efflux of this agent. HP1184 encodes a putative protein similar to the multidrug resistance protein NorM (20). Transformation of a hypersensitive E. coli strain with V. parahaemolyticus norM conferred resistance to norfloxacin and ethidium bromide, and elevated efflux of ethidium bromide was observed (20). In Erwinia amylovora NorM mediates resistance to hydrophobic cationic compounds like norfloxacin and ethidium bromide (7). Similarly, expression of Burkholderia vietnamiensis NorM in an E. coli acrAB mutant strain complemented its hypersensitivity to norfloxacin (11). Thus, H. pylori HP1184 encodes a NorM homolog that actively extrudes ethidium bromide in vitro, similar to the homolog found in other bacteria (7, 11, 20). However, the H. pylori HP1184-knockout mutant was not more susceptible to norfloxacin, indicating a difference in substrate specificity.
On the basis of in silico analyses, four putative OEPs (HP0605, HP0971, HP1327, and HP1489) were identified. As in many other bacteria, the number of OEPs in H. pylori 26695 is low compared to the number of putative translocases with which they could possibly interact to form efflux systems (16). Inactivation of HP0605 or HP1489 resulted in H. pylori being more susceptible to novobiocin and deoxycholate or to ethidium bromide, respectively. In addition, the HP0605 and HP0971 double-knockout strain was also more susceptible to MTZ. These results are consistent with the hypothesis that inactivation of one of the putative OEPs would abrogate the function of a group of efflux systems and, thus, would have more of an impact on antimicrobial efflux than inactivation of 1 of the 26 translocases other than HP1184.
The locations of the four OEP-encoding genes in H. pylori, adjacent to genes encoding putative IEPs (ArcA homologs) and PEPs (AcrB homologs), which may function as adaptors and translocators, respectively, is supportive of their actual TolC-like function (15, 27). In E. coli, TolC has been implicated in type I protein secretion as well as drug efflux (2, 8). The export of the hemolysin HlyA is a well-characterized example of type I protein secretion in E. coli (15). TolC operates in protein export in many other bacteria with different protein substrates (15). Usually, genes encoding the protein substrate and the export system are located in the same operon (15). Of the four TolC homologs in H. pylori, only HP1489 is found adjacent to a gene, HP1490, that might encode a substrate for type I secretion. HP1490 encodes a putative protein with a high degree of similarity (BLASTP E value, 3e−98) to the hemolysin TlyC of Rickettsia typhi (24). HP1489 is a TolC homolog and was shown to be active in efflux. Therefore, it is tempting to speculate that the outer membrane efflux protein encoded by HP1489 extrudes the product HP1490, presumably a toxin. Besides type I protein secretion, TolC is also involved in the efflux of a large variety of small molecules, including antimicrobials. As mentioned above, TolC is shared by different translocases (15). For example, in E. coli the TolC-AcrAB export system is responsible for the efflux of many antibiotics, dyes, detergents, fatty acids, bile salts, and organic solvents, whereas the TolC-EmrAB efflux system exports hydrophobic uncouplers of oxidative phosphorylation, organomercurials, and antibacterial drugs like nalidixic acid and thiolactomycin (15). E. coli TolC-knockout mutants are hypersensitive to a variety of compounds, including detergents, bile salts, and hydrophobic antibiotics (4). However, in H. pylori, knockout mutants in which one of the TolC homologs was inactivated displayed different susceptibility profiles. The HP0605-knockout mutant appeared to be more susceptible to novobiocin and sodium deoxycholate than the wild type, while the HP1489-knockout mutant was more susceptible to ethidium bromide than the wild type. These results are consistent with the findings obtained with a Haemophilus influenzae TolC-knockout mutant, in which the levels of susceptibility to β-lactams, CHL, TET, and fluoroquinolones were also not increased (25, 29). Moreover, the H. influenzae TolC-knockout mutant was more susceptible to other compounds, including novobiocin and sodium cholate, than its wild-type strain (29).
Current treatment for H. pylori infection includes two or more antibiotics but will ultimately not be sufficient due to increasing rates of antibiotic resistance. Indeed, bacterial resistance to MTZ or clarithromycin hampers current treatments for H. pylori infections. Besides chromosomally encoded drug resistance, intrinsic resistance to toxic compounds through either reduced uptake or increased export might be of importance in multidrug resistance in H. pylori (26). H. pylori strains in which one of the four OEPs was inactivated did not display increased susceptibilities to most of the antimicrobials tested. It is possible that, similar to translocases, their absence could be functionally compensated for by the presence of one of the other TolC-like proteins. Therefore, a double-knockout mutant was constructed in which both HP0605 and HP0971 were inactivated. This double-knockout strain displayed not only increased susceptibility to novobiocin and sodium deoxycholate, like the H. pylori HP0605-knockout mutant, but was also more susceptible to MTZ than wild-type strain H. pylori 1061.
Up to 50% of all strains are resistant to MTZ, including H. pylori 1061 (19). Resistance to MTZ in H. pylori is associated with mutations in rdxA and frxA, which encode an NADPH nitroreductase and oxidoreductase, respectively (17). Hence, the altered susceptibility to MTZ of the HP0605 and HP0971 double-knockout strain could be caused by secondary mutations in one of these two genes or, alternatively, in a gene not yet associated with MTZ susceptibility (17). However, the possibility of secondary mutations in either rdxA or frxA was excluded, since in the wild-type strain, the two single-knockout strains, and the double-knockout strain, the sequences of rdxA and those of frxA were identical. Alteration of MTZ susceptibility due to mutations outside these genes was unlikely, because independent transformation events, as well as transformations in which either single-knockout strain acted as the acceptor strain, all resulted in double-knockout mutants with increased susceptibilities to MTZ. Our findings indicate the presence of efflux systems that act in parallel and that comprise both the putative TolC-like proteins encoded by HP0605 and HP0971, which contribute to decreased MTZ susceptibility.
To our knowledge, this study demonstrates for the first time in H. pylori the involvement of active efflux systems in susceptibility to MTZ, an antibiotic important in anti-H. pylori therapy, which may have clinical relevance. The efflux systems were found to be expressed in multiple H. pylori isolates in vitro. In contrast to our study, Bina and coworkers (6) did not detect the hefGHI operon mRNA in vitro. The in vitro conditions for culture of the samples differed between the two studies, which may explain this difference.
Construction of H. pylori mutants by inactivation of two genes is laborious, whereas the construction of H. pylori mutants in which three or more genes are inactivated may be even more difficult. Nevertheless, inactivation of more than two of the four TolC homologs will possibly reveal their involvement in susceptibilities to other antibiotics as well.
Acknowledgments
We thank Monique Feller for excellent technical assistance and Arnoud van Vliet for providing plasmid pJMK30.
This research was financially supported by The Netherlands Digestive Diseases Foundation.
REFERENCES
- 1.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20:5137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, C. 2003. Channel-tunnels: outer membrane components of type I secretion systems and multidrug efflux pumps of gram-negative bacteria. Rev. Physiol. Biochem. Pharmacol. 147:122-165. [DOI] [PubMed] [Google Scholar]
- 3.Apweiler, R., A. Bairoch, C. H. Wu, W. C. Barker, B. Boeckmann, S. Ferro, E. Gasteiger, H. Huang, R. Lopez, M. Magrane, M. J. Martin, D. A. Natale, C. O'Donovan, N. Redaschi, and L. S. Yeh. 2004. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 32(Database issue):D115-D119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustus, A. M., T. Celaya, F. Husain, M. Humbard, and R. Misra. 2004. Antibiotic-sensitive TolC mutants and their suppressors. J. Bacteriol. 186:1851-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel, F. M. 1999. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 6.Bina, J. E., R. A. Alm, M. Uria-Nickelsen, S. R. Thomas, T. J. Trust, and R. E. Hancock. 2000. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob. Agents Chemother. 44:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burse, A., H. Weingart, and M. S. Ullrich. 2004. NorM, an Erwinia amylovora multidrug efflux pump involved in in vitro competition with other epiphytic bacteria. Appl. Environ. Microbiol. 70:693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva, F. G., Y. Shen, C. Dardick, S. Burdman, R. C. Yadav, A. L. de Leon, and P. C. Ronald. 2004. Bacterial genes involved in type I secretion and sulfation are required to elicit the rice Xa21-mediated innate immune response. Mol. Plant-Microbe Interact. 17:593-601. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton, K. A., D. Kersulyte, M. Mefford, S. J. Danon, S. Krakowka, and D. E. Berg. 2001. Role of Helicobacter pylori cag region genes in colonization and gastritis in two animal models. Infect. Immun. 69:2902-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehlner-Gardiner, C. C., and M. A. Valvano. 2002. Cloning and characterization of the Burkholderia vietnamiensis norM gene encoding a multi-drug efflux protein. FEMS Microbiol. Lett. 215:279-283. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin, A., D. Kersulyte, G. Sisson, S. J. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, J. M., and G. M. Church. 1999. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J. Mol. Biol. 287:695-715. [DOI] [PubMed] [Google Scholar]
- 14.Kearney, D. J. 2004. Helicobacter pylori infection. Curr. Options Infect. Dis. 5:197-206. [Google Scholar]
- 15.Koronakis, V. 2003. TolC—the bacterial exit duct for proteins and drugs. FEBS Lett. 555:66-71. [DOI] [PubMed] [Google Scholar]
- 16.Koronakis, V., J. Eswaran, and C. Hughes. 2004. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu. Rev. Biochem. 73:467-489. [DOI] [PubMed] [Google Scholar]
- 17.Marais, A., C. Bilardi, F. Cantet, G. L. Mendz, and F. Megraud. 2003. Characterization of the genes rdxA and frxA involved in metronidazole resistance in Helicobacter pylori. Res. Microbiol. 154:137-144. [DOI] [PubMed] [Google Scholar]
- 18.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Megraud, F. 1998. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology 115:1278-1282. [DOI] [PubMed] [Google Scholar]
- 20.Morita, Y., K. Kodama, S. Shiota, T. Mine, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 42:1778-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, S., A. Ward, C. J. Hoyle, and P. J. Henderson. 2003. Cloning, expression, purification and properties of a putative multidrug resistance efflux protein from Helicobacter pylori. Int. J. Antimicrob. Agents 22:242-249. [DOI] [PubMed] [Google Scholar]
- 22.Nikaido, H. 1998. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin. Infect. Dis. 27(Suppl. 1):S32-S41. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen, I. T., J. Chen, K. E. Nelson, and M. H. Saier, Jr. 2001. Comparative genomics of microbial drug efflux systems. J. Mol. Microbiol. Biotechnol. 3:145-150. [PubMed] [Google Scholar]
- 24.Radulovic, S., J. M. Troyer, M. S. Beier, A. O. Lau, and A. F. Azad. 1999. Identification and molecular analysis of the gene encoding Rickettsia typhi hemolysin. Infect. Immun. 67:6104-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez, L., W. Pan, M. Vinas, and H. Nikaido. 1997. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J. Bacteriol. 179:6855-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweizer, H. P. 2003. Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genet. Mol. Res. 2:48-62. [PubMed] [Google Scholar]
- 27.Tikhonova, E. B., and H. I. Zgurskaya. 2004. AcrA, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J. Biol. Chem. 279:32116-32124. [DOI] [PubMed] [Google Scholar]
- 28.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fuji, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karpk, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 29.Trepod, C. M., and J. E. Mott. 2004. Identification of the Haemophilus influenzae tolC gene by susceptibility profiles of insertionally inactivated efflux pump mutants. Antimicrob. Agents Chemother. 48:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Vliet, A. H., K. G. Wooldridge, and J. M. Ketley. 1998. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J. Bacteriol. 180:5291-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y., K. P. Roos, and D. E. Taylor. 1993. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. Gen. Microbiol. 139:2485-2493. [DOI] [PubMed] [Google Scholar]